Abstract
Increasing soil salinization is an enormous cause of crop productivity reduction in many areas of the world. It has a deep backlash on crop yields because it reduces leaf growth and induces leaf senescence, this in turn reduces plant assimilation activity, impairing its capacity for producing further growth or harvestable biomass. Plant has the capacity for adaptation to the environmental conditions likely involving long-distance signals between different organs (e.g., between root and shoot) through phytohormones. Abscisic acid (ABA) between them, surely, has an important action in the whole plant responses to drought and salt stresses. Processes that control leaf growth and shoot development during the osmotic phase of salinity are still not well known and several different opinions exist on cross-talk between other hormones and ABA in the process of biomass allocation under salinity conditions.
Moreover root system likely plays an important role to cope up with salts, and how salts affect root growth and architecture is of great importance to understand plant adaptation process to this abiotic stress. Root architecture modification under salinity seems to be a crucial aspect of crop response to salinity on which significance there are still many aspect to clarify.
Our experimental activity suggests that the amount of ABA and ion increase in tomato leaves significantly regulates both growth and gas exchange in tomato. ABA seems to be involved in tomato salt response and could have a crucial action in regulation of dry matter partition between root and shoot of tomato plants subjected to salinity. The significance of root architecture in tomato processes to salt adaptation is still uncertain, but it likely plays an important role. Source–sink control and root-to-shoot signaling are interconnected mechanisms that allow tomato plants to increase salt tolerance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Introduction
Soil salinity can emerge under different environmental conditions and vary according to chemical, physical and biological properties of the soil itself. Those different conditions can markedly modify the possible indications about the use of the soil, the most suitable techniques to adopt for its agronomic management or the most appropriate actions to apply for its correction and recovery. In particular, an in-depth knowledge of the origin and nature of the processes leading to soil salinization and a clear analysis of the soil changes is very important for a correct soil use planning at land level (Monteleone 2006).
At the same time understanding crop tolerance and adaptation to salinity is very important (Koyro et al. 2012) and forms one of the major research fields on crops in agronomic sector since agricultural productivity is deeply affected by salinization (Yadav et al. 2011). Salinity and drought conditions are the major limiting factors for yields in agriculture (Gregory 2006). Actually they are enormous problems if we consider the global increasing population and climate change. In agriculture it is important and no more postponed to increase productivity also in salinity conditions in order to achieve a sustainable use of environmental resources, to reach food security and finally in order to increase profitability of farms in using production factors.
For classification purposes plants have been organized into two groups: the salt sensitive glycophytes and the salt tolerant halophytes, unluckily all crops are belonging to the first group (Flowers and Flowers 2005). Plants react to stress environmental conditions at different levels, whole plant, root, leaves, reproductive organs, cellular and molecular levels (Jacobsen et al. 2012). Still today any effort to enhance salt tolerance of crops have met low results for the complexity of the crop mechanisms of adaptation to salinity from the physiological and genetic point of view (Flowers and Flowers 2005). In any case crop physiology may explain structure–function relationship of crop characters and modifications in plant function that are caused by stress environmental conditions (Jacobsen et al. 2012; Miflin 2000).
10.2 Tomato Response to Salinity
Salinity is a significant environmental stress for crops. Currently, soil salinization is one of the main causes of crop yield reduction in many areas of the world (Paranychianakis and Chartzoulakis 2005). It was reported that about 20 % of irrigated surface is compromised by increasing salinity according to the United Nations Food and Agriculture Organization (Rozema and Flowers 2008). Soil salinization may arise from intrinsic soil components, use of low quality water for irrigation, or excessive use of fertilizers. The increasing scarcity of good quality water has focused attention on the problem of using brackish waters for irrigation. Solving salt stress problem in agriculture cannot be postponed due to irrigation with saline water and utilization of saline soils to handle the request of the global increasing population (Koushafar et al. 2011; Munns 2002). Agriculture widening to semiarid and arid regions with the practice of modern irrigation will exacerbate secondary salinization because hydrologic balance of the soil between water applied (irrigation and precipitation) and water consumed by crops (transpiration) will change (Chaves et al. 2009). Moreover a number of researchers have suggested that significant impacts of climate change are likely in the Mediterranean area, where in summer season warming greater than the average is expected, with a further increase in heat waves and a significant rainfall reduction (IPCC et al. 2007; Olesen and Bindi 2002; Vitale et al. 2010; Lovelli et al. 2010).
From a physiological point of view the plant response to salinity is complex, since it varies with the species, the salt concentration, the environmental factors and the growth stage. Actually breeding approaches showed that stress tolerance characters are in main part quantitative trait loci (QTLs), this in turn makes genetic selection of these traits very hard (Bartels and Sunkar 2005), even if in some cases stress tolerant genotypes have been likewise obtained, by inserting traits using as genes source wild relatives (Bartels and Sunkar 2005).
Undoubtedly there are a lot of information on plant response to salinity obtained from several researches on different crops made on different approaches, but it is necessary to integrate information regarding aspects of plant salt adaptation derived from physiological studies with those obtained from other approaches since our knowledge for the processes that ensures salinity tolerance is still today unclear (Paranychianakis and Chartzoulakis 2005).
Tomato is a widespread crop in the Mediterranean area where soil salinization is currently a serious problem (Paranychianakis and Chartzoulakis 2005). It is an important annual vegetable crop and it is usually utilised fresh, cooked or after processing (Cuartero and Fernandez-Munoz 1999). Tomato is well adapted to several climates; nevertheless a great part of world tomato production is localized in dry areas such as Mediterranean and California, where cultivation must be necessarily under irrigation (Cuartero and Fernandez-Munoz 1999).
