Abstract
The term “apheresis” is derived from a Greek word meaning “removal.” In its most general sense, apheresis refers to techniques for large-scale removal of selected components of the blood. “Plasmapheresis” refers to removal of plasma, “erythrocytapheresis” to removal of red blood cells, and “leukapheresis” to removal of white blood cells. In the first part of this chapter we (SLG, DFF, HCK) will give an overview of apheresis techniques in general as currently practiced in the United States, describe some of the issues that are unique to the application of apheresis techniques in pediatrics, and will review indications for use of apheresis in patients with kidney disease. The latter portion of the chapter (GK) is devoted to an in-depth description of low-density lipoprotein (LDL) apheresis, a specialized application of apheresis technology, as it is currently practiced in Europe.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The term “apheresis” is derived from a Greek word meaning “removal.” In its most general sense, apheresis refers to techniques for large-scale removal of selected components of the blood. “Plasmapheresis” refers to removal of plasma, “erythrocytapheresis” to removal of red blood cells, and “leukapheresis” to removal of white blood cells. In the first part of this chapter we (SLG, DFF, HCK) will give an overview of apheresis techniques in general as currently practiced in the United States, describe some of the issues that are unique to the application of apheresis techniques in pediatrics, and will review indications for use of apheresis in patients with kidney disease. The latter portion of the chapter (GK) is devoted to an in-depth description of low-density lipoprotein (LDL) apheresis, a specialized application of apheresis technology, as it is currently practiced in Europe.
Although the majority of this chapter will be devoted to automated apheresis used for therapeutic purposes, the technique of apheresis is commonly used in other situations. The original automated cell separators were designed in the 1960s for donor apheresis, specifically for drawing transfusable single-donor platelet products from normal volunteer donors. It remains true today that the majority of automated apheresis procedures performed in the United States are donor procedures, to produce either platelets or plasma. Furthermore, the term “apheresis” need not be restricted to procedures that use automated cell separator instruments. Manual apheresis procedures using syringes, tubing, stopcocks, and blood bags can be designed to perform whole blood exchanges in neonates with hyperbilirubinemia, to perform therapeutic phlebotomies for adults with idiopathic hemochromatosis, to harvest whole blood from donors to provide T-cell infusions or even to prepare small volumes of plasma or packed red blood cells for neonates. In most cases, however, the automated cell separators offer significant advantages over manual procedures in speed, sterility, and overall safety.
Automated Apheresis Technology
Principle of Separation
Since apheresis technology is based on the use of automated cell separators, it is helpful to understand how these instruments work. The basic task of automated cell separators is to separate red blood cells, buffy coat, and plasma while maintaining sterility such that one or more of the components can be returned to the patient or donor. In most instruments, this separation is accomplished by mechanical centrifugation. It is also possible to separate plasma from cells by filtration across a membrane, but machines based on this principle are used predominantly for collecting plasma from adult donors or in the intensive care unit setting for treatment of sepsis-associated microangiopathies [1]. Centrifugal devices separate whole blood into components on the basis of density differences, while membrane separators work on the basis of differences in particle size.
The configuration of the centrifugal separation chamber differs among instrument manufacturers, but all have certain design requirements in common. All apheresis systems have single use disposable plasticware that will maintain sterility during centrifugation and that incorporates safety features such as air traps to prevent embolism, filters to prevent reinfusion of aggregates, pressure monitors for access pressure, and a means to infuse an anticoagulant to prevent clot formation in the extracorporeal circulation. All automated separators have an obligate extracorporeal volume (ECV) and an obligate extracorporeal red cell mass (ECRCM), which must be in the instrument’s tubing during the apheresis procedure. ECV and ECRCM vary depending on both the type of apheresis device used and the type of procedure being performed. For example, using the COBE Spectra, the ECV and ECRCM for the leukapheresis set are 285 and 114 mL, respectively, but they are 170 and 68 mL for the plasma/RBC exchange procedure. The temporary loss of these volumes, typically 200–400 mL as shown in Table 41.1, is usually well tolerated by adults, but volume and red cell balance must be taken into careful consideration when automated apheresis is performed in small children, especially if the ECV represents >10–15% of the patient’s blood volume
The Apheresis Process
Number of Vascular Access Points
Cell separators are designed to perform “discontinuous” or “continuous” procedures, and some can be modified to do both. Discontinuous apheresis procedures consist of cycles of three separate phases: drawing blood, separating the components, and returning. Since the drawing of blood and returning of blood are done in distinct phases, discontinuous procedures require only one point of vascular access. For this reason, they are often called “single arm” procedures. In contrast, “continuous” apheresis procedures carry out the drawing, separation, and returning of blood simultaneously and continuously. This requires two points of vascular access, one for drawing and one for returning, and thus these procedures are called “two arm procedures.” One feature of continuous procedures is that there is no cyclic removal and administration of volume to the patient, a decided advantage when the ECV of the machine makes up a significant fraction of the patient’s blood volume. For therapeutic apheresis in pediatrics, especially for children younger than 10 years old, the safety advantages of a continuous circuit are the main consideration, and one must accept the need for “two arms.” In contrast, the “single arm” procedures are well suited to donor procedures in healthy adults who can tolerate a small fluctuation in their blood volume, and who would prefer to have only one venipuncture.
Quantification of Removal
Plasmapheresis is the most commonly indicated apheresis treatment for kidney disease. The general rationale for plasmapheresis is to remove, safely and efficiently, those soluble substances in the plasma that might play a role in the patient’s disease process, for example, the pathogenic anti-glomerular basement membrane antibody in patients with Goodpasture syndrome. Plasmapheresis is not as selective as dialysis (see comparison below) since whole plasma is removed. As plasma is removed from the patient, a replacement fluid must be given to maintain intravascular volume and oncotic pressure. This replacement fluid becomes admixed with the patient’s plasma, and some of it is subsequently removed as the plasmapheresis proceeds. At the start of a plasmapheresis, most of what is removed is the patient’s plasma, whereas at the end of the plasmapheresis, much of what is removed is replacement fluid. The relationship of the amount of plasma removed (expressed as multiples of the patient’s plasma volume) in a plasmapheresis to the fraction of the original plasma remaining is given in Fig. 41.1 [2]. A plasmapheresis procedure that removes a volume equal to the patient’s plasma volume will achieve about 63% removal of the original plasma, with 37% remaining in the patient, as shown in the figure. Removal of twice the patient’s plasma volume will remove 86% of the original plasma. From the figure, it is apparent that the additional benefit of prolonging a plasmapheresis past two volumes is marginal. Finally, the overall efficiency of a single plasmapheresis procedure, or of a series of treatments is also affected by the distribution between intra-and extravascular compartments of the targeted substance and on other metabolic characteristics such as rate of resynthesis and degradation [2].
Control of Volume and Red Cell Mass
The rates at which blood is drawn, processed, and returned during an apheresis procedure are determined by computerized algorithms that control the peristaltic pumps that move the blood through the tubing. While it is beyond the scope of this chapter to discuss these algorithms in detail, a few general points are worthwhile. First, within certain limits, the patient’s net balance of volume and the net balance of red cell mass can be manipulated independently during an apheresis procedure. This means, for example, that it is possible to administer a red cell transfusion during a plasmapheresis with no net increase in the patient’s intravascular volume, a maneuver that can be very advantageous for a patient with anemia and oliguric kidney failure. Second, it is possible to perform a plasmapheresis procedure that results in a net removal of plasma volume from the patient, or in a net fluid gain.
Anticoagulation
An anticoagulant must be added to the blood as it enters the extracorporeal circuit in order to prevent clotting in the machine’s tubing. Sodium citrate is the most commonly used anticoagulant for apheresis; sodium citrate is the anticoagulant in blood products as well. It acts to chelate calcium to prevent in vitro activation of the clotting cascade. When infused into the patient, the citrate may cause transient hypocalcemia. The severity of this side effect depends on the rate of infusion, the capacity for hepatic metabolism of citrate, and the patient’s state of calcium homeostasis (i.e., baseline hypocalcemia or hypoparathyroidism). In many apheresis protocols, the rate of citrate infusion to the patient is the limiting safety factor in determining how rapidly blood can be drawn and returned, and ultimately how long the procedure will last.
