Abstract
It is difficult if not impossible to determine when mankind first systematically applied color to a textile substrate. The first colored fabrics were probably nonwoven felts painted in imitation of animal skins. The first dyeings were probably actually little more than stains from the juice of berries. Ancient Greek writers described painted fabrics worn by the tribes of Asia Minor. But just where did the ancient craft have its origins? Was there one original birthplace or were there a number of simultaneous beginnings around the world?
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Dyeing
It is difficult if not impossible to determine when mankind first systematically applied color to a textile substrate. The first colored fabrics were probably nonwoven felts painted in imitation of animal skins. The first dyeings were probably actually little more than stains from the juice of berries. Ancient Greek writers described painted fabrics worn by the tribes of Asia Minor. But just where did the ancient craft have its origins? Was there one original birthplace or were there a number of simultaneous beginnings around the world?
Although it is difficult to determine just when each respective civilization began to use dyes, it is possible to date textile fragments and temple paintings, which have survived the ensuing centuries. The ancient Egyptians wove linen as early as 5000 bc, and paintings on tomb walls infer that colored wall hangings were in use by 3000 bc. By 2500 bc, dyer’s thistle and safflower were used to produce red and yellow shades. Egyptian dyers developed a full range of colors by 1450 bc.
Another cradle of civilization was the Indian subcontinent where religious and social records dating to 2500 bc refer to dyed silk and woven brocades of dyed yarn. Cotton, first cultivated in the Indus valley of Pakistan was woven as early as 2000 bc. A book written around 300 bc included a chapter on dyes. It is believed that systematic dyeing occurred in China as early as 3000 bc near the city of Xian in the Huang (Yellow) River Valley, although there is no conclusive proof. Empress Si-Ling-Chi is credited with the discovery of silk about 2640 bc. Kermes and indigo were used as dyes as early as 2000 bc. Fragments of silk have been found in the corrosive patina of bronze swords of the Shang dynasty (1523–1027 bc), but most assuredly these samples are not the oldest [1].
The New World was similarly active in developing the textile art. With help from the desert climate in the high Andes of Peru, dyed samples of wool have been preserved and recovered from burial sites. These fragments have been dated to the millennium before the Christian era. The western and southwestern regions of the United States provided homes for the Anasazi, or ancient ones, who dwelt in the region of Mesa Verde National Park in southwestern Colorado, northern Arizona and New Mexico, and eastern Utah. Again the dry climate has helped to preserve samples from these early civilizations.
Very little in the nature of large, intact textile samples has survived in Europe. Remains of a large woolen robe, the Thorsberg Robe, found in northern Germany and dated prior to 750 bc, indicate a highly developed dyeing and weaving technology [2]. Indirect evidence is more plentiful: for example, a tombstone of a purpurarius, a Roman purple dyer, was found near Parma, northern Italy, and a dyer’s workshop excavated in Pompeii. This great center of the Roman Empire was destroyed by the eruption of Mount Vesuvius in 79 ad. Similar stone vats for dyeing have been excavated in the tells of Israel and in present-day Turkey.
The Dark Ages following the fall of the Roman Empire were dark indeed, with little development of the dyer’s art. The robes of a number of the monastic orders were brown and black, surely a dark age. By the end of the 1300s, however, civilization began making the swift and certain strides that have led to our present level of development. In 1371, the dyers of Florence, a city famous for its Renaissance art, formed a guild, or association of like merchants and craftsmen, which lasted for 11 years. Other guilds were being formed in other centers across Europe. Some of these guilds exist to this day. The Worshipful Company of Dyers was formed in 1471 in London. One of the legacies of this guild is a Publications Trust, which has underwritten the publication of a number of books on dyeing in cooperation with The Society of Dyers and Colourists (SDC).
The art and craft of dyeing was largely passed down from father to son or from craftsman to apprentice by word of mouth and example until the early 1500s. The Plictho of Gioanventura Rosetti, a Venetian armory superintendent, is believed to be the first published book on dyeing. It certainly is the oldest surviving European text to have come down to us in the twenty-first century. Five known Italian editions were published between 1548 and 1672. A French edition appeared in 1716. It is interesting that no known English translation was made until 1968 [3] when Sidney Edelstein of the Dexter Chemical Company and Hector Borghetty collaborated to reproduce a facsimile of the original 1548 edition along with a complete translation into English. During his extensive travels, Rosetti collected dyeing recipes and processes used in the flourishing city states of Venice, Genoa, and Florence. He published: Plictho de L’arte de Tentori che insegna tenger pani telle banbasi et sede si per larthe magiore come per la comune or Instructions in the Art of the Dyers, Which Teaches the Dyeing of Woolen Cloths, Linens, Cottons, and Silk by the Great Art as Well as by the Common or simply Instruction in the Art of Dyeing. The book was divided into four sections: the first and second sections were devoted to the dyeing of wool, cotton, and linen; the third to the dyeing of silk and the use of fugitive colors; and the fourth to the dyeing of leather and skins. Approximately 160 complete recipes were preserved in the first three sections. Edelstein and Borghetty labored diligently in determining the meanings of terms in recipes written in the dialect of sixteenth century Italy.
Synthetic Dyes
The father of modern synthetic dyes was William Henry Perkin (1838–1907), who synthesized mauve, or aniline purple, in 1856. The story behind this great story bears telling. William’s father was a builder who wanted him to become an architect, but like many others, Perkin did not follow his father’s chosen profession. Perkin studied at the City of London School where he became interested in chemistry at the age of 12. A teacher, Mr. Hall, gave him work in the laboratory, which in turn, inspired Perkin to follow his natural curiosity. At age 15, Perkin entered the Royal College of Science and listened to the lectures of the great German chemist, August Wilhelm von Hofmann (1818–1892). He was granted an assistantship under von Hofmann at age 17. Because his work did not allow time for his own research, he set up a separate laboratory at home and it was there that he discovered aniline purple, the first dyestuff to be commercially produced. Another dye, based on naphthalene, and prepared in collaboration with Arthur H. Church, actually preceded aniline purple, but was not commercially produced before aniline purple. Aniline purple was discovered at this home during Easter vacation while looking for quinine, an antimalarial drug. After oxidizing aniline with potassium dichromate and getting a black precipitate, extraction with ethanol gave a brilliant purple solution. Almost immediately, he sent a sample of this dye to a dyer in Perth with a request to dye silk fabric. The dyer’s report read: “If your discovery does not make the goods too expensive, it is decidedly one of the most valuable that has come out for a long time.” Trials on cotton were not as successful because the need for a mordant was not realized. Perkin later reported, “The value of mauve was first realized in France, in 1859. English and Scotch calico printers did not show any interest in it until it appeared on French patterns, although some of them had printed cloth for me with that colour.” [4]
Since that beginning, thousands of dyes have been synthesized; some 1,500–2,000 are commercially successful today. Until 1884, however, all synthetic dyes required a mordant to give acceptable wash fastness on the textile substrate. In 1884, Böttiger produced Congo Red, which could dye cotton directly without a mordant. These dyes were commonly called direct dyes. In order to improve washfastness, the path taken in some synthetic dye chemistry was to build the dye from two or more components, directly in the fiber, or in situ. By building a large molecule without solubilizing groups within the fiber, washfastness was markedly improved. The first practical development along these lines was by A. G. Green who synthesized primuline, a dye that because of poor light fastness was not commercially important but later led the way to many important commercial dyes. Table 13.1 lists a number of classes of dyes along with the date of discovery. Worldwide, 80% of all dyes go into textiles and 20% into paper, leather, food, and the like.
The Development of the US Dyestuff Industry
The natural dyes industry was more than just a cottage industry in Colonial America. Indigo was a very important cash crop in South Carolina among the coastal islands and for some distance inland. Plantations existed well into the early 1900s despite the growth of the synthetic dyestuff industry.
The modern synthetic dye industry in the United States dates from World War I. However, in 1864, Thomas Holliday of Great Britain, and in 1868, the Albany (NY) Aniline Company with participation of Bayer of Germany began coal-tar dye manufacture. In the early 1900s, most synthetic dyes used in the United States were imported from Germany and Switzerland. With the outbreak of World War I, the British naval blockade of Germany prevented export of dyes from Europe. In spite of the blockade, the German submarine, Deutschland, ran the British blockade and sailed into American ports twice with dyestuffs and drugs. The Germans needed critical war material and export moneys; the United States and others needed dyes. Ironically, in 1914, German dyes were used by French dyers to dye the official French Army uniforms. The outbreak of war and ensuing blockade showed the United States how important dyes were to the American economy. Several companies began investigative work that would lead to dye synthesis; they found that dyestuffs were very difficult to make; the chemistry was much more complex than imagined. A real boost to the US industry came after World War I, when the German patents were given over to the Allies via the Alien Property Custodian. According to Lehner, DuPont reportedly spend $43 million, a tremendous sum of money in the early twentieth century, before ever showing a profit [5]. Obviously, only financially strong companies could afford to enter the business. The early pioneers included Allied (formed by merging five companies), American Cyanamid, and DuPont, to name only a few who survived to become major factors later in the twentieth century. In 1938, others included Dow, German-owned General Aniline and Film (GAF), and Swiss-owned Cincinnati Chemical Company (Ciba, Geigy, and Sandoz). In the 1960s, 50–60% of all US manufacturing was in the hands of four principal US companies:
-
Allied Chemical (later sold to Bayer of Germany and to independent investors as Buffalo Color, 1977)
-
American Cyanamid
-
GAF, the result of the break-up of the German cartel, I. G. Farben, which was nationalized during World War II and sold to BASF in 1978
-
DuPont (sold in 1980–1981 with various lines going to Crompton & Knowles, Ciba, and Blackman-Uhler)
Today, there are at least 42 dyestuff manufacturers, distributors, and repackaging agents in the United States [6]. Of the major companies, Swiss and German-based companies tend to dominate the US market. Those companies include:
-
Ciba, formerly Ciba-Geigy, Switzerland
-
Clariant (split off from Sandoz in 1995), Switzerland
-
DyStar, formed by the merger of Hoechst and Bayer (1995), acquisition of BASF textile colors which include the former Zeneca, and Mitsubishi of Japan, Germany
-
Crompton & Knowles sold to Yorkshire Group Plc and is now Yorkshire Americas
There is no major surviving the US-based company. Raghavan [7] and Mock [8] give an interesting description of these mergers and the reasons behind them.
Most of the international companies have limited manufacturing facilities in the United States and major facilities in other countries where environmental laws are not as stringent or where the parent companies have a modern integrated low-pollution facility. These facilities in the USA minimize the tariffs paid and also allow quicker response to the marketplace. Ciba has a manufacturing facility in St. Gabriel, LA; Clariant in Martin, SC; DyStar near Charleston, SC; and Yorkshire Americas at Lowell, NC.
Today well over 1,500 dyes are produced in commercial quantities, although only a select handful in each class are the true “workhorse” colors found in virtually every dyehouse dyeing a particular substrate for a particular end-use. Approximately two-thirds of the dyes and pigments consumed in the United States are used by the textile industry. One-sixth of the dyes and pigments are used for coloring paper, and the rest are used chiefly in the production of organic pigments and in the dyeing of leather and plastics.
Dyes are catalogued and grouped under a set of rules established by the Colour Index committee, consisting of representatives from the SDC, Bradford, England, and the American Association of Textile Chemists and Colorists (AATCC), Research Triangle Park, NC. Table 13.2 shows how over 9,000 dyes are enumerated in the current Index [9]. The Colour Index, now in its fourth edition, is updated periodically with newly released information and is available in book form and on CD-ROM. Volumes 1–3, published in 1971, contain the C. I. name and number, chemical class, fastness properties, hue indication, application, and usage. Volume 4, also published in 1971, contains the structures of all disclosed structures. Volume 5, last published as part of Volume 9 in 1993, contains the commercial names of all known dyes and pigments. Volumes 6–8 are supplements with updates to information in Volumes 1–4 up to 1976, 1981, and 1987, respectively. An online version of the Colour Index containing a significant number of new entries was made available in 2002.
In addition to the Colour Index, AATCC publishes a Buyer’s Guide annually in July [6]. Part A lists dyes, pigments, and resin-bonded pigment colors available from companies who choose to list this information.
The textile industry uses a large number of dyestuffs from each of the dye categories, the choice depending on the shade, fiber, and dyeing process, end-use of the textile product, requirements for fastness, and economic considerations. To provide an understanding of the interrelationships that exist among the various dye classes and fiber types, a brief survey of the major fibers follows.
Textile Fibers
In this survey, commercially important textile fibers are grouped by their origin. First there are the natural fibers from plant sources, cotton and flax, and those from animal sources, wool and silk. A second group consists of those fibers that are regenerated or chemically modified natural materials, the rayon and acetate fibers. The final group consists of synthetic fibers, which include polyester, nylon, acrylics, polyolefins, and elastane.
Natural Fibers
Cotton. Cotton fibers comprise mainly cellulose, a long-chain polymer of anhydroglucose units connected by ether linkages. The polymer has primary and secondary alcohol groups uniformly distributed throughout the length of the polymer chain. These hydroxyl groups impart high water absorption characteristics to the fiber and can act as reactive sites. The morphology of the cotton fiber is a complex series of reversing spiral fibrils. The fiber in total is a convoluted collapsed tube with a high degree of twist occurring along the length of the fiber. This staple fiber occurs in nature in lengths of ½ to 2 in., depending on the variety and growing conditions. The diameter ranges from 16 to 21 μm (1 μm is 1 × 10−6 m).