Tomato as crop is classified as “moderately sensitive” to salinity (Foolad 2004) and, undoubtedly, it holds an important position in agriculture section (Koushafar et al. 2011). Water deficit and low water quality are surely the most important factors able to reduce yield and quality of tomato from nutritional value and food safety point of view (Favati et al. 2009; Dorais et al. 2008). Irrigation with saline water may increase sugar and organic acid content of cherry tomatoes (De Pascale et al. 2007) and the flavour of processed tomatoes (Mitchell et al. 1991). All the desirable quality aspects for the processed tomato industry such as dry matter, soluble solids and titratable acidity seem to increase with salinity (Mitchell et al. 1991). From agronomic and physiological point of view as regards salinity response of this crop there are several studies (see review of Cuartero and Fernandez-Munoz 1999, and most recent papers Maggio et al. 2007, Albacete et al. 2008, Perez-Alfocea et al. 2010, Ghanem et al. 2008, Okhovatian-Ardakani et al. 2010, Ghanem et al. 2011b, Lovelli et al. 2012). From the most recent papers it was pointed out that crucial points that are assuming great relevance in the understanding of tomato response to salinity conditions are substantially three:
-
1.
Plant biomass partitioning;
-
2.
ABA signal involved;
-
3.
Tomato root architecture.
Our recent paper (Lovelli et al. 2012) confirmed the critical role for biomass partitioning and for root growth and morphology in tomato process adaptation to salts. Our findings provided important elements for elucidation of crucial mechanisms regarding tomato salt tolerance. Previously it was accepted the idea that in tomato, salinity does not change the usual distribution of dry matter between plant organs (fruits, shoot and root) even when there is a yield decrease (Ehret and Ho 1986). Recently we showed the contrary in agree with other recent papers (Albacete et al. 2008). On tomato we showed the high root-to-shoot ratio under salinity in tomato and the close relationship to high abscisic acid (ABA) root concentration (Lovelli et al. 2012). In tomato under high salinity level, the increase of ABA tissue concentration could regulate plant adaptation processes, such as dry matter partitioning (Albacete et al. 2008) and in particular way the root/shoot ratio (Maggio et al. 2007; Zhang and Blumwald 2001; Lovelli et al. 2012).
10.3 Gas Exchange, Plant Growth and Biomass Partitioning Under Salinity
In general, the negative effect of salt excess in soil water on glycophytic plants is mainly due to three phenomena:
-
Osmotic stress, markedly increasing the osmotic potential of soil water stress resulting in a difficulty of plant to uptake water (directly proportional to salt concentration) with consequences similar to those caused by a water deficit (physiological drought);
-
Toxic stress, consisting in the toxic effect and denaturing that some excess ions, especially Na+, cause to cytoplasm enzymatic activities;
-
Nutritional stress caused by an unbalanced ion uptake, given the antagonism among certain useful ions against those being in excess in soil water.
These effects change hormonal status and impair plant metabolic processes. As a consequence of those three stresses a reduction of plant growth and yield occurs (Yeo 2007). It was hypothesized that salinity response of tomato, as for other plants, happens in two phases (“biphasic model”; Munns 1993): during the first phase (days to weeks) the osmotic effect is prevalent, while during the second one (weeks to months) growth is controlled by toxic actions of the high salt accumulation in leaf tissues. In other words in plant adaptation to salts it is essential the time scale of the response. During the first phase (osmotic one) plant growth could be hormonal regulated while during the second phase, toxic effect of high salt concentration at tissue level are prevalent on plant growth reduction. Hormonal regulation of growth during the first phase is actually the main field of scientific debate on salinity.
Photosynthesis and the rhythm of cell growth are the first processes to be compromised by salinity (Chaves et al. 2009; Munns et al. 2006). In fact, it is frequently reported that with salt stress as the stomatal resistance rises, due to leaf water potential reduction, photosynthetic assimilation decreases (Prior et al. 1992; Munns 2002; Lovelli et al. 2012; Rivelli et al. 2002). The observed reduction is caused by the effect of salts on each single photosynthetic sub-process (diffusion, photochemical, biochemical processes). The stress determined by the high concentration of solutes in soil water can determine both an increase of stomatal resistance and mesophyll resistance to gas flows, with a subsequent limitation of photosynthetic activity (Flexas et al. 2004, 2007; Lawlor and Cornic 2002). Salt stress effect on photosynthetic non-stomatal components have been studied also, but precise information on the topic are still few (Rivelli et al. 2002; Seemann and Critchely 1985). With “non stomatal limitations” words we usually consider both physical limitations, mesophyll resistances to CO2 diffusion in the gas and liquid phase, and bio-chemical limitations, mainly carboxylation rate and efficiency, to assimilation rate (Centritto et al. 2003). In addition as regards non-stomatal limitations to photosynthesis, in some cases they may generate confusion of interpretations (Centritto et al. 2003). The difficulty comes from the fact that, being numerous the factors regulating photosynthetic activity, it is particularly difficult to assess whether stomatal or non-stomatal effects prevail in response to salinity. Some studies pointed out that the assimilation activity drop, as a consequence of salt distribution to the crop, should be caused not only by stomatal closure, but mainly by ion actions at biochemical level. Na+ and Cl– ions can have a direct effect on photosynthetic apparatus because they reduce the efficiency of ribulose-1 5-bisphosphate carboxylase (Rubisco) in the Calvin’s cycle (Bethke and Drew 1992; Martin and Ruiz-Torres 1992). Many studies showed the strict correlations between increased salt concentration, such as Cl− and photosynthesis decrease (Paranychianakis and Chartzoulakis 2005; Lovelli et al. 2012; Lloyd et al. 1989; Walker et al. 1981; Chartzoulakis et al. 2002). Actually, it was shown that CO2 concentration in intercellular spaces does not change as an effect of salinity but it stay more or less the same, while stomatal opening decreases; that suggests that both stomata conductance and especially the non stomatal ones are reduced by the salts accumulated in the tissues. Actually understanding the nature of non stomatal limitations of photosynthesis under salinity is an heated field of photosynthesis research (Paranychianakis and Chartzoulakis 2005; Centritto et al. 2003).
