Abstract
Hyponatremia is a common complication in patients with cirrhosis that is related to impairment in the renal capacity to eliminate solute-free water. The main pathogenic factor associated with hyponatremia is a non-osmotic hypersecretion of vasopressin which acts in the V2-receptors in the renal collecting ducts. A decrease in serum sodium concentration is associated with an increased risk of hepatic encephalopathy. Hyponatremia is associated with poor prognosis in patients with cirrhosis. There is evidence that hyponatremia also represents a risk factor for liver transplantation, as it is associated with an increased frequency of complications and a decreased short-term survival after transplantation. Classical treatment of hyponatremia is based on fluid restriction, but is poorly effective. The current pharmacological approach to hyponatremia has advanced with vaptans, a new family of drugs which antagonize V2-receptors in the renal collecting ducts. Short-term treatment with vaptans is associated with an increase in serum sodium concentration and improvement in health-related quality of life. Nevertheless, information on the use of vaptans in cirrhosis is limited and further long-term studies are needed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hepatic Encephalopathy
- Serum Sodium
- Serum Sodium Level
- Serum Sodium Concentration
- Await Liver Transplantation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Patients with end-stage liver disease and ascites have a functional renal impairment that render the kidney susceptible to retain sodium and solute-free water [1]. In some patients, this disorder leads to a disproportionate retention of water relative to sodium which leads to a dilutional state where water is retained out of proportion to sodium causing hyponatremia and hypoosmolality. Hyponatremia in the general population is defined as a serum sodium level below 135 mEq/L [2]. However, hyponatremia in cirrhosis has been defined as a serum sodium concentration of less than 130 mEq/L in the presence of ascites or edema [3–5]. A significant proportion of patients with cirrhosis have a serum sodium concentration above 130 mEq/L and below 135 mEq/L; however these patients may display pathogenic and clinical features similar, yet less pronounced, to those of patients with serum sodium below 130 mEq/l. In patients with cirrhosis and ascites the 5-year probability of developing hyponatremia is 37 % with a 25 % probability of survival at 1 year [6]. It is estimated that 22 % of patients with advanced cirrhosis have serum sodium levels <130 mEq/L; however in patients with refractory ascites or HRS, this proportion may increase to more than 50 % [7]. In the majority of patients, hyponatremia occurs in close association with an impairment of renal function and correlates with poor prognosis. Recent studies also indicate that hyponatremia is an important marker of prognosis in patients with cirrhosis awaiting liver transplantation and may be associated with an increased morbidity, particularly neurological complications, and reduced survival after transplantation [8–11]. In addition a number of studies have also demonstrated that the incorporation of serum sodium can improve the predictive accuracy of the Model for End-Stage liver disease (MELD) score in patients listed for liver transplantation [12–14].
Types of Hyponatremia
Patients with cirrhosis may develop either hypervolemic or hypovolemic hyponatremia. Hypervolemic or dilutional hyponatremia, is by far the most common type that occurs in patients with cirrhosis and it occurs in the setting of an expanded extracellular fluid and plasma volume. Hypervolemic hyponatremia in cirrhosis is due to a marked impairment in the renal capacity to eliminate solute-free water leading to disproportionate water retention with respect to sodium retention. It may occur spontaneously or as a consequence of excessive hypotonic fluids (for example, by giving an undue amount of iv hypotonic fluids—5 % dextrose—during a hospitalization) or other complications of cirrhosis such as in the setting of some bacterial infections [15]. By contrast, hypovolemic hyponatremia is less common and is due to significant losses of extracellular fluid, particularly from the kidney due to overdiuresis from diuretic treatment or from gastrointestinal tract. Hypovolemic hyponatremia is characterized by a reduction of plasma volume, lack of ascites and/or edema, signs and dehydration, and prerenal renal failure. Most patients with hypovolemic hyponatremia show an improvement of serum sodium levels after the administration of normal saline or by increasing sodium content in the diet temporarily. In this chapter we will focus on the pathogenesis and treatment of hypervolemic hyponatremia.