The symptoms of reduced ionized calcium related to citrate [3] are usually referred to as “citrate toxicity.” The mildest and most common symptoms are perioral or hand and foot tingling and paresthesias. Some patients experience nausea, an unusual taste in the mouth, or lightheadedness. More severe hypocalcemia may lead to tremors, twitching, muscular spasm, tetany, seizures, arrhythmias, and hypotension related to myocardial dysfunction [4–6]. Patients undergoing apheresis should be monitored for early signs of citrate toxicity either by clinical questioning or measurement of ionized calcium levels. In small children or sedated or unconscious patients who cannot verbalize their discomfort, frequent vital signs including blood pressure and EKG monitoring are necessary.
Prevention of hypocalcemia can also be achieved using a regional anticoagulation protocol, adapted from continuous renal replacement therapy protocols [7], in which a calcium chloride (8 g/L of NS) is infused in the return line at 1.5–2 times the blood pump rate in mL/h. For example, if the blood pump rate is 60 mL/min, the calcium chloride rate would be 90 mL/h. In general, mild symptoms can be relieved by reducing the rate of citrate infusion or by stopping the procedure temporarily until symptoms subside. Oral calcium supplements are often used to treat mild subjective symptoms although their efficacy has not been proven. For severe reactions, such as seizures, tetany, or EKG changes, it is advisable to terminate the procedure altogether and administer parenteral calcium supplements.
Heparin alone, or in combination with sodium citrate, can also be used as the anticoagulant for apheresis procedures. The patient will usually receive the equivalent of a therapeutic dose of heparin during the procedure and would be expected to have an elevated activated clotting time (ACT) and an anticoagulant effect afterward. Some centers that use heparin as the anticoagulant monitor the degree of heparinization during the procedure and adjust the infusion rate. The reason to use a combination of citrate and heparin is to reduce the net dose of citrate required to prevent clotting in the machine, reduce the dose of citrate delivered to the patient, and permit the blood processing to speed up. The decision to use heparin must take into account the effect of the apheresis procedure on the coagulation system, and the patient’s underlying risk for hemorrhage.
Procedures
There are three basic therapeutic apheresis procedures: plasmapheresis, erythrocytapheresis, and leukapheresis. These three procedures are modified in various ways for the therapeutic goal at hand and for safety considerations in small children.
Plasmapheresis
Plasmapheresis involves separation of the plasma from the cellular elements of blood, collecting the patient’s plasma into a waste bag, and returning to the patient his own cells mixed with a fluid to replace the discarded plasma. The replacement fluid must contain colloid to maintain the patient’s intravascular oncotic pressure. When 5% albumin is used as the only replacement fluid, the plasmapheresis procedure can be performed with minimal concern for transfusion-transmitted infectious disease or transfusion-associated lung injury (TRALI) [8]. Removal of plasma and replacement with 5% albumin will result in depletion of most plasma proteins including immunoglobulins and the components of the coagulation cascade. As shown in Fig. 41.1, plasmapheresis of one plasma volume will reduce the levels of coagulation proteins by about 70%, which can be associated with a fibrinogen level below 100 mg/dL and prolongation of the PT and aPTT but not usually with clinical bleeding. If the rate of hepatic regeneration of these lost coagulation factors is normal, a schedule of plasmapheresis procedures every other day generally does not require exogenous replacement with fresh frozen plasma (FFP). However, if daily plasmapheresis is necessary or if the patient has a concomitant coagulopathy, the replacement fluids must include FFP. If the pre-plasmapheresis fibrinogen level is less than 100 mg/dL, FFP should also be included as part of the replacement fluids. If FFP is used as the replacement fluid, the patient’s plasma proteins and coagulation parameters will remain within normal limits. Plasmapheresis using FFP as replacement fluid is more properly termed “automated plasma exchange.”
Plasmapheresis with Staphylococcal Protein A Immunoadsorption
Since plasmapheresis removes all plasma proteins and requires a large volume of replacement fluids, selective removal of specific plasma constituents is an attractive therapeutic approach. Selective removal can be accomplished by immunologic, chemical, or physical means depending on the specific pathogenic substance to be targeted. One example is specific removal of immunoglobulin G (IgG) using immunoadsorption columns. The advantages of immunoadsorption columns over simple plasmapheresis are as follows: (1) large quantities of replacement fluids are not needed, (2) removal is targeted to antibodies and does not affect other plasma constituents, and (3) there is a potential for greater overall efficiency because a larger volume of plasma may be treated than by simple plasmapheresis.
Two techniques have been developed to remove IgG and IgG-containing circulating immune complexes from plasma after it has been separated from the cellular elements by plasmapheresis. The staphylococcal protein A-silica column (Prosorba® column, Fresenius HemoCare, Inc., Redmond, WA) utilizes a solid phase of silica gel beads to which staphylococcal protein A has been bound. The staphylococcal protein A binds human IgG selectively, permitting other plasma proteins to pass through into the column eluate and to be returned to the patient. While this is theoretically attractive as a means of achieving specific removal of IgG, the amount of IgG actually removed is limited by the binding capacity of the column, which is about 2 g of human IgG. The clinical value of immunoadsorption is limited, and is often attributed to “immunomodulation” rather than quantitative removal of IgG. The Prosorba column has been approved by the FDA for the treatment of ITP [9, 10] and rheumatoid arthritis [11, 12].
A variation of this technique, staphylococcal protein A-agarose column (Immunosorba® column, Excorim AB, Lund, Sweden) which was recently acquired by Fresenius HemoCare, employs the intermittent renewal of two staphylococcal A columns to increase the quantity of IgG removed. One column is stripped and regenerated while a second column is in use, and then the flow of plasma is diverted to the newly stripped column when the first column is saturated. This is the only column which has regulatory approval in the United States for the treatment of patients with hemophilia A and B with inhibitors [13–15]. However, the use of both protein A immunoadsorption column techniques has been reported in patients with kidney diseases, such as, hemolytic uremic syndrome (HUS), Goodpasture syndrome, rapidly progressive glomerulonephritis (RPGN) including lupus nephritis, nephrotic syndrome, and kidney allograft rejection [16–32].
As will be discussed later in this chapter, column technology is also available for use in conjunction with plasmapheresis for selective removal of plasma lipids, with return of the remaining plasma proteins. Two such techniques are available in the United States for the treatment of hyperlipidemia, primarily in adults [33–35]. In pediatric practice, this therapy is indicated for rare congenital hyperlipidemia syndromes that are associated with extremely elevated plasma lipid or cholesterol levels and premature atherosclerosis. While standard plasmapheresis can also be used to remove blood lipids, the specialized techniques and equipment currently in use in Europe to treat these children using low-density lipoprotein (LDL) apheresis will be described later in this chapter.
Erythrocytapheresis
Erythrocytapheresis involves separation of the plasma from the cellular elements, collecting primarily the patient’s red cells into the waste bag and returning the patient’s own plasma mixed with donor-packed red blood cells. This technique can be of great value for hemoglobinopathies, and occasionally for diseases caused by intra-erythrocytic parasites such as malaria. The principal applications of erythrocytapheresis are in sickle cell disease. The pheresis machines can be programmed to calculate the volume of packed red blood cells needed to achieve a desired post-procedure hemoglobin S level, as long as the patient’s pre-procedure hematocrit, hemoglobin S, and packed red cell hematocrit concentrations are known. The patient’s total hemoglobin can also be raised without a large volume of intravascular fluid. Common indicators for erythrocytapheresis in sickle cell disease include emergent preparation for surgery, severe acute chest syndrome, or cerebrovascular event. Typically, a post-procedure hemoglobin S of 25% is desired in these acute situations. Erythrocytapheresis may also be used to deliver chronic transfusion therapy in sickle cell disease for primary or secondary stroke prevention, and for other indications which require chronic transfusion therapy. The typical post-procedure hemoglobin S concentration is 15% in this chronic situation, with a schedule of treatments every 4–6 weeks to maintain hemoglobin S less than 30–40%. The principal advantage of erythrocytapheresis in this setting is that iron overload associated with regular RBC transfusions can he reduced or prevented [36, 37].
