Abstract
The purpose of this chapter is to provide a practical and theoretical framework for the study of the ecophysiology of mycoheterotrophic plants. We accomplish this by providing a comparative overview of our current knowledge on carbon and nitrogen isotope natural abundance in partially and fully mycoheterotrophic plants associated with ectomycorrhizal, wood- and litter-decomposer saprotrophic, and arbuscular mycorrhizal fungi, and discuss their ecophysiological implications. We present a meta-analysis of all stable carbon and nitrogen isotope values from the majority of species of partially and fully mycoheterotrophic plants investigated thus far. We summarize our current understanding of the ecophysiology of fully mycoheterotrophic plants in the families Orchidaceae and Ericaceae as well as nonvascular plants, and species from the tropics that associate with arbuscular mycorrhizal fungi. We also review the occurrence of initial mycoheterotrophy among orchids and ericaceous plants that are autotrophic upon reaching adulthood. We highlight current studies of cryptic or partial mycoheterotrophy in green plants that appear to be fully autotrophic, but meet some portion of their C demands via fungi in a mixotrophic nutrition. Furthermore, we explore the utility of ecophysiological methods such as radioactive and stable isotope probing, measuring plant assimilatory and respiratory responses to environmental gradients such as light availability, and natural abundance stable isotope analysis for future studies of mycoheterotrophic food webs. Finally, methodological limitations and considerations for the study of physiological ecology of mycoheterotrophy are also outlined in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
Mycoheterotrophic (MH) plants have been centerpieces for the study of the physiological aspects of the mycorrhizal symbiosis for over 150 years. A.B. Frank who in 1885 coined the term “mycorrhiza” conducted research on the anatomy and physiology of the fully MH species Hypopitys monotropa (syn. Monotropa hypopitys) (Frank 1885). Due to the charismatic, often highly derived, appearance of many MH plants, it is no surprise that they have captured the interest of researchers throughout the centuries. These plants continue to draw attention from various realms of science because they consistently require that our fundamental understanding of many ecological, evolutionary, and physiological theories be expanded upon. Both the ecology and evolution of mycoheterotrophy are tightly coupled to plant–fungal interactions or ecophysiology, and this subject is the focus of this chapter. From an ecological perspective, MH plants that associate with mycorrhizal fungi represent the best-known examples of mycorrhizal networks, where unrelated plants transfer elemental compounds via shared fungal symbionts (Chap. 1; Simard and Durall 2004; Selosse et al. 2006). From an evolutionary perspective, fully MH plants that associate with mycorrhizal fungi are the primary example of one extreme in the mycorrhizal continuum, ranging from plants giving carbon (C), to plants receiving C from their fungal symbionts (Chap. 1).
Within the plant kingdom the MH strategy has arisen numerous times throughout evolutionary history and involves not only mycorrhizal fungi, but saprotrophic (SAP) fungi as well. The physiology of mycoheterotrophs and their mycorrhizal fungi represent the only clear example of a complete reversal in the normal flow of nutrients in the mycorrhizal symbiosis, where instead of plant-derived C being traded for nutrients acquired by fungi (Kiers et al. 2011; Selosse and Rousset 2011), both C and other nutrients have a net unidirectional flow from fungus to plant. Furthermore, fully MH plants that depend on SAP fungi are the only known examples of plants whose primary C source is derived solely from complex dead organic substrates. Their existence reopens the important, but debated question of reentry of organic C into plants via mycorrhizal interactions (Baldrian 2009; Selosse et al. 2010). Sometimes it is these exceptions to conventional biological systems that provide the greatest insights into ecosystem function as a whole, and we approach here the ecophysiology of mycoheterotrophy with this broader context in mind.
The purpose of this chapter is to provide a practical and theoretical framework for the study of MH plants’ ecophysiology. We will highlight the findings from recent studies that have provided inroads into unraveling the curious functioning of MH plants. Of all the chapters in this book, this chapter will most likely be the quickest to become outdated, because at the time of writing, the field of MH plant ecophysiology is in rapid development. Techniques for high-throughput sample analysis especially for elemental and isotope studies are gaining momentum, as are new techniques for studying source–sink relationships between plants, fungi, and the environment in situ. However, with the application of new methodologies, it is important to remember that within the specific field of MH research, the questions being asked have remained fundamentally the same for over 200 years (Rayner 1927 and references therein). These questions include, who are the players in MH associations? What are the plant and fungal trade-offs for MH interactions? What factors, environmental or otherwise, select for mycoheterotrophy? And more specifically, what forms of C and other nutrients are transferred from fungi to mycoheterotrophs? To date, the majority of research on mycoheterotrophy has addressed the first question and we now have a substantial amount of information on the functional and phylogenetic diversity of fungi that associate with mycoheterotrophs (see Chaps. 5, 6, and 7 for more detail).
A frequent pattern among most MH species is specificity for particular fungal hosts. However, these fungi have a wide phylogenetic breadth within the kingdom Fungi. This indicates that rather than phylogenetic conservatism, it is either physiological or evolutionary pressures that select the fungal hosts for mycoheterotrophs (Hynson and Bruns 2010). To date, the most in-depth research has been focused on fully MH orchids and other taxa that are dependent on mycorrhizal fungi, particularly ectomycorrhizal (EM) fungi, to meet their nutrient demands. Although some fully MH species subsist on compounds derived from SAP fungi, they appear to occur only among the orchids (Selosse et al. 2010). However, the vast majority of fully MH species associate with arbuscular mycorrhizal (AM) fungi in the tropics, but due to a bias of field research to temperate regions, much of what we understand about the ecophysiology of mycoheterotrophy comes from temperate species.
What are the potential ecophysiological determinants of mycoheterotrophy? The physiological dependency of mycoheterotrophs on fungi starts with the earliest stages of seedling development when there are chemical cues between seed and fungus that trigger germination (Bruns and Read 2000). However, germination also seems to be the tightest bottleneck for MH plant survival (Bidartondo and Read 2008; Eriksson and Kainulainen 2011; Tesitelova et al. 2012; Chap. 5). This is because the seeds of many MH species will only germinate in the presence of fungi that are closely related to those associated with adult plants, but upon reaching maturity, no plants have been found with the “wrong” fungal host. However, we cannot rule out the possibility that there are rare instances of individuals surviving with alternative hosts, and that this may lead to permanent host switching, explaining jumps from one fungal partner to another.
At the early developmental stages, securing a source of C and other nutrients is of paramount importance to the plant. It has been argued that for C and other nutrient transfer to occur from fungus to MH plant, the plant must create a concentration gradient that has a draw-down or sink effect on the plant-fungal network (Finlay and Read 1986). This gradient could be created by the rapid transformation of compounds received from the fungus by the mycoheterotroph into forms that are unavailable for fungal use, or by storage apart from the plant–fungal interface. This has been somewhat demonstrated in the mutualistic interactions of mycorrhizal fungi with autotrophic host plants where plant carbohydrates derived from photosynthesis are converted by fungi into trehalose and polyols that are largely unavailable for plant uptake (Smith and Read 2008). The primary example of fungal assimilated C compounds being converted to plant carbohydrates by a mycoheterotroph is from a study by Smith (1966). In her laboratory experiments two rhizoctonia fungi first colonized the MH seedlings of the orchid Dactylorhiza purpurella, then fungi were fed 14C labeled sucrose, which they transformed into trehalose, a portion of which was transferred to the orchid seedlings which transformed it into glucose, fructose, and sucrose (Box 8.1). Interestingly, invertases are absent from at least some EM fungi (Martin and Selosse 2008; Parrent et al. 2009) and AM partners (Tisserant et al. 2012), so that sucrose may well be unavailable for fungal use. Similar to autotrophic plant-to-plant C transfer via shared mycorrhizal fungi, in MH plants, the demand or sink strength could be life stage dependent, as well as seasonally and environmentally variable (Lerat et al. 2002; Simard and Durall 2004).
The recent revelation of cryptic or partial mycoheterotrophy in green plants has highlighted the variation in plants’ dependency on fungi to meet their C demands. Partial mycoheterotrophs are green plants that appear to be fully autotrophic, but meet some portion of their C demands via fungi in a mixotrophic nutrition that will be explored in detail in this chapter. Since these findings, partial mycoheterotrophy has been proposed as a potential evolutionary pathway to full mycoheterotrophy. However, the underlying determinants, geographical and phylogenetic extent of partial mycoheterotrophy are unknown and currently an area of active research. Ecophysiological methods such as radioactive and stable isotope probing, measuring plant assimilatory and respiratory responses to environmental gradients such as light availability, and natural abundance stable isotope analysis are all critical tools for the study of mycoheterotrophy and their applications are discussed in detail in this chapter. Methodological limitations and considerations for the study of physiological ecology of mycoheterotrophy will also be outlined.
The final section of this chapter will address areas for future research and draw attention to the gaps in our current knowledge of MH ecophysiology. This field is ripe for the undertaking by new methods and researchers. In the years to come, many of the current limitations to studying mycoheterotrophs, and plant–fungal interactions in general, will become obsolete as we continue to develop new quantitative and noninvasive methods to study these systems in situ or perhaps even ex situ (Yagame et al. 2012). What is critical now is that robust theoretical frameworks be established a priori that will engage researchers from across fields and provide a sound foundation for the interpretation of forthcoming data on MH plants. In future research, MH plants will continue to be model systems for the study of the ecophysiology of plant–fungal interactions. This is due to their complete dependency on often a sole fungus to meet their nutrient demands, the fact that they are phylogenetically and geographically widespread, and many represent a profound modification of the most common and abundant mutualism on earth, the mycorrhizal symbiosis.
8.3 From Mutualism to Parasitism: Deconstructing the Continuum of Plant–Fungal Interactions
8.3.1 Mycorrhizal Networks
Since the definitive experiments of Erik Björkman in the 1960s where the transfer of a 14C label applied to the phloem of pines was traced to surrounding individuals of the fully MH species Hypopitys monotropa, researchers continue to test for the presence and extent of mycorrhizal networks in nature. In its simplest form, a mycorrhizal network consists of two plant individuals of the same species connected via a shared mycorrhizal fungus. Due to the diffusivity of the mycorrhizal symbiosis and the large size of fungal individuals in some species (some genetic individuals can cover several m2; Douhan et al. 2011) it is almost certain that these connections exist in nature, but to what extent they actually link different plants and act as conduits for C and mineral nutrient exchange between plants is the subject of much debate.
Fully MH plants that associate with mycorrhizal fungi provide the best examples of C transfer from unrelated plants via shared fungi. Because this tripartite network of autotrophic host plants, mycorrhizal fungus, and mycoheterotroph involves only unidirectional C and mineral nutrient flow to the mycoheterotroph, it is often referred to as an epiparasitism rather than a mutualism. However, the impact of mycoheterotrophy on partners’ fitness (especially on the autotrophic host plant) is currently unknown. The evidence for mycorrhizal networks where there is transfer of C or other nutrients among plants that engage in a mutualism with their mycorrhizal fungi is less clear-cut. The first quantitative ex situ laboratory studies to show significant transfer of C between autotrophic plants via shared AM or EM fungi were conducted by Francis and Read in 1984 and Finlay and Read in 1986. Since then, numerous field manipulation and laboratory experiments have taken place to test the significance of mycorrhizal networks in plant establishment, survival, and below-ground resource sharing. These studies have had mixed results, leading some researchers to question the overall importance of mycorrhizal networks (Fitter et al. 1998; Robinson and Fitter 1999; Wu et al. 2001; Pfeffer et al. 2004). However, there is mounting evidence that (1) mycorrhizal networks are common in nature (Simard and Durall 2004; Selosse et al. 2006), (2) there is the potential for bidirectional C, nitrogen (N), and phosphorus (P) movement between plants (Lerat et al. 2002; Teste et al. 2009), and (3) depending on environmental factors such as light availability the sink strength of “receiver” plants in mycorrhizal networks can increase (Finlay and Read 1986; Simard et al. 1997). All these factors could have profound effects on interplant competition, plant and fungal diversity, and community dynamics (Simard and Durall 2004; Selosse et al. 2006). Recent field studies of mycorrhizal networks have focused on their role in seedling establishment (Nara 2006; Teste et al. 2009), survival (McGuire 2007), and growth (Booth 2004). These studies provided strong support that mycorrhizal networks can be critically important in early forest succession stages and tree recruitment. However, they suffer from similar limitations such as the difficulties in assessing the physical presence of fungal connections between plants, measuring long-term net C flow from donor to receiver plants, and the contributions of mycorrhizal networks to plant fitness over temporal and life stage gradients. Future efforts in the study of mycorrhizal networks should be focused in these areas as well as gaining a better understanding of the environmental plasticity of mycorrhizal networks; if they are controlled by plants and/or fungi; if the payoffs of networking are stronger selective forces than the benefits of competition; and finally, whether plants receiving benefits from networking are true “cheaters,” providing no reciprocity, or if they somehow compensate for what they receive.
It is becoming undoubtedly clear that the mycorrhizal symbiosis is far less static than it was historically thought to be. Furthermore, where a particular plant or fungus falls along the continuum of mutualistic to parasitic in relation to its symbiotic partner(s) appears to be potentially life-stage, environmentally, and community driven. Fully MH plants may provide one exception to this plasticity due to their absolute dependency on fungi. Thus, quantifying complete C budgets for mycoheterotrophs over the course of their lifecycles, as well as the fitness costs to their fungi (and perhaps autotrophic host plants) will provide much needed constraints for modeling plant–fungal interactions.
8.3.2 Determination of Full Mycoheterotrophy
8.3.2.1 Evidence Based on Radioisotope and Stable Isotope Labeling
In 1881 it was hypothesized that Hypopitys monotropa shares a symbiotic fungus with neighboring forest trees and is nourished by these trees through a common mycelial network (Kamienski 1881). However, at that time this hypothesis was not widely accepted. It took almost 80 years until Kamienski’s hypothesis was for the first time experimentally confirmed based on radioisotope labeling experiments. It was Björkman (1960) who demonstrated in field experiments that 14C-labeled glucose and 32P-labeled phosphate injected into the phloem of spruce and pine trees were translocated within 5 days to adjacent Hypopitys monotropa plants. Other neighboring understory plants, like Vaccinium myrtillus, V. vitis-idaea, and Calluna vulgaris, remained unlabeled (for details on isotope labeling see Box 8.1). Björkman (1960) furthermore demonstrated that a trenching of H. monotropa plants from adjacent tree roots by metal sheets severely reduced their development. He concluded that these observations confirm the existence of hyphal connections between H. monotropa and neighboring trees and indicate a selective C and P transfer from the trees to H. monotropa plants through shared fungal hyphae.
It took another 40 years until further substantial evidence on a selective C transfer from trees to an MH plant through linked fungal mycelia was successfully documented—again using radiocarbon as a tracer. In microcosm experiments McKendrick et al. (2000) fed shoots of Betula pendula and Salix repens plants growing in association with the leafless orchid Corallorhiza trifida with 14CO2 and traced the movement of the isotope by a combination of digital autoradiography and scintillation counting. Direct C transfer assimilated by both of the autotrophs to Corallorhiza plants occurred only in those cases where plants had already been connected to a shared mycorrhizal fungus. C. trifida seedlings introduced to the microcosms as controls immediately before isotope labeling and thus lacking these hyphal connections failed to assimilate significant C amounts. McKendrick et al. (2000) furthermore documented that C. trifida plants linked to B. pendula and S. repens through mycorrhizal hyphae gained 6–14% in biomass during the 25–28 weeks period of the microcosm experiment, while C. trifida plants growing in microcosms with Pinus sylvestris failed to develop hyphal links and lost 13% of their weight over the same period. Nearly at the same time, fungal ribosomal DNA also provided evidence that the same fungal individuals occurred in the roots of surrounding trees and of the MH orchids Cephalanthera austinae (Taylor and Bruns 1997) and Neottia nidus-avis (Selosse et al. 2002), supporting a link to trees by individual fungal mycelia. In another microcosm labeling experiment it was shown that a 14C label provided as CO2 to Betula pendula seedlings was transferred to the non-photosynthetic liverwort Aneura (Cryptothallus) mirabilis through a shared mycorrhizal fungus (Bidartondo et al. 2003). In this case a Tulasnella sp. was identified to form simultaneously an EM association with trees and a connection with Aneura mirabilis.
Also using the microcosm approach, but stable isotope labels (13C and 15N) instead of radioactive isotopes, Bougoure et al. (2010) investigated the tripartite matter exchange between the fully subterranean orchid Rhizanthella gardneri, a mycorrhizal fungus from the genus Ceratobasidium and the photosynthetic shrub Melaleuca scalena. They demonstrated that up to 5% of the C applied as 13CO2 to the autotrophic shrub was transferred to R. gardneri. Rhizanthella gardneri also gained 6% of the C and 22% of the N fed as [13C-15N]glycine to the soil through the mycorrhizal fungus. The non-stoichiometric C and N transfer from the glycine source through the fungus to R. gardneri is explained by fungal glycine transformation and respiratory 13C-loss (see also Taylor et al. 2004).
Isotope labeling experiments are not only used to trace matter fluxes between ecosystem compartments, but also to document a lack of matter fluxes or reduced fluxes, for example due to missing or reduced metabolic activity. Cameron et al. (2009) provide an example for this kind of tracer application. They compared the potential for CO2 assimilation by the MH orchid Neottia nidus-avis to that of the leafless but chlorophyll containing orchid Corallorhiza trifida. CO2 assimilation of these two orchid species was further compared to the leafy and green (chlorophyllous) orchid Cephalanthera damasonium and to Fagus sylvatica seedlings using 13C isotope tracers in the field. The 13CO2 assimilation rates decreased in the order Fagus > Cephalanthera >> Corrallorhiza > Neottia. These results indicated that the photosynthetic capacity of the Corallorhiza trifida individuals on the day of this experiment was closer to the fully MH Neottia nidus-avis than to the autotrophic Fagus sylvatica or the apparently partially MH (PMH) Cephalanthera damasonium (for further details on this tracer experiment see Sect. 8.5.1).
8.3.2.2 Evidence Based on Natural Abundance 13C and 15N
Independent investigations by Gebauer and Meyer (2003) and Trudell et al. (2003) discovered a considerable enrichment of heavy C and N isotopes in the tissues of fully MH orchids and monotropoids (Ericaceae) in comparison to surrounding autotrophic plants (for further details on the stable isotope natural abundance approach see Box 8.2). This enrichment in 13C in the investigated fully MH plants was explained by these species tapping into alternative C sources to atmospheric CO2 utilized by autotrophic plants in photosynthesis. Fully MH plants enrichment in 15N was thought to be due to these plants receiving compounds enriched in 15N compared to surrounding autotrophic plants that share the same mycorrhizal fungi. Owing to MH plants’ obligate association with various functional groups of fungi (see Chap. 7), and based on the findings from earlier isotope labeling studies, fungi were proposed as the most likely alternative C and N source of fully MH plants. As further support for this, many fungi were already known to be enriched in the heavy isotopes 13C and 15N in comparison to autotrophic plants from the same habitat due to their specific physiology and access to C and N sources also enriched in heavy isotopes (see review by Mayor et al. 2009). Following the food chain concept (Fry 2006) the C and N isotope signatures of MH plants should be similar to, or even more enriched in heavy isotopes than in their fungal source (Trudell et al. 2003). Moreover, the relative enrichment in heavy isotopes in fungi is not a uniform feature, but is specific to different functional and taxonomic fungal groups. For example, fungi forming EM associations tend to be more enriched in heavy C and N isotopes than the majority of SAP fungi (Kohzu et al. 1999; Taylor et al.; 2003; Mayor et al. 2009). In contrast, AM fungi tend to be more depleted in 15N than EM fungi, and are apparently not enriched in 13C compared to their autotrophic host plants (Courty et al. 2011 and references therein). The relative 13C enrichment in EM fungi is related to the gain of 13C-enriched carbohydrates from photosynthetic plant partners (Gleixner et al. 1993). Among EM fungi, species associated with overstory trees are more enriched in 13C than species associated with understory trees (Högberg et al. 1999), and species capable of decomposing recalcitrant soil organic compounds are more enriched in 15N than species with a preference for inorganic N compounds (Gebauer and Taylor 1999). For SAP fungi their respective C and N source (wood, leaf litter, humus, etc.) determines their isotope signature (Gebauer and Taylor 1999; Kohzu et al. 1999). Thus, according to the isotope food chain concept, the differing patterns in the isotope signatures of the various functional groups of fungi is expected to be mirrored by MH plants associated with them. In the following section we provide a comparative overview of our current knowledge on C and N isotope natural abundance in fully MH plants associated with EM, wood- and litter-decomposer SAP, and AM fungi and discuss their ecophysiological implications.
