Abstract
When deciding which programs to invest in, public health decision makers face a number of challenges including limited resources, competing objectives (e.g., maximize health, achieve equity), and limited information about uncertain events. Despite these difficulties, public health planners must make choices about which programs they will invest in—and the quality of these choices affects the health benefits achieved in the population. To support good decisions, information about the likely costs and health consequences of alternative interventions is needed. This is where OR-based modeling can play a role: by providing a structured framework that uses the best available evidence, imperfect as it may be, and that captures relevant uncertainties, complexities, and interactions, OR-based models can be used to evaluate the potential impact of alternative public health programs. This chapter describes modeling efforts in which OR has played and can play a role in informing public health decision making. We describe work in three areas: hepatitis B control, HIV control, and bioterrorism preparedness and response. We conclude with a discussion of lessons learned.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Public Health Decision
- Ring Vaccination
- Public Health Decision Maker
- Anthrax Attack
- Public Health Planner
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The goal of public health is to improve lives through the prevention and treatment of disease. Specifically, public health focuses on population-level aspects of health, including disease prevention, infection control, prolongation of life, and promotion of healthy lifestyles. Early efforts in public health focused on sanitation and hygiene (e.g., clean water, sewers, garbage collection), nutrition, and mass inoculation (e.g., for smallpox). Modern public health efforts focus on these and other activities including control of the local and global spread of infectious diseases (e.g., influenza, malaria), control of chronic diseases (e.g., type 2 diabetes), and promotion of healthy behaviors (e.g., antismoking and obesity reduction campaigns).
When deciding which programs to invest in, public health decision makers face a number of challenges. They typically have limited resources to invest among many potential programs. It is never possible to achieve perfect health for everyone in the population, so they must choose where to focus their efforts. Moreover, when evaluating the worth of potential public health programs, they must consider not only the likely costs and health effects of such programs (e.g., cases of disease prevented, lives saved, or quality-adjusted life years gained as a function of resources expended) but also issues of equity and fairness. For example, it may be more expensive and less effective to target programs to certain impoverished or marginalized population groups compared to other segments of the population—but it is likely not acceptable (either politically or ethically) to ignore such groups. Additionally, public health planners frequently must make decisions with limited information about uncertain events. For example, plans for response to pandemic influenza must be made before it is even known whether such a pandemic will occur, what its magnitude may be, and what strain of influenza will predominate. Despite these difficulties, public health planners must make choices about which programs they will invest in—and the quality of these choices affects the health benefits achieved in the population.
To make good decisions, public health decision makers need information about the likely costs and health consequences of alternative interventions. The gold standard for evaluating health interventions is a randomized clinical trial. However, such trials are very often time consuming, expensive, infeasible, or unethical. This is where OR-based modeling can play a role: by providing a structured framework that uses the best available evidence, imperfect as it may be, and that captures relevant uncertainties, complexities, and interactions, OR-based models can be used to evaluate the potential impact of alternative public health programs. Of course, perfect prediction of the impact of interventions is not possible. Thus, the goal of OR-based modeling of potential health decisions must instead be to identify which alternatives are better than others—in other words, to inform good decisions.
This chapter describes modeling efforts in which OR has played and can play a role in informing public health decision making. We describe work in three areas: hepatitis B control, HIV control in Eastern Europe, and bioterrorism preparedness and response. For each area, we describe key policy questions, the types of models we used to inform decision making, and the process of dissemination of results to policy makers. We conclude with a discussion of lessons learned.
2 Hepatitis B Control
Hepatitis B is a blood-borne viral infection that, if untreated, can cause liver disease and cancer [1]. Individuals can acquire the infection at birth (if born to an infected mother), through sharing of blood (e.g., cuts and scrapes, sharing of toothbrushes), through unsafe blood transfusions, or through sexual contact, among other means. Some individuals who are exposed to hepatitis B can resolve the infection (their immune system generates sufficient antibodies such that they become immune to it), but some individuals go on to develop lifelong, chronic infection. Children are particularly vulnerable because the chance that an acute infection becomes chronic is higher for young children than for older children and adults. The chance of an infection becoming chronic for a newborn is approximately 90 %, whereas a 10-year-old exposed to the infection has approximately a 15 % chance of developing chronic infection and for a 20-year-old the chance is 9 % [2]. Approximately one-fourth of chronically infected individuals will die from hepatitis B-related liver disease (cirrhosis or liver cancer). Chronic hepatitis B is a silent infection: infected individuals are typically asymptomatic for decades before symptoms of the disease appear, so they can unknowingly spread the disease to others for many years.
A vaccine for hepatitis B has been available since the mid-1980s. Despite this, approximately 350 million people worldwide are infected with hepatitis B—more than ten times as many as are infected with HIV [3].
2.1 Hepatitis B in China
One-fourth of the world’s hepatitis B cases occur in China, where an estimated 95 million people are chronically infected with hepatitis B [4, 5]. In 2002, the Chinese government included free hepatitis B vaccination for newborns in its national immunization program [4]. Although newborn vaccination rates have been relatively high in urban areas, vaccination coverage in rural areas has lagged behind [6]. It is estimated that 150 million children in China up to the age of 18 remain unprotected against hepatitis B (they have not been vaccinated, nor have they developed antibodies through exposure to the virus) [7].