Flax. Flax is also a cellulosic fiber but has a greater degree of crystallinity than cotton. The morphology of flax is quite different from that of cotton. Flax fibers have a long cylindrical shape with a hollow core. The fibers range in length from ½ to 2½ inches, with a diameter of 12–16 μm. Flax staple comprises bundles of individual fibers. Historians believe that flax was among the first fibers to be used as a textile fiber. In recent years, its commercial importance as a textile fiber has decreased significantly.
Wool. Wool fibers comprise mainly proteins: the polypeptide polymers in wool are produced from some 20 alpha-amino acids. The major chemical features of the polypeptide polymer are the amide links, which occur between the amino acids along the polymer chain, and the cystine (sulfur to sulfur) cross-links, which occur in a random spacing between the polymer chains. The polymer contains many amine, carboxylic acid, and amide groups, which contribute in part to the water-absorbent nature of the fiber.
The morphology of wool is complex. There is an outer covering over the fiber, the cortical. There are also overlapping scales having a ratchet configuration that causes shrinkage and felting. The coefficient of friction in wool fibers is vastly different between the tip and the root, depending on which way the scales point. Wool can be made washable by chemically abrading the scales or coating the fibers with another polymer.
Wool fibers are not round but are oval in cross-section. The cortical cells constitute the major component of the fiber, and are aligned along the axis of the fiber. There is a medulla section at the center region of the fiber. Each fiber has a bicomponent longitudinal crystalline arrangement. One side of the fiber contains alpha-keratin crystalline regions, and the other contains beta-keratin crystalline regions. Alpha-keratin and beta-keratin have different moisture absorption characteristics, and this difference is what gives wool fibers crimp and springiness. It is also the reason why wool fibers kink in conditions of high humidity.
Wool fibers are sheared from about 30 major sheep breeds. The length of the wool fibers varies from 1 to 14 in. and depends on the breed, the climate, and the location on the sheep’s anatomy. The fibers can be very fine to very coarse, ranging from 10 to 50 μm in diameter. The longer, coarser fibers normally are used for woolen fabrics, whereas the shorter finer ones are used for worsted fabrics.
Silk. Silk, like wool, is a protein fiber, but of much simpler chemical and morphological makeup. It comprises six alpha-amino acids, and is the only continuous-filament natural fiber. Historians claim that silk was discovered in China in 2640 bc. Silk fiber is spun by the silkworm as a smooth double-strand, each part having a trilobal cross-section. This configuration helps give silk its lustrous appearance. The fiber is unwound from the cocoon the silkworm spins as it prepares its chrysalis. The filaments are smooth and have no twists in their length, which can vary from 300 to 1,800 yards. The diameter of silk is very fine, ranging from 2 to 5 μm. Because of the labor-intensiveness of sericulture and subsequent preparation of the fiber, silk remains a luxury fiber.
Regenerated Fibers
Rayon. Viscose rayon, like cotton, comprises cellulose. In the manufacturing process, wood pulp is treated with alkali and carbon disulfide to form cellulose xanthate. Subsequently, the reaction mass is forced through a spinneret and precipitated in an acid coagulation bath as it is formed into a continuous filament. The fiber has a round striated cross-section. Rayon staple is made by “breaking” the continuous strands into staple-length fibers. Viscose rayon is conventionally produced in diameters varying from 9 to 43 μm.
Acetate. Triacetate and diacetate fibers are manufactured by the chemical treatment of cellulose obtained from refined wood pulp or purified cotton lint. Most of the hydroxyl groups are acetylated (esterified) by treating the cellulose with acetic acid. This determines the chemical configuration of triacetate. Acetate or diacetate is made by the saponification of one of the acetylated groups, thus restoring a hydroxyl to each cellulosic monomer unit. Theoretically, then, diacetate has two acetylated groups in each glycoside unit. The conversion of the hydroxyl groups causes these fibers to be hydrophobic and changes the dyeing characteristics drastically from those of the normal cellulosic fibers. Triacetate fibers are spun by mixing the isolated reaction product (flake) with methylene chloride and alcohol. The spinning solution (dope) is forced through a spinneret and dry-spun into continuous filaments.
An alternate way of wet spinning is also possible. Acetate fibers are spun by mixing the isolated reaction product with acetone and water. The spinning solution is formed into filaments by evaporating the solvent and coagulating the acetate in a manner similar to that for triacetate (i.e., by the dry-spinning method).
Synthetic Fibers
Nylon. In 1939 the DuPont Company introduced the first truly synthetic textile fiber. Dr. Wallace Carothers invented nylon as a result of his basic research into polymer science. Chemically, nylon is a polyamide fiber. The two major types of nylon polymer are used in textiles: type 6,6 which is made by using hexamethylene glycol and adipic acid, and type 6, which is made by polymerizing ε-caprolactam. Nylon fibers are made by melt-spinning the molten polymer. The result is a continuous filament fiber of indeterminate length. It is spun in many deniers, with its diameter varying from 10 to 50 μm. The cross-section usually is round, trilobal, or square with hollow channels when used as carpet fiber.
Polyester. Polyester is made by the polymerization reaction of a diol and a diester. The main commercial polymer is formed by a condensation reaction using ethylene glycol and terephthalic acid. Fibers are formed by melt-spinning. Commercially introduced in 1953 by the DuPont Company as Dacron, polyester fibers have high strength, and very low moisture absorbance. The fiber is usually spun with a round cross-section. Polyester is the most-used synthetic fiber around the world.
Acrylics. The DuPont Company introduced the first commercial acrylic fiber, Orlon, in 1950. Acrylics are made from the polymerization of acrylonitrile and other comonomers to allow dyeability and to open the internal structure. The fibers are produced by either solvent-spinning (Orlon), or wet-spinning (Acrilan). In the solvent-spinning process, the polymer is dissolved in a low-boiling liquid solvent such as dimethyl formamide and extruded in a warm air chamber. In wet-spinning, the polymer is dissolved in a suitable solvent, extruded into a coagulation bath, dried, crimped, and collected. Although the acrylic fibers are extruded as continuous filaments, they subsequently are cut into staple-length fibers. Acrylics have found a niche market as a substitute for wool or in wool blends (blankets, sweaters, etc.) and in awnings and boat covers. The cross-section of the filament varies among manufacturers, Orlon having a dog-bone configuration and Acrilan having a lima-bean shape. Acrylic fibers are quick drying and wrinkle resistant.
Polyolefins. Polyolefin fibers are produced from the polymerization of ethylene or propylene gas. The catalysis research of Ziegler and Natta led to the development of these polymers to form crystalline polymers of high molecular weight. Hercules Inc. produced the first commercial fibers in 1961. The fibers made from these polymers are melt-spun. The cross-sections are round, and the fibers are smooth. They have extremely low dye affinity and moisture absorbance. Colored fiber is normally produced by mixing pigments in the melt polymer prior to extrusion.
Elastane. The DuPont Company commercialized the first manufactured elastic fiber, Lycra, in 1958. Originally categorized as a spandex fiber, the name “elastane” has become more common around the world. This specialty fiber is described as a segmented polyurethane that contains “hard” and “soft” segments; their ratio determines the amount of stretch built into the fiber. Elastane fibers are formed by dry-spinning or solvent-spinning. The continuous filaments can be coalesced multifilaments or monofilaments, depending on the manufacturer. Because most dyeings are applied from water solutions or dispersions, the effect of water absorption by the fiber is an important criterion. Table 13.3 shows the hydrophobic/hydrophilic characteristics of the important fibers. The cellulosic and natural fibers are the most hydrophilic, and polyolefin is the most hydrophobic.
Microdenier fibers. The first commercial production of microfiber in the United States was in 1989 by the DuPont Company. Today microfibers are produced in a variety of synthetic fibers (i.e., polyester, nylon, acrylic, etc.). A microfiber is a fiber that is less than one denier per filament. Yarns made from microdenier filaments are able to give silk-like hand to fabrics.
Dye Classification
This section covers structural features that govern the classification and application of various dye classes. In this regard, the chemistry of acid, azoic, basic, direct, disperse, reactive, sulfur, and vat dyes is presented. With regard to the application of synthetic yes to textiles, it is well known that dyeing of textile fibers from an aqueous dyebath involves four steps: exhaustion, diffusion, migration, and fixation. In step 1, individual dye molecules move from the dyebath to the fiber surface and in step 2, dye molecules move from the fiber surface into the amorphous regions of the fiber. In step 3, dye molecules move from regions of high concentration to regions of low concentration

(i.e., migrate) to become uniformly distributed within the polymer matrix. In step 4, dye molecules interact with groups along the polymer chain via primary or secondary valency forces. Dye–polymer interactions can involve ionic bonding (e.g., acid dyes on nylon or wool), covalent bonding (e.g., reactive dyes on cotton), mechanical entrapment (e.g., vat dyes and sulfur dyes on cotton), secondary valency forces (direct dyes on cotton), or solid–solid solution (e.g., disperse dyes on polyester).
Acid Dyes
Acid dyes derive their name from the conditions associated with their application, in that they are typically applied to textile fibers from dyebaths containing acid [9]. Most acid dyes have one or two sodium sulfonate (−SO3Na) groups and, therefore, are water soluble and capable of bonding with fibers having cationic sites (cf. Fig. 13.1). They give a wide range of bright colors on textiles, especially when monoazo and anthraquinone structures are used.
Acid dyes vary widely in molecular structure and in the level of acid required for dye application. They include relatively low molecular weight dyes such as C.I. Acid Orange 7 and C.I. Acid Blue 45, both of which are readily applied to polyamide and protein fibers and are known as level dyeing acid dyes. As the name suggests, these dyes are characterized by good migration and, therefore, readily produce level dyeings with time. In addition they give reasonably good lightfastness and barré coverage. The application of level dyeing acid dyes to nylon and wool utilizes weak acid and strong acid, respectively. For applications requiring good washfastness, milling acid dyes or super-milling acid dyes are employed. Both of these dye types afford relatively poor barré coverage, however. The former type dyes are applied from weakly acidic dyebaths whereas the latter are generally applied at neutral pH, with molecular size increasing as acid strength decreases. Examples of milling acid dyes are C.I. Acid Yellow 42 and C.I. Acid Red 151, and supermilling acid dyes include C.I. Acid Blue 138 and C.I. Acid Red 138.


Because it is well known that azo dyes derived from naphthol and pyrazolone intermediates exist predominantly in the hydrazone form, this tautomeric form is given for Acid Yellow 42, Acid Red 151, Acid Red 138, and for the appropriate dyes that follow in this chapter.
Acid dyes include metal-complexed azo structures, where the metals used are cobalt, chromium, and iron [10, 11]. Examples are 1:1 and 2:3 chromium complexes and 1:2 cobalt complexes, where the numbers employed represent the ratio of metal atoms to dye molecules. Metal-complexed dyes can be formed inside textile fibers by treating suitably dyed fibers with a solution containing metal ions [12]. In this case, the metal-free forms of these azo dyes are known as mordant dyes and contain mainly ortho, ortho′-bis-hydroxy or ortho-carboxy, ortho′-hydroxy groups (e.g., C.I. Mordant Black 11, Mordant Yellow 8, and Mordant Orange 6). When the metal complexes are formed prior to the dye application process, the resultant dyes are known as premetallized acid dyes and vary in the acid strength required in the application step [13]. The 1:1 chromium complexes (e.g., C.I. Acid Blue 158) are stable only in very strong acid, making them suitable for wool but not nylon. Neutral dyeing premetallized acid dyes contain –SO2NH2 or –SO2CH3 groups in lieu of –SO3Na groups (see Acid Black 172 vs. Acid Red 182). In this case, dye–fiber fixation occurs because the combination of trivalent metal ion (e.g., Cr3+) and four attached negatively charged ligands gives the complex a net negative charge.
Metallization of azo dyes enhances lightfastness, reduces water solubility, causes a bathochromic shift in color, and dulls the shade. Iron complexes generally give brown shades (e.g., C.I. Acid Brown 98) and are most often used to dye leather.
Azoic Dyes
Azoic dyes are mainly bright orange and red monoazo dyes for cotton, with dull violet and blue colors also possible [14]. They are water insoluble and consequently give high washfastness. They are also referred to as azoic “combinations” rather than “dyes” because they do not exist as colorants until they are formed inside the pores of cotton fibers [15]. They are quite important for printing on cotton and often give good lightfastness in heavy depths. Their bleachfastness is better than direct and sulfur dyes and good crockfastness requires efficient soaping after the application step. The formation of these dyes requires two constituents: an azoic coupling component and an azoic diazo component, examples of which are shown in Figs. 13.2 and 13.3. The azoic coupling components are beta-naphthol and β-oxynaphthoic (BON) acid derivatives and the azoic diazo components are substituted anilines.
Azoic dyes are also known as naphthol dyes, because all employ a naphthol component in their formation, and they can be produced in batch or continuous processes. Because they have a limited shade range, they are best known for their ability to provide economical wetfast orange and red shades on cotton. A generic azoic dye structure is shown in Fig. 13.4.
Basic or Cationic Dyes
Basic dyes were developed to dye negatively charged acrylic fibers, forming ionic bonds in the fixation step (Fig. 13.5) [16]. They owe their name to the presence of aromatic amino (basic) groups, and in this case a cationic amino group is present. Generally, they have excellent brightness and color strength, especially among the triarylmethane types. However, their lightfastness is often low, when they are applied to fibers other than acrylics. Basic dyes include those containing a fixed cation, examples of which are C.I. Basic Blue 22, and C.I. Basic Red 18. The triarylmethane dye C.I. Basic Violet 3 has a mobile cation that produces resonance structures of comparable energy.