8.5 Isotopic Patterns in Full Mycoheterotrophs Based on Fungal Host
8.5.1 Fully Mycoheterotrophic Plants Associated with Ectomycorrhizal Fungi
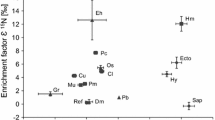
C and N stable isotope natural abundance data of MH plants associated with ectomycorrhizal fungi (EM-MH plants) are already available for a considerable number of species collected in a broad range of habitats of wide geographic distribution (Fig. 8.1). These data include field collections from Europe (Gebauer and Meyer 2003; Bidartondo et al. 2004; Julou et al. 2005; Abadie et al. 2006; Tedersoo et al. 2007; Zimmer et al. 2007, 2008; Liebel et al. 2010; Liebel and Gebauer 2011), North America (Trudell et al. 2003; Zimmer et al. 2007; Hynson et al. 2009b), and Asia (Ogura-Tsujita et al. 2009; Roy et al. 2009a; Motomura et al. 2010) covering habitats from boreal coniferous forests, deciduous or mixed temperate forests, evergreen forests in Mediterranean climates to tropical forests. All of these habitats are forests at least partially composed of tree species known to form EM—an essential prerequisite for a tripartite matter flux between trees, EM fungi, and EM-MH plants. The C and N stable isotope natural abundance data of EM-MH plants currently available from the literature mostly also contains stable isotope natural abundance data of accompanying autotrophic plants. Using these autotrophic plants as references for C gains independent of fungi, site-independent differences between autotrophic “reference” and MH “target” species can be calculated. These calculations allow for comparisons of isotope enrichment between species across broad geographic regions (enrichment factors ε, see Box 8.3). Figure 8.1 compiles the entire currently available C and N stable isotope natural abundance data for EM-MH plants suited to calculate enrichment factors. The data set is composed of 11 orchid species belonging to five different tribes within the subfamily Epidendroideae and five species of Ericaceae belonging to three different tribes within the subfamily Monotropoideae. Among this data set are EM-MH species that have served as model organisms to elucidate matter fluxes between trees, EM fungi, and mycoheterotrophs (like Hypopitys monotropa: Björkman 1960) or to identify the specificity towards certain EM fungi (like Corallorhiza maculata and Neottia nidus-avis: Taylor and Bruns 1997; McKendrick et al. 2002; Selosse et al. 2002). Also among this data set are, however, also species that were just recently identified as EM-MH species based on isotope abundance analyses (like Pyrola aphylla, the only known fully EM-MH species among the tribe Pyroleae: Zimmer et al. 2007; Hynson et al. 2009b), vegetative albino forms of usually chlorophyllous orchid species (Cephalanthera damasonium and C. longifolia: Julou et al. 2005; Abadie et al. 2006; see Sects. 8.4.1 and 8.4.3 for further information on albino phenotypes), and MH species with taxonomically close relatives associated with functional types of fungi other than EM fungi (like the relatives of Epipogium aphyllum: Roy et al. 2009b; Liebel and Gebauer 2011). Furthermore, the data set contains species strictly specialized on narrow EM fungal strains (like Corallorhiza maculata, Neottia nidus-avis and Hypopitys monotropa: Taylor and Bruns 1997; McKendrick et al. 2002; Bidartondo and Bruns 2005) and species associated with a fairly constant set of EM fungi (like Epipogium aphyllum and the albino variants of Cephalanthera damasonium and C. longifolia: Julou et al. 2005; Abadie et al. 2006; Roy et al. 2009b; Liebel and Gebauer 2011) or even with multiple EM fungi (like Pyrola aphylla, Aphyllorchis caudata and A. montana: Hynson and Bruns 2009; Roy et al. 2009a).
Mean enrichment factors (ε, see Box 8.3) for 13C and 15N of fully mycoheterotrophic (MH) plants associated with fungi forming ectomycorrhizas (EM-MH Orchidaceae and EM-MH Ericaceae), with saprotrophic wood-decomposer fungi (SAP-MH Orchidaceae) and with fungi forming arbuscular mycorrhizas (AM-MH plants). The boxes represent one SD of the mean ε values for the four significantly distinguished groups of MH plants and for the photosynthetic reference plants (Ref, n = 659) collected together with each of the mycoheterotrophs. Abbreviations of the respective species and numbers of replicates (n) as following: Ac = Aphyllorchis caudata (n = 3); Am = A. montana (n = 4); Cd(a) = Cephalanthera damasonium albino (n = 10); Ce = C. exigua (n = 5); Cl(a) = C. longifolia albino (n = 9); Com = Corallorhiza maculata (n = 10); Cs = Cyrtosia septentrionalis (n = 1); Cya = Cymbidium aberrans (n = 3); Cym = C. macrorhizon (n = 6); Do = Dictyostega orobanchoides (n = 8); Ea = Epipogium aphyllum (n = 5); Gc = Gastrodia confusa (n = 5); Gs = G. similis (n = 10); Hm = Hypopitys monotropa (n = 23); Ln = Lecanorchis nigricans (n = 3); Mu = Monotropa uniflora (n = 8); Nn = Neottia nidus-avis (n = 36); Pa = Pyrola aphylla (n = 39); Pta = Pterospora andromedea (n = 10); Ss = Sarcodes sanguinea (n = 15); Va = Voyria aphylla (n = 8). Data compiled from Gebauer and Meyer (2003), Bidartondo et al. (2004), Julou et al. (2005), Abadie et al. (2006), Tedersoo et al. (2007), Zimmer et al. (2007, 2008), Hynson et al. (2009b), Martos et al. (2009), Ogura-Tsujita et al. (2009), Roy et al. (2009a), Liebel et al. (2010), Merckx et al. (2010), Motomura et al. (2010) and Liebel and Gebauer (2011)
Irrespective of their wide geographical distribution, phylogenetic breadth within the plant kingdom, their occurrence in different forest types, and their varying degree of EM fungal specificity, what all of these EM-MH species have in common is that they are significantly enriched in the heavy C and N isotopes compared to neighboring reference plants. However, the enrichment factors for both C and N are not constant between MH species, presumably due to variations in the isotope signature of their respective fungal associates. The mean enrichment ranges from 5.3 ± 1.2(SD)‰ (Sarcodes sanguinea, Ericaceae) to 10.8 ± 0.6(SD)‰ (Cymbidium aberrans, Orchidaceae) for 13C and from 6.0 ± 2.1 ‰ (Epipogium aphyllum, Orchidaceae) to 17.8 ± 2.7 ‰ (Pyrola aphylla, Ericaceae) for 15N. Thus, the variation between EM-MH species in heavy isotope enrichment is greater for N than for C and probably reflects the broader variation in fungal N isotope abundance than fungal—and specifically EM fungal—C isotope abundance (Mayor et al. 2009). Current data furthermore indicate a difference in the heavy isotope enrichment of EM-MH species belonging to the Orchidaceae and to the Ericaceae (Fig. 8.1). The investigated 11 EM-MH species belonging to the Orchidaceae are significantly (Mann–Whitney U-test: U = 1731.0, p < 0.001) more enriched in 13C (mean ε13C = 8.2 ± 1.3 ‰, n = 94) than the five EM-MH species belonging to the Ericaceae (mean ε13C = 6.5 ± 1.5 ‰, n = 95), and the EM-MH Orchidaceae (mean ε15N = 11.6 ± 3.1 ‰, n = 94) are significantly (U = 2941.5, p < 0.001) less enriched in 15N than the EM-MH Ericaceae (mean ε15N = 14.2 ± 4.0 ‰, n = 95). The significant difference in C and N stable isotope enrichment between the investigated EM-MH representatives of the two plant families becomes obvious from this meta-analysis thanks to a continuously increasing number of data points available from the literature. Though yet to be confirmed, these differences between orchids and ericaceous MH plants may also relate to the evolution of different mechanisms for MH nutrition in these two families.
8.5.2 Fully Mycoheterotrophic Plants Associated with Saprotrophic Fungi
Besides the tripartite associations with trees and EM fungi, some fully MH orchids associate with free-living wood-decomposer or litter decaying fungi. Interestingly, all of these fully MH plants are orchids and belong mostly to the subfamily Epidendroideae, similar to many EM-MH Orchidaceae. Such saprotrophic-MH (SAP-MH) orchids are typically found in the litter-rich forest floor or beside decomposed woody materials such as decayed tree trunks, stumps, logs, and pruned branches. In 1911, Kusano reported on an association of the MH orchid Gastrodia elata with the normally plant pathogenic wood-decomposing fungus Armillaria, and Hamada (1939) also found this fungus associating with the MH orchid Cyrtosia sepentrionalis. Subsequently, various Armillaria species have been isolated and identified from these orchids (Terashita and Chuman 1987, 1989; Cha and Igarashi 1995, 1996; Matsushita et al. 1996; Terashima et al. 1998; Ota et al. 2000; Kikuchi et al. 2008a, b; Sekizaki et al. 2008). In the world’s largest MH climbing orchid, Erythrorchis ochobiensis, an association with the SAP Erythromyces crocicreas (Basidiomycota) was reported by Hamada and Nakamura (1963) and, later, a wide range of wood-decomposing fungi, such as Lentinula edodes and Pleurotus ostreatus, were shown to induce seed germination and plantlet formation in symbiotic culture (Umata 1995, 1997, 1998a, b, 1999). The MH orchid Epipogium roseum, the sister species of the EM-MH orchid Epipogium aphyllum, has been shown to associate with fungi from the SAP family Psathyrellaceae (Basidiomycota), including Coprinellus disseminatus (Yamato et al. 2005; Yagame et al. 2008a). Notable is that Epipogium roseum (Yagame et al. 2007) can be cultivated for its whole lifecycle with wood-decomposer fungi under laboratory conditions. Such instances of in vitro cultivation are also known for other SAP-MH orchids (Burgeff 1932, 1936; Umata et al. 2007), and Gastrodia elata is now routinely cultivated symbiotically with Armillaria spp. for commercial use of its tubers (Chou 1974; Sung et al. 1995; Xu and Guo 2000). In addition to these reports, several other SAP decomposer fungal genera such as Fomes, Marasmius, Xerotus, Campanella and Gymnopus have been reported to be associated with MH Gastrodia species, its related MH genus Didymoplexis and some species of the MH genera Galeola and Erythrorchis (Burgeff 1932; Campbell 1962, 1964; Dearnaley 2006; Dearnaley and Bougoure 2010). Although information about fungal specificity of SAP-MH orchids is still rather scarce, recent reports showed a wide range of specificities, from a strict association between Eulophia zollingeri and the Psathyrella candolleana species group (Ogura-Tsujita and Yukawa 2008), to an association with a constant set of fungal partners, like Gastrodia confusa and representatives of the Mycenaceae (Ogura-Tsujita et al. 2009), to a broad spectrum of associated fungi in the case of Wullschlaegelia aphylla or Gastrodia similis and multiple Basidiomycetes related to the genera Mycena, Marasmius, Psathyrella and Resinicium (Martos et al. 2009). Apparently different lineages within Psathyrellaceae and Mycenaceae have been repetitively targeted by independent MH orchid lineages (Selosse et al. 2010).
Associations between decomposer SAP fungi and fully MH plants as well as laboratory cultivation experiments provide a hint that fully MH plants also can cover their C and N demand through associations with these fungi in nature. As with EM-MH plants, stable isotope natural abundance analysis is a powerful tool to elucidate the nutritional sources of MH plants associated with decomposer fungi. The still very small stable isotope natural abundance data set available for SAP-MH orchids and accompanying reference plants indicates a significant enrichment in 13C and 15N due to C and N gain from the fungal source, but with a different pattern than found for EM-MH plants (Fig. 8.1). The so far investigated SAP-MH orchids (Gastrodia confusa from a wet-temperate bamboo forest in Japan and associated with several species of wood-decomposer Mycena fungi (Ogura-Tsujita et al. 2009), Gastrodia similis from tropical rainforests and secondary forests in La Réunion and associated with the wood-decomposer Resinicium (Martos et al. 2009), and one individual of Cyrtosia septentrionalis collected in a warm-temperate evergreen broadleaf forest in Japan (Motomura et al. 2010)) indicate a 13C enrichment (mean ε13C = 9.7 ± 2.4 ‰, n = 16) that is significantly higher than EM-MH (vs. EM-MH orchids U = 456, p = 0.012; vs. EM-MH Ericaceae U = 212, p < 0.001) and a 15N enrichment (mean ε15N = 2.7 ± 1.1 ‰, n = 16) that is significantly lower than found for EM-MH orchids (U = 2, p < 0.001) and EM-MH Ericaceae (U = 0, p < 0.001). Though not suited for the ε approach due to the absence of true reference plant data, the C and N isotope signature of one other MH orchid species associated with wood-decomposer fungi (Gastrodia sesamoides from open woodland in Queensland, Australia; (Dearnaley and Bougoure 2010) also points towards a considerably high 13C enrichment and a 15N enrichment lower than found for EM-MH plants. The most likely reason for the different pattern of C and N isotope natural abundance between SAP-MH plants and EM-MH plants is a difference in the isotope signature of the C and N sources utilized by their respective fungal hosts. These few investigated SAP-MH plants are consistently associated with wood-decomposer fungi, and wood is known to be enriched in 13C and depleted in 15N (Gebauer and Schulze 1991; Gebauer and Dietrich 1993; Gebauer and Taylor 1999). Presently, stable isotope natural abundance data are available only for one SAP-MH orchid associated with litter decaying fungi (Wullschlaegelia aphylla from a tropical rainforest in Guadeloupe; Martos et al. 2009). These data point towards lower 13C enrichment in SAP-MH orchids associated with litter decaying fungi than in SAP-MH orchids with wood-decomposer fungi. Undoubtedly more data on stable isotope signatures of SAP-MH orchids (especially associated to litter decaying fungi) and accompanying reference plants are required to confirm these preliminary conclusions. Also, the geographic distribution of SAP-MH orchids and their apparent preference for wood-decomposers among the SAP fungi require further investigation. At present we only can conclude that SAP-MH orchids obviously prefer humid climate conditions under which wood or litter decomposition rates are high.
8.5.3 Fully Mycoheterotrophic Plants Associated with Arbuscular Mycorrhizal Fungi
The AM symbiosis is one of the oldest plant symbioses on earth, estimated to be at least 400 million years old (Smith and Read 2008). It is therefore not surprising that this lineage of fungi has been infiltrated by the cheating strategy of fully MH plants on many more independent occasions than EM or SAP fungi. The majority of fully MH plants grow in tropical forests dominated by photosynthetic plants that associate with AM fungi. For this reason it was assumed in analogy to the EM-MH plants that among these fully MH plants in AM dominated forests an association with AM fungi occurs. Based on fungal DNA analysis from the mycorrhizal roots of several fully MH plants, five Voyria and one Voyriella species (Gentianaceae) from tropical forests in French Guiana and Arachnitis uniflora (Corsiaceae) from three subantarctic forest sites in Argentina, and neighboring green plants, Bidartondo et al. (2002) showed that these plants indeed associate with AM fungi and display a fungal host specificity similar to many EM-MH plants. This finding suggested that AM fungi mediate a C transfer between autotrophic AM plants and AM-MH plants. Recent stable isotope natural abundance data confirm this suggestion (Fig. 8.1). Though phylogenetically distant, the fully MH plants Voyria aphylla (Gentianaceae) and Dictyostega orobanchoides (Burmanniaceae) also collected in a tropical forest in French Guiana are both significantly enriched in the heavy isotope 13C in comparison to neighboring photosynthetic plants (Merckx et al. 2010). The relative 13C enrichment of these AM-MH plants (mean ε13C = 5.5 ± 0.8 ‰, n = 16) is, however, significantly lower than in EM-MH orchids (U = 40.5, p < 0.001), EM-MH Ericaceae (U = 393.5, p = 0.002), and SAP-MH orchids (U = 4, p < 0.001). Similar trends were found by Courty et al. (2011) for V. aphylla and V. tenella (Gentianaceae) as well as Aptera aphylla and Gymnosiphon sphaerocarpus (Burmanniaceae) from a Caribbean island, La Guadeloupe. In addition, the latter study recovered spores from soil to investigate the isotope signature of AM fungal propagules, which proved similar to that of leaves of canopy trees. Thus, while the relative 13C enrichment of EM-MH plants compared to surrounding autotrophic plants is primarily related to the 13C enrichment of their fungal associates, the enrichment of AM-MH plants compared to surrounding understory autotrophic plants is likely unrelated to differences in AM fungal C acquisition and rather due to differences in photosynthetic rates between canopy trees and understory plants. Because of low photosynthetic rates and more humid conditions supporting longer and more numerous stomatal openings, understory plants are more depleted in 13C than canopy trees (see Gebauer and Schulze 1991). Noteworthy is the finding of no significant 15N enrichment in the AM-MH plants by both Merckx et al. (2010) (mean ε15N = −0.4 ± 3.3 ‰, n = 16; see Fig. 8.1) and Courty et al. (2011) who, additionally, reported that the 15N signature was similar in AM fungi. Courty et al. (2011) also showed that, compared to their accompanying reference plants, the investigated AM-MH plants have similar total N concentrations as compared to green plants and fungi, whereas higher total N concentrations are common among EM-MH plants (Gebauer and Meyer 2003; Julou et al. 2005; Liebel et al. 2010; Stöckel et al. 2011; see also Sect. 8.6.1). Lack of differentiation in 15N natural abundance and total N concentrations between AM-MH plants and reference plants suggests utilization by all of these plants of similar N sources, presumably inorganic N compounds obtained through their AM fungal partners. Taken together, these observations, which deserve replicates from other sites and taxa, suggest that the matter exchanged between fungus and host plant differs in EM-MH vs. AM-MH plants, a situation not fully unexpected owing to the ancient divergence between AM Glomeromycota and EM-forming taxa of Asco- and Basidiomycota.
8.7 Partial Mycoheterotrophy
8.7.1 Evolution of Partial and Full Mycoheterotrophy
PMH plant lineages add to a long list of taxa where heterotrophic abilities evolved from autotrophic ancestors. In the broadest sense, such a strategy is a kind of mixotrophy. The word mixotrophy has been used as a synonym for partial mycoheterotrophy, but is a more encompassing term than partial mycoheterotrophy, because mixotrophic strategies and mechanisms are very diverse. For example, many independent phyla of planktonic algae are mixotrophic, either by uptake of dissolved organic matter (Kamjunke and Tittel 2009) or by phagotrophy on unicellular preys (Jones 2000). Uptake of C or phagotrophy is a plesiomorphic condition in these algae, since ancestors of plastid-bearing taxa are considered to have been heterotrophs (phagotrophy likely being the process through which plastids were indeed acquired). In contrast, mixotrophy in land plants is secondarily evolved, i.e., represents subversion from full autotrophy, and its ecological relevance is yet to be estimated in terrestrial ecosystems. Here we use the phrase “partial mycoheterotrophy” whenever mixotrophy is achieved in a green plant by partial use of C from a mycorrhizal fungus.
