Abstract
Biological processes are highly structured in time as endogenously derived rhythms of short, intermediate, and long periods, with the circadian (24h) time structure most studied. Staging of key physiological and biochemical circadian rhythms gives rise to 24-h patterns in the exacerbation of chronic medical conditions, including arthritis, asthma, ulcer, and hypertension, plus manifestation of acute severe morbid and mortal events, such as myocardial infarction, stroke, and sudden cardiac death. Body rhythms may also significantly affect patient response to diagnostic tests and pharmacokinetics, pharmacodynamics, and toxicities of diverse classes of medications. This chapter reviews circadian and other period biological rhythm dependencies of the pathophysiology of disease and pharmacology of medications as the basis for chronotherapeutics and development of time-modulated drug-delivery systems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
This chapter introduces the concepts of medical chronobiology and chronotherapetics to biomedical engineers and pharmaceutical scientists, particularly those involved in drug-delivery system design. Chronobiology, the study of biological rhythms and the mechanisms of biological time keeping, is of fundamental importance to drug-delivery. Herein, we present the perspectives of (1) chronopathology, i.e., rhythms in the manifestation and severity of medical conditions and diseases; (2) chronopharmacology, i.e., biological rhythm dependent differences in the pharmacokinetics (PK) and pharmacodynamics (PD) of medications; (3) time-qualified reference values as they relate to improved clinical laboratory diagnostics and assessment of new drug-delivery pharmaceutical products; (4) chronotherapeutics, i.e., synchronization of therapeutic agents to time patterns in medical conditions and disease pathology; (5) chronotoxicology, i.e., rhythms in tolerance to chemical, physical, and biological interventions; and (6) chronoprevention, i.e., application of biological rhythm-based strategies to minimize, and even avert, risk to health and well being.
The understanding of biological rhythms and biological clocks and applications to medicine and therapeutics are rather recent developments. There are several explanations for this. First, instrumentation and analytical tools, including sophisticated hardware and software to gather and process time series data (data collected over time), to conduct biological rhythm research were not developed until after the middle of the twentieth century. Second, the concept of biological rhythmicity was viewed as being inconsistent with the long-held, primary principle of homeostasis that alleges relative constancy of the milieu intérieur (English: internal environment). The concept of homeostasis is based primarily on research conducted by Claude Bernard in France late in the nineteenth century and Walter Cannon in the USA early in the twentieth century. Technology did not exist then to continuously monitor biological parameters, e.g., heart rate, blood pressure, body temperature, and activity level, and at that time methods to determine constituents and their concentrations in biological fluids were slow and bulky, requiring as much as a pint of blood to conduct even a single time-of-day analysis of some variables. Data analysis and mining were difficult and tedious, since computer based methods to detect and quantify time series data for rhythms did not yet exist. Furthermore, most biological and medical research was conducted during the light of the day, at the convenience of diurnally active investigators and staff. Thus, the understanding of animal and human biology in the nineteenth and early twentieth century, and even to a great extent today, is based largely on findings of daytime investigations performed on nocturnally active laboratory mice and rats – a time of day that corresponds to the animals’ sleep span – and on diurnally active human beings during their wake span. The results of such single time-of-day studies are representative only of one particular biological time, which might not be appropriately representative.
Many thousands of articles have been published in highly respected scientific, medical, and pharmacology journals over the past several decades documenting relevant high-frequency or pulsatile oscillations (tenths of seconds to 1–2 h), ultradian (roughly 2–20 h), circadian (~24 h), circaseptan (~7 day), circamensual (~1 month), and circannual (~1 year) rhythms in humans and animals [1, 2]. Nonetheless, the concept of homeostasis continues to be taught as the governing doctrine of the life sciences Thus, it is not surprising that homeostasis continues to be the foundation for conceptualizing most biological, medical, and pharmaceutical research and applications. Perhaps, this explains why highly accomplished scientists assume, a priori, that the time during the day, month, and year when biomedical research is performed and when preclinical and clinical studies of candidate medications or other medical interventions are trialed is of little or no importance. Perhaps this also explains why the vast majority of drug delivery systems are designed for zero-order release, as it is assumed constancy in drug concentration ensures constancy in therapeutic effect and/or drug safety. However, homeostasis and rhythmicity are compatible concepts, as endogenous biological rhythms give rise to high frequency, 24-h, menstrual, and annual oscillations in the set points of homeostatic feedback mechanisms.
2 Concepts and Terminology of Chronobiology
2.1 Definition and Characteristics of Biological Rhythms
2.1.1 Biological Rhythm
A biological rhythm is a self-sustaining oscillation of endogenous origin defined by its period, level, amplitude, and phase as illustrated in Fig. 13.1 for the 24-h rhythm in plasma cortisol.
Plasma cortisol 24-h pattern of one diurnally active (wake span ~06:30 to ~22:30) healthy subject assessed by blood sampling at 20-min intervals during a single 24-h span. Left: Time plot (chronogram) of cortisol time series data. Apparent are the prominent, high-frequency pulses commencing during mid-sleep and continuing until ~12:00. Rhythm parameters derived by the Cosinor procedure [84], approximation of the time series data by a 24-h in period cosine curve by the least squares technique, are MESOR, rhythm-adjusted time series mean; amplitude, one-half the peak–trough difference or distance from MESOR to peak (or trough) of the approximating waveform; and acrophase, timing of peak of the rhythm in relation to the chosen phase reference, here local midnight. The less than ideal sinusoidality of the time series data and infrequent sampling are potential pitfalls of the Cosinor method. Black and white portions of the bottom time axis indicate the subject’s usual span of nighttime sleep and daytime activity. Right: Polar cosinor plot. The period length (here 24 h) is depicted as a full circle, with local midnight (phase reference of acrophase) located at top of the circle. Rest span is indicated by darkened band. Vector extending from the center points to the acrophase (expressed as a negative value [delay] in degrees [360º = 24 h; 15º = 1 h] from local midnight), and its length is proportional to the amplitude of the rhythm. Error ellipse of vector indicates 95% confidence region for amplitude and acrophase [3]
2.1.2 Period
Period is the duration of time required to complete a single cycle of a biological rhythm. The spectrum of biological rhythms is broad. Short period rhythms, exemplified by electrical impulses of the central and autonomic nervous systems, cardiac tissue, and intracellular calcium fluxes, exhibit a period of a second or so. Circhoral (~1 h in period) or ultradian (from a few to 20 h in period) rhythms are exemplified by the prominent secretions of the endocrine and neuroendocrine systems. Circadian rhythms, which exhibit a period of ~24 h, have been most explored for their importance to clinical medicine. Infradian rhythms, oscillations of 28 h or longer, include those of roughly a week (circaseptan), month (circamensual), and year (circannual). Individual biological variables and processes are typically organized all across this multifrequency time structure [3].
2.1.3 Level
Level is the baseline, i.e., mean value of the rhythm, around which predictable in time variation is manifested. The level of ultradian rhythms may be modulated in a predictable-in-time manner over the 24 h as a circadian rhythm, which in turn may be modulated in a predictable-in-time manner over the month as a menstrual rhythm, and also over the year as a circannual rhythm.
2.1.4 Amplitude
Amplitude is a measure of the magnitude of the predictable-in-time variability ascribable specifically to biological rhythmicity of a given period. Many rhythms are of high amplitude, accounting for 50% or more of the total variability observed during the time period. Amplitudes of rhythms may change, e.g., with aging, in disease, and by work pattern (shift work). For example, the circadian rhythm in antidiuretic hormone (ADH), which regulates urine volume, is of very high amplitude in young adults. Peak ADH concentration occurs during the nighttime to ensure reduced urine formation and volume during sleep, so that urine formation and volume are much greater while awake than asleep. However, the amplitude of the ADH circadian rhythm declines with age. Commencing around the 4th to 5th decade of life, the peak of the 24-h rhythm in urine volume shifts toward the middle of the night, the result being nocturia, with frequent disturbances of nighttime sleep [4, 5]. As a second example, the amplitude of the circadian rhythm in airway caliber of normal lungs is very small, ~5% of the 24-h mean level. However, in persons with mild asthma, amplitude is typically increased to 25%, and in severe asthma it can be as high as 50–60% [6].
2.1.5 Phase
Phase refers to the clocking of specific features, such as the peak and trough, of a rhythm relative to a reference point of a given time scale, e.g., for circadian rhythms local midnight, or more appropriately the sleep onset, mid-sleep, or sleep offset time of the 24-h sleep–wake cycle or acrophase (peak time) of another concomitantly studied circadian rhythm. The phasing of the high-amplitude circadian rhythm of serum cortisol concentration relative to local midnight in diurnally active individuals is marked by its prominent peak of ~20 μg/dl around 08:00 and trough of ~0 μg/dl around midnight (Fig. 13.1). Phasing of the same circadian rhythm in persons completely adapted to night-shift work is marked, again relative to local midnight clock time, by its peak ~18:00 and trough ~10:00. If one were to average together the time-of-day data series of cortisol values of both day and night-shift workers, differences in phasing of the cortisol rhythm between the two groups would likely obscure circadian rhythmicity as a group phenomenon. However, if the 24-h time series data were instead referenced to a relevant biological time reference, such as the habitual time of wakening, for each person adapted to his/her daytime or nighttime routine and then averaged, the prominent circadian rhythm would be obvious, with the peak time around usual wake up time and trough around or a few hours after habitual bedtime, no matter the clock time [7–9].
3 Mechanisms of Biological Time-Keeping
3.1 Master Biological Clock, the Suprachiasmatic Nuclei
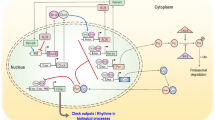
Circadian rhythms are controlled by an inherited master clock network composed of the paired suprachiasmatic nuclei (SCN) situated in the hypothalamus and pineal gland [10–13]. Rhythmic activities in the SCN of the so-called clock genes, Per1, Per2, Per3, Bmal, Clock, and Cry, and their gene products comprise the central time-keeping mechanism. The transcription factors CLOCK and BMAL1 drive the expression of Per1, Per2, Cry1, Cry2 plus a variety of clock controlled genes via E-box sequences in their promoters. PER and CRY proteins negatively feedback on the transcriptional activity of CLOCK:BMAL1, which results in a circadian rhythm in expression of the CLOCK:BMAL1 driven clock and clock controlled genes. The rhythm is stabilized by accessory feedback loops involving the genes Rev-erbα and Rora. The precision of the period of circadian rhythms is achieved via post-translational modulation of the clock proteins by cyclic environmental time cues, the most important being the 24-h environmental light–dark cycle [14]. The biological time-keeping system also includes the multitude of peripheral circadian clocks located in cells, tissues, and organs, which are regulated by the master SCN clock [11]. The output of the central and peripheral circadian clocks is mediated by various clock-controlled genes, giving rise to the body’s circadian time structure (CTS).
The proper phasing of individual circadian rhythms to meet the external cyclic environmental and societal demands and achieve optimal external synchronization of the CTS is conveyed by ambient time cues termed zeitgebers (English: time givers and synonymous with the terms of synchronizers and entraining agents), the light–dark cycle being most powerful under ordinary circumstances [14, 15]. Time cues in the form of ambient light signals sensed by specific non-rod and non-cone cells of the retina are transmitted by the retino-hypothalamic neural projection directly to the SCN and thereafter to the pineal gland by relays involving the paraventricular nucleus and superior cervical ganglion [12]. The major secretory product of the pineal gland is the hormone melatonin. Its synthesis and secretion are highly circadian rhythmic, occurring only during the darkness of night in the diurnally active human species, and for this reason it is termed the hormone of darkness. Melatonin, being water and fat soluble, freely circulates to the cells, tissues, and organs throughout the body, and it also binds to specific melatonin receptor types to induce particular actions [12]. Information of environmental time, specifically, the duration of ambient darkness (time span between sunset and sunrise) daily is conveyed throughout the body by the melatonin onset and offset times. Changes in the duration of time between sunrise and sunset from one day to the next over the course of the year communicate time of year information to the organism via changes in melatonin onset and offset times, i.e., changing duration of melatonin secretion. Exposure to artificial light during the biological nighttime, either in the home, work, or social setting, or to natural environmental light of a new geographic locality following rapid transmeridian displacement by aircraft, alters melatonin synthesis and secretion (Sect. 13.4.2), which by means of feedback to the SCN results over several days in rephrasing of the CTS.
3.2 Synchronizers of Biological Rhythms
A synchronizer or zeitgeber is an environmental time cue that affects the period and/or phase of biological rhythms. The inherited period of circadian clocks, for as yet unknown reasons, is not exactly 24 h; it is a few tenths of an hour longer in most human beings and slightly shorter in some [15]. The master and subservient peripheral circadian clocks are synchronized in period to 24.00 h and in phase with ambient and social cycles by environmental time cues. For both rodents and human beings, the major zeitgeber is the ambient 24-h light–dark cycle [12]. Others include meal schedule, an especially powerful time cue for laboratory animals, and cyclic social phenomena and routines, especially powerful time cues for humans [7, 16]. Features of the natural light–dark cycle vary predictably over the 24 h, month, and year. In human beings, the central circadian clock network relies on the ambient (natural or artificial) daily light–dark cycle to titrate its period to exactly 24 h and to determine phase so as to best meet predictable-in-time environmental demands [17]. The network also registers the duration (sunrise or lights on until sunset or lights off) of the daily environmental photoperiod to adjust the biology seasonally, giving rise to circannual rhythms. The importance of the 28-day lunar cycle on the menstrual cycle in women or on the biology of men is yet to be appropriately explored.
We wish to emphasize that the sleep–wake and environmental light–dark synchronizer cycles are not the source or cause of biological rhythms; rather, they serve only as time cues that synchronize the period and phase of endogenous genetically based circadian clock mechanisms and the oscillations they drive. This distinction is of critical importance in clinical medicine and pharmacology. The phase of circadian rhythms of persons whose time organization is adjusted to a routine of nocturnal activity and work alternating with diurnal sleep will be completely opposite to that of persons whose time organization is adjusted to a routine of diurnal work and activity alternating with nocturnal sleep [8, 9]. This means that clock time, per se, need not be representative of biological time. Review of the methods sections of published human research studies and medication trials reveals that the activity–sleep synchronizer routine is rarely contemplated or stipulated as an inclusion or exclusion criterion for subject selection, except in publications authored by chronobiologists. Similarly, the time of day when investigative procedures are conducted or when a medication is routinely dosed, relative to the sleep in darkness–activity in light synchronizer schedule of subjects, is seldom specified in published research, except in publications authored by chronobiologists. Time of year of investigations is seldom conveyed, and this may be of great importance in certain medication trials, as shown, for example, for human growth hormone and adrenocorticotropic hormone, ACTH [18–20]. Inconsistencies in findings between different human research studies and drug trials can be due to discrepancies in the synchronizer routine of subject samples and/or the chosen timing of procedures, including drug dosing (Sects. 13.5, 13.7, and 13.8).
Timing of the peaks and troughs of circadian rhythms is quite predictable from one day to the next in the majority of people who adhere to a fairly regular activity–sleep routine. However, the phase, and sometimes even exact period, of circadian rhythms in those who are employed in rotating shift work, those who have recently traveled across multiple time zones, or those who have a variable rest–activity routine, are less predictable [8, 9]. This point is of research and clinical importance. The activity in light and sleep in darkness routine determine when the peak and trough of circadian rhythms will occur with reference to the 24-h time scale, which, in turn, determines when diseases and their symptoms are likely to manifest or exacerbate. It also determines, qualitatively and quantitatively, responses to diagnostic tests (Sect. 13.5) and the efficacy and safety of therapeutic interventions according to their timing (Sects. 13.7 and 13.8).
4 Biological Time Structure
The biological time structure consists of the spectrum of periodicities and phase relationships within each. Results of numerous biological rhythm studies help define the temporal organization of human beings. The CTS, which is of particular importance to medical and pharmaceutical sciences, is the major focus of this chapter.
4.1 Circadian Time Structure
The CTS encompasses the entirety of the body’s circadian biological rhythms. One means of illustrating the human CTS is to depict the peak times of selected 24-h rhythms as a clock-like diagram, such as shown in Fig. 13.2, in relation to the synchronizer routine, i.e., sleep in darkness from ~22:30 to ~06:30 and activity during daylight and early night between ~06:30 and ~22:30 [21]. As depicted in the figure, the peak of the circadian rhythms of basal gastric acid secretion, white blood cell count (WBC), calcitonin gene related protein, and atrial natriuretic peptide occurs late at night or early in sleep. The crest of the circadian rhythms in blood lymphocyte and eosinophil number, and plasma concentrations of melatonin, prolactin, growth hormone, thyroid stimulating hormone (TSH), ACTH, follicle stimulating hormone (FSH), and luteinizing hormone (LH) occurs mainly early in sleep. Rhythms of plasma cortisol, renin activity, angiotensin, and aldosterone peak toward the end of the sleep span or commencement of the diurnal activity span, as do those of arterial compliance, vascular resistance, platelet aggregation, and blood viscosity. Hemoglobin and insulin concentrations peak in the afternoon, as do the spirometric measures of airways caliber, FEV1 (forced expiratory volume in one second) and PEF (peak expiratory flow). The circadian rhythms of serum cholesterol and triglycerides and urinary diuresis (young adults) crest early in the evening.
Clock-like diagram illustrating the circadian rhythmic organization of the acrophases (peak times) of selected biological variables. In diurnally active individuals, thyroid stimulating hormone (TSH), melatonin, prolactin, growth hormone, atrial natriuretic peptide, and lymphocyte and eosinopil numbers peak during first half of sleep (shaded portion of the circular diagram). Other shown variables peak just before or after the usual time of morning awakening, i.e., follicle stimulating hormone (FSH); luteinizing hormone (LH); adrenocorticotropic hormone (ACTH); cortisol; testosterone; and plasma renin, angiotensin, aldosterone, and catecholamines. In the morning, most persons experience sudden rise in systolic and diastolic blood pressure and heart rate, and arterial compliance and vascular resistance are greatest as are platelet adhesiveness and blood viscosity. Hemoglobin and serum iron levels peak around midday, and serum total proteins, airway patency (spirometric measures of one second forced expiratory volume, i.e., FEV1, and peak expiratory flow, i.e., PEF), plus insulin level peak in the afternoon. Body temperature and respiratory rate circadian rhythms peak in late afternoon or early evening and cholesterol and triglyceride synthesis rhythms peak in early evening. Urine volume is greatest in late afternoon and evening (in young adults), and neutrophil count, basal gastric acid secretion, calcitonin gene-related protein concentration (a vasodilator), and white blood count (WBC) peak late in the evening or around bedtime (reproduced from Smolensky and Peppas [21])
The information conveyed in Fig. 13.2 illustrates the nature of the CTS and its internal and external synchronization. Clearly, the biochemistry and physiology of human beings are not constant. Rather, they vary in a predictable and coordinated manner during the 24 h. It is worth considering that certain high amplitude circadian rhythmic variables, found in health and disease, might be useful biomarkers to automatically trigger measured medication release from sophisticated biomimetic drug delivery systems.
In individuals who are completely adapted to a schedule of night work, say from 22:00 to 06:00, and daytime sleep, say from 08:00 to 16:00, the clock time entries shown in the diagram would be shifted (delayed) by some 9–10 h; however, the findings of recent studies reveal the majority of night and shift workers do not adapt to such work schedules because of competing social, environmental, and other diurnal zeitgebers [22].
4.1.1 Individual differences in CTS
Human beings, because of the genetics of their inherited circadian clock or due to age, sex, lifestyle, or disease, differ in their biological preference for the times of sleep and wakefulness. Chronotype refers to the time preference of sleep and activity of individuals and associated minor differences in the exact circadian phasing of their CTS.
4.1.2 Circadian Chronotypes
Three different phenotypes of circadian phasing, i.e., chronotypes, can be distinguished using validated questionnaires, such as the Morningness–Eveningness Questionnaire of Horne and Östberg [23]. Morning types, commonly referred to as larks, are most alert and efficient during the morning hours. They express strong preference for early morning waking and early evening bed times, as early as 04:00 and 19:00–21:00, respectively, in extreme morning types. Evening types, commonly referred to owls, are most alert and efficient late in the day and night. They express strong preference for late night bed and late morning or afternoon waking times, as late as 02:00–04:00 and midday or later, respectively, in extreme evening types [24, 25]. The remaining intermediate types constitute the vast majority, perhaps 70–85%, of the population. With reference to the CTS of intermediate types, the clock-time phasing of circadian rhythms, e.g., body temperature, cortisol, and melatonin, of extreme morning types is likely to be advanced on average by ~2 h, while that of extreme evening types is likely to be delayed on average by ~2 h [26–28]. Nonetheless, the CTS of the different chronotypes in most cases shows an internal synchronization, with phasing adjusted to the circadian sleep–wake rhythm, although in extreme owls this may be not the case because of too great a conflict between usual environmental light–dark cycle and societal, school, and work synchronizer schedules versus endogenous biological clock driven preference for very late sleep and activity timings.
4.2 Phase–Response of Circadian Rhythms
Pharmacologists, toxicologists, and other biological scientists are well acquainted with the concept of dose–response. An important, yet less known, chronobiologic concept is phase–response. Phase–response refers to the difference of effect, advance or delay of individual circadian rhythms or the entire CTS, elicited by environmental time signals, chemicals, or other agents. As previously discussed, phase and period of circadian clocks and rhythms are maintained from day to day by entraining cues provided by the onset and offset times of the natural environmental photoperiod. Figure 13.3 depicts the phase–response curves for both light pulses and melatonin administrations when delivered at different circadian times. A single brief exposure of diurnally active human beings to bright artificial light at unusual biological times of the late night or early sleep (dark) span causes phase delay of the CTS by up to 1 h the ensuing 24 h. On the other hand, exposure to the same identical artificial bright light signal very early in the morning, before sunrise and prior to the end of the nocturnal sleep span, causes phase advance of the CTS. In contrast, identical light exposure during the middle of day, when the ambient environment is normally brightly lit, results in no alteration of circadian phase. The phase–response curve for melatonin is opposite the one for light [29–31]. Melatonin administration in the morning is phase delaying, in the early evening phase advancing, and overnight without effect (Fig. 13.3).
Phase–response (delay or advance of circadian time structure) curves for light (solid line) and melatonin (dashed line) in relation to circadian time (expressed relative to the usual time of awakening from nighttime sleep). Exposure of human beings to light of sufficient intensity before customary bedtime and/or during initial hours of sleep results in phase-delay of the circadian rhythm of melatonin and other circadian rhythms the ensuing 24-h, while the same light exposure when timed during the last hours of sleep or initial hours of waking results in phase-advance. The phase–response curve for melatonin is opposite that for light. Ingestion of a physiologic dose (0.25–0.50 mg) of melatonin in the afternoon or early evening results in phase-advance of the melatonin and other circadian rhythms the ensuing 24-h, while ingestion of the same dose of melatonin in the morning results in phase-delay. Indicated at the bottom is circadian time, which represents the expected endogenous phasing of the circadian melatonin rhythm and 24-h time structure of the studied subjects (modified from Burgess et al. [29])
Synthesis and secretion of melatonin in the pineal gland are governed by signals from the SCN in the form of sympathetic input, the neurotransmitter being noradrenalin, which acts via pineal gland ß1-receptors and also α-receptors. Acute, mainly single-dose studies show that both ß1-receptor antagonists, especially the (S)-enantiomers of atenolol, propranolol, metropolol, and bisoprolol, and α-receptor antagonists, especially when administered in the evening, inhibit melatonin synthesis and secretion, resulting in alteration or abolition of its circadian rhythm [32–35]. The clinical consequences of chronic alteration or inhibition of melatonin rhythmicity can include CTS alteration or desynchronization, biological and cognitive inefficiency, sleep and mood disorder, and perhaps even certain cancers [36–39]. Thus, it is critical that the administration of ß1- and α-receptor agonists and other classes of medications not disrupt the melatonin circadian rhythm and CTS. The impact of dosing medications at different times of the day or night on the phasing of circadian clocks and rhythms, as an adverse effect of pharmacotherapy, has not been assessed in clinical trials. Nonetheless, a goal of pharmacotherapy ought to be avoidance of phase alterations of the circadian system, the exception being the use of certain chemical (melatonin), physical (artificial bright light), or other therapies to restore abnormal circadian clock function and CTS to normal [40–42].
4.3 Impact of Transmeridian Travel and Rotating Shift and Permanent Night Work on CTS
Integrity of the CTS is critical for efficient biological and cognitive functioning and maintenance of health. Millions of people each year are either exposed acutely to transient disruptions of their sleep–wake cycle and CTS by rapid travel across time zones or chronically at regular intervals when working rotating or permanent night shift schedules. In the USA, ~15–20% of the adult labor force is likely to be engaged in some type of shift work at any given time, and in developing countries the proportion is likely to be even greater [43]. Disruption of the CTS due to rapid travel across time zones or rotating work schedule typically results in a set of acute and transient symptoms during the several days of adjustment to the new activity–rest cycle and differently timed environmental synchronizers, including light–dark, social, and meal cycles, among others.
These “jet lag” symptoms, so-called even though they occur in nontravelers as a consequence of rotating between day and night work shifts, include fatigue and sleepiness, difficulty in initiating and maintaining sleep, cognitive and physical deficits, changed mood (melancholy/anxiety), altered appetite, digestive complaints, and disrupted digestive track function [9]. Night and rotating shift workers experience disruption of the CTS and several or all of the same symptoms to some degree with each shift change between day and night work, which occurs at regular, typically weekly or shorter, intervals [8, 9]. Moreover, shifting of the sleep–wake pattern and/or regular exposure to light while at work during the night disrupts the CTS and alters or suppresses the melatonin circadian rhythm [8, 22]. Repetition of these biological insults over one’s shift work career poses health risks, such as sleep/mood disorder, peptic ulcer disease (PUD), hypertension, coronary heart disease, plus elevated risk of breast and colorectal cancer in women and prostate cancer in men [9, 36, 38, 39, 44–48]. Substantiation of these health risks in career shift workers supports the integrity of the CTS as a most important aspect of health, and again indicates that therapeutic interventions by drug delivery systems must avoid disturbance of the circadian time keeping system.
5 Medical Chronobiology: Application of Biological Rhythms to Clinical Medicine
5.1 Circadian Rhythms and Clinical Diagnostic Tests
5.1.1 Allergic Rhinitis and Bronchial Asthma
Responses to a variety of common diagnostic tests may be affected by circadian rhythms. The erythema and induration response to intradermally injected allergens, a clinical test for allergies, is two- to threefold greater when performed in the late afternoon and early evening (in diurnally active persons) than morning [49, 50]. Diagnosis of the reversible airway disease asthma, its severity, and its differentiation from fixed airway diseases, namely chronic bronchitis and emphysema, is best accomplished when pulmonary function tests (FEV1 and PEF) are performed as early as feasible after commencement of the diurnal activity span [6, 50]. The airway response to short-acting ß2-agonist bronchodilator aerosol medications, a test to determine the extent to which airway obstruction is reversible, is circadian rhythmic, the response being much stronger in diurnally active individuals when administered in the early morning than afternoon [51]. Thus, early morning so-called reversibility spirometric studies best determine the extent to whether a patient’s chronic obstructive pulmonary disease is reversible, as opposed to nonreversible in the case of chronic bronchitis and emphysema, critical information needed for deciding exact pharmacotherapy [50].
