Abstract
Mitral paravalvular leakage is more frequent than aortic valve. Patient’s symptoms are dependent of the size of the defect and the severity of regurgitation. In severe type, they usually are symptomatic and finally leads to biventricular failure. The recommended treatment is surgical repair but in those with prohibited surgical risk, trans-catheter closure is an option. This intervention is very complex and should be evaluated completely by experience team for defining the anatomical feature of the defect. Various devices are available for closure (off-label), depending on the defect shape and size.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
History
The patient was a 70 years old man, the known case of Mechanical Mitral Valve Replacement (MVR) 16 years ago, who was referred with refractory dyspnea F/C III–IV since 3 months ago despite high dose diuretic therapy. In TEE severe paravalvular leakage in the medial side of the swing ring was detected.
Percutaneous paravalvular closure is a complex procedure that requires multiple imaging modalities to visualize the 3D relationship of intracardiac structures and the operator’ ability. Three approaches can be employed to cross the defect and the delivery device: transseptal, retrograde trans-aortic, and direct trans-apical. There is no dedicated device for this procedure. Paravalvular leakage in the Mitral prosthetic valve has occurred more than Aortic valve (17 vs. 10%) [1]. PVL can affect any valve in any position but is more common with mitral mechanical prostheses, supra-annular aortic prostheses and use of sutures without pledgets or continuous sutures in the mitral position [2]. Although surgical repair is the gold standard treatment for severe paravalvular regurgitation in those with intractable symptoms (right and left side congestive symptoms, hemolytic anemia), but in high-risk patients (STS risk score >8% or at a >15% risk of mortality at 30 days) with refractory symptoms and anatomically suitable for percutaneous closure at a center with expertise in this field, this is a better option (Class IIa) which has been appeared to have superior outcomes and less complications in compared with open surgery.
For the description of paravalvular leaks an adaptation of the accepted surgical nomenclature can be used. Each valve can be compared to a clock, so there are two clocks horizontally inverted one to another: one is in the aortic valve position and the other in the mitral valve position as seen from a cephalad position. The 12 o’clock in both clocks corresponds to the mitral-aortic fibrous continuity, and from there each location is named in a clockwise fashion (Fig. 77.1).
For the mitral valve, the 3 o’clock corresponds to the area of the interatrial septum, the 6 o’clock corresponds to the posterolateral free wall and the 9 o’clock corresponds to the LAA. Mitral PVL is most located between 10 and 2 o’clock (mitro-aortic fibrous continuity) and between 6 and 10 o’clock (posterior wall) [3] (Fig. 77.1 and 77.2).
Diagnostic Work-Up
TTE is the first modality for evaluation of LV, RV size and functions, mechanical valve evaluation, the severity of the valvular or paravalvular leak, any thrombus, or vegetation. The 3D TEE can accurately demonstrate the location, shape, size, number of the defects, dehiscence, any rocking motion and surrounding structures and differentiated PVL from valvular regurgitations. Another modalities like CT Angiography and CMR can also give more information for better evaluations of the defects.
Closure Strategy
It is clear that PVLs of the MVR are more challenging to treat. This procedure should only be undertaken by a team of structural and imaging cardiologists with experience in advanced structural interventions.
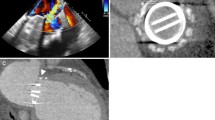
After insertion of right femoral artery and vein sheaths (6F) and IV Heparin injection to achieve ACT > 250 s, LV injections were done in LAO and RAO projections to localize the defect site and paravalvular leakage severity. Under general anesthesia and 3D TEE guidance, after complete assessment of the mechanical valve, the degree of trans-valvular and paravalvular regurgitation, defect numbers, shape, location, and rule outing any thrombus, vegetation or dehiscence, according to the defect location (clock number), transseptal or trans-apical approach is utilized. In this patient, the largest defect was crescent-shaped, 6 mm and at 2 o’clock corresponds to mitro-aortic fibrous continuity. Because of the absent hybrid room in our center, the transseptal approach was chosen.
