Abstract
Objective
Because retina-damaging angiogenesis is controlled by vascular endothelial growth factor (VEGF) and people with higher glucose intakes are more susceptible to retinal complications that may be due to increased VEGF, it is crucial to elucidate relations between glucose exposure and VEGF expression. We aimed to determine if a carbohydrate response element binding protein (ChREBP) plays a role in the transcriptional up-regulation of hypoxia-inducible factor-1α (HIF-1α) and the downstream VEGF expression in retinal pigment epithelial (RPE) cells exposed to high glucose under normoxic conditions.
Methods
ARPE19 cells were exposed to 5.6, 11, 17, 25 and 30 mM glucose for 48 h in serum-free culture media under normoxic (21 % O2) conditions. Protein and mRNA expression of indicated genes were determined by immunoblot analyses and real-time RT-PCR, respectively. An enzyme-linked immunosorbent assay (ELISA) was used to detect the concentrations of VEGF in the media. Immunofluorescence (IF) and chromatin immunoprecipitation (ChIP) for ChREBP were used to demonstrate nuclear translocation and HIF-1α gene promoter association, respectively.
Results
Immunoblot analyses showed that HIF-1α levels were positively related to levels of glucose exposure between 5.6–25 mM in the RPE cells, indicating the induction and stabilization of HIF-1α by elevated glucose under normoxic conditions. Human lens epithelial cells and HeLa cells did not respond to high glucose, implying that this phenomenon is cell type-specific. Real-time RT-PCR for HIF-1α and VEGF and ELISA for VEGF indicated that high glucose is associated with elevated production of HIF-1α-induced VEGF, an established inducer of neovascularization, in the RPE cells. IF analyses showed that, although ChREBP was expressed under both low (5.6 mM) and high (25 mM) glucose conditions, it appeared more in the nuclear region than in the cytosol of the RPE cells after the high glucose treatment. ChIP analyses suggested a HIF-1α gene promoter association with ChREBP under the high glucose condition. These results imply that RPE cells use cytosolic ChREBP as a glucose sensor to up-regulate HIF-1α expression.
Conclusion
These results suggest a high glucose-induced, ChREBP-mediated, and normoxic HIF-1α activation that may be partially responsible for neovascularization in both diabetic and age-related retinopathy.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Glucose
- Hyperglycemia
- Carbohydrate response element binding protein (ChREBP)
- Hypoxia-inducible factor-1α (HIF-1α)
- Vascular endothelial growth factor (VEGF)
- Retinal pigment epithelial (RPE)
- Neovascularization
- Normoxia
- Age-related macular degeneration (AMD)
- Diabetic retinopathy (DR)
1 Introduction
Age-related macular degeneration (AMD) is a progressive disease, the advanced forms of which account for over 50 % (500,000 cases per year) of legal blindness in the USA [1]. Because the proportion of aged in our population is increasing, it is estimated that the number of individuals affected with advanced AMD in the USA will increase more than 50 % from 1.75 million in 2,000 to 2.95 million in 2020 [2]. There is no cure for this devastating disease, so there is a high premium on preventing it or delaying its progress to debilitating stages. It is believed that dietary may be the most cost-effective strategy to address this health issue [3, 4].
In recent studies, we observed a link between glycemic index (GI) and increased risk for AMD in two American cohorts, the Nutrition and Vision Project (NVP) sub-study of the Nurses’ Health Study (NHS) and the Age Related Eye Diseases Study (AREDS) of the NEI, NIH [5–9]. Both studies indicate that consuming diets that cause higher blood glucose loads (i.e. diets with higher GI) is associated with higher risk for AMD in otherwise healthy, non-diabetic individuals. The findings were corroborated in the Blue Mountains Eye Study (BMES) cohort, Australia [10]. However, the mechanism of this association remains poorly understood. Elucidating the biochemical mechanisms is critical for designing nutritional intervention strategies, furthering our understanding of the underlying pathogenesis, and would inform about the designs of new therapeutics for AMD [11].