5.1.2 Systemic Hypertension
The diagnosis of arterial hypertension, a medical condition rather than a disease, which when not properly treated can result in cardiovascular, renal, and other pathologies, is typically based on systolic and diastolic blood pressure (SBP and DBP) measurements made in the doctor’s office at a single time of day and interpreted using fixed homeostatic criteria (Table 13.1 [52]). However, as shown by many thousands of around the clock ambulatory blood pressure monitoring (ABPM) studies, SBP and DBP vary considerably during the 24 h and in different individuals as distinctly different circadian patterns (Fig. 13.4). In normotensive persons, BP rises rapidly from reduced sleep-time levels (generally by at least 20 mmHg for SBP and 10–15 mmHg for DBP) with commencement of morning activity. In normotensives, SBP and DBP peak during the day, decline in the evening, and are lowest during sleep. The BP pattern in uncomplicated essential (primary) hypertension in most, although not all, persons is similar to that seen in normotension, although there is abnormal elevation of the 24-h mean, amplitude of variation, and/or reduced sleep-time decline of BP.
Types of 24-h blood pressure (BP) rhythms determined by ambulatory blood pressure monitoring (ABPM). Systolic (S) and diastolic (D) BP of most healthy normotensive and essential (primary) hypertensive persons typically are lowest, by 10–20%, during nighttime sleep relative to diurnal activity. In diurnally active persons, ordinarily SBP and DBP begin to rise just before the end of nighttime sleep, showing peak or near peak levels in the morning or early afternoon; they remain elevated until late evening when they begin to decline, reaching lowest levels during sleep. Nondipping and rising SBP and DBP 24-h patterns are becoming more prevalent. Persons who are obese, have metabolic syndrome and/or diabetes, and those who are elderly, have a sleep-disorder, or have hypertension secondary to an existing medical condition are likely to have an attenuated decline of SBP and DBP (i.e., less than expected 10–20% decrease during nighttime sleep relative to daytime activity) or even experience highest SBP and DBP during sleep. Finally, some persons (extreme dippers) exhibit greater than 10–20% decline in the sleep-time SBP and/or DBP. Abnormal, in particular nondipping and riser, SBP and DBP 24-h patterns are risk factors for cardiovascular disease, as discussed in the text, and can only be diagnosed by 24-h ABPM; clinic cuff assessments done during daytime office hours are indicative only of SBP and DBP at that specific time of the day, and even these values may not be properly representative, since many patients are stressed by the clinical setting causing BP to rise above true values (Michael Smolensky, unpublished)
The BP profile of secondary hypertension, i.e., hypertension that is the consequence of another medical condition, such as renal insufficiency, diabetes, sleep apnea, congestive heart failure, and salt sensitivity, however, often is very different. Typically, there is blunting of the nocturnal decline or even increase in BP during sleep relative to daytime activity. Differences in the extent of circadian variation and phase of BP rhythmicity in primary compared to secondary hypertension complicate the differential diagnosis of normotension versus hypertension when based solely on a few daytime measurements made in the clinic, since seldom, if ever, are they representative of the SBP and DBP levels at other times of the day and night. Use of around the clock ABPM is required to make the correct diagnosis – normotension or daytime, night time, or 24-h hypertension or hypotension – and avoid “white coat” effects (nonrepresentative elevated SBP and DBP values due to novelty or anxiety effects of the clinical setting) and masked hypertension (lower than usual SBP and DBP values in the clinic than typical at work and/or home due to stresses external to the doctor’s office).
5.1.3 Other Routine Clinical Diagnostic Tests
A broad variety of other medical tests can also be affected by body rhythms. Intraocular pressure, measured to make the diagnosis of intraocular hypertension (glaucoma), is circadian rhythmic. In diurnally active persons, intraocular pressure is typically highest nocturnally, between 02:00–04:00, and lowest in the afternoon [53, 54]. The insulin response to the standard oral glucose tolerance tests (GTT) is greater, resulting in lower blood sugar concentrations, when performed in the morning than evening [55, 56]. The findings of certain hematology, coagulation, and hormone studies can vary greatly with the time during the 24 h of blood sampling as discussed in the next section. Although the emphasis of this illustrative discussion has been upon the CTS, day of the menstrual cycle and month of the year may additionally affect the findings of some diagnostic tests.
5.1.4 Chronobiologic (Rhythm-Qualified) Chemical Laboratory Reference Values
A clinical measurement for a laboratory sample obtained at one given time of the day, month, or year constitutes only a very limited spot check, since the variable may be rhythmic across several frequencies modulated by environmental factors, which for some variables may explain the great variability encountered in the free-living human population and, in turn, the large range of values considered normal in laboratory medicine diagnostics. In laboratory medicine, biological rhythms represent both a challenge and an opportunity for improved diagnostic accuracy, in addition to better assessment of drug tolerance and therapeutic efficiency. In the case of high amplitude rhythms, time qualified (with regard to biological rhythms) reference ranges are required to make the correct clinical diagnosis. This is because the value obtained at one time of sampling may be above, at, or below a conventional “reference range” established around a nonperiodic postulated homeostatic “middle value.” In addition to improving diagnostic accuracy by establishing time qualified reference values, the parameters of biological rhythms as such may contribute a set of additional reference values describing the human time organization, such as phase and amplitude, so as to allow recognition of temporal changes that may be related to functional disturbances and pathology as well as adverse drug effects.
5.1.4.1 Establishment of Chronobiologic Reference Values
A number of biological and environmental factors have to be considered in establishing representative chronobiologic reference values, some of which pertain to the establishment of conventional laboratory medicine values [57, 58]. However, some are especially important in regard to chronobiologic investigations, as detailed elsewhere [59]. Chronobiologic reference values have to be derived from clinically healthy subjects comparable in their population characteristics with the studied subjects or patients, and they have to be obtained under comparable conditions. Time-qualified reference ranges, so-called chronodesms [ 60], can be developed for a single individual by repeated measurements over numerous periods, or they can be determined for groups of comparable subjects by repeated measurement of individuals over a single or limited number of periods (Fig. 13.5).
Circadian chronodesm of plasma cortisol. Top: Individual chronodesm in a clinically healthy, diurnally active young adult woman sampled at 20-min intervals over a single 24-h span (72 blood samples in total). Shown is the calculated tolerance interval (determined separately for each 3-h span of the 24 h), indicating the limits within which 90% of measurements is expected to fall with 90% confidence. Bottom: Group circadian chronodesm (based on study of a group of diurnally active 15–21-year-old women sampled at 20-min intervals during a single 24-h span). The group circadian chronodesm shows a wider range in the time-qualified tolerance intervals, reflecting individual variation in mesor, amplitude, and/or acrophase of the cortisol circadian rhythm. Background gray shading indicates conventionally considered normal range of plasma cortisol values. In both individual and group chronodesms, the same plasma cortisol value, e.g., ~7 μg/dl (represented by asterisks), when evaluated without regard to the time of sampling relative to the person’s sleep–wake synchronizer routine could be below, within, or above the “usual time-qualified range” of normal. Black and white portions of bottom time axis indicate usual span of subjects’ nighttime sleep and daytime activity from whom the cortisol data were obtained (figure constructed from data of Haus and Touitou [59])
Choice of peer population will determine the validity of the reference range for a given individual when using a group chronodesm for a given laboratory variable. The number of subjects required for a valid reference population will vary from variable to variable with the prominence and stability of the rhythm, extent of compatibility of the reference group with the subjects to be studied, and degree of desired statistical power for decision making [59, 61, 62]. Depending upon the Gaussian and (very often) non-Gaussian distribution of the data, reference range limits are presented in parametric or nonparametric statistics, e.g., percentiles or confidence and/or tolerance intervals. Time qualified reference ranges in different populations and in different geographic locations and for different frequencies have been presented as chronograms (graphic time plot of data) and/or in their statistically quantified rhythm parameters by numerous investigators [63–76].
5.1.4.2 Chronobiologic Reference Values for Accurate Medical Diagnoses
Chronodesms coupled with optimal sampling protocols are indispensable for making the correct diagnosis of medical conditions and disease states. For example, to diagnose adrenal insufficiency, which is characterized by abnormally low plasma cortisol concentration, it is inappropriate to sample blood late at night from habitually day-active subjects. As shown by the chronodesm of Fig. 13.5 for plasma cortisol of healthy subjects, cortisol values are minimally detectable at this time of day. Blood samples that are drawn in the morning, when cortisol is highest, will be of greatest diagnostic utility. Likewise, it would be inappropriate to conduct a diagnostic test for Cushing’s syndrome, which is characterized by excessive plasma cortisol concentration due to adrenal hyperfunction, by drawing blood samples in the morning when hormone concentration is normally highest. Samples drawn late in the evening will best reveal the correct diagnosis.
5.1.4.3 Chronobiologic Reference Values for Assessing Abnormalities of Period, Phase, and Peak Time
Parameters of biological rhythms, in particular, period, phase, and amplitude, constitute additional references of the time of organization of an individual or a group of subjects. The first step in the evaluation of biological rhythms is inspection of chronograms of the raw data plotted as a function of time. The data of Fig. 13.6 were derived from a group of diurnally active, clinically healthy residents of Minnesota, USA. The temporal variation and waveform can easily be recognized in each of the five different blood cell parameters routinely assessed in patient care. Analysis of variance and t-tests indicate only whether time is a statistically significant source of variation. The period of a rhythm can be determined from sufficiently long time series of repeated measurements using periodogram analysis, which can be applied to equal [77–79] or unequal interval [62, 80] data series. Power spectrum analysis can also be used for rhythm detection and validation of period, however, only for equal-interval data series [81, 82].
Circadian rhythm of circulating neutrophils, lymphocytes, monocytes, platelets, and eosinophils in clinically healthy men and women (24 ± 10 years of age). A total of 150 diurnally active, Caucasian subjects (79 men and 71 women) were sampled every 4 h for 24 h, except in the case of platelets, when 55 subjects (30 men and 25 women) were sampled. Chronograms (time plots) show average count (±SEM) for each variable, except eosinophils, was lowest at 08:00 and highest at night or during sleep. Peak in eosinophils occurred at 04:00 and trough at 12:00. Black and white portions of bottom time axis indicate usual nighttime sleep and daytime activity routine of subjects (figure redrafted using data from Haus and Touitou [374])
Curve fitting procedures are typically used in chronobiology to identify the rhythm’s period by determining, using least squares techniques, the cosine waveform best approximating the time series data and also to derive its peak time (acrophase) and amplitude. “Cosinor” procedures of this nature, introduced and developed by Halberg [83, 84], are suitable for the detection of rhythms in relatively short and noisy time series, even if the data are of unequal interval. However, these methods have limitations [59, 62, 80]. If rhythm parameters like phase and amplitude and their alterations are to be used as quantitative endpoints in single subjects, there may be substantial sampling requirements [59, 61]. The Population Cosinor procedure summarizes rhythm parameters obtained for different individuals belonging to the same population [84, 85] and enables derivation of confidence intervals (95% or other) relating to the entire population. Moreover, rhythm parameters obtained by the Population Cosinor procedure for different groups of individuals, i.e., healthy versus diseased, treated versus nontreated, men versus women, etc., can be compared statistically [86].
Acrophases and amplitudes (with 95% confidence intervals) derived by the Population Cosinor procedure for the blood cell rhythms of Fig. 13.6 are presented in Fig. 13.7. Acrophases of the red blood cell variables occur around midday, while those of the white blood cell variables occur in the early or late evening. Amplitude of the rhythmic variation, i.e., total peak-to-trough difference, derived by the Population Cosinor procedure is rather small. The full extent of the circadian variation only comes to the fore by comparison of the actual values at the peak and trough of the 24-h patterns. When this is done, the range of variation in the raw data (highest value/lowest value × 100) is much more striking, especially in circulating polymorphonuclear leukocytes and lymphocytes (Table 13.2). Ignoring this clinically and highly significant range of variation can lead to diagnostic and therapeutic mistakes.
Circadian acrophase with 95% confidence interval (95% CI) and double amplitude (entire peak-to-trough 24-h variation) expressed as percent of MESOR for hematologic parameters, circulating blood cells, and platelets of the same group of 150 clinically healthy adults as in Fig. 13.6. Mesor, amplitude, and acrophase determined by population mean Cosinor procedure. Acrophase chart (center) indicates the peak time (with 95% CI) of the group circadian rhythm for each variable and amplitude chart (right) indicates the extent of group circadian (peak-to-trough) variation relative to the group 24-h mean (MESOR) +95% CI. Black and white portions of time axis for acrophase at bottom of center plot indicate usual span of nighttime sleep and daytime activity of subjects (figure drawn from data of E. Haus)
Urinary variables are also useful as marker rhythms of the CTS. Urinary sampling is advantageous for variables that show high-amplitude pulsatile or ultradian variation, since they are integrated over the time-interval of sampling. Urinary sampling can be accomplished in babies (by collection vessels fixed to the skin), children, and middle-aged adults by collection of sequential spontaneous voidings. However, it may not be appropriate for elderly subjects who are prone to urinary retention. A weakness of urinary sampling to derive marker rhythms of the CTS is the slight phase difference between the urinary and plasma circadian rhythms of some variables, which in certain cases may be a function of the duration of the intervals between sample collections. A urinary variable which can be used as a reliable phase reference for CTS is the main metabolite of the pineal hormone melatonin, 6-sulfatoxy-melatonin [87–89], which in diurnally active individuals consistently shows highest concentration during the night (Fig. 13.8). The first morning urine contains the major amount of 6-sulfatoxy-melatonin excreted during the 24 h [90, 91]. However, this biomarker can be altered by exposure to artificial (greater than dim level intensity) light at night, even when asleep, thereby limiting its usefulness in lighted environments [92].
Circadian variation of salivary and serum melatonin and cortisol and urinary excretion of 6-sulfatoxy melatonin (metabolite of melatonin) and cortisol in 20 diurnally active, healthy adult men (21 ± 2 years of age). Samples were collected every 4 h during a single 24-h span. Circadian patterns of serum, saliva, and urine cortisol concentration and of serum and saliva melatonin and urine 6-sulfatoxy melatonin concentration are remarkably similar, with only slight difference in exact peak and trough times. Peak cortisol concentration in the three biological fluids occurs in morning and peak melatonin and 6-sulfatoxy melatonin concentration occurs during sleep. Black and white portions of bottom time axis indicate subjects’ usual span of nighttime sleep and daytime activity (unpublished data of E. Haus)
The constituents of saliva are also suitable for use as marker rhythms of the CTS, and saliva can even be sampled in babies while asleep. Numerous saliva solutes mirror their plasma concentration, while others are salivary gland secretory products. Steroid hormones can be measured in saliva [72, 93], with the acrophase of salivary cortisol and/or melatonin serving as useful circadian phase markers [94], as shown in Fig. 13.8. There are, however, some peculiarities in the collection and use of saliva measures for chronobiologic studies, for example, whether samples are collected by natural flow or stimulation, which have to be understood to obtain meaningful results and avoid pitfalls [59].
In accessible tissues, the study of clock gene expression profiles allows direct access to an individual’s circadian phenotype and CTS phasing. Circulating blood mononuclear cells (PBMC) show robust cycling of circadian clock genes [95–99], which are phase-adapted to habitual sleep timing [95, 97], but altered in patients with circadian sleep disorders [98] and cancer [100]. The circadian clock in the PBMC represents a peripheral oscillator usually linked, presumably by humoral factors, to the central brain (SCN) oscillator, thereby representing an integral marker of the CTS. Alteration of the phase relationship between the central brain clock and PBMC peripheral clock has not been reported in human subjects. Development of a rapid, inexpensive means of determining clock gene expression in PMBC would be useful to identify stages of the circadian clock.
5.1.4.4 Chronobiologic Reference Values for Drug-Delivery Systems and Outcomes Assessment of Chronotherapy Trials
Identification of rhythm stage (biologic time) at a given astronomic time, e.g., clock hour, day of week, etc., may be of importance in choosing the time for optimizing desired and/or minimizing adverse drug effects. Marker rhythms are used to denote the stage of a patient’s endogenous time organization. Habitual awakening and bed times are the simplest, noninvasive, and least expensive markers of the CTS. Body temperature and activity circadian rhythms, which can be easily measured by noninvasive automatic instrumentation, are other useful markers of the CTS [101, 102].
A variety of circadian marker rhythms are useful to evaluate the outcomes of drug-delivery systems. For example, substitution therapy for adrenal insufficiency conventionally entails oral cortisol administration (25–35 mg/24 h), with or without 9-α-fluorocortisol. Taking the daytime activity–nighttime sleep cycle as the marker rhythm of reference for the CTS leads to the expectation that plasma cortisol be highest in the morning (Fig. 13.5). Cortisol substitution therapy entailing the typical three equal doses per day (at breakfast, lunch, and dinner/bedtime) homeostatic-type schedule greatly alters the CTS from normal relative to pertinent urinary circadian marker rhythms (Fig. 13.9). The acrophases of the circadian rhythm of grip strength and urine concentrations of 17-hydroxycorticosteroids (metabolite of cortisol), 17-ketosteroids (metabolite of sex hormones), potassium, and sodium are abnormally displaced to later phasing by up to 6 h. In contrast, when therapy is applied so most, i.e., 2/3 or 3/4, of the daily dose is ingested in the morning and the rest at bedtime, so as to mimic the normal circadian rhythm of plasma cortisol, the CTS is normalized with reference to the circadian urinary and strength (grip strength) marker rhythms, and patient performance status is best improved [103].
Circadian acrophase chart with 95% confidence intervals (95% CI) for several physiologic variables in diurnally active (~07:00 to ~23:00) healthy subjects and patients with adrenal insufficiency (AI) treated by different cortisol substitution schedule. Urine and biological measurements were collected at ~4-h intervals during 48-h study spans when following a self-selected diet. Acrophases of circadian rhythms in grip strength and urinary excretion of 17-OHCS (urinary metabolite of cortisol), 17-KS (urinary metabolite of adrenal androgens), K+, and Na+ in patients treated with three equal doses of cortisol (Schedule B, homeostatic substitution schedule: ingestions at roughly equal intervals – 08:00, 13:00, and 20:00) show abnormal phasing, with acrophases lagging by ~6 h behind those of controls and giving rise to a misaligned and biologically inefficient circadian time structure. In contrast, treatment of the same patients with 2/3 or 3/4 (Schedule A, chronotherapy substitution schedule:) of the daily cortisol dose at 07:00 and the remaining fraction at 23:00 preserves the circadian time structure, i.e., circadian acrophases of rhythms in these same variables are comparable to those of healthy subjects. Black and white portions of bottom time axis indicate usual span of nighttime sleep and daytime activity of the AI patients and healthy controls (drawn using data of Reinberg et al. [103])
Another example concerns chronic synthetic corticotherapy for inflammatory conditions such as rheumatoid arthritis and bronchial asthma. Determining the best circadian time of methylprednisolone (MP) administration, qualified by minimizing adrenal suppression as an adverse effect, can be judged using time qualified reference values provided by the circadian rhythm of urinary 17-hydroxycorticosteroids. Single 4-h MP infusion at a rate of 660 μg/h between midnight and 04:00, the approximate trough time of the circadian rhythm of cortisol in day-active persons, results in profound adrenal suppression (Fig. 13.10). In comparison, MP infusion in twice the dose, i.e., as an 8-h infusion, between 08:00 and 16:00 causes no adrenal suppression. Finally, 4-h MP infusion commencing either at 04:00 or 16:00 results in intermediate level of adrenal suppression [104]. Table 13.3 also shows how the plasma cortisol time qualified reference 08:00 h concentration can be used to assess differences in patient tolerance (absence of plasma cortisol suppression) according to tablet triamcinolone (8 mg/24 h) drug delivery schedule [105]. Accordingly, the first chronotherapy widely applied in clinical medicine, in the 1960s, was the alternate day, morning time schedule of MP tablets [106]. The original clinical trials showed that this MP chronotherapy resulted in significantly better patient tolerance, i.e., reduced adverse effects, and high therapeutic benefit. Recently, a European pharmaceutical company introduced a new chronotherapy, a delayed release synthetic corticosteroid dosage form designed for ingestion at bedtime to achieve highest serum concentration in the morning so as to minimize or avoid completely the adverse effects of this class of medications [107, 108].
Circadian rhythm-dependent differences in induction of adverse effect of adrenal suppression, i.e., inhibition of cortisol synthesis and secretion, from methylprednisolone (MP) infused at a rate of 660 μg/h at different circadian times. Urine samples were collected from diurnally active young adult subjects at 2-h intervals and analyzed for concentration of the urinary metabolite of cortisol, 17-OHCS (solid circles = control, nontreatment placebo patterns; open squares = MP-affected cortisol patterns). Eight-h MP (660 μg/h) infusion during the time of day when endogenous secretion of cortisol is highest, between 08:00 and 16:00 (lower left panel) exerts no adrenal suppression; however, MP infusion (660 μg/h) for only 4 h at circadian times when cortisol synthesis and secretion are minimal, between 00:00 and 04:00 (upper left panel) or reduced, between 04:00 and 08:00 (upper right panel) or 16:00 and 20:00 (lower right panel), induces severe to moderate adrenal suppression, respectively. Black and white portions of bottom time axis indicate usual span of subjects’ nighttime sleep and daytime activity (figure drawn using data from Angeli [104])
Time qualified reference values might also be useful for the development of future biomimetic drug delivery systems. For example, the circadian rhythm of tumor necrosis factor-alpha (TNF-α) seems to be a key biomarker for timing methotrexate (MTX) chronotherapy for rheumatoid arthritis [109]. Cytokines play an important role in the pathogenesis of rheumatoid arthritis and show 24-h rhythms, both in animal models and patients. Studies on animal models, which develop autoimmune disorders that share similarities with human rheumatoid arthritis, found MTX administration exerted best effect when synchronized with the TNF-α 24-h rhythm [109, 110]. Specifically, in the MRL/lpr mouse animal model, inflammation and TNF-α were best reduced when MTX dosing coincided with the circadian time of TNF-α increase. These findings have been trialed in an initial small pilot study on rheumatoid arthritis patients. Patients were transferred from the standard MTX three times/week treatment schedule, entailing dosing after breakfast and supper on day 1 and after breakfast day 2, to a chronotherapy schedule, entailing the same dose and number of treatments/week but with the MTX administration times changed to bedtime on treatment days so as to coincide with the expected TNF-α rise time. Disease activity scores and health assessment questionnaire ratings were significantly improved by the chronotherapy MTX schedule. Significant symptom relief was observed in 41.2% of patients, and 23.5% of patients achieved clinical remission without significant adverse effects [109, 110]. This example illustrates the value of time qualified reference criteria as circadian rhythm biomarkers of disease activity in animal modeling and patient studies to improve therapeutic outcome and to potentially develop chronotherapeutic drug delivery systems.
5.1.4.5 Other Than Circadian Time-Qualified Reference Values
This chapter emphasizes circadian as well as short-period oscillations. In many, but not in all, periodic variables of clinical interest and of potential importance for timed drug delivery, the circadian rhythm is of highest amplitude [59, 68, 111]. However, circadian rhythms are modulated by superimposed rhythms of higher frequencies as well as pulsatile variations which may lead to spurious results and aliasing. Some variables exhibit rhythms of ~7 days (circaseptan) or multiples thereof. An acrophase chart of circaseptan rhythms of some laboratory variables is shown in Fig. 13.11. In the immune system, in particular, a prominent circaseptan periodicity determines in part the host response to introduced antigen, e.g., in transplantation biology [112, 113]. Circadian/infradian (bioperiodicities >28 h) interactions in the effect of chemical carcinogens also have been identified in animal studies [114, 115]. Circaseptan rhythms in drug effects should be expected in human beings and may be of importance in some settings, but they have yet to be much explored.
Circaseptan (~7-day) acrophase chart, with 95% confidence intervals (95% CI), of selected clinical laboratory parameters of blood, plasma, and serum determined in groups ranging in size from 11 to 20 clinically healthy, diurnally active subjects (21–46 years of age) sampled three times/week between 07:30 and 08:00 for several weeks during a 90-day span. Seven-day temporal patterns with acrophases generally on week days are apparent for each variable. Rather large 95% CIs result from relatively infrequent sampling scheme (only three samples/week) and relatively small sample size (figure drawn using data from Haus and Touitou [59])
6 Rhythm-Dependent Patterns of Acute and Chronic Medical Events and Conditions
Many biological and chemical processes inherent to disease pathophysiology are rhythmic, giving rise to multifrequency temporal patterns in morbid and mortal events and symptom intensity. In general, circadian patterns in disease have been substantiated by cross sectional, population based epidemiology investigations and by both cross-sectional and longitudinal clinical case series studies.
6.1 Circadian Rhythms in the Manifestation and Severity of Disease
The manifestation and severity of many acute and chronic medical conditions and the occurrence of several life threatening medical events exhibit rather precise timings as depicted in Fig. 13.12. Gout [116, 117], gallbladder [118], renal [119], fibromyalgia [120, 121], and PUD (peptic ulcer disease) attacks [122] are most frequent late at night or initial hours of sleep. Acute pulmonary edema [123], congestive heart failure [124], bronchial asthma and COPD (chronic obstructive pulmonary disease) [125, 126], atopic dermatitis [127], claudication of the legs [128], vagontic atrial fibrillation [129], and nocturia [130] manifest or worsen nocturnally as do sleep apnea [131], restless leg syndrome and periodic limb movement disorders [132], and BP elevation of secondary hypertension [133]. Sudden infant death (SIDS) [134], allergic rhinitis, acute of upper respiratory infectious disease [50, 135, 136], and rheumatoid arthritis [137] are either most intense overnight or in the morning. Migraine headache [138, 139], angina pectoris [140, 141], ventricular arrhythmia [129], acute myocardial infarction [142], sudden cardiac death [142, 143], ischemic and hemorrhagic stroke [144], fatal pulmonary embolism, and hypertensive crises [145, 146] are most frequent in the morning, as are the symptoms and crises of certain other cardiovascular disease (CVD) conditions, such as adrenergic fibrillation [129], aortic aneurysm rupture, third degree atrial–ventricular heart block, and acute arterial limb occlusion [129]. Depression is most severe in the morning [147, 148], as are alcohol and tobacco cravings [149, 150]. Symptoms of osteoarthritis (OA) worsen during the course of daily activity, typically being most intense in the evening [151, 152]. Perforated and bleeding ulcer is reported to be most common in the afternoon [153, 154], and intraocular pressure of glaucoma rises to peak level during sleep [155, 156]. Some seizure disorders are triggered by specific sleep stages and/or transitions between sleep and wakefulness [157, 158]. Finally, advanced and delayed sleep phase disorders (ASPD and DSPD) manifest in the early evening and middle of the nighttime, respectively [159].