Transseptal Approach: This approach is usually used for mitral PVL between 6 and 11 o’clock. For defects away from the septum, the location of the puncture is less critical. However, for medial defects near the IAS, a posterior and slightly superior puncture provides the appropriate working height within the LA. After septostomy by Brockenbrough needle and contrast injection to be sure of being in LA (Fig. 77.3a), Spiral wire was inserted in LA for more support and LA borders localization and septal dilatation was performed. Through a 125-cm 6F multipurpose diagnostic catheter telescoped through a steerable transseptal sheath (Agilis sheath), after several attempts, a 0.035 inch straight hydrophilic wire was manipulated by TEE and fluoroscopy guidance to cross the defect and advanced to the ascending aorta, then the catheter was passed over the wire (Fig. 77.3b). After that, the wire was exchanged with Amplatzer Superstiff guidewire and hydrophilic kink resistance delivery sheath (Epsylar 7F) was advanced gently over the wire in to the LV. (In case of significant difficulty in crossing the delivery sheath, more support can be obtained by building an arterio-venous rail). In this case, based on the defect characteristics, Muscular VSD 8 mm (Occlutech) was chosen. After loading the device and connected to the delivery cable, the device passed over the delivery sheath and under TEE and fluoroscopy guidance, after partially exposed the Distal Disk (DD), the whole system was pulled back, then the DD fully deployed in the LV, after reassurance about the correct position of the DD by TEE and LV injection, the waist, and proximal disk was deployed in LA (Fig. 77.4a). Before releasing the device, TEE should show mobile mitral leaflets, open pulmonary veins, the residual leakage, and the stability of the device. After final angiography and TEE evaluation, the stability of the device was confirmed and the device was released (Fig. 77.4b). The residual leakage was minimal.
(a, b) In this case, based on the defect characteristics, Muscular VSD 8 mm (Occlutech) was chosen. Under TEE and fluoroscopy guidance, after partially exposed the Distal Disk (DD), the whole system was pulled back, then the DD fully deployed in the LV, after reassurance about the correct position of the DD by TEE and LV injection, the waist and proximal disk was deployed in LA. Before releasing the device, TEE should show mobile mitral leaflets, open pulmonary veins, the residual leakage, and the stability of the device
Trans-Apical Approach: This approach can be a good alternative for mitral PLV between 11 and 6 o’clock (posterior or septal defects) to avoid complications associated with excessive forces on the septal wall from the delivery catheter. The advantage is less difficult wiring of the PVL.
Device Selection
Most of the devices are used off-label for this procedure. PVLs are variable in size, shape (crescentic, oval, serpiginous, or cylindrical), so, one device is not fit in all PVLs.
Usually, for a small cylindrical PVL, AVP II or PDA Occluders are used, for oval types AVP III and for small and angulated ones AVP IV Occluders can be considered [4] (Fig. 77.5).
Most of the devices are used off-label for this procedure. PVLs are variable in size, shape (crescentic, oval, serpiginous, or cylindrical), so, one device is not fit in all PVLs. Usually, for a small cylindrical PVL, AVP II or PDA Occluders are used, for oval types AVP III and for small and angulated ones AVP IV Occluders can be considered
Conclusion
TPVL closure in symptomatic patients is a less invasive option than surgery (most complications are: device embolization, cardiac perforation, vascular complications, prosthetic valve impingement, and stroke), with lower morbidity and mortality. To continue to improve procedural success and outcome, new advancements in device design are necessary.
References
Ionescu A, Fraser AG, Butchart EG. Prevalence and clinical significance of incidental paraprosthetic valvular regurgitation: a prospective study using transoesophageal echocardiography. Heart. 2003;89:1316–21.
Meloni L, Aru G, Abbruzzese PA, Cardu G, Ricchi A, Cattolica FS, Martelli V, Cherchi A. Regurgitant flow of mitral valve prostheses: an intraoperative transesophageal echocardiographic study. J Am Soc Echocardiogr. 1994;7:36–46.
Ruiz CE, Jelnin V, Kronzon I, et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J Am Coll Cardiol. 2011;58:2210–7.
Shapira Y, Hirscg R, Kornowski R, et al. Percutaneous closure of perivalvular leaks with Amplatzer occluders: feasibility, safety, and shortterm results. J Heart Valve Dis. 2007;16:305–13.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer-Verlag London Ltd., part of Springer Nature
About this chapter
Cite this chapter
Firouzi, A., Hosseini, Z. (2021). Transcatheter Closure of Mitral Paravalvular Leakage (PVL). In: Maleki, M., Alizadehasl, A. (eds) Case-Based Clinical Cardiology. Springer, London. https://doi.org/10.1007/978-1-4471-7496-7_77
Download citation
DOI: https://doi.org/10.1007/978-1-4471-7496-7_77
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-7495-0
Online ISBN: 978-1-4471-7496-7
eBook Packages: MedicineMedicine (R0)