A demonstration program implemented in the rural Qinghai province in China provided free hepatitis B catch-up vaccination to nearly 500,000 school children between 2006 and 2008 [8]. China’s health ministry wanted to know whether such free catch-up vaccination would be economical to extend to the rest of the 150 million unprotected children in the country.
To inform this decision, we developed a model to evaluate the costs and health benefits of such catch-up vaccination [9, 10]. We considered a representative cohort of 10,000 children of a given age, and we considered different ages from 1 to 19 years old. We considered three strategies: no catch-up vaccination (which is the status quo); catch-up vaccination with no screening (children would be vaccinated unless they could provide evidence of vaccination); and catch-up vaccination with antibody screening (children with no evidence of vaccination would first be screened for hepatitis B antibodies and, if not immune to hepatitis B, would be vaccinated). For each of these strategies, we modeled costs incurred and health outcomes achieved over the lifetime of all children in the cohort. Costs included the cost of the vaccination program and all future healthcare costs for the cohort. Following standard practice in health economics [11], we measured health outcomes in terms of quality-adjusted life years (QALYs) gained, and we discounted all costs and benefits to the present.
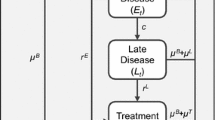
We used an age-structured Markov model to model health states and associated costs over the lifetimes of the children in the cohort (Fig. 2.1). We developed this model in collaboration with hepatitis B experts at the US Centers for Disease Control and Prevention, at the Asian Liver Center at Stanford University, and elsewhere. Susceptible children can become immune to hepatitis B through vaccination. Because of the large reservoir of chronically infected individuals in the population of China, we assumed that vaccination efforts would not change the chance that a child is exposed to hepatitis B; thus, we assumed a constant annual chance of a child in the cohort being exposed to hepatitis B (i.e., constant disease incidence). Children who are exposed to hepatitis B can either resolve the infection and become immune, or develop chronic infection. The first sign of liver dysfunction is an elevated level of alanine aminotransferase (ALT). If the individual is successfully treated with antiviral drugs, then a so-called durable response is achieved. Otherwise, the disease may progress further to cirrhosis and possibly liver cancer (hepatocellular carcinoma). Some individuals may receive a liver transplant. Death can occur from any state, with the rate determined by the health state and the age of the individual (for simplicity, these transitions are not shown in Fig. 2.1).
We modeled age and health state transitions in 1-year time increments. Thus, for example, a susceptible child aged 1 whose health state does not change within 1 year moves to the state for susceptible children aged 2 in the next year. Transition probabilities associated with hepatitis B disease progression were obtained from the literature and from informed judgment of hepatitis experts in China and elsewhere.
Associated with each health state is a quality multiplier reflecting the quality of life in that state. These quality multipliers, which in general can range from 0 (death) to 1 (perfect health), were drawn from the literature on hepatitis B infection. Also associated with each health state is an annual healthcare cost. These costs were obtained from demographic data and from recent studies of hepatitis B-related healthcare costs in China.
We implemented the model in an Excel spreadsheet. We simulated the cohort of 10,000 individuals in annual increments over a 100-year time horizon (reflecting the total possible lifetime of all individuals). For each year and each health state, we calculated costs incurred and QALYs experienced, and then discounted these values back to the present to calculate total costs and QALYs.
Using the model, we found that hepatitis B catch-up vaccination for children up to age 19 in China is cost-saving: the cost of the vaccination program (which is incurred now) is less than the net present savings in healthcare costs, when compared to the strategy of no catch-up vaccination. This finding was robust in sensitivity analysis: even in regions of the country where newborn vaccination coverage is already high and health care costs are low, catch-up vaccination is still cost-saving. We also found that screening before vaccination is not cost-effective: it costs more to screen a child for antibodies than to vaccinate the child, so it is cheaper to just vaccinate all children who have not been vaccinated or whose vaccination status is unknown (extra-vaccination is not harmful).
To disseminate this work, we published it in an international liver journal [9], and one member of our team (So) met multiple times with academics and health officials in China and members of the World Health Organization to share our interim results. Partly as a result of our study, in 2009 China instituted a policy of free hepatitis B catch-up vaccination to all children under the age of 15. Although a significant current expenditure of funds is required to implement the program, the future savings in healthcare costs will be quite large. We estimate that such vaccination could avert some 400,000 cases of chronic hepatitis B infection and almost 70,000 deaths due to hepatitis B, and would save China nearly $1 billion in healthcare costs [12]—a substantial impact on public health. Additionally, many individuals with chronic hepatitis B infection in China face significant discrimination in education and employment, so another benefit of the vaccination program is that it will spare hundreds of thousands of children from a lifetime of discrimination.
2.2 Hepatitis B in the USA
In the USA, which has high childhood vaccination coverage, hepatitis B infection among children is uncommon. However, the prevalence of chronic hepatitis B infection among adult Asian and Pacific Islanders (APIs) in the USA is quite high, partly because many APIs in the USA are foreign-born. Approximately 10 % of APIs in the USA are chronically infected with hepatitis B, as compared to 0.5 % of the general population [13, 14]. Because of this health disparity, a number of ad hoc hepatitis B screening, vaccination, and treatment programs have been implemented for APIs in various US cities. The US Centers for Disease Control and Prevention (CDC), which issues immunization and treatment guidelines for hepatitis B (and other diseases), wanted to decide what nationwide strategy they should recommend for hepatitis B control among APIs.
