Abstract
We investigated whether taurine chloramine (TauCl), which is endogenously produced by immune cells such as macrophages that infiltrate adipose tissue, affects the differentiation of preadipocytes into adipocytes or modulates the expression of adipokines in adipocytes. To study the physiological effects of TauCl on human adipocyte differentiation and adipokine expression, preadipocytes were cultured under differentiation conditions for 14 days in the presence or the absence of TauCl. Differentiated adipocytes were also treated with TauCl in the presence or the absence of IL-1β (1 ng/ml) for 7 days. The culture supernatants were analyzed for adipokines such as adiponectin, leptin, IL-6, and IL-8. At concentrations of 400–600 μM, TauCl significantly inhibited the differentiation of human preadipocytes into adipocytes in a dose-dependent manner. It did not induce the dedifferentiation of adipocytes or inhibit fat accumulation in adipocytes. Expression of major transcription factors of adipogenesis and adipocyte marker genes was decreased after treatment with TauCl, in agreement with its inhibition of differentiation. These results suggest that TauCl may inhibit the differentiation of preadipocytes into adipocytes. Thus, TauCl or more stable derivatives of TauCl could potentially be a safe drug therapy for obesity-related diseases.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Adipose Tissue
- Adipocyte Differentiation
- Major Transcription Factor
- Intracellular Lipid Accumulation
- Taurine Chloramine
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The inhibition of adipogenesis is one of the most important mechanisms involved in reducing body fat, which also includes apoptosis, lipolysis, and fatty acid oxidation. Recent studies have proposed that obesity is a state of systemic, chronic low-grade inflammation (Itoh et al. 2011). During the course of obesity, adipose tissue is characterized by the infiltration of immune cells such as macrophages.
The formation of adipose tissue involves the commitment of mesenchymal stem cells (MSCs) (which have the potential to differentiate into various cell lineages) to the preadipocyte lineage and the differentiation of preadipocytes into mature adipocytes (Gesta et al. 2007; Roufosse et al. 2004). In vitro, MSCs proliferate and develop into preadipocytes after reaching confluency. Preadipocytes remain competent for proliferation but, when allowed to become confluent and treated with substances such as insulin and glucocorticoids, they cease cell division and differentiate into adipocytes (MacDougald and Mandrup 2002). During adipogenesis, a number of morphological and physiological changes occur. The cells change from fibroblast-like preadipocytes to spherical adipocytes and accumulate large fat droplets containing triglycerides (Jessen and Stevens 2002). Then, upstream regulators, including CCAAT/enhancer-binding protein β (C/EBP-β), C/EBP-δ, and sterol-regulatory element-binding protein 1c (SREBP1c), regulate the expression of peroxisome proliferator-activated receptor γ (PPARγ) and C/EBP-α, which are major transcription factors for adipogenesis. The concerted action of these adipogenic transcription factors ultimately drives the expression of adipocyte marker genes, such as fatty acid-binding protein (FABP) 4, lipoprotein lipase (LPL), fatty acid synthetase, adiponectin, glucose transporter 4 (GLUT4), leptin, and others, which are responsible for the synthesis and storage of triglycerides in lipid droplets (Rosen et al. 2002; Rosen and MacDougald 2006).
Taurine (2-aminoethanesulfonic acid) is a simple sulfur-containing amino acid; it is one of the most abundant intracellular free amino acids in mammalian tissues and blood cells. It modulates a variety of cellular functions, including antioxidation, modulation of ion movement, osmoregulation, modulation of neurotransmitters, and conjugation of bile acids (Ito et al. 2011). Decreased tissue taurine concentrations are characteristic of many pathological states (Szymanski and Winiarska 2008). Furthermore, it was reported that taurine deficiency creates a vicious circle that may promote obesity (Tsuboyama-Kasaoka et al. 2006). Both neutrophils and monocytes contain high levels of taurine. Taurine acts as a scavenger of hypochlorous acid (HOCl), which is produced by the myeloperoxidase/hydrogen peroxide/chloride system of activated neutrophils and monocytes (Thomas et al. 1985). It reacts with HOCl to form taurine chloramine (TauCl). TauCl has been shown to play a major role in downregulating the expression of inflammatory mediators such as chemokines, cytokines, cyclooxygenase-2, and inducible nitric oxide synthase in various types of cells (Schuller-Levis and Park 2004).