Pathogenesis
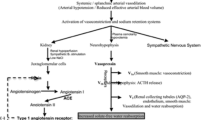
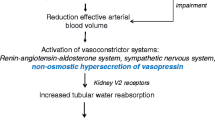
The pathogenesis of increased solute-free water retention in cirrhosis is intricate and involves several factors, including high levels of arginine vasopressin (AVP), reduced synthesis of renal prostaglandins, and reduced delivery of filtrate to the ascending limb of the loop of Henle [1, 3, 4]. Among these, AVP is the most important factor in the pathogenesis of water retention in patients with cirrhosis and ascites [16]. In cirrhosis, splanchnic vasodilation leads to arterial underfilling which unloads high-pressure baroreceptors that stimulate a non-osmotic hypersecretion of AVP leading to solute-free water retention and hyponatremia (Fig. 8.1) [16]. The physiological actions of AVP are exerted through three types of receptors present in target cells throughout the body [17]. These receptors are G protein-coupled receptors known as V1a, V1b, and V2 receptors. V1a and V1b are associated to the phosphoinositol signaling pathway with intracellular calcium as second messenger. V1a is responsible for vascular smooth muscle cell contraction, platelet aggregation, and hepatic glycogenolysis and V1b is expressed in the anterior pituitary where it intervenes in adrenocorticotropin release [17].
Proposed pathogenesis of hypervolemic hyponatremia in cirrhosis. There is activation of the renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system (SNS) and a non-osmotic hypersecretion of arginine vasopressin (AVP) due to decreased effective arterial blood volume that activates baroreceptors and stimulates the hypothalamic release of AVP causing renal solute-free water retention through the action of V2 receptors and arterial vasoconstriction through the action of V1 receptors
The V2 receptors are located on the basolateral (capillary) membrane of the principal cells of the kidney collecting ducts and are responsible for the AVP-induced solute-free water reabsorption [3, 16, 17]. The effect of AVP in the kidney collecting duct occurs by means of specific water channels called aquaporins (AQP). The most important one in solute-free water retention is AQP2. This water channel has been characterized in human and rat kidneys and is expressed almost exclusively in the principal cells of the collecting ducts [18, 19]. The binding of AVP to the V2 receptor stimulates adenyl cyclase via the stimulatory G protein and promotes the formation of cyclic AMP (cAMP). This cAMP binds to a regulatory subunit of protein kinase A, which in turn phosphorylates AQP2, which is then translocated from vesicular bodies present in the cytosol to the luminal (apical) plasma membrane of the collecting duct cells and acts as a water channel thereby increasing water permeability [3]. The water entering the cell by the luminal plasma membrane leaves the cell through the basolateral membrane and enters the capillaries in contact with the tubular cells. Data from patients with cirrhosis and hypervolemic hyponatremia in whom V2 receptor antagonists of AVP (vaptans) were administered indicate that hypersecretion of AVP plays a major role in the development of hyponatremia because these drugs induced an increase in serum sodium concentration in a large proportion (60–70 %) of patients [20]. However, there are a number of patients in whom serum sodium levels do not increase with vaptans which suggests that other mechansims involved in solute-free water retetion play an important role in the pathogenesis of hypervolemic hyponatremia in cirrhosis.
Clinical Features
There is not a lot of information on the clinical consequences of hypervolemic hyponatremia in cirrhosis. This is because hyponatremia occurs in the setting of advanced liver failure and patients may present with a range of nonspecific symptoms attributed to their underlying cirrhosis. Therefore, the precise recognition of specific clinical consequences due to hyponatremia in cirrhosis has not been possible. This has been further flawed by the lack of effective treatments for hyponatremia.
Neurological Features
In patients without liver disease, hyponatremia is primarily associated with a wide range of neurological manifestations related to the existence of brain edema, such as headache, confusion, focal neurological deficits, seizures, and, in some cases, death due to cerebral herniation [2]. The severity of neurological symptoms in patients with hyponatremia without liver disease correlates with the levels of osmolality and sodium in the extracellular fluid. Nevertheless, rather than the absolute reduction in serum sodium levels, the most important factor in determining the severity of neurological symptoms is the rate of fall in serum sodium levels [2]. Patients with acute hyponatremia have a much higher incidence of neurological symptoms than those with chronic hyponatremia.