We used an age-structured Markov model similar to that in Fig. 2.1 to analyze the likely costs and health benefits of various strategies for controlling hepatitis B among APIs in the USA [15]. We obtained data for the model from the literature and from the informed judgment of our collaborator (So) and other hepatitis experts [12]. We implemented the model in an Excel spreadsheet, and instantiated and calibrated the model using an iterative process with input from hepatitis experts at the CDC and elsewhere.
We considered the following strategies: the status quo (no incremental screening, vaccination, or treatment); universal vaccination of all adult APIs; screening and treatment (screening to identify chronic infection, followed by antiviral treatment for those found to be infected); screening, treatment, and ring vaccination (screening to identify chronic infection, followed by antiviral treatment for those found to be infected and vaccination of the close contacts of infected individuals); and screening, treatment, and vaccination (screening to identify chronic infection or immunity, followed by vaccination of susceptibles and antiviral treatment for infected individuals). We considered a cohort of 10,000 adult APIs aged 40, and used the age-structured Markov model to simulate net present costs incurred and QALYs experienced over the lifetime of the cohort.
This analysis showed that the most cost-effective strategies are screening and treatment, which costs approximately $36,000 per QALY gained; and screening, treatment, and ring vaccination, which costs approximately $39,000 per QALY gained. Interventions in the USA that cost less than $50,000 per QALY gained are generally considered highly cost-effective [16–18]. In sensitivity analysis, we showed that the screen and treat and the screen, treat, and ring vaccinate strategies are cost-effective for any population in the USA for which the prevalence of chronic hepatitis B infection is 2 % or higher (e.g., individuals born in countries with endemic hepatitis B prevalence of 2 % or more). The analysis showed that the two strategies that involve vaccination of adult APIs are dominated: they cost more and yield fewer QALYs than the other strategies. This is because the probability of exposure to hepatitis B in the USA is relatively low and, for adults who do get exposed to the virus, the chance that they will develop chronic hepatitis B infection is low.
The key insight from the analysis is that there is substantial benefit to be gained from identifying adults who are chronically infected with hepatitis B, because they can then be started on antiviral treatments which can significantly reduce morbidity and mortality, but there is little benefit to be gained from vaccination of adult APIs. We published the results of this study in a widely read medical journal [15]. Additionally, we shared our results with the CDC throughout the process of developing the model and performing the analyses.
Consistent with our findings, the CDC issued updated hepatitis B recommendations in 2008 that call for hepatitis B screening of all adult APIs in the USA, as well as screening of all adults born in countries where the prevalence of chronic hepatitis B infection is 2–7 % [19, 20]. We estimate that there are approximately 600,000 APIs in the USA who are chronically infected with hepatitis B but unaware of their disease status. If all of these individuals were identified and treated, some 50,000 premature deaths from hepatitis B-related liver disease could be prevented [12]. Thus, this strategy can have a significant impact on improving public health.
3 HIV Control in Eastern Europe
With an estimated 33 million people worldwide infected with HIV, and 2.6 million new infections per year (an average of 7,100 new infections per day), the HIV epidemic presents a serious global challenge [21]. Prevalence of HIV is highest in sub-Saharan Africa, where two-thirds of the world’s cases have occurred. However, HIV incidence (the rate of new infection) is significantly higher in other parts of the world, particularly in certain countries of Eastern Europe and Central Asia, where it has grown significantly in the past decade [21]. An estimated 1.4 million people are living with HIV in Eastern Europe and Central Asia, with approximately 90 % of them in Russia and Ukraine [21]. Since 2001, HIV prevalence in the region has doubled: an estimated 1 % of the population of Russia and 1.1 % of the population of Ukraine is now infected [21]. The rapid growth in HIV infections in this region was spurred by collapse of the Soviet Union and subsequent social and economic disruption in the mid-1990s, which led to increasing levels of injection drug use. Originally occurring mainly in injection drug users (IDUs), the epidemic has now begun to spread heterosexually to sex partners of IDUs and to sex workers.
HIV control efforts in Eastern Europe have been somewhat limited to date. Prevention programs targeted to IDUs include “harm reduction” programs such as needle exchanges and opiate substitution therapy (with methadone or buprenorphine), as well as general education about risk reduction (e.g., safer sex, prevention of mother-to-child transmission). However, it is estimated that at most 10 % of IDUs in Eastern Europe have access to harm reduction programs [22]. In Russia, which has an estimated two million IDUs and an estimated 980,000 persons living with HIV, opiate substitution therapy is illegal, and there are only about 80 needle exchange programs in the country. Treatment coverage is also low: only 19 % of eligible individuals in Eastern Europe received lifesaving antiretroviral therapy (ART) by the end of 2009 [21]. Moreover, fewer than 14 % of treatment slots are currently allocated to IDUs, despite the fact that injection drug use accounts for 80–90 % of new HIV cases in Eastern Europe [22]. Recently, many countries in the region have focused on scaling up their prevention and treatment efforts.