Basic dyes are applied from weakly acidic dyebaths (pH = 4.5–5.5) and often require the use of anionic or cationic retarding agents to control the rate of dye strike and give level dyeing. Suitable retarding agents either form a weak bond with dyesites along the polymer chain or interact with the dye in the dyebath. In the former case, a significant fraction of the cationic retarder employed is displaced by the dye as dyeing progresses because the dye has higher affinity for the fiber. When anionic retarding agents are used, the dye–retarder bond is broken by increasing the dyebath temperature, giving controlled release of dye molecules to facilitate leveling.
To help determine which basic dyes can be combined for shade matching, key dyebath parameters have been developed [17]. The first parameter pertains to the dyes themselves and is known as the combinability constant (k). This value provides a measure of how fast a basic dye will dye the fiber, and the dyes are rated on a scale of 1 (fast) to 5 (slow). The second parameter pertains to the fiber type involved and is known as the fiber saturation value (S F). This value provides an indication of how much dye the fiber will hold at the saturation point. In this regard, the dye used is C.I. Basic Green 4 and typical saturation levels are 1.0–4.0% based on the weight of the fibers (owf) for light to deep dyeing. The third parameter is the dye saturation factor (f), which is a measure of the capacity of a basic dye for saturating a fiber. This factor is influenced by the molecular size and purity of the dye. In this case, the goal is to avoid placing more dye on the fiber than the number of dye sites, and the standard is C.I. Basic Green 4 (f = 1%).
Direct Dyes
Direct dyes are anionic colorants that have affinity for cellulosic fibers [18]. They were the first dyes that could be used to dye cotton in the absence of a mordanting agent, giving rise to the term direct-cotton dyes. Like acid dyes, direct dyes contain one or more –SO3Na groups, making them water-soluble. Unlike acid dyes, they interact with cellulose (Cell–OH) chains via secondary valency forces (e.g., H-bonding and dipole–dipole interactions), as illustrated in Fig. 13.6. The combined effects of these rather weak forces and sulfonated structures cause direct dyes to have low intrinsic washfastness. Direct dye structures are based on four main chromophores: azo (e.g., C.I. Direct Red 81, C.I. Direct Yellow 28, and C.I. Direct Black 22), stilbene (e.g., C.I. Direct Yellow 12 and C.I. Direct Yellow 11), oxazine (e.g., C.I. Direct Blue 106 and C.I. Direct Blue 108), and phthalocyanine (e.g., C.I. Direct Blue 86 and C.I. Direct Blue 199). About 82% of all direct dyes have disazo or polyazo structures, with stilbene and monoazo structures occupying about 5% each and thiazole, phthalocyanine, and dioxazine structures covering the remaining few percent [19].

Suitably substituted direct dyes can be converted to metal complexes. In this regard, Cu is the metal of choice and examples are C.I. Direct Blue 218, C.I. Direct Red 83, and C.I. Direct Brown 95. About 5% of all azo direct dyes are metal complexes and unlike most direct dyes, these dyes have good lightfastness, as would be anticipated.


Direct dyes are subdivided into three classes (A, B, and C), to assist the dyer in selecting appropriate combinations for color matching [20]. Class A direct dyes give good migration and leveling with time. The dyer employs 5–20% salt for their application and in this case all of the salt may be added at the beginning of the dyeing cycle. An example of this direct dye class is C.I. Direct Yellow 12. Class B direct dyes have poor migration and leveling properties and require the controlled addition of salt to afford level dyeing. They are larger than the former types and have better washfastness. An example is C.I. Direct Blue 1. Class C dyes are the largest of the direct dyes and, consequently, have the best washfastness but poorest leveling properties. Leveling requires careful control of the rate of temperature rise during the dyeing process. Some salt may be added but less than the amount used with classes A and B. An example of this dye class is C.I. Direct Black 22.

The high temperature stability of direct dyes is an important consideration if one wishes to use these dyes as the colorant for cotton when dyeing a polyester/cotton blend at 130 °C [21]. The key to success is to choose dyes that are resistant to hydrolysis. Suitable dyes include C.I. Direct Yellow 105, C.I. Direct Orange 39, and C.I. Direct Blue 80, whereas unsuitable dyes include C.I. Direct Yellow 44, C.I. Direct Red 80, and C.I. Direct Red 83. A quick examination of the structures of the unsuitable dyes reveals that they have groups which are subject to hydrolysis. In the case of Direct Red 83, hydrolysis essentially cuts the molecule in half, eliminating fiber affinity (Fig. 13.7).
Because many direct dyes do not have good washfastness and lightfastness, their dyeing on cotton are often treated with a chemical agent, in what is commonly known as an aftertreatment process. The most widely used aftertreatment methods involve (1) cationic fixatives, (2) copper sulfate, or (3) diazotization and coupling reactions. The first and third methods are designed to enhance washfastness and are illustrated in Figs. 13.8 and 13.9. The use of cationic fixatives ties up sodium sulfonate groups, reducing the water solubility of the treated dye. Diazotization and coupling enlarges the size of the dye, making desorption more difficult, and simultaneously makes the dye less hydrophilic. This process requires the presence of at least one diazotizable primary arylamino (Ar–NH2) group in the dye structure. In this two-step process, the amino group is diazotized by treatment with nitrous acid (HNO2) and the resultant diazonium groups are coupled with a naphthoxide to give new azo groups. It should be pointed out that the addition of new azo groups can also affect dye color. Therefore, this process is most often used for navy and black shades, where the differences in shade variations from batch to batch are less objectionable.
Although copper sulfate aftertreatments are designed mainly to enhance lightfastness, the reduction in water solubility that accompanies Cu-complex formation can have a beneficial effect on washfastness. This treatment also dulls the fabric shade and causes a shift in dye color, so that the resultant color must be the one the dyer is seeking.
The dye used as an example in Fig. 13.10 is C.I. Direct Black 38. It is worthwhile to note that this dye is one of many that were synthesized from benzidine, an established human carcinogen [22]. Nowadays, such dyes are regarded as cancer-suspect agents because of their potential to generate free benzidine upon metabolic breakdown [23]. With this point in mind, regulations preventing the use of azo dyes derived from benzidine and 20 other aromatic amines in textiles have appeared [24]. This requires dye chemists to consider the genotoxicity of potential metabolites in the design of new azo dyes [25].
Disperse Dyes
Disperse dyes were invented to dye the first hydrophobic fiber developed, namely cellulose acetate, and were initially called acetate dyes [26]. The term disperse dyes is more appropriate, because these dyes are suitable for a variety of hydrophobic fibers and it is descriptive of their physical state in the dyebath. Disperse dyes have extremely low water solubility and to be applied from this medium they must be (1) dispersed in water using a surfactant (dispersing agent) and (2) milled to a very low particle size (1–3 μm). These nonionic hydrophobic dyes can be used on acetate, triacetate, polyester, nylon, acrylic, and polyolefin fibers, and their mechanism of fixation involves solid–solid solution formation.
Disperse dyes provide a wide range of bright colors on textiles and many have excellent build-up and barré coverage properties. In addition, they have good washfastness properties but their lightfastness varies with structure. They are suitable for continuous dyeing, a process that takes advantage of their sublimation properties. Disperse dye end-use applications are often based on their classification. The classification system employed is shown in Table 13.4. Low-energy disperse dyes are the easiest to exhaust under atmospheric dyeing conditions but have the lowest thermostability, with the latter property making them unsuitable for automotive applications. They are used to dye acetate, triacetate, and nylon fibers. On the other hand, the high energy dyes are best applied under pressure (T = 130 °C) and are most appropriate for polyester body cloth for automobile interiors. Medium energy dyes are also used to dye polyester and can be applied at atmospheric pressure using a carrier.
Disperse dyes vary in the type of chromophore present and include azo, anthraquinone, nitro, methane, benzodifuranone, and quinoline-based structures. Examples of the first three types are given in Table 13.4, and representative of the latter three types are C.I. Disperse Blue 354, C.I. Disperse Yellow 64, and C.I. Disperse Red 356. Most disperse dyes have azo (~59%) or anthraquinone (~32%) structures. Azo disperse dyes cover the entire color spectrum, whereas the important anthraquinone disperse dyes are mainly red, violet, and blue. The azo types offer the advantages of higher extinction coefficients (ε max = 30,000−60,000) and ease of synthesis, and the anthraquinones are generally brighter and have better photostability (lightfastness). The key weaknesses associated with the anthraquinone dyes are their low extinction coefficients (ε max = 10,000−15,000) and less environmentally friendly synthesis.

To produce disperse dyes having the brightness of the anthraquinone system and the color strength of the azo system, azo dyes based on heteroaromatic amines were developed [27–29]. Examples are C.I. Disperse Red 145, Disperse Blue 148, Disperse Red 156, and C.I. Disperse Blue 339. These dyes employ aminated thiazoles, benzothiazoles, benzisothiazoles, and thiadiazoles in their synthesis. They have ε max = 50,000−80,000, good brightness, and good washfastness. Another key feature of disperse dyes with heteroaromatic systems is their less complex structures. Compare, for example, the fewer number of substituent groups in the diazo component (left side of the azo bond) of Disperse Red 167 vs. Disperse Red 156 and Disperse Blue 165 vs. Disperse Blue 102. However, these dyes are more expensive than disperse dyes derived from benzeneamines, owing to their low reaction yields, and have lower lightfastness than the anthraquinone dyes.
The use of disperse dyes in applications requiring high lightfastness involves the coapplication of photostabilizers. These agents enhance dye stability by quenching the excited states of disperse dyes, probably via energy transfer, or by preferential absorption (screening) of UV radiation. They are also known as UV absorbers and exhaust from the dyebath like disperse dyes. They encompass benzophenone, benzotriazole, oxalanilide, and hindered amine/phenol structures (see Fig. 13.11) [30].

Reactive Dyes
Reactive dyes are used mainly as colorants for cotton, although they are also suitable for nylon and wool [31]. They are water soluble, due to the presence of one or more –SO3Na groups, and undergo fixation to polymer chains via covalent bond formation. Reactive dyes have very high washfastness and are used for leisure wear and other applications requiring stability to repeating laundering. Each dye is composed of five basic parts:
In this regard, SG = water solubilizing group (−SO3Na), C = chromogen (e.g., azo, anthraquinone), B = bridging or linking group (e.g., –NH–), RG = reactive group (e.g., chlorotriazine, vinylsulfone), and LG = leaving group (e.g., –Cl, –F, –SO4H). These parts are illustrated for the structure in Fig. 13.12. This structure also shows that reactive dye structures can be quite small, much smaller in fact than those characterizing direct dyes. As a consequence, reactive dyes have significantly lower inherent affinity for cotton and can require high levels of salt (200–300 g/L) in their dyebaths to promote exhaustion [32].
In addition to giving high washfastness on cotton, reactive dyes usually give bright shades. The latter property arises from the fact that reactive dyes are often acid dye structures linked to reactive groups, as shown in Fig. 13.13. Reactive dyes have moderate-to-good lightfastness and fair-to-poor chlorine fastness.
Although the most commonly used reactive systems involve the halotriazine and sulfatoethyl sulfone (vinyl sulfone) groups, halogenated pyrimidines, phthalazines, and quinoxalines are also available (Fig. 13.14). For all of these systems, alkali is used to facilitate dye–fiber fixation, and fixation occurs either by nucleophilic substitution or addition (Figs. 13.15 and 13.16).
The requirement for alkali in the application of reactive dyes to cotton leads to an undesirable side reaction, namely hydrolysis of the reactive groups before dye–fiber fixation can occur (Fig. 13.17). Because the hydrolyzed dye cannot react with the fiber, this leads to wasted dye and the need to treat the residual color in the wastewater prior to dyehouse discharges. To improve percentage fixation, dyes with two or more reactive groups were developed (Fig. 13.18). This makes it possible for dye–fiber fixation to occur even when one reactive group undergoes hydrolysis [33].
Sulfur Dyes
Sulfur dyes are water-insoluble dyes that are applied to cotton [34]. They are used primarily for their economy and high washfastness, are easy to apply, and give mainly dull shades. Yellow, red, brown, olive, and blue colors can be produced; however, sulfur dyes are most important for their ability to delivery washfast black shades on cotton. In this regard, C.I. Sulfur Black 1 is the main dye used commercially. Sulfur dyes have acceptable lightfastness but poor bleachfastness.
Due to extremely low solubility, the precise structures of most sulfur dyes remain unknown. Much of what we know about sulfur dye structures arises from the characterization of certain degradation products or reaction precursors [35]. Based on such work, it has been possible to determine that structures of the type shown in Fig. 13.19 are covered in this dye class. A key common feature of sulfur dyes is the presence of sulfide (−S n –) bonds, and it is this feature that makes dye application from an aqueous medium possible.