There are no studies that have specifically evaluated neurological symptoms in patients with cirrhosis and hyponatremia. However, clinical experience indicates that neurological manifestations such as headache, focal deficits, seizures, and cerebral herniation are very uncommon. It is likely that the relatively low incidence of neurological manifestations in patients with cirrhosis and dilutional hyponatremia is related to the fact that most of these patients have chronic hyponatremia, and this gives sufficient time for brain adaptation to hypoosmolality. In most patients with cirrhosis, hyponatremia is asymptomatic, but some data indicate that hyponatremia is associated with a higher risk of hepatic encephalopathy [21–23]. The mechanism by which hyponatremia is associated with hepatic encephalopathy is likely due to changes in serum osmolality that lead to astrocyte swelling and then cellular release of solutes as a response to prevent cell swelling and cerebral edema (Fig. 8.2). These changes are relevant because the underlying pathogenesis of hepatic encephalopathy in cirrhosis is felt to be based on the fact that ammonia and other toxins induce a low-grade cerebral edema due to astrocyte swelling secondary to increased intracellular levels of glutamine that alter astrocyte function [24]. Consequences of astrocyte swelling include alterations in gene expression and oxidative stress that alter glioneuronal communication and disturb neurological function, leading to encephalopathy [24, 25]. Thus the presence of hyponatremia in combination with hyperammonemia, by favoring astrocyte swelling, may increase the risk of hepatic encephalopathy.
Proposed interaction between hyperammonemia and hyponatremia on brain astrocytes and possible pathogenic relationship with hepatic encephalopathy. Reproduced from reference [4] with permission from John Wiley & Sons, Inc.
Complications of Cirrhosis
Aside from hepatic encephalopathy, hyponatremia is also associated with other complications of cirrhosis, yet information is limited. Hyponatremia is a frequent finding in patients with cirrhosis and bacterial infections. In the majority of patients, hyponatremia occurs in close association with renal failure and correlates with poor prognosis [15, 26]. Moreover, it is important to note that patients with ascites and hyponatremia constitute a population with a very high risk of developing hepatorenal syndrome [27]. On the other hand, low serum sodium levels are a very common finding in patients with hepatorenal syndrome.
Information on the impact of hyponatremia on health-related quality of life in patients both with and without liver disease is limited. In patients with cirrhosis, hyponatremia impairs quality of life because patients require a restriction of daily fluid intake to prevent further reductions in serum sodium concentration, and this is usually poorly tolerated. Moreover, in a recent study in a large population of patients with cirrhosis and ascites, hyponatremia was an independent predictive factor of the impaired health-related quality of life [28].
Management of Hyponatremia
The first step in the management of hyponatremia in cirrhosis is to identify whether hyponatremia is hypovolemic or hypervolemic, because the management is completely different according to the type of hyponatremia. Diuretic treatment should be stopped in all patients, because diuretics may reduce serum sodium levels [5]. The management of hypovolemic hyponatremia consists on the identification and treatment of the cause of sodium loss together with the administration of sodium (either regular saline i.v. or diet with normal sodium content) [5].
A key aspect in the management of hypervolemic hyponatremia is to increase renal solute-free water excretion with the aim of reducing the increased total body water. The advantages of treating hypervolemic hyponatremia in cirrhosis are the following: (1) the reversal of hyponatremia may allow to avoid fluid restriction, (2) since hyponatremia has been shown to be a predisposing factor to hepatic encephalopathy, the improvement of serum sodium concentration may help reduce the risk of encephalopathy and (3) in patients awaiting liver transplantation, the normalization of serum sodium concentration before transplantation may reduce the risk of neurological complications after transplantation. The available therapeutic methods for the management of hypervolemic hyponatremia are summarized below.
Fluid and Water Restriction
Fluid restriction is still considered the first step in the management of hypervolemic hyponatremia [5]. There are no studies specifically assessing the effectiveness of fluid restriction in this setting; but in some cases it is helpful in preventing a progressive decrease in serum sodium levels. Clinical experience indicates that it rarely increases serum sodium concentration in a significant manner. This lack of efficacy is likely due to the fact that in practice total daily fluid intake cannot be restricted to less than 1 L per day, an amount that is generally insufficient to cause a markedly negative fluid balance.
Sodium Chloride
The use of intravenous hypertonic sodium chloride in cirrhosis has not been investigated in randomized studies. Hypertonic sodium chloride has a very partial and short-lived effect in improving serum sodium concentration in cirrhosis perhaps because it has no effect on renal solute-free water excretion. Moreover, it has a major drawback; that is, increasing ascites and edema due to the severe sodium retention present in these patients because of the large amount of sodium given.