We recently postulated that hyperglycemia (e.g. the high postprandial hyperglycemia induced by high-GI diets) may affect the risk for AMD, diabetic retinopathy (DR) , and other diseases, through the activation of hypoxia-inducible factor (HIF)-inducible genes, such as vascular endothelial growth factor (VEGF) , even under normoxia [12]. Uncontrolled VEGF is a major cause of blinding AMD , and contributes to proliferative DR.
Carbohydrate response element binding protein (ChREBP) is a key regulator of glucose metabolism , which is activated in response to high glucose and up-regulates more than 15 genes involved in the metabolic conversion of glucose to fat, such as the pyruvate kinase and lipogenesis enzyme genes, by binding to a carbohydrate response element (ChRE) of these genes [13]. Through controlling transcription of lipogenic enzyme genes in response to nutritional and hormonal inputs, ChREBP may play an important role in disease states, such as diabetes, obesity, hypertension, etc. Recently, the ChREBP has also been shown to be able to up-regulate the transcription of HIF-1α and, therefore, downstream HIF-inducible gene expression in human renal mesangial cells exposed to normoxia and high glucose, and it is suggested that this phenomenon is tissue (cell type)-specific [14]. However, this phenomenon has never been investigated in the human retina. In this study, we tested this hypothesis by determining if a ChREBP plays a role in the transcriptional up-regulation of hypoxia-inducible factor-1α (HIF-1α) and downstream VEGF expression in retinal pigment epithelial (RPE) cells exposed to normoxia and high glucose.
2 Materials and Methods
2.1 Cell Culture
ARPE-19 cells, human lens epithelial (HLE) cells and HeLa cells (ATCC, Manassas, VA) were maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10 % fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. Before each assay, the cells were starved for 16 h in the media containing 0.2 % fetal bovine serum albumin. After washing with PBS twice, the cells were incubated with serum-free culture media containing different glucose concentrations for 48 h.
2.2 Western Blot Analysis
Whole-cells were lysed directly with SDS-gel loading buffer. Total proteins were resolved by 10 % SDS-PAGE and transferred into nitrocellulose membrane (BioRad, USA). Primary antibodies were incubated in Tris-buffered saline with 2.5 % milk. Secondary antibodies conjugated with horseradish peroxidase (Jackson immuno research, USA) and theSuperSignal West Pico detection system (ThermoFisher Scientific) were used for visualization. For semiquantitative analyses, the band densities were measured using Image-J software. The following primary antibodies were used: mouse anti-HIF-1α (Novus Biologicals, USA), rabbit anti-CHREBP (Novus Biologicals, USA), and mouse beta-actin (Sigma, USA).
2.3 Quantitative Real-Time PCR
Total RNA was purified according to the manufacturer’s instructions using an RNeasy mini kit (Qiagen, Valencia, CA). SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA), random hexadeoxynucleotide primers and oligo dT primers were used to synthesize cDNA. The real time PCR analyses were conducted on a MX3000P quantitative PCR system (Agilent technology, Willmington, DE, USA). SYBR Green PCR master mix (Qiagen, USA) was used to quantify human HIF-1α and VEGF mRNA. cDNA amplification was performed using the following set of primers: VEGF forward 5′-CAGAATCATCACGAAGTG-3′; VEGF reverse 5′-TCTGCATGGTGATGTTGGAC-3′. Quantitative RT-PCR was performed and HIF-1α mRNA or VEGF mRNA levels were normalized to beta-actin levels.
2.4 VEGF ELISA
The concentration of VEGF in the cell culture media was measured by a VEGF ELISA kit (R&D Systems, USA), using monoclonal antibodies directed against human VEGF . Absorbance was measured at 450 nm, with wavelength correction at 570 nm.
