Abstract
The most critical problem with commercial scale geological CO2 sequestration is management of displaced fluids. All of the high quality numerical simulations of carbon capture, utilization, and storage (CCUS) on the Rock Springs Uplift (RSU), utilizing realistic 3-D reservoir models, demonstrate that commercial-scale geological CO2 storage will require the removal of formation brines in approximately 1:1 ratio of injected CO2 to displaced fluid. Without the production of formation brines the simulations suggest that very quickly injected CO2 will cause pressures in the storage domain to exceed fracture pressures. To solve this problem, Carbon Management Institute (CMI) proposed a strategy that includes integration of fluid production/treatment with injection of CO2. The treatment of the brines involved three important steps: (1) use of the temperature of the produced brines (~100 °C) to produce electricity via a heat exchanger to power the treatment facility, (2) to separate fresh water from the brines via nanofiltration and reverse osmosis, and (3) to recover metals, notably lithium, from the residual brines after partial evaporation. The impact of this approach; production of electricity, fresh water, and metals such as lithium from produced brines transform an anticipated carbon storage penalty into a revenue center.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
As described in Chap. 2, the University of Wyoming Carbon Management Institute and Wyoming State Geological Survey completed a thorough inventory and prioritization of all Wyoming stratigraphic units and geologic sites capable of sequestering commercial quantities of CO2 (5–15 Mt CO2/yr) (Surdam and Jiao 2007; Surdam 2011). These studies identified the Paleozoic Weber/Tensleep Sandstone and Madison Limestone (and stratigraphic equivalent units) as the leading clastic and carbonate reservoir candidates for commercial-scale geological CO2 sequestration in Wyoming. This conclusion was based on unit thickness, overlying low-permeability lithofacies, reservoir storage and continuity properties, regional distribution patterns, formation fluid chemistry characteristics, and preliminary fluid-flow modeling. This inventory also identified the Rock Springs Uplift in southwestern Wyoming as the most promising geological CO2 sequestration site in Wyoming and probably in any Rocky Mountain basin. This ranking of the Rock Springs Uplift was based on the following attributes (Surdam and Jiao 2007):
-
A thick saline aquifer sequence (700 ft of Weber Sandstone and 400 ft of Madison Limestone) overlain by a think sequence of stacked sealing lithologies
-
A double-plunging anticline with more than 12,000 ft of closed structural relief
-
A huge structural element (50 × 35 mi)
-
Targeted reservoir units (Weber Sandstone and Madison Limestone) with characteristics required for CO2 sequestration, including fluid chemistry, porosity, fluid-flow attributes, and burial history (relatively recent basin inversion resulting in reservoir rock/fluid systems formerly buried more than 20,000 ft deep now lying at depths between 6000 ft and 10,000 ft)
The results of the geological sequestration inventory led CMI to collect available geologic, petrophysical, geochemical, and geophysical data for the Rock Springs Uplift and to build a regional 3-D geologic framework model of the uplift. From the results of these tasks and using the FutureGen protocol, they showed that on the Rock Springs Uplift, the Madison Limestone has sufficient pore space to sequester 8 Gt of CO2 and the Weber Sandstone has the capacity to store an additional 18.4 Gt of CO2 (Surdam and Jiao 2007).
In cooperation with the Los Alamos National Laboratory (LANL), the CMI team combined these geologic databases with numerical models to improve estimates of the CO2 sequestration potential of the Rock Springs Uplift. The 3-D geologic model was constructed using EarthVision® software and was gridded using LaGrit software. Shallow and deep sequestration sites on the Rock Springs Uplift were evaluated using the LANL-PENS software (Stauffer et al. 2009).
