Abstract
The biogeochemistry of the world oceans has been studied for many decades, and major advances in understanding have been linked with development of new techniques and tools that allow the accurate representation of various organic and inorganic pools within the water. The classic study of Redfield 1 showed that some critical bioactive compounds (carbon, nitrogen, phosphorus, oxygen) occur in particular ratios to one another that are relatively invariant over space and time and provided a description of the relationship between the ratio of nitrogen to phosphorus (N:P) for inorganic and plankton pools. The processes that control these compounds were assessed, and it was concluded that phosphorus concentrations are largely controlled by terrestrial inputs, whereas nitrogen is under biological control.
This chapter was originally published as part of the Encyclopedia of Sustainability Science and Technology edited by Robert A. Meyers. DOI:10.1007/978-1-4419-0851-3
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Definition of the Subject and Its Importance
The biogeochemistry of the world oceans has been studied for many decades, and major advances in understanding have been linked with development of new techniques and tools that allow the accurate representation of various organic and inorganic pools within the water. The classic study of Redfield [1] showed that some critical bioactive compounds (carbon, nitrogen, phosphorus, oxygen) occur in particular ratios to one another that are relatively invariant over space and time and provided a description of the relationship between the ratio of nitrogen to phosphorus (N:P) for inorganic and plankton pools. The processes that control these compounds were assessed, and it was concluded that phosphorus concentrations are largely controlled by terrestrial inputs, whereas nitrogen is under biological control.
Subsequent studies have provided more detailed investigations of the processes controlling these ratios. These studies benefited from the development and standardization of methods for accurately measuring dissolved organic carbon (DOC) and dissolved organic nitrogen (DON). The improved methodology, mostly developed during the 1980s, allowed the spatial (vertical and horizontal) and temporal changes of both DOC and DON to be quantitatively described.
Recognition of the importance of the flux of organic carbon to depth in mediating the marine response to increased atmospheric carbon dioxide concentrations stimulated development of technical approaches and instruments for assessing and quantifying the biological pump. This component of marine biogeochemical cycles is still a poorly constrained component of numerical models developed for simulation of ocean carbon cycling and climate, and technological approaches that result in better assessment of the flux of organic matter to depth continue to be developed and refined. Also, numerical models of biogeochemical processes are providing insights into critical processes and provide frameworks that allow measurements to be projected over larger space and timescales. Continued measurement and modeling of oceanic biogeochemical cycles is essential for understanding and projecting responses to natural and anthropogenic-induced climate change.
Introduction
The ocean is the dominant surface feature that has controlled much of the evolution, distribution, and success of life on earth, and the changes in ocean chemistry reflect the interaction with biota throughout geological time. The oceans were originally anoxic, but the evolution of organisms with oxygen-generating processes (photosynthesis) resulted in the conversion of the oceans to an oxygenated environment, which greatly altered the availability of some elements for those organisms. The cycling of elements within the earth’s oceans and the complex relationships among the biological, chemical, and geological processes are the core of the study of marine biogeochemistry. Understanding these relationships is difficult and is further complicated by the space and time variability of the dominant processes that control the cycling of the different elements. Understanding the interactions and linkages among and between the cycles of biogeochemical elements is critically important for assessing and projecting the nature, degree, and direction of changes in ocean processes that may result from changes induced by natural and/or anthropogenic activities.
Elements in the ocean have characteristic vertical and horizontal distributions that result from the processes that regulate their long-term source/sink relationships. For example, oceanic carbon dioxide (CO2) distributions are characterized by a horizontal concentration gradient that increases from the equator to the poles, which results from the greater dissolution of CO2 in colder water. Carbon dioxide concentrations generally increase with depth due to remineralization in the deeper, older waters relative to its removal at the surface. Other elements may be controlled by different factors (e.g., sources from the sediments or hydrothermal vents; atmospheric sources) and have different vertical and horizontal patterns, but all interact to create the observed vertical distributions in the ocean. Understanding marine biogeochemistry requires knowledge not only of specific processes regulating a particular element, but also an understanding of the interdisciplinary aspects that control these cycles.
Nutrients are the biogeochemical elements that are required for biological activity. Some elements are greatly reduced in their concentrations by chemical or biological processes and can reach such low concentrations that they subsequently limit the growth of organisms in the sea. Such elements are thought of as limiting nutrients in the sense of the German agricultural chemist, Justus von Liebig, who suggested that the growth of plants is limited not by the total amount of resources, but by the resource in lowest abundance relative to the others.
Plant growth in the ocean is known to be limited by a small number of nutrients that include nitrogen, phosphorus, iron, silicic acid, and inorganic carbon. The cycling and processes that control the concentrations of these limiting nutrients are critical in the regulation of carbon cycling in the ocean, and hence their study forms the basis for most biogeochemical research.
The biogeochemical cycles described in subsequent sections use carbon as a “common denominator.” Carbon is the basic component of organic matter, and with the advent of industrialization is being added to the atmosphere at an unprecedented, rapid rate, which is changing atmospheric temperatures and impacting the thermal equilibrium of the ocean. Also, carbon is absorbed from the atmosphere at the ocean surface where it reacts with ocean water to produce carbonic acid, thereby making ocean waters more acidic (reducing the pH), which has profound impacts on oceanic chemistry and biological activity. Thus, the production and oxidation of organic matter in the ocean has numerous critical interactions with all other elemental cycles, and is a major regulator of all marine biogeochemical cycles.
The Biogeochemical Cycle of Carbon
Carbon is the primary building block for all life because of its chemical ability to form a myriad of covalent bonds with itself and numerous other elements. As a result, the numerous complex organic compounds that form the basis of life systems are based on carbon. In the present-day ocean, synthesis of organic molecules (photosynthesis) is done largely by phytoplankton, which converts the radiant energy from the sun into chemical energy in the form of adenosine triphosphate (ATP). The ATP, along with reductant, is used to reduce CO2 into simple sugars, which are in turn modified into all of the compounds required for cellular metabolism, growth, and division. Photosynthesis is dependent on energy from the sun, thereby confining this process to the euphotic zone, which is the part of the upper water column that receives at least 1% of the irradiance that reaches the sea surface. Phytoplankton require energy for the uptake and assimilation of nearly all elements. This dependence on light generally results in vertical distributions of nutrients that are characterized by reduced concentrations in the euphotic zone, where photosynthesis and growth are most active, and increased concentrations at depth, where photosynthesis and growth are reduced or absent (Fig. 12.1). This vertical profile is a typical of nutrient distributions throughout the oceans. The organic matter generated by photosynthesis and growth has roughly an inverse relationship to that of the inorganic building blocks (Fig. 12.1).