Leukapheresis
Leukapheresis involves separation of the whole blood into three fractions: plasma, red cells, and white cells from the buffy coat. The plasma and red cells are returned, and only the leukocyte fraction is retained as a leukocyte product. With the standard leukapheresis procedure using the automated cell separators, a replacement fluid is not needed since both donor and therapeutic leukapheresis are collection procedures, not exchange procedures. For any leukapheresis procedure, a replacement fluid may be needed to compensate for the volume of leukocytes and red cells removed in the waste or collected product, especially in small children. This technique can be applied as therapeutic leukocyte depletion to patients with hyperleukocytosis from leukemia as a rapid means of reducing blood viscosity associated with extremely high peripheral white blood cell counts [38–40]. In, general, two blood volumes are processed, and the procedure may he expected to remove approximately 50% of the circulating platelets along with the leukocytes [41]. Variations of this leukapheresis technique can be used to harvest peripheral blood mononuclear cells from an allogeneic or autologous donor, as sources of either hematopoietic stem cells for stem cell transplantation [42–48], dendritic cells, T-lymphocytes for donor lymphocyte infusions [49–53], and other cell-based therapies. Another variation of the leukapheresis procedure is termed “photopheresis,” in which the mononuclear cells harvested by leukapheresis are treated with a photoactivatable chemical (a psoralen), subjected to irradiation under ultraviolet-A light (UVA), and returned to the patient. This therapy is used for cutaneous T-cell lymphoma [54–59] and may have broader applications as immunologic therapy for other autoimmune diseases [60, 61], solid organ graft rejection [62, 63] including kidney allograft rejection [62, 63], and graft-versus-host disease [54, 64, 65].
Plateletpheresis
Plateletpheresis involves separation of the whole blood from healthy donors into three fractions: platelet-poor plasma (PPP), platelet-rich plasma (PRP), and red cells. The PRP is retained as a single-donor platelet concentrate (more accurately termed “apheresis platelets”) while the PPP and red cells are returned to the donor. This is the single most frequent application of apheresis technology. Plateletpheresis is indicated as a therapeutic procedure to remove excess platelets from the circulation in patients with symptomatic thrombocytosis [66–70].
Comparison of Apheresis and Dialysis
Both plasmapheresis and hemodialysis are therapeutic techniques involving extracorporeal circuits for selective removal of components of the blood. For this reason, apheresis and dialysis are sometimes confused, and are occasionally considered as alternative therapeutic options in a patient with kidney disease. In fact, plasmapheresis has been performed safely and efficiently using hemodialysis equipment after modification of the procedure [71, 72]. However, the fundamental mechanisms and clinical utility of plasmapheresis and dialysis are entirely different.
Plasmapheresis employs centrifugation to separate whole plasma from the cellular components of blood. Whether the therapeutic goal is to reduce levels of pathogenic immunoglobulins, lipids, paraproteins, or other substances in the plasma, whole plasma is removed during plasmapheresis, and the proteins of the clotting cascade, normal immunoglobulins, and other plasma proteins are lost. The efficiency of plasmapheresis in removing these substances depends primarily on the volume of plasma removed, but also on their distribution between intra- and extravascular compartments, rate of equilibration between compartments, and other metabolic characteristics [72].
On the other hand, dialysis employs a semipermeable membrane and a dialysis fluid to alter the solute concentrations and free water content of the patient’s plasma. Ions, salts, small molecules, and free water may be removed, but the plasma proteins are unaffected. Thus, dialysis is suitable for treating the electrolyte disturbances, waste product accumulation, water intoxication, and volume overload of kidney failure. Dialysis can also be used to remove toxins if the toxin molecule is small enough and dialyzable, whereas plasmapheresis is suited to removal of antibodies and other pathogenic proteins and lipids, and to large-scale replacement with FFP. Plasmapheresis can be used to remove some toxins, especially if they are predominantly bound to proteins in the plasma. Plasmapheresis does not alter the electrolyte content of plasma, and has only very transient effects on the plasma levels of small molecules such as urea or ammonia. Plasmapheresis can be used to a limited extent to treat fluid overload, but the fluid removed is plasma from the intravascular space not free water or extravascular fluid. The differences between plasmapheresis and dialysis are summarized in Table 41.2.
Pediatric Issues
Use of apheresis in children is feasible regardless of the size of the patient, as long as adequate vascular access can be established. However, apheresis procedures in young children must be customized to the situation and to the size of the patient because apheresis equipment and the software that controls it are, in general, designed for use in adults.
Vascular Access
Most children smaller than 30 kg will not have antecubital veins with large enough diameter to permit successful use of peripheral venous access for apheresis procedures. The access for drawing blood into the cell separator is the most critical, requiring a vein large enough to admit a 16-gauge steel needle and resilient enough to withstand a flow rate as high as 2 mL/kg/min. A 20-gauge flexible IV catheter can be used for returning. A double lumen catheter is usually used for smaller patients or patients with unusable veins so that both draw and return can use the same central access. It is preferable to draw from the proximal ports and reinfuse at the distal point to minimize recirculation, although in practice the better functioning port is usually chosen for the drawing access. The length, gauge, and positioning of the tip of the catheter will depend on the child’s size. However, the wall of the catheter must be resilient enough to withstand the negative pressure generated during the apheresis procedure. In practice, catheters designed for use in dialysis also work well for apheresis procedures, but the softer single and double lumen catheters, such as the Broviac catheter, commonly used in oncology patients and in intensive care units, are not suitable as the draw line, although they can be used for returning.
Extracorporeal Volume
Extracorporeal volume is the most important consideration in adapting apheresis instruments designed for adults to use in children. The ECV for cell separators in clinical use varies from 200 to 400 mL depending on the machine and the procedure to be performed as shown in Table 41.1. Unless specific measures are taken to compensate for this volume, the patient’s blood volume will be depleted by this amount during the apheresis procedure. While an adult may easily tolerate the temporary loss of 200–400 mL of whole blood, this ECV may be too much for a small child. As a general guideline, modification of the procedure in the interest of patient safety is required if the ECV exceeds 15% of the patient’s total blood volume (TBV) and should he considered if the ECV exceeds 10% of the TBV.
The ECV for an apheresis procedure is a fixed specification of the instrument and tubing, and can be determined precisely. The patient’s TBV, however, must be estimated in order to plan the apheresis procedure. The TBV estimate is a basic parameter for the algorithms that control the pumps on an automated apheresis instrument. The traditional formula used by most pediatricians to estimate TBV is 70–75 cm3/kg. More complex, empirically derived formulae [73] for blood volume estimation that take into account gender, weight, and height are shown in Table 41.3. These formulae are programmed into the software of some automated apheresis instruments. While these formulae may be more accurate than a weight-based TBV estimate in adults, they may yield overestimates of TBV in children, especially prepubertal males.
Blood Priming
In addition to the ECV, there is an obligate ECRCM, a volume of packed red blood cells which must be held in the apheresis instrument in order to achieve the separation of plasma from red cells. Two decisions arise with respect to this ECRCM. First, can the patient tolerate the temporary loss of this red cell mass during the procedure? The answer to this question depends not only on the patient’s total blood volume, but also on the patient’s hematocrit and cardiovascular and pulmonary reserve. Second, can the patient tolerate the bolus of fluid which is associated with returning the red cells, or “rinsing back” the red cells from the machine to the patient at the end of the apheresis procedure? The answer to this question also depends on a clinical assessment of the patient’s blood volume, cardiopulmonary reserve, and kidney function.
The procedure modifications that compensate for the ECV and ECRCM for young children undergoing apheresis are often referred to as “priming.” While it is possible to prime the apheresis instrument by filling all of the tubing with red blood cells at a predetermined hematocrit before starting, priming is usually accomplished by infusing additional red cells or fluids at the start of the procedure during the time that the machine is filling with blood from the patient. With proper planning, it is possible to perform an apheresis procedure in a small child with no change in the patient’s blood volume or red cell mass during the procedure. The technical details of priming for pediatric apheresis procedures are discussed in detail in one of the references [74].
In general, the method of priming for an apheresis procedure affects the patient’s blood volume during the procedure and also the final amount of fluid administered at the end of the procedure. The patient’s ability to tolerate volume depletion, loss of red cell mass, and volume overload must be assessed as part of the planning before the procedure is started. For children weighing <20 kg or for patients who are anemic or hemodynamically unstable, red cell priming is usually indicated. From a practical standpoint, this means that half to one unit of packed red cells must be ordered and available before the apheresis procedure can be started.