3.1 HIV Treatment in Russia
In 2005, virtually no IDUs in Russia and only about 1 % of non-IDUs received ART [23, 24]. HIV treatment resources were targeted almost exclusively to non-IDUs, partly because of concerns that IDUs would not adhere to the medications [25]. At the time, plans had been made to significantly scale up the level of HIV treatment in the country. We performed an analysis to determine whether the country’s non-IDU-focused treatment strategy would be successful in slowing the epidemic and to evaluate the impact of alternate allocations of the incremental HIV treatment resources [26].
For this analysis, we developed a dynamic compartmental model of the HIV epidemic, illustrated in Fig. 2.2. In this model, the population (of adults aged 15–49) is divided into mutually exclusive, collectively exhaustive compartments, distinguished by injection drug use status (IDUs, non-IDUs), HIV infection status (uninfected, HIV infected and asymptomatic, HIV infected and symptomatic, and AIDS), and HIV treatment status (untreated, treated). We modeled the transmission of HIV via injection drug use (between IDUs) and via sexual contact (between any members of the population).
The arrows in the diagram represent transitions. Individuals who age into the population enter as HIV-uninfected. Uninfected individuals who acquire HIV infection move to an HIV+, asymptomatic compartment. Uninfected IDUs (compartment 1) can acquire HIV through injection drug use (risky needle sharing with other IDUs) or risky sexual contacts (with IDUs or non-IDUs), while uninfected non-IDUs (compartment 7) acquire HIV only through risky sexual contacts (with IDUs or non-IDUs). Rates of infection transmission are a nonlinear function of the number of infected and uninfected individuals in the population; thus, the model is a nonlinear dynamic system. Individuals with asymptomatic HIV infection may develop symptomatic HIV infection, and may further progress to AIDS. HIV-infected individuals with symptomatic disease or AIDS can enter treatment. Additionally, IDUs may cease injection drug use and transition to the non-IDU population, and vice versa. Deaths can occur from any compartment, as shown by the diagonal dashed arrows.
Use of a dynamic model of the HIV epidemic allowed us to capture both the individual-level and population-level effects of ART: individuals treated with ART live longer (individual benefit) and are less infectious (population benefit). The dynamic model also allowed us to capture the effects of ART on different modes of transmission (injection drug use, sexual contact) as a function of treatment levels in the IDU and non-IDU populations.
We implemented the model in an Excel spreadsheet and simulated the system over a 20-year time horizon in 1/10 year increments. In the base case, we used data for the city of Saint Petersburg, Russia, where HIV prevalence was approximately 35 % among IDUs and 0.6 % among non-IDUs. In sensitivity analysis, we used data for Barnaul, a Russian city in southwestern Siberia with an earlier-stage HIV epidemic (1.7 % HIV prevalence among IDUs and 0.06 % among non-IDUs). We obtained data for the analysis from published literature, from our expert collaborators (Galvin and Vinichenko), and directly from HIV and drug abuse experts in Russia: in 2005 our research team traveled to Russia and met with numerous individuals in governmental and non-governmental organizations to obtain data for the study.
For each compartment, we measured all health care-related costs incurred and all QALYs experienced over a 20-year time horizon. We discounted both costs and QALYs to the present at 3 % annually. We also included the future discounted costs and QALYs accruing from individuals alive in the modeled population at the end of the 20-year time horizon.
The analysis showed that targeting expanded ART to non-IDUs is far less effective than a strategy that expands ART without regard to IDU status (i.e., an untargeted treatment strategy). The most effective and cost-effective strategy would be to target ART to IDUs only, but such a strategy would not be politically acceptable because it would steer treatment resources away from HIV-infected individuals in the general population. An untargeted treatment strategy would be highly cost-effective, costing $1,800 per QALY gained, and could significantly decrease the spread of HIV. This result was unchanged in extensive sensitivity analyses of uncertain parameters. All of the strategies considered had cost-effectiveness ratios less than the gross domestic product (GDP) per capita in Russia, a threshold cited as “very cost effective” by the World Health Organization’s Commission on Macroeconomics and Health [18].
For this example, the most efficient strategy—targeting ART to IDUs—is not the most equitable strategy—providing ART to all eligible individuals. By quantifying the costs and health benefits of alternative strategies, the model informs decision makers about the loss in health benefits of more equitable strategies (or indeed any strategy) compared to the most efficient strategy. Public health planners make decisions based on many considerations in addition to cost and health benefit, such as social, political, and ethical factors (e.g., see [27]). An OR-based model can quantify the likely costs and health benefits of potential decisions.
The key insight from the analysis is that providing IDUs with ART helps reduce HIV transmission not only to the IDU population but also to the non-IDU population—and neglecting IDUs when scaling up treatment is the least effective and least cost-effective strategy. We published the results of this work in an international AIDS journal as a means of informing the debate about HIV policy in the region [26] and translated the article into Russian for broader dissemination [28].
3.2 Harm Reduction and HIV Treatment in Ukraine
With an estimated 350,000 persons living with HIV, Ukraine has the highest HIV prevalence in Europe [21]. Originally confined to IDUs, HIV in Ukraine has recently begun to transition to other members of the population. In 2007, some 40 % of new HIV infections occurred due to risky injection practices and 40 % accrued from heterosexual transmission (often due to contact with an infected IDU) [29]. At the same time, efforts to control HIV have been limited. In 2007, virtually none of the estimated 400,000 IDUs in Ukraine received methadone substitution therapy, and only about 10 % of eligible patients received ART [29].