Generalized vertical distributions of dissolved inorganic elements and particulate matter produced by phytoplankton photosynthesis. Particulate matter concentrations are less than those expected from the disappearance of inorganic elements because of removal by various processes to depth (see Fig. 12.2). Similarly, the particulate matter vertical distribution is less uniform because the time-scale of redistribution of particles is much faster than that of the inorganic elements
Redfield [1] suggested that organic matter (carbon, C) production in the sea occurs in relatively constant elemental ratios given by the relationship:
This relationship describes the reaction of \( C{O_2} \) with hydrogen (H), nitrate (\( NO_3^{-} \)), phosphate (\( {H_3}P{O_4} \)), and water (\( {H_2}O \)) within the photosynthetic process to produce (↔) organic carbon-nitrogen-phosphorus compounds \( \left[ {{{(C{H_2}O)}_{106}}{{(N{H_3})}_{16}}{H_3}P{O_4}} \right] \) and gaseous oxygen (\( {O_2} \)). The numbers preceding the compounds indicate the amount of each. The relationship is reversible (↔) because metabolism (oxidation) of the organic matter produced by photosynthesis regenerates inorganic C, nitrogen (N), and phosphorus (P) in the same ratio and utilizes oxygen. The C:N:P ratio of 106:16:1 obtained from the above relationship is a basic paradigm of marine biogeochemistry. However, Redfield recognized that the C:N:P ratios vary within plankton types and with time, a fact that has been further established in more recent studies. The departure from the basic ratio provides insights in how marine ecosystems change and/or adapt to modified environmental or biological conditions.
All marine organisms contribute to the carbon cycle by moving carbon between organic and inorganic forms, but some marine organisms are able to use calcification to transform inorganic carbon, using bicarbonate and dissolved calcium from the water column to produce calcium carbonate (CaCO3), which is then used to form a skeleton or protective shell [2]. The dissolution of calcium carbonate back into its original components is one of the primary means by which the particulate components reenter the water column, keeping the inorganic carbon cycle running. Although some of the calcium carbonate dissolved back into the water column comes from dead organisms, a large portion is contributed by phytoplankton from coccolithophorids, the genus coccolithophorid, which produce and shed calcium carbonate shells, making them a major contributor to the inorganic carbon cycle [2]. The calcium carbonate not immediately dissolved back into the water column is removed by sinking, with coccolithophorids comprising a major component of the carbon found in marine sediments. A by-product of calcification is CO2, which either remains in the water column or reenters the biological pump through photosynthesis [2].
The importance of iron (Fe) and silicic acid (Si(OH)4) in regulating carbon production in oceanic systems has also been established. Iron is required by all living organisms for a variety of metabolic processes, and silicon (Si) is needed by an important phytoplankton functional group, the diatoms, which are characterized by a hard silica shell. Diatoms remove silicic acid in approximately a 1:1 ratio to N, and the P:Fe ratio is approximately 1,000:1. Both ratios show considerable plasticity and their uptake ratios are related to other environmental variables as well [3, 4].
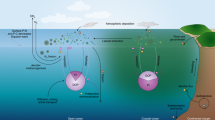
The organic material produced in the upper water column via photosynthesis is used by heterotrophic organisms (e.g., bacteria, zooplankton) and transformed by their metabolism and growth processes. The unassimilated ingestion of these organisms (fecal pellet production) sinks and is oxidized below the euphotic zone by a host of heterotrophic organisms (from bacteria to ciliates to scavenging, mobile animals), thereby converting the organic matter to CO2. Also, particle aggregates formed from phytoplankton cells, detritus, and dead organisms sink from the euphotic zone and are oxidized. The unidirectional movement of large particles to depth and their remineralization defines the biological pump (Fig. 12.2), which also contributes to the generation of “nutrient-like” profiles in the ocean. The processes that contribute to the fluxes within the biological pump are critical to understanding the marine carbon cycle.
Schematic of the biological pump showing the biological and chemical components and processes involved in the transformation of carbon dioxide (CO2) to organic matter, and the subsequent transformation, movement, and oxidation of particulate organic carbon (POC) and dissolved organic carbon (DOC). The CO2 is absorbed from the atmosphere across the air-ocean interface (wavy lines) and is transformed by processes in the euphotic (above dashed line) and aphotic zone (below dashed line). The migration of zooplankton and higher trophic levels within the water column (light blue lines) and unidirectional passive sinking of particles of different sizes to depth (green dot-dashed line) redistribute organic material. Processes of grazing/ingestion (red dashed line), aggregate formation (red line), respiration and CO2 generation (orange line), physical mixing (heavy blue line), and solubilization, and DOC generation (dark blue line) modify the rate at which POC is exported to depth from the surface waters. The POC pool at depth is generally composed of unidentifiable, small particles, whereas the POC pool in the surface is composed of recognizable biota (bacteria, phytoplankton, zooplankton) and variable amounts of detritus
Atmospheric fluxes of CO2 into and out of the ocean vary spatially. In general, equatorial waters tend to be large sources of CO2 (net fluxes are from the ocean to the atmosphere). The equatorial Pacific is a large source because it is the site of large-scale upwelling, a process which brings cold water from depth to the surface. These waters are in turn heated by solar radiation, and because the solubility of CO2 is strongly temperature dependent (CO2 is less soluble in warm water), it is lost to the atmosphere. Conversely, polar waters are in general sinks for CO2. Waters there lose heat to the atmosphere, and thus are able to absorb more CO2. A topic of intense debate is the possible decrease in carbon flux to the waters of the Southern Ocean resulting from recent increases in wind strength, which may have altered the ocean’s ability to remove CO2 [5]. Such changes potentially would have profound impacts on the global carbon budget. At the present time the ocean is a net sink for atmospheric carbon dioxide, and has sequestered at least 25% of all anthropogenic emissions to date.