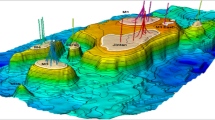
The results of this research are significant in the global effort to accomplish substantial commercial-scale CO2 sequestration. For example, one evaluated scenario was the sequestration of 15 million tons (Mt) of CO2 per year for 50 years into the Weber Sandstone in a nine-point injector pattern within a 16 × 16-km (10-mi × 10-mi) area on the Rock Spring Uplift (Fig. 11.1). These parameters were chosen because the Jim Bridger power plant (2200 MW) is located on the uplift and emits 15 Mt/yr of CO2. The modeled nine-point injection pattern was located near the power plant on the east flank of the RSU. The nine simulated injection wells, each injecting 1.7 Mt/yr of CO2 were spaced approximately 1 mi apart. For the commercial-scale sequestration scenario, no fluid flow was allowed down-dip and the initial pressure up-dip was specified at below fracture pressure. After 50 years of injection, the CO2 plumes around the injection wells barely impinged on one another. All the CO2 (750 Mt was contained within the 16 × 16-km storage area (area of review). Moreover, the preliminary modeling in this scenario demonstrates that once injection stops, the pressure buildup in the individual injection wells decreases to near initial pressure in 25 years (Surdam and Jiao 2007; Surdam et al. 2009, 2011).
CO2 injection simulation results utilizing Los Alamos National Laboratory’s FEHM simulators for the Weber Sandstone, Rock Springs Uplift, Wyoming. (Modified from Surdam et al. 2009)
The most critical problem in this geological CO2 sequestration simulation is the relationship between the volume of injection CO2 and the displaced fluid that must leave the storage area (Fig. 11.2). In the example cited above, 750 Mt of CO2 is sequestered in the storage domain and 1 km3 of fluid must leave the domain over a 75-year period (50 years of CO2 injection and 25 years post-injection). The key questions are as follows: Can the accommodation space be found within the geologic site to accept this huge volume of fluid that must leave the storage domain? If so, given the heterogeneity of most geological settings (fluid-flow compartmentalization), can fluid migration pathways be maintained so that the displaced fluid can migrate from the storage domain to some external accommodation space without disrupting the confining units and destroying the integrity of the rock/fluid system?
CO2 injection simulator results reveal that 750 Mt of CO2 is sequestered in the storage domain and 1 km3 of fluid must leave the storage domain (migrate out or be produced) to manage the reservoir pressure under the fracture pressure of the Weber Sandstone at the injection site. (Modified from Stauffer et al. 2009 and Surdam et al. 2009)
These difficult questions plague not only the RSU project but many other CCS projects as well. Much of the uncertainty raised by these questions can be avoided with proper management of displaced fluids in a CCS project. To manage the displaced fluids in the RSU CO2 storage project, the CMI/WSGS team proposed a strategy that includes integration of fluid production/water treatment with CO2 injection (Surdam et al. 2009). If the ratio of produced fluids to injected CO2 remains near 1:1, this strategy greatly reduces the probability of creating damaging pressure effects and unsuspected CO2 migration beyond the defined storage area. Most importantly, a major objective in designing a successful carbon storage project on the RSU is minimization of the domain outside of the CO2 storage area that is affected by potential fluid plume migration. Without displaced fluid management, carbon storage on the RSU would be highly problematic.
Using this strategy in the RSU project, it will be possible to inject 750 Mt of CO2 into the Paleozoic Weber and Madison formations and to sequester the CO2 in a 16 × 16-km storage domain over a 50-year period (Fig. 11.1). It is noteworthy that the injection of the 750 Mt of CO2 will displace 1 km3 of fluid over a 75-year period (Fig. 11.2).
If 15 million tonnes of CO2 is injected annually into the CO2 storage reservoirs, approximately 80 million barrels of fluid will be displaced each year for 75 years (a total of 6 billion barrels). Lindner-Lunsford et al. (1989) showed that the salinity concentrations (TDS) in groundwater in the targeted Paleozoic aquifers in southwestern Wyoming generally exceed 30,000 mg/L, so any produced fluids must be treated. Aines et al. (2010) showed that at an osmotic pressure limit of 1200 psi, a Tensleep/Weber brine (Na–Cl–SO4; 25,000 mg/L) can be treated via reverse osmosis (RO) at 50 °C, achieving up to 80 % H2O removal. Also, they showed that osmotic pressure, not mineral scaling, is the most important constraint on RO treatment of brines (Aines et al. 2010).