2.5 ChIP Analysis
Chromatin immunoprecipitation (ChIP) analysis was performed according to the instructions of EpiTect ChIP OneDay kit (QIAGEN, USA). Briefly, fragments of the chromatin were incubated with rabbit anti-ChREBP antibodies (Novus Biologicals, USA) or rabbit polyclonal immunoglobulin G (Jackson Immune Research) and then precipitated by protein G-Sepharose. After wash and purification, PCR was performed using primers flanking the ChRE within the HIF-1α gene promoter: forward, 5’-CTCTTTCCTCCGCCGCTAAAC-3’ and reverse, 5’-GGTTCCTCGAGATCCAATGGC-3’.
2.6 Immunofluorescence Staining
Cells were grown on four-well chamber slides (BD Biosciences). Cells were washed with PBS, fixed for 10 min with 4 % paraformaldehyde in PBS and treated with 0.1 % Triton X-100. The slides were blocked with PBS containing 0.1 % TX-100 and 0.5 % BSA for 30 min. Staining with primary rabbit anti-CHREBP (Novus Biologicals, USA) for 2 h, and the slides were then washed with PBS and incubated with FITC conjugated secondary anti-rabbit antibody (Jackson Immuno Research) for 1 h. Slides were again washed with PBS and counterstained with DAPI for 5 min, rinsed, and briefly dried before mounting.
2.7 Statistical Analysis
Data are reported as the mean ± 95 % confidence interval of at least three independent experiments. For comparison between two groups, the two-sided t test was used. In all cases, p < 0.05 was considered significant.
3 Results
3.1 High Glucose Induces Expression of HIF-1α in Human RPE Cells Under Normoxia
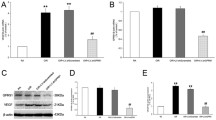
ARPE-19 cells were incubated in medium containing 5.6, 11, 17, 25, and 30 mM glucose under normoxic (21 % O2) condition for 48 h (Fig. 77.1). HIF-1α expression was then determined by immunoblot analyses and β-actin was used as a protein loading control. We observed that RPE cells maintained in the medium with normal level of glucose (5.6 mM D-glucose) under normoxic (21 % O2) conditions showed weak basal HIF-1α expression, and HIF-1α levels were positively related to glucose concentrations between 5.6–25 mM in the RPE cells whereas at 5.6 mM glucose little HIF-1α was identified, HIF-1α was visible at 11 mM and became evident at above 17 mM. 25 mM D-glucose seemed to be a threshold for this relationship since the HIF-1α level under 30 mM D-glucose did not differ from that under 25 mM D-glucose. These results indicate that HIF-1α is induced and stabilized by glucose under normoxic conditions in RPE cells. To rule out the effect of osmolarity on this glucose-induced HIF-1α expression, the RPE cells were incubated in 5.6 mM D-glucose (NG), 25 mM D-glucose (HG), or osmolarity equivalent medium (OEM: 5.6 mM D-glucose and 19.4 mM L-glucose) for 48 h. HIF-1α and β-actin were then determined by Western blotting. The results showed that increasing osmolarity to levels that would be created by 25 mM glucose failed to enhance the HIF-1α expression (Fig. 77.2). This data suggest that glucose is responsible for the upregulation of HIF-1α.
Dose-response of the induction and stabilization of HIF-1α by glucose under normoxia in RPE cells. ARPE-19 cells were incubated in medium containing the indicated concentrations of glucose under normoxic (21 % O2) conditions for 48 h. HIF-1α expression was determined by Western blotting. β-actin was used as a protein loading control
We also compared this glucose-induced HIF-1α stabilization among HeLa, ARPE-19, and HLE cells. The Western blots showed stabilized HIF-1 α in the RPE cells , but not in HeLa and HLE under normoxic conditions, implying that this phenomenon is cell type-specific (Fig. 77.3).