Ocean Acidification
Recently, great concern has been expressed about the increasing concentrations of CO2 in the ocean, since its absorption decreases the pH, leading to ocean acidification [5, 6]. A decrease in pH would seriously impact calcification, likely increasing dissolution of CaCO3 found in skeletons and shells because the material is unprotected from seawater, and decreasing the rate at which calcification can occur by altering the concentrations of the necessary minerals in the water column. As a result, decreased pH has a great capacity to alter the ecology of marine systems such as coral reefs. In addition, decreased pH levels have been shown to alter the growth, reproduction, efficiency, and survival of those organisms that require CaCO3 to survive, and these effects vary among organisms, suggesting that substantial and unexpected impacts on biodiversity could occur [7].
It is now recognized that many phytoplankton can remove only CO2 for use in photosynthesis. Under preindustrial pH levels, free CO2 levels could have been at limiting levels, particularly for conditions that produced high concentrations of algae, because photosynthesis naturally increases the pH level. Decreased pH and increased absolute CO2 levels arising from current conditions might reduce this limitation. Because there is substantial variability among species of phytoplankton in their response to increased CO2, planktonic biodiversity is at risk [8]. However, certain algal functional groups, such as nitrogen-fixing cyanobacteria, positively respond to increased CO2 concentrations by increasing their growth and photosynthesis, whereas others can not. Similarly, at least one species of toxin-producing dinoflagellate demonstrated increased growth and modified elemental ratios under increased CO2 conditions [9], suggesting the possibility of an enhancement of occurrences of harmful algal blooms in the future. Because the marine carbon cycle is intimately linked with the biogeochemical cycles of nitrogen, phosphorus, silicon, and iron, these interactive effects make it extremely difficult to predict what future decreases in oceanic pH will generate. Oceanographers have recognized that increased inorganic carbon levels can have subtle effects on the biota, and much work is being done to document and quantify these effects.
The Biogeochemical Cycles of Nitrogen and Phosphorus
Although the early work of Redfield [1] clearly differentiated between the sources of nitrogen and phosphorus and the regulation of their turnover, they are linked in nature by the processes operating in the biological pump (Fig. 12.2). Despite this coupling, as well as their linkage to carbon, there are a number of features that distinguish them.
Nitrogen occurs in three reactive, inorganic forms in the ocean: nitrate (\( NO_3^{-} \)), nitrite (\( NO_2^{-} \)), and ammonium (\( NH_4^{+} \)) and the processes that transform and modify these forms make up the nitrogen cycle (Fig. 12.3). The nitrogen cycle has five major pathways that result in changes in the availability of nitrogen that can be used by plants. Nitrogen fixation removes gaseous nitrogen from the atmosphere, which is then converted by a series of reactions to forms that can be used for plant growth. In the ocean this process occurs primarily in tropical and semitropical environments, and the major algal species responsible for this transformation is Trichodesmium. Denitrification results in the reduction of \( NO_3^{-} \) to gaseous nitrogen, usually mediated by bacteria, and results in the loss of nitrogen available for phytoplankton in oceanic systems. These two processes are the primary means by which the ocean biota controls nitrogen biogeochemistry.
Schematic of the nitrogen cycle showing the nine key mechanisms by which nitrogen moves through the water column, which are denitrification (dark blue line), nitrification (green dash-dot line), nitrogen assimilation (red dashed line), nitrogen fixation (brown line), ammonification (purple line), annamox (orange line), mixing (light blue line), diffusion (pink line), and sinking (maroon line)
Nitrogen assimilation is the process by which nitrate (\( NO_3^{-} \)) and ammonium \( (NH_4^{+} \)) are removed from the water by phytoplankton. Ammonium is energetically favored for uptake because it does not have to be reduced intracellularly, but nitrate often occurs in greater concentrations, particularly in areas of upwelling or deep vertical mixing. Ammonium inhibits nitrate uptake, but the degree of inhibition varies with the relative concentration of the two nutrients. Ammonification generates \( NH_4^{+} \) by the cleaving of amine groups from organic nitrogen. Because many marine organisms excrete ammonium, the vertical distribution of \( NH_4^{+} \) can depend on the distribution of heterotrophs, such as copepods, which is variable. Nitrification is the production of \( NO_3^{-} \) from ammonium. Earlier work suggested that this was a relatively slow process, but more recent investigations suggest that the oxidation of \( NH_4^{+} \) and production of nitrate is quite rapid, particularly in tropical waters.
The different transformations result from different organisms and some require specific types of environmental conditions (Table 12.1). Denitrification and nitrogen fixation are anaerobic processes, which occur only in the absence of oxygen. Oceanic systems, ranging from estuarine to open ocean, provide sites for denitrification and as a result are depleted of oxygen. These oxygen-minimum regions are characterized by large vertical fluxes of organic matter, which heterotrophic bacteria oxidize and release nitrogen, consuming the available oxygen in the process. Anoxic conditions also occur in sediments where oxygen is depleted by aerobic metabolism. An unusual biological adaptation allows for nitrogen fixation (an anaerobic process) to occur in surface waters with high levels of oxygen. Some organisms (e.g., the cyanobacterium Trichodesmium) form extensive patches or tufts. These tufts, by virtue of their own metabolism, unusually thick cell walls and biochemical modifications of specialized cells where N2 fixation occurs, create a microzone of very low oxygen, thus allowing nitrogen fixation to proceed. Other, smaller cyanobacteria have unusual biochemical adaptations that allow them to fix N2 as well, despite living in oxygen-saturated water.
Recently a new nitrogen transformation, the annamox pathway, has been described in which anaerobic bacteria oxidize ammonium and nitrite directly to gaseous nitrogen, providing a second means by which nitrogen is “lost” from the nitrogen cycle [10]. This pathway has been found to be quantitatively important in regions such as the Peruvian and Arabian Sea oxygen-minimum zones [11, 12]. Because 30–50% of global nitrogen “losses” occur in these types of regions, elucidation of this process, its oceanographic controls, and the absolute rates, have important implications for the global nitrogen cycle.
