Abstract
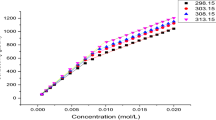
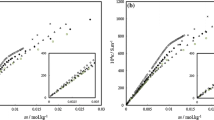
In this paper we have studied the thermodynamic behavior in aqueous solution of three anionic surfactants: Sodium dodecylsulphate (SDS), sodium tetra- decylsulfate (STS and sodium deoxycholate (SDC). From osmotic pressure data and theoretical association models WE have calculated several excess functions (SE , GE and HE ) in the temperature range 298–363 K.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
B. D. Sykes and E. E. Hull, Ann. N.Y. Acad. Sci., 226, 6 (1973).
I. Katime and F. Aguilar, An. Quim. (Madrid), 75, 61 (1979).
I. Katime and F. Aguilar, Thermochim. Acta, 49, 139 (1981).
D. Voisin, A. Huot and B. Gastambide, Ann. Chim.,3, 497 (1968).
J. M Madariaga, M. Aurrekoetxea and I. Katime, Thermochim. Acta, 62, 163 (1983).
I. Katime and J. L. Allende, Thermochim. Acta, 74, 215 (1984).
H. G. Elias in “Light Scattering from Polymer Solutions”. M. B. Huglin, Editor, Academic Press, London, 1972.
O. O. P. T.s’O and S. I. Chan, J. Am. Chem. Soc, 86, 4176 (1964)
P. O. P. Ts’O, I. S. Melvin and O. C. Olson, J. Am. Chem. Soc., 85, 1289 (1963).
S. Hayashi, S. Ikeda, J. Phys. Chem., 84, 744 (1980)
Y. Mori, K. Motomura and R. Matuura, Bull. Chem. Soc. Japan, 44, 2078 (1971)
A. K. Ghosh and P. Mukerjee, J. Am. Chem. Soc., 92,6403 (1970).
H. S. Harned and L. F. Nims, J. Am. Chem. Soc., 54, 423 (1932).
H. S. Harned and M. A. Cook, J. Am. Chem. Soc., 61, 495 (1939).
G. J. Janz and A. R. Gordon, J. Am. Chem. Soc., 65, 218 (1943).
A. H. Truesdel, Science, 161, 884 (1968).
P. Mukerjee and A. K. Ghosh, J. Am. Chem. Soc., 92, 6403 (1970).
J. M. Madariaga, M. Aurrekoetxea and I. Katime, Thermochim. Acta, 63, 163 (1983).
R. F. Steiner, Biochemistry, 7, 2201 (1968).
R. F. Steiner, Biochemistry, 9, 1375 (1970).
J. Kreuzer, Z. Phys. Chem. (Leipzig), B53, 213 (1943).
I Katime “Quimica Fisica Macromolecular”. Editorial Del Castillo, Madrid, 1979.
E. R. Jones and C. R. Bury, Phil. Mag., j4, 841 (1927).
E..A. Gugenheim and R. H. Stokes “Equilibrium Properties” Pergamon Press, Oxford, 1969
H. G. Elias and H. Lys, Makromol. Chem., 92, 1 (1966)
H. G. Elias and H. Lys, Makromol. Chem., 96, 64 (1966).
J. M. Corkill, J. F. Goodman, T. Walker, and J. Wyer, Proc Roy. Soc., 312 ,243 (1969).
P. H. Elworthy and K. J. Mysels, J. Colloid Sci.,_21, 331 (1966).
R. H. Aranow, J. Phys. Chem., 67, 556 (1963).
E. A. Guggenheim, J. Phys. Chem., 33, 842 (1929)
E. A. Guggenheim, ibid, 34, 1541 (1930).
E. A. Guggenheim, “Mixtures” Oxford, 1952.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1986 Plenum Press, New York
About this chapter
Cite this chapter
Katime, I., Allende, J.L. (1986). Thermodynamic Behaviour of Sodium Deoxycholate, Sodium Dodecylsulphate and Sodium Tetradecylsulphate Micellar Solutions. In: Mittal, K.L., Bothorel, P. (eds) Surfactants in Solution. Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-1831-6_5
Download citation
DOI: https://doi.org/10.1007/978-1-4613-1831-6_5
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4612-9023-0
Online ISBN: 978-1-4613-1831-6
eBook Packages: Springer Book Archive