Approximate time(s) during the 24 h in diurnally active individuals (waking span from ~06:30 to ~22:30 alternating with nighttime sleep from ~22:30 to ~06:30) in the manifestation of the most severe signs and symptoms of various chronic medical conditions, acute severe life-threatening (morbid and mortal) events, and acute infectious and other nonserious medical ailments. Apparent is the large number of medical conditions and events that evidence predictable-in-time (24 h) patterns in symptoms or risk of life-threatening events. Times of greatest risk are approximate, varying to some extent between morning and evening chronotypes, i.e., larks and owls. Some conditions show more than one time of elevated risk, e.g., angina pectoris, acute myocardial infarct (AMI), sudden cardiac death (SCD), epistaxis, and certain epileptic seizure disorders. Such 24-h patterns constitute one important basis for chronotherapeutics. Sleep and activity spans indicated, respectively, as the darkened and white portions of the circle (Smolensky and Haus, unpublished)
6.2 Medical Conditions Manifesting as a Disrupted CTS
It is assumed, even by seasoned chronobiologists, that the CTS is normal and more or less comparable among human beings, excluding differences in phasing seen in the small proportion of extreme morning and evening chronotypes. This assumption is not always valid. Some persons exhibit significant alteration and disruption of the CTS without negative effects, while others are significantly affected, suggesting there may be genetic differences in tolerance to disruption of the CTS, thus the need to develop special therapeutic interventions to reset it to normal.
Certain sleep disorders are directly representative of abnormalities of the circadian time-keeping system [160, 161]. For example, DSPD syndrome is characterized by severe sleep onset insomnia. Typically, sleep is impossible to achieve until 03:00 or later in affected children and adults, and consequently there is great difficulty in awakening the next morning at the normal time. The underlying mechanism of DSPD may be abnormal sensitivity to evening light, causing the clock controlling the sleep–wake cycle to reset to a later time by means of a phase response mechanism [162]. ASPD is characterized by early evening sleep onset, as early as 19:00–20:00 and very early morning awakening. The underlying mechanism of ASPD in some individuals and families involves a genetic difference in the circadian time keeping system [163]. Non-24-h sleep–wake syndrome, a relatively uncommon condition, is characterized by free-running of the activity–rest rhythm from the normal 24-h period. Diagnostic studies show sleep-onset and sleep-offset times from one day to next are progressively delayed in some patients and advanced in others by as much as ~2 h. The period of the inherited biological clock controlling the sleep–wake cycle is abnormal in these individuals, being as long as 26–27 h in some patients and as short as ~23 h in others.
Shift work intolerance is a medical condition that may be manifested in career rotating or permanent night shift workers, typically around the age of 45–50 years. It is characterized by poor quality and inadequate duration of daytime sleep when on the night shift, mild to severe depression and/or irritability, compromised work performance, digestive or PUD, and often hypertension [8, 9]. It appears that the pathology of this condition involves CTS desynchronization, with the period, amplitude, and staging of circadian rhythms altered significantly [164]. Transfer of affected employees from shift to day work will eventually alleviate the disrupted CTS and the associated medical complaints. Currently, no so-called chronobiotics, medications or other interventions capable or resetting and normalizing the CTS are known. Although melatonin and bright-light therapy, depending on their biological timing, are able to shift (delay or advance) or stabilize the CTS in a phase response manner (Fig. 13.3), they are yet to be endorsed by the medical community to treat shift work intolerance.
Blind individuals, who are unable to perceive environmental synchronizing light cues, often show desynchronized CTS, and manifest free running circadian rhythms, chronic sleep problems, and depression. A series of studies have found that physiologic low dose melatonin administered at the right circadian phase can, over time, restore normal CTS to totally blind persons and relieve medical complaints [40, 165]. It is of interest that certain mood disorders, such as seasonal affective mood disorder (SAD), premenstrual dysphoric disorder (PMDD), and even regular endogenous depression, have been associated with abnormalities of the circadian time keeping system [166–169].
7 Chronopharmacology: Biological Rhythms and Medications
The biological time when medications are administered may affect their pharmacokinetics (PK) and pharmacodynamics (PD), no matter their route of delivery.
7.1 Chronopharmacology: Definition and Concepts
Chronopharmacology is the study of the manner and extent to which the PK and PD of medications are affected by endogenous biological rhythms, and also how the time of dosing affects biological time keeping and CTS, i.e., period, level, amplitude, and phase [20, 170–174]. The concept of chronopharmacology is in direct conflict with that of homeostasis. The theory of homeostasis promotes as a major goal for drug delivery systems constancy in medication levels, since it is assumed that constancy in drug levels translates to constancy in drug effects and avoidance of adverse effects. The fields of chronopharmacology and chronotherapy challenge these long held concepts and goals. Indeed, numerous studies clearly indicate the time of ingestion, inhalation, injection, infusion, or cutaneous application of medications, especially with reference to circadian rhythms, can affect PK and PD, and sometimes markedly.
7.2 Chronokinetics
Chronokinetics refers to dosing-time (i.e., biological rhythm) dependent differences in absorption, distribution, metabolism, and elimination of medications [20, 170, 171]. This is revealed, for example, by administration time differences in PK parameters of various types and classes of therapeutic agents, including time to peak concentration, peak height, elimination rate, volume of distribution, and area under the time–concentration curve [20, 175–178]. These differences result from circadian rhythms in gastrointestinal pH affecting drug dissolution plus circadian rhythms in gastric emptying, motility, and blood flow affecting the rate, and sometimes amount, of drug absorption [179]. Circadian rhythms in hepatic blood flow and enzyme activity affect drug biotransformation and metabolism. Hepatic and kidney rhythms, in bile function and flow and renal glomerular filtration and tubular function, affect drug elimination [175]. Many examples of dosing time differences in the PK of commonly prescribed medications can be found in previous published reviews [176, 178, 180].
7.3 Chronodynamics
Chronodynamics refers to dosing time (i.e., rhythm dependent) differences in the effects of medications that cannot be attributed to their PK [20, 170]. Such administration time differences result from rhythms in free versus bound drug fraction, number and conformation of drug-specific receptors, second messenger and ion channel dynamics, and rate limiting steps in metabolic pathways [20, 181]. Beneficial and adverse effects of medications may both vary significantly according to their administration time.
Many examples of chronodynamics can be cited. One is the differential effect of constant rate infusion of H2-receptor blocker medication during the 24 h. Gastric acidity exhibits significant circadian rhythmicity, both in healthy subjects and peptic ulcer patients. Under fasting condition, basal (nonfood stimulated) gastric hydrogen ion concentration of diurnally active subjects is higher around and just after bedtime at night than in the morning when awakening (Fig. 13.13a) [122]. Constant rate 24-h infusion of therapeutic doses of the H2 antagonist famotidine exerts differential day–night efficacy, i.e. suppression of gastric acid secretion (Fig. 13.13b) [179]. Drug effect is attenuated in the evening and at night, indicating partial resistance to H2-receptor blockade at this time [182–184].
(a) Circadian pattern in basal (fasting) gastric acid secretory rate in 14 active healthy (closed circles) and 21 diurnally active, peptic ulcer disease (closed squares) subjects. Dashed horizontal line represents mean 24-h secretory rate for ulcer group (5.76 ± 0.98 mEq H+/h) and solid horizontal line represents mean rate for healthy group (4.12 ± 0.40 mEq H+/h). Note reduced morning and elevated evening gastric acid secretory rate in both groups. Black and white portions of bottom time axis indicate subjects’ usual span of nighttime sleep and daytime activity (figure redrawn using data of Moore and Halberg [122]). (b) Median 24-h intragastric pH profiles of 12, ordinarily diurnally active, fed duodenal ulcer patients. Dashed line represents control (placebo) 24-h study; solid line represents IV continuous infusion of H2-receptor antagonist famotidine at a rate of 3.2 mg/h for 24 h; dash–dot line represents IV continuous infusion of famotidine to same subjects at a higher rate of 4.0 mg/h for 24 h. Meals and drink are shown at bottom by arrows: L = lunch, T = tea, D = dinner, and S = snack. In spite of constant infusion of the H2-receptor antagonist, intragastric pH exhibits pronounced decline (higher acidity) commencing late afternoon/evening and lasting to the initial hours of usual sleep span, when pH is lowest (placebo curve) and rate of gastric acid secretion is highest (as shown in Fig. 13.13a). Black and white portions of bottom time axis indicate subjects’ usual nighttime sleep and daytime activity spans (figure redrawn using data from Moore and Merki [179])
A second example is the differential anticoagulant effect during the 24 h of constant rate infusion of standard (nonlow-molecular weight) heparin on deep vein thrombosis patients [185, 186]. The effect may be too great overnight, posing risk of hemorrhage, while in the morning it may be subtherapeutic, risking aggravation of the medical condition (Fig. 13.14). These circadian rhythm dependent effects are also found when heparin is administered by other routes [187].
Circadian variation in three measures of blood coagulation – Activated Partial Thromboplastin Time (aPTT), Thrombin Time (TT), and Factor Anti-Xa inhibition – in six ordinarily diurnally active venous thrombo-embolism patients administered unfractionated heparin by constant-rate continuous intravenous infusion for 48 consecutive hours. Initial daily dose of heparin was adjusted on an individual patient basis to maintain aPPT between 1.5 and 2.5 times the before-treatment 08:00 level. Top: Circadian variation in heparin effect on coagulation parameters shown in standard laboratory units. Bottom: Circadian variation of the same coagulation parameters after data re-expressed as percent of each subjects’ time series mean. Maximal anticoagulation effect was achieved ~04:00 and minimum effect ~08:00. Differences between night and morning values amounted to ~50% for aPTT, 60% for TT, and 40% for Factor Anti-Xa inhibition. In four patients, the nocturnal peak in aPTT exceeded the upper desired limits of anticoagulation and the heparin effect was too great, while during the wake span in some patients heparin produced too weak an anticoagulation effect. Sleep–wake pattern of group is represented at bottom of each figure; shaded portion represents nighttime sleep span and white portion represents diurnal wake span (figure drawn using the data of Decousus [185, 186])
Other examples involve oral dosage forms. For example, clinical trials of nonsteroidal anti-inflammatory drugs (NSAIDs) demonstrate better therapeutic effect on the characteristic morning symptoms of pain, stiffness, and inflammation of rheumatoid arthritis and with less side effects when ingested in the evening or at bedtime than morning [188]. On the other hand, NSAIDs are more effective in reducing the characteristic afternoon and evening peak intensity of OA symptoms when ingested in the morning or around lunch time, although with elevated risk of adverse events compared to evening dosing [188].
Another important set of examples, given the large number of people worldwide diagnosed with hypertension, e.g. an estimated 63 million in the USA and 163 million in China, are the differential ingestion time dependent effects of BP-lowering monotherapies, including angiotension converting enzyme inhibitors (ACEIs), angiotension receptor blockers (ARBs), calcium channel blockers (CCBs), ß-blockers, α-blockers, and diuretics [189]. As shown in Table 13.4, the average enhancement relative to baseline of the bedtime versus upon-awakening regimen of the diuretic torasemide (5 mg/day) amounted to 8.4/6.1 mmHg in the 48-h SBP/DBP means, 8.3/6.2 mmHg in the awake SBP/DBP means, and 8.2/5.5 mmHg in the asleep SBP/DBP means. Further, bedtime compared to conventional upon-awakening scheduling of the ACEIs ramipril (5 mg/day) and spirapril (6 mg/day) resulted in greater reduction of asleep SBP/DBP means relative to baseline of 9.0/7.4 and 7.1/4.0 mmHg, respectively. Finally, in two studies on different cohorts of hypertension patients, bedtime compared to conventional upon-awakening dosing of the ARB valsartan (160 mg/day) better attenuated the asleep SBP/DBP means from baseline by 9.6/5.6 and 8.2/5.8 mmHg, respectively. The differential effect of the timing of BP-lowering medications is not simply dependent on drug half life, as illustrated by findings for the long-half life (24 h) ARB telmisartan (80 mg/day) medication. When taken at bedtime, rather than upon awakening, reduction from baseline of the asleep SBP/DBP means was improved by 5.5/3.3 mmHg. The findings of studies summarized in Table 13.4 are consistent; bedtime as opposed to morning ingestion of different hypertension therapies exerts significantly greater reduction of sleep-time BP and better normalization, restoration, and preservation of the BP circadian pattern.
Normalization of sleep-time BP level seems to be a new and important clinical target for hypertension therapies for at least two reasons. First, recently completed clinical outcome studies indicate the nondipping BP pattern (i.e., absence of 10–20% decline in SBP and DBP during sleep relative to daytime levels) is associated with increased risk of injury to heart, brain, blood vessel, and kidney tissue, plus heightened 5-year risk of CVD mortality as reviewed in Portaluppi and Smolensky [190]. Second, a recently published 5.6-year clinical outcomes trial (Sect. 13.8.2.2) documents that regular ingestion of at least one BP-lowering medication at bedtime, as opposed to ingestion of all such prescribed medications in the morning upon awakening, results in significantly better protection against heart attack and stroke [191]. Results such as these form the basis for the development of hypertension chronotherapy drug delivery systems.
A growing trend in the treatment of some medical conditions is combination therapy of two or more complementary acting medications. Polytherapies thus far developed and marketed for hypertension entail a morning-time indication with simultaneous release of both medications during the 24-h dosing interval. Rhythm dependencies in the PK and PD of the individual constituents should be suspected and may be especially important in optimizing their synergistic efficacy and safety. One illustrative example is the differential magnitude of therapeutic effect exerted by CCB amlodipine and ARB valsartan when ingested in combination in the morning versus at bedtime [192]. Hypertension patients were randomized across one of four treatment schedules: (1) ingestion of both medications in the morning upon awakening; (2) ingestion of both medications at bedtime; (3) ingestion of amlodipine upon awakening and valsartan at bedtime; and (4) ingestion of valsartan upon awakening and amlodipine at bedtime. The BP-lowering effect upon the daytime, sleep time, and 48-h SBP and DBP means derived by ABPM was strong no matter the scheduling of the two medications in combination (Fig. 13.15). However, the synergetic effect when both were routinely ingested together at bedtime as compared to upon awakening resulted in a nearly 50% greater mean reduction in daytime BP, more than doubling of the mean reduction in sleep time BP, and more than 50% greater mean reduction in 48-h BP.
Changes from before-treatment baseline in awake (active hours), asleep (nighttime sleep span), and 48-h means of systolic blood pressure (SBP) in 203 hypertension persons after 12 weeks of differently scheduled 160 mg valsartan/5 mg amlopidine (angiotensin receptor antagonist/calcium channel blocker) combination therapy: valsartan (V) + amlodipine (A) ingested together on awakening, V ingested on awakening + A ingested at bedtime, V ingested at bedtime + A ingested on awakening, and V + A ingested together at bedtime. Different timings of the V + A combination therapy exerted statistically significant differences in effect on the awake, asleep, and 48-h SBP means. Strongest effect was exerted when V and A were ingested together at bedtime, i.e., the asleep SBP mean (relative to the pre-treatment baseline level) was reduced on average by about twice the amount compared to when V was ingested on awakening and A at bedtime or when V + A were ingested together on awakening. These findings illustrate the potential prominent circadian rhythm-dependent effect of medications used in combination and importance of such phenomena to drug-delivery (figure drawn using data from Hermida et al. [192])
While strong BP-lowering effect is a desired clinical outcome, over-correction of BP during nighttime sleep could be problematic for certain patients, since too low nocturnal BP may increase the risk of anterior ischemic optic neuropathy in hypertensive glaucoma patients, and in elderly patients nighttime falls with bone fracture and even nocturnal stroke. Obviously, the development of combination polytherapies should take into account possible strong circadian rhythm dependencies in synergetic effects of constituent medications, which might enable the use of lower doses, thereby being a potential means of reducing cost of therapy.
7.3.1 Administration Time Dependent Differences Between Men and Women in PD of Medications
An increasing number of reports show differences, including treatment-time ones, between men and women in the PD of various classes of medications. Women develop cough related reactions to the ACEI lisinopril three times more often than men [193]. ß-Blockers tend to be less effective in women than men, particularly in stroke prevention, while diuretics may be of more value in older women because of decreased bone loss and reduced risk of hip fracture [194]. Moreover, women tend to show a stronger BP response than men to amlodipine (CCB) [195], candesartan (ARB) [196], and the combination of irbesartan (ARB)/hydrochlorothiazide (diuretic) [197].
Administration-time differences between men and women in therapeutic responses to blood pressure medications also have been detected. The first example involves low-dose (5 mg/day) amlodipine. In this as yet unpublished study lead by one of the authors (R. Hermida), 193 diurnally active hypertensive subjects, 101 men, and 93 women, were randomized to two groups; subjects of one group ingested amlodipine daily upon awakening in the morning, and those of the other group took amlodipine daily at bedtime. Subjects underwent 48-h ABPM before and at the end of treatment. Morning treatment revealed difference between men and women only in the amount of sleep time BP reduction, with the average decrease in sleep time SBP being nearly 50% greater in men than in women (Fig. 13.16). Bedtime treatment, on the other hand, resulted in a much more prominent difference in effect between men and women; average reduction of the wake-time, sleep-time, and 48-h SBP means was ~5 mmHg greater in women than men.
Sex differences in blood pressure-lowering effects of amlopidine (5 mg) when ingested at different times of the day. Hypertensive subjects, 101 men and 93 women, were randomized to two groups; subjects of one group ingested the medication daily in the morning upon awakening from nighttime sleep, and those of the other group ingested it daily at bedtime. Subjects underwent 48-h ambulatory blood pressure monitoring (ABPM) before and after 12 weeks of treatment. Morning treatment (Top, a) revealed difference between men and women only in the amount of the sleep-time BP reduction, with average decrease in sleep-time systolic blood pressure (SBP) being ~50% greater in men than women. Bedtime treatment (Bottom, b) resulted in much greater statistically significant reduction of the wake-time, sleep-time, and 48-h SBP means, on average by ~5 mmHg, in women than men (figure drafted using the data of Hermida et al., unpublished)
The second example involves the little known effect of low-dose (100 mg/daily) aspirin on SBP and DBP [198]. In a Spanish study, 130 men and 186 women with untreated mild hypertension were randomized to take aspirin either on awakening or at bedtime daily for 3 months. ABPM was performed for 48 h before and after treatment. In men who routinely ingested aspirin upon awakening, the effect relative to baseline on the awake time, sleep time, and 48-h SBP/DBP means was nil; in women aspirin slightly elevated BP levels. In contrast, aspirin ingestion at bedtime significantly reduced all SBP/DBP means, and more so in women than men (Fig. 13.17). Factors influencing the stronger response of BP to low dose aspirin at bedtime included female gender, elevated fasting glucose, and high glomerular filtration rate. This study corroborates significant administration-time-dependent effect of low dose aspirin on ambulatory BP in Spanish subjects with untreated mild hypertension, and further illustrates the significant differences between men and women in the circadian-time-related variation in responses to medications [198]. Additional studies are needed on men and women of other ethnic/racial groups to explore potential sex and racial interactions relative to the circadian dosing time of aspirin and other classes of medications.
Differential effect of low-dose (100 mg/daily) aspirin on systolic blood pressure (SBP) reduction when ingested at different times of the day. A group of 316 untreated Spanish (130 men and 186 women) mild hypertension subjects (44.1 ± 13.2 years of age) was randomized to ingest low-dose aspirin either on awakening or at bedtime daily for 3 months. ABPM was performed for 48 h before and after treatment. In both men and women who ingested low-dose aspirin daily upon awakening, SBP wake-time, sleep-time, and 48-h means were unchanged from baseline. In constast, those who ingested low-dose aspirin daily at bedtime, the three SBP means were reduced and significantly more so in women than men (figure redrawn from data published by Ayala and Hermida [198])
The examples presented here call attention to the fact that drug-delivery systems need to be sensitive to potential differences between women and men in the circadian rhythm dependencies of the PD of therapeutic agents. However, not all medications, for example, those used to treat hypertension, are prone to circadian-time/sex interactions, for example, as demonstrated by results of studies with the ARB valsartan [199]. Bedtime dosing of valsartan (160 mg/day) better reduced sleep time SBP and DBP than did morning dosing and without administration time differences between women and men.
7.3.2 Chronotoxicology: Rhythm Dependencies in Adverse Effects of Medications
Chronotoxicology, an aspect of chronodynamics, refers to dosing-time (rhythm-dependent) differences in the susceptibility/resistance to potentially noxious exposures to biological, chemical, or physical agents, including infectious, therapeutic, and radioactive agents [200, 201]. The concept was initially demonstrated 60 years ago through a series of around-the-clock LD50 challenge studies of different groups of rodents [202]. Circadian rhythm-dependencies of adverse effects of medication are rarely explored in preclinical animal investigations and clinical trials. Knowledge of such dependencies comes from studies designed and conducted by chronobiologists. In humans, administration-time differences in the occurrence and severity of adverse effects have been reported, for instance, for synthetic hormone, NSAID, anticoagulant, aminoglycoside antibiotic, and hypertension medications [104, 185, 186, 188, 200, 201, 203].
One example entails the manifestation and severity of adrenocortical suppression, i.e., inhibition of cortisol synthesis and secretion, a potential undesired effect of synthetic anti-inflammatory glucocorticoid medications, such as dexamethasone, prednisolone, methylprednisolone, and triamcinolone. The risk and severity of adrenocortical suppression differ not only with dose, but also with time of ingestion, infusion, or injection of the medications, as demonstrated by a substantial number of animal and clinical studies commencing 50 years ago [50, 104, 105, 204–207]. As discussed in Sect. 5.1.4.4 and shown in Fig. 13.10 and Table 13.3, these medications are best tolerated, i.e., cause least adrenocortical suppression, when the entire dose is administered in the morning at the commencement of daily activity, which corresponds in time to the peak of the cortisol circadian rhythm. They are least tolerated, i.e., cause greatest adrenocortical suppression, when the entire or a significant portion of the daily dose is administered late in the day at supper or bedtime. These chronotoxicological findings have significantly impacted how synthetic corticosteroids are used in clinical practice. Since the 1960s, tablet MP and other such corticotherapies have been recommended as single-daily or alternate-day morning doses to minimize adrenal suppression, especially in patients requiring these potent anti-inflammatory medications chronically [106].
Other clinical examples of chronotoxicity are gastric effects caused by NSAIDs and pedal edema induced by the dihydropyridine CCB nifedipine GITS system. The likelihood of adverse gastric and neurologic effects of the slow release formulation of the NSAID indomethacin is greater when routinely ingested once-daily in the morning as opposed to evening. It is worthy of mention that discontinuance of indomethacin therapy in one large scale study was much more common when patients ingested the medication once-a-day in the morning or midday than at bedtime [188]. Risk of nifedipine-induced pedal edema varies greatly according to treatment time. In one study, the incidence of pedal edema was 13% when the medication was ingested upon-awakening, but only 1% when ingested at bedtime [208].
Medications with high risk of adverse effects and relatively narrow therapeutic range are especially prone to significant dosing time differences in patient tolerance. This is clearly exemplified by the findings of numerous circadian rhythm studies on animal models and patient trials involving cancer therapies [209–213]. Circadian chronotoxicities have been demonstrated for roughly 40 highly prescribed antitumor medications, including arabinosylcytosine, cisplantin, carboplatin, oxaliplatin, cyclophosphamide, docetaxel, doxorubicin, etoposide, 5-fluorouracil, and methotrexate. Moreover, animal studies also show vulnerability to fetal growth defects, malformations, and death due to teratogens, such as cortisone, dexamethasone, hydroxyurea, 5-fluorouracil, cyclophosphamide, cyctosine arabinoside, and ethanol, can sometimes differ greatly with their circadian timing [214].
8 Chronotherapeutics
Collectively, the examples cited in Sect. 13.7 substantiate the importance of body rhythms in determining the extent of therapeutic effect and safety of medications. They also reveal opportunities for the design of drug delivery systems to improve both desired outcomes and patient tolerance of pharmacotherapies by taking into consideration their specific circadian chonokinetics, chronodynamics, and chronotoxicologies.
8.1 Definition and Concepts
Chronotherapeutics is the purposeful delivery of medications in time to meet biological-time determinants of disease pathophysiology (chronopathology) and chronopharmacology (chronokinetics, chronodynamics, and chronotoxicology) of medications to optimize outcomes and minimize/avoid adverse effects [20, 170]. Chronotherapeutics may involve improved delivery of established therapies or new medicines. In certain instances, chronotherapeutics is achieved by unequal morning and evening dosing schedules of conventional sustained release 12-h tablet and capsule systems, optimal timing of conventional once-a-day delivery systems, or application of special drug-delivery systems to proportion medications over the 24-h cycle in order to meet rhythm determined requirements. Current first generation chronotherapeutic drug delivery systems demand strict adherence by patients to recommended dosing time(s), with reference to the sleep–wake cycle, to achieve desired outcomes. Success of these chronotherapies also requires appropriate understanding by the clinical community of the concepts of chronobiology and chronotherapeutics to ensure their proper application [215].
Revision of dosing schedule, reformulation of the drug-delivery system, and use of programmable infusion pumps to deliver medications at biologically opportune times are some simple chronotherapeutic improvements that may reap enormous benefits. In some instances, chronotherapeutics may entail delivery of medication, especially endocrine and neuroendocrine analogues, in a high frequency mode to mimic the “language” of the endocrine and neuroendocrine systems. Chronotherapeutics may also entail resetting or reorganizing a disordered or desynchronized CTS by a special class of medications termed “chronobiotics,” an example of which is melatonin. Judicious choice of ingestion time of a physiologic dose of melatonin by diurnally active persons can result either in a phase advance of the CTS, when dosed in the afternoon or early evening, or phase delay, when dosed in the morning after awakening (Fig. 13.3). When properly timed, melatonin accelerates adjustment (phase shift) of the circadian system of persons rapidly displaced by aircraft across time zones and lessens the duration and severity of jet lag symptoms [92, 216].
8.2 Chronotherapeutics: History and Early Applications
The first widely applied chronotherapy, introduced in the 1960s, entailed alternate-day morning dosing of tablet corticosteroid MP medication [106]. Other chronotherapies have since been widely used in clinical medicine in the USA, Europe, and Asia as summarized in Fig. 13.18. These include special evening theophylline and ß2-agonist tablet and capsule systems for nocturnal asthma [50, 217, 218], conventional evening H2-receptor antagonists and proton pump therapy for PUD [179, 219], conventional evening tablet statin medications for hyperlipidemia [220], and several delayed onset hypertension formulations [221–225]. In certain centers, based on the results of local clinical trials, some medications, for example, H1-receptor blockers, NIADS, thyroid supplementation, low-dose aspirin, and melatonin, are timed as chronotherapies.