Induction and stabilization of HIF-1α by glucose under normoxia is cell-type specific. a HeLa, ARPE-19, and human lens epithelial (HLE) cells were treated with 5.6 mM glucose (NG) or 25 mM glucose (HG) for 48 h. HIF-1α and β-actin were detected by Western blotting. b Summary of three independent experiments. Desitometry was determined by Image J. HIF-1α values were normalized to β-actin. Data are means (n = 3). *p < 0.05 for 25 mM glucose versus control 5.6 mM glucose
To begin to explore the mechanism underlying high glucose-mediated upregulation of HIF-1α, HLE and ARPE-19 were incubated for 48 h in NG or HG medium and HIF-1α mRNA expression was determined by real-time PCR and normalized by β-actin. Interestingly, HIF-1α mRNA induction was increased by HG in the ARPE-19 cells but not in HLE cells (Fig. 77.4). Taken together, we observe a cell-type specific glucose-dependent transcriptional regulation of HIF-1α under normoxia .
3.2 High Glucose Induces VEGF Under Normoxia in RPE Cells
To determine if VEGF , an established inducer of neovascularization , also respond to glucose-dependent transcriptional regulation, HLE and ARPE-19 cells were incubated in NG or HG medium for 48 h. VEGF mRNA expression was determined by real-time PCR and normalized by β-actin. Similar to the results from HIF-1α, VEGF mRNA induction was increased by HG in the ARPE-19 cells but not in HLE cells (Fig. 77.5). To further determine if this glucose-dependent regulation of VEGF corresponded to the VEGF secretion, we measured VEGF secretion by ELISA. There was an over 60 % increase of secreted VEGF by the RPE cells in the HG medium than in the NG. No significant difference was noted for the HLE cells (Fig. 77.6). Thus, like HIF-1α , VEGF levels are regulated in RPE cells in response to altering glucose , and the effect is cell-specific.
High glucose induces VEGF secretion by RPE cells under normoxia. VEGF secretions by ARPE-19 cells were measured by ELISA after incubation in the medium containing the indicated concentrations of glucose under normoxic (21 % O2) condition for 48 h. Human lens epithelial (HLE). Error bar indicates 95 % CI, and *p-value < 0.05 comparing 25 mM glucose (HG) to 5.6 mM (NG) glucose
3.3 ChREBP has a Role in HIF-1α mRNA Induction by High Glucose
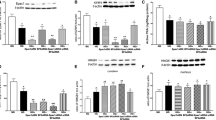
To determine if the variations in levels of HIF-1α are a downstream effect of glucose-induced ChREBP , HLE cells and RPE cells were treated with NG or HG medium for 48 h and ChREBP protein expression was monitored by immunofluorescence. Rabbit polyclonal IgG was applied as a negative control, and input lysate was used as a positive control. We observed that, while ChREBP was expressed in the RPE cells under both NG and HG conditions, it tended to aggregate around the nucleoli under the HG condition (Fig. 77.7). We did not observe a distributional difference of ChREBP between NG and HG conditions in the HLE cells. Protein levels of ChREBP in the nucleus of the RPE cells appeared to be higher than in HLE cells, which might correlate with the cell-type specificity for HIF-1α induction by high glucose .
To demonstrate a role of ChREBP on the transcriptional regulation of HIF-1α gene in RPE cells , we carried out ChIP assays. PCR products amplifying indicated region of HIF-1α gene promoter by 40 cycles was then separated by 2.5 % agarose gel electrophoresis. PCR product was confirmed by DNA sequencing. Anti-ChREBP antibodies efficiently precipitated the DNA fragments containing ChRE-like sequence in HIF-1α promoter from the ARPE-19 cells treated with HG (Fig. 77.8), indicating the binding of ChREBP to the HIF-1α promoter in the RPE cells exposed to HG under nomaxia. In conclusion, HIF-1α uses ChREBP as a glucose sensor for regulation of its expression to participate in gene regulation in the RPE cells in response to high glucose.