The Biogeochemical Cycle of Iron
The understanding of the role of iron in the ocean has undergone a dramatic revision in the past few decades. Until recently data on absolute iron concentrations were seriously compromised by the difficulty of obtaining samples without contamination. As the collection and sampling aspects were greatly improved, the ability to quantify concentrations of iron in the oxygenated waters of the ocean decreased dramatically. Coincident with increased realization and acceptance of the vanishingly low concentrations of iron was the hypothesis that iron could, and does, regulate phytoplankton growth and productivity over large areas of the ocean [13]. Indeed, the hypothesis appeared to explain a number of oceanic features that were only partially explained. For example, large areas of the ocean, such as the Southern Ocean, the equatorial Pacific, and the north Pacific, have substantial standing stocks of nitrate and phosphate, as well as adequate irradiance, but exhibit very low standing stocks of phytoplankton (high-nutrient, low-chlorophyll regions, or HNLCs). During glacial-interglacial periods, atmospheric concentrations of CO2 showed substantial variations and were strongly negatively correlated with iron deposition [14]. Thus iron limitation could explain CO2 variations over geological time as well. Given that iron is the fourth most abundant element on earth, how can such low concentrations exist in the ocean, and how did oceanographers unequivocally demonstrate the ecological importance of iron?
Iron is derived from terrestrial and hydrothermal sources, but upon entry into oxygenated, saline waters, it rapidly forms iron oxides. The precipitates are largely insoluble under aerobic conditions, and attach to particles or remain in the water as colloids. The colloids can be solubilized by irradiance, contributing to a pool of dissolved inorganic iron, which consists of two forms, Fe+2 and Fe+3. Both of these ions can be removed by plankton for their growth, although Fe+2 is generally oxidized to Fe+3 and kept at low levels. The mean ocean concentration of dissolved inorganic iron in the upper 200 m of the ocean is 0.07 nmol kg−1 [12]. Both forms can also be chelated by organic molecules, and thus become part of the dissolved ferro-organic pool. In general, there are two classes of organic ligands that bind with iron, a strong-binding ligand and a weak-binding ligand. The latter exchanges iron easily with biota, and thus makes iron bioavailable. There is also a class of special ligands called siderophores, which are low molecular weight organics that are produced and excreted primarily by prokaryotic organisms (bacteria, cyanobacteria) and that bind dissolved inorganic iron [15]. The ferro-ligand complex can be assimilated by bacteria, phytoplankton, and cyanobacteria, and the iron incorporated into a variety of cellular processes. Transformations among all of these pools are both biologically and irradiance mediated; entirely different transformations and equilibria are established in anoxic waters and sediments.
Iron in ocean surface waters derives from either atmospheric or deep ocean sources. Atmospheric deposition varies by latitude (proximity to terrestrial sources) and temporally (dependent on source region wind variability). Aerosols can be measured by satellite-borne sensors, which have shown that some oceanic systems receive substantial periodic depositions of iron from industrial sources (the North Atlantic) and from dust derived from terrestrial deserts in China (the western Pacific) and the Sahara in Africa (the coast of North Africa). Dissolution of aerosols in ocean water (fractional solubility) depends on the type of mineral in the aerosol, and can range from <1–90% [16, 17]. Small aerosol particles can rapidly aggregate with biological particles and exit the surface layer by sinking. Residence times for particulate iron can be as short as 6 days [17]. Conversely, other regions are rarely impacted by atmospheric deposition events (e.g., the Southern Ocean, the equatorial Pacific) by virtue of large-scale wind patterns that isolate them from terrestrial sources. These regions have their iron inputs driven by oceanographic processes such as deep vertical mixing and upwelling. Given the spatial and temporal variability in both of these processes, it is not surprising that surface water concentrations of iron are also highly variable.
Mesoscale Iron Fertilization Experiments
In the 1990s a series of large-scale ocean manipulations were undertaken to test the hypothesis that iron limited phytoplankton growth in the tropical Pacific. Two competing hypotheses were offered to explain the equilibrium concentrations of high concentrations of nitrate and low phytoplankton biomass which were (1) limiting levels of bioavailable iron and (2) rates of loss processes from grazing kept phytoplankton standing stocks at low levels. To test these, in situ additions of iron were planned for limited regions of the ocean. The passive tracer sulfur hexafluoride (SF6), which can be detected at very low levels, was added with the iron so that the enriched water could be followed over time. The first iron enrichment experiment produced contradictory results. The photosynthetic capacity of phytoplankton showed a clear enhancement that was correlated with iron additions, but nitrate and CO2 concentrations were unaffected [18, 19]. Further analysis showed that upon initial iron enrichment, the iron dropped to extremely low levels because colloid formation rapidly converted soluble iron to insoluble iron oxides, and the fertilized water patch was subducted to depth, which removed the iron-enriched water from the high irradiance euphotic zone required for nutrient assimilation. To further test the two hypotheses, the experiment was repeated, and this experiment clearly demonstrated the critical role of iron in limiting phytoplankton growth in high-nutrient, low-chlorophyll waters. Iron was added repeatedly to the patch of water at 3-day intervals for almost 2 weeks [20], and the response of the surface water was clear, showing decreased nitrate (which dropped to zero), decreased CO2, increased phytoplankton biomass and photosynthetic activity, and a quantifiable decrease in iron concentrations. That is, the concentration and supply of iron was nevertheless the essential feature in driving the carbon and nitrogen cycles of the equatorial Pacific Ocean.
Subsequent similar iron enrichment experiments have been conducted in other HNLC regions in the Southern Ocean and the North Pacific. The former is extremely important to global biogeochemical cycles, as it is the site of deep and intermediate water mass formation, and thus regulates the concentrations of inorganic nutrients in much of the world’s surface waters. As an example, models suggested that if all the inorganic nutrients were utilized (by iron fertilization) in the Southern Ocean that within 300 years the waters being upwelled in the eastern tropical Pacific would be greatly reduced in nutrient levels, and thus decrease productivity of commercially important higher trophic levels and marine mammals dependent on ecosystem processes in that region [21]. In all iron enrichment experiments to date, substantial and positive responses to additions of inorganic iron were observed, and while the details among experiments differ (and the causes debated), it is now accepted that iron plays a major role in the biogeochemistry of the ocean [22].