Registered and experimental chronotherapies. Since the 1960s, various chronotherapies have been introduced into clinical medicine. Some are recommended for administration before dinner, such as the H2-receptor antagonist and proton-pump inhibitor medications for peptic ulcer disease and gastroeosphageal reflux disorder. Statin medications exert best cholesterol-lowering effect when ingested in the evening, and special theophylline and ß2-agonist tablet and capsule formulations for asthma and other chronic obstructive pulmonary diseases are intended for evening ingestion. Evening bright light therapy is recommended to normalize the sleep-wake cycle of advanced sleep phase disorder (ASPD) patients. Other chronotherapies are intended for bedtime use, such as nitroglycerin patch application for angina pectoris, testosterone patch or cream substitution therapies, conventional tablet corticotherapy for Cushing’s disease, sleep medications for sleep disorders, controlled onset, extended release calcium channel and ß-blocker hypertension and angina pectoris chronotherapies, and modified-release prednisone chronotherapy. Finally, conventional tablet corticosteroid chronotherapy for inflammatory conditions and as substitution therapy for Addison’s disease, and bright light chronotherapy for seasonal affective disorder (SAD) and delayed sleep phase disorder (DSPD) are recommend for morning application. Unregistered or experimental chronotherapies include bedtime tablet H1-receptor antagonists for control of sleep-time and morning peak symptom intensity of allergic rhinitis, levothyroxine for hypothyroidism, low-dose aspirin for prevention of pregnancy-induced hypertension and preeclampsia, and late evening methotrexate therapy for rheumatoid arthritis. Others include bedtime NSAIDS for rheumatoid arthritis and breakfast or lunch NSAIDS for osteoarthritis. Sleep and activity spans are indicated, respectively, as the darkened and white portions of the circle (Smolensky and Haus, unpublished)
8.2.1 Chronotherapy of Nocturnal Asthma
Asthma is a relatively common chronic medical condition affecting in the USA alone, an estimated 6.5 million children and 15.7 million adults. It is characterized by persistent airway inflammation, heightened airway hyperreactivity to antigens and various environmental agents, markedly reduced airway caliber, and often excessive mucus production [50]. Symptoms include dyspnea (difficulty in breathing), wheezy chest, and croupy cough. It is seldom a problem when very mild, but it can significantly affect lifestyle and well being and even be life-threatening when severe.
Asthma is greatly affected by the CTS [50]. Symptoms and attacks of breathing distress occur only at night in most cases. A large multiple center study of more than 3,000 presumably diurnally active asthma patients found crises of breathing distress 70- to 100-fold greater in number between 04:00 and 05:00, during intended nighttime sleep, than between 14:00 and 15:00, middle of daytime activity span (Fig. 13.19) [126]. Turner-Warwick [125], in a 1980s study of a large group of 7,729 noninstitutionalized British patients, reported that 94% experienced disruption of their nighttime sleep by asthma at least once per month, 74% at least once per week, 64% three nights per week, and 39% every night, even though most were medicated with equal interval, equal dose bronchodilator, and anti-inflammatory medications. Perhaps it is because asthma exhibits such an obvious and profound day–night pattern in symptom intensity that it was the first medical condition to be aggressively investigated for circadian rhythm related mechanisms and chronotherapeutic interventions.
Day–night difference in asthma (1,632 episodes of dyspnea, i.e., difficult breathing) per hour of the day and night in 3,129 untreated asthma patients, i.e., washed-out of prescribed medications in preparation for trialing of a new asthma therapy. Episodes of dyspnea, signaling asthma, were 70- to 100-fold more common between 04:00 and 05:00, causing disruption of nighttime sleep, than early afternoon between 14:00 and 15:00. This 24-h pattern in asthma risk is the basis for chronotherapy with tablet, capsule, and aerosol bronchodilator and anti-inflammatory medication. Shaded portion of bottom time axis represents patients’ nighttime sleep span and white portion represents diurnal wake span (redrawn after Dethlefsen and Repges [126])
The signs and symptoms of asthma are at least partially reversible with bronchodilator and anti-inflammatory medication, plus proper environmental control of exposure to triggering agents. The goals of asthma therapy are (a) prevention of acute and chronic symptoms during night and day, (b) maintenance of normal or near normal pulmonary function (airway caliber), lifestyle, activity, and sleep, and (c) avoidance of medication-induced adverse effects. Specific treatment algorithms have been published by medical societies and governmental agencies [50] to guide patient management and to achieve therapeutic goals, and national and international guidelines exist for grading asthma severity and implementing treatment strategies. Grading of asthma severity is based in part on the frequency of disease-induced awakenings from nighttime sleep and the mean and amplitude of the circadian rhythm in airway caliber, i.e., PEF, self-measured by the patient.
Chronotherapy of nocturnal asthma is based on the day–night pattern in the occurrence and intensity of symptoms, identified underlying rhythm dependencies, and findings that equal interval, equal dose schedules of therapy are not always optimal to avert nighttime episodes of breathing distress. A large variety of medications, such as tablet β2-adrenergic agonists, anticholinergics, tablet and capsule theophyllines, and aerosol and tablet anti-inflammatory corticosteroids have been studied for differences in their PK and/or PD as a function of the circadian time of administration [50]. Here, we illustrate the earlier developed theophylline chronotherapies for nocturnal asthma. Chronotherapies of other medications, including aerosol ones, are reviewed in detail elsewhere [50].
During the 1980s, theophylline was a first-line treatment for asthma, and although it is much less prescribed today, it constitutes a useful case study of applied clinical chronotherapeutics. Theophylline chronotherapy entails the purposeful delivery of medication in unequal amounts during the 24 h such that elevated concentration is achieved during nighttime sleep, when the risk of breathing crises is greatest, and reduced concentration occurs during daytime, when risk is lowest. The German pharmaceutical company Byk Gulden was probably the first to embrace the concept of theophylline chronotherapy. It recommended that Euphyllin® be ingested twice daily in unequal doses, with one third the daily dose in the morning at the commencement of diurnal activity and remaining two thirds in the evening [226]. Similar asymmetric morning–evening dosing schedules have been used with other twice-daily sustained release 12-h theophylline (and also ß2-agonist terbutaline tablet [227]) preparations to better manage nocturnal asthma, and occasionally conventional twice-daily sustained release theophyllines were trialed as evening-only high dose chronotherapies [50]. Although asymmetrical 12-h dosing regimens improved the beneficial effects of some conventionally formulated asthma medications, they posed difficulties for patient adherence and often caused adverse effects, especially when prescribed in high dose to ensure elevated daytime as well as nighttime drug concentrations.
Several once-a-day theophylline chronotherapies, relying on special tablet and capsule (tablet or bead coating) technologies were developed during the 1980s by pharmaceutical companies in Europe and USA. Two popular ones were Euphylong® (Byk Gulden, Germany) and Uniphyl®/Uniphyllin® (Purdue Frederick, USA/Mundipharma, Germany) [217, 218]. Figure 13.20 presents the so-called steady state PK and PD of one theophylline chronotherapy ingested in the evening as recommended, relative to the PK and PD of a popular conventional slow release theophylline medication ingested twice daily at 12-h intervals as recommended [218]. The drug delivery systems and dosing schedules of this conventional medication were intended to achieve the homeostatic goal of constancy in serum theophylline concentration, while the drug delivery system and bedtime dosing schedule of the chronotherapy were intended to achieve unequal levels during the 24 h, with peak drug concentration overnight when asthma risk is greatest.
Pharmacokinetics and pharmacodynamics of comparable doses of conventional sustained-release (SR) twice daily, open triangles and dashed line, and evening chronotherapeutic (Uniphyl®: Purdue Frederick, USA/Uniphyllin®: Mundipharma, Germany), closed circles and solid line, theophylline medications. Top: Steady-state serum theophylline concentration for the conventional SR preparation ingested daily at 08:00 and 20:00 and chronotherapeutic preparation ingested daily at 20:00. Theophylline concentration is relatively constant over the 24 h with twice-daily conventional drug dosing; evening theophylline chronotherapy in contrast achieves much higher drug concentration during the sleep span, when asthma risk is greatest. Bottom: Effect on airway caliber of the conventional and chronotherapeutic theophylline time-concentration patterns. Conventional equal-interval, equal-dose theophylline regimen fails to avert significant nighttime decline of airway caliber (i.e., PEF or peak expiratory flow, expressed as % of 24-h group mean, designated as 100%), while theophylline chronotherapy does. Greater stability of the 24-h rhythm in airway caliber translates into enhanced protection against breakthrough asthma nocturnally. During the 1980s, when this study was conducted, high theophylline doses were prescribed, as it was believed then high serum concentrations (10–20 mg/ml) were required to achieve sufficient bronchodilation. Today, it is recognized that theophylline exerts anti-inflammatory effect on the airways at considerably lower doses, as now prescribed. Shaded portion of bottom time axis represents subjects’ nighttime sleep span and white portion represents diurnal wake span (figure redrawn after Neukirchen et al [218])
Results demonstrated that conventionally formulated theophylline preparations do indeed achieve near-constant serum drug concentration, but as shown in Fig. 13.20 they fail to avert significant nocturnal decline of airway caliber. Studies show that the greater the nighttime decline of airway patency, the greater the risk of nocturnal asthma crises [6]. Thus, the extent of nocturnal decline in PEF, a measure of airway patency and ease of breathng, is a key biomarker of the robustness of therapeutic effect of asthma medications. Theophylline chronotherapies of approximately the same dose/24 h, in contrast, achieve considerably higher serum drug concentration during nighttime sleep, while still maintaining daytime therapeutic levels. The high nocturnal serum theophylline concentration achieved by chronotherapy results in substantial moderation of the overnight decline in PEF, leading to better control of symptoms and reduced risk of nighttime asthma, without loss of daytime drug effectiveness.
8.2.2 Hypertension Chronotherapy
A second example of clinical chronotherapeutics entails hypertension medications. Toward the end of the twentieth century, special bedtime tablet and capsule BP-lowering medication systems were introduced that proportioned drug concentration in synchrony with a priori assumed day–night patterning in SBP, DBP, and heart rate (HR) of hypertension patients (see Fig. 13.4). In the USA, four delayed onset, controlled release chronotherapeutic systems – Covera HS® (Searle/Pfizer), Verelan PM® (Schwarz), Cardizem LA® (Biovail), and Innopran XL® (Reliant) – were introduced to improve hypertension therapy and reduce morning-time risk of angina pectoris, myocardial infarction, and stroke (Fig. 13.21) [221–225]. Myocardial infarction and stroke are 30–40% higher in incidence during the initial hours of the activity span [142, 144], and it is hypothesized they are triggered in at-risk patients by the marked morning raise in SBP, DBP, and HR, coincident with peak platelet and blood coagulation level and minimum fibrinolytic activity [228].
Steady-state pharmacokinetics of the R- and S-enantiomers of first generation controlled-onset, extended-release hypertension chronotherapies: (a) 240 mg COVERA HS® (calcium channel blocker verapamil; Searle/Pfizer), (b) 200 mg Verelan PM® (calcium channel blocker verapamil; Schwarz Pharma), (c) 360 mg Cardizem LA® (calcium channel blocker diltiazem; Biovail Pharmaceuticals), and (d) 160 mg Innopran XL® (ß-agonist propranolol; Reliant Pharmaceuticals). When these chronotherapies are ingested at bedtime as intended, they produce highest drug concentrations in the morning between 06:00 and 12:00 (shaded rectangle), when in dipper hypertension patients systolic and diastolic blood pressure (BP) reaches peak or near-peak level and when angina pectoris, acute myocardial infarct, and sudden cardiac death are of greatest risk, presumably as a consequence of the sudden surge in BP and heart rate at this time of day. In part c, the steady-state pharmacokinetics of 360 mg Cardizem LA® is shown when ingested as intended at bedtime, in accordance with the design of the drug-delivery system, and also in the morning upon awakening. Dosing of this hypertension chronotherapy in the morning at the wrong time results in lowest concentration of diltiazem in the morning, when BP in essential hypertension patients is likely to be greatest, and highest concentrations in the late afternoon and evening, when BP is expected to be declining from daytime elevated levels. Shaded portion of bottom time axis represents assumed subjects’ nighttime sleep span and white portion represents assumed diurnal wake span (figure redrawn from Smolensky et al. [224])
The drug delivery system for each of these special products is designed to retard release of medication for ~4 h following bedtime ingestion and to ensure peak or near-peak drug concentrations during morning and daytime activity, in order to maximally control SBP, DBP, and HR, when they are all anticipated to be highest, and to ensure lower, but nonetheless therapeutic, concentrations during the evening and nighttime sleep, when they are anticipated to be lowest. Clinical trials of these special drug delivery medication systems substantiated improved control of morning-time HR and BP compared to conventional medications of the same class and dose. However, complaints of adverse effects, such as daytime fatigue and dizziness, signs suggestive of hypotension, were expressed by some patients. These drug-delivery forms are specifically designed to inhibit discharge of medication for ~4 h following ingestion at bedtime; thus, the entire daily dose is released over 20 rather than 24 h, posing risk of over-correction of BP and daytime hypotension when patients are transferred from the same dose of the same medication formulated for conventional constant-release over the full 24-h dosing interval.
The first intended prospective, large scale assessment of the preventive effects of hypertension chronotherapy was the 5-year international multicenter Controlled Onset Verapamil INvestigation of Cardiovascular Endpoints (CONVINCE) outcomes trial involving 15,000 hypertensive patients with identified CVD risk. This trial was designed to compare the degree of BP control and protection against CVD events afforded by verapamil (Covera HS®) chronotherapy compared to the then considered standard conventional treatment, i.e., β-blocker and diuretic medications [229]. Unfortunately, this community-based outcomes study was terminated prematurely before sufficient number of CVD events had occurred to enable valid assessment of the bedtime chronotherapy [230, 231]. Thus, the merit of delayed-onset, constant release chronotherapeutic systems, relative to conventional therapy, in preventing CVD events as a consequence of improved control of morning-time SBP, DBP, and HR remained unresolved.
In view of the paucity of available data, the prospective MAPEC (Spanish: Monitorización Ambulatoria para Predicción de Eventos Cardiovasculares; English: Ambulatory Blood Pressure Monitoring for Prediction of Cardiovascular Events) study was conducted in Spain by Hermida and collaborators [191, 232]. This recently completed study was specifically designed to test the hypothesis that bedtime chronotherapy with one or more hypertension medications exerts better BP control and better reduces CVD risk than conventional morning dosing of all such medications. All 2,156 hypertensive subjects were evaluated by 48-h ABPM at baseline and thereafter annually, if not more frequently (quarterly) if adjustment of hypertension treatment was required.
At baseline, the two treatment time groups were comparable in their clinic and mean ambulatory SBP and DBP, and prevalence of nondipping 24-h BP pattern. Subjects who ingested the entire daily dose of one or more of their hypertension medications at bedtime, compared to subjects who ingested all their hypertension medications in the morning, showed at their last available ABPM evaluation significantly lower mean sleep time BP, higher sleep time relative BP decline (an index of normal BP dipping 24-h pattern), reduced prevalence of nondipping BP circadian pattern (34% versus 62%), and higher prevalence of controlled ambulatory BP (62% versus 53%). After a median follow-up of 5.6 years, subjects who consistently ingested at least one prescribed BP-lowering medication at bedtime showed highly statistically significant reduced risk of CVD events, in particular, myocardial infarction and stroke, compared to subjects who ingested all such medications upon awakening (relative risk 0.39 with 95% confidence interval 0.29–0.51). The progressive decrease in the asleep BP and increase in the sleep-time relative BP decline, both indicative of a more normal dipping 24-h pattern, were best achieved when one or more hypertension medications were taken at bedtime, and these two indicators of BP control were the most significant predictors of CVD event-free survival. Results of the prospective MAPEC study thus indicate bedtime chronotherapy with one or more hypertension medications, compared to conventional upon-waking treatment of all medications, more effectively achieves BP control, better decreases the prevalence of BP nondipping, and better normalizes the 24-h BP pattern and, most importantly, significantly reduces CVD morbidity and mortality [191].
8.2.3 Nitroglycerin Chronotherapy
Nitroglycerin (glyceryl trinitrate, GTN) is a smooth muscle relaxant that has been used in the treatment of hypertension and, most notably, angina attacks. GTN, upon uptake into cells, is metabolized and converted to nitric oxide, which activates adenylate cyclase and increases cyclic AMP production, which signals smooth muscle relaxation [233–236]. As a small lipophilic molecule, GTN readily permeates epithelial membranes such as those of the skin and cheek. Traditionally, it is prepared in ampule form. During or in anticipation of an angina attack, the ampule is placed in the mouth by the cheek and broken, permitting rapid absorption across the buccal membrane, and providing fast relief. Because of its ease in formulation, permeability properties, and low plasma concentration required for activity (~10 nm), GTN was an early candidate for transdermal patch delivery. It was hoped that GTN patch therapy would exert prophylactic effect and reduce incidence of angina. Several such patches received regulatory approval based on PK studies that demonstrated sustained maintenance of GTN at therapeutic levels. Unfortunately, it was soon observed that biological tolerance to GTN developed after ~12 h. (Interestingly, this tolerance could have been anticipated based on the “Monday rebound” effect reported in munitions workers in the nineteenth century [237, 238].) Numerous hypotheses regarding the mechanism of tolerance have been under investigation for several decades, some attributing it to depletion of cofactors required for GTN metabolism, and others to down-regulation of metabolizing enzymes or adenylate cyclase activation [234, 239–242]. Regardless of mechanism, patches are now prescribed to be worn for 12 h, followed by a patch-free period of equal duration during which sensitivity to GTN is regenerated [243]. The notion of tolerance followed by resensitization finds parallel in explanations for pulsatility of neuroendocrine hormones, to be discussed later.
Optimal use of GTN patch therapy has been tied to the circadian rhythm in risk of both exertion- and nonexertion triggered (variant or Prinzmetal) forms of angina (Fig. 13.22), with or without (silent angina pectoris) chest pain. Thus, GTN patch chronotherapy entails evening or bedtime application and midday removal to cover the time span when risk of angina due to physical exertion, coronary vasospasm (variant angina), and cardiac stress induced by hypoxia of sleep apnea is greatest [140, 141, 244, 245].
Twenty-four-hour pattern of exertion-induced angina pectoris (984 ischemic events denoted as ST-segment depression of the electrocardiolographic [ECG] tracings obtained by ambulatory holter monitoring study) recorded in 235 diurnally active, nonmedicated ischemic heart disease patients (Top) and also angina pectoris (234 events in total) recorded in a group of diurnally active vasospastic (Prinzmetal, nonexertion variant) patients (Bottom). Bouts of conventional angina pectoris are more common during daytime activity, in particular between awakening (~06:00) and noon (12:00). In contrast, bouts of vasospastic angina typically occur during rest, especially during nighttime sleep, between 01:00 and 04:00. Chronotherapy of angina pectoris must take into consideration the circadian pattern in risk during the 24 h and associated temporal requirement for medication. Shaded portion of bottom time axis represents presumed nighttime sleep span of subjects and white portion represents diurnal wake span (top graph created using unpublished data of first author; bottom graph created using data of Kimura and Kuroiwa [244, 245])
8.2.4 Cancer Chronotherapy
Chronotherapy of cancer medications is based on knowledge that their chronotoxicity varies markedly by cell cycle stage, both in normal and tumor tissue [209–213]. Programmable in time, light weight ambulatory infusion pumps can be used to infuse cancer medications at the desired circadian time when healthy, noncancerous cells are in a nonvulnerable stage. Cancer cells may lose their circadian periodicity and multiply at a faster rate, i.e., with a period <24 h, or at random and, therefore, are likely to be more vulnerable to therapy at a time when noncancerous cells are least susceptible. Results of both laboratory animal and European multicenter patient trials clearly show that proper circadian delivery and timing of cancer medications improve tolerance to therapy, thereby enabling more aggressive treatment [210–212]. However, significant advances in improving long term cancer-free interval and survival, relative to conventional treatment entailing constant infusion of cancer medications during the daytime, have not yet been consistently demonstrated. Nonetheless, clear opportunities exist for the application of advanced drug delivery systems to improve both patient tolerance and outcomes of cancer medications.
8.3 Noncircadian Chronotherapeutics
Many endocrine and neuroendocrine secretions exhibit prominent high-frequency and pulsatile variability over time, which may be relevant to the effectiveness of certain neuroendocrine analogues. Indeed, the frequency-modulated or pulsatile mode of drug-delivery systems may be of greater importance than the dose of drug delivered.
8.3.1 Gonadotropin Releasing Hormone (GnRH: aka Luteinizing Hormone Releasing Hormone, LHRH) Chronobiology and Chronotherapy
GnRH is a decapeptide, synthesized and secreted by the hypothalamus into the pituitary portal circulation in an intermittent fashion, stimulating pituitary gonadotropes to synthesize and secrete LH and FSH [246, 247]. In a series of exquisite studies on female Rhesus monkeys [248–250], Knobil and coworkers established that the frequency of GnRH delivery determines its biological effect. Lesioning of the hypothalamus abolishes GnRH secretion, and continuous infusion of the decapeptide fails to reinitiate endogenous LH and FSH production. However, frequency modulated GnRH infusion as a single 6-min pulse/h reestablishes normal pituitary function. Of practical interest is that GnRH delivery as five 12-min pulses/h produces an effect similar to constant rate infusion, as it fails to reinitiate endogenous LH and FSH production. An infusion frequency of two or three pulses of GnRH/h is less efficient or exerts inhibitory effect on FSH and LH, while an infusion frequency to one pulse/3 h changes the LH/FSH ratio.
Pulsatile GnRH secretion is essential for its effect on the pituitary, as confirmed by clinical trials. Pulse pattern and circadian variation of plasma LH in sexually mature women are modulated by the menstrual cycle [251, 252]. During luteal–follicular transition, LH pulse frequency increases markedly, and this accompanies selective FSH rise leading to normal folliculogenesis [253]. Continuous and/or closely repetitive GnRH stimulation leads to blunted gonadotropin response [254, 255] due to pituitary desensitization (analogous to tolerance developed to nitroglycerin, as discussed in Sect. 13.8.2.3, but by a different mechanism) [246]; thus, continuous infusion of constant dose GnRH, or administration of long-acting GnRH analogs, inhibits LH secretion [246, 256]. This knowledge can be used for contraception and to manage hormone-dependent cancers, such as those of the breast and prostate, and sustained-release depot injections of biodegradable microspheres containing GnRH (LHRH) analogs are marketed for this purpose, e.g. Lupron® depot. Pulsatile delivery of GnRH every 60–90 min, on the other hand, is an effective means of obtaining ova for in vitro fertilization [257, 258], and for treating supra-hypophyseal hypogonadotropic hypogonadal anovulation [258–260] and arrested puberty [261–263].
8.3.2 Growth Hormone
Growth hormone (GH) is critically important for growth, metabolism, and tissue and organ maintenance. It is used to treat abnormally short stature and dwarfism in children, and increasingly to slow aging of adults. In animal husbandry, GH is commonly used to increase milk yield of dairy cows and enhance muscle mass of pigs and other animals. Prominent pulsatile, ultradian, circadian, and perhaps other periodicities in GH secretion and suspected rhythm dependencies in target tissue responsiveness constitute significant challenges, but also potential opportunities, for drug delivery scientists to achieve or improve desired treatment outcomes and/or avoid negative consequences.
8.3.2.1 Growth Hormone Synthesis and Secretion
GH is synthesized and secreted by somatotrophs of the anterior pituitary and plays a major role in many processes, most notably growth and metabolism. GH is synthesized and secreted in a pulsatile manner, generally with plasma peaks ranging from 5 to 45 ng/mL Greatest GH secretion takes place primarily during sleep in a sex-dependent pattern, with largest and most predictable GH peaks found ~1 h after sleep onset and with ~50% of GH secretion occurring during the third and fourth REM sleep episodes. Between peaks, basal GH levels are low, usually less than 5 ng/mL. Its effects are exerted directly, by binding to its receptors, and indirectly, by stimulation of mediators, primarily insulin-like growth factor-1 (IGF-1), in liver and other tissues. Growth of organs and tissues is mediated via IGF-1. For example, IGF-1 causes proliferation of chondrocytes (cartilage cells) and thus bone growth, and it induces muscle growth by stimulating differentiation and proliferation of myoblasts. GH participates in blood glucose regulation through its anti-insulin activity, i.e., by suppressing glucose uptake in peripheral tissues and promoting hepatic glucose synthesis. It also promotes protein anabolism and synthesis by enhancing amino acid uptake and slowing protein oxidation, and it stimulates adipocyte fat utilization, inducing triglyceride breakdown and oxidation. Other important actions of GH include bone calcium retention and mineralization, pancreatic islet cell maintenance and function, and immune system maintenance [264–266].
GH is secreted at an elevated rate of ~700 μg/day in young adolescents, but at a lower rate of ~400 μg/day in mature healthy adults. The primary controllers of GH synthesis and secretion are two hypothalamic hormones, growth hormone-releasing hormone (GHRH), which stimulates synthesis and secretion of GH, and somatostatin (SS), which inhibits GH release in response to GHRH and other stimulatory factors (e.g., low blood glucose concentration). The stomach growth hormone secretagogue (GHS) ghrelin binds to receptors on somatotrophs to robustly stimulate GH secretion. GH secretion is regulated by a negative feedback loop involving IGF-1. Elevated blood IGF-1 concentration inhibits GH secretion by suppressing somatotrophs and by stimulating SS release from the hypothalamus. GH also feeds back to inhibit GHRH secretion to exert a direct (autocrine) inhibitory action on its own secretion from somatotrophs.
Regulation of the somatotropic axis, however, is much more complex than overviewed above, since GH synthesis is influenced and moderated by many factors, for example, sex hormones in pubertal boys and girls, androgen secretion (respectively, by the testis and adrenal cortex), and estrogen secretion (respectively, by the adrenal cortex and ovaries). Hypoglycemia, exercise, and deep sleep stimulate GH secretion, while hyperglycemia, free-fatty acids, glucocorticosteroids, and dihydrotestosterone inhibit it. GH secretion levels may also be affected by medications, being stimulated by L-DOPA and clonidine and inhibited by estrogen and testosterone. It may also be modified by estrogen disruptors.
Somatotropic axis regulation is highly organized in time as circadian, ultradian, and pulsatile oscillations [205, 265, 267]. Sex, age, body mass index, and IGF-1, individually and jointly, determine distinct GH dynamics [267]. In the resulting complex pattern of GH secretion, rhythmic and nonrhythmic variations in plasma concentrations determine circadian mean, peak values, and/or trough values, which mediate metabolic effects in target organs [268, 269]. Furthermore, regulation of GH secretion and effects also depends upon interactions with other neuroendocrine and endocrine constituents, including GHS ghrelin [270], sex hormones [271], and other factors [265], many of which are rhythmic. GH effects are also determined by rhythms in target tissues [271, 272], the temporal control of which in the circadian frequency range may be centrally mediated by the hypothalamic body clock (SCN) plus other periodic phenomena, like the time of food uptake that can synchronize peripheral oscillators, especially in the liver. The complex pattern of GH secretion and plasma concentrations encountered in the periphery consists of regularly recurring rhythmic and pulsatile variations, the amplitude, shape, and spacing of which may be regular or variable [273]. The placement in time of clusters of pulses and variation in their amplitude [274] over the 24 h may represent expression of circadian and/or ultradian oscillations in GH, which may differ between males and females and with age, but are of fundamental importance.