In this study, we investigated whether TauCl, which is endogenously produced by immune cells such as macrophages that have infiltrated adipose tissue, affects the differentiation of preadipocytes into adipocytes.
2 Methods
2.1 Preadipocyte Cell Culture and Differentiation into Adipocytes
Human preadipocytes were purchased from Cell Applications (San Diego, CA, USA) and maintained in Preadipocyte Growth Medium Kit (Cell Applications). Preadipocytes were seeded into six-well plates (1.5 × 105 cell/well in 2 ml of medium) or 60-mM dishes (2.5 × 105 cell/60 mM dish in 2 ml of medium) and cultured until confluent. For differentiation, the culture medium was changed to Adipocyte Differentiation Medium (Cell Applications) and cultured for 2 weeks by changing the medium every 2 days in the presence or the absence of TauCl at different concentrations.
2.2 Preparation of TauCl
TauCl was synthesized by mixing equimolar amounts of sodium hypochlorite (Aldrich Chemical, Milwaukee, MI, USA) and taurine (Sigma, St. Louis, MO, USA) as described somewhere (Kim et al. 2007). TauCl formation was verified by UV absorption (200–400 nM). Endotoxin-free or low-endotoxin-grade water and buffers were used. Stock solutions of TauCl were kept at 4°C and used within 3 days.
2.3 Oil Red O Staining
Lipid accumulation was examined with oil red O staining (Ramirez-Zacarias et al. 1992). Cultured cells were rinsed twice with phosphate-buffered saline (PBS) and fixed in 10% (v/v) formaldehyde for 1 h. After the formaldehyde was removed, cells were rinsed three times with deionized water and stained with a saturated solution of oil red O in 60% isopropanol solution for 2 h at room temperature. Microscopic images (Olympus, Tokyo, Japan) of the stained cells were obtained after removing the staining solution. Finally, the dye retained in the cells was eluted with isopropanol and quantified by measuring the optical absorbance at 500 nm.
2.4 Semiquantitative and Real-Time Reverse Transcription Polymerase Chain Reaction
Complementary DNA was synthesized from 1 μg total RNA in 20 μl reverse transcription reaction mixture containing 5 mmol/l MgCl2, 1× RT buffer, 1 mmol/l dNTP, 1 U/μl RNase inhibitor, 0.25 U/μl AMV reverse transcriptase, and 2.5 μmol/l random 9-mers as described previously (Kim et al. 2007). For semiquantitative PCR, aliquots of cDNA were amplified with the primers in a 25 μl PCR mixture containing 1× PCR buffer, 0.625 units of TaKaRa Ex Taq™ HS, and 0.2 μmol/l of specific upstream primers, in accordance with the manufacturer’s protocol (TaKaRa Bio, Kyoto, Japan). The PCR conditions for the Leptin, GLUT-4, and β-actin were as follows: 25–30 cycles at 95°C for 45 s, 55–60°C for 45 s, and 72°C for 45 s. PCR products were subjected to electrophoresis in 1.5% agarose gels containing ethidium bromide, and the bands were visualized under UV light. The primers were synthesized by Bioneer Co. Ltd (Seoul, Republic of Korea), and their sequences are as follows: leptin (5′-CGCAGTCAGTCTCCTCCAAA-3′, 5′-GGTTCTCCAGGTCGTTGGAT-3′), GLUT4 (5′-TGGCTGAGCTGAAGGATGAG-3′, 5′-CCAACAACACCGAGACCAAG-3′), and β-actin (5′-TCATGAGGTAGTCAGTCAGG-3′, 5′-CTTCTACAATGAGCTGCGTG-3′).