Numerical simulations, using real rock/fluid system characteristics, demonstrate that it is feasible to inject 15 Mt of CO2 per year for 50 years into the Weber/Madison formations on the Rock Springs Uplift (a total of 750 Mt of CO2) in relatively small areas of the RSU. This volume of CO2 can be contained beneath a 16 × 16-km area (the storage domain; Fig. 11.3). Over a 75-year period, this volume of CO2 will displace one cubic kilometer of fluid. Without management, this huge volume of displaced fluid will further cause unwanted fractures within the storage domains, or will migrate out of the storage domain, probably up-dip toward the crest of the structure, 30 km to the west. Numerical simulations for the RSU 50-year storage scenario (with unmanaged displaced fluids) suggests that pressure effects will occur 30–40 km up-dip from the site of CO2 injection, necessitating a huge area of review (1200 km2) under proposed regulations. Under new EPA regulations and Wyoming state statutes governing CO2 sequestration, commercial-scale storage of CO2 on the RSU (10 Mt CO2 or greater) will definitely require displaced fluid-management. At present, the fluid management strategy of choice is fluid production, RO treatment, with the water and metal recovery driven by reservoir temperature and pressure.
11.2 Fluid Treatment Strategy
This section highlights a proposed produced fluid management study. Recent site- specific fluid analysis from RSU #1 measured TDS concentrations ranging from 80,000 mg/L to 100,000 mg/L. Research has shown that the lower range of these waters could be treated with standard RO with low recovery, ~10 % (Aines et al. 2010). By combining multistage RO or adding nanofiltration membranes at the front end of RO treatment, makes the treatment of these waters probable (Aines et al. 2010; Bourcier et al. 2011).
In conventional applications (i.e., treatment of sea water), much of the cost of the procedure is the energy required to pressurize the treatment system. Pressures of 700–1200 psi are needed to create the reverse osmosatic pressure gradient between the saline and fresh water (Fritzmann et al. 2007). Reverse osmosis is limited by the maximum pressure difference that the membrane spacers can withstand without collapsing and causing the membrane to become impermeable (Matsuura 2001). The current limit for commercially available membrane spacers is 1200 psi (Bourcier 2011); presently the membrane itself can withstand higher pressures. Higher-pressure membranes have been developed for osmatic pressures of 1500 psi (Baker 2004) but have not been demonstrated commercially. This is likely due to the large amount of energy required to pressurize seawater to above 1200 psi; thus, seawater treatment is not economical at 1500 psi.
However, in treating displaced fluids, pressure is an asset rather than a problem because the fluid arrives at the membrane pressurized (the in situ formation fluids in the Weber/Madison formations on the Rock Springs Uplift come in at 4800–5900 psi). The energy needed to drive the RO process would be harvested directly from the pressure of the formation. Consequently, treating these produced fluids would cost half as much as treating sea water with RO (Aines at al. 2010). Estimated recovery for the RSU brines are approximately 10 % using osmotic pressures of 1200 psi, but could exceed 20 % by incorporating nanofiltration and increasing osmatic pressure to 1500 psi (Aines et al. 2010; Bourcier et al. 2011).