GH secretion is governed by the facilitative and antagonistic effects of GHRH and SS, respectively, and depends upon the relative timing of their releases, with differences in regulation due to sexual dimorphism and age [265, 275, 276]. Sex steroid hormones modulate GH synthesis, release, and actions [266, 277], leading to sex-dependent dimorphism of GH secretion and plasma levels. GHRH and GHS ghrelin amplify GH signals. GHS ghrelin is secreted predominantly, but not exclusively, in the stomach and may amplify, but not set, the timing of this regulatory circle of GHRH/GH pulses [264, 270]. Reciprocal control by GHRH-SS may constitute the oscillator of the somatotropic axis [265] for some, but not all, observed variations. The stimulatory effect of GHRH depends upon age, sex, body composition, and nutritional status and augments the outcome of GHS ghrelin stimulation, which may be altered by underlying diseases [266, 278].
8.3.2.2 Sex Differences in GH Secretion and Patterns
Sex hormones determine the pattern of GH secretion and lead to a dimorphic form of plasma GH and, as a consequence, IGF-1 concentrations. Average 24-h GH concentration is higher in women than in men largely due to higher daily trough values between secretory bursts [267]. Irregularity of pulses and narrow sharp elevations (“spikes”) are more pronounced in women than in men [267]. Major (eight to tenfold) variation in mean GH pulse size occurs in puberty and during the menstrual cycle caused by a variety of internal and external factors [271]. In men, highest GH pulse, amounting to ~70% of the 24-h secretory output, occurs shortly after sleep onset, coinciding with the first episode of slow-wave sleep (SWS) [279]. In normally cycling women, there is a wider distribution of GH pulses during the 24 h. The sleep onset-associated pulse is also found in most women, but it accounts for a smaller fraction of total 24-h secretory output [280]. During sleep, healthy young men show overnight GH pulses every 35–60 min superimposed on which frequent sampling (30-s intervals) reveals diminutive pulses linked with sleep stage [281]. This association is based on the hypothalamic relationship between GHRH and brain areas involved in sleep regulation [282].
Suppression of endogenous GHRH action by specific antagonists or by immunoneutralization inhibits both sleep and GH secretion [283]. Conversely, substances that promote SWS increase nocturnal GH section [284, 285]. The linkage between the major GH pulse and sleep onset leads to an immediate shift in the circadian rhythm in GH with any alteration of the sleep–wake cycle, e.g., in workers rotating between day and night shifts and in travelers rapidly displaced across time zones. This linkage also leads to alteration of GH secretions with day-to-day sleep pattern irregularities [279].
8.3.2.3 GH Receptors
The presence and action of hormone receptors in peripheral tissues is organotypic, as is their interaction with other hormones. Type of sex steroid and specific target site determine the regulation of GH and IGF-1 receptors at that level [271, 286, 287]. In animal models, GH pulsatility mediates a sexually bimorphic regulation of hepatic and muscle gene expression, somatic growth, and negative feedback upon the hypothalamus [265, 271]. Moreover, the GH pulse pattern determines target tissue responses. For example, exposure of female rats to seven or fewer distinct GH pulses per 24 h induces masculine growth and gene expression patterning in different organs [288]. In contrast, exposure of hypophysectomized male rats to more frequent pulses or continuous infusion of GH evokes feminine growth and gene expression patterning [288, 289]. Furthermore, different target organs appear to have different absolute pulse amplitude dose response dependencies [273]. In subjects with inactivity of human GHRH receptor, i.e., Laron syndrome [290], there is profound reduction in secretory burst mass and disorderliness of GH pulses. Nonetheless, most pulses occur at a normal frequency and in a circadian rhythmic distribution with sex difference in the pattern maintained [271, 291].
8.3.2.4 Factors Influencing GH Secretion and Consequences of GH Abnormalities
Aging is associated with reduced mean 24-h GH concentration due to decreased pulse amplitude rather than pulse frequency [292, 293]. Such age associated changes in GH secretion are thought to be due to diminished GHRH responsiveness and increased SS secretion [294].
Abnormalities and lesions of the hypothalamus and pituitary that interfere with GH level and pulse characteristics and/or effects in peripheral target cells result in specific disease states. The consequence of GH deficiency depends upon age of onset. GH deficiency and receptor binding defects during childhood manifest as retarded growth and dwarfism. Excessive GH secretion in young children or adolescents may result in giantism, typically due to somatotroph tumor. In adults, excessive GH secretion is typically due to a benign pituitary tumor and results in acromegaly.
Acromegaly is typically an insidious condition, developing over several years in middle-age adults, causing extremity overgrowth, soft-tissue inflammation, skin thickening and oiliness, jaw and facial abnormalities, hypertension, cardiac hypertrophy, and metabolic derangements, including hyperglycemia and diabetes, among other symptoms and complaints. This disorder is accompanied by alteration of both the amount and pulse pattern of GH secretion. Hormone secretion is irregular in pattern and timing, with irregularly shaped frequent small peaks superimposed upon high baseline interpulse GH concentrations [295, 296]. High basal values are the likely cause of the characteristic elevation of IGF-1 observed in acromegaly [297]. Difference in GH secretory pattern, rather than in absolute level, determines GH effect, and this explains finding normal overall GH levels but characteristically elevated IGF-1 in a recent study of acromegalic patients [298]. Surgical micro-adenomectomy restores physiologic secretion patterning in most acromegalic patients [299].
Basal GH levels and pulsatile secretory patterns impact body fat metabolism and distribution. Both increased and decreased GH concentrations persisting over a prolonged time span lead to CVD risk. GH deficiency is associated with an increase in CVD risk factors, such as elevated mean total and LDL cholesterol plus C-reactive protein [300, 301], reduced HDL levels, but higher TNF-alpha receptors I and II. GH-deficient patients tend to be overweight or obese and show increased carotid media thickening as evidence of increased vascular complications [301, 302]. GH deficiency is also associated with high incidence of nonalcoholic fatty liver disease [303], which can be reversed by GH administration [304]. C-reactive protein (CRP) and free fatty acids have been found to be significantly elevated in hypopituitary patients with fatty liver [303]. GH replacement therapy improves the lipid profile with the exception of lipoprotein(a) concentrations, which tend to increase after GH therapy [305]. GH replacement therapy in this respect is complementary with HMG-CoA reductase inhibitor (statin) therapy [306].
In obese premenopausal women (BMI >34 kg/m [2]) with abdominal obesity pattern, plasma GH concentration is markedly reduced, due both to diminished basal hormone secretion and disordered pulsatile patterning. This abnormal GH level and secretory pattern persists after substantial weight loss (~40% of visceral fat); thus it is viewed as a cause rather than consequence of the condition, a conclusion supported by the finding that obese women of the same BMI, but with small visceral fat area, show normal GH level and secretory patterning [307]. Increased concentrations of GH in active acromegaly are also associated with increased atherogenic risk factors. For example, increased levels of oxidized low density lipoproteins are probably related to increased levels of pro-oxidants, such as ceruloplasmin, and biomarkers of inflammation, which are linked to increased CVD mortality [308, 309].
8.3.2.5 Role of Delivery Pattern on GH Effect
Continuous or pulsatile GH delivery differently affects various parameters of GH action [269, 297, 310, 311]. Continuous, rather than intermittent, GH administration is regarded as the preferential pattern for induction of plasma IGF-1 and muscle IGF-1 mRNA [311], both in pituitary deficient and clinically healthy human subjects. Continuous GH delivery to hypopituitary patients maintains liver-derived IGF-1 and lipoprotein(a) concentrations to a greater degree than repeated injections [297, 312–315]. Conversely, bolus GH injection stimulates visceral lipolysis and elevates HDL concentrations more effectively than constant infusion [273, 310, 314]. Only pulsatile GH augments rate of lipolysis [311].
In young diabetics, GH pulsing increases insulin requirements more than continuous GH infusion [273], while in healthy young men GH pulses are more effective in promoting SWS [316], indicating organ specificity in effects of GH delivery pattern. Treatment protocols of continuous infusion and of infusions of eight equal boluses every 3 h were most effective in increasing IGF-1 and IGFBP-3 (insulin growth factor-binding protein-3), whereas pulsatile administration had greatest effect on markers of bone formation and resorption [269]. All GH treatments decrease cytochrome P1A2 activity studied by erythromycin breath tests, with greatest effect for pulsatile GH. Pulsatile GH infusion decreases, whereas continuous GH infusion increases, cytochrome P3A4 activity [269]. These cytochromes are mixed function oxidases, which, among others, catalyze many reactions involved in drug metabolism [317, 318].
8.3.2.6 Pharmaceutical Uses of GH and GHRH
Treatment of severe childhood growth retardation in the past relied upon GH purified from human cadaver pituitaries. Nowadays, recombinant GH (rhGH) or GHRH may be used. GHRH treatment of children with pituitary insufficiency was most effective when bolus injections were delivered by pump at 3-h intervals [319]. When GH was given by continuous infusion over a 4-week span to adult GH-deficient patients, it was more effective than when given as a once-a-day injection [320]. GHRH is absorbed nasally, but its effectiveness by this route is still not widely confirmed [321]. Of interest for noninvasive timed administration of GH is the nasal delivery of recombinant hGH together with a polymeric absorption enhancer [322]. The current clinical guidelines for evaluation and treatment of childhood [323] and adult GH deficiency by the Endocrine Society [324] do not address optimal time and pattern for GH and GHRH treatment, which may vary in different clinical situations.
The role of GH supplementation in aging remains poorly understood, but some cosmetic symptoms of aging appear to be amenable to therapy. This is an active area of research, and additional information and recommendations concerning risks and benefits, as well as biological timing and patterning during the 24 h, will undoubtedly surface in the near future. Sensitivity to treatment with rhGH is sex-dependent. Women require a substantially higher dose than men to achieve comparable effect on IGF-1 level [325–327] and bone mass [327], and much higher doses are required by women receiving oral estrogen replacement [326]. Older patients show increased susceptibility to GH-related side effects [324] and rhGH dose for GH-deficient patients should be individually titrated according to desired effect, i.e., to maintain serum IGF-1 within the physiologic range and avoid undesirable effects. However, it is not yet resolved how to best deliver rhGH, as a continuous infusion or as intermittent pulses, and if the latter at what frequency, concentration (height/amplitude), and circadian time. Different disorders may require different temporal patterns of dosing. GH secretion patterns are an independent regulator of GH action in humans [269], which are thus far grossly underexplored and underutilized.
GH is approved and marketed for use in animal husbandry. Administration of bovine somatotropin to lactating cows increases milk yield, and seems to be cost-effective. Administration of porcine GH to growing pigs significantly stimulates muscle growth and reduces fat deposition. However, it is not known whether GH effects in animals can be improved by delivery in a chronotherapeutic mode, which takes into consideration the temporal patterns observed in nature, and if so whether it will be cost-competitive.
Analogs of GHRH and other peptidergic and nonpeptidergic compounds including ghrelin, collectively designated GH secretagogues, have been used as diagnostic tools in GH-deficient states and in relation to aging [328–331]. Treatment with GHRH analogs in single doses in the morning or without regard to circadian phase [329, 332] and the orally active GH secretagogue MK-677, a ghrelin mimetic [328, 331], enhance GH basal interpulse concentrations and GH pulse height in adults and elderly subjects without change in pulse number and pattern. The complex time organization of the somatotropic axis suggests the possibility of drug delivery patterns tailored according to age, sex, and condition to be treated. This is a wide open field which will require further research.
8.3.3 Insulin and Glucose Chronobiology and Chronopharmacology
In nondiabetic individuals, insulin is secreted by the pancreas at a low basal rate between meals and rapidly secreted at meal times. The purpose of this switch is to maintain glucose homeostasis in the normoglycemic range of 70–120 mg/dl. The nature of the temporal variation in glucose and insulin concentrations during the 24 h in healthy individuals, based on blood samplings every 5 min during meals and 30 min at other times, is shown in Fig. 13.23 [333]. This figure highlights the substantial rise of the two constituents following each meal of the day and also at the end of the night, the so-called “dawn phenomenon”, as discussed below. The temporal variation, however, is primarily of a high frequency mode. Insulin exerts its control by inducing transport of excess blood glucose into the liver, where it is stored in its polymerized glycogen form, and by mobilizing uptake of glucose into tissues. This automatic control system is disabled in Type I diabetes due to autoimmune destruction of insulin-secreting pancreatic ß cells. For diabetic individuals prescribed insulin, delivery rate should mimic, as closely as possible, that provided by a normally functioning pancreas.
Average 24-h patterning of blood glucose (Top) and plasma insulin (Bottom) concentration of five diurnal active, clinically healthy subjects sampled at 5 to 30-min intervals during a single 24-h span. Spikes in glucose and insulin are evident after each meal (breakfast ~08:00, lunch ~12:30, and dinner ~19:00) and in addition ~04:00–05:00, so-called dawn effect. Shaded portion of bottom time axis represents subjects’ nighttime sleep span and white portion represents diurnal wake span (figure redrawn from data published by Mèjean et al. [333])
Failure to maintain normoglycemia entails two risks. If insulin is overdosed, then blood sugar levels fall below normal range, exposing the diabetic patient to hypoglycemia. Symptoms of hypoglycemia are typically acute, and may include dizziness, confusion, and sometimes seizures and/or coma. Underinsulination, on the other hand, leads to hyperglycemia. An acute symptom of hyperglycemia is ketoacidosis, which occurs with metabolic shifts due to deficient glucose transport into metabolizing tissues. Longer term consequences of sustained hyperglycemia include myopathy, retinopathy, nephropathy, and neural and connective tissue degeneration.
Since the isolation of insulin by Banting and Best in the 1920s, extensive effort has been extended to develop and improve insulin-based therapies for Type I diabetes. Slow (insulin glargine, Lantus®) and fast-acting (Lyspro/Humalog®) modified insulins have been developed, respectively, to provide basal levels, especially overnight, and to rapidly respond to postprandial rises in glucose [334]. These insulins can be administered by injection or wearable pump. Glucose monitoring, either discretely by finger sticks or continuously by an in-dwelling glucose sensor, can be utilized to improve timing and dosing decisions. Automatic closed loop systems, in which sensed glucose time series concentration data are used to instruct insulin pumping rates, are the object of continued research [335–339]. Such systems will optimally mimic the proportional, integral, and derivative (PDI) aspects of control by a normally functioning pancreas.
The goal of insulin therapy is to mimic a functioning pancreas and in the words of traditionalists “maintain glucose homeostasis.” This result can only be achieved by delivering insulin at a decidedly nonconstant rate and in a chronotherapeutic fashion. Strictly speaking this kind of chronotherapy is not designed primarily around a circadian or ultradian rhythm, however, since demand for insulin at any given time is governed by many factors, the most important ones being the timing, content, and size of meals, counter-regulatory and other hormonal effects, and energy expenditure. There are, however, well established circadian and ultradian effects of insulin on blood glucose level that are not driven by feeding times [340–345]. For example, both glucose and insulin levels exhibit morning-time peaks (Fig. 13.23). The concurrency of these peaks suggests decreased insulin efficiency in the morning, which has been attributed to the rise of counter-regulatory molecules nocturnally, especially growth hormone. Proper inclusion of this and other circadian and ultradian components of insulin sensitivity into insulin pump algorithms may improve regulation of normal glucose levels and reduce long term morbidities associated with hyperglycemia.
In addition to “slow” wave chronotherapeutic aspects of insulin’s regulation of glucose level, attention is required to the fine structure of insulin release from the normally functioning pancreas. Release of insulin from the healthy pancreas occurs in bursts of 10–15-min intervals [346–349]. This rapid, pulsatile behavior appears to be due to entrained intrinsic oscillators of pancreatic ß cells. It has been demonstrated that less overall insulin is required when delivered exogenously with this bursting pattern than when delivered in a continuous manner. Incorporation of such fine structure into pumps, while technically challenging and perhaps engendering added expense, may lead to reduction in side effects that result from prolonged exposure to increased average insulin levels.
Glucose regulation also entails circadian rhythmicity in insulin PK and PD in response to change in blood glucose level and also blood glucose PK and PD in response to change in insulin level. Reduction of blood glucose level by a standardized dosage of insulin (0.05 units/kg body weight) in diurnally active nondiabetic subjects under controlled conditions is ~30% greater when administered at 08:00 than 17:00 (Fig. 13.24) [350]. Moreover, insulin response to 50 g oral glucose loading under controlled and fasting conditions is significantly more rapid and extensive at 09:00 than either 15:00 or 20:00 (Fig. 13.25) [55]. The lower insulin response in the afternoon and evening versus morning needed to regulate blood glucose level when glucose challenged is consistent with the observation that the dose of insulin required by Type I diabetics progressively declines during the course of the day [343, 344, 351, 352].
Mean blood glucose levels in 14 diurnal active, healthy subjects following timed IV insulin infusions (0.05 units/kg body weight). Morning (08:00) and evening (17:00) insulin infusion studies were conducted on different test days. Eight hours prior to each infusion, at 08:00 or 17:00, a 50-g oral glucose meal was given, respectively at 00:00 and 09:00. Subjects fasted until blood glucose was sampled. Effect of morning (08:00, solid line) insulin infusion on blood glucose concentration was considerably greater than the afternoon one (17:00, dashed line). Findings of this study suggest the pharmacodyamic effect of insulin on blood glucose concentration varies according to circadian time (figure drawn using data of Gibson et al. [350])
Mean plasma insulin response of 24 presumably diurnally active healthy subjects following 50 g oral glucose tolerance testing at different circadian times. Three different test times (09:00, 15:00, and 20:00) were explored, each on different days. Insulin response to glucose loading under controlled conditions of this study was greatest and most rapid in morning at 09:00 and slowest and weakest in evening at 20:00. This and other such glucose loading/tolerance studies indicate the pharmacodynamics of the insulin response is circadian, and perhaps other period, rhythmic (figure redrawn using data of Jarrett [55])
Differences in the PD of insulin upon blood glucose levels and the latter on the former take place not only during the 24 h but also over the year [205, 353], and perhaps menstrual cycle in young women [354]. The hypoglycemic effects of exercise in Type I diabetics may also differ according to its biological timing in that hypoglycemic risk is reported to be higher for exercise done in the evening than in morning by diurnally active subjects [355].
8.3.3.1 Pump-Delivery of Insulin for Diabetes
Recent glucose sensor and pump technologies provide for improvement of treatment of type 1 (DM1) and resistant type 2 (DM2) diabetes. Insulin pump treatment is considered by many as the most physiologic way to imitate the healthy body’s insulin profile [356–359]. To approximate circadian and postprandial/ultradian variations in blood glucose and insulin sensitivity, circadian synchronization of the patient has to be considered [205]. Pumps should be programmed and bolus injection timed and quantitated according to need [359, 360]. Use of a bolus calculator may improve glucose control [361]. Hypoglycemic response to insulin is markedly more pronounced in the morning than later in the day [340]. In pump administration studies, greatest insulin requirement was found in the late morning or early afternoon [343, 344]. Since high frequency pulses may provide an advantage in insulin utilization and effect plus avoidance of hypoglycemic episodes, the chronotherapy of insulin-dependent DM requires that the optimal pulse frequency of drug delivery be determined [345, 346]. This combined with an automated reasoning system used to monitor life events with weekly situation assessment can help detect and prevent problems in the use of insulin pumps [362].
Combination of an insulin pump with continuous glucose monitoring can automate the periodic adjustments. Insulin pumps and real time continuous glucose monitoring devices combined can provide a sensor augmented pump (SAP) system and can achieve better control and decrease in insulin requirements as compared to conventional insulin pumps without the sensor. Blood glucose concentration in patients carrying a closed system of sensor and pump spend more time in the target range [363–365]. A FDA approved system providing this combination is presently available (MiniMed Paradigm® REAL-Time, Medtronic). The newest RevelTM model carries, in addition, low and high-glucose alerts that signal up to 30 min before the preset low or high limit of glucose concentration is reached, allowing the pump system to suspend insulin delivery if hypoglycemic threshold is too closely approached or achieved [336, 337]. This feature substantially reduces hypoglycemic episodes. The system also provides time trend graphs of 3, 6, 12, and 24-h, which will enable recognition of circadian and ultradian variations and correction of treatment, if needed [336, 366].
8.3.4 High Frequency and Pulsatile Hormone Delivery: Chronotherapeutic Implications
The above examples illustrate the role that can be played by pulse frequency, as opposed to dose, of drug delivery systems, particularly for hormones. Choice of delivery mode, ultradian pulsatile versus continuous, for GnRH and its analogues is decisive and depends on desired effect, i.e., induction/restoration of reproductive function versus treatment of sex hormone-related cancers. Proper pulsatile and ultradian delivery of insulin, GH, and other neuroendocrine analogues, and perhaps other medications, may enable better outcomes and reduced doses. However, the clinical and pharmacoeconomic advantages of such chronotherapeutic approaches are yet to be completely assessed. The added compliance burden demanded by precise drug timings and frequency oscillations cannot be ignored, and may slow patient acceptance. Nonetheless, recognition of the critical dependence of high frequency modulation of certain medications, including insulin, to achieve therapeutic outcomes calls for application of new drug-delivery technologies to achieve practical and cost-effective clinical applications.
9 Chronoprevention: A Complementary Aspect of Chronotherapeutics
Chronoprevention is the timing of medications or other interventions according to biological rhythm criteria to avert disease or decline in health status. Chronopreventive strategies take into account the same factors as do chronotherapeutic strategies. However, the goal of chronoprevention is avoidance of disease, pathology, and other deleterious phenomena, while the goal of chronotherapeutics is curative management of existing medical conditions. For example, hypertension is a medical condition, not a disease, but if left untreated or treated inappropriately will result in blood vessel injury, culminating in renal, CVD, and other serious pathologies. Thus, the rationale underlying hypertension chronotherapy is their prevention, which as verifiied by the MAPEC outcomes study (sec. 13.8.2.2) is best achieved by bedtime chronotherapy of one or more hypertension medications (191). Similarly, hyperglycemia, in itself, is not a disease, but its long-term associated morbidities can be reduced or avoided in both type I and type II diabetes by proper management of glycemia, which involves accurate and timely dosing of insulin. Bedtime GTN chronotherapy is also intended to prevent angina attacks that are of greatest risk during nighttime sleep (Prinzmetal or sleep-apnea induced angina) and in the morning (exertion triggered angina) (Fig. 13.22).
The concept of chronoprevention is well illustrated by findings of trials involving low-dose aspirin (ASA) to minimize or avert risk of preeclampsia, a form of toxemia of pregnancy characterized by hypertension, fluid retention, and abnormal albumin level. While initial investigations established the safety of low dose ASA in pregnancy, findings of initial clinical trials were inconsistent in preventing preeclampsia. Review of published studies revealed several potential methodological flaws: trials did not always involve high risk obstetric patients, low dose ASA intervention was not always begun early in pregnancy, and none specified the clock time of dosing.
Hermida et al. [367] wondered whether the disparity in findings between published investigations could be due to differences in when ASA was ingested daily. A prospective double blind, randomized controlled trial of administration time dependent differences in the chronopreventive effect of low dose ASA was initiated to explore this possibility. Diurnally active pregnant women were recruited, 341 in total (181 primipara) who were normotensive but at elevated risk of developing gestational hypertension and preeclampsia. They were randomized into one of six different groups, each composed of 55–59 participants. Groups were specified by the two treatments, placebo or 100 mg ASA, and three dosing times: upon awakening in the morning, 8 h after awakening (lunch time in Spain), or before sleep at night. SBP and DBP were automatically assessed by ABPM for 48 h, commencing at 12–16 weeks gestation and repeated thereafter at 4-week intervals until term.
The effect of low dose ASA on SBP and DBP was nil when ingested daily upon awakening throughout pregnancy. In contrast, those who ingested ASA in the afternoon exhibited significantly lower 48-h mean SBP and DBP after the first month of treatment, with the difference at term relative to the pretreatment baseline amounting to 4.4 and 3.5 mmHg, respectively. However, the effect of ASA was best, almost twice as strong, when ingested daily at bedtime, with reduced 48-h mean SBP and DBP again apparent after the first month of treatment and with differences at term relative to baseline levels averaging, respectively, 9.7 and 6.5 mmHg.
The preventive effect of ASA against preeclampsia and gestational hypertension in the high-risk pregnant women and their negative consequences on fetal development and well-being also differed dramatically according to treatment time. Average incidence of preeclampsia in the three placebo-treated groups was ~12%. Daily morning low dose ASA was not at all preventive (15% incidence), while afternoon and bedtime ASA was (1% incidence). Gestational hypertension was common, affecting on average ~30% of pregnant women randomized to the three placebo groups. Again, morning ASA dosing offered essentially no protection (25% incidence), whereas afternoon and bedtime dosing did (9 and 7% incidence, respectively). Average incidence of intrauterine growth retardation in the three placebo-treated groups was ~18%. Once again, morning ASA dosing exerted little prevention (16% incidence), while afternoon and, especially, bedtime dosing did (7 and 3% incidence, respectively). Finally, average incidence of preterm birth was ~14% in the three placebo groups. Daily morning low-dose ASA provided no meaningful protection against preterm birth (12% incidence), while afternoon and, in particular, bedtime dosing provided marked and clinically significant protection (3 and 0% incidence, respectively).
10 Discussion
Living organisms are precisely organized in time as evident by the multifrequency spectrum of biological processes and functions. The range of oscillations is broad, from very short periods and pulses of seconds, minutes, or a few hours to longer periodicities including ultradian (<20 h), circadian (~24 h), and infradian (>28 h) with periods of a week, month, and year. Such temporal variabilities are observed in almost all biological process from the subcellular to organ-system level. A given biological process may simultaneously exhibit many different periodicities, although the prominence, i.e., amplitude, of each may differ. For example, cortisol simultaneously exhibits very high amplitude pulsatile (minutes to a few hours) oscillations as well as ultradian, circadian, circamensual, and circannual rhythmicities. These diverse rhythmicities, and the unique phase relationships of the multitude of oscillatory variables within each given frequency domain, constitute the biological time structure.
The prominent CTS of human beings gives rise to substantial biological time-dependent differences during the 24 h in one’s response to a variety of clinical and laboratory diagnostic tests, risk of severe and life threatening medical events, symptom severity of a great number of common acute and chronic medical conditions, and PK and PD of medications when ingested, injected, infused, inhaled, or applied transcutaneously. Indeed, a medication that proves to be therapeutic and safe when applied at a one biological time may conceivably lack efficacy and/or be poorly tolerated at another. The reverse situation also is plausible; a medication that proves to be subtherapeutic, nontherapeutic, or unsafe at a one biological time may be efficacious and well tolerated at another. This idea was illustrated by several examples in this chapter, among them glucocorticoid, hypertension, NSAID, including low-dose ASA, and cancer medications.
Pharmacotherapy remains essentially guided by the concept of homeostasis. Therefore, it is not surprising that almost all monotherapy and polytherapy drug-delivery systems are designed to achieve, as an assumed goal, constancy of medication levels as a presumed means of optimizing and achieving consistency of therapeutic effect. This long-held conceptual dogma of pharmacology and drug delivery is counter to the rhythmic organization of biology that gives rise to predictable-in-time variability in the pathophysiology of medical ailments and conditions, and the PK and PD of therapies. Studies cited in this chapter, particularly with respect to rheumatoid arthritis, nocturnal asthma, and hypertension, demonstrate improvement of therapy and reduction of unwanted side effects achievable by timing medications in synchrony with the patient’s CTS. For medications that have a narrow therapeutic range and high risk of adverse effect, such as cancer agents, constant rate delivery may actually potentiate side effects, since they may be delivered in too great a concentration at a wrong biological time.