Anticoagulation (Dose)
The need for anticoagulation to prevent clotting in the extracorporeal circuit was discussed above. For apheresis procedures in pediatrics, one must pay particular attention to the dose rate at which the anticoagulant is administered to the patient. Since the anticoagulant is added to the blood drawn from the patient in a constant ratio of volume of anticoagulant per volume of blood, the rate of blood draw determines the dose of anticoagulant that the patient ultimately receives. Apheresis procedures in children are often performed at higher flow rates than adults, when the rate is expressed on a per kilogram basis. Using typical values as an example, a 70-kg adult undergoing plasmapheresis with flow rates of 90–120 mL/min experiences blood draw rates in the range of 1.3–1.7 mL/kg/min, but a 20-kg child undergoing plasmapheresis using a central line that permits a flow of 40 mL/min experiences a draw rate of 2.0 mL/kg/min. Thus, the dose rate of anticoagulant, citrate, heparin, or a combination will be higher in the child than in the adult. For many apheresis protocols, the dose rate of citrate is the limiting parameter for how fast the procedure can be run. Procedure modifications including calcium supplementation based on regional citrate anticoagulation protocols used in CRRT [7] to prevent citrate toxicity are commonly used in pediatric plasmapheresis.
Hypothermia
Children and adults experience some degree of hypothermia during apheresis procedures because of cooling of blood in the extracorporeal circuit. This side effect may be more pronounced in younger children since the flow rate per kilogram is higher than for adults, as discussed above. A blood warmer is commonly incorporated into the return line in most pediatric apheresis procedures. Depending on the model used, the warmer increases the ECV by 20–50 mL.
Cooperation
The aspects of apheresis that children tolerate least well are the needles, the need to remain seated and still, the restriction of one or both arms, the operation of the blood pressure cuff, and boredom. The apheresis staff must be expert in phlebotomy and IV placement to gain the trust and cooperation of young patients. The staff must also be able to provide age-appropriate explanations of what is going on, and should encourage parental involvement wherever possible. Space and resources to provide distracting entertainment for children undergoing apheresis are a necessity. With a sensitive and experienced apheresis staff, it is rare that children are so frightened, inconsolable, or uncooperative that sedation must be used.
Application to Kidney Diseases
The evidence that demonstrates the clinical efficacy of apheresis-based treatments is compelling in some disease states and marginal in others. For this reason, the Journal of Clinical Apheresis has published, most recently in 2010 [75], a categorized listing of the indications for therapeutic apheresis. The indications are placed into one of four categories, as shown in Table 41.4, based on the strength of evidence that therapeutic apheresis is effective for that disease process. Although this system of categories is imperfect, it is helpful in guiding clinical decisions about the use of apheresis. When therapeutic apheresis is applied to diseases of the kidney, either plasmapheresis or plasma exchange is most commonly indicated. The kidney diseases for which therapeutic apheresis may be indicated are shown in Table 41.5, along with commonly used treatment schedules. Of course, these schedules must be individualized based on the patient’s clinical condition. It is important to establish at the start of a course of apheresis how the success or failure of the therapy will be monitored and judged. This is often difficult to determine with certainty, because many diseases do not have a discrete identifiable marker with which to follow clinical response to treatment.
Apheresis and ACE Inhibitors
One unusual interaction of medications with apheresis therapy is relevant to the care of patients with kidney disease. Antihypertensive agents of the angiotensin-converting enzyme (ACE) inhibitor class have been associated with an atypical and potentially severe reaction occurring shortly after the start of apheresis procedures. The symptoms include flushing and hypotension in most patients, and abdominal cramping, diarrhea, nausea, and diaphoresis in some. The reactions were first reported in patients taking ACE inhibitors who underwent staphylococcal protein A column therapy, but have been associated with plasmapheresis [76] and other therapies involving extracorporeal circuits. The postulated mechanism of these reactions is that during an apheresis procedure elevated levels of bradykinin are generated. In most apheresis patients this is inconsequential because of rapid degradation of bradykinin by kininase II. However, if the patient is receiving ACE inhibitors, the degradation mechanism may be blocked by the drug, and the vasodilatory and gastrointestinal effects of bradykinin give rise to the symptoms. Many ACE inhibitors have been implicated, and it is recommended that ACE inhibitors be withheld at least 24 h before an apheresis procedure.
Low-Density Lipoprotein (LDL) Apheresis
Background
Familial hypercholesterolemia (FH) is an autosomal dominant hereditary metabolic disease due to inactivating mutations in the low-density lipoprotein receptor (LDLR) gene. This results in grossly elevated plasma LDL cholesterol. Clinical manifestations are severe and include premature atherosclerosis and a high risk of myocardial infarction before the age of 30. Because lipid-lowering drug therapy often is insufficient, LDL apheresis has been a mainstay of FH treatment for the past 20 years. LDL apheresis in combination with drug therapy lowers LDL cholesterol plasma levels by 40–70%, and earlier treatment is more likely to prevent the complications of premature atherosclerosis. Pediatric reports are scarce and often refer to patients above the age of 10 years, despite the fact that treatment is recommended below this age. Different techniques for LDL apheresis are available, including chemoadsorption, precipitation, cascade filtration, and direct adsorption. However, most commercial systems are not suitable for children below 10 years due to large extracorporeal volume requirements. The following section will review the experience and technique with LDL apheresis in the pediatric population.
Homozygous familial hypercholesterolemia (FH) affects one subject per million inhabitants. It is characterized by grossly elevated LDL cholesterol plasma levels (>15.5 mmol/L). Clinical signs and symptoms include tuberous xanthomas over the extensor surfaces, thickened Achilles tendons, and stenosis of the carotid artery and aortic valve developing during the first 10 years of life. Untreated, this results in myocardial infarction and/or sudden death due to cardiovascular complications during the first or second decade of life [77, 78]. Brown and Goldstein discovered that FH is caused by mutations within the LDL receptor gene [79]. The LDL receptor is essential for uptake of LDL into the cells by receptor-mediated endocytosis. This occurs mainly in hepatocytes and accounts for the clearance of about 70% of all plasma circulating LDL [79]. The gene for the LDLR is located on chromosome 19, and more than 800 different mutations have been described ([80], http://www.ucl.ac.uk/fh). Heterozygous patients rarely develop clinical signs during childhood other than elevated LDL cholesterol plasma levels, but left untreated, their relative risk of death is increased three- to fourfold [81].
Treatment options for FH are limited. Conventional cholesterol-lowering therapy includes dietary interventions, intestinal bile-acid or cholesterol binding agents (cholestyramine), specific cholesterol absorption inhibitors (ezetimib), and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (HMGCoA-reductase-inhibitors, statins). However, in FH patients cholesterol-lowering drug therapy often is not sufficient to lower the plasma LDL cholesterol level to recommended levels below 100–140 mg/dL. In FH patients resistant to dietetic and drug therapy, extracorporeal cholesterol elimination by LDL apheresis is indicated. LDL apheresis lowers cholesterol by approximately 50% long term [82] and has been proven effective to prevent future cardiovascular events [83, 84].
Indication for LDL Apheresis
The diagnosis of FH should be validated. The American Heart Association has published a set of diagnostic criteria and LDL apheresis indications focused on cardiovascular risk reduction in high-risk pediatric patients [85].
In patients with HF LDL apheresis is indicated if the following take place:
-
A LDLR deficiency is demonstrated functionally or genetically. These patients have LDL cholesterol plasma levels above 15 mmol/L and face a high risk of cardiovascular morbidity and mortality already in childhood. Therefore LDL apheresis is indicated as prophylaxis for these devastating complications.
-
Plasma cholesterol cannot be lowered below 160 mg/dL despite dietetic and medical therapy.
-
In heterozygous FH LDL apheresis is only indicated as a secondary treatment option; most patients will respond to drug therapy.