In land plants, mixotrophic strategies encompasses partial mycoheterotrophy, but also the use of C from prey (Adamec 1997) and of the host sap in hemiparasitic plants (Schulze et al. 1991; Press and Graves 1995; Těšitel et al. 2010). The latter are green plants that obtain some nourishment, especially mineral nutrients and sometimes C, by parasitizing other plants: C is obtained from connection to the xylem sap, or even to the phloem sap. For example, mistletoes (Loranthaceae) derive up to 63% of their C from their host (Schulze et al. 1991; Bannister and Strong 2001); Olax phyllanthi (Olacaceae) derives 19–30% (Tennakoon and Pate 1996); Rhinanthus alectorolophus (Orobanchaceae) derives up to 50% (Těšitel et al. 2011). In the latter case, as is true for some PMH species (see Sect. 8.4.3), shading enhanced the contribution of host-derived C; moreover, achlorophyllous variants of Striga hermonthica (Orobanchaceae) can survive (Press and Graves 1995).
When considering mycoheterotrophy in a phylogenetic framework, some fully MH species are nested within PMH lineages, e.g., in the tribe Neottieae (Fig. 8.2a; Abadie et al. 2006; Selosse and Roy 2009) and in the genus Cymbidium (Motomura et al. 2010); and the same may have happened in the genus Platanthera (Yagame et al. 2012). Pyroloids (= tribe Pyroleae) are closely related to the MH Monotropoideae (Monotropeae and Pterosporeae; Kron et al. 2002), suggesting a similar scenario (Tedersoo et al. 2007); however, the subtribe relationships in Monotropoideae deserve new analyses and a basal position of Pyroleae remains uncertain. Interestingly, in pyroloids, there are two well-supported clades (Freudenstein 1999; Kron et al. 2002), Pyrola + Orthilia on the one hand and Moneses + Chimaphila on the other; the second clade tends to encompass less frequent reports of partial MH than the first, which harbors the fully MH P. aphylla (Zimmer et al. 2007; Hynson et al. 2009b) and PMH P. japonica (Matsuda et al. 2012).
(a) A phylogeny of Neottieae (based on ITS + rbcL phylogenies, inferred by Maximum Likelihood and 1,000 bootstrap repetitions, using a GTR model, from Roy et al. 2009a) with reconstruction inferred ancestral trophic status, assuming the most parsimonious scenario for its evolution. Green, autotrophic nutrition; yellow, partially mycoheterotrophic (PMH) nutrition; red, mycoheterotrophic nutrition. Stars indicate node supported by >80% bootstrap support and (a) indicates green species for which albino, i.e., fully achlorophyllous variants are reported. (b) CO2 exchanges at various light levels in Cephalanthera damasonium, a green species where rare fully non-chlorophyllous albinos exist. Each curve is the mean of five individuals (M. Roy and M.-A. Selosse, unpublished data; see Julou et al. 2005 for site, material and methods). On the right a typical green C. damasonium individual together with a non-chlorophyllous variant
The evolution to full mycoheterotrophy through partial mycoheterotrophy is thus reminiscent of the evolution of holoparasitic plants (= full heterotrophs) from hemiparasitic ancestors, as reported in the Orobanchaceae (Bennett and Mathews 2006) and Convolvulaceae (McNeal et al. 2007). A common scenario for evolution of plant heterotrophy can be suggested (Table 8.1; Cameron and Leake 2007; Selosse and Roy 2009), where biological interactions which only formerly selected for mineral nutrition allow (1) some indirect mixotrophy, and then (2) select for emergences of full heterotrophy, directly targeting C. Basically, this is an exaptation from mineral to C nutrition by “baiting the feeding hand.” This scenario awaits confirmation from other taxa such as AM-MH plants that associate with one of the oldest extant mycorrhizal lineages of fungi on earth, the Glomeromycota (Selosse and Roy 2009).
In terms of selective mechanisms, evolutionary transition to mycoheterotrophy can be fuelled by the fact that some PMH plants, at least in some environments, grow in young forests whose initially open canopies allows photosynthesis but tend to close due to ongoing succession. Thus, they undergo increasing shading that selects for more light-independent C supply (Selosse and Roy 2009); indeed, this may explain the convergent evolution to full MH observed within the Neottieae (Fig. 8.2a; Roy et al. 2009a). In this tribe, full MH is never shown to be evolutionarily reversible, maybe due to loss of photosynthetic genes both in the plastid and nucleus. However, there are possible reversions from partial mycoheterotrophy at adulthood to autotrophy at adulthood. For example, the Epipactis palustris-gigantea clade (Fig. 8.2a) is autotrophic at adulthood, as shown by survival upon transplantation (Sadovsky 1965), 13C abundances indicative of autotrophy (Fig. 8.3), and association with rhizoctonia fungi that are common associates of initially MH orchids (Bidartondo et al. 2004; Zimmer et al. 2007; Illyés et al. 2009). Similarly, some green Listera species, despite close taxonomic relatedness to Neottia nidus-avis and Asian MH species (Roy et al. 2009a), are likely autotrophic as adults based on their 13C abundances (Fig. 8.3) and associations with rhizoctonias (Bidartondo et al. 2004). Although Neottieae phylogenies remain poorly resolved, these clades are unlikely to be basal (Pridgeon et al. 2008; Roy et al. 2009a), making reversion from partial mycoheterotrophy to autotrophy at adulthood the most parsimonious scenario, while multiple shifts to partial mycoherotrophy remain a possible, but less parsimonious, alternative. Reversion to autotrophy at adulthood is not unexpected, since photosynthetic abilities remain in partial mycoheterotrophs.
Mean enrichment factors (ε, see Box 8.3) for 13C and 15N of (a) 16 green orchid species (black symbols) and 14 fully mycoheterotrophic (MH) orchid species (grey symbols, for further details see Fig. 8.1) belonging to the subfamily Epidendroideae and associated either with fungi from the polyphyletic rhizoctonia group or, fungi simultaneously forming ectomycorrhizas with surrounding trees (EM-PMH and EM-MH) or with saprotrophic wood-decomposer fungi (SAP-MH) and of autotrophic reference plants (Ref, n = 765) collected together with each of the respective orchids and (b) of 44 green orchid species belonging to the subfamily Orchidoideae and of autotrophic reference plants (Ref, n = 863) collected together with each of the respective orchids. The boxes represent one SD of the mean ε values for the different groups of green and MH orchids and for the autotrophic reference plants. Abbreviations of the green orchid species belonging to the Epidendroideae and numbers of replicates (n) as following: Cd = Cephalanthera damasonium (n = 21); Ce = C. erecta (n = 3); Cyl = C. longifolia (n = 19); Cr = C. rubra (n = 7); Cot = Corallorhiza trifida (n = 9); Cyg = Cymbidium goeringii (n = 7); Cl = C. lancifolium (n = 6); Ea = Epipactis atrorubens (n = 11); Ed = E. distans (n = 4); Eg = E. gigantea (n = 5); Eh = E. helleborine (n = 18); Ep = E. palustris (n = 4); La = Limodorum abortivum (n = 10); Lt = L. trabutianum (n = 5); Ln = Liparis nervosa (n = 3); Lo = Listera ovata (n = 25). The data set of the green orchids belonging to the Orchidoideae includes the following species and replicates (n): Aceras anthropophorum (n = 10), Anacamptis laxiflora (n = 5), Barlia metlesicsiana (n = 5), B. robertiana (n = 5), Cheirostylis montana (n = 2), Dactylorhiza majalis (n = 4), D. sambucina (n = 11), Gennaria diphylla (n = 10), Goodyera oblongifolia (n = 18), G. repens (n = 5), G. schlechtendaliana (n = 1), Gymnadenia conopsea (n = 8), Habenaria tridactylites (n = 5), Ludisia discolour (n = 5), Neotinea maculata (n = 5), Ophrys fuciflora (n = 9), O. insectifera (n = 12), O. apifera (n = 5), O. incubacea (n = 5), O. sicula (n = 5), O. sphegodes (n = 5), Orchis brancifortii (n = 5), O. canariensis (n = 5), O. ichnusae (n = 5), O. laxiflora (n = 5), O. longicornu (n = 5), O. mascula (n = 18), O. morio (n = 5), O. papilionacea (n = 5), O. pauciflora (n = 5), O. provincialis (n = 5), O. purpurea (n = 10), O. tridentata (n = 5), O. ustulata (n = 5), Platanthera bifolia (n = 7), P. chlorantha (n = 4), P. leucostachys (n = 13), Serapias cordigera (n = 5), S. lingua (n = 5), S. nurrica (n = 5), S. parviflora (n = 10), S. vomeracea (n = 10), Spiranthes spiralis (n = 5), and Zeuxine agyokuana (n = 1). Data compiled from Gebauer and Meyer (2003), Bidartondo et al. (2004), Julou et al. (2005), Abadie et al. (2006), Tedersoo et al. (2007), Zimmer et al. (2007, 2008), Hynson et al. (2009a), Roy et al. (2009a), Liebel et al. (2010), Motomura et al. (2010) and Girlanda et al. (2011)
Despite their successful survival, albinos observed in some orchid species show reduced fitness (Salmia 1986, 1989b; Roy et al. 2012), reduced demography as compared to green individuals (Abadie et al. 2006; Tranchida-Lombardo et al. 2010), and more or less impaired vegetative traits (Salmia 1989b; Julou et al. 2005). Their lower basal metabolic rates (Julou et al. 2005; see CO2 evolution in the dark on Fig. 8.2b) suggest C limitation. They often display higher rates of mycorrhizal colonization (Salmia 1989a; Selosse et al. 2004; Abadie et al. 2006), a fact that could compensate for absence of photosynthesis, but it is unknown if more extensive colonization is linked to greater carbon gains. In a study of two C. damasonium populations with albino and green individuals (Roy et al. 2012), albinos comparatively displayed (1) more frequent shoot drying at time of fruiting, possibly due to stomatal dysfunctions, (2) higher frequency of dormancy, and (3) fewer seeds, with lower germination capacity. This results in a 500–1,000× fitness reduction as compared to green individuals. Among other factors, two observed features were proposed to cause a C limitation and fitness reduction in these albinos: they displayed higher pathogen and herbivore load, and a sharp reduction of mycorrhizal colonization at time of fruiting, which would likely be compensated by photosynthesis in green individuals, but may be critical for the survival of albinos (Roy et al. 2012). Albinos likely represent unique snapshots of failed transitions from partial mycoheterotrophy to full mycoheterotrophy, and the analysis by Roy et al. (2012) suggests that successful transitions at least require degeneration of leaves and stomata, optimization of the temporal pattern of fungal colonization and shoot sprouting, and new defences against pathogens and herbivores. In Neottieae, albinos suggest that the transition from partial to full mycoheterotrophy cannot be sudden, and that additional traits are required to become successfully MH. Moreover, their absence in PMH Ericaceae suggests that the transition to full mycoheterotrophy can occur in lineages devoid of albinos, so that they are not a necessary step toward full mycoheterotrophy. Albinos are ecological equivalents to mutants in genetics, i.e., their dysfunctions may suggest what makes mycoheterotrophy successful. Although their determinism remains unknown, they offer fascinating models for comparing the physiology of mixo- and autotrophs within very similar genetic backgrounds. The options for physiological investigations on the transition from autotrophy to full mycoheterotrophy become even wider when including MH species possessing variegated leaves. In addition to the frequent green and rare albino forms of some orchid species, individuals with a continuous range between these extremes have episodically been found (Renner 1938; Salmia 1989b; Stöckel et al. 2011).
8.7.2 Initial Mycoheterotrophy-Autotrophy
In contrast with full mycoheterotrophs, many plants are MH during and after seed/spore germination, but eventually develop into autotrophic individuals. While the duration of mycoheterotrophy in such species is relatively short, it remains an obligate and critical part of their life cycle. Most orchids (aside from a few hundred species that remain fully or PMH as adults) appear to be initially mycoheterotrophic-autotrophic (IMH), although direct evidence is available for only a small number of species. With over 20,000 species in the Orchidaceae, the number of IMH species is likely far greater than all fully and PMH species combined. Initial mycoheterotrophy-autotrophy is not limited to the orchid family; however, additional taxa within the Ericaceae (Pyroleae), Lycopodiaceae, and several fern families appear to be IMH, as well (see Chap. 2).
MH seedlings and sporelings of IMH plants are typically subterranean and non-photosynthetic; however, despite their cryptic nature, such germlings belonging to the Orchidaceae (Salisbury 1804), Pyroleae (Irmisch 1855), Ophioglossaceae (Hofmeister 1857), and Lycopodiaceae (Mettenius 1856) had already been observed by the nineteenth century. Like fully MH plants, they were often erroneously described as “saprophytic” (Leake 2005). The understanding of IMH orchid seedlings advanced in the late nineteenth to early twentieth centuries, with discoveries by Noël Bernard (Bernard 1899) that fungal symbionts are necessary for the heterotrophic growth of orchid seedlings, Beau (1920) that growth of symbiotic orchid seedlings occurs only when the fungi have access to a C source, and Bernard (1908) and Knudson (1922) that orchid seedlings exhibit asymbiotic growth on media enriched with simple sugars. More recently, studies involving labeled and naturally abundant isotopes have indicated that adult plants of photosynthetic orchid spp., as has generally been assumed, are usually autotrophic (Gebauer and Meyer 2003; Cameron et al. 2006, 2008; Hynson et al. 2009a; Liebel et al. 2010; Girlanda et al. 2011). Autotrophy at adulthood is indicated by 13C natural abundance for the majority of green species from the subfamily Orchidoideae investigated thus far (Fig. 8.3b) and for species from the subfamily Epidendroideae that are solely associated with fungi of the polyphyletic rhizoctonia group (Fig. 8.3a). While isotopic evidence for initial mycoheterotrophy-autotrophy is not yet available for most putatively IMH taxa, this lifestyle can be inferred by characteristic dust seed morphology (in angiosperms), subterranean and non-photosynthetic germling development, consistent association of germlings with fungi, absence of hemiparasitic interactions, and photosynthetic, readily cultivated adults.
8.7.2.1 Initial Mycoheterotrophy-Autotrophy in the Orchidaceae
Like fully MH angiosperms, initially mycoheterotrophic-autotrophic orchids (IMHOs) have highly reduced “dust seeds” which contain an embryo but no endosperm (see Chap. 5). Germination occurs when the embryo enlarges enough to rupture the testa, with the seedling at this stage known as a protocorm. Seeds of IMHOs may be stimulated to germinate by host or non-host fungi, or may germinate in the absence of fungi all together (Downie 1959; Hadley 1970; Warcup 1973; Rasmussen 1995); however, further protocorm growth is usually dependent on mycoheterotrophy. Protocorms of most terrestrial IMHOs are subterranean, non-photosynthetic, and unambiguously MH. While protocorms of many epiphytic and some terrestrial species are superficial and green, they usually cannot progress beyond germination without a fungal host (or exogenous supply of sugar). A small number of terrestrial species (e.g., in the genera Disa (Section Disa, Bletilla, and Sobralia) have been observed to germinate and develop to the leafy stage without host fungi (Burgeff 1959). However, it has been suggested that seedling development in some of these taxa occurs more rapidly with fungal symbionts than without.
Shoot production in symbiotically cultured seedlings frequently occurs within several months of germination (Warcup 1973; Muir 1989; reviewed in Rasmussen 1995), though the first leaf may not emerge until the second growing season in some temperate spp. (e.g., Zettler et al. 2001; Sharma et al. 2003); production of the first root usually occurs concurrently or shortly thereafter. Early field reports suggesting IMHO seedlings remain underground and MH for several years (e.g., Curtis 1943) were based on conjectural interpretation of seedling “growth segments”; as MH periods of similar duration have not been observed in symbiotic cultural studies, it would appear that such claims are exaggerated. Whether seedlings become fully autotrophic upon emergence of the first leaf or remain PMH for a period of time thereafter is not known. However, asymbiotic seedlings propagated following Knudson’s (1922) protocol are commonly removed from sugar-enriched media when they have well-developed shoots and roots, apparently transitioning readily from partial/full heterotrophy to full autotrophy.
With very few exceptions (e.g., McCormick et al. 2004), host fungi of IMHO seedlings belong to Ceratobasidium sensu lato (incl. Thanatephorus), Tulasnella, and the Sebacinales (Dearnaley et al. 2012). Before Warcup and Talbot (1967) identified the perfect states of host fungi in culture, the identity of such fungi was hidden behind the veil of the morphologically defined, asexual genus Rhizoctonia, now known to be polyphyletic. Identification of host fungi has at times been further confounded by the propensity of IMHO seeds to germinate—and even for protocorms to undergo limited MH development—with non-host fungi, and of adult plants to allow peloton-formation of such fungi in their roots. These fungi may include rhizoctonia strains capable of hosting seedlings of other orchid species (Harvais and Hadley 1967), as well as Basidio- and Ascomycota not known to host seedlings of any orchid species (Currah et al. 1997; Vujanovic et al. 2000). Consequently, true host fungi of IMHOs are most appropriately identified as those supporting seedling development to the first leaf stage (e.g., Warcup 1973; Zettler and Hofer 1998).
Host specificity of IMHO seedlings is variable; while some IMHO species are highly host-specific (e.g., Wright et al. 2010; Phillips et al. 2011), compatibility with multiple strains of Ceratobasidium and/or Tulasnella is not uncommon (Hadley 1970). However, compatibility with Tulasnella and Sebacinales has only been observed in Microtis spp. (Warcup 1981; Milligan and Williams 1988; Bonnardeaux et al. 2007). Ceratobasidium and Tulasnella, by far the most common hosts of IMHOs, are thought to be predominantly SAP and/or pathogenic (Rasmussen 2002), though some lineages can be EM (Bidartondo et al. 2003; Yagame et al. 2012), and the ecology of these genera deserves further study. Sebacinales are known as hosts of IMHO seedlings within the Caladeniinae and occasionally the Prasophyllinae and Acianthinae (three closely related and predominantly Australian subtribes within the Diuridae). Sebacinales have been implicated in a wide variety of mycorrhizal and non-mycorrhizal (endophytic) interactions with plants (Selosse et al. 2009; Weiss et al. 2011), though notably, almost all such hosts of IMHOs belong to Sebacinales clade B, a group that is endophytic in many plants (Weiss et al. 2004; Selosse et al. 2009).
In symbiotic culture, growth of IMHO seedlings commonly occurs when C is available to host fungi in the form of starch or cellulose, and there is field evidence that organic matter may enhance germination in the presence of host fungi (McCormick et al. 2012). The extent to which C from living plants—acquired by orchid host fungi as pathogens or endophytes—may contribute to IMHO seedling development in nature is unknown. Unlike Ceratobasidium and Tulasnella spp., Sebacinales are often difficult to culture, suggesting relatively poor SAP capability. Nevertheless, they may consume dead plant tissues (Zuccaro et al. 2011) and are capable of supporting IMHO seedling development in vitro via utilization of starch (Warcup 1981; Ramsay et al. 1986; Bonnardeaux et al. 2007; Wright et al. 2009). Seedlings of Microtis spp., which are sometimes compatible with EM sebacinoid hosts (Warcup 1988), are the only known examples of EM-hosted IMHOs. However, given the recent discovery of EM Ceratobasidium and Tulasnella strains hosting fully and PMH orchids (Mursidawati 2004; Bougoure et al. 2009, 2010; Yagame et al. 2008b, 2012) and a fully MH liverwort (Bidartondo et al. 2003), respectively, it is possible that IMHO seedlings are hosted by EM fungi in additional genera.