At the end of 2007, Ukraine approved the use of methadone for substitution therapy and announced plans to enroll 11,000 IDUs in methadone treatment by 2011. The country also announced plans to scale up ART coverage to 90 % of eligible individuals. Because these two interventions may potentially compete for scarce resources, it is essential to determine the most cost-effective combination of these programs. We performed an analysis to determine the effectiveness and cost-effectiveness of different levels and combinations of methadone and ART scale up [30]. We analyzed strategies that focus on increasing methadone slots, ART slots, or both.
We used a dynamic compartmental model, schematically illustrated in Fig. 2.3, to evaluate costs and health outcomes of various scale-up strategies. This model is similar to that shown in Fig. 2.2 except that it also includes compartments for IDUs receiving methadone treatment. IDUs can transition into and out of methadone treatment. On leaving treatment, IDUs can return to untreated injection drug use or can become non-IDUs. Additionally, IDUs not receiving methadone treatment can stop injecting drugs and enter the non-IDU population, and vice versa. IDUs on methadone treatment may continue to inject drugs, but at a lower rate and with fewer shared injections than untreated IDUs, so their chance of acquiring or transmitting HIV is lower than for untreated IDUs, as is their mortality rate.
Similar to our analysis evaluating ART scale up in Russia, we implemented this model in Microsoft Excel and simulated the system for 20 years in 1/10 year increments, discounting all costs and health benefits back to the present, including those estimated to accrue beyond the end of the time horizon. We obtained data for the model from published literature and from information provided by governmental and nongovernmental organizations that work in Ukraine and Eastern Europe.
The analysis showed that expansion of methadone therapy is the most cost-effective strategy, followed by a strategy of methadone and ART expansion. Both strategies—expansion of methadone only or expansion of methadone and ART simultaneously—are highly cost-effective. Expansion of ART only, without expanding methadone, is also cost-effective, but less so. However, expansion of ART, if it is only offered to non-IDUs, is not cost-effective.
Methadone has not been widely adopted in Eastern Europe, primarily for social and political reasons. Some politicians in the region fear that making methadone available to IDUs will encourage drug addiction. Others believe that it is inappropriate to treat drug addicts with an addictive substance (methadone). Our analysis quantifies the loss in health benefits associated with not adopting methadone, thus informing decision makers about the cost of this political constraint.
A key insight from the analysis is that even modest levels of methadone treatment can substantially reduce the HIV epidemic in Ukraine and would be highly cost-effective. A second important insight is that methadone treatment averts the most infections, but expanded ART along with expanded methadone treatment provides the largest total increase in QALYs. This result highlights the complementary nature of these interventions. Thus, when expanding ART in Ukraine, a simultaneous expansion of methadone treatment can significantly increase the number of infections averted in a highly cost-effective manner. Because the HIV epidemic in Ukraine is representative of the HIV epidemic in Eastern Europe and Central Asia, these findings can inform HIV policy in Ukraine as well as in other countries in the region. To disseminate the results of this work, we published it in a widely read international medical journal [30]. We also translated the article into Russian for broader dissemination [31].
4 Bioterrorism Preparedness and Response
Although it has long been known that biological agents can be used as weapons, the 2001 anthrax attacks in the USA focused new attention on the threat of bioterrorism. Biological agents are thought to be a particularly dangerous form of terrorism because, if properly deployed, they can become weapons of mass destruction, killing large numbers of people and potentially creating mass disruption.
In 2001, the newly formed Department of Homeland Security set about developing enhanced plans to prepare for and respond to potential terrorist attacks. As part of this effort, the US Strategic National Stockpile was created. This nationally held repository of medical, pharmaceutical, and other supplies is intended for use in any type of public health emergency, including a terrorist or bioterror attack, when local supplies are insufficient [32]. The Strategic National Stockpile has two components: Push Packs and Vendor-Managed Inventories (VMI). Push Packs contain antibiotics, antidotes, and other medical supplies necessary to treat a wide range of possible biological or chemical agents and are reportedly available for local distribution within 12 h after being requested. VMI consist of additional supplies of antibiotics and medical equipment tailored to the specific needs of communities and are reported to be able to arrive at local distribution and/or dispensing sites within 36 h following the detection of an attack.
Local communities may also hold inventories of supplies for response. However, there is no consensus about the amount and type of local supplies that should be held. Some communities stock only enough supplies for first responders, whereas other communities stock enough supplies so as to be self sufficient for several days after an attack [33, 34].
In addition to the question of how much local inventory should be held, a 2003 review [35] identified the following unresolved questions regarding bioterrorism response logistics: What strategy should be used for dispensing Push Packs and VMI? How much dispensing capacity should local communities have for emergency response to a bioterror attack? What dispensing strategies should be used at local dispensing centers? To what extent will quicker detection of a bioterror attack save lives? What is the effect of large numbers of unexposed individuals requiring prophylaxis?