High glucose induces nuclear translocation of cytosolic ChREBP to up-regulate HIF-1α expression. RPE cells were treated in either 5.6 mM glucose (NG) or 25 mM glucose (HG) medium for 48 h, and chromatin immunoprecipitation (ChIP) assays using anti-ChREBP antibody were then performed. Rabbit polyclonal IgG was applied as a negative control, and input lysate was used as a positive control. PCR products amplifying indicated region of HIF-1α gene promoter by 40 cycles was then separated by 2.5 % agarose gel electrophoresis. PCR product was confirmed by DNA sequencing
4 Discussion
From a clinical point of view, VEGF is one of the most important HIF-inducible genes, because it induces postnatal neovascularization and angiogenesis seen after ischemic events in both DR and AMD patients [15]. It has been shown that hypoxia-induced HIF-1α mediates VEGF expression by increased binding of the active HIF-1α to the HRE of the VEGF promoter and by increasing the stability of the VEGF mRNA transcript through mitogen-activated protein kinase and Akt pathways, respectively [16]. This mechanism at least partially contributes to the pathogenesis of DR and AMD, especially for the proliferative and neovascular types of the diseases. Anti-VEGF, such as Lucentis®, Avastin®, and Macugen®, has been used in clinics to treat exudative AMD [17, 18] and is currently being evaluated for the treatment of proliferative DR and neovascular glaucoma [19]. Recently, it has been shown that intravitreal ranibizumab (anti-VEGF therapy, trade name Lucentis®) with prompt or deferred laser is more effective through at least 1 year compared with prompt laser alone for the treatment of diabetic macular edema involving the central macula [20]. However, these applications of anti-VEGF therapeutics are limited to the late stages of the diseases and can only arrest the progression but not restore the compromised physiological functions. In addition to VEGF , recent studies have also explored the possibility of directly targeting HIF for a new therapeutic option for both DR and AMD [21–24]. Nevertheless, caution is advised because suppressing of HIF may be a double-edged sword because, by serving as one of the major regulators in glucose metabolism, HIF is necessary for maintaining physiological homeostasis.
Other challenges than hypoxia could also stabilize HIF and activate HIF-inducible proteins to contribute to retinal pathogenesis. It has been shown that RPE cells cultured with high concentrations of glucose enhance synthesis and accumulation of HIF under nomoxia, and the expression of VEGF is increased [25, 26]. Because cell respiration depends on the balance between glucose homeostasis and oxygen homeostasis, hyperglycemia increases the risk of oxygen depletion, which is similar to a hypoxic environment. Therefore, oxygen depletion can be viewed as frequently coincident events with hyperglycemia, even under physiological conditions [12]. This is especially true for the retina because the retina is the most metabolically active tissue in the human body, with dual blood supplies and rapid consumption of glucose and oxygen [27]. It has been proposed that even under normal tissue partial oxygen pressure diabetes-related hyperglycemia mimics the effects of true hypoxia on vascular and neural function and plays an important role in the pathogenesis of diabetic complications, including DR [28, 29]. Despite of these observations, it is unclear how the molecular mechanism works for these hyperglycemic retinal pathogeneses. In this study, we show that high glucose levels induced a ChREBP-mediated normoxic HIF-1α stabilization and VEGF expression.
Although ChREBP is a well-characterized transcription factor and playing a pivotal role in the glycolytic and lipogenic gene regulation in liver and adipose tissues [30], the role of ChREBP in the retina remains unclear. Our study is the first to demonstrate the expression of ChREBP and its role in mediating hyperglycemia-induced HIF expression in the RPE cells under normoxia . Notably, unlike hypoxia, the nomoxic hyperglycemia-induced, ChREBP-mediated HIF is regulated by increasing mRNA levels, and the phenomenon is cell-type specific, i.e., we only observed the phenomenon in the RPE cells but not in either HLE cells or HeLa cells. It has been proposed that high glucose facilitates nuclear translocation of dephosphorylated ChREBP by protein phosphatase 2A (PP2A) , which is upregulated by an intermediate, xylulose-5-P, in the pentose phosphate pathway [31]. Since the pentose phosphate pathway is an alternative to glycolysis and only activated in specific cell types, this may offer an explanation for the cell-type specific glucose-induced ChREBP-mediated HIF activation under normoxia . Further deciphering this biochemical mechanism will advance our understanding of the underlying pathogenesis and enhance therapeutic options for metabolic retinal diseases, such as DR and AMD , preferably in the early stages of the diseases.