8.7.2.2 Isotopic Evidence for Initial Mycoheterotrophy-Autotrophy in Orchidaceae
Cameron et al. (2006, 2008) demonstrated reciprocal transport of labeled C between adult, photosynthetic plants of Goodyera repens and their Ceratobasidium symbiont, with more than five times as much C transferred from plant to fungus as in the opposite direction. These findings suggest the direction of net C flow may reverse as seedlings transition from mycoheterotrophy to autotrophy. Although Cameron et al. did not confirm that the fungal symbiont associated with adult plants is capable of hosting MH development of conspecific seedlings, this has frequently been found in other studies of Goodyera spp. (Rasmussen 1995; McCormick et al. 2004). It should be noted, however, that the placement within the subfamily Orchidoideae of taxa capable of reciprocal C transfer is not sufficient to support that the trait is ancestral to the orchid family; isotopic data from taxa in the Apostasioideae, Vanilloideae, and Cypripedioideae are needed in order to determine the evolutionary polarity of this trait. Further, the repeated acquisition of EM hosts by partially and fully MH orchids, as stated above, suggests an innate ability to exploit C of fungi with which they had no apparent preexisting mutualism.
Natural abundance 13C and 15N data indicate that adult IMHOs may, as expected, exhibit 13C and 15N abundances equivalent to autotrophic reference plants (Fig. 8.3). More often, however, they are significantly depleted in 13C and/or enriched in 15N (Fig. 8.3). Hynson et al. (2009a) suggest that because adult individuals of Goodyera spp. exhibit such 13C depletion (Fig. 8.3b), it may result from the net C transfer from plant to fungus, as documented in G. repens by Cameron et al. (2006, 2008). Given that 13C depletion has been found in other spp. within the Orchideae (Liebel et al. 2010), it is possible that reciprocal C transfer occurs in additional taxa.
Why C transfer from orchids to fungi might result in 13C depletion relative to autotrophic reference plants (which also participate in such transfer) is not known. It is possible that physiological differences in C transfer pathways between orchids and SAP Ceratobasidium/Tulasnella fungi and non-orchid reference plants and EM fungi result in different patterns of 13C natural abundance in these groups of plants. The mechanism by which adults of some IMHOs are enriched in 15N, typically to a level intermediate between autotrophic and MH reference plants (Fig. 8.3), is also unknown. Liebel et al. (2010) suggest such species may continue to obtain N via a pathway analogous to that in fully MH plants, resulting in 15N enrichment, but that concurrent 13C enrichment of plant tissue may be counteracted by simultaneous transfer of 13C from plant to fungus. This suggestion is supported by the finding of significantly increased total N concentrations in many IMHOs in comparison to accompanying reference plants (Gebauer and Meyer 2003; Abadie et al. 2006; Liebel et al. 2010; see also Sect. 8.6.1).
While it has been assumed that cultivable, Ceratobasidium/Tulasnella-hosted orchid species are invariably IMH, Liebel et al. (2010) and Girlanda et al. (2011) found levels of natural 13C enrichment consistent with partial mycoheterotrophy in adult plants of several such species (Fig. 8.3b). While these taxa represent a minority of the orchids investigated thus far, the discovery of their trophic status suggests the total number of IMHO species may be significantly smaller than previously expected. Further, the known cultivability of some of the study taxa suggests that partial mycoheterotrophy in the adults is facultative rather than obligate, in which case initial mycoheterotrophy-autotrophy and initial mycoheterotrophy-partial mycoheterotrophy may not represent mutually exclusive categories. Additionally, the association of these orchids with Ceratobasidium and Tulasnella suggest that partial mycoheterotrophy in adult orchids may not always be accompanied by a switch to EM hosts, though the ability to form mycorrhizal networks among the strains identified by Liebel et al. (2010) and Girlanda et al. (2011) is not yet known.
Among putatively IMHOs, natural abundance stable isotope analyses have largely been limited to species that are (1) hosted by Ceratobasidium and/or Tulasnella, (2) members of the Orchidoideae and Epidendroideae subfamilies, and (3) terrestrial. Given the utility of these analyses in confirming the trophic status of photosynthetic adults, they deserve to be more widely applied to species that are hosted by Sebacinales members of the Apostasioideae, Vanilloideae, and Cypripedioideae subfamilies; and/or epiphytic species. However, a potentially confounding factor for examining partial mycoheterotrophy among epiphytic orchids is that many of these species are either obligate or facultative CAM (crassulacean acid metabolism) plants (Neales and Hew 1975; Motomura et al. 2008; Silvera et al. 2010). Plants that rely on the CAM photosynthetic pathway are enriched in 13C compared to those that utilize C3 photosynthesis. Thus, based on their C stable isotope profiles some epiphytic CAM orchids may, like PMH orchids, be enriched in 13C even though they do not rely on fungi to meet their C demands. While analyses of IMHO seedlings have not yet been published, it would seem that these seedlings are likely to exhibit enrichment in 13C and 15N similar to fully MH plants. Enrichment in 13C and 15N compared to surrounding autotrophs has been observed in some adult orchids associated with Ceratobasidium and Tulasnella (Liebel et al. 2010; Girlanda et al. 2011), the most common hosts of IMHO seedlings.
8.7.2.3 Initial Mycoheterotrophy-Autotrophy in Pyroleae
The Pyroleae have dust seeds that, like most terrestrial orchids, germinate underground and develop into MH seedlings. While such subterranean, non-photosynthetic seedlings, made up of root-like organs, have been observed in culture (Lihnell 1942) and in the field (Irmisch 1855; Velenovsky 1892), the duration of initial mycoheterotrophy remains unknown. The few data available indicate that individual seedlings of two species of Pyroleae, Pyrola chlorantha and Orthilia secunda are hosted by fungi in Sebacinales clade B (sensu Weiss et al. 2004), and a suite of EM fungi (N.A. Hynson unpublished; Smith and Read 2008). When investigating the fungal hosts to seedlings of P. asarifolia in Japan, Hashimoto et al. (2012) found a higher degree of host specificity for fungi only in Sebacinales clade B. However, seedlings of Pyrola chlorantha and Orthilia secunda have also been found to associate with ectomycorrhizal Sebacinales fungi from clade A (N.A. Hynson unpublished). It is surprising that single seedlings associate with single fungal hosts, and that some appear to be rather specific to non-EM fungi given that adult Pyroleae commonly associate with a diversity of EM fungi (Tedersoo et al. 2007; Zimmer et al. 2007; Vincenot et al. 2008; Hynson and Bruns 2009; Toftegaard et al. 2010).
With the exception of the fully MH Pyrola aphylla (Zimmer et al. 2007; Hynson et al. 2009b), many adult Pyroleae are leafy and primarily dependent on photosynthesis. Partial mycoheterotrophy in adult plants is frequently inconsistent between conspecific populations, with some individuals significantly more enriched in 13C than autotrophic reference plants and others not (see Sect. 8.4.3; Tedersoo et al. 2007; Zimmer et al. 2007; Hynson et al. 2009b). Nevertheless, the populations that appear to be primarily dependent on photosynthetic C gains upon reaching adulthood, together with the survival of adult plants in cultivation (e.g., Hunt and Hope-Simpson 1990), suggests that some adult Pyroleae may be facultatively, if not consistently, autotrophic. Clearly, this group of plants deserves further investigation into the trophic status of adult individuals.
8.7.2.4 Initial Mycoheterotrophy-Autotrophy in Other Taxa
A number of taxa in the Lycopodiaceae, Psilotaceae, Ophioglossaceae, Schizaeaceae, and Gleicheniaceae have unambiguously MH gametophytes and preemergent sporophytes (Boullard 1979). With few exceptions, adult sporophytes are consistently photosynthetic, and many can be cultivated. It appears that many of these taxa may be IMH, although the trophic status of adult sporophytes under field conditions has yet to be investigated (see Sect. 8.4.3).
8.7.3 Initial Mycoheterotrophy- Partial Mycoheterotrophy
Contrasting with the previous scenario where adult plants are fully autotrophic, several species that turn green at adulthood after MH seedling development were discovered to remain PMH, i.e., maintain a C flow from the fungus to the plant over their whole lifespan. Although orchids were instrumental in the emergence of the concept, the phenomenon is now suspected, and partly demonstrated, to be more widespread (Tedersoo et al. 2007; Zimmer et al. 2007; Cameron and Bolin 2010; reviewed in Selosse and Roy 2009).
8.7.3.1 Discovery of Partial Mycoheterotrophy in Adult Orchids
The suspicion of partial mycoheterotrophy came from two lines of observation in species of the Neottieae orchid tribe: unique C and N isotope natural abundances compared to autotrophic reference plants, and existence of achlorophyllous, albino individuals in otherwise green species. Gebauer and Meyer (2003) discovered unexpected isotope abundances in some forest orchids, with 13C and 15N abundances intermediate between those of autotrophic reference plants and full mycoheterotrophs from the same site. This was confirmed for additional European, North American and Asian species by several studies (Fig. 8.3a; Bidartondo et al. 2004; Julou et al. 2005; Abadie et al. 2006; Tedersoo et al. 2007; Zimmer et al. 2007; Liebel et al. 2010; Motomura et al. 2010). Stable isotope analyses from putative PMH plants have also been used to calculate these plants degree of heterotrophy (Table 8.2, Box 8.4).
Independent observations that some fully achlorophyllous individuals (= albinos), with colors ranging from white to pinkish due to anthocyanins (Fig. 8.4a, b), indicated partial mycoheterotrophy in some orchids. The surviving of such individuals in species that are normally green, especially from the Neottieae tribe, hinted at partial heterotrophy in these species. Albinos occur especially frequently in the genera Epipactis (Beau 1920; Renner 1938; Salmia 1986, 1989a, b; Selosse et al. 2004) and Cephalanthera (Renner 1938; Julou et al. 2005; Abadie et al. 2006; Stöckel et al. 2011). In some populations, the phenotype remains stable for green individuals and nearby albinos over many years (Renner 1938; Tranchida-Lombardo et al. 2010), up to 14 years for albinos (Abadie et al. 2006), while in others, variegated individuals or reversion to green shoots can occur (Salmia 1986; Stöckel et al. 2011; Fig. 8.4a, b). Although they tend to perform less well than green individuals (as stated above, see Sect. 8.4.1), some albinos form flowers and fruits (Salmia 1986, 1989a, b; Julou et al. 2005; Tranchida-Lombardo et al. 2010). Albinos were suggested to depend on their mycorrhizal fungi for C nutrition (Selosse et al. 2004): Beau (1920), having observed albino Epipactis and Cephalanthera spp. nearly one century ago, wrote that “the exact complementation of the photosynthetic function by the symbiosis permits us to understand how green orchids can exceptionally grow and flower in more or less etiolating conditions” (see also Renner 1938). Indeed, most of these species tend to inhabit shaded forest sites. Albinos’ mycoheterotrophy is now further corroborated by the demonstration of their low chlorophyll content and lack of CO2 absorption in the light (Fig. 8.4b; Julou et al. 2005); congruently, they display 13C enrichment similar to that of fully MH plants (Fig. 8.1; Julou et al. 2005; Abadie et al. 2006). This supported the likelihood of partial mycoheterotrophy in green conspecifics (Selosse et al. 2004; Julou et al. 2005). Accordingly, survival of albinos is also reported from green parasitic plants such as Striga hermonthica (Press et al. 1991) that use other plants’ sap to support part of their C needs (see Sect. 8.4.1 and Table 8.1).
(a) Albino Cephalanthera rubra (courtesy of J.-P. Amardeihl); see also Fig. 8.2b for albino C. damasonium. (b) Variegated individual of Epipactis helleborine (photo M.-A. Selosse). (c) Correlation between relative enrichments in 13C (ε, see Box 8.3) and mean light availability for two PMH and one fully mycoheterotrophic (MH) orchid species and for autotrophic reference plants. Regression lines (±95% confidence intervals) represent the range of isotope signatures of autotrophic plants including the initially mycoheterotrophic (IMH) orchid Cypripedium calceolus (green), of the two PMH orchids Cephalanthera damasonium (light red) and C. rubra (dark red) and of the MH orchid Neottia nidus-avis (blue). Arrows indicate the variable proportions of C derived from fungi (Cdf) or photosynthesis (Cdp), respectively (reproduced with permission from Preiss et al. 2010)
Moreover, some green orchids were found to have high 13C abundance, which correlates with the potential for mycoheterotrophy via their EM fungal partners (Bidartondo et al. 2004; see also Dearnaley et al. 2012, for review). These orchid species also have a trend to low or no colonization by rhizoctonias. However, many orchids from more or less open environments associate with rhizoctonias mainly but occasionally display EM fungi, e.g. Cypripedium (Shefferson et al. 2005, 2007), Gymnadenia (Stark et al. 2009), or Orchis (Liebel et al. 2010; Lievens et al. 2010; Girlanda et al. 2011). These associates were likely hidden in studies based on fungal cultivation, because EM fungi do not grow easily in culture, and may even be discarded as “molecular scraps” in some molecular studies (Selosse et al. 2010), but, as suggested by Girlanda et al. (2011), they could allow a C flow to the plant, at least in some light environments.
PMH Neottieae display various levels of specificity to non-rhizoctonia fungi that are known to form EM associations with forest trees: most Epipactis species show a preference for Pezizomycetes related to truffles, sometimes with additional fungi (Bidartondo et al. 2004; Selosse et al. 2004; Ouanphanivanh et al. 2008; Ogura-Tsujita and Yukawa 2008; Shefferson et al. 2008; Liebel et al. 2010); Cephalanthera species display a large fungal spectrum including Cortinariaceae, Hymenogastraceae and Thelephoraceae (Bidartondo et al. 2004; Julou et al. 2005; Abadie et al. 2006; Matsuda et al. 2008; Yamato and Iwase 2008) whereas Russula are specific associates of Limodorum species (Girlanda et al. 2006; Liebel et al. 2010). This is much reminiscent of the EM fungal associates in many fully MH orchids (see Sect. 8.3.1). A similar feature occurs in Japanese Cymbidium species (Motomura et al. 2010; Ogura-Tsujita et al. 2012). The MH C. macrorhizon and C. aberrans exclusively associate with EM taxa (Russulaceae, Thelephoraceae, and Sebacinaceae), the green C. lancifolium and C. goeringii, which have 13C abundances indicative of partial mycoheterotrophy (Fig. 8.3a), associate simultaneously with EM taxa and Tulasnellaceae. However, there is evidence that Ceratobasidiaceae species alone can support the ex situ growth of C. goeringii, although the heterotrophy level of these individuals and ecology of these fungi remain unknown Wu et al. 2010). The pattern is further complicated by the fact that some orchids associated with potentially EM taxa have not demonstrated partial mycoheterotrophy (Waterman et al. 2011), and that a few rhizoctonia lineages within the Sebacinales (Selosse et al. 2004), the Tulasnellaceae (Bidartondo et al. 2003) or the Ceratobasidiaceae (Bougoure et al. 2009) have EM abilities. Indeed, EM Ceratobasidium associate with the PMH Plantanthera minor and autotrophic trees such as Pinus densiflora (Yagame et al. 2008b, 2012). Surrounding trees are thus likely to be the ultimate C source of all known PMH orchids, and the availability of EM fungi could be a limitation for the geographic distribution of partial mycoheterotrophy (Liebel et al. 2010). The reason why non-EM rhizoctonia, which support MH seedling development in green orchids, only support minor MH gains in adult orchids (Liebel et al. 2010; Girlanda et al. 2011) remains unclear: one interpretation is that they may not be capable of transferring a sufficient amount of C to support adult orchid individualss (Taylor and Bruns 1997; Martos et al. 2009), but there is no direct evidence for this.
Experiments carried out by Sadovsky (1965), at a time European protection laws did not forbid destructive manipulation of native orchids, and in repeated attempts to transplant various orchids, he listed some species that did not survive the process. The list interestingly mixes full mycoheterotrophs (such as Neottia nidus-avis) and PMH species (Corallorhiza trifida, Cephalanthera, Limodorum, and Epipactis spp.), suggesting that in these cases, disconnecting the fungus from its resources (=nearby mycorrhizal tree roots) entailed plant death.
Within the Epidendroideae (Fig. 8.3a), partial mycoheterotrophy is not limited to the Neottieae. It also occurs in the genus Cymbidium (tribe Cymbidieae) as stated above, and in the interesting case of Corallorhiza trifida (tribe Calypsoeae). Despite being leafless and having only chlorophyllous stems and seed capsules Corallorhiza trifida, in contrast to all other species of its genus (Freudenstein and Doyle 1994), is not fully MH upon reaching adulthood (Zimmer et al. 2008; see also Sect. 8.5.1). PMH species with apparently low heterotrophy levels were found among Mediterranean and Macaronesian orchids from the tribe Orchideae (such as Barlia robertiana, Gennaria diphylla, Habenaria tridactylites, and Orchis purpurea; Fig. 8.3b; Liebel et al. 2010; Girlanda et al. 2011). Interestingly, G. diphylla is associated with EM fungi (Liebel et al. 2010), and it is possible that some Tulasnellaceae and Ceratobasidiaceae found in association with other orchid taxa (Liebel et al. 2010; Girlanda et al. 2011) are EM as well.
The situation is more complicated in Orchidoideae (Fig. 8.3b), where some green species show 13C depletion compared to surrounding autotrophs (Hynson et al. 2009a). This is especially pronounced for Goodyera repens (Fig. 8.3b), which is demonstrated to be autotrophic and to transfer C to its fungal associate (Cameron et al. 2008). Finding a relevant autotrophic baseline in terms of 13C abundance is thus difficult for Orchidoideae phylogenetically related to Goodyera spp.: using the surrounding green plants that are enriched in 13C relative to autotrophic Goodyera spp. may underestimate the heterotrophy level of adult PMH plants in some Orchidoideae. Nevertheless, even using such a baseline, partial mycoheterotrophy was successfully demonstrated in the Japanese Platanthera minor (Yagame et al. 2012), and in Cheirostylis montana from Thailand (Roy et al. 2009a; Fig. 8.3b), suggesting the need for more investigations to find PMH Orchidoideae within North America and Europe. Thus, many, if not all, orchid taxa appear predisposed to partial mycoheterotrophy (as well as to full mycoheterotrophy), perhaps because of their required MH germination.
We currently have no data on the impact of adult PMH plants on their associated fungi (exact load of the C loss, existence of any reward such as nutrients and/or protection). If one assumes, as suggested by C flow, that the plant is parasitic on the fungus, then some conflicts may occur and fungi may undergo selection to avoid this parasitism. In initial mycoheterotrophy-autotrophy, the interaction maybe positively selected on the fungal side, since investing C in a seedling may later allow it to recover C from the adult roots (see below) and thus compensate the C flow to germinating seedlings. Thus, one may speculate that initial mycoheterotrophy-autotrophy systems are more evolutionarily stable, which is corroborated by this strategy being more ancient in orchids than initial mycoheterotrophy-partial mycoheterotrophy. However, one might also speculate that initial mycoheterotrophy-autotrophy is more evolutionarily stable because the plant—by being MH only at the seedling stage—limits the duration and magnitude of the parasitism, thereby reducing the selective pressure on host fungi to evolve resistance.