2.5 Western Blot Analysis
The cells are prepared for Western blot analysis and the samples were separated using 12 or 15% SDS-PAGE, and were then transferred to Hybond-ECL membranes (Amersham, Arlington Heights, IL, USA) as described previously (Choi et al. 2009). The membranes were first blocked with 6% nonfat milk dissolved in TBST buffer (10 mM Tris–Cl [pH 8.0], 150 mM NaCl, 0.05% Tween 20). The blots were then probed with various rabbit polyclonal antibodies for C/EBP-α (Cell Applications, Inc), PPAR-γ (Cell Applications, Inc), SREBP1 (Santa Cruz BioTechnology), and β-actin (Santa Cruz BioTechnology) diluted according to the manufacturer’s protocol in TBS at 4°C overnight, and incubated with 1:1,000 dilutions of goat anti-rabbit IgG secondary antibody coupled with horseradish peroxidase. The blots were developed using the ECL method (Amersham). For re-probing, the blots were incubated in the stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris–HCl [pH 6.7]) at 50°C for 30 min with occasional agitation.
2.6 Statistical Analysis
The in vitro experimental data are expressed as the mean ± standard error of the mean (SEM) of quadruplicate samples. Differences between groups were compared with the Mann–Whitney test. Prism software 4 (Graphpad Software, San Diego, CA) was used for statistical analysis and graphing. Differences were considered significant at P < 0.05.
3 Results
3.1 Effect of TauCl on Differentiation of Preadipocytes into Adipocytes
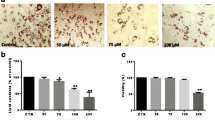
To study the physiological effects of TauCl on human adipocyte differentiation, preadipocytes were cultured under differentiation conditions for 14 days in the presence or the absence of TauCl. As shown in Fig. 21.1a, the preadipocytes differentiated into adipocytes in the absence of TauCl, and differentiation was inhibited by TauCl in a dose-dependent manner. In addition, the differentiated adipocytes showed intracellular lipid accumulation. The accumulated lipid droplets were examined by oil red O staining. In accordance with the degree of differentiation, the degree of oil red O staining was dose dependently decreased by TauCl (Fig. 21.1b, c). Fat droplet formation was almost completely blocked by treatment with 600 μM TauCl. To test the cytotoxic effects of TauCl on preadipocytes, MTT assays were conducted after cells were treated with 1 mM TauCl for 14 days. Cell viability was not affected by TauCl at a concentration of 1 mM, suggesting that the inhibitory effect of TauCl on cell differentiation and lipid accumulation is not due to cytotoxicity (data not shown).
Effect of TauCl on differentiation of preadipocytes into adipocytes. Human preadipocytes were seeded into six-well plates and cultured until confluent. For differentiation, the culture medium was changed to adipocyte differentiation medium and cells were cultured for 2 weeks while changing the medium every 2 days with or without TauCl at different concentrations. (a) Microscopic image of differentiated adipocytes before (top row) and after (bottom row) oil red O staining. (b) Image of differentiated adipocytes in six-well plate in the absence or the presence of different concentrations of TauCl. (c) Optical absorbance at 500 nm of the dye retained in adipocytes. Three independent experiments were performed in triplicate. The data shown are representative of three independent experiments, and similar results were obtained from all three. Values are expressed as mean ± standard error of the mean (SEM). **P < 0.01 versus no treatment with TauCl
3.2 Effect of TauCl on Expression of Adipocyte-Specific Proteins
To determine whether the inhibition of cell differentiation and fat accumulation resulted from TauCl-mediated alterations in the differentiation program, we examined the expression of a number of adipogenic proteins by Western blot analysis. As shown in Fig. 21.2a, TauCl treatment reduced the protein levels of the major adipogenic transcription factors PPAR-γ and C/EBP-α in preadipocytes. TauCl treatment at a concentration of 600 μM significantly reduced the protein levels of the major adipogenic transcription factors PPAR-γ and C/EBP-α. In addition, SREBP1, the upstream regulator of these transcription factors, was also downregulated by TauCl treatment in a dose-dependent manner. The effect of TauCl on these factors was specific, because the levels of β-actin were unaffected. Furthermore, the expression of GLUT4, a protein specific to adipocytes, was significantly reduced in the presence of TauCl in a dose-dependent manner (Fig. 21.2b). These data suggest that TauCl downregulates the expression of SREBP1 and the subsequent expression of PPAR-γ and C/EBP-α, resulting in inhibition of adipocyte differentiation.