Nanofiltration membranes resemble RO membranes but discriminate against larger-molecular-weight ions and divalent ions due to larger pore sizes and a surface charge on the membrane. Hilal et al. (2004) suggest that nanofiltration membranes reject > 90 % of the divalent ions while letting monovalent ions pass. Nanofiltration is used to partially lower the salinity of the brine in order to increase the efficiency of the RO process and is especially attractive in fluids that have a hardness of > 10,000 mg/L. Though RSU brines are relatively soft (2000–5000 mg/L), the nanofiltration phase could be utilized as a pressure-reduction step, decreasing scaling potential in the RO process by removing Ca, Mg, and SO4, and lastly to begin the separation process for potential mineral recovery (Sect. )
The temperature of the brines provides another means for further energy/cost reduction benefit when compared to seawater treatment. The brines of the RSU have a measured temperature of 92–100 °C. Modern binary power generating units have demonstrated success with temperatures as low as 73 °C (Green and Nix 2006). Geothermal heat recovered from produced brine could generate enough electricity to defray the cost of water treatment, and perhaps, to sell an excess. Aines et al. (2010) report that the lifetime of RO membranes is greatest when fluid temperatures range between 40 °C and 50 °C. Thus RSU brines would need to undergo a cooling phase prior to treatment; it simply makes sense to harvest that heat via a heat exchanger for electricity generation.
Figure 11.4 illustrates a potential course of operations for the water treatment of RSU brines. First, the water is produced from the formation, arriving to the surface at about 100 °C, 5000 psi, and 80,000 mg/L TDS, a strongly sodium-chloride brine, with lesser amounts of Ca, Mg, and SO4. The first step in the operation is a geothermal heat exchanger: this step not only harvests thermal energy for electricity generation, but decreases the temperature of the brine to optimize the RO treatment. Next, the brine is passed through a nanofiltration membrane, and as a result the overall TDS is reduced to about 75,000 mg/L, pressure is reduced to 1500 psi, and more than 90 % of the calcium, magnesium, and sulfate are removed. Removing the divalent ions greatly reduces the risk of mineral scale during RO treatment, also effectively lowering the lithium-to-magnesium ration. The feed is now ready for RO treatment. The permeate from the RO process is estimated to be 20–40 % of the original feed stream while 60–80 % is further concentrated in the brine. The permeate water is ready to be sold to market. The TDS concentration of the residual brine is expected to increase from 75,000 mg/L to 98,000 mg/L–126,000 mg/L (Fig. 11.5). By concentrating the brine the lithium concentration is also increased from 95 mg/L to 110 mg/L–150 mg/L (Fig. 11.6). The residual brine is then moved to evaporation ponds for mineral recovery. The last step is to dispose of any remaining brine back into the subsurface.
11.3 Value Added Products
A treatment scenario as described above produces value-added products: fresh water, electricity, and metals. In arid Wyoming, the need and demand for potable water is very high: local coal-to-power and coal-to-chemical plants, agriculture operations, hydraulic fracturing water, residential users, and downstream users in the Colorado River drainage, among others, all require increasing amounts of water. Bourcier et al. (2011) suggest that treatment cost for reservoir pressure driven RO with 20 % recovery might range between US $450 to US $600 per acre-ft of permeate. Agriculture users in some areas of the Colorado River drainage pay more than US $700 per acre-ft. Therefore it is conceivable that the sale of fresh water to market could in fact cover the cost of treating the produced fluid.
Treating these formation fluids at a reverse osmosis treatment plant, or other desalination facility, would yield approximately 10,000 acre-ft of portable water per year, which could be used by an adjacent power plant; by the community of Rock Springs, Wyoming; as replacement water in the Upper Colorado river drainage; or perhaps by communities along the Colorado Front Range. The heat associated with the produced brines (100–130 °C) could be used to generate power for the power plant or the water treatment plant or both.
The feasibility of such a large-scale desalination endeavor is demonstrated by desalination projects in Israel, where desalination plants produce 2 million barrels of potable water per day −13 % of the country’s annual water consumption (water-technology.net 2012).