Under severe salt stress, photosynthesis of tomato was deeply reduced, so in this way stressed plants had a lower amount of fixed carbon to utilize for plant growth (Lovelli et al. 2012). Lower stomatal conductance and photosynthesis observed in salt stressed tomato plants explain the lower leaf growth and consequently the smaller accumulation of dry matter (Lovelli et al. 2012). During osmotic stress (during the first phase) ABA contributes to salt response through an effective stomatal control (Hassine and Lutts 2010). Indeed, a strong relation between ABA tomato leaf concentration and stomatal conductance occurred (Lovelli et al. 2012).
One consequence of reduced photosynthesis is the overall plant growth reduction, but different parts of the tomato plant grow in different way. In fact we observed an unbalanced growth rhythm of root and shoot under salinity (Lovelli et al. 2012), in particular we showed the high root-to-shoot ratio and the close relationship to high abscisic acid (ABA) root concentration (Lovelli et al. 2012). As said before we refer to the biphasic model of Munns (1993) that considers the physiological and agronomic adaptation of plants to salts as temporal changes in both osmotic and ionic stress (Perez-Alfocea et al. 2010). Actually processes that regulate leaf growth and shoot development under the osmotic phase of salinity are under debate (Albacete et al. 2008), as said before.
During the first osmotic phase it has been hypothesized that inhibition of plant growth could be controlled by hormones or their precursors (Munns and Tester 2008; Lovelli et al. 2012), while later (ionic phase), plant growth is mainly reduced by high leaf salt (Na+ and Cl-) build up that in turn involves to whole plant photosynthesis reduction and partly induces premature leaf senescence (Perez-Alfocea et al. 2010).
If from one side stomatal control by ABA increase in leaf tissues was an important research field for long time, actually there are very few data on ABA partitioning among the different plant organs (Assmann 2004; Zhang et al. 2004; Lovelli et al. 2012). Actually the ABA function in growth control is particularly controversy (Albacete et al. 2008), as according to some authors it holds up plant growth (Dodd and Davies 1996, Zhang and Davies 1990), while according to others it favours it (Sharp and LeNoble 2002). Therefore, several contrasting opinions exist on ABA function in the biomass allocation under salt stress (Sachs 2005). Modifications of plant growth under salinity could be controlled by changes in phytohormone tissues concentrations controlling assimilate partitioning from source to sink organs (Perez-Alfocea et al. 2010; van der Werf and Nagel 1996; Hartig and Beck 2006). We known that the ratio between root and shoot dry matter is usually constant, since root system and epigeous plant part grow at the same rate (Lovelli et al. 2012). The balance of width between each part of the plant is provided by assimilation rate and carbohydrate partitioning, but it can be highly modified by stress conditions (Erice et al. 2010). The ratio between root and shoot dry matter increased with the rise of salt concentration in the nutrient solution. In tomato it was showed that salinity does not affect the usual partitioning of dry matter between fruits, shoot and root even when yield decrease reductions is about close to 25 % of the control (Ehret and Ho 1986). On tomato we showed the contrary (Lovelli et al. 2012). Our results on plant growth are similar to that obtained from other authors always on tomato under salinity (Albacete et al. 2008; Maggio et al. 2007). It is clear now that salinity slows down cellular division and growth (Albacete et al. 2008). It happened that some morphological characters of crucial interest in stress adaptation, such as root growth and stomatal behaviour, have been less studied (Maggio et al. 2007). Sharp and LeNoble (2002) and Spollen et al. (2000) showed that higher root growth in conditions of low water potential is strictly related to ABA increase in the root tissues, and that the keeping of root growth under very negative water potential is controlled by ABA accumulation in roots.
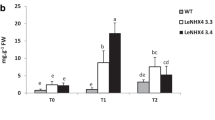
All these results strengthened the idea that photosynthate utilisation rather than its availability is the main factor that limit plant growth under salt stress and assign an important function to hormonal signalling between plant organs (Perez-Alfocea et al. 2010). Moreover channelling assimilates from leaves to the roots (Perez-Alfocea et al. 2010) could be considered a particular choice of the plant, without meaning. At the same time this adaptive plant behaviour allows the plant roots to take out more water and uptake nutrients from the soil and allows to maintain ionic homeostasis, so it have a clear significance from the ecological point of view within salinised environment (Perez-Alfocea et al. 2010). The increase of root/shoot ratio in tomato is likely an effective physiological process that allow to regulate ion increase into tissues under salt stress (Fig. 10.1; Lovelli et al. 2012).