For certain endocrine and neuroendocrine analogues, desired effectiveness may only be achievable when delivered in a frequency-modulated mode. Indeed, oscillation frequency of drug delivery, rather than dose, can be pivotal in determining therapeutic outcome with some peptide analogues. The pulsatile nature of some peptides may be modulated by circadian and other periodic systems; thus, the expression of pulsatile behavior, itself, maybe restricted to one or only a limited number of phases of circadian, menstrual, and perhaps even annual cycles [205]. This is another very important consideration in the design of drug delivery systems. Thus, it is imperative that the chronobiology of targeted systems be thoroughly known and properly incorporated into the design of drug delivery systems.
Knowledge of (a) CTS and clocks that control it, (b) rhythms in disease pathophysiology and/or associated 24-h patterns in symptom intensity of acute and chronic medical conditions, and (c) chronopharmacology (chronokinetics and chronodynamics) of medications, plus (d) emerging drug delivery technologies will facilitate development of chronotherapeutic dosage forms so as to better attain desired outcomes while minimizing adverse effects. Some early chronotherapies simply involved unequal morning and evening dosing of conventional, sustained release capsule and tablet (12-h activity) systems or prudent selection of dosing time of conventional once-a-day, ultraslow release therapies. This was illustrated in this chapter by the chronotherapy of rheumatoid arthritis and osteoarthritis with NSAIDs, nocturnal asthma with theophylline, and hypertension with different classes of medications via the MAPEC trial. Such simple chronotherapeutic approaches can greatly improve disease management, for example, allergic rhinitis, asthma, PUD, rheumatoid arthritis/osteoarthritis hypertension, and hypercholesterolemia. Nonetheless, the philosophy and practice of clinical pharmacology continues to be dominated by homeostatic dogma in the design of drug delivery systems and goal to achieve constancy of medication concentrations during the 24 h.
A small number of antihypertensive and coronary heart disease medications that have been termed and marketed as chronotherapies by the pharmaceutical industry have been designed as once-daily, delayed onset drug delivery systems [224]. When ingested at bedtime as recommended, these rather simple systems are able to synchronize the concentration of medication in time during the 24 h to the presumed, but unverified by 24-h ABPM, BP and HR circadian rhythms of hypertensive and coronary heart disease patients. Only around-the-clock ABPM can determine the circadian BP and HR pattern for which the medication is intended. The recently completed MAPEC trial attests to the very significant advantage of the chronotherapeutic as opposed to conventional scheduling of BP lowering medications, not only to better control SBP an DBP and normalize the circadian BP rhythm, but also to (chrono)prevent CVD morbidity and mortality [191]. Development and validation of noninvasive and patient friendly technologies for monitoring direct or surrogate biomarkers of BP temporal variation, and predicting proper medication times, are challenges for future research.
From a chronopharmacologic perspective, combination therapy may pose special problems, since each component medication may exhibit different circadian times of best efficacy and safety. If the patient elects to change the ingestion time from the one recommended, the effect may be different from predicted and desired, as illustrated in this chapter by administration-time difference in synergistic effects on BP by amlodipine–valsartan combination hypertension therapy; when the two medications are ingested together at bedtime, reduction of SBP and DBP is greatly magnified, perhaps enhancing risk of hypotension as an adverse effect in some patients [192].
Chronotherapies thus far developed and used in treatment have required a high degree of participation and attention by the patient. Patients with multiple comorbidities, a common situation of the aged, typically are dependent on several classes of medications. In such cases, the particular dosing-time requirements of chronotherapeutic preparations may be impossible to meet with current, conventional drug delivery systems. Delayed or multiple bolus release, implantable, or transcutaneous pump, patch, spray, dry particle, and hydrogel technologies can be adopted and used in the design of chronotherapeutic oral, buccal, nasal, inhalation, subcutaneous, transdermal, rectal, or vaginal systems [368–370]. More advanced systems based on biomimetic schemes or implantable chips with multiple addressable depots that can be individually commanded to release their contents in a proper time sequence are also under investigation [371–373]. Fully implantable systems are limited by the volume of required dose and stability of drug at body temperature. On the other hand, route of administration and intrinsic pharmacokinetics may place dynamical constraints on obtainable drug concentration profiles. In this regard, PK parameters, such as time to peak blood concentration following a single dose, and multiple dosing half-life, become important determinants of chrono-efficacy and chronotoxicity.
New and innovative drug delivery systems are needed to ensure future development and application of chronotherapeutic interventions. Optimally, next generation drug delivery systems should be configured so they (a) require minimal volitional adherence or at least minimize patient resistance to compliance, (b) respond to one or more sensitive biomarkers of disease activity that vary predictably in time, and that are also inducible by nonperiodic environmental phenomena that exacerbate disease, to release medication to targeted tissue(s) on an as-needed real-time basis, and (c) are cost-effective. Further, new generation systems should be able to deliver multiple medications, each responsive to unique biomarkers and each capable of meeting circadian and other bioperiodic determinants of efficacy and safety as a comprehensive polychronotherapy of disease states. The complexity of such systems presents quality assurance and regulatory challenges, particularly when combination therapies are contemplated. Overcoming such challenges is expected to lead to significant advances in treatment outcomes and enhanced quality of life.
Additional relevant information regarding chronopharmacology and chronotherapeutics can be found in two theme issues of Advanced Drug Delivery Reviews (vol 59, issues 9-10, 2007; vol 62, issues 9-10, 2010), and in recently published reviews [375–378].
References
Reinberg A, Smolensky MH (1983) Biologic rhythms and medicine. cellular, metabolic, pathophysiologic, and pharmacologic aspects. Springer, Heidelberg
Touitou Y, Haus E (eds) (1992) Biologic rhythms in clinical and laboratory medicine. Springer, Heidelberg
Haus E, Touitou Y (1992) Principles of clinical chronobiology. In: Touitou Y Haus E (eds) Biological rhythms in clinical and laboratory medicine. New York, Springer, pp 6–34
Donahue JL, Lowenthal DT (1997) Nocturnal polyuria in the elderly person. Am J Med Sci 314:232–238
Kallas HE, Chintanadilok J, Maruenda J, Donahue JL, Lowenthal DT (1999) A clinical investigation of nocturnal polyuria in patients with nocturia: a diurnal variation in arginine vasopressin secretion and its relevance to mean blood pressure. Drugs Aging 15:429–437
Smolensky MH, Halberg F (1977) Circadian rhythm in airway patency and lung volumes. In: McGovern JP, Smolensky MH, Reinberg A, Thomas CC (eds) Chronobiology in allergy and immunology. Springfield, Illinois, pp 117–138
Halberg F, Simpson H (1967) Circadian acrophases of human 17-hydroxycorticosteroid excretion referred to midsleep rather than midnight. Hum Biol 39:405–413
Reinberg A (1979) Chronobiologic field trials of oil refinery shift workers. Chronobiologia 6(Suppl 1):1–119
Reinberg A, Smolensky MH (1992) Night and shift work and transmeridian and space flights. In: Touitou Y, Haus E (eds) Biologic rhythms in clinical and laboratory medicine. Springer, Heidelberg, pp 242–255
Dardente H, Cermakian N (2007) Review: molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol Int 24:195–214
Duguay D, Cermakian N (2009) The crosstalk between physiology and circadian clock proteins. Chronobiol Int 26:1479–1513
Hanifin JP, Brainard GC (2007) Photoreception for circadian, neuroendocrine, and neurobehavioral regulation. J Physiol Anthropol 26:87–94
Maronde E, Stehle JH (2007) The mammalian pineal gland: known facts, unknown facets. Trends Endocrinol Metab 18:142–149
Golombek DA, Rosenstein RE (2010) Physiology of circadian entrainment. Physiol Rev 90:1063–1102
Wever RA (1979) The circadian system of man, results of experiments under temporal isolation. Springer, New York
Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M (2010) Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms 25:432–441
Khalsa SB, Jewett ME, Cajochen C, Czeisler CA (2003) A phase response curve to single bright light pulses in human subjects. J Physiol 549:945–952
Lagoguey M, Reinberg A, Legrand JC (1981) Variations chronobiologiques de la response testiculaire a l’HCG chez l’homme adulte sain. Ann Endocrinol 41:59–60
Lagoguey M, Reinberg A (1981) Circadian and circannual changes of pituitary hormones in healthy human males. In: Van Cauter E, Copinschi G (eds) Human pituitary hormones. Martinus Nijhoff Publ, The Hague, pp 261–285
Reinberg A (1983) Clinical chronopharmacology: an experimental basis for chronotherapy. In: Reinberg A, Smolensky MH (eds) Biologic rhythms and medicine. cellular, metabolic, pathophysiologic, and pharmacologic aspects. Springer, Heidelberg, pp 243–248
Smolensky MH, Peppas N (2007) Chronobiology, drug delivery, and chronotherapeutics. Adv Drug Deliv Rev 59:828–851
Folkard S (2008) Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int 25:215–224
Horne JA, Östberg O (1976) A self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. Int J Chronobiol 4:97–110
Duffy JF, Rimmer DW, Czeisler CA (2001) Association of intrinsic circadian period with morningness–eveningness, usual wake time, and circadian phase. Behav Neurosci 115:895–899
Roenneberg T, Wirtz-Justice A, Merrow M (2003) Life between the clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 18:80–90
Baehr EK, Revelle W, Eastman CI (2000) Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness–eveningness. J Sleep Res 9:117–127
Bailey SL, Heitkemper MM (2001) Circadian rhythmicity of cortisol and body temperature: morningness–eveningness effects. Chronobiol Int 18:249–261
Duffy JF, Dijk DJ, Hall EF, Czeisler CA (1999) Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med 47:141–150
Burgess HJ, Sharkey KM, Eastman CI (2002) Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev 6:407–420
Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL (1998) The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int 15:71–83
Minors DS, Waterhouse JM, Wirz-Justice A (1991) A human phase-response curve to light. Neurosci Lett 133:36–40
Brismar K, Hylander B, Eliasson K, Rössner S, Wetterberg L (1988) Melatonin secretion related to side-effects of beta-blockers from the central nervous system. Acta Med Scand 223:525–530
Nathan PJ, Maguire KP, Burrows GD, Norman TR (1997) The effect of atenolol, a beta1-adrenergic antagonist, on nocturnal plasma melatonin secretion: evidence for a dose-response relationship in humans. J Pineal Res 23:131–135
Stoschitzky K, Sakotnik A, Lercher P, Zweiker R, Maier R, Liebmann P, Lindner W (1999) Influence of beta-blockers on melatonin release. Eur J Clin Pharmacol 55:111–115
Stoschitzky K, Stoschitzky G, Brussee H, Bonelli C, Dobnig H (2006) Comparing beta-blocking effects of bisoprolol, carvedilol and nebivolol. Cardiology 106:199–206
Conlon M, Lightfoot N, Kreiger N (2007) Rotating shift work and risk of prostate cancer. Epidemiology 18:182–183
Deacon S, English J, Tate J, Arendt J (1998) Atenolol facilitates light-induced phase shifts in humans. Neurosci Lett 242:53–56
Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA (2002) Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst 93:1563–1568
Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA (2003) Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst 95:825–828
Lewy A, Emens JS, Lefler BJ, Yuhas K, Jackman AR (2005) Melatonin entrains free-running blind people according to a physiological dose–response curve. Chronobiol Int 22:1093–1106
Lewy AJ, Emens J, Jackman A, Yuhas K (2006) Circadian uses of melatonin in humans. Chronobiol Int 23:403–412
Hoban TM, Sack RL, Lewy AJ, Miller LS, Singer CM (1989) Entrainment of a free-running human with bright light? Chronobiol Int 6:347–353
Costa G, Di Milia L (2008) Aging and shift work. A complex problem to face. Chronobiol Int 25:165–181
Haus E, Smolensky MH (2006) Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control 17:489–500
Knutsson A (2003) Health disorders of shift workers. Occup Med 53:103–108
Morikawa Y, Nakagawa H, Miura K, Ishizaki M, Tabata M, Nishijo M, Higashiguchi K, Yoshita K, Sagara T, Kido T, Naruse Y, Nogawa K (1999) Relationship between shift work and onset of hypertension in a cohort of manual workers. Scand J Work Environ Health 25:100–104
Oishi M, Suwazono Y, Sakata K, Okubo Y, Harada H, Kobayashi E, Uetani M, Nogawa K (2005) A longitudinal study on the relationship between shift work and the progression of hypertension in Japanese male workers. J Hypertens 23:2173–2178
WHO-IARC (2010) Painting, firefighting, and shiftwork/IARC working group on the evaluation of carcinogenic risks to humans (2007: Lyon, France). v. 98
Lee RE, Smolensky MH, Leach CS, Mc Govern JP (1997) Circadian rhythms in the cutaneous reactivity to histamine and selected antigens, including phase relationship to urinary cortisol excretion. Ann Allergy 38:231–236
Smolensky MH, Lemmer B, Reinberg A (2007) The chronobiology and chronotherapy of allergic rhinitis and bronchial asthma. Adv Drug Deliv Rev 59:852–882
Gaultier C, Reinberg A, Girard F (1975) Circadian changes in lung resisatnce and dynamic compliance in healthy and asthmatic children. Effects of two bronchodilators. Respir Physiol 31:169–182
JNC 7 (2003) The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure, National Heart, Lung, and Blood Institute. JAMA 289:2560-2671
Drance SM (1960) The significance of the diurnal tension variations in normal and glaucomatous eyes. Arch Ophthalmol 64:494–501
Saccà SC, Rolando M, Marletta A, Macrí A, Cerqueti P, Ciurlo G (1998) Fluctuations of intraocular pressure during the day in open glaucoma, normal-tension glaucoma and normal subjects. Opthalmologica 212:115–119
Jarrett RJ (1972) Circadian variations in blood glucose levels, in glucose tolerance and in plasma immunoreactive insulin levels. Acta Diabetol Lat 9:263–275
Zimmet PZ, Wall JR, Rome R, Stimmler L, Jarrett RJ (1974) Diurnal variation in glucose tolerance: associated changes in plasma insulin, growth hormone and non-esterified fatty acids. Br Med J 1:485–488
Solberg HE (1987) Approved recommendation (1986) on the theory of reference values, Part 1. The concept of reference values. Report of Expert Panel on Theory of Reference Values (EPTRV) of the International Federation of Clinical Chemistry (IFCC). Clin Chim Acta 165:111–118
PetitClerc C, Solberg HE (1987) Approved recommendation on the theory of reference values, Part 2. Selection of individuals for the production of reference values. Report of Expert Panel on Theory of Reference Values (EPTRV) of the International Federation of Clinical Chemistry (IFCC). J Clin Chem Clin Biochem 25:639–644
Haus E, Touitou Y (1992) Chronobiology in laboratory medicine. In: Touitou Y, Haus E (eds) Biological rhythms in clinical and laboratory medicine. Heidelberg, Springer, pp 673–708
Halberg F, Lee JK, Nelson WL (1978) Time-qualified reference intervals – chronodesms. Experientia 34:713–716
Haus E (1987) Requirements for chronobiotechnology and chronobiologic engineering in laboratory medicine. In: Scheving LE, Halberg F, Ehret CF (eds) Chronobiotechnology and chronobiological engineering. Appl Sci 120:331–372
DePrins J, Hecquet B (1992) Data processing in chronobiological studies. In: Touitou Y Haus E(eds) Biologic rhythms in clinical and laboratory medicine. Springer, Heidelberg, pp 90–113
Halberg F, Cornelissen G, Sothern RB, Wallach LA, Halberg E, Ahlgren A, Kuzel M, Radke A, Barbosa J, Goetz F, Buckley J, Mandel J, Schuman L, Haus E, Lakatua D, Sackett L, Berg H, Kawasaki T, Ueno M, Uezono K, Matsouka M, Omae T, Tarquini B, Cagnoni M, Garcia Sainz M, Perez Vega E, Griffiths K, Wilson D, Donati L, Tatti P, Vasta M, Locatelli I, Camagna A, Lauro R, Tritsch G, Wendt HW (1981) International geographic studies of oncological interest on chronobiological variables. In: Kaiser HN (ed) Neoplasms-comparative pathology of growth in animals, plants, and man. Wiley, New York, pp 553–596
Halberg F, Lagoguey A, Reinberg A (1983) Human circannual rhythms over a broad spectrum of physiological processes. Int J Chronobiol 8:225–268
Haus E, Lakatua DJ, Halberg F, Halberg E, Cornelissen G, Sackett LL, Berg HG, Kawasaki T, Ueno M, Uezono K, Matsouka M, Omae T (1980) Chronobiological studies of plasma prolactin in women and Kyuishu, Japan and Minnesota USA. J Clin Endocrinol Metabol 51:632–640
Haus E, Lakatua DJ, Sackett-Lundeen L, Swoyer J (1984) Chronobiology in laboratory medicine. In: Reitveld WT (ed) Clinical aspects of chronobiology. Baarn, Bakker, pp 13–82
Haus E, Lakatua DJ, Swoyer J, Sackett-Lundeen L (1983) Chronobiology in hematology and immunology. Am J Anatomy 168:467–517
Haus E, Nicolau GY, Lakatua DJ, Sackett-Lundeen L (1988) Reference values for chronopharmacology. Annu Rev Chronopharmacol 4:333–424
Kanabrocki EL, Sothern RB, Scheving LE, Vesely DL, Tsai TH, Shelstad J, Cournoyer C, Greco J, Mermall H, Ferlin H, Nemchausky BM, Bushnell DL, Kaplan E, Kahn S, Augustine G, Holmes E, Rumbyrt J, Sturtevant RP, Sturtevant F, Bremer F, Third JLHG, McCormick JB, Mudd CA, Dawson S, Sackett-Lundeen L, Haus E, Halberg F, Pauly JE, Olwin JH (1990) Reference values for circadian rhythms of 98 variables in clinically healthy men in the fifth decade of life. Chronobiol Int 7:445–461
Kanabrocki EL, Sothern RB, Scheving LE, Vesely DL, Tsai TH, Shelstad J, Cournoyer C, Greco J, Mermall H, Nemchausky BM, Bushnell DL, Kaplan E, Kahn S, Augustine G, Holmes E, Rumbyrt J, Sturtevant RP, Sturtevant F, Bremer F, Third JLHG, McCormick JB, Mudd CA, Dawson S, Olwin JH, Sackett-Lundeen L, Haus E, Halberg F, Pauly JE, Hrushesky WJM (1990) Circadian reference data for men in fifth decade of life. In: Hayes, DK, Pauly, JE, Reiter, RE (eds) Chronobiology: its role in clinical medicine, general biology and agriculture. Prog Clin Biol Res, Wiley/Liss, New York, 341A:771–781
Touitou Y, Fèvre M, Lagoguey M, Carayon A, Bogdan A, Reinberg A, Beck H, Cesselin F, Touitou C (1981) Age- and mental health-related circadian rhythms of plasma levels of melatonin, prolactin, luteinizing hormone and follicle-stimulating hormone in man. J Endocrinol 91:467–475
Touitou Y, Motohashi Y, Pati A, Lévi F, Reinberg A, Ferment O (1986) Comparison of cortical circadian rhythms documented in samples of saliva, capillary (fingertips) and venous blood from healthy subjects. Annu Rev Chronopharmacol 3:297–299
Touitou Y, Sulon J, Bogdan A, Touitou C, Reinberg A, Beck H, Sodoyez JC, Demey-Ponsart E, Van Cauwenberge H (1982) Adrenal circadian system in young and elderly human subjects: a comparative study. J Endocrinol 93:201–210
Touitou Y, Touitou C, Bogdan A, Reinberg A, Auzeby A, Beck H, Guillet PH (1986) Differences between young and elderly subjects in seasonal and circadian variations of total plasma proteins and blood volume as reflected by hemoglobin, hematocrit and erythrocyte counts. Clin Chem 32:801–804
Touitou Y, Touitou C, Bogdan A, Reinberg A, Motohashi Y, Auzeby A, Beck H (1989) Circadian and seasonal variations of electrolytes in aging humans. Clin Chil Acta 180:245–254
Reinberg A, Lagoguey M, Cesselin F, Touitou Y, Legrand JC, Delassalle A, Antreassian J, Lagoguey A (1978) Circadian and circannual rhythms in plasma hormones and other variables in five healthy young human males. Acta Endocrinol 88:417–427
Hanson EJ (1970) Multiple time series. Wiley, New York
MacNeill IB (1974) Tests for periodic components in multiple time series. Biometrika 61:57–70
Van Cauter E (1979) Method for characterization of 24-hr temporal variations of blood components. Am J Physiol 237:E255–E264
DePrins J, Cornelissen G, Malberg W (1986) Statistical procedures in chronobiology and chronotherapeutics. Annu Rev Chronopharmacol 2:27–141
Halberg F, Panofsky H (1961) I. Thermo-variance spectra; method and clinical illustrations. Exp Med Surg 19:284–309
Panofsky H, Halberg F (1961) II. Thermo-variance spectra; simplified computational example and other methodology. Exp Med Surg 19:323–338
Halberg F, Engeli M, Hamburger C, Hillman D (1965) Spectral resolution of low-frequency, small amplitude rhythms in excreted ketosteroids; probable androgen-induced circaseptan desynchronization. Acta Endocrinol 103(Suppl):5–54
Nelson WL, Tong YL, Lee JK, Halberg F (1979) Methods for cosinor rhythmometry. Chronobiologia 6:305–323
Halberg F, Tong YL, Johnson EA (1967) Circadian system phase – an aspect of temporal morphology; procedures and illustrative examples. In: von Mayersbach H (ed) The cellular aspects of biorhythms. Heidelberg, Springer, pp 20–48
Bingham C, Arbogast B, Cornelissen-Guillaume G, Lee JK, Halberg F (1982) Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia 9:397–439
Arendt J (1995) Melatonin and the mammalian pineal gland. Chapman Hill, London
Mirick DK, Davis S (2008) Melatonin as a biomarker of circadian dysregulation (review). Cancer Epidemiol Biomarkers Prev 17:3306–3313
Touitou Y, Motohashi Y, Reinberg A, Touitou C, Bourdeleau P, Bogdan A, Auzéby A (1990) Effect of shift work on the night-time secretory patterns of melatonin, prolactin, cortisol, and testosterone. Eur J Appl Physiol Occup Physiol 60:288–292
Schernhammer ES, Hankinson SE (2009) Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses’ Health Study cohort. Cancer Epidemiol Biomarkers Prev 18:74–79
Schernhammer ES, Rosner B, Willett WC, Laden F, Colditz GA, Hankinson SE (2004) Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol Biomarkers Prev 13:936–943
Arendt J (1992) The pineal. In: Touitou Y, Haus E (eds) Biologic rhythms in clinical and laboratory medicine. Springer, Heidelberg, pp 348–362
Walker RF, Read GF, Wilson DW, Riad-Fahmy D, Griffiths K (1990) Chronobiology in laboratory medicine: principles and clinical applications illustrated from measurements of neutral steroids in saliva. In: Hayes DK, Pauly JE, Reiter RE (eds) Chronobiology: its role in clinical medicine, general biology and agriculture. Prog Clin Biol Res, Wiley/Liss, New York, 341A:105–117
Miles A, Philbrick DRS, Thomas DR, Grey J (1987) Diagnostic and clinical implications of plasma and salivary melatonin assay. Clin Chem 33:1295–1297
Archer SN, Viola AU, Kyriakopoulou V, von Schantz M, Dijk DJ (2008) Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep 31:608–617
Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS (2003) Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 102:4143–4145
Hida A, Kusanagi H, Satoh K, Kato T, Matsumoto Y, Echizenya M, Shimizu T, Higuchi S, Mishima K (2009) Expression profiles of PERIOD 1, 2 and 3 in peripheral blood mononuclear cells from older subjects. Life Sci 84:33–37
Takimoto M, Hamada A, Tomoda A, Ohdo S, Omura T, Sakato H, Kawatani J, Jodoi T, Nakagawa H, Terazono H, Koyanagi S, Higuchi S, Kimura M, Tukikawa H, Irie S, Saito H, Miike T (2005) Daily expression of clock genes in whole blood cells in healthy subjects and a patient with circadian rhythm sleep disorder. Am J Physiol Regul Integr Comp Physiol 289:1273–1279
Teboul M, Barrat-Petit MA, Li XM, Claustrat B, Formento JL, Delaunay F, Lévi F, Milano G (2005) Atypical patterns of circadian clock gene expression in human peripheral blood mononuclear cells. J Mol Med 83:693–699
Azama T, Yano M, Oishi K, Kadota K, Hyun K, Tokura H, Nishimura S, Matsunaga T, Iwanaga H, Miki H, Okada K, Hiraoka N, Miyata H, Takiguchi S, Fujiwara Y, Yasuda T, Ishida N, Monden M (2007) Altered expression profiles of clock genes hPer1 and hPer2 in peripheral blood mononuclear cells of cancer patients undergoing surgery. Life Sci 80:1100–1108
Waterhouse J, Edwards B, Mugarza J, Flemming R, Minors D, Calbraith D, Williams G, Atkinson G, Reilly T (1999) Purification of masked temperature data from humans: some preliminary observations on a comparison of the use of an activity diary, wrist actimetry, and heart rate monitoring. Chronobiol Int 16:461–475
Waterhouse J, Weinert D, Minors D, Atkinson G, Reilly T, Folkard S, Owens D, Macdonald I, Sytnik N, Tucker P (1999) The effect of activity on the waking temperature rhythm in humans. Chronobiol Int 16:343–357