LDL Apheresis Technique
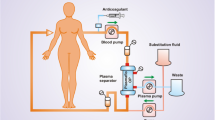
For extracorporeal removal of cholesterol, direct absorption from whole blood can be obtained (Fig. 41.2). In most systems, plasma is separated from the cellular blood components by membrane or centrifuge separation. The separated plasma then passes through the absorber unit, in which LDL is removed by different methods, before the cleared plasma is given back to the patient without major volume change (Fig. 41.3). The disadvantage of membrane-plasma separation is the limited capacity of the plasma filter membrane. However, with modern plasma filters, this is no longer a significant clinical problem in LDL apheresis. With centrifuge plasma separation, the amount of plasma generated is not limited.
For membrane-plasma separation, a plasma filter with a membrane surface of 0.2 m2 is recommended for children of 10–20 kg body weight, and 0.5 m2 for children >20 kg, assuming the treatment is designed to process approximately 1.5 times the plasma volume (see Table 41.3 and Ref. [73] for plasma volume determination).
Venous Access
Venous access can be obtained by peripheral veins, central-venous catheter (dialysis catheter) or arteriovenous fistula. The blood flow needed is considerably lower than in hemodialysis, but should be more than 10 mL/min even in small children. In older children, blood flow of 60–80 mL/min is sufficient. Peripheral veins are the preferred access in adult patients. Several studies have shown that peripheral access is sufficient in more than 50% of adolescent patients [82, 86]. However, because both antecubital veins often must be used, patients become totally dependent on the assistance of hospital or apheresis personal or accompanying persons for sniffing, eating, and almost all activities during the procedure. This is reported to be discomforting, especially with longer duration of treatment [82]. The antecubital veins should not be used for blood sampling or infusions unrelated to LDL apheresis. For these reasons, an arteriovenous fistula or central line might be advantageous with respect to both patient comfort and treatment adherence. In our experience, patients have discontinued treatment due to discomfort associated with bilateral antecubital venipuncture and taping for the LDL apheresis procedure. Due to the long treatment period expected with FH, an arteriovenous fistula is the preferred access.
Anticoagulation
For anticoagulation the same medications are principally applicable as for hemodialysis or plasma separation. However, in membrane-plasma separation a higher level of anticoagulation is needed as in hemodialysis. The choice of anticoagulation is influenced by the method of LDL apheresis: for dextran-sulfate absorption (Liposorber®, Fa Kaneka) no citrate anticoagulation is needed, whereas for the cascade filtration (CascadeFlow®, Fa. ASHAI Kassei Kurray) and direct adsorption (DALI®, Fa Fresenius, Germany) citrate anticoagulation is recommended. The anticoagulation is aimed at sufficient anticoagulation in the external blood circuit with the best biocompatibility, and without activation of the coagulation system, complement system, or cellular blood components. Use of citrate-based anticoagulation has been shown to have the advantage of inhibition of calcium-dependent complement activation [87], albeit this was not confirmed in other studies [88]. For control of anticoagulation, measurement of activated clotting time or ionized calcium in case of citrate anticoagulation should be immediately available.
LDL Apheresis Systems
Different techniques for removal of LDL are available (Table 41.6). When choosing an LDL apheresis system, the size of the child, i.e., his/her circulating blood volume should be calculated, as many commercially available systems require large priming volumes. The extracorporeal blood volume should not exceed 5–7% of circulating blood volume. In children below the age of 6 years priming of the extracorporeal circuit is often performed using albumin [89]. However, this is not ideal due to increased costs, risk of adverse events, and increased risk of transmitted infections. Most published pediatric series report on use of the Liposorber®-system, but cascade filtration and direct absorption have also been described, whereas precipitation techniques are infrequently used in pediatric patients.
Chemoadsorption (Liposorber®, Fa. Kaneka)
The Liposorber®-System adsorbs LDL to dextran-sulfate-cellulose from plasma separated from the cellular blood components. The mechanism of adsorption is the result of electrostatic forces between negatively charged sulfate-groups on dextran-sulfate and positively charged Apo B of LDL and Lp(a). Immunoglobulins, HDL, and albumin are adsorbed by this system at a very low level (http://www.liposorber.com/physician/prescribe/prescribe.htm) [83, 86, 89–91]. The commercially available system uses two dextran-sulfate columns, which are alternately being loaded and regenerating. The ECV of the system is 400 mL; therefore this system cannot be used safely in small children. Because bradykinin can be generated in the plasma filter as well as in the dextran-sulfate columns, bradykinin-related symptoms can be observed when transition time is too short for bradykinin inactivation. Furthermore, ACE inhibitors decrease bradykinin inactivation and should therefore not be used with dextran-sulfate adsorption or stopped at least 24 h before treatment.
Dextran-sulfate columns can be run with a pediatric blood pump monitor and a volume-regulated plasma dialysis device (BM-25, Fa. Baxter) using a custom-made tubing system (Päd. Lipidapherese-Set I09.4, Fa Meise, Germany). With such a system, the volume of the extracorporeal circuit can be reduced to 60 mL in the blood compartment and 100 mL in the plasma compartment, making this system suitable for children of 10–20 kg without the need of priming with albumin [92] (Fig. 41.4).
Double/Cascade Filtration
The double/cascade filtration (CascadeFlow EX 50 W Lipidfilter (Fa ASAHI Kasei Kuraray, Tokyo, Fa DIAMED Cologne)) depletes plasma components nonspecifically according to their size. After separation from the cellular blood components, plasma is run on a plasma fractionator (ethylene vinyl alcohol copolymer) that retains LDL by 98% and fibrinogen by 69% (data given by manufacturer [93]), which is discarded. The reduction in fibrinogen might exclude patients with low fibrinogen plasma levels. For anticoagulation, citrate is used. The system has a priming volume of 165 mL blood and 180 or 240 mL plasma according to the size of the plasma fractionator used. Therefore it is suitable for children above about 20 kg body weight. However, due to its lack of specificity, this technique is reported to be used decreasingly [86].
Precipitation Techniques
In heparin-induced extracorporeal LDL precipitation (H.E.L.P.® Fa Braun, Melsungen, Germany, http://www.help-therapie.de) LDL, Lp(a), and fibrinogen are precipitated from plasma by heparin in acidic buffer with a pH of 5.12 (sodium acetate). The cleared plasma then passes a heparin adsorber (DEA mod. polyamide, fill volume 150 mL, adsorption capacity ≥ 300.000 IE heparin). This is followed by a single pass dialysis with a cellulose membrane (ultrafilter SMC 1.8, 1.84 m² surface, fill volume 117 mL, max. TMP 600 mmHg) for removing the sodium acetate and normalizing the pH. The system is complex but the procedure is well tolerated (<3% adverse events) [94]. However, published pediatric experience is not available. In contrast to the dextran-sulfate adsorption, H.E.L.P. additionally reduces fibrinogen plasma levels. This system may not be usable in patients with low fibrinogen levels. Co-medication with ACE inhibitors is possible. Due to its large extracorporeal volume, the system is not recommended for patients <30 kg body weight. The H.E.L.P. system also removes complement. Therefore the indication in patients with low C3 or C1 esterase inhibitor deficiency should be thoroughly evaluated. For anticoagulation, no citrate must be used. An initial heparin bolus of 2,000 IE/m2 body surface often is sufficient.
Whole Blood Apheresis
Direct adsorption of lipoproteins on whole blood is obtained with the DALI®-system (Fa Fresenius Medical Care, Germany [95]). This technique lowers both LDL and Lp(a). The system does not need plasma separation. The blood is run over the adsorber unit, which contains negatively charged polyacrylate ligands immobilized on polyacrylamide. The DALI adsorber is available in five different sizes (300, 500, 750, 1,000, and 1,250 mL). Anticoagulation is performed by citrate. The DALI system is reported to be increasingly used in France [86].
Infrastructure for Pediatric LDL Apheresis
The application of LDL apheresis in children and adolescents needs specific structural conditions and staff qualification. The physician should be trained in pediatric extracorporeal therapies as well as in intensive care medicine, because acute life-threatening complications can occur. The unit should also have emergency equipment. The nursing staff must be experienced in maintaining extracorporeal circuits in children. It is recommended that at least one physician and one to two nurses are present during the entire procedure. The availability of social workers, teachers, or play therapists may increase pediatric patient tolerance of apheresis, especially if the patient is immobilized due to blood lines in both antecubital veins. For control of anticoagulation, measurement of activated clotting time or ionized calcium in case of citrate anticoagulation should be immediately available. Pediatric dialysis units offer qualified staff and appropriate equipment, but due to high costs, in most countries health insurance systems must authorize LDL apheresis in the individual patient prior to the start of treatment.