Albumin
Two short-term studies including a low number of patients suggest that the administration of albumin improves serum sodium concentration in patients with hypervolemic hyponatremia [29, 30]. This beneficial effect of albumin is probably related to an improvement in circulatory function with suppression of several sodium and water-retaining systems, including AVP. Although an attractive therapy, the effects were studied in only 1 week and the changes therefore short lived. The use of albumin for hyponatremia, although probably impractical due to the need of daily intravenous administration, could be further investigated in a subset of patients that would benefit from a short-term therapy (i.e., patients with very advanced liver disease awaiting liver transplantation). Further studies in larger series of patients and for prolonged periods of time are needed to assess the potential benefits of albumin administration on hypervolemic hyponatremia in cirrhosis.
AVP Antagonists: The Vaptans
The pharmacological approach to treatment of hypervolemic hyponatremia was revamped with the introduction of vaptans. These drugs are active orally and cause a selective blockade of the V2-receptors of AVP in the principal cells of the collecting ducts [31]. In healthy subjects, the administration of vaptans induces a marked and dose-dependent increase in urine volume with low urine osmolality due to a marked increase in solute-free water excretion, but without an increase in urinary sodium excretion. Randomized, double-blind, comparative studies indicate that treatment with vaptans for a short period of time (up to 1 month), including tolvaptan, lixivaptan, and satavaptan, improves serum sodium concentration in patients with cirrhosis and hypervolemic hyponatremia [32–36]. A small study suggests that intravenous conivaptan, a vaptan that is not only an antagonist of the V2 receptors but also of the V1 receptors of AVP, is also effective in patients with cirrhosis and hyponatremia [37]. The increase in serum sodium concentration occurs within the first 7 days of treatment and normalization of serum sodium concentration has been observed in up to 80 % of patients [32–36] (Table 8.1). Moreover, in approximately one-third of additional patients, serum sodium increases more than 5 mEq/L but does not reach values >130 mEq/L. Therefore, vaptans are effective in the short-term treatment of hypervolemic hyponatremia in patients with cirrhosis.
It should be mentioned that treatment with vaptans has been assessed for the management of ascites in cirrhosis. Specifically, satavaptan was evaluated for the treatment of ascites in association with diuretics with the rationale that by increasing diuresis the vaptan would help manage ascites and prevent its recurrence. Although results of phase-2 studies were promising [38], phase-3 long-term treatment studies in three different populations of patients with cirrhosis and ascites demonstrated a lack of efficacy in both, ascites management and prevention of its recurrence [39]. Moreover, use of satavaptan was associated with an increased mortality in one of the studies but not in the other two and the drug was withdrawn from development. The reason for this increased mortality could not be elucidated. It is not known if this increased mortality during long-term treatment is a class effect or is exclusively related to satavaptan. A small study in 18 patients with cirrhosis and ascites without hyponatremia showed that the administration of tolvaptan dose dependently decreased body weight and improved ascites and edema, however the results of this observation needs to be further studied in large cohorts of patients before considering this agent a treatment for patients with ascites [40].
The most frequent side-effect reported in studies evaluating the vaptans in patients with hyponatremia is thirst, which is related to the pharmacodynamic actions of these drugs. Potential theoretical concerns of the administration of vaptans in patients with cirrhosis are dehydration and hypernatremia and renal failure due to depletion of the intravascular volume. In short-term studies, hypernatremia (serum sodium >145 mmol/l) occurred in only 2–4 % of patients with cirrhosis treated with vaptans [32–36]. Nevertheless, the frequency of this complication may be higher if patients are treated with high doses of vaptans. An important concern is to avoid a rapid increase in serum sodium that could lead to neurological complications due to osmotic demyelination syndrome. In double-blind studies, an increase greater than 8 mEq/L per day within the first days of therapy has been reported with low and similar frequency in patients treated with vaptans compared to patients treated with placebo, ranging from 4 to 14 % in different studies [32–34]. More importantly, osmotic demyelination syndrome has not been reported. It should be noted, however, that in all studies patients were treated in the hospital for the first 2 days of therapy, had free access to water, and followed strict protocols with daily measurement of serum sodium during the first days of therapy and temporary interruption of drug administration in patients in whom serum sodium increased more than 8 mEq/L per day. In short-term studies, no significant impairment of renal and circulatory function was found in vaptan-treated groups compared to placebo [35, 36]. Nonetheless, it should be pointed out that in these studies patients were treated for short periods of time, under strict clinical and analytical surveillance, and with low doses of diuretics. Therefore, it is not known whether the frequency of renal impairment could be higher under different conditions. Finally, vaptans are metabolized by CYP3A enzymes in the liver; therefore drugs or substances that are strong inhibitors of CYP3A such as ketoconazole, grapefruit juice, and clarythromycin among others, increase the exposure to vaptans and may be associated with larger increases in serum sodium concentration. By contrast, drugs that are inducers of the CYP3A system, such as rifampicin, barbiturates, and phenytoin, may decrease the effectiveness of vaptans.