Reinberg A, Ghata J, Halberg F, Apelbaum M, Gervais P, Boudon P, Abulker C, Dupont J (1974) Treatment schedules modify circadian timing in human adrenocortical insufficiency. In: Scheving LE, Halberg F, Pauly JE (eds) Chronobiology. Igaku Shoin Ltd, Tokyo, pp 168–173
Angeli A (1974) Circadian ACTH-adrenal rhythm in man. Chronobiologia 1(Suppl):253–268
Grant PH, Forsham PH, DiRaimando VC (1965) Suppression of 17-hydroxycorticosteroids in plasma and urine after single and divided doses of triamcinolone. N Engl J Med 273:1115–1118
Harter JG, Reddy WJ, Thorn GW (1963) Studies on an intermittent corticosteroid dosage regimen. N Engl J Med 296:591–595
Alten R, Döring G, Cutolo M, Gromnica-Ihle E, Witte S, Straub R, Buttgereit F (2010) Hypothalamus-pituitary-adrenal axis function in patients with rheumatoid arthritis treated with nighttime-release prednisone. J Rheumatol 37:2025–2031
Buttgereit F, Döring G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, Jeka S, Krueger K, Szechinski J, Alten R (2008) Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): A double-blind randomised controlled trial. Lancet 371:205–214
To H, Irie S, Tomonari M, Watanabe Y, Kitahara T, Sasaki H (2009) Therapeutic index of methotrexate depends on circadian cycling of tumor necrosis factor-α in collagen-induced arthritis rats and mice. J Pharm Pharmacol 61:1333–1338
To H, Yoshimatsu H, Tomonari M, Ida H, Tsurumoto T, Tsuji Y, Sonemoto E, Shimasaki N, Koyanagi S, Sasaki H, Ieiri I, Higuchi S, Kawakami A, Ueki Y, Eguchi K (2011) Methotrexate chronotherapy is effective against rheumatoid arthritis. Chronobiol Int 28:267–274
Haus E, Cusulos M, Sackett-Lundeen L, Swoyer J (1990) Circadian variations in blood coagulation parameters, alpha-antitrypsin antigen and platelet aggregation and retention in clinically healthy subjects. Chronobiol Int 7:203–216
Haus E, Smolensky MH (1999) Biologic rhythms in the immune system. Chronobiol Int 16:581–622
Fernandes G (1992) Chronobiology of immune functions: cellular and humoral aspects. In: Touitou Y, Haus E (eds) Biologic rhythms in clinical and laboratory medicine. Heidelberg, Springer, pp 493–503
Wrba H, Dutter A, Sánchez de la Peña S, Wu J, Carandente F, Cornélissen G, Halberg F (1990) Secular or circadian effects of placebo and melatonin on murine breast cancer? In: Hayes DK, Pauly JE, Reiter RE (eds) Chronobiology: its role in clinical medicine, general biology, and agriculture. Wiley/Liss, Washington, pp 31–40
Wrba H, Halberg F, Dutter A (1986) Melatonin circadian-stage-dependently delays breast tumor development in mice injected daily for several months. Chronobiologia 13:123–128
Harris MD, Siegel LB, Alloway JA (1999) Gout and hyperuricemia. Am Fam Physician 59:925–934
Sydenham T (1850) The works of Thomas Sydenham. Translated from the Latin by R.G. Lathan
Rigas B, Torosis J, McDougall CJ, Vener KJ, Spiro HM (1990) The circadian rhythm of biliary colic. J Clin Gastroenterol 12:409–414
Manfredini R, Gallerani K, Cecilia O, Boari B, Fersini C, Portaluppi F (2002) Circadian pattern in occurrence of renal colic in an emergency department; an analaysis of patients notes. BMJ 324:767
Bellamy N, Sothern RB, Campbell J (2004) Aspects of diurnal rhythmicity in pain, stiffness, and fatigue in patients with fibromyalgia. J Rheumatol 31:379–389
Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL (1981) Primary fibromyalgia: clinical study of 50 patients with matched controls. Semin Arthritis Rheum 11:151–171
Moore JG, Halberg F (1987) Circadian rhythm of gastric acid secretion in active duodenal ulcer: Chronobiological statistical characteristics and comparison of acid sectretory and plasma gastrin patterns with healthy subjects and post-vagotomy and pyloroplasty patients. Chronobiol Int 4:101–110
Cugini P, Di Palma L, Battisti P, Leone G, Materia E, Parenzi A, Romano M, Ferrera U, Moretti M (1990) Ultradian, circadian and infradian periodicity of some cardiovascular emergencies. Am J Cardiol 66:240–243
Kroetz C (1940) Ein biologiescher 24-Stunden-Rhythmus des Blutkreislaufs bei Gesundheit und bei Herzschwache zugleich ein Beitrag zur tageszeitlichen Haufung einiger akuter Kreislaufstorungen. Munch Med Wschr 87:314–317
Turner-Warwick M (1998) Epidemiology of nocturnal asthma. Am J Med 85:6–8
Dethlefsen U, Repges R (1985) Ein neues Therapieprinzip bei nachtlichem Asthma. Med Klin 80:44–47
Ebata T, Aizawa H, Kamide R, Niimura M (1999) The characteristics of nocturnal scratching in adults with atopic dermatitis. Br J Dermatol 141:82–86
Smolensky MH, Tatar SE, Bergman SA, Losman JG, Barnard CN, Dasco CC, Kraft IA (1976) Circadian rhythmic aspects of human cardiovascular function: A review by chronobiologic statistical methods. Chronobiologia 3:337–371
Portaluppi F, Hermida RC (2007) Circadian rhythms in cardiac arrhythmias and opportunities for their chronotherapy. Adv Drug Deliv Rev 59:940–951
Tikkinen KA, Johnson TMn, Tammela TL, Sintonen H, Huhtala H, Auvinen A (2010) Nocturia frequency, bother, and quality of life: how much is too often? A population-based study in Finland. Eur Urol 57:488–496
Cruz IA, Drummond M, Wimck JC (2011) Obstructive sleep apnea symptoms beyond sleepiness and snoring: effects of nasal APAP therapy. Sleep Breath doi.10.1007/s11325-011-0502-4
Natarajan R (2010) Review of periodic limb movement and restless leg syndrome. J Postgrad Med 56:157–162
Portaluppi F, Cortelli P, Buonaura GC, Smolensky MH, Fabbain F (2009) Do restless legs syndrome and periodic limb movements of sleep play a role in nocturnal hypertension and increased cardiovascular disease risk in renal patients. Chronobiol Int 26:1206–1221
Kelmanson IA (1991) Circadian variation of the frequency of sudden infant death syndrome and of sudden death from life-threatening conditions in infants. Chronobiologia 18:181–186
Reinberg AE, Gervais P, Levi F, Smolensky M, Del Cerro L, Ugolini C (1988) Circadian and circannual rhythms of allergic rhinitis: an epidemiologic study involving chronobiologic methods. J Allergy Clin Immunol 81:51–62
Smolensky MH, Reinberg A, Labrecque G (1995) Twenty-four hour pattern in symptom intensity of viral and allergic rhinitis: Treatment implications. J Allergy Immunol 95:1084–1096
Bellamy N, Sothern RB, Campbell J, Buchanan WW (1991) Circadian rhythm in pain, stiffness, and manual dexterity in rheumatoid arthritis: relation between discomfort and disability. Ann Rhuem Dis 50:243–348
Fox AW, Davis RL (1998) Migraine chronobiology. Headache 38:436–441
Solomon GD (1992) Circadian rhythms and migraine. Cleveland Clin J Med 59:326–329
Mulcahy D, Keegan J, Cunningham D, Quyyumi A, Crean P, Park A, Wright C, Fox K (1998) Circadian variation of total ischemic burden and its alteration with anti-anginal agents. Lancet 2:755–759
Rocco MB, Barry J, Campbell S, Nabel E, Cook EF, Goldman L, Selwyn AP (1987) Circadian variation of transient myocardial ischemia in patients with coronary artery disease. Circulation 75:395–400
Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA (1997) Meta analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol 79:1512–1516
Shaw E, Tofler GH (2009) Circadian rhythm and cardiovascular disease. Curr Atheroscler Rep 11:289–295
Elliott WJ (1998) Circadian variation in the timing of stoke onset. A meta-analysis. Stroke 29:992–996
Gallerani M, Manfredini R, Fersini C (1993) Chronoepidemiology in human disease. Ann Inst Super Sanita 29:569–579
Gallerani M, Manfredini R, Ricci L, Grandi E, Cappato R, Calò G, Pareschi PL, Fersini C (1992) Sudden death from pulmonary thromboembolism: chronobiological aspects. Eur Heart J 6:305–323
Rossenwasser AM, Wirz-Justice A (1997) Circadian rhythms and depression: clinical and experimental models. In: Redfern PH, Lemmer B (eds) Physiology and pharmacology of biological rhythms, handbook of experimental pharmacology, vol 125. Springer, Berlin, pp 457–486
Wehr TA (1982) Circadian rhythm disturbances in depression and mania. In: Brown FM, Graeber RC (eds) Rhythmic aspects of behavior. Lawrence Erlbaum Ass, New Jersey, pp 399–428
Mooney M, Green C, Hatsukami D (2006) Nicotine self-administration: cigarette versus nicotine gum diurnal topography. Hum Psychopharamcol 21:539–548
Mützell S (1998) Alcohol consumption, clinical findings and retrospective psycho social data in a random sample of men in suburban Stockholm. Scand J Prim Health Care 6:185–192
Bellamy N, Sothern RB, Campbell J (1990) Rhythmic variations in pain perception in osteoarthritis of the knee. J Rheumatol 17:364–372
Bellamy N, Sothern RB, Campbell J, Buchanan WW (2002) Rhythmic variations in pain, stiffness, and manual dexterity in osteoarthristis. Ann Rheum Dis 61:1075–1080
Manfredini R, Gallerani M, Salmi R, Calò G, Pasin M, Bigoni M, Fersini C (1994) Circadian variation in the time of onset of acute intestinal bleeding. J Emerg Med 12:5–9
Svanes C, Sothern RB, Sorbye H (1998) Rhythmic patterns in incidence of peptic ulcer perforation over 5.5 decades in Norway. Chronobiol Int 15:241–264
Kim YK, Oh WH, Park KH, Kim JM, Kim DH (2010) Circadian blood pressure and intraocular pressure patterns in normal tension glaucoma patients with undisturbed sleep. Korean J Opththalmol 24:23–28
Liu JH, Bouligny RP, Kripke DF, Weinreb RN (2003) Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Opththalmol Vis Sci 44(10):4439–4442
Baxil CW, Walczak TS (1997) Effects of sleep and sleep stage on epileptic and nonepileptic seizures. Epilepsia 38:56–62
Langdon-Down M, Brain WR (1929) Time of day in relation to convulsion in epilepsy. Lancet 1:1029–1032
Bjorvatn B, Pallesen S (2009) A practical approach to circadian rhythm sleep disorders. Sleep Med Rev 13:47–60
Pandi-Perumal SR, Trakht I, Spence DW, Srinivasan V, Dagan Y, Cardinali DP (2008) The roles of melatonin and light in the pathophysiology and treatment of circadian rhythm sleep disorders. Nat Clin Pract Neurol 4:436–447
Lamont EW, James FO, Boivin DB, Cermakian N (2007) From circadian clock genes to pathologies. Sleep Med Rev 8:547–556
Aoki H, Ozeki Y, Yamada N (2001) Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int 18:263–271
Shanware NP, Hutchinson JA, Kim SH, Zhan L, Bowler MJ, Tibbetts RS (2011) Casein kinease1-dependent phosphorylation of familial advanced sleep phase syndrome-associated residues controls PERIOD 2 stability. J Biochem 286:12766–12774
Reinberg A, Ashkanazi I (2008) Internal desynchronization of circadian rhythms and tolerance to shift work. Chronobiol Int 25:625–643
Hack LM, Lockley SW, Arendt J, Skene DJ (2003) The effects of low-dose melatonin on the free-running rhythm of blind subjects. J Biol Rhythms 18:420–429
Kennaway DJ (2010) Clock genes at the heart of depression. J Pyschopharmacol 24:5–14
Kripke D, Drennan MD, Elliott JA (1992) The complex circadian pacemaker in affective disorder. In: Touitou Y Haus E (eds) Biologic rhythms in clinic and laboratory medicine. Springer, Heidelberg, pp 265–276
Parry BL, Meliska CJ, Sorenson DL, Martínez LF, López AM, Elliott JA, Hauger RL (2011) Reduced phase-advance of plasma melatonin after bright morning light in the luteal, but not follicular, menstrual cycle phase in premenstrual dysphoric disorder: an extended study. Chronobiol Int 28:415–424
Utge SJ, Soronen P, Loukola A, Kronholm E, Ollilia HM, Pirkola S, Porkka-Heiskanen T, Partonen T, Paunio T (2010) Systemic analysis of circadian genes in a population-based sample reveals association of TIMELESS with depression and sleep disturbance. PLos One 5:e9259
Reinberg AE (1991) Concepts of circadian chronopharmacology. In: Hrushesky WJM, Langer R, Theeuwes F (eds) Temporal control of drug delivery. Ann N Y Acad Sci 618:102–115
Lemmer B (ed) (1989) Chronopharmacology: cellular and biochemical interactions. Marcel Dekker, New York
Lemmer B (2005) Chronopharmacology and controlled drug release. Expert Opin Drug Deliv 2:667–681
Redfern PH, Lemmer B (eds) (1997) Physiology and pharmacology of biological rhythms. Springer, Heidelberg
Bélanger PM (1993) Chronopharmacology in drug research and therapy. Adv Drug Res 24:1–80
Bélanger PM, Bruguerolle B, Labrecque G (1997) Rhythms in pharmacokinetics: absorption, distribution, metabolism, and excretion. In: Redfern PH, Lemmer B (eds) Physiology and pharmacology of biological rhythms: handbook of experimental pharmacology, vol 125. Springer, Berlin, pp 177–204
Bruguerolle B (1998) Chronopharmacokinetics. Current status. Clin Pharmacokinet 35:83–94
Lemmer B (2006) Clinical chronopharmacology of the cardiovascular system: hypertension and coronary heart disease. Clin Ther 157:41–52
Lemmer B, Bruguerolle B (1994) Chronopharmacokinetics. Are they clinically relevant? Clin Pharmacokinet 26:419–427
Moore J, Merki H (1997) Gastrointestinal tract. In: Redfern PH, Lemmer B (eds) Physiology and pharmacology of biological rhythms, handbook of experimental pharmacology, vol 125. Berlin, Springer, pp 351–373
Reinberg AE, Smolensky MH (1982) Circadian changes in drug disposition in man. Clin Pharmacokinet 7:401–420
Witte K, Lemmer B (1997) Rhythms in second message mechanism. In: Redfern PH, Lemmer B (eds) Physiology and pharmacology of biological rhythms, handbook of experimental pharmacology, vol 125. Berlin, Springer, pp 135–156
Sanders SW, Moore JG, Buchi KN, Bishop AL (1988) Circadian variation in the pharmacodynamic effect of intrvenous ranitidine. Annu Rev Chronopharmacol 5:335–338
Sanders SW, Moore JG, Buchi KN, Bishop AL (1989) Pharmacodynamics of intravenous ranitidine after bolus and continuous infusion in patients with healed duodenal ulcer. Clin Pharmacol Ther 46:545–551
White C, Smolensky MH, Sanders SW, Buchi KN, Moore JG (1991) Day-night and individual differences in response to constant-rate ranitidine infusion. Chronobiol Int 8:56–66
Decousus H (1992) Chronobiology in hemostasis. In: Touitou Y, Haus E (eds) Biologic rhythms in clinical and laboratory medicine. Springer, Heidelberg, pp 554–565
Decousus H, Croze M, Lévi F, Perpoint B, Jaubert J, Bonadona JF, Reinberg A, Queneau P (1985) Circadian changes in anticoagulant effect of heparin infused at a constant rate. Br Med J 290:341–344
Haus E (2007) Chronobiology of hemostasis and inferences for the chronotherapy of coagulation disorders and thrombosis prevention. Adv Drug Deliv Rev 59:966–984
Bruguerolle B, Labrecque G (2007) Rhythm patterns in pain and their chronotherapy. Adv Drug Deliv Rev 59:883–895
Smolensky MH, Hermida R, Ayala DE, Portaluppi F (2010) Administration-time-dependent effects of antihypertension medications: basis for the chronotherapy of hypertension (review). Blood Press Monitor 15:173–180
Portaluppi F, Smolensky MH (2010) Perspectives on the chronotherapy of hypertension based on the results of the MAPEC study. Chronobiol Int 27:1652–1667
Hermida RC, Ayala DE, Mojón A, Fernández JR (2010) Influence of circadian time of hypertension treatment on cardiovascular risk: Results of the MAPEC study. Chronobiol Int 27:1629–1651
Hermida RC, Ayala DE, Fontao MJ, Mojón A, Fernández JR (2010) Chronotherapy with valsartan/amlodipine fixed combination: improved blood pressure control of essential hypertension with bedtime dosing. Chronobiol Int 27:1287–1303
Os I, Bratland B, Dahlhöf B, Syvertsen JO, Tretli S (1994) Female preponderance for lisinopril cough in hypertension. Am J Hypertens 7:1012–1015
Oparil S, Miller AP (2005) Gender and hypertension. J Clin Hypertens 7:305–309
Kloner RA, Sowers JR, DiBona GF, Gaffney M, Wein M (1996) Sex- and age-related antihypertensive effects of amlodipine. The Amlodipine Cardiovascular Community Trial Study Group. Am J Cardiol 77:713–722
Canzanello VJ, Baranco-Pryor E, Rahbari-Oskoui F, Schwartz GL, Boerwinkle E, Turner ST, Chapman AB (2008) Predictors of blood pressure response to the angiotensin receptor blocker candesartan in essential hypertension. Am J Hypertens 21:61–66
Saunders E, Cable G, Neutel J (2008) Predictors of blood pressure response to angiotensin receptor blocker/diuretic combination therapy: a secondary analysis of the irbesartan/hydrochlorothiazide blood pressure reductions in diverse populations (INCLUSIVE) study. J Clin Hypertens 10:27–33
Ayala DE, Hermida RC (2010) Sex differences in the administration-time-dependent effects of low-dose aspirin on ambulatory blood pressure in hypertensive subjects. Chronobiol Int 27:354–362
Hermida RC, Calvo C, Ayala DE, Domínquez MJ, Covelo M, Fernández JR, Mojón A, López JE (2003) Administration time-dependent effects of valsartan on ambulatory blood pressure of hypertensive subjects. Hypertension 42:283–290
Cambar J, L’Azou B, Cal C (1992) Chronotoxicology. In: Touitou Y, Haus E (eds) Biologic rhythms in clinical and laboratory medicine. Springer, Heidelberg, pp 139–150
Cambar J, Pons M (1997) New trends in chronotoxicology. In: Redfern PH, Lemmer B (eds) Physiology and pharmacology of biological rhythms, handbook of experimental pharmacology, vol 125. Springer, Berlin, pp 557–588
Halberg F (1960) Temporal coordination of physiologic function. Cold Spring Harbor Symp Quant Biol 25:289–310
Beauchamp D, Labrecque G (2007) Chronobiology and chronotoxicology of antibiotics and aminoglycosides. Adv Drug Deliv Rev 59:896–903
Ceresa F, Angeli A, Buccuzzi G, Molino G (1969) Once-a-day neurally stimulated and basal ACTH secretion phases in man and their response to corticoid inhibition. J Clin Endocrinol 29:1074–1082
Haus E (2007) Chronobiology in the endocrine system. Adv Drug Deliv Rev 59:985–1014
McHugh RB, Smolensky MH, Halberg F (1975) Biological rhythm experimentation: a longitudinal design and analysis. Chronobiologia 2:1–12
Smolensky MH, Reinberg A (1976) The chronotherapy of corticosteroids: a practical application of chronobiological findings to clinical and hospital nursing. J Nursing Clinics 11:609–620
Hermida RC, Ayala DC, Mojón A, Fernández JR (2008) Chronotherapy with nifedipin GITS in hypertensive patients: improved efficacy and safety with bedtime dosing. Am J Hypertens 21:948–954
Bernard S, Cajavec Bernard B, Lévi F, Herzel H (2010) Tumor growth rate determines the timing of optimal chronomodulated treatment schedules. PLOS Comput Biol 6(3):e1000712
Hrushesky WJM, März WJ (1992) Chronochemotherapy of malignant tumors: Temporal aspects of antineoplastic drug toxicity. In: Touitou Y, Haus E (eds) Biologic rhythms in clinical and laboratory medicine. Springer, Heidelberg, pp 611–634
Lévi F (1997) Chronopharmacology of anticancer agents. In: Redfern PH, Lemmer B (eds) Physiology and pharmacology of biological rhythms, handbook of experimental pharmacology, vol 125. Springer, Berlin, pp 299–350
Lévi F, Focan C, Karaboué A, de la Valette V, Focan-Henrard D, Baron B, Kreutz F, Giacchetti S (2007) Implications of circadian clocks for the rhythmic delivery of cancer medications. Adv Drug Deliv Rev 59:1015–1035
Mormont C, Boughattas N, Lévi F (1989) Mechanisms of circadian rhythms in the toxicity and efficacy of anticancer drugs: relevance for the development of new analogues In: Lemmer B (ed) Chronopharmacology: cellular and biochemical interactions. Marcel Dekker Inc, New York, pp 395–437
Sauerbier I (1992) Rhythms in drug-induced teratogensis. In: Touitou Y, Haus E (eds) Biologic rhythms in clinical and laboratory medicine. Heidelberg, Springer, pp 151–157
Smolensky MH (1998) Knowledge and attitudes of American physicians and public about medical chronobiology and chronotherapeutics. Findings of two 1996 Gallup surveys. Chronobiol Int 15:377–394
Arendt J, Aldhous M, Marks V (1986) Alleviation of jet-lag by melatonin: preliminary results of a controlled double-blind trial. Br Med J 292:1170
D'Alonzo GE, Smolensky MH, Feldman S, Gianotti LA, Emerson MB, Staudinger H, Steinijans VM (1990) Twenty-four-h lung function in adult patients with asthma: chronoptimized theophylline therapy once-daily dosing in the evening versus conventional twice-daily dosing. Am Rev Respir Dis 142:84–90
Neuenkirchen H, Wilkens JH, Oellerich M, Sybrecht GW (1985) Nocturnal asthma: effect of a once per evening dose of sustained-release theophylline. Eur J Respir Dis 66:196–204
Merki HS, Witzel L, Hare K, Scheurle E, Bauerfeind P, Blum AL (1987) Single dose treatment with H2-receptor antagonists: Is bedtime too late? Gut 28:451–454
Stalenhoff AFH, Mol MJTM, Stuyt PMJ (1989) Efficacy and tolerability of simvastatin. Am J Med 87:39s–43s
Sica D, Frishman WH, Manowitz N (2003) Pharmacokinetics of propranolol after single and multiple dosing with sustained released propranolol or propranolol CR (Innopran XL™), a new chronotherapeutic formulation. Heart Dis 5:176–181
Sista S, Lai J, Eradiri O, Albert K (2003) Pharmacokinetics of a novel diltiazem HCL extended-release tablet formulation for evening administration. J Clin Pharmacol 43:1149–1157
Smith DHG, Neutel JM, Weber MA (2001) A new chronotherapeutic oral drug absorption system for verapamil optimizes blood pressure control in the morning. Am J Hypertens 14:14–19
Smolensky MH, Hermida R, Portaluppi F, Haus E, Reinberg A (2005) Chronotherapeutics in the treatment of hypertersion. In: Oparil S, Weber MA (eds) Hypertension: a companion to Brenner and Rector’s the kidney, 2nd edn. Philadelphia, Elsevier Saunders, pp 530–542
White WB, Anders RJ, MacInyre JM, Black HR, Sica DA (1995) Nocturnal dosing of a novel delivery system of verapamil for systemic hypertension. Am J Cardiol 76:375–380
Kunkel G, Steinijans VW, Borner K (1987) Chrono-optimization of the time of evening administration of theophylline with unequally divided twice daily dosing. Chronobiol Int 4:364–368
Postma DS, Koëter GH, vd Mark TW, Reig RP, Sluiter HJ (1985) The effects of oral slow-release terbutaline on the circadian variation in spirometry and arterial blood gas levels in patients with chronic air flow obstruction. Chest 87:653–657
Portaluppi F, Manfredini R, Fersini C (1999) From a static to a dynamic concept of risk: the circadian epidemiology of cardiovascular risk. Chronobiol Int 16:33–50
Black HR, Elliott WJ, Neaton JD, Grandits G, Grambsch P, Grimm RH, Hansson L, Lacoucière Y, Muller J, Sleight P, Weber MA, White WB, Williams G, Wittes J, Zanchetti A, Fakouhi TD (1998) Rationale and design for the Controlled ONset Verapamil INvestigation of Cardiovascular Endpoints (CONVINCE) trial. Control Clin Trials 19:370–390
Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, Neaton JD, Grimm RH, Hansson L, Lacourciere Y, Muller J, Sleight P, Weber MA, Williams G, Wittes J, Zanchetti A, Anders RJ, Group CR (2003) Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA 289:2073–2082
Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, Neaton JD, Grimm RH, Hansson L, Lacourcière Y, Muller JE, Sleight P, Weber MA, White WB, Williams GH, Wittes J, Zanchett A, Anders RJ, Group CR (2005) Results of the Controlled ONset Verapamil INvestigation of Cardiovascular Endpoints (CONVINCE) trial by geographical region. J Hypertens 23:1099–1106
Hermida RC (2007) Ambulatory blood pressure monitoring in the prediction of cardiovascular events of chronotherapy: rationale and design of the MAPEC study. Chronobiol Int 24:749–775
Bennett BM, Leitman DC, Schroeder H, Kawamoto JH, Nakatsu K, Murad F (1989) Relationship between biotransformation of glyceryl trinitrate and cyclic GMP accumulation in various cultured cell lines. J Pharmacol Exp Therap 250:316–322
Fung H-L, Chung S-J, Bauer JA, Chong S, Kowaluk EA (1992) Biochemical mechanisms of organic nitrate action. Am J Cardiol 70:4B–10B
Salvemini D, Pistelli A, Vane J (1993) Conversion of glyceryl trinitrate to nitric oxide in tolerant and non-tolerant smooth muscle cells. Br J Pharmacol 108:162–169
Waldman SA, Rapoport RM, Ginsburg R, Murad F (1986) Densitization to nitroglycerin in vascular smooth muscle from rat and human. Biochem Pharmacol 35:3525–3531
Enbright GE (1914) The effects of nitroglycerin on those engaged in its manufacture. J Am Med Assoc 62:201–202
Stewart D (1888) Remarkable tolerance to nitroglycerin. Philadelphia Polyclinic 6:43
Chen Z, Zhang J, Stamler JS (2002) Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A 99:8306–8311
Katz RJ (1990) Mechanism of nitrate tolerance: a review. Cardiovasc Drugs Therapy 4:247–252
Hinz B, Schroeder H (1998) Nitrate tolerance is specific for nitric acid esters and its recovery requires intact protein synthesis. Biochem Biophys Res Commun 252:232–235
Kenkare S, Benet LZ (1996) Tolerance to nitroglycerin in rabbit aorta. Biochem Pharmacol 51:1357–1361
Thadhani U (1992) Role of nitrates in angina pectoris. Am J Cardiol 70:43B–53B
Kimura E, Hosoda S, Katoh K, Endo M, Yasue H, Asada S, Kuroiwa A (1978) Panel discussion on the variant form of angina pectoris. Jpn Circ J 42:455–476
Kuroiwa A (1978) Symptomology of variant angina. Jpn Circ J 42:459–478
Conn PM, Crowley WFJ (1994) Gonadotropin-releasing hormone and its analogs. Annu Rev Med 45:391–405
Huirne JA, Lambalk CB (2001) Gonadotropin releasing hormone receptor antagonists. Lancet 358:1793–1803
Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E (1978) Hypophysial response to continuous and intermittent delivery of hypopthalamic gonadotropin releasing hormone. Science 202:632–633
Knobil E (1980) The role of signal pattern in the hypothalamic control of gonadotropin secretion. In: Ortavant R, Reinberg A (eds) Rhythmes et reproduction. Paris, Masson, pp 75–80
Nakai Y, Plant TM, Hess DL, Keoch EJ, Knobil E (1978) On the sites of negative and positive feedback action of estradiol in the control of gonadotropin secretion in the Rhesus monkey. Endocrinology 102:1008–1014