Dry matter (a) root shoot ratio (b) measured in tomato plants subjected to two levels of salt stress (100 and 150 mM of NaCl, respectively), (c) ABA concentration in leaves and roots of tomato plants under two levels of salt stress. Mean value (n = 5) within a column followed by different capital and lower case letters are significantly different at P < 0.01 and P < 0.05, respectively according to Duncan’s multiple range test
10.4 Role of Abscisic Acid (ABA) and Other Phytohormones Under Salinity
Since plants are sessile organisms, for them having and efficient system of response to the changing environment is crucial for surviving. First discovered group of plant hormones includes auxin, gibberellins (GAs), cytokinins, abscisic acid (ABA) and ethylene. Only recently another group of compounds such as brassinosteroids (BRs), jasmonate (JA), salicylate (SA), strigolactones (SLs), nitric oxide (NO), polyamines, and some oligopeptides were recognized as new families of plant hormones (Javid et al. 2011; Santner and Estelle 2010). Notwithstanding phythormones were studied for many years, interactions that occur between them are still unclear (Ross and O’Neill 2001). Vanguard of the research on this field regards the modalities through which plant hormones are involved in multiple processes and if the so-called “cross- talk” between different hormones results in synergetic or antagonistic interactions in response of plants to abiotic stress (Peleg and Blumwold 2011; Zhu et al. 2012; Gemes et al. 2011).
Under salinity plant response is triggered by osmotic signals (Chaves et al. 2003) or by other compounds (hormones, reactive oxygen species and intracellular second messengers) (Chaves et al. 2009). Surely between them abscisic acid (ABA) has an important function in the whole plant responses to salt stress (Zhang et al. 2006). Generally, ABA operates as a general inhibitor of growth and metabolism, and negatively affects the synthesis of proteins and nucleic acids, even if these actions vary with tissue, developmental stage and the concentrations of this hormone increase substantially under stress conditions (Sofo et al. 2011; Yuan et al. 2011; Kobashi et al. 2001; Srivastava 2002). The changes of the endogenous levels of ABA also stimulate different metabolic and physiological events that increase the level of tolerance to salts (Munns and Tester 2008; Xiong et al. 2002). However, in many stress conditions other hormones (ethylene, cytokinins, auxins) are involved also, and in particular, their biosynthesis could be considered an appropriate indicator of the plant health. Under salinity other hormones such as gibberellins can interact with ABA and other stress metabolites including antioxidants and ROS scavengers (Achard et al. 2006).
Recently it was underlined that all too often ABA is considered “the stress hormones”, while other phytohormones such as cytokinins and auxins seems involved in explaining changes in plant biomass partitioning (Albacete et al. 2008; Javid et al. 2011). Notwithstanding it is frequently reported that salinity triggers off ABA synthesis in roots which is relocated to the shoots where it causes stomatal closure (Chaves et al. 2009). ABA can also be produced in leaf cells and then transported in other part of the plant (Wilkinson and Davies 2002). With regards to this aspect, recently it was showed that xylem and apoplastic pH affects ABA movements into plant tissues and in this way it seems to control the levels of ABA reaching the stomata (Jia and Davies 2007). The “alkaline trapping” of ABA may be triggered by salts also (Jia and Davies 2007). ABA concurs in salt response during the osmotic phase through an effective enhancement of stomatal control (Hassine and Lutts 2010). Stomatal control by ABA accumulation in leaf tissues was an important research field for years but data on ABA effects on dry matter partitioning among the different plant organs are lacking (Assmann 2004; Zhang et al. 2004). Modifications of ABA concentrations between leaves and roots may be accountable for the relative changes in growth ratios and biomass partitioning caused by salt stress (Lovelli et al. 2012). Leaf and stem dry matter decrease can be related to a redistribution of photosynthetates to the root system (Maggio et al. 2007) mediated by ABA signaling (Albacete et al. 2008). The few available experimental data on tomato (Albacete et al. 2008; Ghanem et al. 2008) are in agreement with our results (Lovelli et al. 2012), but they disagree with results of other authors (Mulholland et al. 2003; Maggio et al. 2007). In tomato under advanced salinization, the high ABA root levels could regulate organ adaptation, such as dry matter partitioning (Albacete et al. 2008) and usual alteration of the root/shoot ratio (Maggio et al. 2007). It is possible to suppose that similar to its action in the shoot, ABA accumulation may also be useful to keep root growth in salt stressed plants (Albacete et al. 2008). Sharp and LeNoble (2002) and Spollen et al. (2000) clearly showed that higher root growth under low water potential is associated with high ABA levels in the roots, and that the maintaining of root growth under low water potential is controlled by ABA accumulation in roots. Build up of ABA root concentration and root/shoot ratio observed under salt conditions (Lovelli et al. 2012) seems to strength our hypothesis. Some authors (Albacete et al. 2008) also supposed that ABA is related with inhibition of ethylene production, which is sometimes considered a growth inhibitor under stress. It is a clear example of cross-talk between plant hormones during plant response to salt stress, as said before. It could justify how a single hormone produces different effects in different plant organs, in other words in each organ this compound interacts in different modality with the other hormones that are at the sometime present (Ross and O’Neill 2001). Some authors (Ghanem et al. 2011b) observed that the root cytokinin production deeply reduces both ABA and Na+ build up in the root and other organs without modifying root dry matter under moderate salinity (100 mM NaCl). Another possible explanation could be that given by Zhang et al. (2006). According to this author it is possible to give to ABA a dual function in plant physiological control. That is an inhibitive function when it accumulated at high concentration under stress, and a promoting function when it is at low amount in plant tissues. We observed high ABA root tissue amount in correspondence of high Na+ and Cl– root level (Lovelli et al. 2012), so it is possible to hypotize its inhibition function, as other authors reported (Sharp and LeNoble 2002).
In any case, higher root/shoot growth might be interpreted as part of an adaptation behaviour in which plant physiological and metabolic modifications evolve together with plant development, soil salinization and atmospheric parameters during the crop cycle (Maggio et al. 2011). Different tissue root and leaf ABA may have an important function in controlling growth, leaf gas exchange and dry matter partitioning of salinized tomato plants (Lovelli et al. 2012).