To address these questions, we developed a model that focuses on the case of a potential large-scale anthrax attack in an urban area [36, 37]. We focused on anthrax because it is thought to be a particularly dangerous threat: it may be possible to dispense large amounts of aerosolized anthrax without immediate detection, and the resulting pulmonary anthrax infection, if untreated, is almost uniformly fatal.
The model is designed to evaluate the costs and benefits of various strategies for pre-attack stockpiling and post-attack distribution and dispensing of medical and pharmaceutical supplies, as well as the benefits of rapid attack detection. A schematic of the model is shown in Fig. 2.4.
We assumed the following sequence of events after a large-scale anthrax attack: The attack is detected and announced to the public, and the order is given to dispense antibiotics to affected members of the public. Local dispensing centers are set up and antibiotics and other supplies are requested from the Strategic National Stockpile. Over time, exposed and potentially exposed individuals learn of the attack and go to the local dispensing centers to receive oral antibiotics. At the local dispensing centers, they are given a supply of one of two prophylactic antibiotics, ciprofloxacin or doxycycline (“prophylaxis”). Locally held inventories are dispensed until supplies from the Strategic National Stockpile arrive; these supplies may later be augmented by VMI. Symptomatic individuals are admitted to intensive care unit (ICU) beds in local hospitals (“treatment”), where they must be given intravenous antibiotics, put on a respirator, and monitored by a respiratory technician. Queues may arise for both prophylaxis and treatment.
Our model has two interconnected components: a dynamic model of disease progression, prophylaxis, and treatment in the population (disease model), and a model of local dispensing and hospital capacity and the supply chain of available inventories (logistics model). The disease model, illustrated in Fig. 2.5, is a dynamic compartmental model that incorporates five states for anthrax disease (not exposed, potentially exposed and requiring prophylaxis, infected and in the incubation period of disease, prodromal disease, and fulminant disease) and four states for individuals’ awareness and care (unaware of exposure, aware of exposure or potential exposure but not receiving antibiotics, in prophylaxis, and in treatment). The incubation period of anthrax, which is asymptomatic, lasts approximately 9–13 days. This can progress to prodromal infection, which manifests with flulike symptoms and lasts 3–4 days. Prodromal infection can progress to fulminant infection, which is associated with extreme respiratory distress, lasts approximately 1 day, followed almost always by death. The probability of progression through the different disease stages depends on when (and if) the infected individual receives antibiotics to treat anthrax.
The rates at which exposed and potentially exposed individuals receive antibiotics are governed by the logistics model. Specifically, the rate at which individuals can enter prophylaxis is a function of the dispensing centers’ capacity and the level of available oral antibiotics. The rate at which hospitals can accept patients for treatment is a function of the number of available ICU beds, doses of intravenous antibiotics, and respiratory technicians.
We implemented the model in an Excel spreadsheet, using data for a typical US city of five million people. Data for the disease model came from published studies, including a systematic review of inhalational anthrax cases in the USA [38]. We obtained data on costs, national antibiotic inventories, and Strategic National Stockpile response times from published sources. We used illustrative values for variables such as local hospital capacity, local dispensing capacity, and levels of local antibiotic inventories.
We considered different scenarios for attack size (small, exposing 50,000 people, or large, exposing 250,000 people) and fraction of people in the unexposed population who are potentially exposed and thus require prophylaxis (ranging from 2 to 95 %). Then we examined the effects of different levels of local inventory and dispensing capacity, and different times to event detection. For each scenario, the model calculates total expected mortality. In addition, to evaluate the cost-effectiveness of local inventory and dispensing capacity expansion, the model calculates total local costs (the costs of inventories in the Strategic National Stockpile were not considered in the analysis, as they are a sunk cost).
A key insight from the analyses is that the constraining factor in an anthrax response is likely to be local dispensing capacity, not the availability of antibiotics and other needed inventories. This suggests that stockpiling local inventories of medical and pharmaceutical supplies is unlikely to be the most effective (or cost-effective) means of reducing mortality from an anthrax attack. Instead, the development of plans for extensive dispensing capacity will likely have a much greater impact on reducing mortality in the event of a large-scale anthrax attack. Another key insight is that improved surveillance systems that can lead to quicker attack detection can avert deaths, but only if the local community has sufficient dispensing capacity. Finally, factors related to behavior of the public, including the rate at which people in the affected community become aware of the attack and seek treatment and their rate of adherence to prophylaxis, have a significant impact on mortality. This suggests that, in the event of such an attack, effective strategies for communicating with the public will be essential.
To maximize the impact of this work, we disseminated our findings in journals in two different fields. We published some of the key findings from our analyses in a bioterror journal [36] and published a more detailed description of the model and its capabilities in a medical journal [37]. The visibility of these publications led to a subsequent invited consultation with planners at the Strategic National Stockpile regarding design of the supply chain for anthrax response. Additionally, this work led to membership of the author (Brandeau) on an Institute of Medicine Committee charged with examining the costs and benefits of prepositioned medical supplies for bioterror response in local communities [39]. Although it is not known if, when, or where an anthrax attack will occur in the USA, information about the potential costs and benefits of alternative preparedness plans can help planners now in creating effective, and cost-effective, preparedness plans.