Filicori M, Santoro N, Merriam GR, Crowley WFJ (1986) Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab 62:1136–1144
Reame N, Sauder SE, Kelch RP, Marshall JC (1984) Pulsatile gonadotropin secretion during the human menstrual cycle: evidence for altered frequency of gonadotropin-releasing hormone secretion. J Clin Endocrinol Metab 59:328–337
Hall JE, Schoenfeld DA, Martin KA, Crowley WFJ (1992) Hypothalamic gonadotropin-releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab 74:600–607
Knobil E (1980) The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res 36:53–88
Crowley WFJ, Vale W, Rivier J, MacArthur JW (1981) LH and RH in hypogonadotropic hypogonadism. In: Zatuchini GI, Schelter JD, Sciarra JJ (eds) LH-RH peptides as female and male contraceptives. Philadelphia, Harper, pp 321–333
Santen RJ, Manni A, Harvey H (1986) Gonadotropin releasing hormone (GnRH) analogs for the treatment of breast and prostatic carcinoma. Breast Cancer Res Treat 7:129–145
Macklon NS, Stouffer RL, Giudice LC, Fauser BC (2006) The Science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev 27:170–207
Gompel A, Poitout P (1997) Inducteurs de l’ovulation. In: Maurais-Jarvis P, Schaison P, Touraine P (eds) Médecine de la reproduction. Paris, Flammarion, pp 604–616
Gompel A (2003) Induction de l’ovulation par administration pulsatile de Gn-RH par pompe portable. In: Reinberg AE (ed) Chronobiologie Médicale et Chronothérapeutique. Paris, Flammarion, pp 177–180
Leyendecker G, Wildt L, Hansmann M (1980) Pregnancies following chronic intermittent (pulsatile) administration of Gn-Rh by means of a portable pump (“Zyklomat”) – a new approach to the treatment of infertility in hypothalamic amenorrhea. J Clin Endocrinol Metab 51:1214–1216
Hayes FJ, Seminara SB, Crowley WF Jr (1998) Hypogonadotropic hypogonadism. Endocrinol Metab Clin N Am 27:739–763
Santoro N, Filicori M, Crowley WF Jr (1986) Hypogonadotropic disorders in men and women: diagnosis and therapy with pulsatile gonadotropin-releasing hormone. Endocr Rev 7:11–23
Spratt DI, Crowley WF Jr, Butler JP, Hoffman AR, Conn PM, Badger TM (1985) Pituitary luteinizing hormone responses to intravenous and subcutaneous administration of gonadotropin-releasing hormone in men. JCE & M 61:890–895
Farhy LS, Veldhuis JD (2005) Deterministic construct of amplifying actions of ghrelin on pulsatile growth hormone secretion. Am J Physiol Regul Integr Comp Physiol 288:R1649–R1663
Giustina A, Veldhuis JD (1998) Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797
Veldhuis JD, Bowers CY (2003) Three-peptide control of pulsatile and entropic feedback-sensitive modes of growth hormone secretion: modulation by estrogen and aromatizable androgen (review). J Pediatr Endocrinol Metab 16(Suppl 3):587–605
Veldhuis JD, Roelfsema F, Keenan DM, Pincus S (2011) Gender, age, body mass index, and IGF-1 individually and jointly determine distinct GH dynamics: analyses in one hundred healthy adults. J Clin Endocrinol Metab 96:115–121
Isgaard J, Carlsson L, Isaksson O, Jansson J (1988) Pulsatile intravenous growth hormone (GH) infusion to hypophysectomized rats increases insulin-like growth factor I messenger ribonucleic acid in skeletal tissues more effectively than continuous GH infusion. Endocrinology 123:2605–2610
Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, Watkins PB (2002) Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol Endocrinol Metab 283:E1008–E1015
Veldhuis JD, Bowers CY (2010) Integrating GHS into the ghrelin system (Review). Int J Peptides 2010:879503
Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY (2006) Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27:101–140
Litman T, Halberg F, Ellis S, Bittner JJ (1958) Pituitary growth hormone and mitoses in immature mouse liver. Endocrinology 62:361–364
Veldhuis JD, Keenan DM, Pincus SM (2008) Motivations and methods for analyzing pulsatile hormone secretion (review). Endocr Rev 29:823–864
Wu FC, Butler GE, Kelnar CJ, Huhtaniemi I, Veldhuis JD (1996) Ontogeny of pulsatile gonadotropin releasing hormone secretion from midchildhood, through puberty, to adulthood in the human male: a study using deconvolution analysis and an ultrasensitive immunofluorometric assay. J Clin Endocrinol Metab 81:1798–1805
Farhy LS, Veldhuis JD (2004) Putative GH pulse renewal: periventricular somatostatinergic control of an arcuate-nuclear somatostatin and GH-releasing hormone oscillator. Am J Physiol Regul Integr Comp Physiol 286:R1030–R1042
Farhy LS, Straume M, Johnson ML, Kovatchev B, Veldhuis JD (2002) Unequal autonegative feedback by GH models the sexual dimorphism in GH secretory dynamics. Am J Physiol Regul Integr Comp Physiol 282:R753–R764
Veldhuis JD, Evans WS, Shah N, Story S, Bray MJ, Anderson SM (1999) Proposed mechanisms of sex-steroid hormone neuromodulation of the human GH-IGP-I axis. In: Veldhuis JD, Giustina A (eds) Sex-steroid interactions with growth hormone. Springer, New York, pp 93–121
Arvat E, Ceda GP, Di Vito L, Ramunni J, Gianotti L, Broglio F, Deghenghi R, Ghigo E (1998) Age-related variations in the neuroendocrine control, more than impaired receptor sensitivity, cause the reduction in the GH-releasing activity of GHRPs in human aging. Pituitary 1:51–58
Van Cauter E, Plat L, Copinschi G (1998) Interrelations between sleep and the somatotropic axis. Sleep 21:553–566
Ho KY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO (1987) Effects of sex and age on the 24-h profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 64:51–58
Holl RW, Hartman ML, Veldhuis JD, Taylor WM, Thorner MO (1991) Thirty-second sampling of plasma growth hormone in man: correlation with sleep stages. J Clin Endocrinol Metab 72:854–861
Krueger JM, Obál F Jr (1993) Growth hormone-releasing hormone and interleukin-1 in sleep regulation. FASEB J 7:645–652
Ocampo-Lim B, Guo W, DeMott-Friberg R, Barkan AL, Jaffe CA (1996) Nocturnal growth hormone (GH) secretion is eliminated by infusion of GH-releasing hormone antagonist. J Clin Endocrinol Metab 81:4396–4399
Gronfier C, Luthringer R, Follenius M, Schaltenbrand N, Macher JP, Muzet A, Brandenberger G (1996) A quantitative evaluation of the relationships between growth hormone secretion and delta wave electroencephalographic activity during normal sleep and after enrichment in delta waves. Sleep 19:817–824
Van Cauter E, Plat L, Scharf MB, Leproult R, Cespedes S, L’Hermite-Balériaux M, Copinschi G (1997) Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young men. J Clin Invest 100:745–753
Anderson LA, McTernan PG, Barnett AH, Kumar S (2001) The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: influence of gender and site. J Clin Endocrinol Metab 86:5045–5051
Leung KC, Johannsson G, Leong GM, Ho KK (2004) Estrogen regulation of growth hormone action. Endocr Rev 25:693–721
Davey HW, Wilkins RJ, Waxman DJ (1999) STAT5 signaling in sexually dimorphic gene expression and growth patterns. Am J Hum Genet 65:959–965
Rudling M, Norstedt G, Olivecrona H, Reihner E, Gustafsson JA, Angelin B (1992) Importance of growth hormone for the induction of hepatic low density lipoprotein receptors. Proc Natl Acad Sci U S A 89:6983–6987
Laron Z (2004) Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958–2003. J Clin Endocrinol Metab 89:1031–1044
Roelfsema F, Biermasz NR, Veldman RG, Veldhuis JD, Frölich M, Stokvis-Brantsma WH, Wit JM (2000) Growth hormone (GH) secretion in patients with an inactivating defect of the GH-releasing hormone (GHRH) receptor is pulsatile: evidence for a role for non-GHRH inputs into the generation of GH pulses. J Clin Endocrinol Metab 86:2459–2464
van Coevorden A, Mockel J, Laurent E, Kerkhofs M, L’Hermite-Balériaux M, Decoster C, Nève P, Van Cauter E (1991) Neuroendocrine rhythms and sleep in aging men. Am J Physiol 260(4 Pt 1):E651–E661
Veldhuis JD, Liem AY, South S, Weltman A, Weltman J, Clemmons DA, Abbott R, Mulligan T, Johnson ML, Pincus S, Straume M, Iranmanesh A (1995) Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab 80:3209–3222
Martin FC, Yeo AL, Sonksen PH (1997) Growth hormone secretion in the elderly: aging and the somatopause. Baillieres Clin Endocrinol Metab 11:223–250
Biermasz NR, Pereira AM, Frölich M, Romijn JA, Veldhuis JD, Roelfsema F (2004) Octreotide represses secretory-burst mass and nonpulsatile secretion but does not restore event frequency or orderly GH secretion in acromegaly. J Clin Endocrinol Metab 286:E25–E30
Hartman ML, Pincus SM, Johnson ML, Matthews DH, Faunt LM, Vance ML, Thorner MO, Veldhuis JD (1994) Enhanced basal and disorderly growth hormone secretion distinguish acromegalic from normal pulsatile growth hormone release. J Clin Invest 95:1277–1288
Faje AT, Barkan AL (2010) Basal, but not pulsatile, growth hormone secretion determines the ambient circulating levels of insulin-like growth factor-1. J Clin Endocrinol Metab 95:2486–2491
Dimaraki EV, Jaffe CA, DeMott-Friberg R, Chandler WF, Barkan AL (2002) Acromegaly with apparently normal GH secretion: implications for diagnosis and follow-up. J Clin Endocrinol Metab 87:3537–3542
van den Berg G, Pincus SM, Frölich M, Veldhuis JD, Roelfsema F (1998) Reduced disorderliness of growth hormone release in biochemically inactive acromegaly after pituitary surgery. Eur J Endocrinol 138:164–169
Cordido F, Garcia-Buela J, Sangiao-Alvarellos S, Martinez T, Vidal O (2010) The decreased growth hormone response to growth hormone releasing hormone in obesity is associated to cardiometabolic risk factors. Mediators Inflamm 2010:1–8
Utz AL, Yamamoto A, Hemphill L, Miller KK (2008) Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab 93:2507–2514
Sen F, Demirturk M, Abaci N, Golcuk E, Oflaz H, Elitok A, Kutluturk F, Issever H, Unaltuna NE, Ozbey NC (2008) Endothelial nitric oxide synthase intron 4a/b polymorphism and early atherosclerotic changes in hypopituitary GH-deficient adult patients. Eur J Endocrinol 158:615–622
Hong JW, Kim JY, Kim YE, Lee EJ (2011) Metabolic parameters and nonalcoholic fatty liver disease in hypopituitary men. Horm Metab Res 43:48–54
Barclay JL, Nelson CN, Ishikawa M, Murray LA, Kerr LM, McPhee TR, Powell EE, Waters MJ (2011) GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology 152:181–192
Christ ER, Cummings MH, Russell-Jones DL (1998) Dyslipidaemia in adult growth hormone (GH) deficiency and the effect of GH replacement therapy: a review. Trends Endocrinol Metab 9:200–206
Monson JP, Jönsson P, Koltowska-Häggström M, Kourides I (2007) Growth hormone (GH) replacement decreases serum total and LDL-cholesterol in hypopituitary patients on maintenance HMG CoA reductase inhibitor (statin) therapy. Clin Endocrinol (Oxf) 67:623–628
Pijl H, Langendonk JG, Burggraaf J, Frölich M, Cohen AF, Veldhuis JD, Meinders AE (2001) Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab 86:5509–5515
Boero L, Cuniberti L, Magnani N, Manavela M, Yapur V, Bustos M, Rosso LG, Meroño T, Marziali L, Viale L, Evelson P, Negri G, Brites F (2010) Increased oxidized low density lipoprotein associated with high ceruloplasmin activity in patients with active acromegaly. Clin Endocrinol (Oxf) 72:654–660
Mihailescu DV, Vora A, Mazzone T (2011) Lipid effects of endocrine medications. Curr Atheroscler, Rep, 13
Cersosimo E, Danou F, Persson M, Miles JM (1996) Effects of pulsatile delivery of basal growth hormone on lipolysis in humans. Am J Physiol 271:E123–E126
Surya S, Horowitz JF, Goldenberg N, Sakharova A, Harber M, Cornford AS, Symons K, Barkan AL (2009) The pattern of growth hormone delivery to peripheral tissues determines insulin-like growth factor-1 and lipolytic responses in obese subjects. J Clin Endocrinol Metab 94:2828–2834
Johansson JO, Oscarsson J, Bjarnason R, Bengtsson BA (1996) Two weeks of daily injections and continuous infusion of recombinant human growth hormone (GH) in GH-deficient adults. I. Effects on insulin-like growth factor-I (IGF-I), GH and IGF binding proteins, and glucose homeostasis. Metabolism 45:362–369
Jørgensen JO, Møller N, Lauritzen T, Christiansen JS (1990) Pulsatile versus continuous intravenous administration of growth hormone (GH) in GH-deficient patients: effects on circulating insulin-like growth factor-I and metabolic indices. J Clin Endocrinol Metab 70:1616–1623
Laursen T, Lemming L, Jorgensen JO, Klausen IC, Christiansen JS (1998) Different effects of continuous and intermittent patterns of growth hormone administration on lipoprotein levels in growth hormone-deficient patients. Horm Res 50:284–291
Oscarsson J, Ottosson M, Johansson JO, Wiklund O, Mårin P, Björntorp P, Bengtsson BA (1996) Two weeks of daily injections and continuous infusion of recombinant human growth hormone (GH) in GH-deficient adults. II. Effects on serum lipoproteins and lipoprotein and hepatic lipase activity. Metabolism 45:370–377
Marshall L, Mölle M, Böschen G, Steiger A, Fehm HL, Born J (1996) Greater efficacy of episodic than continuous growth hormone-releasing hormone (GHRH) administration in promoting slow-wave sleep (SWS). J Clin Endocrinol Metab 81:1009–1013
Johnson TN, Rostami-Hodjegan A, Tucker GT (2006) Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet 45:931–956
Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14:1–18
Thorner MO, Rogol AD, Blizzard RM, Klingensmith GJ, Najjar J, Misra R, Burr I, Chao G, Martha P, Mc Donald J (1988) Acceleration of growth rate in growth hormone-deficient children treated with human growth hormone-releasing hormone. Pediatr Res 24:145–151
Laursen T, Jørgensen JO, Jakobsen G, Hansen BL, Christiansen JS (1995) Continuous infusion versus daily injections of growth hormone (GH) for 4 weeks in GH-deficient patients. J Clin Endocrinol Metab 80:2410–2418
Hümmelink R, Sippell WG, Benoit KG, Danielson K, Faijerson Y (1993) Intranasal administration of growth hormone-releasing hormone (1–29)-NH2 in children with growth hormone deficiency: effects on growth hormone secretion and growth. Acta Pediatr 388:23–26
Steyn D, du Plessis L, Kotzé A (2010) Nasal delivery of recombinant human growth hormone: in vivo evaluation with Pheroid technology and N-trimethyl chitosan chloride. J Pharm Pharm Sci 13:263–273
Takeda A, Copper K, Bird A, Baxter L, Frampton GK, Gospodarevskaya E, Welch K, Bryant J (2010) Recombinant human growth hormone for the treatment of growth disorders in children: a systemic review and economic evaluation (Review). Health Technol Assess 14:1–209
Endocrine Society (2011) Evaluation and treatment of adult growth hormone deficiency: An endocrine society clinical practice guideline, J. Clin Endocrinol Metabol 96:1587–1609
Burman P, Johansson AG, Siegbahn A, Vessby B, Karlsson FA (1997) Growth hormone (GH)-deficient men are more responsive to GH replacement than women. J Clin Endocrinol Metab 82:550–555
Cook DM, Ludlam WH, Cook MB (1999) Route of estrogen administration helps to determine growth hormone (GH) replacement dose in GH-deficient adults. J Clin Endocrinol Metab 84:3956–3960
Johansson AG, Engström BE, Ljunghall S, Karlsson FA, Burman P (1999) Gender differences in the effects of long term growth hormone (GH) treatment on bone in adults with GH deficiency. J Clin Endocrinol Metab 84:2002–2007
Chapman IM, Bach MA, Van Cauter E, Farmer M, Krupa D, Taylor AM, Schilling LM, Cole KY, Skiles EH, Pezzoli SS, Hartman ML, Veldhuis JD, Gormley GJ, Thorner MO (1996) Stimulation of the growth hormone (GH)-insulin-like growth factor I axis by daily oral administration of a GH secretogogue (MK-677) in healthy elderly subjects. J Clin Endocrinol Metab 81:4249–4257
Ionescu M, Frohman LA (2006) Pulsatile secretion of growth hormone (GH) persists during continuous stimulation by CJC-1295, a long-acting GH-releasing hormone analog. J Clin Endocroniol Metab 91:4792–4797
Micic D, Casabiell X, Gualillo O, Pombo M, Dieguez C, Casanueva FF (1999) Growth hormone secretagogues: the clinical future. Horm Res 51:29–33
Nass R, Pezzoli SS, Oliveri MC, Patrie JT, Harrell FEJ, Clasey JL, Heymsfield SB, Bach MA, Vance ML, Thorner MO (2008) Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized, controlled trial. Ann Intern Med 149:601–611
Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK (2011) Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab 96:150–158
Méjean L, Kolopp M, Drouin P (1992) Chronobiology, nutrition, and diabetes. In: Touitou Y, Haus E (eds) Biologic rhythms in clinical and laboratory medicine. Springer, Heidelberg, pp 375–385
Gualandi-Signorini AM, Giorgi G (2001) Insulin formulations – a review. Eur Rev Med Pharmacol Sci 5:73–83
Dessau E, Cameron F, Lee HB, Bequette BW, Zisser H, Jovanovic L, Chase HP, Wilson DM, Buckingham BA, Doyle FJ (2010) Real-time hypoglycemia prediction suite using continuous glucose monitoring. Diabetes Care 33:1249–1254
Keenan DB, Cartaya R, Mastrototaro JJ (2010) Accuracy of a new real-time continuous glucose monitoring algorithm. J Diabetes Sci Technol 4:111–118
Keenan DB, Cartaya R, Mastrototaro JJ (2010) The pathway to the closed-loop artificial pancreas: research and commercial perspectives. Pediatr Endocrinol Rev 7:445–451
Lee H, Buckingham BA, Wilson DM, Bequette BW (2009) A closed-loop artificial pancreas using model predictive control and a sliding meal size estimator. J Diabetes Sci Technol 3:1082–1090
Parker RS, Doyle FJ, Peppas NA (1999) A model-based algorithm for blood glucose control in type I diabetes. IEEE Trans Biomed Eng 46:148–157
Bratusch-Marrain PR, Komjati M, Waldhäusl WK (1986) Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes 35:922–926
Courtney CH, Atkinson AB, Ennis CN, Sheridan B, Bell PM (2003) Comparison of the priming effects of pulsatile and continuous insulin delivery on insulin action in man. Metabolism 52:1050–1055
Meier JJ, Veldhuis JD, Butler PC (2005) Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 54:1649–1656
Mirouze J, Selam JL, Pham TC (1977) Le pancréas artificial extra-corporel; nouvelle orientation du traitement insulinique. In: XIV Congrès Internat. Thérapeutique, Expansion Scientifique, Montpellier, France, pp 79–91
Mirouze J, Selam JL, Pham TC, Orsetti A (1977) Evaluation of exogenous insulin homeostatis by the artificial pancrease in insulin dependent diabetes. Diabetologia 13:273–278
Paolisso G, Scheen AJ, Giugliano D, Sgambato S, Albert A, Varricchio M, D'Onofrio F, Lefèbvre PJ (1991) Pulsatile insulin delivery has greater metabolic effects than continuous hormone administration in man; importance of pulse frequency. J Clin Endocrinol Metab 72:607–615
Matthews DR, Naylor BA, Jones RG, Ward GM, Turner RC (1983) Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes 32:617–621
Berman N, Chou HF, Berman A, Ipp E (1993) A mathematical model of oscillatory insulin secretion. Am J Physiol 264:R839–R851
Jaspan JB, Lever E, Polonsky KS, Van Cauter E (1986) In vivo pulsatility of pancreatic islet peptides. Am J Physiol 251:E215–E226
Lefebvre PJ, Paolisso G, Scheen AJ, Henquin JC (1987) Pulsatility of insulin and glucagon release: physiological significance and pharmacological implications. Diabetologia 30:443–452
Gibson T, Stimmler L, Jarrett RJ, Rutland P, Shiu M (1975) Diurnal variation in the effects of insulin in blood glucose, plasma non-esterified fatty acids and growth hormone. Diabetologia 11:83–88
Debry G, Mejean L, Villaume C, Drouin P, Martin JM, Pointel JP, Gay G (1977) Chronobiologie et nutrition humaine. In: XIV Congrès Internat. Thérapeutique, Expansion Scientifique, Montpellier, France, pp 225–245
Mirouze J, Collard F (1973) Continous blood glucose monitoring in brittle diabetes. In: Proceedings 8th Int’l. Congr. Diabetes Fed., pp 532–545
Haus E, Nicolau G, Halberg F, Lakatua D, Sackett-Lundeen L (1983) Circannual variations in plasma insulin and C-peptide in clinically healthy subjects. Chronobiologia 10:132
Cawood EH, Bancroft J, Steel JM (1993) Perimenstrual symptoms in women with diabetes mellitus and the relationship to diabetic control. Diabet Med 10:444–448
Ruegemer JJ, Squires RW, Marsh HM, Haymond MW, Cryer PE, Rizza RA, Miles JM (1990) Differences between pre-breakfast and late afternoon glycemic response to exercise in IDDM patients. Diabetes Care 13:104–110
Aye T, Block J, Buckingham B (2010) Toward closing the loop: an update on insulin pumps and continuous glucose monitoring systems. Endocrinol Metab Clin North Am 39:609–624
Linkeschova R, Raoul M, Bott U, Berger M, Spraul M (2002) Less severe hypoglycemia, better metabolic control, and improved quality of life in type I diabetes mellitus with continuous subcutaneous insulin infusion (CSII) therapy; an observational study of 100 consecutive patients followed for a mean of 2 years. Diabet Med 19:746–751
Olinder AL, Nyhlin KT, Smide B (2011) Clarifying responsibility for self-management of diabetes in adolescents using insulin pumps – a qualitative study. J Adv Nurs 67:1547–1557
Revert A, Rossetti P, Calm R, Vehí J, Bondia J (2010) Combining basal-bolus insulin infusion for tight postprandial glucose control: an in silico evaluation in adults, children, and adolescents. J Diabetes Sci Technol 4:1424–1437
Walsh J, Roberts R, Bailey T (2010) Guidelines for insulin dosing in continuous subcutaneous insulin infusion using new formulas from a retrospective study of individuals with optimal glucose levels. J Diabetes Sci Technol 4:1174–1181
Cukierman-Yaffe T, Konvalina N, Cohen O (2011) Key elements for successful intensive insulin pump therapy in individuals with type 1 diabetes. Diabetes Res Clin Pract 92:69–73
Schwartz FL, Vernier SJ, Shubrook JH, Marling CR (2010) Evaluating the automated blood glucose pattern detection and case-retrieval modules of the 4 Diabetes Support System. J Diabetes Sci Technol 4:1563–1569
Dudde R, Vering T, Piechotta G, Hintsche R (2006) Computer-aided continuous drug infusion: setup and test of a mobile closed-loop system for the continuous automated infusion of insulin. IEEE Trans Inf Technol Biomed 10:395–402
Scaramuzza AE, Iafusco D, Rabbone I, Bonfanti R, Lombardo F, Schiaffini R, Buono P, Toni S, Cherubini V, Zuccoti GV, Diabetes Study Group of the Italian Society of S. Paediatric Endocrinology and Diabetology T (2011) Use of integrated real-time continuous glucose monitoring/insulin pump system in children and adolescents with type 1 diabetes: a 3-year follow-up study. Diabetes Technol Ther 13:99–103
Welsh JB, Kannard B, Nogueira K, Kaufman FR, Shah R (2010) Insights from a large observational database of continuous glucose monitoring adoption, insulin pump usage and glycemic control: the CareLinkTM database. Pediatr Endocrinol Rev 7:413–416
Mastrototaro J, Shin J, Marcus A, Sulur G (2008) The accuracy and efficacy of real-time continuous glucose monitoring sensor in patients with type 1 diabetes. Diabetes Technol Ther 10:385–390
Hermida RC, Ayala DE, Iglesias M (2003) Administration time-dependent influence of aspirin on blood pressure in pregnant women. Hypertension 41:651–656
Peppas N, Leobandung W (2004) Stimuli-sensitive hydrogels: ideal carriers for chronobiology and chronotherapy. J Biomater Sci Polym Ed 15:124–144
Rathbone MJ, Hadgraft J, Roberts MS (eds) (2003) Modified-release drug delivery technology. Marcel Dekker, New York
Youan B-BC (ed) (2009) Chronopharmaceutics. Wiley, Hoboken
Moschou EA, Peteu SF, Bachas LG, Madou MJ, Daunert S (2004) Artificial muscle material with fast electroactuation under neutral pH conditions. Chem Mater 16:2499–2502
Prescott JH, Lipka S, Baldwin S, Sheppard NFJ, Maloney JM, Coppeta J, Yomtov B, Staples MA, Satini JTJ (2006) Chronic, programmed polypeptide delivery from an implanted, multireservoir microchip device. Nat Biotechnol 24:437–438
Santini JT, Cima MJ, Langer R (1999) A controlled release microchip. Nat Biotechnol 397:335–338
Haus E, Touitou Y (1992) Chronobiology in circulating blood cells and platelets. In: Touitou Y, Haus E (eds) Chronobiology in laboratory medicine. Heidelberg, Springer, pp 504–526
Ohdo S, Koyanagi S, Matsunaga N, Hamdan A (2011) Molecular basis of chronopharmaceutics. J Pharm Sci 100:3560–3576
Mandal AS, Biswas N, Karim KM, Guha A, Chatterjee S, Behera M, Kuotsu K (2010) Drug delivery systems based on chronobiology--a review. J Control Release 147:314–325
Khan Z, Pillay V, Choonara YE, du Toit L (2010) Drug delivery technologies for therapeutic applications. Pharmaceut Develop Technol 14:602–612
Sewlall S, Pillay V, Danckwerts MP, Choonara YE, Ndesendo VM, du Toit LC (2010) A timely review of state-of-the-art chronopharmaceuticals synchronized with biological rhythms. Curr Drug Deliv 7:370–388
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer US
About this chapter
Cite this chapter
Smolensky, M.H., Siegel, R.A., Haus, E., Hermida, R., Portaluppi, F. (2012). Biological Rhythms, Drug Delivery, and Chronotherapeutics. In: Siepmann, J., Siegel, R., Rathbone, M. (eds) Fundamentals and Applications of Controlled Release Drug Delivery. Advances in Delivery Science and Technology. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-0881-9_13
Download citation
DOI: https://doi.org/10.1007/978-1-4614-0881-9_13
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-0880-2
Online ISBN: 978-1-4614-0881-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)