The reaction of sulfur dyes with sodium sulfide (Na2S) at pH >10 effects the reduction of the sulfide bonds, giving their water soluble (leuco) forms. The reduced forms behave like direct dyes, in that they exhaust onto cotton in the presence of salt. Once applied, the reduced dyes are reoxidized to their water-insoluble forms, giving dyeing with good washfastness. This chemistry is illustrated in Fig. 13.20. Although the oxygen in air can be used for the oxidation step, an agent such as hydrogen peroxide is used because it works faster. Sulfur dyes have also been marketed in their prereduced form (Dye-S−Na+), as ready-to-use C.I. Leuco Sulfur dyes. Dye exhaustion in the presence of salt is followed by oxidation. Similarly, water-soluble sulfur dyes containing thiosulfate groups are sold as C.I. Solubilized Sulfur dyes. They are known as “Bunte salts” [36], have better leveling properties than the C.I. Sulfur dyes, and are attractive for package dyeing. The C.I. Solubilized sulfur dyes are applied with Na2S and the chemistry associated with their two-step application is summarized in Fig. 13.21, along with a representative dye structure.
Vat Dyes
Like sulfur dyes, vat dyes are water-insoluble colorants for cotton that must be reduced to their soluble “leuco” forms to be applied from an aqueous dyebath [37, 38]. Their name originates from their early application from wooden vessels known as vats. The term “vatting” is used to refer to the application of these dyes via chemical reduction followed by oxidation. Vat dyes are easier to reoxidize than sulfur dyes and the oxygen in air is often the agent used. As would be anticipated, most vat dyes display high washfastness. As a class, they have the best lightfastness and bleach fastness among the dyes families suitable for cotton. Some cause catalytic fading or phototendering on cotton [39].
Vat dyes have mainly anthraquinone (82%) or indigoid/thioindigoid (9%) structures, with the former having much better fastness properties. The anthraquinone vat dyes exhibit a bathochromic color shift (λ max of higher wavelength) upon reduction to their leuco forms, whereas the indigoids exhibit a hypsochromic shift. Examples of the two structural types are shown in Figs. 13.13 and 13.22. Anthraquinone vat dyes having a single anthraquinone unit exist; however, those with the best fastness properties seem to have the equivalent of two anthraquinone units.
No doubt the best-known and biggest volume vat dye is C.I. Vat Blue 1, indigo, the denim blue dye. Closely related structures are the thioindigoids (4%), which have a sulfur atom in lieu of the –NH– group (Fig. 13.23). The thioindigoids are used mainly as colorants for printing and give orange and red hues. A few dyes having the features of both indigoid types are also known (e.g., Ciba Violet A).
The chemistry associated with the vatting process is illustrated in Fig. 13.24. For the reduction step, a mixture of sodium hydroxide (caustic) and sodium hydrosulfite (hydro, Na2S2O4) is used. Depending upon the amount of caustic and hydro employed one or both of the anthraquinone rings may undergo reduction.
Vat dyes are also available in prereduced forms (3%), an example of which is the leuco sulfuric acid ester C.I. Solubilized Vat Blue 4. These water-soluble forms have affinity for cellulose and exhaust like direct dyes. They are oxidized to the insoluble form using hydrogen peroxide.

Vat dyes are brighter than direct and sulfur dyes but less so than reactive dyes. They are the colorants of choice when dye bleachfastness on cotton is important. They span the entire color spectrum and can be applied to cotton using a variety of methods. With regard to the latter point, they can be further classified based on the temperatures involved in their application. Accordingly, there are hot (50–60 °C), warm (40–50 °C), and cold (25–30 °C) dyeing vat dyes. The hot dyeing types are large planar leuco forms having high affinity and no salt is required for their application. The cold types are small molecules with low affinity and require repeated application to get good build-up. Indigo falls into the cold dyeing category.
The Application of Dyes
The process of dyeing may be carried out in batches or on a continuous basis. The fiber may be dyed as stock, yarn, or fabric. However, no matter how the dyeing is done, the process is always fundamentally the same: dye must be transferred from a bath—usually aqueous—to the fiber itself. The basic operations of dyeing include: (1) preparation of the fiber, (2) preparation of the dyebath, (3) application of the dye, and (4) finishing. There are many variations of these operations, depending on the kind of dye. The dyeing process is complicated by the fact that single dyes seldom are used. The matching of a specified shade may require from two to a dozen dyes.
Fiber Preparation
Fiber preparation ordinarily involves scouring to remove foreign materials and ensure even access to dye liquor. Some natural fibers are contaminated with fatty materials and dirt, and synthetic fibers may have been treated with spinning lubricants or sizing that must be removed. Some fibers also may require bleaching before they are ready for use.
Dyebath Preparation
Preparation of the dyebath may involve simply dissolving the dye in water, or it may be necessary to carry out more involved operations such as reducing the vat dyes. Wetting agents, salts, “carriers,” retarders, and other dyeing assistants also may be added. Carriers are swelling agents that improve the dyeing rate of very hydrophobic fibers such as the polyesters. Examples are o-phenylphenol and biphenyl. Retarders are colorless substances that compete with dyes for dye sites or form a complex with the dye in the bath and act to slow the dyeing rate. Their use is necessary when too-rapid dyeing tends to cause unevenness in the dyeings.
Finishing
The finishing steps for many dyes, such as the direct dyes, are very simple: the dyed material merely is rinsed and dried. Vat-dyed materials, on the other hand, must be rinsed to remove the reducing agent, oxidized, rinsed again, and soaped before the final rinsing and drying steps are carried out. Generally, the finishing steps must fix the color (if fixation has not occurred during application) and remove any loose dye from the surface of the colored substrate. Residual dyeing assistants such as carriers also must be removed.
The types of textile structures that lend themselves to continuous dyeing methods are woven and tufted carpets. Continuous dyeing is designed for long runs of similar product; it is a high-output method of dye application.
The first volume-yardage continuous process was the continuous pad-steam process for vat dyes on cotton. The vat dye dispersion was padded onto the cloth and dried; this was followed by passage through a reducing bath, steaming for 30 s, passage through an oxidizing bath and, finally, washing. When it was discovered that disperse dyes could be thermosoled into polyesters by treatment with dry heat for 60 s and 400 °F, this procedure was readily adapted to continuous processing. The advent of large volumes of dyed polyester-cotton-blend fabrics in the late 1960s made it possible to combine these two processes into one thermosol pad-steam system.
Tufted nylon carpet grew to be the number-one floor covering in the United States in recent decades. Continuous open-width ranges were developed but not without a great deal of ingenuity to deliver the precise loading of liquid to the tufted surface. This was accomplished by a dye applicator that flooded the dye solution onto the carpet surface. The advancing technology in continuous, metered dyeing systems has created a need for dyes in liquid form, both dispersions and solution. The dyes used in carpet dyeing, for the most part, are supplied by the dye manufacturers as liquids. See Fig. 13.25.
Dyeing Methods/Batch
Virtually all types of textile structures are dyed by batch (or exhaust) methods of dyeing, such as stock, yarn, circular knits, warp knits, woven fabrics, garments, carpets, and so on. Batch methods include beck dyeing, jig dyeing, pad-batch, beam dyeing, and others. These methods are dictated primarily by the physical structure of the textile product and the type of fiber(s) it contains. Each of these batch methods employs a different type of machine. As an example, a circular knit fabric comprises cotton could be dyed in a beck, whereas the same structure comprises polyester most likely would be dyed in a high-pressure jet machine, and a garment constructed from the circular knit cotton likely would be dyed in a garment machine.
Stock dyeing often is carried out in large heated kettles made of stainless steel or other corrosion-resistant metal. These kettles can be sealed and used for dyeing at temperatures somewhat above the boiling point of water at atmospheric pressure.
Yarns are dyed in package machines. In this arrangement the yarn is wound onto perforated dye tubes and placed on spindles that are fit into a closed kettle. The dye solution is heated and pumped through the spindle and yarn package. A cycle of inside-outside flow usually is used to provide level dyeing by equal exposure of the dye to yarns. Although the basis of package dyeing has not changed, a number of refinements have been introduced in recent years. Precision winding of the yarn has improved quality by giving a more uniform package density. Horizontal machines and valving between chambers to allow reconfiguration of the dye machine to control the size of the dyeing have changed the way package dyehouses are built. Robotization has been widely utilized to load and unload machines. Also lower-ratio dyebaths with higher flow rates have improved the energy efficiency of the newer machines.
Fabrics are dyed in machines that move them through the dye liquor either under tension (jig) or relaxed (beck). Fabrics also can be dyed in full width by winding them on a perforated beam through which hot dye liquor is pumped. This is the principle of the beam dyeing machine.
The pressure-jet dyeing machine is unique in that it has no moving parts. The cloth, in rope form, is introduced into a unidirectional liquid stream enclosed in a pipe. Liquor is pumped through a specially designed xanthen jet imparting a driving force that moves the fabric. The two fabric ends are sewn together to form a continuous loop.
The first jet machine was introduced in 1965. There are two major types of jet dyeing machines: the vertical kier and the elongated horizontal kier (see Fig. 13.26). In general, the kier uses small water volumes, whereas the elongated types use larger-volume ratios in dyeing. The kier types normally are used for more substantial fabrics, and the elongated types are suited for fine or delicate fabric styles. Important features in today’s machines are improved corrosion-resistant alloys and the ability to operate at higher efficiencies with minimum energy consumption. The control systems have been refined; there is simultaneous loading and unloading. Larger-capacity machines also are being built; a jet dye machine has been developed for carpet dyeing.
Printing
Printing is a special kind of localized dyeing that produces patterns. Four kinds of printing have long been recognized: (1) direct, (2) dyed, (3) discharge, and (4) resist. In direct printing, a thickened paste of the dye is printed on the fabric to produce a pattern. The fabric then is steamed to fix the dye and is finished by washing and drying. Dyed printing requires that the pattern be printed on the fabric with a mordant. The entire piece then is placed in a dyebath containing a mordant dye, but only the mordanted areas are dyeable. Washing then clears the dye from the unmordanted areas, leaving the pattern in color.
In discharge printing, the cloth is dyed all over and then printed with a substance that can destroy the dye by oxidation or reduction, leaving the pattern in white. When a reducing agent such as sodium hydrosulfite is used to destroy the dye, the paste may contain a reduced vat dye. Finishing the goods by oxidation and soaping then produces the pattern in color. In resist printing, certain colorless substances are printed on the fabric. The whole piece then is dyed, but the dye is repelled from the printed areas, thus producing a colored ground with the pattern in white.
Printing is most often done with rotary screens etched in the design to be printed. Printing paste is fed constantly to the center of the rotating screen from a nearby supply, and a squeegee pushes the colored paste through the holes in the screen, leaving the dye paste only in the intended areas, a separate screen is required for each color in the pattern. See Fig. 13.27.
An important recent advance in the pattern-coloring of textiles is ink-jet or digital printing. Milliken’s Millitron and Zimmer’s ChromoJet have been successfully used for carpet and upholstery markets for over two decades. Finer-resolution machines began to emerge in the late 1980s when Stork introduced a prototype machine. During the past 5 years a number of manufacturers have introduced digital ink-jet printers that use either CYMK (Cyan, Yellow, Magenta, and Black) to make a composite color or true-color machines that use mixed pigment systems. The current machines are very successful at furnishing one of a kind and for use in rapid prototyping.
Pigment Dyeing and Printing
Pigment dyeing and printing are processes that compete with the more conventional means of dyeing and printing described above. These processes use water-insoluble dyes or pigments that are bound to the surfaces of fabrics with resins. A paste or an emulsion, containing pigment and resin or a resin-former, is applied to the fabric. The goods then are dried and cured by heat to produce the finished dyeing or print. During the heating or curing, fabric, resin, and pigment become firmly bonded together. This method of color application is economical and produces good results. It should be noted that the pigment is confined to the surface of the fabric and can be selected without regard for fiber affinity.
Nontextile Uses of Dyes
Colorants for nontextile use have been developed mainly for use in hair dyeing, photography, biomedical application, and electronics and reprographics [34–41]. Dye application areas involving the latter areas include ink-jet printing, thermal or pressure dye transfer, laser printing, liquid crystal displays, optical data storage, and nonlinear optics. In several nontextile applications, dyes are not used for their ability to deliver color. Instead, they are used because of their potential electrical properties, such as photoconduction and electrostatic charging of toners, and in some cases they are used because they absorb IR radiation, which induces heating effects. The latter property is important in optical data recording.
Liquid Crystal Dyes
Dyes for liquid crystalline media typically have (1) nonionic structures, (2) high purity, (3) solubility and compatibility with the medium, (4) a transition dipole that is parallel with the alignment axis of the molecular structure, and (5) good alignment with the liquid crystal molecule [42]. Examples include the disazo and anthraquinone dyes in Fig. 13.28.
Ink-Jet Dyes
Dyes used in this area must have the following properties: (1) very good water solubility; (2) low toxicity; (3) good stability to UV light, heat, and moisture; (4) quick fixation to paper following application (deposition); and (5) good color strength [43]. To achieve high resistance to removal by water (wetfastness), ink-jet dyes often contain fewer sulfonate groups and one or more carboxylate groups. This change in structural features allows the dyes to have good solubility in alkaline ink formulations but high wetfastness following deposition. This change in solubility behavior is known as differential solubility [44]. Structures in Fig. 13.29 illustrate the type changes made to the early ink-jet dye C.I. Food Black 2 to enhance wetfastness.
New water-soluble yellow dyes for ink-jet printing are similar to the initially used dye C.I. Direct Yellow 86, except that they are smaller (Fig. 13.30). The size change is designed to provide the solubility needed for high throughput ink cartridge systems, without clogging the ink-jet nozzles.
Most of the new water-soluble magenta dyes are based on H-acid. Examples include dyes that contain a fluorocyanophenyl group (Fig. 13.31). To improve the lightfastness of magenta dyes, gamma acid can be used as the coupling component. For very bright magenta prints, dyes based on xanthene structures can be used. Examples include C.I. Acid Red 52,

which has low photostability, and a carboxylated analog, which has better photostability and wetfastness (Fig. 13.32).