The Biogeochemical Cycle of Silicon
Silicon, despite being a nutrient for only one major functional group of phytoplankton (diatoms), is a major factor in regulating other biogeochemical cycles, such as carbon. This is because diatoms are extremely important primary producers, generating approximately as much oxygen on an annual basis as do pine trees in terrestrial systems. In addition, diatoms are among the largest forms of phytoplankton, and hence can sink passively to depth. Diatoms also produce transparent exopolymer particles, which serve as the primary mechanism for aggregating particles in the ocean’s surface layer, thus producing large, rapidly sinking particles that are the major component of organic carbon and nitrogen flux to deeper water (Fig. 12.2). Finally, diatoms are also heavily grazed by herbivorous organisms, and serve as a means to transfer photosynthate to the large organism-based food web. All of these characteristics contribute to the substantial importance of diatoms in the ocean.
Silicon is a major component of rocks and terrestrial minerals, and as a result the inputs to the ocean in riverine waters are substantial. However, silicon is not readily dissolvable, and dissolved silicon, which occurs as Si(OH)4, remains at relatively low levels. Aeolian and oceanic weathering of seafloor rocks also constitutes a significant source of dissolved silicon. Silicon also is found in high concentrations in waters exiting hydrothermal vents, and while quantitative estimates are uncertain, the contribution of this source to total silicon inputs is likely to be significant.
Silicon is incorporated into diatoms and other marine organisms as opal (\( Si{(OH)_4}n{H_2}O \)), which is slightly more soluble than pure SiO2 and undersaturated in all ocean waters. Opal is found in the sediments as siliceous deposits of biogenic origin; these deposits are largely focused in the Southern Ocean’s polar front region [23]. Silicon is recycled within the water column, but rates of this cycling are modest, and silicon regeneration is often markedly uncoupled from that of carbon and nitrogen in some regions. The reason for this appears to result from the different controls of each: organic matter regeneration is largely biologically mediated (by heterotrophic processes), whereas silicon regeneration is regulated by temperature [24]. As a result, in polar regions a large fraction of the organic matter that sinks from the euphotic zone is regenerated in the upper 250 m, whereas a substantial amount of silicon sinks to a greater depth as biogenic particles. This uncoupling contributes to the formation of large zones of biogenic silica deposits in polar regions and are reflective of surface layer diatomaceous productivity. In a more recent reanalysis of the global silicon budget, it was concluded that the deposition of silicon in continental margins may have been greatly underestimated [25]. If this were true, then the coupling between the silicon and organic matter budgets would be even stronger than previously thought.
An additional mechanism to couple the biogeochemistry of silicon and organic carbon is the presence of an organic membrane that covers diatom frustules [26]. Silica dissolution does not begin until this membrane is degraded by bacteria, which decreases the time for the dissolution of opal during the transit of a particle through the water column (ca. 3,000 m). Sinking rates of large aggregates are ca. 200 m day−1, so that a reduction in the already low rate of dissolution by the necessity for organic degradation can decrease dissolution of silica markedly. Similar effects of grazing can occur, as fecal pellets are usually composed of an organic pellicle that must be degraded prior to chemical silica dissolution.
As with other nutrients, silicic acid has substantial interactions with other elements, such as nitrate and iron. Under iron-limiting conditions, diatoms continue to assimilate silicon, but because iron is needed in the enzymes used for nitrate assimilation, nitrate uptake decreases [3]. As a result, Si:N ratios increase by nearly an order of magnitude in diatoms under iron limitation and elevated ratios observed in natural systems have been used to infer iron limitation.
The Biogeochemical Cycle of Sulfur
In marine systems sulfur is largely present in its most stable form, which is sulfate (SO −24 ). Sulfate is present in high concentrations in most marine systems, and relatively low concentrations are required by organisms to survive [27]. As a result, sulfur does not normally become growth limiting. Sulfate concentrations in marine systems are primarily controlled by physical rather than chemical processes. Variations in concentration only have a significant biological impact in anoxic zones where sulfate reduction occurs [2]. Sulfur is also present as other inorganic (H2S) and organic (dimethylsulfoniopropionate (DMSP), dimethylsulfide (DMS), carbonyl sulfide (COS), and methanethiol (MeSH)) forms. Sulfate is transformed into these compounds via the sulfur cycle, which operates primarily in the photic zone of the upper water column, in the sediments, and around hydrothermal vents (Fig. 12.4).
The sulfur cycle involves transformation of sulfur in the water column through physical mechanisms such as diffusion (light blue line), bioturbation (purple line), and sinking (dark blue line); chemical mechanisms of photooxidation (orange line), and precipitation (yellow line); and biological mechanisms of sulfur assimilation and metabolism by phytoplankton (green dash-dot line) and reduction and oxidation by bacteria in the sediment (orange dashed line)
In aerobic environments sulfur is converted between inorganic compounds (sulfate and hydrogen sulfide) and organic sulfur compounds including DMSP, DMS, COS, and amino acids. Most algae and bacteria use sulfur assimilation to form amino acids, such as cysteine and methionine [27]. Some phytoplankton species, particularly prymnesiophytes and dinoflagellates, use methionine to produce DMSP, a compound with antioxidant properties [28, 29]. DMSP can be released into the water and subsequently used to produce amino acids through assimilation by bacteria or phytoplankton, including some species of diatoms and cyanobacteria, demethylated by bacteria to produce MeSH, or oxidized into DMS and acrylic acid [30]. DMS is either broken down in the water into sulfate through bacterial uptake or photooxidation, or is volatilized into the atmosphere, where it can act as an important aerosol [27].