5 Conclusions and Policy Implications
We have described three types of models that we have used to inform public health policy in three areas: Markov models to evaluate hepatitis B control strategies in the USA and China, dynamic compartmental models to evaluate strategies for HIV control in Eastern Europe, and a hybrid logistics/disease model to evaluate strategies for bioterrorism preparedness and response in the USA. Although the impact of all of these studies is not yet fully known, they have provided important information that can inform decision making. They have also provided useful lessons for OR modelers who wish to help improve public health decision making.
First, a successful OR-based policy analysis will focus on a problem of importance. In some cases, a problem may be identified as important by a public health decision maker, as was the case when the CDC asked for evidence about alternative strategies for controlling hepatitis B among adult APIs in the USA. In other cases, a problem of importance may be identified through articles in the media, through discussions with knowledgeable individuals, or through personal observations. For example, our analysis of HIV treatment strategies in Russia was inspired by discussions with members of nongovernmental organizations who were working to implement HIV prevention and treatment programs in Russia, while our analysis of methadone and ART scale up in Ukraine was inspired by articles in the media and by discussions with country-level HIV planners in Eastern Europe. No matter how the problem is identified, an OR model must examine a problem of importance in order to have impact.
Second, successful analyses of public health decisions very often require multidisciplinary expertise. For example, to analyze hepatitis B control strategies we collaborated with a liver surgeon (So) who is an expert on hepatitis B as well as an advocate for hepatitis B control in Asian populations. We also sought advice from and shared our ongoing work with the CDC and with other hepatitis B experts. Our analysis of anthrax preparedness strategies involved an internist (Owens), a pulmonologist (Holty), and an expert in public health disaster response (Bravata). Working with domain experts helps to ensure that models and assumptions are believable and that the most important aspects of the problem are appropriately addressed.
Third, the goal of OR modeling in public health is not to predict the impact of alternative decisions with complete precision, but instead to identify good decisions. A useful—and believable—model will be detailed enough so that it can appropriately evaluate the decisions at hand, but not so detailed that it relies on numerous potentially untenable assumptions and large amounts of unavailable data. One way to achieve an appropriate balance between simplicity and realism is to start with a simple model and only add detail if an essential component of the problem—one that may change the believability or the results of the analysis—has been omitted. Additionally, because uncertainty is an integral part of most public health problems, a key part of any analysis that aims to identify good decisions is sensitivity analysis. Decision makers need to know how values of uncertain parameters affect the findings of the analysis.
Fourth, although an OR model may generate many interesting findings, policy makers are most interested in the key insights from the analysis. For example, in our analysis of anthrax preparedness strategies, we considered many combinations of attack scenario, time to attack detection, local inventory levels, local dispensing capacity, etc., and generated a variety of detailed results. However, we focused our written reports of the work on the few most important findings. Decision makers typically want to know, “What is your main finding and how is this relevant to my decision making?” To have impact, an OR-based analysis will answer these questions concisely and clearly.
Finally, an essential component of successful OR-based modeling in public health is dissemination of findings to decision makers. In public health there is typically no single decision maker. Instead, public health decisions are often made by consensus among groups of individuals including public health and government officials, members of nongovernmental public health organizations, health care providers, advocacy groups, and members of the public. Thus, unlike work done for companies which can be relatively easy to report to the person (or few persons) in charge of the decision, significant effort is often required to disseminate the results of OR analyses for the public sector. An important step in this process is to publish the results in outlets where they are likely to be read by decision makers. For example, decision makers who work in the area of HIV control may read AIDS journals or general medical journals, whereas decision makers in the area of bioterror preparedness may read publications targeted to a general public health audience or a bioterror audience. For international studies, translation of the published paper into other languages may be helpful; for example, our study of hepatitis B in China was translated by the journal into Chinese, in addition to its publication in English, so as to reach a wider audience in China. Additionally, dissemination of results through conference presentations and meetings with interested individuals can increase the audience for the work. Although broad dissemination of results of OR-based public health analyses may require significant effort, such effort is essential if the work is to have impact.
Decision makers in public health face many complex problems for which OR-based analyses can provide valuable insights. Effective OR models of public health problems do not need to be highly complex, as long as they capture the salient aspects of the problem and help to identify good decisions. In this promising new area of OR application, a little help can indeed go a long way.