Inhibition of adipogenic gene expression by TauCl. Human preadipocytes were cultured for 14 days in the absence or the presence of TauCl as described in Fig. 21.1. The cells were prepared for (a) Western blot analysis and (b) semiquantitative RT-PCR. The data shown are representative of three independent experiments, and similar results were obtained from all three
3.3 Effect of TauCl on Fat Accumulation and Adipocyte Dedifferentiation
To check if TauCl inhibits fat accumulation in differentiated adipocytes and induces the dedifferentiation of adipocytes into preadipocytes, TauCl was added to fully differentiated adipocytes and cultured for 14 days. As shown in Fig. 21.3, the degree of adipogenesis did not change; furthermore, oil red O staining showed that TauCl did not inhibit intracellular fat accumulation (Fig. 21.3a). Expression of the adipocyte-specific proteins PPAR-γ, C/EBP-α, SREBP1, and GLUT4 was also not affected, even at a concentration of 600 μM TauCl (Fig. 21.3b, c). These results suggest that TauCl does not lead to the dedifferentiation of adipocytes and does not affect fat accumulation in adipocytes.
Effect of TauCl on fat accumulation and adipocyte dedifferentiation. TauCl was added to differentiated adipocytes, which were then further incubated in starvation medium for 14 days (medium was changed after 7 days of culture in the absence or the presence of different concentrations of TauCl). (a) Dye retained in adipocytes was measured by optical absorbance at 500 nm. (b) Western blot analysis and (c) semiquantitative RT-PCR of adipogenic gene expression. The data shown are representative of three independent experiments, and similar results were obtained from all three
4 Discussion
Adipose tissue, once viewed as simply a storage and release depot for lipids, is now considered to be an endocrine tissue (Halberg et al. 2008; Ronti et al. 2006). Adipose tissue secretes pro- and anti-inflammatory adipokines such as adiponectin, leptin, visfatin, IL-6, and IL-8, which play critical roles in many aspects of the metabolic syndrome (Itoh et al. 2011). Thus, obesity is an important topic in the realm of public health and preventive medicine, since it is considered to be a risk factor associated with the genesis or development of various diseases, including coronary heart disease, hypertension, type 2 diabetes mellitus, cancer, respiratory complications, and osteoarthritis (Shehzad et al. 2011; Sowers and Karvonen-Gutierrez 2010). Increases in fat tissue result in part from an imbalance between energy intake and energy expenditure. It is also a result of increased adipogenesis, which includes adipocyte differentiation. Adipogenesis can occur even in adults, as observed in severe human obesity and rodents fed a high-carbohydrate or a high-fat diet (Kirkland et al. 1990). Conversely, when energy intake is less than output, mobilization of triglycerides leads to a decrease in adipose tissue mass.
There is increasing interest in finding safe and effective antiobesity agents for long-term use. Thus, many research groups have focused on developing antiobesity agents from natural products such as plants (Cho et al. 2008). However, the in vitro antiobesity effects of biomaterials extracted from plants and natural products are sometimes clinically negligible in humans (Gades and Stern 2005; Ho et al. 2001). Obesity is now viewed as a state of systemic, chronic low-grade inflammation, which directly promotes systemic insulin resistance (Apovian et al. 2008; Itoh et al. 2011), which has led to increased interest in anti-inflammatory agents as a means of treating obesity. Some molecules, such as resolvins and protectins, are endogenously generated during the process of inflammation, and are then involved in terminating acute inflammation so that the local tissues can return to homeostasis (Ariel and Serhan 2007).