The sulfur cycle in ocean sediments can be divided into reactions that occur in the upper oxic layer and those that occur in the lower, oxygen-depleted (anoxic) region. In the anoxic sediments, sulfur-reducing bacteria carry out anaerobic respiration using sulfate or sulfur-containing organic compounds to oxidize organic matter, resulting in the production of sulfide, typically as H2S, a form of sulfur that is highly toxic to most organisms. In the deeper layers of the sediment, sulfide reacts with iron and precipitates as iron sulfides such as pyrite (FeS2) [30]. Some sulfide remains in the sediment, and, when mixed back into the oxic zone through processes such as bioturbation, is quickly oxidized by sulfur-oxidizing bacteria into sulfate, which can then remain in the sediment or be released into the overlying water [31]. Sulfur oxidation and reduction by bacteria in the sediment are also important to the functioning of the nitrogen cycle in oxygen-minimum zones [32]. In these environments, sulfate reduction provides a significant amount of the ammonium used in the anammox reaction in anaerobic environments, and nitrate reduction may be coupled to sulfide oxidation, indicating that the anaerobic mechanisms in the sulfur cycle may also be important in the nitrogen cycle [32].
The presence of hydrogen sulfide around hydrothermal vents has resulted in the development of unique organisms with the ability to use the energy contained in hydrothermal fluids to produce organic compounds through chemoautolithotrophy [33]. At hydrothermal vents seawater comes into contact with magma from the earth’s interior, which cools and forms reduced sulfur compounds [2]. The sulfate in seawater then reacts to form hydrogen sulfide as well as sulfur-containing minerals such as pyrite (FeS2), chalcopyrite (CuFeS2), and pyrrhotite (Fe1−xS) [2], which form the surface chimney structure that is characteristic of hydrothermal vents. The hydrogen sulfide provides the energy, rather than light, for the chemoautrophic microorganisms that form the base of the hydrothermal vent food web [33]. Some species of microorganisms can operate in aerobic conditions, using oxygen as the electron acceptor, while others have the ability to carry out this reaction in anaerobic conditions, using nitrate, sulfate, or sulfur as the electron acceptor [2]. These organisms survive in symbiotic relationships with other organisms living near the hydrothermal vents. The microorganisms, which are endemic to hydrothermal vent environments, allow unique communities to develop and help maintain the oceanic sulfur cycle by transforming hydrogen sulfide released by the hydrothermal vents into sulfate [33].
The Biogeochemical Cycle of Oxygen
Oxygen is involved in all nutrient cycles, and its presence or absence dictates the reactions that will occur in a specific marine environment. Oxygen gas can be introduced into marine environments across the air-sea interface (e.g., by diffusion). However, oxygen concentration is controlled by the biological processes of photosynthesis and respiration, and by physical processes such as mixing within the water column. In the euphotic zone, phytoplankton photosynthesis produces oxygen, which is then used as the electron acceptor to conduct aerobic respiration. This process is carried out by both autotrophic and heterotrophic organisms throughout the water column.
Oxygen concentration generally decreases with depth in the ocean. Photosynthesis can only be carried out in the lighted parts of the water column, but respiration continues throughout the water column. As the organic matter from the surface layers sinks, it is taken up by organisms and used to conduct respiration, depleting oxygen levels. Some marine environments, particularly in marine sediments, are suboxic, with oxygen concentrations less than 0.2 ppm (but still detectable), or anoxic, with oxygen concentrations below detectable levels [2]. Organisms survive in these environments by using anaerobic respiration, in which compounds such a nitrate, sulfate, iron, or even organic matter are used as alternative electron acceptors to oxygen [2].
Anoxic zones are not limited to marine sediments, with increasing attention being paid to decreasing oxygen concentrations in previously oxygen-rich areas of the ocean. Hypoxic zones, marine environments with oxygen concentrations below 2 mg L−1, typically form when primary productivity is high, leading to increased organic matter in the system and increased respiration, and when mixing throughout the water column is low, preventing the oxygen in the upper water column from reaching lower layers [34–36]. Hypoxic zones have been increasing in frequency, including the Gulf of Mexico and Chesapeake Bay [34, 35]. Factors such as eutrophication due to increased fertilizer or wastewater runoff have lead to the development of hypoxic conditions in systems already susceptible due to vertical stratification of the water column [34]. Thus, the disruption in the typical oxygen cycle and the lack of an anaerobic respiration mechanism in most marine organisms can result in serious consequences for the composition and productivity of the marine food web community in these hypoxic zones.
Future Directions
Studies of Biogeochemical Cycles
In the past two decades, a number of large, interdisciplinary programs were conducted to obtain biogeochemical data on appropriate time and space scales so that mathematical models of global climate change can accurately represent the complex processes of elemental cycles. One such program, the Joint Global Ocean Flux Study (JGOFS), which occurred from 1987 to 2003, was international in scope, and undertook coordinated, multidisciplinary, international studies in the equatorial Pacific, the north Atlantic, the Arabian Sea, and the Southern Ocean, and coordinated multidisciplinary national programs in a range of coastal and open ocean environments. The JGOFS project was designed to assess the carbon cycle, but because all elemental cycles are closely linked, insights were gained into the understanding of nitrogen, silicon, and iron cycles as well. The JGOFS program also had a significant synthesis and modeling component that was intended to integrate the data sets from the multidisciplinary studies and to develop mathematical models of increased complexity and biological realism. In addition to providing a wealth of publicly available data, the JGOFS program served as a model for large, multidisciplinary studies of ocean processes.
The results and understanding from the JGOFS program provided the basis for the Integrated Marine Biogeochemistry and Ecosystem Research (IMBER) Project, which was initiated in 2001 by the International Geosphere-Biosphere Program and the Scientific Committee on Oceanic Research. The science goals of the IMBER project extend the investigation of marine biogeochemical cycles to include the influence of feedbacks with marine food webs and the consequences for marine ecosystems. Central to the IMBER goal is the development of a predictive understanding of how marine biogeochemical cycles and ecosystems respond to complex forcings, such as large-scale climatic variations, changing physical dynamics, carbon cycle chemistry and nutrient fluxes, and the impacts of marine harvesting. IMBER science is making new advances in understanding marine systems by bringing together the natural and social science communities to study key impacts and feedbacks between the marine and human systems. The emerging recognition of human interactions as integral parts of marine ecosystems is providing the direction for future integrative research designed to understand and sustain ocean systems as environmental change and its associated uncertainties occur.