References
Centers for Disease Control and Prevention (2008) Hepatitis B fact sheet. http://www.cdc.gov/ncidod/diseases/hepatitis/b/fact.htm. Accessed 8 Apr 2008
Edmunds WJ et al. (1993) The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci 253(1337):197–201
World Health Organization (2008) Hepatitis B. Fact Sheet No. 204. World Health Organization, Geneva, Switzerland
Centers for Disease Control and Prevention (2007) Progress in hepatitis B prevention through universal infant vaccination—China, 1997–2006. MMWR Morb Mortal Wkly Rep 56(18):441–445
Liu J, Fan D (2007) Hepatitis B in China. Lancet 369(9573):1582–1583
Chinese Ministry of Health (2008) The Ministry of Health conference on planning and hepatitis B immunization, malaria prevention and control work [Chinese]. http://www.gov.cn/xwfb/2008–04/21/content_950425.htm. Accessed 1 Aug 2008
United States Census Bureau (2008) China international database country summary. http://www.census.gov/ipc/www/idb/country/chportal.html#TAB. Accessed 18 Nov 2008
Chen JJ (2008) A model HBV catch-up immunization and education project in Qinghai, China. In: National Immunization Conference
Hutton DW, So SK, Brandeau ML (2010) Cost effectiveness of nationwide hepatitis B catch-up vaccination among children and adolescents in China. Hepatology 51(2):405–414
Hutton DW, Brandeau ML (2013) Too much of a good thing? When to stop catch-up vaccination. Working paper
Gold MR et al. (1996) Cost-effectiveness in health and medicine. Oxford University Press, New York
Hutton DW, So SK, Brandeau ML (2011) Doing good with good OR: supporting cost-effective hepatitis B interventions. Interfaces 41(3):289–300
Custer B et al. (2004) Global epidemiology of hepatitis B virus. J Clin Gastroenterol 38(10 Suppl):S158–S168
McQuillan GM, Coleman PJ, Kruszon-Moran D (1999) Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health 89(1):14–18
Hutton DW et al. (2007) Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med 147(7):460–469
Murray CJ, Lopez A (2002) World Health Report 2002: reducing risks, promoting healthy life. World Health Organization, Geneva, Switzerland, p 186
Owens DK (1998) Interpretation of cost-effectiveness analyses [Editorial]. J Gen Intern Med 13(10):716–717
World Health Organization (2003) Making choices in health: WHO guide to cost-effectiveness analysis. World Health Organization, Geneva, Switzerland
Weinbaum CM, Mast EE, Ward JW (2009) Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Hepatology 49(5 Suppl):S35–S44
Weinbaum CM et al. (2008) Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep 57(RR-8):1–20
Joint United Nations Programme on HIV/AIDS (UNAIDS) (2010) UNAIDS report on the global AIDS epidemic 2010. UNAIDS, Geneva, Switzerland
Open Society Institute (2008) Harm reduction developments 2008. Countries with injection-driven HIV epidemics. Open Society Institute, New York
Samoilov D (2004) Double discrimination: drug users living with HIV/AIDS. HIV AIDS Policy Law Rev 9(3):83–85
World Health Organization (2005) Summary country profile for HIV/AIDS treatment scale-up: Russian Federation, June 2005. World Health Organization, Geneva, Switzerland
Open Society Institute (2004) Breaking down barriers: lessons on providing HIV treatment to injection drug users. Open Society Institute, New York
Long EF et al. (2006) Effectiveness and cost-effectiveness of strategies to expand antiretroviral therapy in St. Petersburg, Russia. AIDS 20(17):2207–2215
Alistar SS, Brandeau ML (2012) Decision making for HIV prevention and treatment scale up: bridging the gap between theory and practice. Med Decis Making 32(1):105–117
Long EF et al. (2006) Оценка эффективности и экономической эффективности стратегии расширенной антиретровирусной терапии в Санкт-Петербурге, Россия. http://www.stanford.edu/dept/MSandE/cgi-bin/people/faculty/brandeau/brandeau.php. Accessed 31 Oct 2011
Joint United Nations Programme on HIV/AIDS (UNAIDS) (2008) Ukraine—National report on monitoring progress towards the UNGASS declaration of commitment on HIV/AIDS. UNAIDS, Geneva, Switzerland
Alistar SS, Owens DK, Brandeau ML (2011) Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med 8(3):e1000423
Alistar SS, Owens DK, Brandeau ML (2011) Результативность и экономическая эффективность расширения программ снижения вреда и антиретровирусной терапии при «смешанной» эпидемии ВИЧ: анализ модели на примере Украины. http://www.plosmedicine.org/attachments/pmed.1000423_Russian.pdf. Accessed 31 Oct 2011
Koplan J (2001) CDC’s strategic plan for bioterrorism preparedness and response. Public Health Rep 116(Suppl 2):9–16
Benson B, McKinney P (2003) Selecting and stocking antidotes to biological/chemical agents. In: Texas Society of Health-Systems Pharmacists Annual Meeting, Austin, TX
Case GG, West BM, McHugh CJ (2001) Hospital preparedness for biological and chemical terrorism in central New Jersey. N J Med 98(11):23–33
Bravata DM et al. (2003) Regionalization of bioterrorism preparedness and response (Evidence report/technology assessment). Agency for Healthcare Research and Quality, Rockville, MD
Bravata DM et al. (2006) Reducing mortality from anthrax bioterrorism: strategies for stockpiling and dispensing medical and pharmaceutical supplies. Biosecur Bioterror 4(3):244–262
Zaric GS et al. (2008) Modeling the logistics of response to anthrax bioterrorism. Med Decis Making 28(3):332–350
Holty JE et al. (2006) Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med 144(4):270–280
Institute of Medicine Committee on Prepositioned Medical Countermeasures (2011) Prepositioning antibiotics for anthrax. National Academies Press, Washington, DC
Acknowledgment
This work was supported by grant number R01-DA15612 from the National Institute on Drug Abuse.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Brandeau, M.L. (2013). OR in Public Health: A Little Help Can Go a Long Way. In: Zaric, G. (eds) Operations Research and Health Care Policy. International Series in Operations Research & Management Science, vol 190. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6507-2_2
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6507-2_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6506-5
Online ISBN: 978-1-4614-6507-2
eBook Packages: Business and EconomicsBusiness and Management (R0)