In conclusion, our observations suggest that ChREBP plays a role in the transcriptional upregulation of HIF-1α under hyperglycemic, nomoxic conditions. This observation provides support for our hypothesis that HIF can also be considered as a hyperglycemia-inducible factor. Since the hyperglycemic HIF pathway and its interactions with other hyperglycemic pathogenesis pathways, such as hyperglycemic AGE, PKC, polyol, and hexosamine pathways, can affect oxidative stress responses, inflammation, proteolytic mechanisms, etc., all of which are involved in the pathogeneses of DR and AMD [12]. Elucidating this wide range of hyperglycemic cellular effects may open new treatment indications.
Abbreviations
- AMD:
-
Age-related macular degeneration
- AREDS:
-
Age-related eye Diseases study
- BMES:
-
Blue mountains eye study
- ChIP:
-
Chromatin immunoprecipitation
- ChRE:
-
Carbohydrate response element
- ChREBP:
-
Carbohydrate response element binding protein
- DR:
-
Diabetic retinopathy
- GI:
-
Gycemic index
- HIF-1α:
-
Hypoxia-inducible factor-1α
- HLE:
-
Human lens epithelial
- NHS:
-
Nurses’ Health Study
- NVP:
-
Nutrition and Vision Project
- OEM:
-
Osmolarity equivalent medium
- PP2A:
-
Protein phosphatase 2A
- RPE:
-
Retinal pigment epithelial
- VEGF:
-
Vascular endothelial growth factor
References
Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P, Group EDPR (2004) Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 122:477–485
Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J, Group EDPR (2004) Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 122:564–572
Chiu CJ, Taylor A (2007) Nutritional antioxidants and age-related cataract and maculopathy. Exp Eye Res 84:229–245
Weikel KA, Chiu CJ, Taylor A (2012) Nutritional modulation of age-related macular degeneration. Mol Aspects Med 33:318–375
Chiu CJ, Hubbard LD, Armstrong J, Rogers G, Jacques PF, Chylack JLT, Hankinson SE, Willett WC, Taylor A (2006) Dietary glycemic index and carbohydrate in relation to early age-related macular degeneration. Am J Clin Nutr 83:880–886
Chiu CJ, Klein R, Milton RC, Gensler G, Taylor A (2009) Does eating particular diets alter risk of age-related macular degeneration in users of the age-related eye disease study supplements? Br J Ophthalmol 93:1241–1246
Chiu CJ, Milton RC, Gensler G, Taylor A (2007) Association between dietary glycemic index and age-related macular degeneration in the age-related eye disease study. Am J Clin Nutr 86:180–188
Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A (2007) Dietary carbohydrate and progression of age-related macular degeneration, a prospective study from the age-related eye disease study. Am J Clin Nutr 86:1210–1218
Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A (2009) Dietary compound score and risk of age-related macular degeneration in the age-related eye disease study. Ophthalmology 116:939–946
Kaushik S, Wang JJ, Flood V, Tan JS, Barclay AW, Wong TY, Brand-Miller J, Mitchell P (2008) Dietary glycemic index and the risk of age-related macular degeneration. Am J Clin Nutr 88:1104–1110
Chiu CJ, Liu S, Willett WC, Wolever TMS, Brand-Miller JC, Barclay AW, Taylor A (2011) Informing food choices and health outcomes by use of the dietary glycemic index. Nutr Rev 69:231–242
Chiu CJ, Taylor A (2011) Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res 30:18–53
Uyeda K, Yamashita H, Kawaguchi T (2002) Carbohydrate responsive element-binding protein (ChREBP): a key regulator of glucose metabolism and fat storage. Biochem Pharmacol 63:2075–2080
Isoe T, Makino Y, Mizumoto K, Sakagami H, Fujita Y, Honjo J, Takiyama Y, Itoh H, Haneda M (2010) High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int 78:48–59
Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA (2000) Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med 342:626–633
Suzuma K, Naruse K, Suzuma I, Takahara N, Ueki K, Aiello LP, King GL (2000) Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1, and phosphatidylinositol 3-kinase-akt-dependent pathways in retinal vascular cells. J Biol Chem 275:40725–40731
Bressler SB (2009) Introduction: understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology 116(10 Suppl):S 1–7
Bressler NM (2009) Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology 116(10 Suppl):S 15–23
Rodriguez-Fontal M, Alfaro V, Kerrison JB Jablon EP (2009) Ranibizumab for diabetic retinopathy. Curr Diabetes Rev 5:47–51
The Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL III, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK (2010) Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117:1064–1077
Wang X, Wang G, Wang Y (2009) Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol 148:883–889
Zhang P, Wang Y, Hui Y, Hu D, Wang H, Zhou J, Du H (2007) Inhibition of VEGF expression by targeting HIF-1 alpha with small interference RNA in human RPE cells. Ophthalmologica 221:411–417
Arjamaa O, Nikinmaa M (2006) Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res 83:473–483
Arjamaa O, Nikinmaa M, Salminen A, Kaarniranta K (2009) Regulatory role of HIF-1alpha in the pathogenesis of age-related macular degeneration (AMD). Ageing Res Rev 8:349–358
Xiao Q, Zeng S, Ling S, Lv M (2006) Up-regulation of HIF-1alpha and VEGF expression by elevated glucose concentration and hypoxia in cultured human retinal pigment epithelial cells. J Huazhong Univ Sci Technolog Med Sci 26:463–465
Yao Y, Guan M, Zhao XQ, Huang YF (2003) Downregulation of the pigment epithelium derived factor by hypoxia and elevated glucose concentration in cultured human retinal pigment epithelial cells (Article in Chinese). Zhonghua Yi Xue Za Zhi 83:1989–1992
Cohen LH, Noell WK (1965) Relationships between visual function and metabolism. Academic Press, New York
Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, Nyengaard JR, van den Enden M, Kilo C, Tilton RG (1993) Hyperglycemic pseudohypoxia and diabetic complications. Diabetes 42:801–813
Nyengaard JR, Ido Y, Kilo C, Williamson JR (2004) Interactions between hyperglycemia and hypoxia: implications for diabetic retinopathy. Diabetes 53:2931–2938
Uyeda K, Repa JJ (2006) Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab 4:107–110
Haase VH (2010) The sweet side of HIF. Kidney Int 78:10–13
Acknowledgment
The funding sources had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
We declare that we have no conflict of interest. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views or policies of the USA Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the USA Government.
Funding
NEI R01 EY021826, EY021212, EY013250, Ross Aging Initiative, and USDA agreements 1950-5100-060-01A.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media, LLC
About this paper
Cite this paper
Chang, ML., Chiu, CJ., Shang, F., Taylor, A. (2014). High Glucose Activates ChREBP-Mediated HIF-1α and VEGF Expression in Human RPE Cells Under Normoxia. In: Ash, J., Grimm, C., Hollyfield, J., Anderson, R., LaVail, M., Bowes Rickman, C. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 801. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-3209-8_77
Download citation
DOI: https://doi.org/10.1007/978-1-4614-3209-8_77
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-3208-1
Online ISBN: 978-1-4614-3209-8
eBook Packages: MedicineMedicine (R0)