8.7.3.2 Partial Mycoheterotrophy in Adult Pyroloids
Until recently, orchids have been the primary models for investigations of partial mycoheterotrophy, but this nutritional mode is more widespread, and some Ericaceae were essential in demonstrating that this strategy had evolved convergently in other plant lineages. Pyroloids, for instance, are small shade-tolerant perennial shrubs, often with impressive underground rhizome networks, and grow in temperate, alpine and boreal forests. They are candidates for partial mycoheterotrophy for three reasons: (1) they have a close, although not fully resolved, phylogenetic relationship with the MH Monotropeae and Pterosporeae (all members of the Monotropoideae are MH; Kron et al. 2002; Tedersoo et al. 2007; Matsuda et al. 2012). As stated in Sect. 8.3.1, one species of Pyroleae, Pyrola aphylla, is even fully MH (Fig. 8.1; Zimmer et al. 2007; Hynson et al. 2009b), although its recognition as a separate species, or simply a leafless variant of the green P. picta, remains unclear (Camp 1940; Haber 1987; Freudenstein 1999). (2) As in orchids, seeds are very small, with few C reserves (Eriksson and Kainulainen 2011), and pyroloids undergo an MH subterranean germination (Christoph 1921; Lihnell 1942) where various fungi have been shown to provide C nutrition (Smith and Read 2008). (3) As in many other basal Ericaceae (Selosse et al. 2007), pyroloids form EM-like arbutoid associations: the fungus often forms a sheath around the root and a Hartig net, but in addition, hyphal coils penetrate root cells (Khan 1972; Robertson and Robertson 1985; Vincenot et al. 2008; Hynson and Bruns 2009). The fungal taxa involved are also capable of forming typical EM associations with surrounding tree roots (Robertson and Robertson 1985; Tedersoo et al. 2007; Zimmer et al. 2007; Vincenot et al. 2008; Hynson and Bruns 2009; Toftegaard et al. 2010; Matsuda et al. 2012), thus offering a link to autotrophic trees.
Investigations on stable isotope abundances of leafy Pyroleae species have provided variable results. An investigation in two boreal Estonian forests revealed that some pyroloids had higher 13C and 15N abundances and leaf N concentrations than surrounding autotrophs, including other plants from Ericaceae (Fig. 8.5; Tedersoo et al. 2007). Based on a linear mixing model using Hypopitys monotropa as the fully MH end-member (Box 8.4), 10–68% of C was of fungal origin in Orthilia secunda, Pyrola chlorantha, P. rotundifolia, and Chimaphila umbellata. However, the latter species did not differ from autotrophs in one of the investigated sites, and the levels of conspecific heterotrophy did not correlate among sites, indicating a complex regulation of this parameter among species. In a second set of investigations in more southern sites from Germany and California, (Fig. 8.5; Zimmer et al. 2007; Hynson et al. 2009b), 15N abundance was intermediate between autotrophic and MH plants for O. secunda, C. umbellata, P. chlorantha, P. minor and P. picta; however, based on 13C abundance, only O. secunda showed significant C gain via mycoheterotrophy at a single low irradiance site. Given the broad range of irradiances in investigated sites, light availability is unlikely to be the only driver of the heterotrophy level in the study by Zimmer et al. (2007). Conversely, Matsuda et al. (2012) demonstrated that the Japanese P. japonica derived 50% of it’s C from fungi, and that individuals growing in most shaded forest microsites and in darker months (due to canopy closure) tended to display higher 15N and 13C abundances, and higher leaf N concentrations. This was linked to a more specific association to Russula spp. in these conditions, a feature speculated to allow a better supply of fungal C. In a recent study by Hynson et al. (2012), the first truly experimental study on PMH in pyroloids, light and access to mycorrhizal networks, was manipulated in the field for two species of Pyroleae, P. picta and C. umbellata. The C stable isotope values from these species’ leaf soluble sugars were then used to track changes in their relative dependency on MH C gains over the course of a growing season. The major findings of this study were that (1) P. picta and C. umbellata respond differently to a decrease in light availability: due to an increase in 13C abundance over the course of the experiment P. picta showed signs of partial mycoheterotrophy, whereas C. umbellata did not. (2) Assaying the leaf soluble sugars from the two target species rather than bulk tissue revealed subtle changes along the autotrophy-mycoheterotrophy continuum in these species that would have otherwise gone undetected. And (3), the relative dependency of P. picta compared to C. umbellata on MH C gains does not appear to be solely driven by light availability.
Mean enrichment factors (ε, see Box 8.3) for 13C and 15N of six green species belonging to the tribe Pyroleae within the subfamily Monotropoideae of the Ericaceae (black symbols) and five fully mycoheterotrophic (MH) species belonging to three different tribes within the subfamily Monotropoideae of the Ericaceae (grey symbols, for further details see Fig. 8.1) and of autotrophic reference plants (Ref, n = 392) collected together with each of the respective target species. The boxes represent one SD of the mean ε values for the different groups of green and fully MH Monotropoideae and for the autotrophic reference plants. Number of replicates for the six target species as following: Chimaphila umbellata (n = 36), Orthilia secunda (n = 38), Pyrola chlorantha (n = 17), P. minor (n = 9), P. picta (n = 54), P. rotundifolia (n = 6). Data compiled from Tedersoo et al. (2007), Zimmer et al. (2007), Hynson et al. (2009b) and Liebel et al. (2009)
In the cases where light levels do not drive the heterotrophy level, it was suggested that the C gains were not solely based on C needs, but could arise as a “side-product” of the N and P nutrition (Selosse and Roy 2009; see stage #3 in Table 8.1). Some fungal C could move along with organic forms of N, which is the case in mixotrophic algae where C gains arise as a consequence of mineral nutrients from prey (see Sect. 8.4.1). Indeed, a specific strategy to obtain fungal organic N in pyroloids may also explain their unusual 15N abundance (Fig. 8.5), as well as their elimination after anthropogenic N deposition (Allen et al. 2007). A study of two species (O. secunda and P. asarifolia) in Canada confirmed high 15N abundance and leaf N concentrations (Kranabetter and MacKenzie 2010), but the responses of these parameters to an edaphic gradient of N availability strongly suggested difference in N metabolism, not only as compared to other plants, but also between these two species. Much remains to be studied on N and C acquisition and metabolism in pyroloids. Interestingly, no evidence for lysis or degradation of intracellular hyphae is reported in living root cells of pyroloids (Tedersoo et al. 2007; Vincenot et al. 2008), adding to the idea, against previous postulates, that hyphal lysis may not be necessary for C transfer.
Furthermore, a 14C labeling experiment was claimed to show transfer from autotrophs to pyroloids, in dual pot cultures of Larix kaempferi seedlings and Pyrola incarnata from Japan (Kunishi et al. 2004; Hashimoto et al. 2005), but this experiment and its controls remain unpublished. Carbon gains from fungi may explain other ecophysiological features of pyroloids, such as the few C reserves in winter (the fungal C may contribute to development of new organs in spring for P. incarnata; Isogai et al. 2003), the low capacity for adjusting vegetative growth and leaf area after shading (in P. rotundifolia at least; Hunt and Hope-Simpson 1990), or the sensitivity to forest disturbances that affect mycorrhizal networks (logging, burning; Zimmer et al. 2007). However, at least some green pyroloids survive, at least temporarily, outplanting to glasshouse conditions (e.g., Hunt and Hope-Simpson 1990). In contrast with PMH orchids, the use of fungal C in pyroloids may be more facultative, allowing disconnections from mycorrhizal network in some conditions; If one assumes that populations display variable level of dependency on mycorrhizal networks, this may also resolve the discrepancies between studies reporting variable levels of heterotrophy (Tedersoo et al. 2007; Zimmer et al. 2007; Hynson et al. 2009b).
8.7.3.3 Partial Mycoheterotrophy in Plants Associated with Arbuscular Mycorrhizal Fungi
The two lineages investigated above, orchids and Pyroleae, present two unusual features among MH lineages: (1) they associate with Asco- or Basidiomycota (EM or SAP) and (2) are primarily from temperate ecosystems. Yet, the majority of fully MH species are from tropical regions, and associate with the obligatorily biotrophic AM fungi (Leake 1994, 2004). Is there some evidence of partial mycoheterotrophy in these or other families mycorrhizal with Glomeromycota? Indeed, competition for light is high in wet tropical forests, and may select for partial mycoheterotrophy. In these conditions, some families contain both green and MH species, such as Burmanniaceae (Merckx et al. 2006, 2010), Gentianaceae (Struwe et al. 2002; Cameron and Bolin 2010), Polygalaceae (Imhof 2008), Iridaceae (Reeves et al. 2001), Pandanales (Rudall and Bateman 2006), and Petrosaviaceae (Cameron et al. 2003). Also, some green species have reduced leaves that suggest that photosynthesis is not their sole C source (Cameron and Bolin 2010).
There are unique challenges to assessing partial mycoheterotrophy in AM plants. For instance, it is difficult to sample the tiny, submillimetric spores of individual AM fungi (that do not form conspicuous fruitbodies) for stable isotope analyses, although this has sometimes been done (Allen and Allen 1990; Nakano et al. 1999; Courty et al. 2011). Also, 15N abundances and N concentrations are similar in AM-MH plants and nearby green plants (Merckx et al. 2010; Courty et al. 2011; see Sect. 8.3.3): this suggests similar N nutrition and forbids any specific detection of mixotrophy on these parameters only—at least in Burmanniaceae and Gentianaceae. Finally, Courty et al. (2011) found few or no difference between canopy leaves, Glomeromycota spores, and AM-MH plants, so that specific 13C enrichment is not generally expected for AM-partial mycoheterotrophy. Only in dense forests, light-starved understory plants may display some differences with AM-partial mycoheterotrophy (see Sect. 8.3.3). In more open conditions (field or canopy-open forest), similar 13C enrichment in potential AM-PMH and surrounding autotrophic plants can be expected. Moreover, in some non-forest ecosystems, the occurrence of C4 plants as partners of AM fungi may further complicate the pattern, as these have lower 13C depletion than the C3 plants.
Evidence for partial mycoheterotrophy among AM-MH plants, although expected (e.g., Selosse and Roy 2009), thus remains unclear. Investigating two Gentianaceae species with reduced leaves from a Virginia hardwood forest, Cameron and Bolin (2010) found no 13C enrichment, but higher leaf N concentrations in Bartonia virginica and higher 15N abundance in Obolaria virginica as compared to nearby autotrophs. Given the statements above, it is difficult to conclude whether this reflects some heterotrophy or some physiological particularity of N nutrition. Investigating the potential candidate for AM-partial mycoheterotrophy Burmannia capitata from French Guiana forest gaps, Merckx et al. (2010) showed no enrichment in 13C as compared to nearby herbaceous plants. Moreover, this species and three other from the same genus were successfully grown from seeds in isolated pots under controlled conditions (i.e., no mycorrhizal network). Thus, partial mycoheterotrophy is lacking, or at least facultative in these cases.
Green relatives of fully AM-MH plants could provide promising targets for future mechanistic studies on mycoheterotrophy. If partial mycoheterotrophy is found among AM plants, these plants target the most widespread mycorrhizal symbiosis, and could prove ideal for further studies of the ecophysiology of MH plants. Field and laboratory studies of the tripartite symbiosis between autotrophic host plant, mycorrhizal fungus and mycoheterotroph that have been difficult with EM mycoheterotrophs (but see Yagame et al. 2012) may receive new attention among AM mycoheterotrophs. Although many AM fungi cannot be grown axenically in culture, the putative autotrophic C source for AM mycoheterotrophs are relatively smaller than the trees that host EM fungi, and are possibly annuals which can be grown in combination with their AM mutualists. Therefore, these systems lend themselves to both in situ and laboratory experiments that follow the fate of C as it is passed from the autotrophic host, onto its associated fungus and finally the mycoheterotroph. Owing to these factors and, the plasticity of specificity among AM mycoheterotrophs, the physiological pathways and biochemical signaling between mycoheterotrophs and fungi may finally gain traction in the field of MH research.
8.9 Challenges in the Study of Partial Mycoheterotrophy
8.9.1 Gas Exchange Measurements and Photosynthesis in Partially Mycoheterotrophic Plants
Investigations of photosynthesis and in situ gas exchange support partial heterotrophy for some green orchids. CO2 exchanges in C. damasonium revealed that, as expected from their phenotype, albinos were fully heterotrophic and did not respond to light, while green individuals exhibited a normal photosynthetic response to light (Fig. 8.4b; Julou et al. 2005). After in situ 13C labeling, C. damasonium showed reduced CO2 assimilation compared to surrounding green plants, while C. trifida showed nearly no assimilation, close to the level of the fully MH control (Cameron et al. 2009). This was in agreement with chlorophyll content and fluorescence values (reflecting proper assembly of pigments into photosystems): these parameters suggested subnormal and very reduced potential for photosynthesis in C. damasonium and C. trifida respectively (Fig. 8.6; Cameron et al. 2009). In C. trifida, the chlorophyll content is only 1% of that of C. damasonium (Zimmer et al. 2008). However, Montfort and Küsters (1940), measuring CO2 exchange in inflorescences and maturing infrutescences, found that CO2 assimilation was 2.2 times higher than respiration—whether differences in technology or plant tissue origin explains this remains unclear. Intrinsic photosynthetic limitations also exist in Limodorum abortivum, where photosynthetic abilities do not compensate respiration even in full light (Girlanda et al. 2006). In some C. damasonium populations, there is evidence that low light conditions force the plant to survive near its compensation point, where C losses through respiration are equal to C gains from photosynthesis (Julou et al. 2005). Based on variegated albinos, C. damasonium individuals with a mosaic of chlorophyllous and achlorophyllous tissues, Stöckel et al. (2011) found a positive correlation between leaf chlorophyll concentrations and degree of mycoheterotrophy, as shown by 13C abundance. However, the recent finding that some meadow Mediterranean orchids, living in places devoid of canopy, are PMH (Girlanda et al. 2011) demonstrates that light limitation is not the sole driver of this nutritional strategy. Thus, both intrinsic and environmental factors determine partial mycoheterotrophy, depending on the species and site. Partial mycoheterotrophy may also allow buffering against herbivory or shading damage: in a defoliation and shading experiment involving the autotrophic Cypripedium calceolus and the PMH C. longifolia, Shefferson et al. (2006) demonstrated that both treatments led to significant declines in vegetative and reproductive vigor of C. calceolus, but had more limited impacts in C. longifolia.
A comparison of photosynthetic abilities between Neottia nidus-avis (mycoheterotroph) and Corallorhiza trifida (leafless partial mycoheterotroph) and the leaves of Cephalanthera damasonium (partial mycoheterotroph) and Fagus sylvatica (autotroph), reproduced with permission from Cameron et al. (2009). (a) Total chlorophyll content as a function of surface area of the organ (stems for Corallorhiza trifida, which is leafless, and leaves in all other cases). (b) Maximum quantum yield (Fv/Fm). (c) Total amount of 13C present in plant shoots after a 4-h exposure to a 13CO2 atmosphere; mean photosynthetically active radiation (PAR) is given above each bar (values on the right). In each panel, bars with differing letters are significantly different
Measurements of photosynthetic activity and stable isotope natural abundance are both powerful tools for investigating partial mycoheterotrophy. However, the information gained from these techniques differs. Gas exchange measurements provide snapshot information about photosynthetic activity, while stable isotope natural abundance data integrate the sources of C gain over the entire life history of a plant or plant organ (Liebel et al. 2010). This difference may explain the diverging results for C. trifida, where isotopic abundances suggest partial mycoheterotrophy (Zimmer et al. 2008) whereas CO2 fixation was detected (Montfort and Küsters 1940) or not (Cameron et al. 2009). However, one advantage of stable isotope analyses is that they can be used in a quantitative manner way to compare levels of heterotrophy: the percent of C derived from the fungi can be estimated from a linear mixing model (Box 8.4, Table 8.2, but see 8.5.2 for considerations when applying linear mixing models to the study of PMH). Not unexpectedly, this reveals a continuum from autotrophy to full mycoheterotrophy (as also suggested in Fig. 8.3). Moreover, a given species shows a variable heterotrophy level from one site to another: for the well-studied C. damasonium, this level ranges for green individuals from 33% in an open, sunny pine forest to 85% in a dark beech forest (Gebauer and Meyer 2003; Bidartondo et al. 2004; Gebauer 2005).
8.9.2 Considerations for the Application of Linear Isotope Mixing Models to Mycoheterotrophic Food Webs
With the revelation of partial mycoheterotrophy, two primary challenges for researchers have been (1) determining the degree of dependence on fungal C gains in these plants and (2) elucidating the factors that select for partial mycoheterotrophy in nature. Attempts to quantify the percent fungal C and N gains in PMH plants have been made through the application of an isotope mixing model (Box 8.4; Gebauer and Meyer 2003; Bidartondo et al. 2004; Tedersoo et al. 2007; Zimmer et al. 2007). The two end-members of this linear mixing model are the 13C and 15N isotope abundances of fully MH plants that theoretically receive all of their C and mineral nutrients from fungi, and the 13C and 15N isotope abundances of autotrophic plants, which are theoretically not receiving any C from their associated mycorrhizal fungi, but meeting basically all of their N demands via their mycorrhizal fungi (Gebauer and Meyer 2003). However, while this model generates estimates of the potential dependency on fungal derived C, it also involves numerous assumptions about the isotopic behavior of MH plants, many of which are not yet fully understood. For instance, it is unknown if the relationship between the 13C isotope signatures of partial mycoheterotrophs and autotrophs across environmental gradients is linear. Most PMH orchids and some pyroloids have been found living in low-light environments (<20 μmol photons m−2 s−1; Gebauer and Meyer 2003; Bidartondo et al. 2004; Julou et al. 2005; Abadie et al. 2006; Zimmer et al. 2007; Liebel et al. 2010). Under low light conditions, fully autotrophic plants in the understory would theoretically become more depleted in 13C. This depletion is owed to a combination of biochemical and leaf-level processes such as a decrease in photosynthetic rate, along with an increase in Ci/Ca (the ratio of the CO2 concentration inside the leaf to the atmosphere outside the leaf) which would lead to greater discrimination against 13CO2 by the primary caboxylating enzyme for C3 photosynthesis, Rubisco (Farquhar et al. 1989). In contrast, PMH often have significant increases in δ13C in low light conditions (Zimmer et al. 2007; Liebel et al. 2010; Preiss et al. 2010; Hynson et al. 2012; Fig. 8.4b). Thus the assumption that the δ13C of target species and the autotrophic end-members of the mixing model are linearly related may be invalid, especially if they are from different light environments or have differing rates of photosynthesis (Fig. 8.2b). In fact, PMH species are likely have lower photosynthesis rates than autotrophic plants, resulting in better equilibration of 13CO2 concentration between environmental air and stomatal chamber, therefore entailing increased 13C discrimination (= lower 13C abundance). They may also use respired CO2 in relatively larger amounts as compared to plants with higher CO2 need; and this respiratory CO2 is more depleted in 13C than atmospheric CO2. Thus the photosynthetic contributions to PMH’s δ13C may be more depleted in 13C than surrounding autotrophic plants. The linear relationship between leaf chlorophyll concentration (= photosynthetic capacity) and δ13C observed in the variegated leaves of Cephalanthera damasonium (Stöckel et al. 2011) suggests that these mechanisms may have limited effects. However, it should be kept in mind that CO2 equilibrium effects and respiratory effects may increase 13C depletion in leaves, thus potentially reducing the apparent heterotrophy level of some PMH plants.
Furthermore, the factors that lead to interspecific differences in the δ13C and δ15N of fully MH plants are unclear (Fig. 8.1; Kranabetter and MacKenzie 2010). Therefore, the species of fully MH plants used as end-members in the mixing model can greatly affect the estimates of percent C or N gains via mycoheterotrophy. For example, in the case of the MH Pyrola aphylla (Ericaceae), if the percent C and N gains via fungi are calculated (Hynson et al. 2009b) using the mixing model approach with an MH end-member based on the mean relative 13C enrichment of seven fully MH plants associated with EM fungi (Table 8.2, Preiss and Gebauer 2008) the estimated percent C derived from fungi would be 96 ± 12%. This indicates that P. aphylla essentially gains all of its C from a source that is similar to other fully MH plants associated with EM fungi. In contrast, if the same mixing model is used to calculate percent N gain via mycoheterotrophy, P. aphylla would gain over 100% (149 ± 18%) of its N from the source(s) utilized by the MH end-members. Thus, the MH species used as end-members in this scenario do not fully represent the extent of variability in 15N signatures of mycoheterotrophs. However, from the meta-analysis of MH plant enrichment factors (Fig. 8.1), we have found significant differences in C and N stable isotope enrichment between EM-MH Orchidaceae and Ericaceae. In order to improve the resolution of the mixing model approach, we recommend that for future quantifications of mycoheterotrophy among orchidaceous and ericaceous species the MH end-member of the mixing model should be chosen based on the test species’ respective plant family.