Geological CO2 sequestration on the scale required by a large power plant—on the Rock Springs Uplift or elsewhere in the Rocky Mountain region—will require displaced fluid management. Work at the Wyoming State Geological and CMI suggests an integrated CO2 sequestration/fluid production/fluid treatment strategy to accomplish CO2 sequestration on the Rock Spring Uplift: carbon is sequestered on a commercial scale at depth while displaced fluids are produced and treated at the surface. The volume of treated water produced as a result of this strategy represents a valuable commodity in arid southwestern Wyoming. The metals recoverable form the concentrated brine also represent value: recent discoveries at CMI suggest that treatment of displaced brine at a carbon storage facility would result in a substantial economic asset. In fact, recovery of metals from the residual brine may not only pay for the displaced-fluid treatment, but may create a significant profit center. (For the profitable recovery of lithium, for example, see Sect. ). Finally, even the heat contained in the produced brine (92–100 °C) is valuable, as process heat or as a heat exchange source for steam generation
The proposed integrated strategy may be expensive, but there is presently no viable alternative in Rocky Mountain basins. Finally, it is important to realize that this proposed strategy will be a test of the whole idea of CO2 sequestration: if commercial-scale geological CO2 sequestration cannot be accomplished on the Rock Springs Uplift, it probably cannot be accomplished anywhere in the Rocky Mountain region.
11.4 Changing Markets with a New US Lithium Resource—Transitioning from a Significant Lithium Importer to an Independent Lithium Producer
In the global transition to greener economies, demand and competition for lithium resources has significantly intensified. A reliable, abundant, available supply of lithium (Li) is key to accelerating this transition in the United States, particularly with respect to batteries required for energy storage and electronic devices ranging from ordinary cell phones to high-tech military applications. The University of Wyoming Carbon Management Institute (CMI) discovered a vast new lithium resource during its CO2 storage site characterization project, WY-CUSP—a resource that could transform the United States from a net lithium importer to an independent Lithium producer during the next 25 years.
In evaluating formation fluids retrieved from its RSU #1 well on the Rock Springs Uplift, CMI noted that formation fluids from the Weber and Madison formations contain relatively high concentrations of lithium. The lithium concentrations in two sets of samples from both formations, collected in August 2011 and December 2012, ranged from 90 mg/L to 100 mg/L. In sufficient volume, fluids with these lithium concentrations could support a successful lithium recovery industry. The huge volume of Weber/Madison formation fluids available on the Rock Springs Uplift potentially provides the United States with an abundant new source of lithium.
Integrated deployment of carbon capture, utilization, and storage (CCUS) technology together with recovery of a huge new domestic lithium resource would (1) substantially reduce US dependence on foreign lithium imports within 25 years (we currently import up to 80 % of our lithium); (2) lay the foundation for entirely new integrated energy development industries and create a new US lithium market; (3) enable continued use of domestic fossil fuels by storing CO2 (emission reduction) and providing CO2 to enhanced oil recovery projects; (4) create a new source of potable water; and (5) keep the United States at the forefront of emerging advanced lithium and CCUS technologies.
The lithium resource discovered by CMI is a significant component of the formation brines within Wyoming’s premier CO2 storage reservoirs (the Mississippian Madison Limestone and Pennsylvanian Weber/Tensleep Sandstone) at the state’s highest-priority CO2 storage site (the Rock Springs Uplift (RSU) in southeastern Wyoming). The lithium concentration in these formation brines is 90–100 mg/L. CMI proposes to facilitate development of this lithium resource by designing water treatment and lithium production facilities, along with other infrastructure necessary to integrate lithium recovery into a CCUS framework; the CMI strategy would transform an enormous lithium resource into a substantial proven domestic lithium reserve. On the Rock Springs Uplift the potential CO2 storage reservoirs lie at depths of 7000–12,000 ft (2000–3500 m). Due to the depth of the lithium-rich brines, the economic viability of lithium recovery depends on implementation of CCUS at the site. Though CCUS technology and methods for extracting lithium from brines exist and are currently in use, these technologies have never been integrated, and lithium has never been produced on a commercial scale from such deep brines: consequently, the proposed integration of these two technologies is currently a new and untried process.
Numerical simulations and performance assessments of CO2 storage scenarios for RSU site indicate that, concurrent with CO2 injection, pressure management via brine production and surface treatment will be required (1:1 ratio, brine:CO2). Injection of CO2 into the brines could increase the lithium concentration in the brines through dissolution of Li2CO3 in the rock matrix. The brine treatment, designed around reverse osmosis, will increase the lithium concentration of residual brines by 120 % (Fig. 11.4). Presently, the lithium concentration of the brines at Silver Peak, Nevada—the main existing lithium-producing site in the nation—is approximately 230 mg/L.