Role of Modeling
Mathematical models provide an approach for integrating and synthesizing the knowledge and understanding obtained from measurements of oceanic biogeochemical processes. The use of biogeochemical models in ocean research has a long history [37, 38] but their use was advanced significantly in the early 1990s when a model that simulated nitrogen cycling through the lower trophic levels in the oceanic mixed layer became generally available [39], which subsequently has provided the basis for the coupled circulation-biogeochemical models that are now embedded in regional, basin, and global scale models.
The skill of the current generation of biogeochemical models is sufficient to allow projections of future states that may result from climate variability and the oceanic uptake of anthropogenic carbon [40–42]. The patterns and distributions emerging from these simulations show shifts in phytoplankton distributions and marine biomes, alteration of phytoplankton species assemblages, and modified lower trophic level community structure [43–45], all of which have direct and important consequences for biogeochemical cycling. Simulations of the effect of increasing atmospheric CO2 and its uptake by the ocean show reductions in ocean pH and in saturation levels of calcium carbonate, which have serious consequences for many marine organisms [46].
Advances in conceptual understanding, modeling techniques, and data availability have made predictive marine biogeochemical models a feasible goal [47]. However, modeling for prediction is still rapidly developing and much remains to be done in generating appropriate frameworks and in collection of data sets that support predictive modeling for marine biogeochemical cycling [48, 49].
Abbreviations
- Autotrophic:
-
Organisms whose mode of nutrition is photosynthesis.
- Biogeochemistry :
-
The biological and chemical processes that transform and cycle elements over various time and space scales and that determine the composition of the environment.
- Biological pump:
-
The biological processes and transformations that move carbon from the surface to depth.
- Cyanobacteria:
-
Prokaryotic phytoplankton.
- Diatom:
-
Phytoplankton which are encased in frustule consisting of silica.
- Euphotic zone:
-
The surface layer of the ocean where most primary production occurs, generally considered to be the depth to which 1% of surface radiation penetrates.
- Heterotrophic:
-
Organisms who require reduced organic carbon as an energy and carbon source.
- Nutrient:
-
Element that is required for biological activity and growth.
- Oxidation:
-
Chemical reaction in which reactant loses electrons; half-reaction paired with reduction.
- Photosynthesis:
-
The process by which radiant energy from the sun is transformed into chemical energy that can later be used to reduce carbon dioxide to organic sugars, which in turn are coupled to biochemical pathways to produce all compounds necessary for cell growth.
- Phytoplankton:
-
Microscopic, often unicellular, floating autotrophs that live in the ocean’s surface layer and form the base of nearly all marine food webs.
- Reduction:
-
Chemical reaction in which reactant gains electrons; half-reaction paired with oxidation.
Bibliography
Primary Literature
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 64:205–221
Libes SM (2009) Introduction to marine biogeochemistry. Academic, Amsterdam
Hutchins DA, Bruland KW (1998) Iron-limited diatom growth and Si:N uptake ratios in a coastal upwelling regime. Nature 393:561–564
Sunda WG, Huntsman S (1997) Interrelated influence of iron, light, and cell size on growth of marine phytoplankton. Nature 390:389–392
Le Quéré C et al (2007) Saturation of the Southern Ocean CO2 sink due to recent climate change. Science 316:1735–1738
Doney SC, Fabry VF, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Ann Rev Mar Sci 1:169–192
Ries BR, Cohen AL, McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37:131–134
Hutchins DA, Mulholland MR, Fu F (2009) Nutrient cycles and marine microbes in a CO2-enriched ocean. Oceanogr 22:128–145
Fu F-X, Zhang Y, Warner ME, Feng Y, Hutchins DA (2008) A comparison of future increased CO2 and temperature effects on sympatric Heterosigma akashiwo and Prorocentrum minimum. Harm Algae 7:76–90
Devol AH (2003) Solution to a marine mystery. Nature 422:575–576
Lam P et al (2009) Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc Nat Aca Sci 106:4752–4757
Ward B et al (2009) Denitrification as the dominant nitrogen loss process in the Arabian Sea. Nature 451:78–81
Martin JH, Fitzwater S (1988) Iron deficiency limits phytoplankton growth in the northeast Pacific subarctic. Nature 331:341–343
Ducklow HW, Oliver JL, Smith WO (2007) The role of iron as a limiting nutrient for marine plankton processes. In: Melillo JM, Field CB, Moldan B (eds) Interactions of the major biogeochemical cycles. Island Press, Washington, DC, pp 295–310
Vraspir JM, Butler A (2009) Chemistry of marine ligands and siderophores. Ann Rev Mar Sci 1:43–63
Johnson KS, Gordon RM, Coale KH (1997) What controls dissolved iron concentrations in the world ocean? Mar Chem 57:137–161
Baker AR, Croot PR (2010) Atmospheric and marine controls on aerosol iron solubility in seawater. Mar Chem 120:4–13
Martin JH et al (1994) Testing the iron hypothesis in ecosystems of the equatorial Pacific Ocean. Nature 371:123–129
Watson AJ et al (1994) Minimal effect of iron fertilization on sea-surface carbon dioxide concentrations. Nature 371:143–145
Coale KH et al (1996) A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific. Nature 383:495–501
Sarmiento JL, Gruber N, Brzezinski MA, Dunne JP (2004) High-latitude controls of thermocline nutrients and low latitude biological productivity. Nature 427:56–60
Boyd PW et al (2007) Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science 315:612–617