10.5 Tomato Root Architecture Modification Under Salinity
Considering its role function in absorbing water and nutrients, the root system is the main part of the plant to meet soil salinity (Ouyang et al. 2007), and likely plays an important role to cope with salts. In particular how salts affects root growth and architecture is of great importance to elucidate mechanisms for plant adaptation process to salinity.
The role of the roots and their function in mediating shoot responses to abiotic stresses such as salinity, was recently emphasised (Ghanem et al. 2011a). Root morphology such as root system architecture should be thoroughly investigated to improve plant development under environmental stress conditions (Ghanem et al. 2011a) because, currently, there are very few information on root architecture/morphology under salt stress (Maggio et al. 2011).
Root growth traits reduction associated to salinity agree with the results of several authors (Schwarz and Grosch 2003; Kafkafi 1996). On tomato we measured a reduction of total root weight and length in salt treatments and a large increase in specific root length (SRL) compared to the control (Lovelli et al. 2012 submitted). Snapp and Shennan (1992) observed no modification of Root Length Density in hydroponically-grown tomato plants under salinity. Recently both a root fresh weight reduction (30 %) was observed on tomato after 3 weeks under saline conditions (Albacete et al. 2008) and a root dry matter reduction under salinity together with a root/shoot increase (Lovelli et al. 2012). According to Cuartero and Fernandez-Munoz (1999) salinity deeply affects root biomass of tomato, but other authors (Abrisqueta et al. 1991) showed that tomato root biomass grown under salinity conditions, have only a delay in reaching a depth of 80 cm and the end root length density is a quarter than in control plants.
Also on other crops there are contrasting results. Considering faba bean growth on salinized soil root length density and root mass density are deeply reduced as effect of salts (Abdelhamid et al. 2010), while on soil-grown alfalfa some authors (Vaughan et al. 2002) showed that root production was stimulated by salinity. These contrasting results may depend also by confusion that comes from heterogeneous growth condition under salinity on soil-grown plants. This is an enormous problem of salinity experiments, each time we want to impose salinity condition in an artificial we may meet difficulties that can generate confusing results. In fact when plants are grown under salinity soil compaction could affect plant growth by causing increased resistance to root penetration and the resulting different mechanisms of salt damage may be very different as the result of the system under which the plants were grown (Tavakkoli et al. 2011). In order to avoid confusing results it is important to separate salt stress from other soil abiotic stress, eliminating soil component and this can be done only growing plant in hydroponics.
In our experiment on hydroponically-growth plants analyzing root length density along the depth we found a significant interaction between salinity and root depth on specific root length (SRL; Lovelli et al. 2012 submitted). A root system with a high SRL in high salinity conditions could be considered an adaptative response that gives to plants the possibility to growth better in the soil volume (Bazzaz and Morse 1991; Snapp and Shennan 1992), and to delay toxic ions accumulation in plant shoots (Maggio et al. 2007) keeping a right degree of ions homeostasis. Moreover on other crops some authors pointed out that differential rooting was higher in the upper half of the root zone on alfalfa soil grown plants, and that high fibrous rooting in alfalfa is a character to interpret as a salt stress avoidance behaviour (Vaughan et al. 2002). All these recent experiments on root architecture modification under salinity seems to be of great help for elucidation of mechanisms for tomato adaptation to salt stress on whose significance there are still many aspect to clarify.
On tomato it was observed that salt or other abiotic stresses may affect different roots to a different extent (Cuartero and Fernandez-Munoz 1999). Other researches showed that under stress tomato usually grows numerous small lateral feeder roots, which are not present in tomato plants growth in non-stress conditions (Zobel 1975). Moreover in our experiment in salt treatments we observed a particular root diameter distribution. Under severe salt stress we measured a significant amount of tomato roots belonging to the lower diametric class (0–0.5 cm) (Fig. 10.2; Lovelli et al. 2012 submitted). Increased Specific Root Length (SRL) usually associates with low average root diameters (Schwarz et al. 1995; Schwarz and Grosch 2003). These last results are in agreement with other authors (Kurth et al. 1986; Sharp et al. 1990) that observed thinner roots in cotton and maize, respectively, under high level of salinity. In general the increase of Specific Root Length (SRL) under salinity reflects differences in diameter distribution and may be used as an indicator of plant response to management (Basirat et al. 2011) or environmental change (Ostonen et al. 2007).
Moreover modifications in the root class diameter distribution may be considered as a mechanism of adaptation to salinity, thinner roots allow osmotic adjustment without alteration of fixed carbon partitioned to roots (Snapp and Shennan 1992).
10.6 Conclusion and Future Perspective
From recent research activity on tomato, it can be concluded that new elements have emerged that are useful in the elucidation of mechanisms of salt adaptation and tolerance. Source – sink regulation and root-to-shoot signaling are interconnected mechanisms that allow tomato plants to increase salt tolerance since they allow to maintain growth and delay leaf senescence during the first phase of salt response (osmotic one; Perez-Alfocea et al. 2010).
In biomass partitioning plant hormones plays a crucial role. As regards tomato different endogenous ABA at root and leaf level are key aspects in growth control, leaf gas exchange and dry matter partitioning of salt-stressed plants (Lovelli et al. 2012), even if it seems that in the complex plant hormonal network cross-talk between hormones may result in synergetic or antagonic interactions in response to one stress (Peleg and Blumwold 2011).