The water-soluble cyan dyes continue to be based on the copper phthalocyanine (CuPc) system. In this regard, C.I. Direct Blue 199 has proved effective, due to its good color strength and photostability. Carboxylated analogs of this type of dye have been developed to enhance wetfastness on paper (Fig. 13.33).
Thermal and Pressure-Sensitive Printing
In direct thermal printing, a color former (colorless) and a developer (acidic) are brought into contact in the presence of heat, to produce color on paper [45, 46]. The most important color in thermal printing is black and the majority of the color formers are fluorans (Fig. 13.34). The most important application of direct thermal printing is in facsimile machines.
In pressure-sensitive printing technology the color former is dissolved in a solvent and encapsulated [47]. The use of pressure (pen, typewriter key) ruptures microcapsules containing the color former, which generates color upon contacting a developer. Black prints are usually obtained either from fluorans or from color former mixtures. Compounds of the type shown in Fig. 13.35 can be used in two- and three-component mixtures.
Organic Photoconductors and Toners
Photoconductors and toners are used in photocopiers and laser printers to produce images [48]. Organic photoconductors consist of a charge-generating layer and a charge-transporting layer. The former comprises pigments and the latter comprise electron-rich organic compounds that are usually colorless. Suitable organic pigments for charge generation include azo pigments, tetracarboxydiimides, polycyclic quinones, phthalocyanines, perylenes, and squarylium compounds (e.g., Fig. 13.36).
Colorants are used in toners to provide color and control the electrostatic charge on toner particles. Diarylides and monoarylides have been used as the yellow pigments in colored toners. The magenta pigments are often quinacridones and the cyan pigments are CuPcs.
Infrared Absorbing Dyes
Infrared dyes include indoleninecyanines and azulenium compounds, both of which are used in optical recording materials [49]. Other examples are metal (Mn, Fe, Co, Cd, Al, Cu, Pd)-complexed phthalocyanines, quinones, quinonoids, and imminium and diiminium compounds (Fig. 13.37).
Laser Dyes
Lasers in which dyes comprise the active medium have become one of the most widely used types [50]. The key virtue of these systems is their ability to cover virtually the entire fluorescence spectral region. Accordingly, the most commonly used dyes are highly fluorescent and include coumarin, rhodamine, oxazine, and syn-bimane structures (Fig. 13.38). Dye lasers are employed in liquid form, which allows them to dispel excessive heat by recirculating the dye solution. Good photostability and efficient laser action under flashlamp excitation are important properties.
Biomedical Dyes
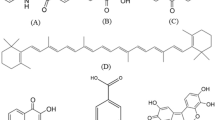
Dyes can be used clinically in bioanalysis and medical diagnostics and in the treatment of certain diseases (cf. Fig. 13.39). For instance, near infrared (NIR) and IR-absorbing dyes can be used in automated DNA sequencing (dye A), fluorescent dyes can be used in cancer detection (dye B), and certain azo and heterocyclic dyes can be used in virus (dye C), cell (dye D), and bacteria (dye E) detection. An in-depth summary of dyes in this area has been published recently [51].
Hair Dyes
About 80% of the dyes used in hair coloring are known as oxidation hair dyes [52, 53]. The remaining 20% of the available hair dyes are mainly synthetic dyes that have affinity for protein substrates. Oxidation dyes are produced directly on hair by oxidizing aromatic diamines (e.g., para-phenylenediamine or 2,5-diaminotoluene) with a suitable oxidizing agent. In this regard, the diamines have been referred to as “primary intermediates” and the oxidizing agents (e.g., hydrogen peroxide) as “developers.” Other suitable primary intermediates are aminodiphenylamines, amino-methylphenols, and para-aminophenol.
When used alone, the primary intermediates give a quite limited shade range following oxidation on hair. To enhance the range of available hair colors, the primary intermediates are oxidized in the presence of suitable “couplers.” Whereas most couplers do not produce colors when exposed to developers alone, they give a wide array of hair shades in combination with primary intermediates. Suitable couplers include 3-aminophenol, resorcinol, and α-naphthol.
The chemistry associated with the oxidation of primary intermediates is now reasonably well known. For para-phenylenediamine and para-aminophenol, this involves the process outlined in Fig. 13.40. It can be seen that dye formation is a two-step process involving oxidation and self-coupling.
C.I. Basic dyes such as Yellow 57, Red 76, Blue 99, Brown 16, and Brown 17 have been used in color refreshener shampoos and conditioners. Similarly, C.I. Acid dyes such as Yellow 3, Orange 7, Red 33, Violet 43, and Blue 9 have been used in shampoos, in this case to deliver highlighting effects [54].
Photographic Dyes
Color photography is still one of the most important and interesting nontextile uses for synthetic dyes. The chemistry employed is comparable to that described above for oxidation hair dyes, in that an oxidizable substrate (e.g., phenylenediamine) is combined with a coupler to produce the target colorant. In this case the diamine is referred to as the “developer,” and it is oxidized by silver halide in the photographic film. The oxidized developer then reacts with the coupler to form the dye. This process produces a negative dye image consisting of yellow, magenta, and cyan dyes in proportion to the amount of red, blue, and green light absorbed by the film [55].
Some widely used developers are shown in Fig. 13.41. They can be used to produce the yellow, magenta, and cyan dyes shown in Fig. 13.42. These dye structures demonstrate that acetoacetanilide, pyrazolone, and indoaniline intermediates are useful for producing yellow, magenta, and cyan colors, respectively.
Dye Intermediates
The raw materials used to synthesize organic dyes are commonly referred to as dye intermediates. Largely, they are derivatives of aromatic compounds obtained from coal tar mixtures. The majority of these derivatives are benzene, naphthalene, and anthracene-based compounds. This section provides an overview of the chemical reactions used to prepare the key intermediates employed in dye synthesis. In this regard, emphasis is placed on halogenated, aminated, hydroxylated, sulfonated, and alkylated derivatives of benzene, naphthalene, and anthraquinone.
Most dye intermediates are prepared by reactions involving electrophilic or nucleophilic substitution processes. The electrophilic processes include nitration, sulfonation, and halogenation reactions, and the nucleophilic processes include hydroxylation and amination reactions. Electrophilic substitution reactions are of the form shown in Fig. 13.43. In this regard, the incoming electrophile (electron-seeking species) reacts with the more electron-rich positions. When the aromatic ring contains ring-activating groups (e.g., hydroxy, alkoxy, amino, alkyl), the incoming group will attack ortho/para positions. If ring-deactivating groups (e.g., nitro, sulfonic acid, carboxylic) are present, the positions meta to the deactivating groups will be attacked.
Other key dye intermediates are prepared by oxidation and reduction processes. Examples of each of these processes are covered in the sections that follow.
Nitration
For dye intermediates, this process involves the introduction of one or more nitro (NO2) groups into aromatic ring systems. Nitro groups serve as chromophores (color bearers, precursors for amino groups, and as auxochromes (color aiders). Because they are meta-directing groups they are also useful in the strategic placement of another incoming group.
Nitric acid (HNO3) is the chemical agent commonly used in nitration reactions. Depending upon the degree of ring activation, HNO3 may be used in combination with other acids. In fact, nitrations are often conducted by using a mixture of HNO3 and sulfuric acid (H2SO4). This combination is known as “nitrating mixture” or “mixed acid,” and it is especially effective when deactivated ring systems are to be nitrated. Dilute HNO3 or a HNO3/acetic acid (CH3CO2H) mixture can be used for nitrating very reactive ring systems. When the former is used there is also the potential for ring oxidation to occur rather than the desired nitration, depending upon the actual compound undergoing nitration. Examples of nitration reactions are shown in Figs. 13.44, 13.45, and 13.46. The nitration of toluene (Fig. 13.44) is selected because it illustrates what can happen when monosubstituted benzenes having a ring-activating group are used. In this case, the principal products reflect a statistical mixture of ortho and para isomers, with only a small amount of the meta isomer obtained. Nitration is conducted near 20 °C and the products are separated by distillation.
Nitration of naphthalene gives mostly the 1-nitro isomer (~90%), initially. Introduction of a second nitro group takes place in the opposite ring because the existing nitro group reduces the reactivity of the ring to which it is attached. Although, nitro groups are meta directors, in this case they can also direct the incoming second (or third) nitro group to a peri position. In the naphthalene ring system, the peri positions are those that are 1,8 and 4,5 to each other (Fig. 13.45).
The nitration of anthraquinone at 50 °C gives, initially, the 1-nitro isomer, and if nitration continues at 80–90 °C, the 1,5 and 1,8 isomers are obtained. Further nitration is impractical and serves to point out that the anthraquinone ring is appreciably less reactive than the naphthalene system. This will be more evident as the chemistry reported in this section continues to unfold.
The nitration of phenols and amines must be conducted with care, as these systems are subject to ring oxidation if the temperature gets too high. For instance, the nitration of phenol itself is conducted near 0 °C using 5% HNO3. This gives a mixture of ortho and para isomers that can be separated by steam distillation (Fig. 13.47). Aromatic amines are often protected by N-acetylation prior to nitration. This reduces both the potential for ring oxidation and the amount of meta isomer that forms when the amino group undergoes protonation. The protonated amino group \( ( - {\text{NH}}_3^{ + }) \) is a meta director, unlike the free amino (NH2) and the acetylated amino (NHAc) groups. This chemistry is illustrated in Fig. 13.47 for 1-naphthylamine. Following nitration, the acetyl group can be removed by hydrolysis.
Reduction
The most important reduction reactions are those leading to aromatic amines that are suitable for azo dye formation. Although this usually involves the reduction of a nitro group to an amino (NH2) group, the reduction of azo groups to amino groups is also an important process. Agents that are commonly used to effect chemical reductions include: Fe + HCl or H2SO4; Na2S; NaSH; Zn + NaOH; H2 + transition metal catalysts; and Na2S2O4. Examples of these reductions are given in Figs. 13.48, 13.49, 13.50, and 13.51. While the reduction of nitrobenzene can be conducted in a number of ways, a key commercial process involves the method in Fig. 13.48, where high-temperature hydrogenation is used.
The reduction of azo compounds using sodium hydrosulfite (Na2S2O4) and NaOH is an important reaction, as it provides an indirect method for the amination of phenols and naphthols (Fig. 13.49). The reduction of nitro groups in anthraquinone compounds works best when a mild reducing agent (e.g., sodium hydrosulfide, NaSH) is used. In this way one avoids reducing the quinoid system.
An example of an important reduction reaction involving Fe + H+ is shown in Fig. 13.50. In this case the sequential use of nitration and reduction is illustrated.
It must also be pointed out that the medium employed in the reduction process can play a major role in the outcome of the reaction. A good example is the reduction of nitrobenzene in the presence of acid or alkali. One should expect the reduction to follow the course shown in Fig. 13.48 under normal conditions, however, in acidic media the product obtained is mainly para-aminophenol. In fact, this has long been the key step in the commercial route to acetaminophen [55], which is obtained by N-acetylation of the reduction product. When the reduction is conducted in the presence of alkali and Zn, the nitro compound is converted to a hydrazo compound via azoxy and azo intermediates. The hydrazo compound is important because it can be treated with acid to form diaminobiphenyls known as benzidines. These reactions are shown in Fig. 13.51. Because benzidine (4,4′-diaminobiphenyl) itself is known to be a human carcinogen, its use as a dye intermediate is substantially curtailed in the western world.
Amination
In as much as the previous section covers the reduction of nitro and azo compounds as a method for introducing amino groups, the focus of this section is direct aminations involving replacement reactions and examples of indirect amination. In the former case, amination via the replacement of activated halogens using an alkyl or arylamine is widely used. The examples given in Fig. 13.52 show that halogens positioned ortho to a nitro group or in an α-position on the anthraquinone ring can replaced by amino groups. The former reaction also works well when the groups are para. However, the reaction is difficult and usually impractical when electron-donating rather than electron-attracting groups are situated ortho and/or para to the halogen. In the case of the anthraquinone system, α-sulfonic acid and α-nitro groups can also be replaced.
An important amination reaction involves hydroxy-substituted naphthalenes (Fig. 13.53). In a process known as the Bucherer reaction, naphthols are heated under pressure with a mixture of ammonia and sodium bisulfite. As the second and third examples indicate, the reaction works with aromatic amines and is selective. Note that the β-hydroxy group reacts preferentially when an α-hydroxy group is also present, and that two hydroxy groups in the same compound can be replaced.
An alternative route to the synthesis of aminoanthraquinones is the two-step sequence shown in Fig. 13.54. In this case, amination occurs via the condensation of para-toluenesulfonamide with chloroanthraquinone followed by hydrolysis of the sulfonamide bond. This method provides a way to introduce an –NH2 group without the use of ammonia gas and the associated high temperatures and pressures.
Another interesting reaction is shown in Fig. 13.55. In this example, amination and sulfonation occur when α-nitronaphthalene is reduced by heating it under pressure with NaHSO3.
Sulfonation
The introduction of one or more sulfonic acid groups (sulfonation) into dye intermediates is often conducted to confer water solubility, to provide fiber affinity, and to direct other incoming groups in the steps that follow sulfonation. In most cases this process employs sulfuric acid but in difficult cases, for example, deactivated ring systems, oleum (an SO3/H2SO4 mixture) is used. This chemistry is illustrated in Fig. 13.56 for benzene. Here we see that benzene can be sulfonated using sulfuric acid and that the introduction of a second sulfonic acid group requires oleum. When a more reactive system is sulfonated, less stringent conditions are required. For example, naphthalene (Fig. 13.57) is readily sulfonated up to four times without using oleum. It is important to note that it is not possible to have sulfonic acid groups that are ortho, para, or peri to each other in the naphthalene system.