TauCl is also endogenously produced during the inflammation process, in which it plays an important role (Schuller-Levis and Park 2004). We thought that some of the endogenous by-products produced by the inflammation process might also be involved in the homeostasis of adipose tissue. Thus, we investigated whether TauCl affects the differentiation of adipocytes and the expression of adipokines, which may play important roles in energy metabolism and inflammation. TauCl inhibited human preadipocyte differentiation into adipocytes and intracellular lipid accumulation in a dose-dependent manner without cytotoxicity. However, taurine alone at concentrations of 10–100 mM did not affect adipogenesis (data not shown), suggesting that TauCl has a specific inhibitory effect on the differentiation of preadipocytes into adipocytes. Future studies seeking to develop TauCl as a therapeutic agent to treat obesity-related diseases or disorders should employ animal models with obesity-related diseases. In addition, it will be necessary to develop taurine derivatives that are more stable and effective than TauCl, which is unstable and easily degraded at room temperature. TauCl derivatives should be safer for long-term use than extracts from natural materials, because TauCl is an endogenously generated molecule.
Some concerns should be raised regarding the anti-adipogenic effects of TauCl when used as an antiobesity drug. Adipose tissue comprises approximately 50% adipocytes and 50% other cell types, including preadipocytes; vascular, neural, and immune cells; and leucocytes (Compher and Badellino 2008). The number of adipoctyes is determined during early adulthood, and changes in fat mass are attributed to changes in adipocyte cell size (Spalding et al. 2008). Thus, most adult-onset obesity appears to be related to the hypertrophy of adipocytes; in addition to insulin resistance, enlarged adipocytes appear to be the factor most closely correlated with obesity (Bjorntorp et al. 1971; Weyer et al. 2000). Therefore, TauCl, which had in vitro anti-adipogenic effects in the present study, may not have any therapeutic effects on obesity-related diseases in adult humans. However, the adipocyte turnover rate in humans was recently established to be about 10% per year (Spalding et al. 2008). If endogenous anti-adipogenic molecules such as TauCl could be taken for long periods of time without side effects, they might reduce the number of adipocytes, thereby leading to loss of fat mass.
Adipose tissue also serves as a source of adipokines and cytokines, which have both local and systemic actions in health and disease. The adipocytes, preadipocytes, and macrophages within adipose tissue secrete a wide range of hormones and cytokines, including IL-6, IL-8, IL-1β, and monocyte chemoattractant protein (MCP-1). It is now evident that many of these adipokines have the ability to influence other tissues, such as liver and muscle. For example, IL-6 promotes inflammation not only in adipose tissue but also in endothelial and liver cells (Klover et al. 2005). IL-6 also promotes insulin resistance by interfering with insulin signaling in adipose tissue (Rotter et al. 2003). In general, pro-inflammatory cytokines have been demonstrated to prevent in vitro adipocyte differentiation from preadipocytes and to enhance lipolytic activity in adipocytes, leading to the so-called dedifferentiation (Coppack 2001; Gregoire et al. 1998).
5 Conclusion
TauCl, which is endogenously produced by immune cells such as macrophages that have infiltrated adipose tissue, may inhibit the differentiation of preadipocytes into adipocytes and modulate the expression of adipokines in adipocytes. Thus, TauCl could potentially be developed into a safe drug therapy for obesity-related diseases.