The role of root architecture in tomato response to salinity is still unclear, but it likely plays an important role. In hydroponically grown tomato plants under high salinity (150 mM NaCl) we observed decrease in root weight, depth and length density but an increase in specific root length, corresponding to an increase in fine roots in the middle part of the root system.
Although this chapter on tomato biomass partitioning under salinity covers only a part of a very complex scientific field of research it is clear that physiological approach is still a powerful tool for analyzing the complex process that is plant adaptation to salts. Several authors (Jacobsen et al. 2012; Boote et al. 2001; Hunt et al. 2003; Martre et al. 2003) think that only integrating knowledge from different approaches (plant physiology, soil science and agrometeorology) into mathematical equations, through models it is possible to forecast plant response and yield in stress conditions. So many efforts would be addressed to create synergies between scientific research groups and to develop a multidisciplinary approach for the salinity stress problematic (Wollenweber et al. 2005) in order to give a further chance to agriculture in areas affected by salinization.
References
Abdelhamid MT, Shokr MMB, Bekheta MA (2010) Growth, root characteristics, and leaf nutrients accumulation of four faba bean (Vicia faba L.) cultivars differing in their broomrape tolerance and the soil properties in relation to salinity. Commun Soil Sci Plan 41:2713–2728
Abrisqueta JM, Hernansaenz A, Alarcon JJ, Lozano MA (1991) Root growth dynamics of two tomato genotypes under saline conditions. Suelo y Planta 1:351–361
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Ven der Straeten D, Peng J, Herberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 6:91–94
Albacete A, Ghanem ME, Martinez-Andujar C, Acosta M, Sanchez-Bravo J, Martinez V, Lutts S, Dodd IC, Perez-Alfocea F (2008) Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 59:4119–4131
Assmann SA (2004) Abscisic acid signal transduction in stomatal responses. In: Davis PJ (ed) Plant hormones: biosynthesis, signal transduction, action. Kluwer, London, pp 291–442
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58
Basirat M, Malboobi MA, Mousavi A, Asgharzadeh A, Samavat S (2011) Effects of phosphorous supply on growth, phosphate distribution and expression of transporter genes in tomato plants. Aust J Coltural Sci 5(5):537–543
Bazzaz FA, Morse SR (1991) Annual plants: potential for response to multiple stresses. In: Mooney HA, Winner WE, Dell EJ (eds) Response of plants to multiple stresses. Academic, New York, pp 283–305
Bethke PC, Drew MC (1992) Stomatal and non stomatal components to inhibition of photosynthesis in leaves of Capsicum annum during progressive exposure to NaCl salinity. Physiol Plant 86:115–123
Boote KJ, Kropff MJ, Bindraban PS (2001) Physiology and modeling of traits in crop plants: implications for genetic improvement. Agric Syst 70:395–420
Centritto M, Loreto F, Chartzoulakis K (2003) The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant Cell Environ 26:585–594
Chartzoulakis K, Loupassaki M, Bertaki M, Androulakis I (2002) Effects of NaCl salinity on growth, ion content and CO2 assimilation rate of six olive cultivars. Sci Hortic 96:235–247
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought – from genes to the whole plant. Funct Plant Biol 30:239–264
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. J Exp Bot 103:551–560
Cuartero J, Fernandez-Munoz R (1999) Tomato and salinity. Sci Hortic 78:83–125
De Pascale S, Martino A, Raimondi G, Maggio A (2007) Comparative analysis of water and salt stress-induced modifications of quality parameters in cherry tomato. J Hort Sci Biotechnol 82:283–289
Dodd IC, Davies WJ (1996) The relationship between leaf growth and ABA accumulation in the grass leaf elongation zone. Plant Cell Environ 19:1047–1056
Dorais M, Ehret DL, Papadopoulos AP (2008) Tomato (Solanum lycopersicum) health components: from the seed to the consumer. Phytochem Rev 7:231–250
Ehret DL, Ho LC (1986) The effects of salinity on dry matter partitioning and fruit growth in tomatoes grown in nutrient film culture. J Horti Sci 61:361–367
Erice G, Louahlia S, Irigoyen JJ, Sanchez-Diaz M, Avice JC (2010) Biomass partitioning, morphology and water status of four alfalfa genotypes submitted to progressive drought and subsequent recovery. J Plant Physiol 167:114–120
Favati F, Lovelli S, Galgano F, Di Tommaso T, Miccolis V, Candido V (2009) Processing tomato quality as affected by irrigation scheduling. Sci Hortic 122:562–571
Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD (2004) Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol 6:269–279
Flexas J, Diaz-Espejo A, Galme’s J, Kaldenhoff H, Medrano A, Ribas-Carbo M (2007) Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ 30:1284–1298
Flowers TJ, Flowers SA (2005) Why does salinity pose such a difficult problem for plant breeders. Agric Water Manage 78:15–24
Foolad MR (2004) Recent advances in genetics of salt tolerance and cold tolerance in tomato. Plant Cell Tiss Org 76:101–119
Gemes K, Poor P, Horvath E, Kolbert Z, Szopko D, Szepesi A, Tari I (2011) Cross-talk between salicylic acid and NaCl-generated reactive oxygen species and nitric oxide in tomato during acclimation to high salinity. Physiol Plant 142:179–192
Ghanem ME, Albacete A, Martínez-Andújar C, Acosta M, Romero-Aranda R, Dodd IC, Lutts S, Pérez-Alfocea F (2008) Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). J Exp Bot 59:3039–3050