LDL Apheresis Treatment Results
Plasma Cholesterol
Despite different characteristics of the currently available techniques, efficacy in lowering plasma cholesterol levels is similar. One single treatment session with the processing of 1.5× plasma volume lowers plasma LDL cholesterol by 50–80% [82, 86, 90, 96, 97]. In the long term, patients have 18–52% lower average plasma LDL cholesterol levels with a treatment frequency of once every 1–2 weeks [82]. The average LDL cholesterol level is calculated as the mean of the LDL cholesterol plasma concentrations before and after the LDL apheresis session. However, recommended targets are often not met.
Atherosclerotic Lesions
In general, a late start of LDL apheresis in FH patients is associated with more severe involvement of the aortic valve and less response to treatment. Atherosclerotic involvement of the aortic valve often is detectable by 6 years of age and shows no or little regression on therapy. Therefore, initiation of LDL apheresis is recommended below the age of 8 years [85] or even 6 years [84]. If the patient has established atherosclerotic lesions, a mean plasma cholesterol <140 mg/dL was found to be associated with regression of the lesions in adult patients [81]. LDL cholesterol was the main determinant for the effect of LDL apheresis. In pediatric FH patients on LDL apheresis followed with angiography every 2 years, Stefanutti reported no development of atherogenic lesions in those free of it at start of treatment. In about 50% of patients lesions were already present before LDL apheresis started. These lesions regressed or stabilized and no increase was observed with biweekly or weekly apheresis sessions aiming at a posttreatment LDL cholesterol of 70–100 mg/dL [84]. After a mean observation period of 12.6 ± 6 years, all FH patients of the pediatric series of Palcoux [86] were alive with normal physical and pubertal development; these patients began LDL apheresis treatment at a mean age of 8.5 years. Cardiac disorders were observed in five children during the treatment period, three of them experiencing angina pectoris. In the series of Hudgkins with biweekly treatment, which resulted in a 48% lowering of baseline plasma cholesterol levels, 60% of patients showed atherosclerotic disease of the coronary artery or aorta or aortic valve on angiography. The lesions progressed in one third of the patients [90].
Xanthomas/Xanthelasmas
Xanthomas or xanthelasmas may develop rapidly and are of significance for the body image of the adolescent patient. On adequate apheresis therapy, the xanthomas and xanthelasmas are reported to resolve completely or decrease in size within 2 years [85]. Even after 12 years, these lesions may resolve [86].
Adverse Events
LDL apheresis in general is well tolerated. In many series including our own, adverse events occurred more often during the development of an apheresis capability in the unit with limited prior experience. In our own series with two FH twins starting weekly LDL apheresis at the age of 3.5 years, a high level of adverse events was recorded (14%) during the first 75 treatment episodes, mainly due to venous access problems and mild hypotension (6.7%): much lower rates were seen with further follow-up. Adverse events can be minimized by careful selection of apheresis modality according to extracorporeal volume and adherence to exclusion criteria. The most commonly reported adverse events are related to hypotension and venous access problems (Table 41.7). Furthermore, anaphylactic reactions were observed in 9 of 27 patients in the series of Palcoux [86]. Most of these patients used whole blood adsorption techniques, but anaphylactic reactions are also found in dextran-sulfate cellulose systems due to bradykinin liberation. In some patients development of iron deficiency has been described ([98], Klaus unpublished), necessitating iron supplements.
LDL Apheresis Recommendations
LDL apheresis is indicated in FH patients not responsive to medical treatment. In summary, the following recommendations are suggested (for details, see text):
-
Start LDL apheresis at age below 6–8 years.
-
Vascular access by peripheral antecubital veins in adolescents, if tolerated; Cimino fistula in younger subjects.
-
Adequately equipped unit and trained staff including psychosocial support is necessary.
-
Selection of modality by adjusted extracorporeal volume and local experience.
-
Initial treatment frequency: every 2 weeks.
-
Reduction in LDL cholesterol should be aimed at the lowest level possible, at least 60% reduction per session.
-
In patients with established atherosclerotic lesions, mean LDL cholesterol should be <100 mg/dL.
-
If targets are not met, frequency should be increased to weekly sessions.
References
Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36(10):2878–87.
Chopek M, McCullough J. Protein and biochemical changes during plasma exchange. Washington: AABB Press; 1980.
Strauss R, McLeod B. Adverse reactions to therapeutic apheresis. Bethesda: AABB Press; 1996.
Dzik WH, Kirkley SA. Citrate toxicity during massive blood transfusion. Transfus Med Rev. 1988;2(2): 76–94.
Olson PR, Cox C, McCullough J. Laboratory and clinical effects of the infusion of ACD solution during plateletpheresis. Vox Sang. 1977;33(2):79–87.
Szymanski IO. Ionized calcium during plateletpheresis. Transfusion. 1978;18(6):701–8.
Bunchman TE, Maxvold NJ, Barnett J, Hutchings A, Benfield MR. Pediatric hemofiltration: Normocarb dialysate solution with citrate anticoagulation. Pediatr Nephrol. 2002;17(3):150–4.
Mair DC, Hirschler N, Eastlund T. Blood donor and component management strategies to prevent transfusion-related acute lung injury (TRALI). Crit Care Med. 2006;34(Suppl 5):S137–43.
Snyder Jr HW, Cochran SK, Balint Jr JP, et al. Experience with protein A-immunoadsorption in treatment-resistant adult immune thrombocytopenic purpura. Blood. 1992;79(9):2237–45.
George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88(1):3–40.
Furst D, Felson D, Thoren G, Gendreau R. Immunoadsorption for the treatment of rheumatoid arthritis: final results of a randomized trial. Prosorba Trial Investigators. Ther Apher. 2000;4(5):363–73.
Hughes LB, Moreland LW. New therapeutic approaches to the management of rheumatoid arthritis. BioDrugs. 2001;15(6):379–93.
Freiburghaus C, Berntorp E, Ekman M, Gunnarsson M, Kjellberg BM, Nilsson IM. Immunoadsorption for removal of inhibitors: update on treatments in Malmo-Lund between 1980 and 1995. Haemophilia. 1998; 4(1):16–20.
Nilsson IM, Freiburghaus C. Apheresis. Adv Exp Med Biol. 1995;386:175–84.
Uehlinger J, Button GR, McCarthy J, Forster A, Watt R, Aledort LM. Immunoadsorption for coagulation factor inhibitors. Transfusion. 1991;31(3):265–9.
Bohmig GA, Regele H, Saemann MD, et al. Role of humoral immune reactions as target for antirejection therapy in recipients of a spousal-donor kidney graft. Am J Kidney Dis. 2000;35(4):667–73.
Braun N, Kadar JG, Risler T. Therapeutic immunoadsorption – its role in clinical practice. Transfus Sci. 1998;19(Suppl):65–9.
Bueno Jr D, Sevigny J, Kaplan AA. Extracorporeal treatment of thrombotic microangiopathy: a ten year experience. Ther Apher. 1999;3(4):294–7.
Bygren P, Freiburghaus C, Lindholm T, Simonsen O, Thysell H, Wieslander J. Goodpasture’s syndrome treated with staphylococcal protein A immunoadsorption. Lancet. 1985;2(8467):1295–6.
Dantal J, Bigot E, Bogers W, et al. Effect of plasma protein adsorption on protein excretion in kidney-transplant recipients with recurrent nephrotic syndrome. N Engl J Med. 1994;330(1):7–14.
Esnault V, Bignon JD, Testa A, Preud’homme JL, Vergracht A, Soulillou JP. Effect of protein A immunoadsorption on panel lymphocyte reactivity in hyperimmunized patients awaiting a kidney graft. Transplantation. 1990;50(3):449–53.
Esnault VL, Besnier D, Testa A, et al. Effect of protein A immunoadsorption in nephrotic syndrome of various etiologies. J Am Soc Nephrol. 1999;10(9): 2014–17.