Other potential issues with using a mixing model approach to quantify the degree of mycoheterotrophy are the types of compounds that are being analyzed for their stable isotope abundances. To date, all mixing model calculations of partial mycoheterotrophy except a single study by Hynson et al. (2012), have been based on structural (bulk) leaf or stem tissue isotope signatures. Because these values represent a time-integrated measurement of C and other nutrient gains over the lifespan of a leaf, they may mask short-term fluctuations of mycoheterotrophy with organ or plant developmental stage, or with season. This factor may be particularly important for long-lived evergreen species such as Pyroleae species or orchids that have long belowground dormancy periods. For example, in the latter case, when the dormancy period ends and aboveground shoots are formed, the C for building these tissues will be derived from a heterotrophic source—either fungal or the mobilization of stored C such as starch. Both these scenarios would lead to 13C enrichment in newly formed orchid leaves, potentially leading to erroneous conclusions regarding the MH status of the plants at time of observation (although the conclusion is exact regarding the C origin). The only study to date to have targeted compounds other than bulk C and N is by Hynson et al. (2012) where the leaf soluble sugars of two Pyroleae species were used to track changes in relative levels of mycoheterotrophy throughout a single growing season. Though the identities of the transfer compounds from fungi to MH plants remain unknown, sugars are reasonable candidates. Sugars are the major transport materials in biological systems, including the mycorrhizal symbiosis, due to their high energy and chemical stability (Smith and Read 2008). The results of the work by Hynson et al. (2012) revealed that indeed sugars were a sensitive assay for shifts of C assimilation over short time periods, whereas bulk C gave an average value for the global origin of the C in the investigated plants’ biomass.
8.9.3 Cryptic Mycoheterotrophy
One additional gap remaining in the current literature are labeling experiments directly demonstrating the C transfer from plant to (1) fungus and (2) PMH orchid: thus, the fact that nearby trees are the ultimate C source remains to be formally demonstrated. There are two indirect additional sources of evidence for the use of mycorrhizal fungi as trophic source: 15N abundance and N concentrations in plant tissues (N content, best expressed as mmol N gdw−1, also expressed as C/N ratios in some papers) are high in PMH orchids as compared to surrounding autotrophs (Gebauer and Meyer 2003; Abadie et al. 2006; Tedersoo et al. 2007; Selosse and Roy 2009; Liebel et al. 2010). However, these values are lower than in fully MH plants, supporting a mixotrophic strategy (see also Sect. 8.6.1). Stöckel et al. (2011) demonstrated a linear relationship between MH C gain and leaf N concentration in Cephalanthera damasonium populations with albinos displaying various level of variegation with green tissues. The same studies also demonstrate that some orchids considered as autotrophic based on their 13C abundance exhibit higher (although less extreme) 15N abundance (Fig. 8.3) and N concentrations than other autotrophs, again raising the possibility of a cryptic partial mycoheterotrophy.
8.10 Future Directions and Unanswered Questions
8.10.1 Nitrogen
Soil N availability is for many plants in natural terrestrial habitats a growth-limiting factor (e.g., Marschner 1995). Therefore, plants repeatedly developed strategies to gain access to N sources alternative to soil-born mineral N. For example, legumes through their symbiosis with N2-fixing bacteria use atmospheric N and carnivorous plants use their preys as alternative N sources. The N availability to plants is mirrored by the N concentration in their tissues (Gebauer et al. 1988). Legumes have N concentrations in their tissues that are up to twice as high as in non-legumes living in the same environment. A major part of N, specifically in photosynthetic plant tissues, is invested in the enzyme Rubisco (Feller et al. 2008), which is directly involved in photosynthetic C fixation. Thus, the capacity for leaf photosynthesis and plant productivity increases with increasing leaf total N concentration (Schulze et al. 2005).
As already mentioned above (see Sects. 8.3.3 and 8.5.3) many MH and PMH plants, specifically those associated with EM fungi, but also orchids associated with rhizoctonias, have surprisingly high total N concentrations in their tissues (Gebauer and Meyer 2003; Abadie et al. 2006). Liebel et al. (2010) investigated orchids and autotrophic reference plant species in the Mediterranean and Macaronesian regions and found that representatives of the tribe Neottieae (subfamily Epidendroideae) mostly associated with EM fungi were PMH or even MH had total N concentrations of 2.9 ± 0.6(SD) mmol N gdw−1 (n = 42). Conversely, species belonging to the subfamily Orchidoideae that were mostly associated with rhizoctonias did not show signs of mycoheterotrophy based on their bulk stable isotope signatures, but had tissue total N concentrations of 1.7 ± 0.4(SD) mmol N gdw−1 (n = 150). Both groups of orchids’ total N concentrations are significantly distinguished from each other and significantly above those found for non-orchid reference plants from the same environments: 1.4 ± 0.5(SD) mmol N gdw−1 (n = 513). The finding of increased tissue N concentrations in orchids associated with rhizoctonias supports the idea of a cryptic C gain from the fungus through organic N compounds (see Sect. 8.5.3). Moreover, the high total N concentrations in PMH and MH orchids raise the question as to the function of these high N concentrations in their tissues. Use of increased photosynthetic activity as in “normal” autotrophic plants seems to be unlikely, because PMH plants mostly live under conditions under which light availability limits their photosynthetic activity (Preiss et al. 2010) and fully MH plants have given up photosynthetic activity altogether. Furthermore, in variegated individuals of Cephalanthera damasonium, Stöckel et al. (2011) documented a linear increase in leaf total N concentration with decreasing chlorophyll concentration and thus, decreasing photosynthetic capacity. At the moment we only can speculate about the reasons why MH and PMH plants accumulate unusually high N concentrations in their tissues. One possible reason may be related to the fact that fungal tissues and specifically those of EM fungi also have total N concentrations far above those known for the majority of autotrophic plants (Gebauer and Dietrich 1993; Gebauer and Taylor 1999) and MH plants may have an N metabolism more similar to their fungal hosts than other plants. Future studies that focus specifically on the N assimilation strategies among MH plants are greatly needed. These studies may in turn elucidate modes of C acquisition among MH plants because the two nutrients are inherently linked in their organic forms.
8.10.2 Liverworts, Lycopods, and Ferns
Gametophytes of most species in the liverwort family Aneuraceae are photosynthetic, readily grown in asymbiotic culture, and lack a morphologically unambiguous MH stage. However, in the field, most species maintain specialized relationships with tulasnelloid fungi, exhibiting patterns of fungal peloton formation and digestion that appear cytologically identical to those in the confamilial MH liverwort Aneura mirabilis and in IMH orchid seedlings (Ligrone et al. 1993). As in other liverworts, the sporophytes of the Aneuraceae are minute, ephemeral, dependent on the gametophytes, and uncolonized by mycorrhizal fungi. While it appears likely that gametophytes of many species in the Aneuraceae exhibit facultative partial mycoheterotrophy, their trophic status has yet to be investigated via isotope analyses.
Most members of the Lycopodiaceae, all members of the Ophioglossaceae and Psilotaceae, some members of the Schizaeaceae, and one member of the Gleicheniaceae have subterranean, non-photosynthetic, and unambiguously MH gametophytes and preemergent sporophytes. Both gametophytes and sporophytes in the Lycopodiaceae (Winther and Friedman 2008), Ophioglossaeae (Winther and Friedman 2007), and Psilotaceae (Winther and Friedman 2009) have specialized associations with Glomeromycota. Adult sporophytes of all species are typically photosynthetic, although albino and/or non-emergent forms have been observed in Lycopodium clavatum (Bruce and Beitel 1979), Psilotum nudum (Whittier 1988), and Botrychium mormo (Johnson-Groh 1998; Johnson-Groh and Lee 2002). In B. paradoxum, sporophyte leaves, while green, are reduced to small, bladeless, sporangia-bearing stipes (Wagner and Wagner 1981). Adult sporophytes of Lycopodiaceae, Ophioglossaceae, and Psilotaceae are commonly cultivated in the absence of AM host plants, although some Botrychium spp. are noted to be uncultivable (Donald Farrar, pers. comm.). In contrast, adult sporophytes of the Gleicheniaceae are difficult to cultivate, and those of the Schizaeaceae are rarely, if ever, cultivated.
Basic life history data, such as the duration of initial mycoheterotrophy, are unknown for most fern and lycopod taxa. The identities of autotrophic plants supporting MH development of ferns and lycopods via AM fungal networks are also largely unknown. Adult sporophytes are apparently autotrophic or PMH, depending on the taxa, but this has yet to be investigated via isotope analyses. Whether surficial, green gametophytes possessed by some taxa in the Lycopodiaceae, Schizaeaceae, and Gleicheniaceae are autotrophic or PMH is also uncertain. While gametophytes belonging to some species in the Lycopodiaceae (Whittier and Renzaglia 2005) and Gleicheniaceae (Stokey 1950) have been cultured asymbiotically on sugar-free media (indicating they are at least facultatively autotrophic), relatively few such taxa have been studied and the trophic status of gametophytes under field conditions remains unknown. While this represents an interesting avenue for further research, collecting isotope data from minute—and in some cases, filamentous—gametophytes may prove difficult.
8.10.3 Fungi
Because the study of PMH and MH plants reveals constraints that we currently are unable to explain, future studies should also focus on the physiology of soil mycelia in regards to carbon and nutrient cycling. For example, apparently only in wet climates can SAP fungi support fully MH orchids. Although, as mentioned above (see Sect. 8.3.2), this may be linked to longer periods of C assimilation (growing seasons) and higher efficiency of fungal exoenzymes, which allow the access to larger C amounts, we must acknowledge these ideas are speculative, and face the reality that current knowledge of fungal ecophysiology may limit our understanding of these processes. Similarly, although SAP, parasitic and endophytic rhizoctonias support MH seedling development in most orchid species and some pyroloids, they are never used by fully MH orchids. Here again, we currently can speculate that the maximal C provided by rhizoctonias is not sufficient (Taylor and Bruns 1997; Hashimoto et al. 2012), but we lack direct evidence for this. In the future, simple designs that are tractable in the lab, or data from the growing number of sequenced fungal genomes, that reveal metabolic abilities (Martin and Selosse 2008) may help understanding these observations through the knowledge gained on the physiology of fungal partners.
8.10.4 An Ecological View
Up to now, as exemplified in this chapter, our approach to the study of partial and full mycoherotrophy is centered on the side of plants receiving C, as well as on the description of the fungal associates. We now need to know more about the overlooked components of the association. First, what is the impact on the fungus? Although widespread opinion holds mycoheterotrophy as a parasitism on the fungus, and EM-MH/AM-MH nutrition as “epiparasitism,” on the autotrophic host plant the final evidence for this would be to show that the fungal or autotrophic host plant fitness, i.e., growth and/or sporulation, is impaired. Again here, tractable ex situ designs, which may be the most plausible on plants using SAP fungi, may help to resolve the costs to fungi associating with MH plants. It may be discovered that there is a variable level of impact of mycoheterotrophy on fungal fitness, or even some compensation by MH plants to their fungi: This compentation could possibly in the form of nutrients, growth factors (as early suggested by Björkman 1960) or shelter provided to the fungi in the roots of MH plants.
Furthermore, future studies should address the impact of PMH and MH plants on surrounding green plant(s) that provide C to these plants, in every case where a mycorrhizal fungus is providing C. From these ideas comes the question of the ecological relevance of MH nutrition. Parasitic plants are well known to impact ecosystem productivity (Westwood et al. 2010) and hemiparasitic plants, by influencing dominance of plants, have important impact on diversity of plant communities (Watson 2009). It is fascinating to observe how common some MH plants are in some tropical and temperate forests while completely absent from forests that appear similar in both their above and belowground biotic and abiotic conditions. Future studies, will hopefully reveal the conditions that select for the presence of MH plants in nature, and discern the impacts that they have on ecosystem dynamics.
References
Abadie J-C, Püttsepp Ü, Gebauer G, Faccio A, Bonfante P, Selosse M-A (2006) Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and non-photosynthetic individuals. Can J Bot 84:1462–1477
Adamec L (1997) Mineral nutrition of carnivorous plants: a review. Bot Rev 63:273–299
Allen EB, Allen MF (1990) The mediation of competition by mycorrhizae in successional and patchy environments. In: Grace JR, Tilman D (eds) Perspectives on plant competition. Academic, New York, pp 367–389
Allen EB, Temple PJ, Bytnerowicz A, Arbaugh MJ, Sirulnik AG, Rao LE (2007) Patterns of understory diversity in mixed coniferous forests of southern California impacted by air pollution. ScientificWorldJournal 7, http://www.thescientificworld.com
Baldrian P (2009) Ectomycorrhizal fungi and their enzymes in soils: is there enough evidence for their role as facultative soil saprotrophs? Oecologia 161:657–660
Bannister P, Strong GL (2001) Carbon and nitrogen isotope ratios, nitrogen content and heterotrophy in New Zealand mistletoes. Oecologia 126:10–20
Beau C (1920) Sur le role trophique des endophytes d’orchid6es. Comp Rend Acad Sci 171:675–677
Bennett JR, Mathews S (2006) Phylogeny of the parasitic plant family Orobanchaceae inferred from phytochrome A. Am J Bot 93:1039–1051
Bernard N (1899) Sur la germination du Neottia nidus-avis. C R Hebd Seances Acad Sci 128:1253–1255
Bernard N (1908) La culture des orchidées dans ses rapports avec la symbiose. Société Royale d’Agriculture et de Botanique de Gand, Gand
Bidartondo MI, Bruns TD (2005) On the origins of extreme mycorrhizal specificity in the Monotropoideae (Ericaceae): performance trade-offs during seed germination and seedling development. Mol Ecol 14:1549–1560
Bidartondo MI, Read DJ (2008) Fungal specificity bottlenecks during orchid germination and development. Mol Ecol 17:3707–3716
Bidartondo MI, Redecker D, Hijri I, Wiemken A, Bruns TD, Dominguez L, Sérsic A, Leake JR, Read DJ (2002) Epiparasitic plants specialized on arbuscular mycorrhizal fungi. Nature 419:389–392
Bidartondo MI, Bruns TD, Weiß M, Sérgio C, Read DJ (2003) Specialized cheating of the ectomycorrhizal symbiosis by a epiparasitic liverwort. Proc Biol Sci 270:835–842
Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ (2004) Changing partners in the dark: Isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc Biol Sci 271:1799–1806
Björkman E (1960) Monotropa hypopitys L.—an epiparasite on tree roots. Physiol Plant 13:308–327
Bonnardeaux Y, Brundrett M, Batty A, Dixon K, Koch J, Sivasithamparam K (2007) Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Mycol Res 111:51–61
Booth MG (2004) Mycorrhizal networks mediate overstorey-understorey competition in a temperate forest. Ecol Lett 7:538–546
Bougoure J, Ludwig M, Brundrett M, Grierson P (2009) Identity and specificity of the fungi forming mycorrhizas with the rare mycoheterotrophic orchid Rhizanthella gardneri. Mycol Res 113:1097–1106
Bougoure JJ, Brundrett MC, Grierson PF (2010) Carbon and nitrogen supply to the underground orchid, Rhizanthella gardneri. New Phytol 186:947–956
Boullard B (1979) Considérations sur la symbiose fongique chez les Ptéridophytes. Syllogeus 19:1–58
Bruce JG, Beitel JM (1979) A community of Lycopodium gametophytes in Michigan. Am Fern J 69:33–41
Bruns TD, Read DJ (2000) In vitro germination of nonphotosynthetic, myco-heterotrophic plants stimulated by fungi isolated from the adult plants. New Phytol 148:335–342
Burgeff H (1932) Saprophytismus und Symbiose. Studien an tropischen Orchideen. Gustav Fischer, Jena, Germany
Burgeff H (1936) Samenkeimung der Orchideen. Gustav Fischer, Jena, Germany
Burgeff H (1959) Mycorrhiza of orchids. In: Withner CL (ed) The orchids. Ronald Press, New York, pp 361–395
Cameron DD, Bolin JF (2010) Isotopic evidence of partial mycoheterotrophy in the Gentianaceae: Bartonia virginica and Obolaria virginica as case studies. Am J Bot 97:1272–1277
Cameron DD, Leake JR (2007) A different kind of parasitic plant: a brief history of mycoheterotrophy and epi-parasitism. Haustorium 50:4–6
Cameron KM, Chase MW, Rudall PJ (2003) Recircumscription of the monocotyledonous family Petrosaviaceae to include Japanolirion. Brittonia 55:214–225
Cameron DD, Leake JR, Read DJ (2006) Mutualistic mycorrhiza in orchids: evidence from the plant-fungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens. New Phytol 171:405–416
Cameron DD, Johnson I, Read DJ, Leake JR (2008) Giving and receiving: measuring the carbon cost of mycorrhizas in the green orchid, Goodyera repens. New Phytol 180:176–184
Cameron DD, Preiss K, Gebauer G, Read DJ (2009) The chlorophyll-containing orchid Corallorhiza trifida derives little carbon through photosynthesis. New Phytol 183:358–364
Camp WH (1940) Aphyllous forms in pyrola. Bull Torrey Bot Club 67:453–465
Campbell EO (1962) The mycorrhiza of Gastrodia cunninghamii Hook. F. Trans R Soc New Zealand 1:289–296
Campbell EO (1964) The fungal association in a colony of Gastrodia sesamoides. R Br Trans R Soc New Zealand 2:237–246