Ghanem ME, Albacete A, Smigocki AC, Frebort I, Pospisilova H, Martinez-Andujar C, Acosta M, Sanchez-Bravo J, Lutts S, Dodd IC, Perez-Alfocea F (2011a) Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 62:125–140
Ghanem ME, Hichri I, Smigocki AC, Albacete A, Fauconnier ML, Diatloff E, Martinez-Andujar C, Lutts S, Dodd IC, Perez-Alfocea F (2011b) Root-targeted biotechnology to mediate hormonal signalling and improve crop stress tolerance. Plant Cell Rep 30:807–823
Gregory PJ (2006) Food production under poor, adverse climatic conditions. In: Proceedings of the IX ESA Congress, Warsaw, 4–7 Sept 2006, 19pp
Hartig K, Beck E (2006) Crosstalk between auxin, cytokinins and sugars in the plant cell cycle. Plant Biol 8:389–396
Hassine AB, Lutts S (2010) Differential responses of saltbush Atriplex halimus L. exposed to salinity and water stress in relation to senescing hormones abscisic acid and ethylene. J Plant Physiol 167:1440–1456
Hunt LA, Reynolds MP, Sayre KD, Rajaram S, White JW, Yan W (2003) Crop modelling and the identification of stable coefficients that may reflect significant groups of genes. Agron J 95:20–31
IPCC, Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (2007) IPCC summary for policymakers. In: Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, UK/New York
Jacobsen S-E, Jensen CR, Liu F (2012) Improving crop production in the arid Mediterranean climate. Field Crop Res 128:34–47
Javid MG, Sorooshzadeh A, Moradi F, Sanavy SAMM, Allahdadi I (2011) The role of phytohormones in alleviating salt stress in crop plants. Austr J Crop Sci 5:726–734
Jia W, Davies WJ (2007) Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals. Plant Physiol 143:68–77
Kafkafi U (1996) Root growth under stress-salinity. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, ed, 2nd edn. Marcel Dekker, New York, pp 375–391
Kobashi K, Sugaya S, Gemma H, Iwahori S (2001) Effect of abscisic acid (ABA) on sugar accumulation in the fresh tissue of peach fruit at the start of the maturation stage. Plant Growth Regul 35:215–223
Koushafar M, Khoshgoftarmanesh AH, Moezzi A, Mobli M (2011) Effect of dynamic unequal distribution of salts in the root environment on performance and crop per drop (CPD) of hydroponic-grown tomato. Sci Hortic 131:1–5
Koyro HW, Ahmad P, Nicole G (2012) Abiotic stress responses in plants: an overview. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer Science+Business Media, New York, pp 1–28
Kurth E, Cramer GR, Lauchli A, Epstein E (1986) Effects of NaCl and CaCl2 on cell enlargement and cell production in cotton roots. Plant Physiol 82:1102–1106
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Lloyd J, Kriedemann PE, Aspinall D (1989) Comparative sensitivity of Prior Lisbon lemon and Valencia orange trees to foliar sodium and chloride concentrations. Plant Cell Environ 12:529–540
Lovelli S, Perniola M, Di Tommaso T, Ventrella D, Moriondo M, Amato M (2010) Effects of rising atmospheric CO2 on crop evapotranspiration in a Mediterranean area. Agric Water Manage 97:1287–1292
Lovelli S, Scopa A, Perniola M, Di Tommaso T, Sofo A (2012) Abscisic acid root and leaf concentration in relation to biomass partitioning in salinized tomato plants. J Plant Physiol 169:226–233
Maggio A, Raimondi G, Martino A, De Pascale S (2007) Salt stress response in tomato beyond the salinity tolerance threshold. Env Exp Bot 59:276–282
Maggio A, De Pascale S, Fagnano M, Barbieri G (2011) Saline agriculture in Mediterranean environments. Ital J Agron 6:36–43
Martin B, Ruiz-Torres N (1992) Effects of water-deficit stress on photosynthesis, its components and component limitations and on water use efficiency in wheat (Triticum aestivum L.). Plant Physiol 100:733–739
Martre P, Porter JR, Jamieson PD, Triboi E (2003) Modeling grain nitrogen accumulation and protein composition to understand the sink/source regulation of nitrogen remobilization for wheat. Plant Physiol 133:1959–1967
Miflin B (2000) Crop improvement in the 21st century. J Exp Bot 51:1–8
Mitchell JP, Shennan C, Grattan SR, May DM (1991) Tomato fruit yields and quality under water deficit and salinity. J Am Soc Hort Sci 116:215–221
Monteleone M (2006) Salinity management in southern Italy irrigation areas. Ital J Agron 1:129–202
Mulholland BJ, Taylor IB, Jackson IC (2003) Can ABA mediate responses of salinity of stressed tomato. Env Exp Bot 50:17–28
Munns R (1993) Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ 16:15–24
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Munns R, James RA, Lauchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043
Okhovatian-Ardakani AR, Mehrabanian M, Dehghani F, Akbarzadeh A (2010) Salt tolerance evaluation and relative comparison in cuttings of different pomegranate cultivars. Plant Soil Environ 56:176–185
Olesen JE, Bindi M (2002) Consequences of climate change for European agricultural productivity, land use and policy. Eur J Agron 16:239–262
Ostonen I, Püttsepp U, Biel C, Alberton O, Bakker MR, Lõhmus K, Majdi H, Metcalfe D, Olsthoorn AFM, Pronk A, Vanguelova E, Weih M, Brunner I (2007) Specific root length as an indicator of environmental change. Plant Biosyst 141(3):426–442
Ouyang B, Yang T, Li H, Zhang L, Zhang Y, Zhang J, Fei Z, Ye Z (2007) Identification of early salt stress response genes in tomato root by suppression subtractive hybridization and microarray analysis. J Exp Bot 58:507–520