By contrast, the sulfonation of anthraquinone requires oleum and no more than two sulfonic acid groups can be introduced. In this system, sulfonation in the α-position requires the use of HgO as a catalyst. Examples of the possible products are shown in the scheme in Fig. 13.58.
The sulfonation of β-naphthol produces several important dye intermediates, the nature of which depends upon the conditions employed (Fig. 13.59). At low temperatures, sulfonation occurs in the α-position to giveoxy-Tobias acid. Under ambient conditions Crocein acid is produced and at elevated temperatures three other products are obtained, including two that are disulfonated.
The sulfonation of aromatic amines such as aniline can give a mixture of products that must be separated prior to dye synthesis. When a single product is sought, the “baking” reaction is often employed (Fig. 13.60). In this process, the sulfate salt of aniline is prepared, dried, and then “baked” in an oven under vacuum. The product in this case is the important dye intermediate, sulfanilic acid. Similarly, naphthylamine sulfonic acid can be produced, and if the para-position is occupied, sulfonation of an ortho-position occurs (Fig. 13.61).
Halogenation
For dye intermediates, halogenation most often involves the incorporation of chloro groups. As pointed out earlier, halogens are important as leaving groups in the amination process, but they can also be used to enhance brightness and influence color. Later, we show that halogens are important as leaving groups in reactive dye chemistry, and in this regard chloro and fluoro groups are used.
Figures 13.62, 13.63, 13.64, and 13.65 provide examples of chlorination reactions. In the first example, the commonly used agent FeCl3/Cl2 is employed for the chlorination of benzene and naphthalene rings. This method is not practical for the chlorination of anthraquinone. In this case the most important reaction is the tetrachlorination process shown in Fig. 13.63.
Because the chlorination of phenols and aromatic amines can be difficult to control, chlorination of these systems usually employs agents that will give a single chloro group when this is the desired outcome. In this regard, NaOCl and SO2Cl2 are quite useful chlorinating agents (see Fig. 13.64). In cases involving amines, often such compounds are protected by acetylation prior to chlorination. If the reactivity of the ring has been reduced by the presence of a deactivating group (e.g., –NO2), acetylation may not be needed.
Halogens are also introduced via indirect methods, three examples of which are shown in Fig. 13.65. In the first case (sequence “A”), aniline is diazotized and the resultant diazonium compound is heated with cuprous chloride to give chlorobenzene, in a process known as the Sandmeyer reaction [56]. Alternatively, the diazonium compound can be converted to the tetrafluoroborate salt, which in turn is heated to give fluorobenzene. In sequence “B,” anthraquinone-2-sulfonic acid is converted to the corresponding chloro compound by treatment with NaClO3/HCl. All three reactions can be used to prepare a wide array of halogenated aromatics.
Chlorination is also an important step in the synthesis of oxygenated aromatic compounds. In this case, chlorination takes place at alkyl groups attached to the rings and is conducted in the absence of iron. The use of UV light speeds up this reaction, which is illustrated for toluene in Fig. 13.66. This free radical chlorination of toluene gives a mixture of benzyl chloride, benzal chloride, and benzotrichloride, which in turn can be hydrolyzed to benzyl alcohol, benzaldehyde, and benzoic acid.
Hydroxylation
The introduction of hydroxy groups is important in dye chemistry because it opens the door to azo dye formation, using phenols and naphthols, and provides an important auxochrome. Hydroxylation methods include alkali fusion, replacement of labile groups, and the reverse Bucherer reaction. In the alkali fusion reaction, naphthalene sulfonic acids are reacted with molten NaOH, KOH, or combinations of the two, as illustrated in Fig. 13.67. When disulfonated naphthalenes are used, the reaction can be stopped at the mono-hydroxylation stage if this is the desired outcome. The second example shows that the α-sulfonic acid group reacts faster.
When sulfonated anthraquinones are used, hydroxylation is conducted with Ca(OH)2 to avoid over oxidation that occurs when hot NaOH is used. Example reactions are shown in Fig. 13.68.
The short sequence in Fig. 13.69 shows that aqueous alkali can also be used in hydroxylation reactions. In both cases, however, elevated temperatures are required.
Other important hydroxylation reactions are shown in Fig. 13.70. Here it can be seen that the Bucherer reaction is reversible, that the fusion reaction works for sulfonated benzene compounds, and that diazonium compounds undergo hydrolysis to produce phenols/naphthols.
Oxidation
Although the oxidation of aromatic methyl groups can be conducted via the two-step sequence shown in Fig. 13.66, a convenient alternative process involves potassium dichromate. In this case, the ring system involved must be stable to the conditions of the reactions. Another important oxidation reaction involves the conversion of naphthalene to phthalic anhydride, which can be accomplished using hot KMnO4 or V2O5. These two reactions are illustrated in Fig. 13.71. Later we show that the oxygen in air can be used as the oxidant for certain organic dyes.
Other Important Reactions
Diazotization. The conversion of a primary aromatic amine to a diazonium compound is known as diazotization. Although this process is covered in more detail in our discussion of azo dye synthesis, it is worthwhile to point out that the diazonium group \( ( - {\text{N}}_2^{ + }) \) is used to produce a wide range of intermediates. As indicated in Fig. 13.72 diazotization is often achieved through the action of nitrous acid (HNO2) and the resultant diazonium group can be replaced by various groups or reduced to give arylhydrazines.
Carboxylation. The introduction of carboxyl groups into the structures of phenols and naphthols produces some important dye intermediates, including salicylic acid and BON acid. This process is conducted under pressure at elevated temperatures using the sodium salts of phenols/naphthols and in the case of β-naphthol, the carboxyl group enters the 3-position (cf. Fig. 13.73). The free acid (−CO2H) group is produced by acid treatment in the final step.
Dye Manufacture
In this section, we summarize the principal methods of synthesis for different dye classes. Emphasis is placed on dyes presently in commerce and the industrial methods suitable for making them. Before doing so, we review the important principles that set dyes apart from other classes of organic compounds.
Unlike other organic compounds dyes possess color because they (1) absorb light in the visible spectrum (400–700 nm), (2) have at least one chromophore (color bearing group), (3) have a conjugated system (system of alternating double and single bonds), and (4) exhibit resonance (a stabilizing force in organic compounds). Table 13.5 shows the relationships between wavelength of visible light and color absorbed/seen and the other three factors are illustrated in Fig. 13.74, 13.75, and 13.76.
Concerning the various factors responsible for color in organic compounds, it is worthwhile to point out that the chromophore must be part of a conjugated system. This is illustrated through the examples in Fig. 13.77. When the azo group is connected to methyl groups the resultant compound is colorless. When it is attached to aromatic rings, the compound possesses color. Similarly, the structures in Fig. 13.75 illustrate the importance of having an extended conjugated system. In this case, doubling the length of the conjugated system for Vitamin A to give β-carotene causes the λ max value to shift from 325 nm to 466 and 497 nm.
Nitro Dyes
As the name suggests, this very small class of organic dyes has at least one nitro group as the chromophore. Nitro dyes invariably are yellow or orange and are important for their economical cost and good lightfastness. Examples include the dyes shown in Fig. 13.78—C.I. Acid Orange 3 (a), C.I. Disperse Yellow 42 (b), C.I. Acid Yellow 1 (c), and C.I. Disperse Yellow 70 (d). A key disadvantage of nitro dyes is their low color strength (ε max = 5,000–7,000). Improvements in color strength have been achieved by incorporating an azo group, as illustrated in dye D.
Representative syntheses are shown in Figs. 13.79 and 13.80. In the first example, C.I. Disperse Yellow 42 is prepared by condensing two molecules of aniline with one molecule of 4-chloro-3-nitrobenzenesulfonyl chloride, using ethanol as the solvent. In the second example, C.I. Acid Orange is prepared in a 3-step synthesis, starting from 2-chloro-5-nitrobenzenesulfonic acid.
Azo Dyes
Azo dyes are by far the largest family of organic dyes. They play a prominent role in acid, direct, reactive, azoic, and disperse dye structures, as shown previously, and include structures that cover the full color spectrum. Generally, the synthesis of azo dyes involves a process known as diazo coupling. In this process, a diazotized aromatic amine is coupled to a phenol, naphthol, aromatic amine, or a compound that has an active methylene group, as illustrated in the two-step synthesis in Fig. 13.81. Step 1 is the conversion of aniline to benzenediazonium chloride, a process known as diazotization, and step 2 is the reaction of the diazo compound with phenol to produce the corresponding azo dye, a process known as diazo coupling.
Diazotizations are normally conducted in an aqueous medium containing nitrous acid, generated in situ from HCl + NaNO2, and a primary aromatic amine. When weakly basic or heteroaromatic amines are used in azo dye synthesis, H2SO4 is often used as the reaction medium, forming H(NO)SO4 (nitrosylsulfuric acid) as the diazotizing agent [57]. The stoichiometry associated with this reaction is given in Fig. 13.82, and although only 2 moles of acid per mole amine are required, in practice 2.2–2.5 moles are used. Diazotizations are most often conducted at 0–10 °C because the resultant diazo compounds are usually unstable at higher temperatures.
Examples of aromatic amines that can be diazotized are shown in Fig. 13.83. This extremely abbreviated list is designed to show that a wide variety of amines can be used, including hydrophobic, weakly basic, hydrophilic, and heterocyclic compounds. ortho-Diamines are not typically used because of their propensity to undergo triazole formation (Fig. 13.84).
Examples of compounds that can be used as coupling components in azo dye synthesis are shown in Figs. 13.85, 13.86, and 13.87. The first group comprises phenols and naphthols, the second group comprises amines that couple, and the third contains couplers that have an active methylene group (see Fig. 13.87). Compounds in the first and third groups require ionization using alkali, to give sufficient reactivity for diazo coupling, and the pH employed is usually 8–9. Because aromatic amines are appreciably more reactive, they couple at pH 5–6. Arrows have been used to indicate the coupling positions for the various couplers. Compounds such as 1-naphthol or 1-naphthylamine give a mixture of monoazo dyes by coupling in the 2-position or the 4-position. When couplers containing –OH and –NH2 groups are employed (see Fig. 13.85), coupling may occur twice, giving disazo dyes. In such cases, coupling is first conducted in acid, ortho to the –NH2 group, and then in alkali. This is important because the introduction of the first azo group decreases the reactivity of the coupler. The ability to ionize the –OH group provides sufficient ring activation for the second coupling. In the case of gamma acid, one has the lone option of coupling under acidic or alkaline conditions.
When primary amines are used as couplers, coupling can occur on the ring or at the amino group itself unless the amino group is blocked. One good way to block this group is by converting it to the N-sulfomethyl group, as illustrated in Fig. 13.86. The products formed are also known as omega salts [58]. The blocking group can be removed following the coupling step, by treating the resultant azo dye with an alkaline solution.
There are also important examples of phenolic compounds that do not couple (see Fig. 13.88). In these examples, the required coupling positions are blocked, the ring is too deactivated, or the compounds undergo oxidation in the presence of the diazo compound.
The synthesis of azo dyes can be illustrated using the following letter designations:
-
A = Diazotizable amine
-
D = Tetrazotizable diamine
-
E = Coupler that couples once
-
M = 1° Amine that couples once and is diazotized and coupled again
-
Z = Coupler that couples twice
-
Z⋅X⋅Z = Binuclear coupler that couples twice

These designations are used to provide an indication of how a given dye has been assembled, and will be used in describing the azo dye syntheses covered in the subsections that follow.
Monoazo Dyes
Azo dyes of this type are manufactured predominantly by the reaction between a diazotized amine (“A”) and a type “E” or “Z” coupler. The synthesis can be as simple as coupling diazotized aniline to H-acid, in an A → E process, to produce C.I. Acid Red 33. An example of a reactive dye that is manufactured via an A → E process is C.I. Reactive Red 1. In this case, the target dye is manufactured as shown in Fig. 13.89, which shows that the reactive group can be introduced prior to (sequence 1) or after (sequence 2) the coupling step. Similarly, monoazo bireactive dyes are made by this process (Fig. 13.90). This illustrates that a quite complex arylamine can be used as the diazo compound.
Other examples of monoazo dyes that are synthesized via an A → E process are shown in Fig. 13.91, further illustrating the wide range of structural types that can be manufactured this way.
Disazo Dyes
There are four often-used methods for synthesizing dyes containing two azo linkages, each of which requires two diazo coupling reactions. A nontraditional “disazo” dye involves 1:2 metal complex formation.
Type A 1 → Z → A 2 synthesis. Dyes of this type include those shown in Fig. 13.92 (C.I. Acid Black 1 (7), C.I. Mordant Brown 1 (8), and C.I. Acid Black 17 (9), C.I. Direct Orange 18 (10)), in which couplers such as H-acid, resorcinol, and meta-phenylenediamine are coupled twice. Although A1 and A2 are different in the present examples, they need not be different. As pointed out above, coupling ortho to the amino group of H-acid is usually conducted first, under weakly acidic conditions, followed by coupling with diazotized aniline under alkaline conditions. This is also true for the structurally similar dye 9, which is prepared from S-acid. In the case of dye 10, however, coupling with aniline under slightly acidic conditions is the second step. For dye 8, both couplings are conducted under acidic conditions, with 2-amino-4-nitrophenol introduced first.