Treguer P, Nelson DM, van Bennekom AJT, DeMaster DJ, Lynaert A, Queguiner B (1995) The silica balance in the world ocean: a reestimate. Science 268:375–379
Nelson DM et al (1995) Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentations. Global Biogeochem Cycles 9:359–372
DeMaster DJ (2002) The accumulation and cycling of biogenic silica in the Southern Ocean: revisiting the marine silica budget. Deep Sea Res II 49:3155–3167
Biddle KD, Azam F (1999) Accelerated dissolution of diatom silica by natural bacterial assemblages. Nature 397:508–512
Sievert SM, Kiene RP, Schulz-Vogt HN (2007) The sulfur cycle. Oceanography 20:117–123
Andreae MO (1990) Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Mar Chem 30:1–29
Sunda W, Kieber DJ, Kiene RP, Huntsman S (2002) An antioxidant function for DMSP and DMS in marine algae. Nature 418:317–320
Jorgensen BB (1977) The sulfur cycle of a coastal marine sediment (Limfjorden, Denmark). Limnol Oceanogr 22:814–832
Canfield DE, Farquhar J (2009) Animal evolution, bioturbation, and the sulfate concentration of the oceans. Proc Nat Acad Sci 106:8123–8127
Canfield DE, Stewart FJ, Thamdrup B, Brabandere LD, Dalsgaard T, Delong EF, Revsbech NP, Ulloa O (2010) A cryptic sulfur cycle in oxygen-minimum-zone waters off the Chilean coast. Science 330:1375–1378
van Dover CL (2000) The ecology of deep-sea hydrothermal vents. Princeton University Press, Princeton
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Scavia D, Justic D, Bierman VJ (2004) Reducing hypoxia in the Gulf of Mexico: advice from three models. Estuaries 27:419–425
Lam P, Kuypers MMM (2011) Microbial nitrogen cycling processes in oxygen minimum zones. Ann Rev Mar Sci 3:317–345
Riley GA (1946) Factors controlling phytoplankton populations on Georges Bank. J Mar Res 6:54–73
Riley GA (1947) A theoretical analysis of the zooplankton populations of Georges Bank. J Mar Res 6:104–113
Fasham MJR, Ducklow HW, McKelvie SM (1990) A nitrogen-based model of plankton dynamics in the oceanic mixed layer. J Mar Res 48:591–639
Orr JC et al (2001) Estimates of anthropogenic carbon uptake from four three-dimensional global ocean models. Global Biogeochem Cycles 15:43–60
Doney SC et al (2004) Evaluating global ocean carbon models: the importance of realistic physics. Global Biogeochem Cycles 18:GB3017. doi:10.1029/2003GB002150
Boyd PW, Doney SC (2001) Modeling regional response by marine pelagic ecosystems to global climate change. Geophys Res Lett 29. doi:10.10292001GL014130
Follows MJ, Dutkiewicz S, Grant S, Chisholm SW (2007) Emergent biogeography of microbial communities in a model ocean. Science 315:1843–1846
Le Quéré C et al (2005) Ecosystem dynamics based on plankton function types for global ocean biogeochemistry models. Global Change Biol 11:2016–2040. doi:10.111/j.1365-2486.2005.1004.
Sinha B, Buitenhuis ET, Le Quéré C, Anderson TR (2010) Comparison of the emergent behavior of a complex ecosystem model in two ocean general circulation models. Prog Oceanogr 84:204–224
Orr JC et al (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–686
Friedrichs MAM et al (2007) Assessment of skill and portability in regional marine biogeochemical models: role of multiple planktonic groups. J Geophys Res 112:C08001. doi:10.1029/2006JC003852
Anderson TR (2009) Progress in marine ecosystem modeling and the “unreasonable effectiveness of mathematics.” J Mar Sys 81:4–11
Ducklow HW, Doney SC, Steinberg DK (2009) Contributions of long-term research and time-series observation to marine ecology and biogeochemistry. Ann Rev Mar Sci 1:279–302
Books and Reviews
Baliño BM, Fasham MJR, Bowles MC (2001) Ocean biogeochemistry and global change. IGBP Science no 2, Stockholm (A popular summary of JGOFS main achievements)
Benitez-Nelson CR (2000) The biogeochemical cycling of phosphorus in marine systems. Earth-Sci Rev 51:109–135
Brandes JA, Devol AH, Deutsch C (2007) New developments in the marine nitrogen cycle. Chem Rev 107:577–589
Broecker WS (2009) Wally’s quest to understand the ocean’s CaCO3 cycle. Ann Rev Mar Sci 1:1–18
Deutsch C, Brix H, Ito T, Frenzel H, Thompson L (2011) Climate-forced variability of ocean hypoxia. Science 333:336–339
Diaz RJ (2000) Overview of hypoxia around the world. J Environ Quality 30:275–281
Doney SC et al (2001) Marine biogeochemical modeling: recent advances and future challenges. Oceanography 14:93–108
Fasham MJR (ed) (2003) The role of the ocean carbon cycle in global change. Springer, Heidelberg, p 297
Gruber N, Galloway JN (2008) An earth-system perspective of the global nitrogen cycle. Nature 451:293–296
Hanson RB, Ducklow HW, Field JG (eds) (2000) The changing ocean carbon cycle: a midterm synthesis of the joint global ocean flux study, IGBP book series no 5. Cambridge University Press, Cambridge, 520 pp
IMBER Science Plan and Implementation Strategy (2005) IGBP Report N°52. IGBP Secretariat, Stockholm, 76 pp
Jickells TD et al (2005) Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 308:67–71
Keeling RF, Kortzinger A, Gruber N (2010) Oxygen deoxygenation in a warming world. Ann Rev Mar Sci 2:199–229
Paulmier A, Ruiz-Pino D (2009) Oxygen minimum zones (OMZs) in the modern ocean. Prog Oceanogr 80:113–128
Ragueneau O et al (2000) A review of the Si cycle in the modern ocean: recent progress and missing gaps in the application of biogenic opal as a paleoproductivity proxy. Global Planetary Change 26:317–365
Sabine CL, Tanhua T (2010) Estimation of anthropogenic CO2 inventories in the ocean. Ann Rev Mar Sci 2:175–198
Sunda WG (2010) Iron and the carbon pump. Science 327:654–655
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Smith, W.O., Hofmann, E.E., Mosby, A. (2013). Marine Biogeochemistry. In: Leemans, R. (eds) Ecological Systems. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5755-8_12
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5755-8_12
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5754-1
Online ISBN: 978-1-4614-5755-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)