Paranychianakis NV, Chartzoulakis KS (2005) Irrigation of Mediterranean crops with saline water: from physiology to management practices. Agric Ecosyst Environ 106:171–187
Peleg Z, Blumwold E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295
Perez-Alfocea F, Albacete A, Ghanem ME, Dodd IC (2010) Hormonal regulation of source-sink relations to maintain crop productivity under salinity: a case study of root-to-root signalling in tomato. Funct Plant Biol 37:592–603
Prior LD, Grieve AM, Slavish PG, Gullis PR (1992) Sodium chloride and soil texture interactions in irrigated field grown Sultana grapevines. II. Plant mineral content, growth and physiology. Aust J Agric Res 43:1067–1084
Rivelli AR, Lovelli S, Perniola M (2002) Effects of salinity on gas exchange, water relations and growth of sunflower (Helianthus annuus L.). Funct Plant Biol 29:1405–1415
Ross J, O’Neill D (2001) New interactions between classical plant hormones. Trends Plant Sci 6:2–4
Rozema J, Flowers T (2008) Crops for a salinized world. Science 322:1478–1480
Sachs T (2005) Auxin’s role as an example of the mechanisms of shoot/root relations. Plant Soil 268:13–19
Santner A, Estelle M (2010) The ubiquitin-proteasome system regulates plant hormone signalling. Plant J 61:1029–1040
Schwarz D, Grosch R (2003) Influence of nutrient solution concentration and root pathogen (Pythium aphanidermatum) on tomato root growth and morphology. Sci Hortic 97:109–120
Schwarz D, Heinen M, Van Noordiwijk M (1995) Rooting characteristics of lettuce grown in irrigated sand beds. Plant Soil 176:205–217
Seemann JR, Critchely C (1985) Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 164:151–162
Sharp RE, LeNoble ME (2002) ABA ethylene and the control of shoot and root growth under water stress. J Exp Bot 53:33–37
Sharp RE, Hsiao TC, Silk WK (1990) Growth of the maize primary root at low water potentials. II. Role of growth and déposition of hexose and potassium in osmotic adjustment. Plant Physiol 93:1337–1346
Snapp SS, Shennan C (1992) Effects of salinity on root growth and death dynamics of tomato, Lycopersicon esculentum Mill. New Phytol 121:71–79
Sofo A, Scopa A, Manfra M, De Nisco M, Tenore G, Troisi J, Fiori RD, Novellino E (2011) Trichoderma harzianum strain T-22 induces changes in phytohormone levels in cherry rootstocks (Prunus cerasus x P.canescens). Plant Growth Regul 65:421–425
Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE (2000) Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol 122:967–976
Srivastava LM (2002) Plant, growth and development – hormones and environment. Elsevier Academic Press, San Diego, pp 307–314
Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, McDonald GK (2011) Additive effects of Na+ and Cl− ions on barley growth under salinity stress. J Exp Bot 62:2189–2203
Van der Werf A, Nagel OW (1996) Carbon allocation to shoots and roots in relation to nitrogen supply is mediated by cytokinins and sucrose: opinion. Plant Soil 185:21–32
Vaughan LV, MacAdam JW, Smith SE, Dudley LM (2002) Root growth and yield of differing alfalfa rooting populations under increasing salinity and zero leaching. Crop Sci 42:2064–2071
Vitale D, Rana G, Soldo P (2010) Trends and extremes analysis of daily weather data from a site in the Capitanata plain (Southern Italy). Ital J Agron 5:133–143
Walker RP, Torokfalvy E, Scott NS, Kriedemann PE (1981) An analysis of photosynthetic response to salt treatment in Vitis vinifera. Aust J Plant Physiol 8:359–374
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25:195–210
Wollenweber B, Porter JB, Lubberstedt T (2005) Need for multidisciplinary research towards a second green revolution. Curr Opin Plant Biol 8:337–341
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought and salt stress. Plant Cell 14(Suppl 1):165–183
Yadav S, Irfan M, Ahmad A, Hayat S (2011) Causes of salinity and plant manifestations to salt stress: a review. J Environ Biol 32:667–685
Yeo AR (2007) Salinity. In: Yeo AR, Flowers TJ (eds) Plant solute transport. Blackwell, Oxford, pp 340–365
Yuan K, Rashotte AM, Wysocka-Diller JW (2011) ABA and GA signaling pathways interact and regulate seed germination and seedling development under salt stress. Acta Physiol Plant 33:261–271
Zhang H-X, Blumwald E (2001) Transgenic salt tolerant tomato plants accumulate salt in the foliage but not in the fruits. Nat Biotech 19:765–768
Zhang J, Davies WJ (1990) Does ABA in the xylem control the rate of leaf growth in soil-dried maize and sunflower plants. J Exp Bot 41:1125–1132
Zhang J, Jia W, Yang J (2004) ABA in plant water stress signalling. Proc Indian Natl Sci Acad B 70:367–377
Zhang J, Jia W, Yang J, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crop Res 97:111–119
Zhu M, Dai S, Chen S (2012) The stomata frontline of plant interaction with the environment-perspectives from hormone regulation. Front Biol 7:96–112
Zobel RW (1975) The genetics of the root development. In: Torrey JG, Clarkson DF (eds) The development and function of roots. Academic, London, pp 261–275
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Lovelli, S., Sofo, A., Perniola, M., Scopa, A. (2013). Abscisic Acid and Biomass Partitioning in Tomato Under Salinity. In: Ahmad, P., Azooz, M., Prasad, M. (eds) Ecophysiology and Responses of Plants under Salt Stress. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4747-4_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4747-4_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4746-7
Online ISBN: 978-1-4614-4747-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)