Haas M, Bohmig GA, Leko-Mohr Z, et al. Peri-operative immunoadsorption in sensitized renal transplant recipients. Nephrol Dial Transplant. 2002;17(8): 1503–8.
Hickstein H, Korten G, Bast R, Barz D, Nizze H, Schmidt R. Immunoadsorption of sensitized kidney transplant candidates immediately prior to surgery. Clin Transplant. 2002;16(2):97–101.
Hiesse C, Kriaa F, Rousseau P, et al. Immunoadsorption of anti-HLA antibodies for highly sensitized patients awaiting renal transplantation. Nephrol Dial Transplant. 1992;7(9):944–51.
Juckett M, Perry EH, Daniels BS, Weisdorf DJ. Hemolytic uremic syndrome following bone marrow transplantation. Bone Marrow Transplant. 1991;7(5): 405–9.
Mastrangelo F, Pretagostini R, Berloco P, et al. Immunoadsorption with protein A in humoral acute rejection of kidney transplants: multicenter experience. Transplant Proc. 1995;27(1):892–5.
Matic G, Bosch T, Ramlow W. Background and indications for protein A-based extracorporeal immunoadsorption. Ther Apher. 2001;5(5):394–403.
Mittelman A, Bertram J, Henry DH, et al. Treatment of patients with HIV thrombocytopenia and hemolytic uremic syndrome with protein A (Prosorba column) immunoadsorption. Semin Hematol. 1989;26(2 Suppl 1): 15–18.
Pretagostini R, Berloco P, Poli L, et al. Immunoadsorption with protein A in humoral rejection of kidney transplants. ASAIO J. 1996;42(5): M645–8.
Snyder Jr HW, Mittelman A, Oral A, et al. Treatment of cancer chemotherapy-associated thrombotic thrombocytopenic purpura/hemolytic uremic syndrome by protein A immunoadsorption of plasma. Cancer. 1993;71(5):1882–92.
Watson PR, Guthrie Jr TH, Caruana RJ. Cisplatin-associated hemolytic-uremic syndrome. Successful treatment with a staphylococcal protein A column. Cancer. 1989;64(7):1400–3.
Parhofer KG, Geiss HC, Schwandt P. Efficacy of different low-density lipoprotein apheresis methods. Ther Apher. 2000;4(5):382–5.
Schmaldienst S, Banyai S, Stulnig TM, et al. Prospective randomised cross-over comparison of three LDL-apheresis systems in statin pretreated patients with familial hypercholesterolaemia. Atherosclerosis. 2000;151(2):493–9.
Vella A, Pineda AA, O’Brien T. Low-density lipoprotein apheresis for the treatment of refractory hyperlipidemia. Mayo Clin Proc. 2001;76(10):1039–46.
Adams DM, Schultz WH, Ware RE, Kinney TR. Erythrocytapheresis can reduce Iron overload and prevent the need for chelation therapy in chronically transfused pediatric patients. J Pediatr Hematol Oncol. 1996;18(1):46–50.
Kim HC, Dugan NP, Silber JH, et al. Erythrocytapheresis therapy to reduce iron overload in chronically transfused patients with sickle cell disease. Blood. 1994;83(4):1136–42.
Stemmler J, Wittmann GW, Hacker U, Heinemann V. Leukapheresis in chronic myelomonocytic leukemia with leukostasis syndrome: elevated serum lactate levels as an early sign of microcirculation failure. Leuk Lymphoma. 2002;43(7):1427–30.
Rowe JM, Lichtman MA. Hyperleukocytosis and leukostasis: common features of childhood chronic myelogenous leukemia. Blood. 1984;63(5):1230–4.
Maurer HS, Steinherz PG, Gaynon PS, et al. The effect of initial management of hyperleukocytosis on early complications and outcome of children with acute lymphoblastic leukemia. J Clin Oncol. 1988; 6(9):1425–32.
Steeper TA, Smith JA, McCullough J. Therapeutic cytapheresis using the Fenwal CS-3000 blood cell separator. Vox Sang. 1985;48(4):193–200.
Benito AI, Gonzalez-Vicent M, Garcia F, et al. Allogeneic peripheral blood stem cell transplantation (PBSCT) from HLA-identical sibling donors in children with hematological diseases: a single center pilot study. Bone Marrow Transplant. 2001;28(6): 537–43.
Diaz MA, Garcia-Sanchez F, Lillo R, Vicent MG, Vicario JL, Madero L. Large-volume leukapheresis in pediatric patients: pre-apheresis peripheral blood CD34+ cell count predicts progenitor cell yield. Haematologica. 1999;84(1):32–5.
Goldman JM, Horowitz MM. The international bone marrow transplant registry. Int J Hematol. 2002; 76(Suppl 1):393–7.
Gorlin JB, Humphreys D, Kent P, et al. Pediatric large volume peripheral blood progenitor cell collections from patients under 25 kg: a primer. J Clin Apher. 1996;11(4):195–203.
Marson P, Petris MG, Messina C, et al. Peripheral blood stem cell collection in pediatric oncohematology. Experience with patients weighing less than 15 kg. Minerva Pediatr. 2000;52(3):129–35.
Sevilla J, Gonzalez-Vicent M, Madero L, Diaz MA. Peripheral blood progenitor cell collection in low-weight children. J Hematother Stem Cell Res. 2002;11(4):633–42.
Torrabadella M, Olive T, Ortega JJ, Massuet L. Enhanced HPC recruitment in children using LVL and a new automated apheresis system. Transfusion. 2000;40(4):404–10.
Collins Jr RH, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15(2):433–44.
Drobyski WR, Hessner MJ, Klein JP, et al. T-cell depletion plus salvage immunotherapy with donor leukocyte infusions as a strategy to treat chronic-phase chronic myelogenous leukemia patients undergoing HLA-identical sibling marrow transplantation. Blood. 1999;94(2):434–41.
Drobyski WR, Keever CA, Roth MS, et al. Salvage immunotherapy using donor leukocyte infusions as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation: efficacy and toxicity of a defined T-cell dose. Blood. 1993;82(8):2310–18.
Gasparetto C, Gasparetto M, Morse M, et al. Mobilization of dendritic cells from patients with breast cancer into peripheral blood stem cell leukapheresis samples using Flt-3-Ligand and G-CSF or GM-CSF. Cytokine. 2002;18(1):8–19.
Mandanas RA, Saez RA, Selby GB, Confer DL. G-CSF-mobilized donor leukocyte infusions as immunotherapy in acute leukemia relapsing after allogeneic marrow transplantation. J Hematother. 1998;7(5):449–56.
Zic JA, Miller JL, Stricklin GP, King Jr LE. The North American experience with photopheresis. Ther Apher. 1999;3(1):50–62.
Rook AH, Suchin KR, Kao DM, et al. Photopheresis: clinical applications and mechanism of action. J Investig Dermatol Symp Proc. 1999;4(1):85–90.
Knobler R, Jantschitsch C. Extracorporeal photochemoimmunotherapy in cutaneous T-cell lymphoma. Transfus Apher Sci. 2003;28(1):81–9.
Knobler R, Girardi M. Extracorporeal photochemoimmunotherapy in cutaneous T cell lymphomas. Ann N Y Acad Sci. 2001;941:123–38.
Knobler R. Extracorporeal photochemotherapy – Present and future. Vox Sang. 2000;78(Suppl 2): 197–201.
Greinix HT, Volc-Platzer B, Rabitsch W, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92(9):3098–104.
Shinoda T. Photopheresis and leukocytapheresis: cytapheresis treatment against immune-mediated diseases. Ther Apher. 2002;6(4):245–6.
Schneider M. Plasma- and lymphapheresis in autoimmune diseases. Z Rheumatol. 1996;55(2):90–104.
Costanzo-Nordin MR, Hubbell EA, O’Sullivan EJ, et al. Photopheresis versus corticosteroids in the therapy of heart transplant rejection. Preliminary clinical report. Circulation. 1992;86(Suppl 5):II242–50.
Meiser BM, Kur F, Reichenspurner H, et al. Reduction of the incidence of rejection by adjunct immunosuppression with photochemotherapy after heart transplantation. Transplantation. 1994;57(4):563–8.