Cha JY, Igarashi T (1995) Armillaria species associated with Gastrodia elata in Japan. Eur J Forest Pathol 25:319–326
Cha JY, Igarashi T (1996) Armillaria jezoensis, a new symbiont of Galeola septentrionalis (Orchidaceae) in Hokkaido. Mycoscience 37:21–24
Chou H (1974) In propagation of Gastrodia elata Bl. Acta Bot Sin 16:288–290
Christoph H (1921) Untersuchungen über mykotrophen Verhältnisse der “Ericales” und die Keimung von Pirolaceen. Beihefte Botan Centralblatt 38:115–157
Courty P-E, Walder F, Boller T, Ineichen K, Wiemken A, Selosse M-A (2011) C and N metabolism in mycorrhizal networks and mycoheterotrophic plants of tropical forests: a stable isotope analysis. Plant Physiol 156:952–961
Currah RS, Zelmer CD, Hambleton S, Richardson KA (1997) Fungi from orchid mycorrhizas. In: Arditti J, Pridgeon AM (eds) Orchid biology: reviews and perspectives, VII. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 117–170
Curtis JT (1943) Germination and seedling development in five species of Cypripedium. Am J Bot 30:199–206
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–559
Dearnaley JDW (2006) The fungal endophytes of Erythrorchis cassythoides—is this orchid saprophytic or parasitic? Aust Mycol 25:51–57
Dearnaley JDW, Bougoure JJ (2010) Isotopic and molecular evidence for saprotrophic Marasmiaceae mycobionts in rhizomes of Gastrodia sesamoides. Fungal Ecol 3:288–294
Dearnaley JDW, Martos F, Selosse M-A (2012) Orchid mycorrhizas: molecular ecology, physiology, evolution and conservation aspects. In: Hock B (ed) Fungal associations, The mycota IX, 2nd edn. Springer-Verlag, Berlin
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Douhan GW, Vincenot L, Selosse M-A (2011) Population genetics of ectomycorrhizal fungi: from current knowledge to emerging directions. Fungal Biol 115:569–597
Downie DG (1959) Rhisoctonia solani and orchid seed. Trans Bot Soc Edinb 37:279–285
Eriksson O, Kainulainen K (2011) The evolutionary ecology of dust seeds. Perspect Plant Ecol Evol Syst 13:73–87
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Feller U, Anders I, Mae T (2008) Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. J Exp Bot 59:1615–1624
Finlay RD, Read DJ (1986) The structure and function of the vegetative mycelium of ectomycorrhizal plants: 1. Translocation of C-14-labeled carbon between plants interconnected by a common mycelium. New Phytol 103:143–156
Fitter AH, Graves JD, Watkins NK, Robinson D, Scrimgeour C (1998) Carbon transfer between plants and its control in networks of arbuscular mycorrhizas. Funct Ecol 12:406–412
Francis R, Read DJ (1984) Direct transfer of carbon between plants connected by vesicular arbuscular mycorrhizal mycelium. Nature 307:53–56
Frank AB (1885) Neue Mittheilungen über die Mycorrhiza der Bäume und der Monotropa hypopitys. Ber Dtsch Bot Ges 3:27–33
Freudenstein JV (1999) Relationships and character transformation in Pyroloideae (Ericaceae) based on ITS sequences, morphology, and development. Syst Bot 24:398–408
Freudenstein JV, Doyle JJ (1994) Character transformation and relationships in Corallorhiza (Orchidaceae: Epidendroideae). I. Plastid DNA. Am J Bot 81:1449–1457
Fry B (2006) Stable isotope ecology. Springer, New York, 308pp
Gebauer G (2005) Partnertausch im dunklen Wald—Stabile Isotope geben neue Einblicke in das Ernährungsverhalten von Orchideen. In: Bayer (ed) Auf Spurensuche in der Natur: Stabile Isotope in der ökologischen Forschung. Rundgespräche der Kommission für Ökologie Bd. 30. Akademie der Wissenschaften. Verlag Dr. Friedrich Pfeil, München, Germany, pp 55–67
Gebauer G, Dietrich P (1993) Nitrogen isotope ratios in different compartments of a mixed stand of spruce, larch and beech trees and of understorey vegetation including fungi. Isotopenpraxis 29:35–44
Gebauer G, Meyer M (2003) 15N and 13C natural abundance of autotrophic and mycoheterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytol 160:209–223
Gebauer G, Schulze E-D (1991) Carbon and nitrogen isotope ratios in different compartments of a healthy and a declining Picea abies forest in the Fichtelgebirge, NE Bavaria. Oecologia 87:198–207
Gebauer G, Taylor AFS (1999) 15N natural abundance in fruit bodies of different functional groups of fungi in relation to substrate utilization. New Phytol 142:93–101
Gebauer G, Rehder H, Wollenweber B (1988) Nitrate, nitrate reduction and organic nitrogen in plants from different ecological and taxonomic groups of Central Europe. Oecologia 75:371–385
Girlanda M, Selosse M-A, Cafasso D, Brilli F, Delfine S, Fabbian R, Ghignone S, Pinelli P, Segreto R, Loreto F, Cozzolino S, Perotto S (2006) Inefficient photosynthesis in the Mediterranean orchid Limodorum abortivum is mirrored by specific association to ectomycorrhizal Russulaceae. Mol Ecol 15:491–504
Girlanda M, Segreto R, Cafasso D, Liebel HT, Rodda M, Ercole E, Cozzolino S, Gebauer G, Perotto S (2011) Photosynthetic Mediterranean meadow orchids feature partial mycoheterotrophy and specific mycorrhizal associations. Am J Bot 98:1148–1163
Gleixner G, Danier H-J, Werner RA, Schmidt H-L (1993) Correlations between the 13C content of primary and secondary plant products in different cell compartments and that in decomposing fungi. Plant Physiol 102:1287–1290
Haber E (1987) Variability distribution and systematics of pyrola-picta sensu-lato Ericaceae in Western North America. Syst Bot 12:324–335
Hadley G (1970) Non-specificity of symbiotic infection in orchid mycorrhiza. New Phytol 69:1015–1023
Hamada M (1939) Studien über die Mykorrhiza von Galeola septentrionalis Reichb. F.—Ein neuer Fall der Mycorrhizabildung durch intraradicale Rhizomorpha. Jpn J Bot 10:151–211
Hamada M, Nakamura S (1963) Wurzelsymbiose von Galeola altissima Reichb. F., einer chlorophyllfreien Orchidee, mit dem holzzerstörenden Pilz Hymenochaete crocicreas Berk et Br. Science Reports of the Tohoku University, Fourth Series (Biology), vol 29, pp 227–238
Harvais G, Hadley G (1967) The relation between host and endophyte in orchid mycorrhiza. New Phytol 66:205–215
Hashimoto Y, Kunishi A, Hasegawa S (2005) Interspecific C transfers from Larix kaempferi Carr. to Pyrola incarnata Fischer by way of mycorrhizal fungi. Inoculum (Supplement to Mycologia) 56:23–24
Hashimoto Y, Fukukawa S, Kunishi A, Suga H, Richard F, Sauve M, Selosse M-A (2012) Mycoheterotrophic germination of Pyrola asarifolia dust seeds reveals convergences with germination in orchids. New Phytol 195(3):620–630
Hofmeister W (1857) Beiträge zur Entwicklungsgeschichte der Gefässkryptogamen. II. Abh Math Phys Cl Königl Sächs Ges Wiss 3:601–682, Taf. 1–13
Högberg P (1997) Tansley review no. 95. 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Högberg P, Plamboeck AH, Taylor AFS, Fransson PMA (1999) Natural 13C abundance reveals trophic status of fungi and host-origin of carbon in mycorrhizal fungi in mixed forests. Proc Natl Acad Sci U S A 96:8534–8539
Hunt R, Hope-Simpson JF (1990) Growth of Pyrola rotundifolia ssp. maritima in relation to shade. New Phytol 114:129–137
Hynson NA, Bruns TD (2009) Evidence of a myco-heterotroph in the plant family Ericaceae that lacks mycorrhizal specificity. Proc Biol Sci 276:4053–4059
Hynson NA, Bruns TD (2010) Fungal hosts for mycoheterotrophic plants: a nonexclusive, but highly selective club. New Phytol 185:598–601
Hynson NA, Preiss K, Gebauer G (2009a) Is it better to give than receive? A stable isotope perspective to orchid-fungal carbon transport in the green orchid species Goodyera repens and Goodyera oblongifolia. New Phytol 182:8–11
Hynson NA, Preiss K, Gebauer G, Bruns TD (2009b) Isotopic evidence of full and partial myco-heterotrophy in the plant tribe Pyroleae (Ericaceae). New Phytol 182:719–726
Hynson NA, Mambelli S, Amend AS, Dawson TE (2012) Measuring carbon gains from fungal networks in understory plants from the tribe Pyroleae (Ericaceae): a field manipulation and stable isotope approach. Oecologia 169:307–317. doi:10.1007/s00442-011-2198-3
Illyés Z, Halász K, Rudnóy S, Ouanphanivanh N, Garay T, Bratek Z (2009) Changes in the diversity of the mycorrhizal fungi of orchids as a function of the water supply of the habitat. J Appl Bot Food Qual 83:28–36
Imhof S (2008) Specialized mycorrhizal colonization pattern in achlorophyllous Epirixanthes spp. (Polygalaceae). Plant Biol 9:786–792
Irmisch T (1855) Bemerkungen über einige Pflanzen der deutschen Flora. Flora 13:625–638
Isogai N, Yamamura Y, Mariko S, Nakano T (2003) Seasonal pattern of photosynthetic production in a subalpine evergreen herb, Pyrola incarnata. J Plant Res 116:199–206
Johnson-Groh CL (1998) Population demographics, underground ecology and phenology of Botrychium mormo. In: Berlin N, Miller P, Borovansky J, Seal US, Byers O (eds) Population and habitat viability assessment (PHVA) for the goblin fern (Botrychium mormo), Final Report. Conservation Biology Specialist Group, Apple Valley, Minnesota, pp 103–108
Johnson-Groh CL, Lee JM (2002) Phenology and demography of two species of Botrychium (Ophioglossaceae). Am J Bot 89:1624–1633
Jones RI (2000) Mixotrophy in planktonic protists: an overview. Freshwater Biol 45:219–226
Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse M-A (2005) Mixotrophy in orchids: insights from a comparative study of green individuals and non-photosynthetic mutants of Cephalanthera damasonium. New Phytol 166:639–653
Kamienski F (1881) Die Vegetationsorgane der Monotropa hypopitys L. Bot Zeit 29:458–461
Kamjunke N, Tittel J (2009) Mixotrophic algae constrain the loss of organic carbon by exudation. J Phycol 45:807–811
Khan AG (1972) Mycorrhizae in the Pakistan Ericales Pak. J Bot 4:183–194
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Felbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bücking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882
Kikuchi G, Higuchi M, Yoshimura H, Morota T, Suzuki A (2008a) In vitro symbiosis between Gastrodia elata Blume (Orchidaceae) and Armillaria Kummer (Tricholomataceae) species isolated from the orchid tuber. J Jpn Bot 83:77–87
Kikuchi G, Higuchi M, Morota T, Nagasawa E, Suzuki A (2008b) Fungal symbiont and cultivation test of Gastrodia elata Blume (Orchidaceae). J Jpn Bot 83:88–95
Knudson L (1922) Nonsymbiotic germination of orchid seeds. Bot Gaz 73:1–25
Kohzu A, Yoshioka T, Ando T, Takahashi M, Koba K, Wada E (1999) Natural 13C and 15N abundance of field-collected fungi and their ecological implications. New Phytol 144:323–330
Kranabetter JM, MacKenzie WH (2010) Contrasts among mycorrhizal plant guilds in foliar nitrogen concentration and d15N along productivity gradients of a boreal forest. Ecosystems 13:108–117
Kron KA, Judd WS, Stevens PF, Crayn DM, Anderberg AA, Gadek PA, Quinn CJ, Luteyn JL (2002) Phylogenic classification of Ericaceae: molecular and morphological evidence. Bot Rev 68:335–423
Kunishi A, Hasegawa S, Hashimoto Y (2004) Effects of mycorrhiza on Pyrola incarnata growing in dark forest floor. In: Proceedings of the 51st annual meeting of the Ecological Society of Japan (JES51)
Kusano S (1911) Gastrodia elata and its symbiotic association with Armillaria mellea. J Coll Agric Imp Univ Tokyo 4:1–65
Leake JR (1994) Tansley review No. 69. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol 127:171–216
Leake JR (2004) Myco-heterotroph/epiparasitic plant interactions with ectomycorrhizal and arbuscular mycorrhizal fungi. Curr Opin Plant Biol 7:422–428
Leake JR (2005) Plants parasitic on fungi: unearthing the fungi in myco-heterotrophs and debunking the “saprophytic” plant myth. Mycologist 19:113–122
Lerat S, Gauci R, Catford JG, Vierheilig H, Piché Y, Lapointe L (2002) 14C transfer between the spring ephemeral Erythronium americanum and sugar maple saplings via arbuscular mycorrhizal fungi in natural stands. Oecologia 132:181–187
Levin I, Kromer B (1997) Twenty years of atmospheric 14CO2 observations at Schauinsland Station, Germany. Radiocarbon 39:205–218
Liebel HT, Gebauer G (2011) Stable isotope signatures confirm carbon and nitrogen gain through ectomycorrhizas in the ghost orchid Epipogium aphyllum Swartz. Plant Biol 13:270–275
Liebel HT, Preiss K, Gebauer G (2009) Parsiell mycoheterotrofi i norske vintergrønnarter – relevans for vernetiltak av truede vintergrønnarter (partial myco-heterotrophy in Norwegian wintergreen species—relevance for endangered species protection). Blyttia J Norw Bot Soc 67:138–143
Liebel HT, Bidartondo MI, Preiss K, Segreto R, Stöckel M, Rodda M, Gebauer G (2010) C and N isotope signatures reveal constraints to nutritional modes in orchids of the Mediterranean and Macaronesia. Am J Bot 97:903–912
Lievens B, van Kerckhove S, Justé A, Cammue BPA, Honnay O, Jacquemyn H (2010) From extensive clone libraries to comprehensive DNA arrays for the efficient and simultaneous detection and identification of orchid mycorrhizal fungi. J Microbiol Methods 80:76–85
Ligrone R, Pocok K, Duckett JG (1993) A comparative ultrastructural study of endophytic basidiomycetes in the parasitic achlorophyllous hepatic Cryptothallus mirabilis and the closely allied photosynthetic species Aneura pinguis (Metzgeriales). Can J Bot 71:666–679
Lihnell D (1942) Keimungsversuche mit Pyrola-Samen. Symb Bot Upsal 6:1–37
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London, UK
Martin F, Selosse M-A (2008) The Laccaria genome: a symbiont blueprint decoded (Tansley review—introduction to a special issue). New Phytol 180:296–310
Martos F, Dulormne M, Pailler T, Bonfante P, Faccio A, Fournel J, Dubios M-P, Selosse M-A (2009) Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytol 184:668–681
Matsuda Y, Amiya A, Ito S-I (2008) Colonization patterns of mycorrhizal fungi associated with two rare orchids, Cephalanthera falcata and C. erecta.. Ecol Res 24:1023–1031
Matsuda Y, Shimizu S, Mori M, Ito S-I, Selosse M-A (2012) Seasonal and spatial changes of mycorrhizal associations and heterotrophy levels in mixotrophic Pyrola japonica growing under different light environments. In revision for Am. J, Bot
Matsushita N, Fukuda K, Nagasawa E, Terashita T, Suzuki K (1996) Armillaria species in Japan identified by isozyme patterns with special reference to the biological species of the Northern hemisphere. J For Res 1:155–160
Mayor JR, Schuur EAG, Henkel TW (2009) Elucidating the nutritional dynamics of fungi using stable isotopes. Ecol Lett 12:171–183
McCormick MK, Whigham DF, O’Neill J (2004) Mycorrhizal diversity in photosynthetic terrestrial orchids. New Phytol 163:425–438
McCormick MK, Taylor DL, Juhaszova K, Burnett RK, Whigham DF, O’Neill JP (2012) Limitations on orchid recruitment: not a simple picture. Mol Ecol 21:1511–1523
McGuire KL (2007) Common ectomycorrhizal networks may maintain monodominance in a tropical rain forest. Ecology 88:567–574
McKendrick SL, Leake JR, Read DJ (2000) Symbiotic germination and development of myco-heterotrophic plants in nature: transfer of carbon from ectomycorrhizal Salix repens and Betula pendula to the orchid Corallorhiza trifida through shared hyphal connections. New Phytol 145:539–548
McKendrick SL, Leake JR, Taylor DL, Read DJ (2002) Symbiotic germination and development of the myco-heterotrophic orchid Neottia nidus-avis in nature and its requirement for locally distributed Sebacina spp. New Phytol 154:233–247
McNeal JR, Arumugunathan K, Kueh JV, Boore JL, dePamphilis CW (2007) Systematics and plastid genome evolution of the cryptically photosynthetic parasitic plant genus Cuscuta (Convolvulaceae). BMC Biol 5:55
Merckx V, Schols P, Van De Kamer HM, Maas HM, Huysmans S, Smets E (2006) Phylogeny and evolution of Burmaniaceae (Dioscoreales) based on nuclear and mitochondrial data. Am J Bot 93:1684–1698
Merckx V, Stöckel M, Fleischmann A, Bruns TD, Gebauer G (2010) 15N and 13C natural abundance of two mycoheterotrophic and a putative partially mycoheterotrophic species associated with arbuscular mycorrhizal fungi. New Phytol 188:590–596
Mettenius G (1856) Filices Horti Botanici Lipsiensis. L. Voss, Leipzig
Milligan MJ, Williams PG (1988) The mycorrhizal relationship of multinucleate rhizoctonias from non-orchids with Microtis (Orchidaceae). New Phytol 108:205–209
Montfort C, Küsters E (1940) Saprophytismus und Photosynthese. I. Biochemische und physiologische Studien an Humus-Orchideen. Bot Arch 40:571–633
Motomura H, Yukawa T, Ueno O, Kagawa A (2008) The occurrence of Crassulacean acid metabolism in Cymbidium (Orchidaceae) and its ecological and evolutionary implications. J Plant Res 121:163–177
Motomura H, Selosse M-A, Martos F, Kagawa A, Yukawa T (2010) Mycoheterotrophy evolved from mixotrophic ancestors: evidence in Cymbidium (Orchidaceae). Ann Bot 106:573–581
Muir HJ (1989) Germination and mycorrhizal fungus compatibility in European orchids. In: Pritchard HW (ed) Modern methods in orchid conservation: the role of physiology, ecology and management. Cambridge University Press, England, pp 39–56
Mursidawati S (2004) Mycorrhizal association, propagation and conservation of the myco-heterotrophic orchid Rhizanthella gardneri. MSc thesis, The University of Western Australia
Nakano A, Takahashi K, Kimura M (1999) The carbon origin of arbuscular mycorrhizal fungi estimated from δ13C values of individual spores. Mycorrhiza 9:41–47
Nara K (2006) Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol 169:169–178
Neales TF, Hew CS (1975) Two types of carbon fixation in tropical orchids. Planta 123:303–306
Ogura-Tsujita Y, Yukawa T (2008) High mycorrhizal specificity in a widespread mycoheterotrophic plant, Eulophia zollingeri (Orchidaceae). Am J Bot 95:93–97
Ogura-Tsujita Y, Gebauer G, Hashimoto T, Umata H, Yukawa T (2009) Evidence for novel and specialized mycorrhizal parasitism: the orchid Gastrodia confusa gains carbon from saprotrophic Mycena. Proc Biol Sci 276:761–767
Ogura-Tsujita Y, Yokoyama J, Miyoshi K, Yukawa T (2012) Shifts in mycorrhizal fungi during the evolution of autotrophy to mycoheterotrophy in Cymbidium (Orchidaceae). Am J Bot 99(7):1158–1176
Ota Y, Intini M, Hattori T (2000) Genetic characterization of heterothallic and non-heterothallic Armillaria mellea sensu stricto. Mycol Res 104:1046–1054
Ouanphanivanh N, Merényi Z, Orczán ÁK, Bratek Z, Szigeti Z, Illyés Z (2008) Could orchids indicate truffle habitats? Mycorrhizal association between orchids and truffles. Acta Biol Szeg 52:229–232
Parrent JL, James TY, Vasaitis R, Taylor AFS (2009) Friend or foe? Evolutionary history of glycoside hydrolase family 32 genes encoding for sucrolytic activity in fungi and its implications for plant-fungal symbioses. BMC Evol Biol 9:148