Type E 1 → D → E 1 synthesis. Dyes of this type require the conversion of an aryldiamine to a tetrazonium compound (one that has two diazonium groups) in a process know as tetrazotization. See Fig. 13.93 which involves environmentally friendly alternatives to benzidine. Following tetrazotization, one tetrazonium molecule reacts with two coupler molecules to produce the target dye, examples of which are provided in Fig. 13.94 (11: C.I. Direct Red 28 (Congo Red), 12: C.I. Direct Yellow 12, and 13: C.I. Acid Yellow 42). Disazo dyes prepared this way include dye 14 (C.I. Direct Blue 15), which is converted to the important bis-copper complex, C.I. Direct Blue 218 (see Fig. 13.95).
Type A → M → E synthesis. This is one of the largest groups of disazo dyes, as they include acid, disperse, direct, and reactive dye structures. A representative synthesis is shown in Fig. 13.96. The second diazotization and coupling steps can be conducted inside certain textile fibers. For instance, disperse black dyes are produced in the presence of cellulose acetate by conducting the chemistry shown in Fig. 13.97 after dyeing cellulose acetate with the monoazo dye.
Examples of dyes made via an A → M → E synthesis are shown in Fig. 13.98. Although most azo disperse dyes are based on monoazo structures, disazo structures such as 15 (C.I. Disperse Orange 13) and 16 (C.I. Disperse Orange 29) are manufactured. An important direct dye of this type is 17 (C.I. Direct Red 81), a reactive dye is 18 (C.I. Reactive Blue 40), and acid dyes include 19 (C.I. Acid Red 151) and 20 (C.I. Acid Blue 116).
Type A 1 → Z⋅X⋅Z ← A 1 synthesis. Disazo dyes of this type are produced from coupling twice to dye intermediates such as those shown in Fig. 13.99, and are largely direct dyes for cotton. A representative synthesis is shown in Fig. 13.100, for C.I. Direct Red 83. In this case the target dye is prepared by metallization after the coupling step.
Disazo dyes such as C.I. Direct Yellow 44 are prepared according to the sequence shown in Fig. 13.101. In this example, a pair of monoazo dyes is reacted with phosgene.
1:2 Metal complexes. Although somewhat different from the previous examples and methods, dyes containing two azo groups can also be synthesized by forming 1:2 metal complexes of suitably substituted monoazo dyes. The resultant dyes are mostly acid dyes for protein and polyamide substrates and the metals employed are Cr, Co, and Fe. Examples shown in Fig. 13.102 are for C.I. Acid Black 172 (21) and C.I. Acid Yellow 151 (22). In these examples, the corresponding monoazo dye is treated with one-half the molar amount of Cr2(SO4)3 or CoCl3, respectively.
Polyazo Dyes
In this section, we cover the synthesis of dyes containing three or more azo linkages. In this regard, methods for producing trisazo dyes (those having three azo linkages) include E ← D → Z ← A and A → M1 → M2 → E syntheses. Examples are shown in Fig. 13.103 for C.I. Acid Black 234 (23) and C.I. Direct Blue 71 (24). In the synthesis of dye 23, an unsymmetrical dye can be made from diamine 25 because the end of the tetrazonium compound (cf. 26) that is para to the –SO2 moiety is more reactive than the one that is para to the –NH moiety (Fig. 13.104).
Dyes containing four azo linkages are direct dyes for cotton and can be prepared in several ways, including via A → M → Z ← D → E, A1 → Z1 ← D → Z2 ← A2, E1 ← M1 ← D → M2 → E2, E1 ← D1 → Z ← D2 → E2, and E1 ← D → M1 → M2 → E2 sequences. Examples of the second and third methods are shown in Fig. 13.105. Note that both are symmetrical molecules, the first of which (C.I. Direct Brown 44) employs meta-phenylenediamine as a type “Z” coupler and a type “D” diazo component. In the second example (C.I. Direct Black 22), gamma acid is twice used as the “M” moiety, and the dye is synthesized by (1) coupling tetrazotized benzidine disulfonic acid to two molecules of gamma acid, (2) diazotizing the amino groups on the gamma acid moieties, and (3) coupling to two molecules of meta-phenylenediamine.
Triphenylmethane Dyes
Triphenylmethane dyes are usually prepared in two steps: (1) condensation of an N,N-dialkylaniline with a benzaldehyde compound and (2) oxidation of the resultant leuco base (27). The synthesis of C.I. Basic Green 4 (Malachite Green) is given as an example in Fig. 13.106. Alternatively, C.I. Acid Green 50 is prepared in three steps: (1) condensation of N,N-dimethylaniline and para-(N,N-dimethylamino)benzaldehyde to produce Michler’s hydrol (28), condensation with R-acid to give an intermediate leuco base (29), and (3) oxidation to give the target dye. Historically, PbO2 has been used as the oxidizing agent. However, concerns about its toxicity have led to the use of a more environmentally friendly agent such as tetrachloro-para-benzoquinone (chloranil).
In another synthetic variation, C.I. Acid Violet 17 is prepared in the four steps shown in Fig. 13.107. The different steps in this process are the synthesis of the N-arylmethyl intermediate 30 and the diphenylmethane intermediate 31. Oxidation to the intermediate hydrol and condensation with N,N-dimethylaniline produce the target dye.
Structurally related dyes are synthesized by condensing phenols with phthalic anhydride to give a colorless intermediate lactone (32) that reacts with alkali to give the colored form. An example of this dye type is phenolphthalein, the synthesis of which is shown in Fig. 13.108.
Xanthene Dyes
Like phenolphthalein, xanthene dyes are prepared in a condensation reaction involving phthalic anhydride. However, resorcinol is employed instead of phenol. The simplest representative of this family is C.I. Acid Yellow 73 (fluorescein), which is made via the sequence of steps shown in Fig. 13.109. Similarly, C.I. Acid Red 92 is made by the condensation of tetrachlorophthalic anhydride and resorcinol followed by bromination.
Anthraquinone and Related Dyes
The commercial preparation of anthraquinone dyes begins with the synthesis of anthraquinone itself. In this regard, the three-step synthesis involves: (1) the oxidation of naphthalene to phthalic anhydride, (2) Friedel–Crafts acylation of benzene to give a keto acid, and (3) cyclodehydration using H2SO4. See Fig. 13.110. The preparation of 1,4-disubstituted anthraquinones utilizes the intermediates prepared in Fig. 13.111, where R = OH corresponds to quinizarin.
The reduction of quinizarin using sodium hydrosulfite produces leuco quinizarin, which, in turn, undergoes condensation with alkyl- or arylamines and reoxidation to produce blue and green disperse and solvent dyes. Although chemical oxidation can be used, air oxidation is normally sufficient. See steps “A” and “B” in Fig. 13.112 for the general reaction scheme. The use of boric acid in the reduction step follows the course outlined in Fig. 13.113, where the synthesis of C.I. Solvent Green 3 is given as an example [59].
Anthraquinone Disperse Dyes
Examples of dyes prepared using the above methods are shown in Fig. 13.114. The C.I. disperse dyes Red 15, Violet 1, Blue 3, Violet 27, Blue 19, and Blue 23, are prepared from leucoquinizarin. When unsymmetrical dyes such as Disperse Blue 3 are made, the use of a mixture of two amines in the condensation step gives the corresponding symmetrical dyes as by-products. In this case, Disperse Blue 23 would be one of the by-products.
The synthesis of Disperse Red 4 employs the dibromoanthraquinone intermediate 33, which is hydrolyzed to compound 34 and converted to the target dye upon alcoholysis. See Fig. 13.115. The synthesis of Disperse Violet 26 is conducted in two steps: (1) chlorination of Disperse Violet 1 in the 2, 3-positions using SO2Cl2 and (2) condensation with phenol.
Disperse dyes containing substituents in both anthraquinone rings are often prepared from dinitroanthrarufin (DNA) and dinitrochrysazin (DNC), the structures of which are shown in Fig. 13.116. Examples of these dyes are C.I. Disperse Blue 56 and Blue 77. The former dye is made by reduction of DNA followed by bromination, and the latter is made by condensing aniline with DNC. The DNC condensation shows that nitro groups in the α-position can be displaced like a halogen.
The dichlorinated precursor for Disperse Violet 26 can be used to make turquoise blue dyes such as C.I. Disperse Blue 60, as shown in Fig. 13.117. In this sequence, the chloro groups are replaced by cyano groups, using NaCN, and the resultant intermediate (35) is hydrolyzed to give the corresponding imide (36), which in turn is alkylated to give the target dye.
Anthraquinone Acid Dyes
A key intermediate in the synthesis of anthraquinone acid dyes is bromamine acid. This compound is made via the sequence shown in Fig. 13.118. Acid dyes made from this intermediate include C.I. Acid Blue 25, C.I. Acid Blue 40, and C.I. Acid Blue 127.
The synthesis of C.I. Acid Blue 127 takes place according to the route shown in Fig. 13.119. A key step in the synthesis is the formation of diamine 37, which is produced in two steps from N-sulfomethylaniline: (1) condensation with acetone and (2) hydrolysis to remove the protecting group. At this point, one molecule of diamine 37 is condensed with two molecules of bromamine acid to form the dye.
Another important dye is C.I. Acid Green 25. This dye is made by the sulfonation of C.I. Solvent Green 3 (Fig. 13.113). Because the benzene rings are more reactive than the anthraquinone system, sulfonation occurs there preferentially.

Anthraquinone Basic Dyes
Dyes of this type include C.I. Basic Blue 22 and Basic Blue 47. The synthesis of Basic Blue 22 is shown in Fig. 13.120, as an example of the type of chemistry required. The sequence begins with the preparation of N,N-dimethylpropylenediamine, which in turn is combined with methylamine and condensed with leucoquinizarin. Oxidation gives the key intermediate 38, which is alkylated using methyl chloride to produce the dye.
Anthraquinone Reactive Dyes
Three examples of dyes of this type are C.I. Reactive Blue 19 (39), Reactive Blue 2 (40), and Reactive Blue 4 (41). All three dyes can be synthesized by condensing the appropriate arylamine with bromamine acid. In the case of the high-volume dye Reactive Blue 19, arylamine 44 is the key intermediate, and its synthesis is shown in Fig. 13.121. Chlorosulfonation and then reduction of the intermediate sulfonyl chloride produce the sulfinic acid 42 Alternatively, the reduction step can be conducted with Na2S2O4. Alkylation of the sulfinic acid with 2-chloroethanol or ethylene oxide (a more toxic agent) produces Compound 43. Treatment of this compound with hot H2SO4 gives simultaneous hydrolysis of the acetamido (−NHAc) group and sulfonation of the hydroxyethyl (−CH2CH2OH) group to give key intermediate 44.
Similarly, dyes 40 and 41 are prepared by condensing 2,5-diaminobenzenesulfonic acid with bromamine acid, which reacts first at the less hindered amino group, followed by a reaction with cyanuric chloride to introduce the reactive group. These steps produce dye 41 and dye 40 is formed by reacting 41 with a mixture of sulfonated anilines. See Fig. 13.122.
Vat Dyes
The synthesis of vat dyes covers the full gamut of simple to complex chemistry. We have chosen examples to illustrate the broad spectrum of possible structures and synthetic methods. Emphasis is placed on anthraquinone vat dyes, because they dominate the number of commercial dyes.
Anthraquinone
The simplest anthraquinone vat dyes are benzoylated amines such as C.I. Vat Yellow 3 (45) and Vat Yellow 33 (46). The syntheses are shown in Figs. 13.123 and 13.124.
Anthraquinone vat dyes containing a thiazole ring include C.I. Vat Yellow 2, the synthesis of which is shown in Fig. 13.125. In this case, at least two approaches are possible. In the first, 2,6-diaminoanthraquinone is condensed with benzotrichloride in the presence of sulfur and the initial product is oxidized without isolation to give the target dye. Alternatively, the starting diamine can be chlorinated and converted to the corresponding dithiol (47). At this point condensation with benzaldehyde followed by oxidation (e.g., air or dichromate) gives the dye.
Important vat dyes containing a carbazole moiety include C.I. Vat Brown 3 and Vat Black 27. These dyes are made according to the method shown in Fig. 13.126 for Vat Brown 3. The synthesis employs an Ullmann-type condensation reaction between compounds 48 and 49 followed by acid-induced cyclization using H2SO4.
Vat dyes that do not contain all of the elements of the anthraquinone moiety include benzanthrone-based vat dyes such as C.I. Vat Orange 1 and Vat Green 1, which are made according to the routes shown in Figs. 13.127 and 13.128. The first synthesis is a three-step process: (1) dibenzoylation of naphthalene, (2) Lewis acid-induced cyclization to the benzanthrone system, and (3) dibromination. The second synthesis is a four-step process: (1) oxidative-coupling of benzanthrone in the presence of alkali to give compound 50, (2) H2SO4-induced ring closure to give compound 51, (3) reduction to compound 52, and (4) methylation to give the target dye.

Other important anthraquinone vat dyes belong to the family known as indanthrones. Important examples of this structural type are C.I. Vat Blue 4 and Vat Blue 6. Vat Blue 4 is made by heating 1-amino or 2-aminoanthraquinone at 220–230 °C in a KOH/H2O mixture. The Vat Blue 6 synthesis is a much longer process that requires the synthesis of 2-chloro-3-aminoanthraquinone [60]. The resultant amine is brominated and converted to the target dye via an Ullmann reaction.