Rossetti F, Dall’Amico R, Crovetti G, et al. Extracorporeal photochemotherapy for the treatment of graft-versus-host disease. Bone Marrow Transplant. 1996;18(Suppl 2):175–81.
Dall’Amico R, Rossetti F, Zulian F, et al. Photopheresis in paediatric patients with drug-resistant chronic graft-versus-host disease. Br J Haematol. 1997;97(4): 848–54.
Adami R. Therapeutic thrombocytapheresis: a review of 132 patients. Int J Artif Organs. 1993;16(Suppl 5):183–4.
Baron BW, Mick R, Baron JM. Combined plateletpheresis and cytotoxic chemotherapy for symptomatic thrombocytosis in myeloproliferative disorders. Cancer. 1993;72(4):1209–18.
Liumbruno G, Centoni PE, Ceretelli S, Sodini ML. Rapid reduction of platelet numbers in thrombocytosis. Ther Apher. 2000;4(5):374–6.
Renner D, Queisser U, Martinez C, Queisser W. Treatment of excessive thrombocythemia in chronic myeloid leukemia by thrombocytopheresis and intravenous Thio-TEPA. Onkologie. 1987;10(5):324–6.
Ullrich H, Kadar J, Waxenberger Y, et al. Therapeutic thrombocytapheresis in patients with myeloproliferative diseases with the cell separators Fresenius AS 104 and Cobe Spectra: biocompatibility and safety. Beitr Infusionsther. 1992;30:311–14.
Gerhardt RE, Ntoso KA, Koethe JD, Lodge S, Wolf CJ. Acute plasma separation with hemodialysis equipment. J Am Soc Nephrol. 1992;2(9):1455–8.
Price CA. Therapeutic plasma exchange in a dialysis unit. ANNA J. 1987;14(2):103–8.
Nadler S, Hidalgo J, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224.
Kim HC. Therapeutic pediatric apheresis. J Clin Apher. 2000;15(1–2):129–57.
Szczepiorkowski ZM, Winters JL, Bandarenko N, et al. Guidelines on the use of therapeutic apheresis in clinical practice – Evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher. 2010;25(3): 83–177.
Owen HG, Brecher ME. Atypical reactions associated with use of angiotensin-converting enzyme inhibitors and apheresis. Transfusion. 1994;34(10):891–4.
Thompson GR. LDL apheresis. Atherosclerosis. 2003;167(1):1–13. Review.
Marks D, Thorogood M, Neil HA, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168(1):1–14. Review.
Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986; 232(4746):34–47. Review.
Leigh SE, Foster AH, Whittall RA, Hubbart CS, Humphries SE. Update and analysis of the University College London low density lipoprotein receptor familial hypercholesterolemia database. Ann Hum Genet. 2008;72(Pt 4):485–98.
Masaki N, Tatami R, Kumamoto T, Izawa A, Shimada Y, Takamatsu T, Katsushika S, Ishise S, Maruyama Y, Yoshimoto N. Ten-year follow-up of familial hypercholesterolemia patients after intensive cholesterol-lowering therapy. Int Heart J. 2005;46(5): 833–43.
Coker M, Ucar SK, Simsek DG, Darcan S, Bak M, Can S. Low density lipoprotein apheresis in pediatric patients with homozygous familial hypercholesterolemia. Ther Apher Dial. 2009;13(2):121–8.
Mabuchi H, Koizumi J, Shimizu M, Kajinami K, Miyamoto S, Ueda K, Takegoshi T. Long-term efficacy of low-density lipoprotein apheresis on coronary heart disease in familial hypercholesterolemia. Hokuriku-FH-LDL-Apheresis Study Group. Am J Cardiol. 1998;82(12):1489–95.
Stefanutti C, Vivenzio A, Di Giacomo S, Mazzarella B, Bosco G, Berni A. Aorta and coronary angiographic follow-up of children with severe hypercholesterolemia treated with low-density lipoprotein apheresis. Transfusion. 2009;49(7):1461–70. Epub 2009 Mar 23.
Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J; American Heart Association Expert Panel on Population and Prevention Science; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity and Metabolism; American Heart Association Council on High Blood Pressure Research; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on the Kidney in Heart Disease; Interdisciplinary Working Group on Quality of Care and Outcomes Research. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114(24):2710–38. Epub 2006 Nov 27. Review.
Palcoux JB, Atassi-Dumont M, Lefevre P, Hequet O, Schlienger JL, Brignon P, Roussel B. Low-density lipoprotein apheresis in children with familial hypercholesterolemia: follow-up to 21 years. Ther Apher Dial. 2008;12(3):195–201.
Böhler J, Schollmeyer P, Dressel B, Dobos G, Hörl WH. Reduction of granulocyte activation during hemodialysis with regional citrate anticoagulation: dissociation of complement activation and neutropenia from neutrophil degranulation. J Am Soc Nephrol. 1996;7(2):234–41.
Gabutti L, Ferrari N, Mombelli G, Keller F, Marone C. The favorable effect of regional citrate anticoagulation on interleukin-1beta release is dissociated from both coagulation and complement activation. J Nephrol. 2004;17(6):819–25.
Stefanutti C, Di Giacomo S, Vivenzio A, Colloridi V, Bosco G, Berni A, Rabbone I, Cerutti F, Bertolini S. Low-density lipoprotein apheresis in a patient aged 3.5 years. Acta Paediatr. 2001;90(6):694–701.
Hudgins LC, Kleinman B, Scheuer A, White S, Gordon BR. Long-term safety and efficacy of low-density lipoprotein apheresis in childhood for homozygous familial hypercholesterolemia. Am J Cardiol. 2008;102(9):1199–204.
De Silvestro G, Tison T, Vicarioto M, Bagatella P, Stefanutti C, Marson P. The Italian Registry of Pediatric Therapeutic Apheresis: a report on activity during 2005. J Clin Apher. 2009;24(1):1–5.
Soufi M, Kurt B, Schweer H, Sattler AM, Klaus G, Zschocke J, Schaefer JR. Genetics and kinetics of familial hypercholesterolemia, with the special focus on FH-Marburg).W556R. Atheroscler Suppl. 2009; 10(5):5–11.
Geiss HC, Parhofer KG, Donner MG, Schwandt P. Low density lipoprotein apheresis by membrane differential filtration (cascade filtration). Ther Apher. 2000;3(3):199–202.
Seidel D, Armstrong VW, Schuff-Werner P. The HELP-LDL-apheresis multicentre study, an angiographically assessed trial on the role of LDL-apheresis in the secondary prevention of coronary heart disease. I. Evaluation of safety and cholesterol-lowering effects during the first 12 months. HELP Study Group. Eur J Clin Invest. 1991;21(4):375–83.
Bosch T, Gahr S, Belschner U, Schaefer C, Lennertz A, Rammo J, for the DALI Study Group. Direct adsorption of low-density lipoprotein by DALI-LDL-apheresis: results of a prospective long-term multicenter follow-up covering 12,291 sessions. Ther Apher Dial. 2006;10(3):210–18.
Uauy R, Zwiener RJ, Phillips MJ, Petruska ML, Bilheimer DW. Treatment of children with homozygous familial hypercholesterolemia: safety and efficacy of low-density lipoprotein apheresis. J Pediatr. 1992;120(6):892–8.
Zwiener RJ, Uauy R, Petruska ML, Huet BA. Low-density lipoprotein apheresis as long-term treatment for children with homozygous familial hypercholesterolemia. J Pediatr. 1995;126(5 Pt 1):728–35.
Eminoglu TF, Yenicesu I, Tumer L, Okur I, Dilsiz G, Hasanoglu A. Lipid apheresis applications in childhood: experience in the University Hospital of Gazi. Transfus Apher Sci. 2008;39(3):235–40.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Goldstein, S.L., Klaus, G., Friedman, D.F., Friedman, D.F., Kim, H.C. (2012). Pediatric Therapeutic Apheresis. In: Warady, B., Schaefer, F., Alexander, S. (eds) Pediatric Dialysis. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-0721-8_41
Download citation
DOI: https://doi.org/10.1007/978-1-4614-0721-8_41
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-0720-1
Online ISBN: 978-1-4614-0721-8
eBook Packages: MedicineMedicine (R0)