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- C/EBP:
-
CCAAT/enhancer-binding protein
- PPARg:
-
Peroxisome proliferator-activated receptor γ
- FABP:
-
Fatty acid-binding protein
- LPL:
-
Lipoprotein lipase
- GLUT4:
-
Glucose transporter 4
- TauCl:
-
Taurine chloramine
- HOCl:
-
Hypochlorous acid
References
Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N (2008) Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol 28: 1654–1659
Ariel A, Serhan CN (2007) Resolvins and protectins in the termination program of acute inflammation. Trends Immunol 28:176–183
Bjorntorp P, Berchtold P, Tibblin G (1971) Insulin secretion in relation to adipose tissue in men. Diabetes 20:65–70
Cho EJ, Rahman MA, Kim SW, Baek YM, Hwang HJ, Oh JY, Hwang HS, Lee SH, Yun JW (2008) Chitosan oligosaccharides inhibit adipogenesis in 3T3-L1 adipocytes. J Microbiol Biotechnol 18:80–87
Choi HM, Lee YA, Lee SH, Hong SJ, Hahm DH, Choi SY, Yang HI, Yoo MC, Kim KS (2009) Adiponectin may contribute to synovitis and joint destruction in rheumatoid arthritis by stimulating vascular endothelial growth factor, matrix metalloproteinase-1, and matrix metalloproteinase-13 expression in fibroblast-like synoviocytes more than proinflammatory mediators. Arthritis Res Ther 11:R161
Compher C, Badellino KO (2008) Obesity and inflammation: lessons from bariatric surgery. JPEN J Parenter Enteral Nutr 32:645–647
Coppack SW (2001) Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 60:349–356
Gades MD, Stern JS (2005) Chitosan supplementation and fat absorption in men and women. J Am Diet Assoc 105:72–77
Gesta S, Tseng YH, Kahn CR (2007) Developmental origin of fat: tracking obesity to its source. Cell 131:242–256
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78:783–809
Halberg N, Wernstedt-Asterholm I, Scherer PE (2008) The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am 37:753–768, x-xi
Ho SC, Tai ES, Eng PH, Tan CE, Fok AC (2001) In the absence of dietary surveillance, chitosan does not reduce plasma lipids or obesity in hypercholesterolaemic obese Asian subjects. Singapore Med J 42:6–10
Ito T, Schaffer SW, Azuma J (2011) The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids 42(5):1529–1539
Itoh M, Suganami T, Hachiya R, Ogawa Y (2011) Adipose tissue remodeling as homeostatic inflammation. Int J Inflam 2011:720926
Jessen BA, Stevens GJ (2002) Expression profiling during adipocyte differentiation of 3T3-L1 fibroblasts. Gene 299:95–100
Kim KS, Park EK, Ju SM, Jung HS, Bang JS, Kim C, Lee YA, Hong SJ, Lee SH, Yang HI, Yoo MC (2007) Taurine chloramine differentially inhibits matrix metalloproteinase 1 and 13 synthesis in interleukin-1beta stimulated fibroblast-like synoviocytes. Arthritis Res Ther 9:R80
Kirkland JL, Hollenberg CH, Gillon WS (1990) Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am J Physiol 258:C206–C210
Klover PJ, Clementi AH, Mooney RA (2005) Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology 146:3417–3427
MacDougald OA, Mandrup S (2002) Adipogenesis: forces that tip the scales. Trends Endocrinol Metab 13:5–11
Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W (1992) Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 97:493–497
Ronti T, Lupattelli G, Mannarino E (2006) The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 64:355–365
Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM (2002) C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev 16:22–26
Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896
Rotter V, Nagaev I, Smith U (2003) Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 278:45777–45784
Roufosse CA, Direkze NC, Otto WR, Wright NA (2004) Circulating mesenchymal stem cells. Int J Biochem Cell Biol 36:585–597
Schuller-Levis GB, Park E (2004) Taurine and its chloramine: modulators of immunity. Neurochem Res 29:117–126
Shehzad A, Ha T, Subhan F, Lee YS (2011) New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr 50:151–161
Sowers MR, Karvonen-Gutierrez CA (2010) The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol 22:533–537
Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P (2008) Dynamics of fat cell turnover in humans. Nature 453:783–787
Szymanski K, Winiarska K (2008) [Taurine and its potential therapeutic application]. Postepy Hig Med Dosw (Online) 62:75–86
Thomas EL, Grisham MB, Melton DF, Jefferson MM (1985) Evidence for a role of taurine in the in vitro oxidative toxicity of neutrophils toward erythrocytes. J Biol Chem 260:3321–3329
Tsuboyama-Kasaoka N, Shozawa C, Sano K, Kamei Y, Kasaoka S, Hosokawa Y, Ezaki O (2006) Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology 147:3276–3284
Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE (2000) Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 43:1498–1506
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0024089 and 2011-0009061).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this paper
Cite this paper
Kim, K.S. et al. (2013). Effect of Taurine Chloramine on Differentiation of Human Preadipocytes into Adipocytes. In: El Idrissi, A., L'Amoreaux, W. (eds) Taurine 8. Advances in Experimental Medicine and Biology, vol 775. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6130-2_21
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6130-2_21
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6129-6
Online ISBN: 978-1-4614-6130-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)