With injection and storage of 1 million tonnes of CO2 annually and a recovery rate of 65 %, 312 tonnes of Li2CO3 would be recovered per year on the RSU from produced brines. Stationary sources in southwestern Wyoming currently emit 29 Mt of CO2 annually, the injection of which could yield enough lithium to replace annual US imports and cut the state’s annual CO2 emissions by 50 %. Consider the following:
-
Recovery of lithium from produced brines transforms the economic penalty normally associated with pressure management in carbon storage applications into a source of profit.
-
The lithium resource within the Madison Limestone underlying just the 25-mi2 (65-km2) study area is estimated at 76,000 tonnes (assuming 10 % porosity), approximately three years of global lithium production (averaging roughly 25,000 tonnes per year). The RSU and Madison Limestone cover nearly 2000 mi2 (5000 km2), so the lithium resource in the Madison on the RSU is truly unique, huge, and capable of changing the world market.
-
The lithium resource in the Weber Sandstone is even larger: the lithium resource in the Weber Sandstone underlying just the 25-mi2 (65-km2) study area is estimated at 152,000 tonnes (assuming 10 % porosity), approximately six years of global lithium production. The Weber Sandstone on the RSU covers nearly 2000 mi2 (5000 km2): the lithium resource in the Weber Sandstone is also unique, huge, and capable of changing world markets.
-
Combined, the formation fluids in the Madison Limestone and Weber Sandstone within a 100 mi2 area contain 900,000 tonnes of lithium, equivalent to 36 years of present global lithium production. Should these concentrated brines in the Madison and Weber formations be present throughout the RSU (2000 mi2, 5000 km2), these units, with four-way closure and 10,000 ft (3000 m) of structural relief, could contain as much as 18 Mt of lithium.
-
The lithium reserves at Silver Peak, Nevada, the largest producer of lithium in the United States, are 118,000 tonnes from a 20 mi2 (52 km2) area.
-
Before lithium can be precipitated from brine, magnesium salts must be removed; low Mg concentrations are desirable. The magnesium concentrations in brines derived from the Madison Limestone and Weber Sandstone are relatively low, about 150 mg/L and 40 mg/L, respectively. The Mg: Li ratio for the Madison and Weber brines on the RSU is less than 2:1, whereas the Mg:Li ratio in the brines of the “lithium triangle” in South America (home to 70 % of the world’s economic lithium deposits) is 6:1.
-
Southwestern Wyoming hosts the world’s largest soda ash industry. Production of 1 tonne of Li2CO3 from brine (LiCl) requires 1.8 tonnes of soda ash. The RSU is located just 20–30 mi (30–50 km) by railroad or interstate highway from this soda ash source.
-
The scientific tools, experience, and expertise necessary to understand recovery of lithium on the RSU have been gathered by CMI during extensive research into CO2 injection to inform CMI’s CCUS characterization project. This research includes a 5 × 5-mi (8 × 8-km) 3-D seismic survey; a 12,812-ft-deep (3900 m) stratigraphic test well; 916 ft (278 m) of high-quality core; specialized electric log suites; formation fluid samples; continuous visual documentation of core; core flooding tests; continuous permeability scans; and a wide variety of core measurements. Most importantly, CMI now has the ability to accurately simulate CO2 injection and brine production scenarios.
-
The potential impact of the recovery of the RSU lithium resources on the global market is huge: it could transform the US from a significant lithium importer to an independent lithium producer.
-
Risks associated with the project are not technical, but rather hinge on federal greenhouse-gas emissions policy. For instance, recent new USEPA regulations mandating CCUS technologies for new coal-fired power plants should provide additional incentive to create regional CCUS complexes.