The only vaptans currently approved for clinical use are tolvaptan, conivaptan, and mozavaptan. Tolvaptan is approved in USA for the management of severe (<125 mEq/L) hypervolemic hyponatremia and in Europe for the management of SIADH. Conivaptan is also approved in the USA for the short-term (5-day) intravenous treatment of hypervolemic hyponatremia. Treatment of tolvaptan is started with 15 mg/day and titrated progressively to 30 and 60 mg/day, if needed, according to the desired changes in serum sodium concentration. The safety and efficacy of tolvaptan has only been reported for a short-treatment period (30 days) and the results indicate that mean serum sodium levels increased during the first 7 days and were maintained above 130 mEq/L during 30 days and levels dropped after the medication was discontinued (Fig. 8.3) [36]. In addtion, tolvaptan improved health-related quality of life in patients with cirrhosis and hypervolemic hyponatremia [36]. Very limited information exists on the effects of tolvaptan on serum sodium concentration for longer periods of time [41]. In randomized studies, a slightly increased frequency of gastrointestinal bleeding was reported in patients with cirrhosis and hyponatremia receiving tolvaptan compared to that in patients treated with placebo. This would require evaluation in future studies. Thus, studies assessing the efficacy and safety of long-term treatment with tolvaptan in patients with cirrhosis and hyponatremia are needed. On the basis of available evidence, the recommendations for the management of hypervolemic hyponatremia in cirrhosis are summarized in Table 8.2. Candidate patients to treatment with vaptans are patients with severe hyponatremia (<125 mEq/L) awaiting transplantation. Use of vaptans in patients not candidates to transplantation should be individualized in each case.
Observed serum sodium concentration in patients with hyponatremia that received tolvaptan or placebo for 30 days and 7 days after stopping (day 37) Error bars are ± SE. *P < 0.001, tolvaptan vs. placebo; † P < 0.01, tolvaptan vs. placebo; ‡ P < 0.05, tolvaptan vs. placebo. Reproduced from reference [36] with permission from Elsevier
References
Ginès P, Cárdenas A, Schrier R. Liver disease and the kidney. In: Schrier R, editor. Diseases of the kidney and urinary tract. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 2179–205.
Adrogue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–9.
Ginès P, Berl T, Bernardi M, et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology. 1998;28:851–64.
Ginès P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance and management. Hepatology. 2008;48:1002–10.
Ginès P, Angeli P, Lenz K, Möller S, Moore K, Moreau R, et al. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417.
Planas R, Montoliu S, Ballesté B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385–94.
Angeli P, Wong F, Watson H, Gines P. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology. 2006;44:1535–42.
Biggins S, Rodriguez HJ, Bachetti P, et al. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32–9.
Londoño MC, Cardenas A, Guevara M, et al. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut. 2007;56:1283–90.
Londoño MC, Guevara M, Rimola A, et al. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology. 2006;130:1135–43.
Dawwas MF, Lewsey JD, Neuberger J, et al. The impact of serum sodium concentration on mortality after liver transplantation: a cohort multicenter study. Liver Transpl. 2007;13:1115–24.
Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–26.
Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–60.
Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–43.
Pereira G, Guevara M, Fagundes C, Solá E, Rodríguez E, Fernández J, et al. Renal failure and hyponatremia in patients with cirrhosis and skin and soft tissue infection. A retrospective study. J Hepatol. 2012;56:1040–6.