Pfeffer PE, Douds DD, Bucking H, Schwartz DP, Shachar-Hill Y (2004) The fungus does not transfer carbon to or between roots in an arbuscular mycorrhizal symbiosis. New Phytol 163:617–627
Phillips RD, Barrett MD, Dixon KW, Hopper SD (2011) Do mycorrhizal symbioses cause rarity in orchids? J Ecol 99:858–869
Preiss K, Gebauer G (2008) A methodological approach to improve estimates of nutrient gains by partially myco-heterotrophic plants. Isotopes Environ Health Stud 44:393–401
Preiss K, Adam IKU, Gebauer G (2010) Irradiance governs exploitation of fungi: fine-tuning of carbon gain by two partially myco-heterotrophic orchids. Proc Biol Sci 277:1333–1336
Press MC, Graves JD (1995) Parasitic plants. Chapmann & Hall, London
Press MC, Smith JD, Stewart GR (1991) Carbon acquisition and assimilation relations in parasitic plants. Funct Ecol 5:278–283
Pridgeon A, Cribb PJ, Chase MM (2008) Genera orchidacearum: vol 4: Epidendroidae. Oxford University Press, New York
Ramsay RR, Dixon KW, Sivasithamparam K (1986) Patterns of infection and endophytes associated with Western Australian orchids. Lindleyana 1:203–214
Rasmussen HN (1995) Terrestrial orchids from seed to mycotrophic plant. Cambridge University Press, Cambridge, UK
Rasmussen HN (2002) Recent developments in the study of orchid mycorrhiza. Plant Soil 244:149–163
Rayner MC (1927) Mycorrhiza: an account of non-pathogenic infection by fungi in vascular plants and bryophyes. New phytologist reprint no. 15. Wheldon & Wesley, London, UK
Reeves P, Chase MW, Goldblatt P, Rudall P, Fay MF, Cox AV, Lejeune B, Souza-Chies T (2001) Molecular systematics of Iridaceae: evidence from four plastid DNA regions. Am J Bot 88:2074–2087
Renner O (1938) Über blasse, saprophytische Cephalanthera alba und Epipactis latifolia. Flora 132:225–233
Robertson DC, Robertson JA (1985) Ultrastructural aspects of Pyrola mycorrhizae. Can J Bot 63:1089–1098
Robinson D, Fitter A (1999) The magnitude and control of carbon transfer between plants linked by a common mycorrhizal network. J Exp Bot 50:9–13
Roy M, Watthana S, Stier A, Richard F, Vessabutr S, Selosse M-A (2009a) Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biol 7:51
Roy M, Yagame T, Yamato M, Iwase K, Heinz C, Faccio A, Bonfante P, Selosse M-A (2009b) Ectomycorrhizal Incybe species associate with the mycoheterotrophic orchid Epipogium aphyllum but not its asexual propagules. Ann Bot 104:595–610
Roy M, Gonneau C, Rocheteau A, Berveiller D, Thomas J-C, Damesin C, Selosse M-A (in press) Why do mixotrophic plants stay green? A comparison between green and achlorophyllous orchid individuals in situ. Ecol Monographs
Rudall PJ, Bateman R (2006) Morphological phylogenetic analysis of Pandanales: testing contrasting hypotheses of floral evolution. Syst Bot 31:223–238
Sadovsky O (1965) Orchideen im eigenen Garten. Bayerischer Landwirtschaftsverlag, Munich, Germany
Salisbury RA (1804) On the germination of the seeds of Orchidaceae. Trans Linn Soc Lond 7:29–32
Salmia A (1986) Chlorophyll-free form of Epipactis helleborine (Orchidaceae) in SE Finland. Ann Bot Fenn 23:49–57
Salmia A (1989a) Features of endomycorrhizal infection of chlorophyll-free and green forms of Epipactis helleborine (Orchidaceae). Ann Bot Fenn 26:15–26
Salmia A (1989b) General morphology and anatomy of chlorophyll-free and green forms of Epipactis helleborine (Orchidaceae). Ann Bot Fenn 26:95–105
Schimel DS (1993) Theory and application of tracers. Academic, San Diego, USA
Schulze E-D, Lange OL, Ziegler H, Gebauer G (1991) Carbon and nitrogen isotope ratios of mistletoes growing on nitrogen fixing and non-nitrogen fixing hosts and on CAM plants in the Namib desert confirm partial heterotrophy. Oecologia 88:457–462
Schulze E-D, Beck E, Müller-Hohenstein K (2005) Plant ecology. Springer, Berlin
Sekizaki H, Kuninaga S, Yamamoto M, Asazu SN, Sawa S, Kojoma M, Yokosawa R, Yoshida N (2008) Identification of Armillaria nabsnona in Gastrodia tubers. Biol Pharm Bull 31:1410–1414
Selosse M-A, Rousset F (2011) The plant-fungal marketplace. Science 333:828–829
Selosse M-A, Roy M (2009) Green plants eating fungi: facts and questions about mixotrophy. Trends Plant Sci 14:64–70
Selosse M-A, Weiss M, Jany JL, Tillier A (2002) Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis (L.) L.C.M. Rich. and neighbouring treeectomycorrhizae. Mol Ecol 11:1831–1844
Selosse M-A, Faccio A, Scappaticci G, Bonfante P (2004) Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microb Ecol 47:416–426
Selosse M-A, Richard F, He X, Simard SW (2006) Mycorrhizal networks: des liaisons dangereuses? Trends Ecol Evol 22:621–628
Selosse M-A, Setaro S, Glatard F, Richard F, Urcelay C, Weiß M (2007) Sebacinales are common mycorrhizal associates of Ericaceae. New Phytol 174:864–878
Selosse M-A, Dubois M-P, Alvarez N (2009) Do Sebacinales commonly associate with plant roots as endophytes? Mycol Res 113:1062–1069
Selosse M-A, Martos F, Perry BA, Padamsee M, Roy M, Pailler T (2010) Saprotrophic fungal symbionts in tropical achlorophyllous orchids: finding treasures among the ‘molecular scraps’? Plant Signal Behav 5:1–5
Selosse M-A, Boullard B, Richardson D (2011) Noel Bernard (1874–1911): orchids to symbiosis in a dozen years, one century ago. Symbiosis 54:61–68
Sharma J, Zettler LW, Van Sambeek JW, Ellersieck MR, Starbuck CJ (2003) Symbiotic seed germination and mycorrhizae of federally threatened Platanthera praeclara (Orchidaceae). Am Midl Nat 149:104–120
Shefferson RP, Weiß M, Kull T, Taylor DL (2005) High specificity generally characterizes mycorrhizal association in rare lady’s slipper orchids, genus Cypripedium. Mol Ecol 14:613–626
Shefferson RP, Kull T, Tali K (2006) Demographic response to shading and defoliation in two woodland orchids. Folia Geobot 41:95–106
Shefferson RP, Taylor DL, Weiß M, Garnica S, McCormick MK, Adams S, Gray HM, McFarland JW, Kull T, Tali K, Yukawa T, Kawahara T, Miyoshi K, Lee Y-I (2007) The evolutionary history of mycorrhizal specificity among lady’s slipper orchids. Evolution 61:1380–1390
Shefferson RP, Kull T, Tali K (2008) Mycorrhizal interactions of orchids colonizing Estonian mine tailings hills. Am J Bot 95:156–164
Silvera K, Santiago LS, Cushman JC, Winter K (2010) The incidence of crassulacean acid metabolism in Orchidaceae derived from carbon isotope ratios: a checklist of the flora of Panama and Costa Rica. Bot J Linn Soc 163:194–222
Simard SW, Durall DM (2004) Mycorrhizal networks: a review of their extent, function, and importance. Can J Bot 82:1140–1165
Simard SW, Perry DA, Jones MD, Myrold DD, Durall DM, Molina R (1997) Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 388:579–582
Smith SE (1966) Physiology and ecology of orchid mycorrhizal fungi with reference to seedling nutrition. New Phytol 65:488–499
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London, UK
Stark JM (2000) Nutrient transformations. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods in ecosystem science. Springer, New York, pp 215–234
Stark C, Babik W, Durka W (2009) Fungi from the roots of the common terrestrial orchid Gymnadenia conopsea. Mycol Res 113:952–959
Stöckel M, Meyer C, Gebauer G (2011) The degree of mycoheterotrophic carbon gain in green, variegated and vegetative albino individuals of Cephalanthera damasonium is related to leaf chlorophyll concentrations. New Phytol 189:790–796
Stokey AG (1950) The gametophyte of the Gleicheniaceae. Bull Torrey Bot Club 77:323–339
Struwe L, Kadereit J, Klackenberg J, Nilsson S, Thiv M, von Hagen KB, Albert VA (2002) Systematics, character evolution, and biogeography of Gentianaceae, including a new tribal and subtribal classification. In: Struwe L, Albert VA (eds) Gentianaceae—systematics and natural history. Cambridge University Press, Cambridge, pp 21–309
Sung JM, Jung BS, Yang KJ, Lee HK, Harrington TC (1995) Production of Gastrodia elata tuber using Armillaria spp. Korean J Mycol 23:61–70
Taylor DL, Bruns TD (1997) Independent, specialized invasions of ectomycorrhizal mutualism by two nonphotosynthetic orchids. Proc Natl Acad Sci U S A 94:4510–4515
Taylor AFS, Fransson PM, Högberg P, Högberg MN, Plamboeck AH (2003) Species level patterns in 13C and 15N abundance of ectomycorrhizal and saprotrophic fungal sporocarps. New Phytol 159:757–774
Taylor AFS, Gebauer G, Read DJ (2004) Uptake of nitrogen and carbon from double-labelled (15N and 13C) glycine by mycorrhizal pine seedlings. New Phytol 164:383–388
Tedersoo L, Pellet P, Kõljalg U, Selosse M-A (2007) Parallel evolutionary paths to mycohetereotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. Oecologia 151:206–217
Tennakoon KU, Pate JS (1996) Heterotrophic gain of carbon from hosts by the xylem-tapping root hemiparasite Olax phyllanthi (Olacaceae). Oecologia 105:369–376
Terashima K, Kawashima Y, Cha JY, Miura K (1998) Identification of Armillaria species from Hokkaido by analysis of the intergenic spacer (IGS) region of ribosomal DNA using PCR-RFLP. Mycoscience 39:179–183
Terashita T, Chuman S (1987) Fungi inhabiting wild orchids in Japan. IV. Armillariella tabescens, a new symbiont of Galeola septentrionalis. Trans Mycol Soc Jpn 28:145–154
Terashita T, Chuman S (1989) Armillarias, isolated from the wild orchid, Galeola septentrionalis. In: Proceedings of the 7th IUFRO international conference on root and butt rots of forest trees, pp 364–370
Těšitel J, Plavcová L, Cameron DD (2010) Interactions between hemiparasitic plants and their hosts. The importance of organic carbon transfer. Plant Signal Behav 5:1072–1076
Těšitel J, Lepš J, Vráblová M, Cameron DD (2011) The role of heterotrophic carbon acquisition by the hemiparasitic plant Rhinanthus alectorolophus in seedling establishment in natural communities: a physiological perspective. New Phytol 192:188–199
Tesitelova T, Těšitel J, Jersáková J, Říhová G, Selosse M-A (2012) Symbiotic germination capability of four Epipactis species (Orchidaceae) is broader than expected from adult ecology. Am J Bot 99(6):1020–1032
Teste FP, Simard SW, Durall DM, Guy RD, Jones MD, Schoonmaker AL (2009) Access to mycorrhizal networks and roots of trees: importance for seedling survival and resource transfer. Ecology 90:2808–2822
Tisserant E, Kohler A, Dozolme-Seddas P, Balestrini R, Benabdellah K, Colard A, Croll D, Da Silva C, Gomez SK, Koul R, Ferrol N, Fiorilli V, Formey D, Franken P, Helber N, Hijri M, Lanfranco L, Lindquist E, Liu Y, Malbreil M, Morin E, Poulain J, Shapiro H, van Tuinen D, Waschke A, Azcón-Aguilar C, Bécard G, Bonfante P, Harrison MJ, Küster H, Lammers P, Paszkowski U, Requena N, Rensing SA, Roux C, Sanders IR, Shachar-Hill Y, Tuskan G, Young JP, Gianinazzi-Pearson V, Martin F (2012) The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol 193:755–769
Toftegaard T, Iason GR, Alexander IJ, Rosendahl S, Taylor AFS (2010) The threatened plant intermediate wintergreen (Pyrola media) associates with a wide range of biotrophic fungi in native Scottish pine woods. Biodivers Conserv 19:3963–3971
Tranchida-Lombardo V, Roy M, Bugot E, Santoro G, Püttsepp U, Selosse M-A, Cozzolino S (2010) Spatial repartition and genetic relationship of green and albino individuals in mixed populations of Cephalanthera orchids. Plant Biol 12:659–667
Trudell SA, Rygiewicz PT, Edmonds RL (2003) Nitrogen and carbon stable isotope abundances support the myco-heterotrophic nature and host-specificity of certain achlorophyllous plants. New Phytol 160: 391–401
Trumbore SE (2000) Age of soil organic matter and soil respiration: radiocarbon constraints on belowground C dynamics. Ecol Appl 10:399–411
Umata H (1995) Seed germination of Galeola altissima, an achlorophyllous orchid, with aphyllophorales fungi. Mycoscience 36:369–372
Umata H (1997) Formation of endomycorrhizas by an achlorophyllous orchid, Erythrorchis ochobiensis, and Auricularia polytricha. Mycoscience 38:335–339
Umata H (1998a) In vitro symbiotic association of an achlorophyllous orchid, Erythrorchis ochobiensis, with orchid and non-orchid fungi. Memoirs of the Faculty of Agriculture, Kagoshima University, vol 34, pp 97–107
Umata H (1998b) A new biological function of shiitake mushroom, Lentinula edodes, in a myco-heterotrophic orchid, Erythrorchis ochobiensis. Mycoscience 39: 85–88
Umata H (1999) Germination and growth of Erythrorchis ochobiensis (Orchidaceae) accelerated by monokaryons and dikaryons of Lenzites betulinus and Trametes hirsuta. Mycoscience 40:367–371
Umata H, Kaneko M, Miyagi T, Nakahira Y (2007) The application and utilization of fungi for the propagation of the endangered achlorophyllous plant, Erythrorchis ochobiensis (Hayata) Garay (Orchidaceae) in natural situations. Research Bulletin of the Kagoshima University Forests 35:31–48
Velenovsky J (1892) Über die Biologie und Morphologie der Gattung Moneses. Rozpravy Královské české společnosti nauk, řada, matematicko-přírodovědná 11:147–159
Vincenot L, Tedersoo L, Richard F, Horcine H, Kõljalg U, Selosse M-A (2008) Fungal associates of Pyrola rotundifolia, a mixotrophic Ericaceae, from two Estonian boreal forests. Mycorrhiza 19:15–25
Vujanovic V, St-Arnaut M, Barab D, Thibeault G (2000) Viability testing of orchid seed and the promotion of colouration and germination. Ann Bot 86:79–86
Wagner WH Jr, Wagner FS (1981) New species of moonworts, Botrychium subg. Botrychium (Ophioglossaceae), from North America. Am Fern J 71:20–30
Warcup JH (1973) Symbiotic germination of some Australian terrestrial orchids. New Phytol 72:387–392
Warcup JH (1981) The mycorrhizal relationships of Australian orchids. New Phytol 87:371–381
Warcup JH (1988) Mycorrhizal associations of isolates of Sebacina vermifera. New Phytol 110:227–231
Warcup JH, Talbot PHB (1967) Perfect states of rhizoctonias associated with orchids. New Phytol 66:631–641
Waterman RJ, Bidartondo MI, Stofberg J, Combs JK, Gebauer G, Savolainen V, Barraclough TG, Pauw A (2011) The effects of above- and belowground mutualism in orchid speciation and coexistence. Am Nat 177:E54–E68
Watson DM (2009) Parasitic plants as facilitators: more dryad than dracula? J Ecol 97:1151–1159
Weiss M, Selosse M-A, Rexer K, Urban A, Oberwinkler F (2004) Sebacinales: a hitherto overlooked cosm of heterobasidiomycetes with a broad mycorrhizal potential. Mycol Res 108:1003–1010
Weiss M, Sýkorová Z, Garnica S, Riess K, Martos F, Krause C, Oberwinkler F, Bauer R, Redecker D (2011) Sebacinales everywhere: previously overlooked ubiquitous fungal endophytes. PLoS One 6:e16793
Westwood JH, Yoder JI, Timko MP, dePamphilis CW (2010) The evolution of parasitism in plants. Trends Plant Sci 15:227–235
Whittier P (1988) Dark-grown psilotum. Am Fern J 78:109–116
Whittier DP, Renzaglia KS (2005) The young gametophyte of Lycopodiella lateralis and the role of the intermediate shaft in development of Lycopodiella gametophytes. Am Fern J 95:153–159
Winther JL, Friedman WE (2007) Arbuscular mycorrhizal symbionts in Botrychium (Ophioglossaceae). Am J Bot 94:1248–1255
Winther JL, Friedman WE (2008) Arbuscular mycorrhizal associations in Lycopodiaceae. New Phytol 177:790–801
Winther JL, Friedman WE (2009) Phylogenetic affinity of arbuscular mycorrhizal symbionts in Psilotum nudum. J Plant Res 122:485–496
Wright M, Cross R, Dixon K, Huynh T, Lawrie A, Nesbitt L, Pritchard A, Swarts N, Thomson R (2009) Propagation and reintroduction of Caladenia. Aust J Bot 57:373–387
Wright MM, Cross R, Cousens RD, May TW, McLean CB (2010) Taxonomic and functional characterisation of fungi from the Sebacina vermifera complex from common and rare orchids in the genus Caladenia. Mycorrhiza 20:375–390
Wu BY, Nara K, Hogetsu T (2001) Can C-14-labeled photosynthetic products move between Pinus densiflora seedlings linked by ectomycorrhizal mycelia? New Phytol 149:137–146
Wu J, Ma H, Lü M, Han S, Zhu Y, Jin H, Liang J, Liu L, Xu J (2010) Rhizoctonia fungi enhance the growth of the endangered orchid Cymbidium goeringii. Botany 88:20–29
Xu J, Guo S (2000) Retrospect on the research of the cultivation of Gastrodia elata Bl, a rare traditional Chinese medicine. Chin Med J 113:686–692
Yagame T, Yamato M, Mii M, Suzuki A, Iwase K (2007) Developmental processes of achlorophyllous orchid, Epipogium roseum: from seed germination to flowering under symbiotic cultivation with mycorrhizal fungus. J Plant Res 120:229–236
Yagame T, Fukiharu T, Yamato M, Suzuki A, Iwase K (2008a) Identification of a mycorrhizal fungus in Epipogium roseum (Orchidaceae) from morphological characteristics of basidiomata. Mycoscience 49: 147–151
Yagame T, Yamato M, Suzuki A, Iwase K (2008b) Ceratobasidiaceae mycorrhizal fungi isolated from nonphotosynthetic orchid Chamaegastrodia sikokiana. Mycorrhiza 18:97–101
Yagame T, Orihara T, Selosse M-A, Yamato M, Iwase K (2012) Mixotrophy of Platanthera minor, an orchid associated with ectomycorrhiza-forming Ceratobasidiaceae fungi. New Phytol 193:178–187
Yamato M, Iwase K (2008) Introduction of asymbiotically propagated seedlings of Cephalanthera falcata (Orchidaceae) into natural habitat and investigation of colonized mycorrhizal fungi. Ecol Res 23:329–337
Yamato M, Yagame T, Suzuki A, Iwase K (2005) Isolation and identification of mycorrhizal fungi associating with an achlorophyllous plant, Epipogium roseum (Orchidaceae). Mycoscience 46:73–77
Zettler LW, Hofer CJ (1998) Propagation of the little club-spur orchid (Platanthera clavellata) by symbiotic seed germination and its ecological implications. Environ Exp Bot 39:189–195
Zettler LW, Stewart SL, Bowles ML, Jacobs KA (2001) Mycorrhizal fungi and cold-assisted symbiotic germination of federally threatened eastern prairie fringed orchid, Platanthera leucophaea (Nuttall) Lindley. Am Midl Nat 145:168–175
Zimmer K, Hynson NA, Gebauer G, Allen EB, Allen MF, Read DJ (2007) Wide geographical and ecological distribution of nitrogen and carbon gains from fungi in pyroloids and monotropoids (Ericaceae) and in orchids. New Phytol 175:166–175
Zimmer K, Meyer C, Gebauer G (2008) The ectomycorrhizal specialist orchid Corallorhiza trifida is a partial myco-heterotroph. New Phytol 178:395–400
Zuccaro A, Lahrmann U, Güldener U, Langen G, Pfiffi S, Biedenkopf D, Wong P, Samans B, Grimm C, Basiewicz M, Murat C, Martin F, Kogel K-H (2011) Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog 7:e1002290
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Hynson, N.A. et al. (2013). The Physiological Ecology of Mycoheterotrophy. In: Merckx, V. (eds) Mycoheterotrophy. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5209-6_8
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5209-6_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5208-9
Online ISBN: 978-1-4614-5209-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)