CMI’s lithium discovery will create new industries (domestic lithium production), reduce US dependence on foreign lithium and fossil fuel imports, overcome a major hurdle for CCUS by making pressure management profitable, reduce GHG emissions, create an additional market for soda ash, sustain Wyoming’s coal extraction industry by implementing CCUS, and provide CO2 for enhanced oil recovery projects (the 4–8 billion barrels of stranded oil in Wyoming will require 1.8–3.3 billion tonnes of CO2 for tertiary recovery). Importantly, active development of this resource will ensure US technological leadership in developing and deploying advanced lithium and CCUS technologies. For Wyoming, important results will be new industries, increased direct and indirect employment, new or expanded markets, and sustained or expanded traditional economies.
References
Aines R, Wolery T, Bourcier W (2010) Fresh water generation from aquifer-pressured carbon storage. Ninth annual conference on carbon capture and sequestration. Pittsburgh, Pennsylvania
Baker RW (2004) Membrane technology and applications (2nd ed). Wiley, Chichester
Bourcier WL, Wolery TJ, Wolfe T, Haussmann, Buscheck TA, Aines RD (2011) A preliminary cost and engineering estimate for desalinating produced formation water associated with carbon dioxide capture and storage. Int J Greenhouse Gas Control 5:1319–1328
C. Fritzmann, J. Löwenberg, T. Wintgens, T. Melin, State-of-the-art of reverse osmosis desalination, Desalination, Volume 216, Issues 1–3, 5 October 2007, Pages 1–76, ISSN 0011-9164, http://dx.doi.org/10.1016/j.desal.2006.12.009
Green B, Nix RG (2006) Geothermal – the energy under our feet, geothermal resource estimates for the United States: National Renewable Energy Laboratory Technical Report NREL/TP-840–40665
Hilal N, Al-Zoubi H, Darwish NA, Mohammed AW, Arabi MA (2004) A comprehensive review of nanofiltration membranes: treatment, pretreatment, modeling, and atomic force microscopy. Desalination 170:281–308
Lindner-Lunsford, JB, Kimball BA, Chafin DT, Bryant CG (1989) Hydrogeology of aquifers of Paleozoic age, upper Colorado River basin – excluding the San Juan Basin-in Colorado, Utah, Wyoming, and Arizona. U.S. Geological Survey Hydrologic Investigations Atlas HA-702, scale 1:2,500,000 and 1:6,000,000
Matsuura T (2001) Progress in membrane science and technology for seawater desalination – a review. Desalination 134:47–54
Stauffer PH, Surdam RC, Jiao ZS, Miller T (2009) Combining geological data and numerical modeling to improve estimates of the CO2 sequestration potential of the Rock Springs Uplift, Wyoming. In: Proceedings of the 9th greenhouse gas technology conference. Energy Procedia 1(1):2714–2724. Elsevier
Surdam RC, Jiao ZS (2007) The Rock Springs Uplift – an outstanding geological CO2 sequestration site in the southwest Wyoming. Wyoming State Geological Survey Challenges in Geologic Resource Development No. 2
Surdam RC, Jiao ZS, Stauffer P, Miller T (2009) An integrated strategy for carbon management combining geological CO2 sequestration, displaced fluid production and water treatment. Wyoming State Geological Survey Challenges in Geological Resource Development No. 8
Surdam RC, Jiao ZS, Stauffer P, Miller T (2011) The key to commercial-scale CO2 sequestration: displaced fluid management. Energy Procedia 4, pp 4246–4251
water-technology.net (2012) Ashkelon, Israel. Net Resources International. www.water-technology.net/projects/israel. Accessed January, 2013
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Surdam, R., Quillinan, S., Jiao, Z. (2013). Displaced Fluid Management—the Key to Commercial-Scale Geologic CO2 Storage. In: Surdam, R. (eds) Geological CO2 Storage Characterization. Springer Environmental Science and Engineering. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5788-6_11
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5788-6_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5787-9
Online ISBN: 978-1-4614-5788-6
eBook Packages: EnergyEnergy (R0)