Ishikawa S, Schrier RW. Pathogenesis of hyponatremia: the role of arginine vasopressin. In: Ginès P, Arroyo V, Rodes J, Schrier R, editors. Ascites and renal dysfunction in liver disease. 2nd ed. Oxford: Blackwell; 2005. p. 305–14.
Thibonnier M, Conarty DM, Preston JA, et al. Molecular pharmacology of human vasopressin receptors. Adv Exp Med Biol. 1998;449:251–76.
Kwon TH, Hager H, Nejsum LN, et al. Physiology and pathophysiology of renal aquaporins. Semin Nephrol. 2001;21:231–8.
Nielsen S, Frokiaer J, Marples D, et al. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–44.
Elhassan EA, Schrier RW. Hyponatremia: diagnosis, complications, and management including V2 receptor antagonists. Curr Opin Nephrol Hypertens. 2011;20:161–8.
Guevara M, Baccaro ME, Torre A, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. 2009;104:1382–9.
Riggio O, Angeloni S, Salvatori FM, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738–46.
Guevara M, Baccarro ME, Rios J, et al. Risk factors for hepatic encephalopathy in patients with cirrhosis and refractory ascites: relevance of serum sodium concentration. Liver Int. 2010;30:1137–42.
Haussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57:1156–65.
Córdoba J, García-Martinez R, Simón-Talero M. Hyponatremic and hepatic encephalopathies: similarities, differences and coexistence. Metab Brain Dis. 2010;25:73–80.
Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: course, predictive factors and prognosis. Hepatology. 1994;20:1495–501.
Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, et al. Incidence, predictive factors, and prognosis of hepatorenal syndrome in cirrhosis. Gastroenterology. 1993;105:229–36.
Solà E, Guevara M, Rodriguez E, Barreto R, Pavesi M, Arroyo V, et al. Hyponatremia is a major factor of the impaired health-related quality of life in patients with cirrhosis and ascites. J Hepatol. 2012;57:1199–206.
Jalan R, Mookerjee R, Cheshire L, Williams R, et al. Albumin infusion for severe hyponatremia in patients with refractory ascites: a randomized clinical trial. J Hepatol. 2007;46:232A.
McCormick PA, Mistry P, Kaye G, Burroughs AK, McIntyre N. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut. 1990;31:204–7.
Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet. 2008;371:1624–32.
Wong F, Blei AT, Blendis LM, Thuluvath PJ. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: a multicenter, randomized, placebo-controlled trial. Hepatology. 2003;37:182–91.
Gerbes AL, Gulberg V, Ginès P, et al. VPA study group. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology. 2003;124:933–9.
Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–112.
Ginès P, Wong F, Watson H, et al. Effects of satavaptan, a selective vasopressin V2 receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial. Hepatology. 2008;48:204–13.
Cárdenas A, Ginès P, Marotta P, et al. The safety and efficacy of tolvaptan, an oral vasopressin antagonist in the treatment of hyponatremia in cirrhosis. J Hepatol. 2012;56:571–8.
O'Leary JG, Davis GL. Conivaptan increases serum sodium in hyponatremic patients with end-stage liver disease. Liver Transpl. 2009;15:1325–9.
Wong F, Ginès P, Watson H, et al. Effects of a selective vasopressin V2 receptor antagonist, satavaptan, on ascites recurrence after paracentesis in patients with cirrhosis. J Hepatol. 2010;53:283–90.
Wong F, Watson H, Gerbes A, et al. Satavaptan for the management of ascites in cirrhosis. Efficacy and safety across the spectrum of ascites severity. Gut. 2012;61:108–16.
Okita K, Sakaida I, Okada M, Kaneko A, Chayama K, Kato M, et al. A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose-response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol. 2010;45:979–87.
Berl T, Quittnat-Pelletier F, Verbalis JG, Schrier RW, Bichet DG, Ouyang J, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21:705–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Solà, E., Cárdenas, A., Ginès, P. (2013). Hyponatremia in Cirrhosis: Evaluation and Treatment. In: Simon, E. (eds) Hyponatremia. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6645-1_8
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6645-1_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6644-4
Online ISBN: 978-1-4614-6645-1
eBook Packages: MedicineMedicine (R0)