Indigoid and Thioindigoid
By far the most important member of these vat dye families is C.I. Vat Blue 1 (indigo). Its synthesis can be achieved via the four-step method shown in Fig. 13.129. The method shown is known as the Heumann–Pfleger synthesis [61], where the key intermediate, N-carboxymethylaniline, is fused with NaNH2. The cyclic product of the fusion step undergoes air oxidation to give indigo.
Thioindigoids are similarly prepared, in that the synthesis of carboxymethyl intermediates is conducted. The resultant cyclic ketones are much less air sensitive, making oxidation with a chemical agent important. However, this also means that unsymmetrical thioindigoid systems can be synthesized (see Fig. 13.130). Although many have been made, few are in commerce today. Examples are C.I. Vat Red 1 and Vat Red 41.
Sulfur Dyes
Earlier we mentioned that sulfur dye chemistry, although quite old, is still much less well defined than for the other classes of dyes. It is clear, however, that many sulfur dyes are produced by the sulfur bake process and that compounds containing the benzothiazole group (e.g. 53) are formed in route to the final dyes. For instance, the synthesis of C.I. Sulfur Yellow 4 follows a course of the type outlined in Fig. 13.131. In this regard, heating a mixture of para-toluidine and sulfur produces a 2-(para-aminophenyl) benzothiazole. The sulfur bake


process has also been used to make C.I. Sulfur Orange 1, where benzothiazone intermediate 54 is produced along the way [62]. See Fig. 13.132.
Sulfur blue dyes are often made using an organic solvent such as n-butanol, in what is known as the solvent reflux process. Examples are C.I. Sulfur Blue 9 and Sulfur Blue 13. In this case, intermediate structures are indophenols (e.g., 55). See Fig. 13.133. Similarly, sulfur dyes containing benzothiazine groups can be made from tetrahalogenated benzophenones. See Fig. 13.134.
Sulfur black dyes are synthesized according to the methods shown in Fig. 13.135. In these examples sodium polysulfide is the sulfurizing agent employed.
Phthalocyanine Dyes
The synthesis of the CuPc system is achieved as shown in Fig. 13.136. Here it can be seen that any of four precursors can be used. Disulfonation gives C.I. Direct Blue 86 and tetrasulfonation gives C.I. Acid Blue 249.
The chlorosulfonation of the CuPc system opens the door to the synthesis of reactive dyes, as shown in Fig. 13.137. In this case, aminochlorotriazine 56 reacts with a CuPc–SO2Cl intermediate to give a monochlorotriazine reactive dye (57), which in turn can be used to make the cationic reactive dye 58.
Fluorescent Brighteners (Colorless “Dyes”)
Many fluorescent brighteners are derivatives of 4,4′diamino-stilbene-2,2′-disulfonic acid (59), an example of which is C.I. Fluorescent Brightener 32 (Fig. 13.138). In this case, successive reactions involving diamine 59 with two molecules of cyanuric chloride and two molecules of aniline followed by hydrolysis of the final chloro groups give the target compound.
Structurally related fluorescent brighteners containing a benzotriazole moiety are made according to the route shown in Fig. 13.139. In this case, diamine 59 is tetrazotized, coupled to 2 molecules of 1,6-Cleve’s acid, and the intermediate disazo stilbene structure (60) is oxidized to C.I. Fluorescent Brightener 40. Nowadays, monosulfonated benzotriazole brighteners are more important [63]. The synthesis of one example is shown in Fig. 13.140 for C.I. Fluorescent Brightener 46.
Examples of hydrophobic fluorescent brighteners include C.I. Fluorescent Brighteners 199, 130, 236, and 162. The synthesis of these compounds is shown in Figs. 13.141, 13.42, 13.43, and 13.144. In the first of these examples, a bis-stilbene structure is made in two steps from bis-chloromethyl-xylene, using the traditional reaction of a phosphorus ylide with an aldehyde as the key step in the sequence.
In the second example, the synthesis of a coumarin-type fluorescent brightener is illustrated. Here, meta-hydroxy-N,N-diethylaniline is condensed with ethyl acetoacetate followed by cyclization of the intermediate keto ester 61. The latter compound undergoes acid-catalyzed cyclization and dehydration to give C.I. Fluorescent Brightener 130. See Fig. 13.142.
A fluorescent brightener containing coumarin and triazole groups is made according to the method shown in Fig. 13.143. The synthesis begins with the preparation of amino-coumarin 62, which in turn is coupled to Tobias acid with concomitant loss of the SO3H group and then oxidized to give C.I. Fluorescent Brightener 236.
The final example is for a naphthalimide structure that is made from acenaphthene (63) in the four-step sequence shown in Fig. 13.144: (1) sulfonation, (2) chromate oxidation to give the naphthalic anhydride (64), (3) condensation with N-methylamine, and (4) replacement of the sulfonic acid group in a reaction with methoxide. This process gives C.I. Fluorescent Brightener 162.
Production and Sales
During the 1990s, the large international companies began to form alliances with producers around the world. Hoechst AG, which had done little research on disperse dyes since the 1970s, signed an agreement in 1990 with Mitsubishi of Japan and gained access to a strong line of disperse dyes. BASF AG and Mitsui signed agreements for vat dyes. ATIC resulted from a joint venture between ICI and Atul of India. Finally, a major break came in January 1995, when Bayer AG and Hoechst AG, the parent companies in Germany, announced the formation of DyStar, a worldwide consolidation of their textile dye businesses, which included the US Hoechst Celanese, and Bayer. Within a short time, BASF acquired the textile dyes business of ICI/Zeneca. Swiss companies Ciba and Clariant (derived by consolidating Sandoz and portions of Hoechst in 1995) announced a merger of the textile dyes business but canceled the venture in 1998. Crompton & Knowles (C&K) emerged as the sole US-based major company, but the company struggled during the late 1990s and was sold to Yorkshire Group PLC of the United Kingdom. Yorkshire Pat-Chem and C&K became Yorkshire Americas.
Globalization and establishment of NAFTA meant fewer textile dyes were needed and manufactured in the United States during the late 1990s. The market shrank from 232 million pounds ($955 million) in 1994 to 214 million pounds ($689 million) in 1998 with further cuts expected. Imported dyes expanded but prices fell. Some 1.1 million pounds of disperse dyes were brought in with a value of $5 million in 1992. In 1999, 5.7 million pounds with a value of $10 million were imported. For each class of dyes, you can find expansion of imports for fewer and fewer dollars. The latest year when consumption was publicly revealed is given in Table 13.6.
References
Edelstein SM (nd) Historical notes on the wet-processing industry. Dexter Chemical Co., Bronx, NY
Zahn J (1967) Bayer Farben Rev 12:32; 11:75
Edelstein SM, Borghetty HC (1969) The Plictho of Gioanventura Rosetti. MIT Press, Cambridge, MA
Robinson R (1957) The life and work of Sir William Henry Perkin. Proceedings of the Perkin Centennial 1856–1956, AATCC, vol 41
Lehner S. America’s debt to Perkin. ibid. 269
Buyer’s guide. AATCC Rev 1(7):17 (2001)
Raghavan KSS (1982) Text Dyer Printer 2(20):21
Mock GN (2001) AATCC Rev 1(12):18
Compiled from: The Colour Index, 5, 4th edn (1992)
Crossley ML (1938) Am Dyest Rep 27(3):124
Crossley ML (1939) Am Dyest Rep 28(3):487
Welham AC (1986) J Soc Dyes Colour 102:126
Beffa F, Back G (1984) Rev Prog Color 14:33
Allen RLM (1971) Colour chemistry (Chapter 7). Thomas Nelson & Sons, London
Hueckel M (1969) Text Chem Color 1(11):510
Raue R (1984) Rev Prog Color 14:187
Aspland JR (1993) Text Chem Color 25(6):21
Allen RLM (1971) Colour chemistry (Chapter 5). Thomas Nelson & Sons, London
Shore J (1990) Colorants and auxiliaries–organic chemistry, properties and applications, vol 1 (Colorants). Society of Dyers and Colourists, Manchester, England, p 20
Aspland JR (1991) Text Chem Color 21(11):21
Shore J (1991) Rev Prog Color 21:23
Haley TJ (1975) Clin Toxicol 8:13
Prival MJ, Bell SJ, Mitchell VD, Peirl MD, Vaughan VL (1984) Mutat Res 136:33
Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers (1996) ETAD Information Notice No. 6. Text Chem Color 28(6):11
Freeman HS, Esancy MK, Esancy JF, Claxton LD (1991) CHEMTECH 21(7):438
Dawson JF (1984) Rev Prog Color 14:90
Annen O, Egli R, Hasler R, Henzi B, Jakob H, Matzinger P (1987) Rev Prog Color 17:72
Weaver MA, Shuttleworth L (1982) Dyes Pigments 3:81
Egli R (1991) The chemistry of disperse blue dyes, past and present. In: Peters AT, Freeman HS (eds) Colour chemistry (Chapter 1). Elsevier Applied Science, London
Reinert G (1997) Rev Prog Color 27:32
Renfrow AHM, Taylor JA (1990) Rev Prog Color 20:1
Aspland JR (1992) Text Chem Color 24(5):31
Carr K (1995) Reactive dyes, especially bireactive molecules. In: Peters AT, Freeman HS (eds) Modern colorants: syntheses and structures (Chapter 4), vol 3. Blackie Academic and Professional, London
Wood WE (1976) Rev Prog Color 7:80
Guest RA, Wood WE (1989) Rev Prog Color 19:63
Aspland JR (1992) Text Chem Color 24(4):27
Baumgarte U (1987) Rev Prog Color 17:29
Jones F (1989) Rev Prog Color 19:20
Egerton GS (1949) J Soc Dyes Colour 65:764
Freeman HS, Peter AT (eds) (2000) Colorants for non-textile applications. Elsevier, Amsterdam
Gregory P (1991) Colorants for high technology. In: Peters AT, Freeman HS (eds) Colour chemistry. Elsevier Applied Science, London
Freeman HS, Sokolowska J (1999) Rev Prog Color 29:8
Bauer W, Ritter J (1993) Tailoring dyes for ink-jet applications. In: Yoshida Z, Shirota Y (eds) Chemistry of functional dyes (Chapter 8.1). Mita Press, Tokyo, p 649
Carr K (2000) Dyes for ink jet printing. In: Freeman HS, Peter AT (eds) Colorants for non-textile applications (Chapter 1). Elsevier, Amsterdam
Gregory P (1990) Dyes Pigments 13:251
Bradbury R (2000) Thermal transfer printing. In: Freeman HS, Peter AT (eds) Colorants for non-textile applications (Chapter 2). Elsevier, Amsterdam
Bamfield P (2001) Chromic phenomena: technological applications of colour chemistry (Chapter 1). The Royal Society of Chemistry, Cambridge, UK, p 50
Inokuchi H (1993) Organic semiconductors: a still fashionable subject. In: Yoshida Z, Shirota Y (eds) Chemistry of functional dyes (Chapter 7.1). Mita Press, Tokyo, p 521
Bamfield P (2001) Chromic phenomena: technological applications of colour chemistry (Chapter 4). The Royal Society of Chemistry, Cambridge, UK, pp 245–256
Pavlopoulos T (2000) Laser dyes. In: Freeman HS, Peter AT (eds) Colorants for non-textile applications (Chapter 7). Elsevier, Amsterdam
Moura JCVP (2000) Biomedical applications of dyes. In: Freeman HS, Peter AT (eds) Colorants for non-textile applications (Chapter 5). Elsevier, Amsterdam
Corbett JG (1985) Rev Prog Color 15:52
Corbett JG (1967) J Soc Dyes Colour 83:273
Corbett J (2000) Hair dyes. In: Freeman HS, Peter AT (eds) Colorants for non-textile applications (Chapter 10). Elsevier, Amsterdam
Waller D, Zbigniew HJ, Filosa M (2000) Dyes used in photography. In: Freeman HS, Peter AT (eds) Colorants for non-textile applications (Chapter 3). Elsevier, Amsterdam
Moury DT (1948) Chem Rev 42:213
Zollinger H (1991) Color chemistry (Chapter 7), 2nd edn. VCH, Weinheim
Fierz-David HE, Blangey L (1979) Fundamental processes in dye chemistry, 5th edn. Interscience, New York, p 250
Gordon PF, Gregory P (1983) Organic chemistry in colour (Chapter 2). Springer, Berlin
Zollinger H (1991) Color chemistry (Chapter 8), 2nd edn. VCH, Weinheim
Püntener A, Schlesinger U (2000) Natural dyes. In: Freeman HS, Peter AT (eds) Colorants for non-textile applications (Chapter 9). Elsevier, Amsterdam
Hallas G (1990) Chemistry of anthraquinoid, polycyclic, and miscellaneous colorants. In: Shore J (ed) Colorants and auxiliaries–organic chemistry, properties and applications (Chapter 6), vol 1 (Colorants). Society of Dyers and Colourists, Manchester, England, p 20
Siegrist AE, Heffi H, Meyer HR, Schmidt E (1987) Rev Prog Color 17:39
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media New York
About this chapter
Cite this chapter
Freeman, H.S., Mock, G.N. (2012). Dye Application, Manufacture of Dye Intermediates and Dyes. In: Kent, J. (eds) Handbook of Industrial Chemistry and Biotechnology. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-4259-2_13
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4259-2_13
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-4258-5
Online ISBN: 978-1-4614-4259-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)