Abstract
Aortic para-valvular leakage, is a regurgitation jet between swing ring and native valve tissue. The etiology of this side effect depends on the time of surgery (technical issues, endocarditis, severe calcification of the surrounding tissue). Depends on the size of the defects, patient’s symptoms range between asymptomatic to pulmonary edema. The standard treatment in severe leakage is surgical repair, but in those with high surgical mortality, trans-catheter device closure (if anatomically being suitable) is an alternative approach by expert interventionist. Dedicated device is not available, but according the shape and location of the defect in 3-D TEE, various device can be used.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
History
The patient was a 65 years old man, a case of coronary bypass surgery, Aortic Valve Replacement (AVR) and Mitral Valve Replacement (MVR) 15 years ago who was admitted with refractory CHF (Five times admissions within recent 6 months). In TEE, he had severe paravalvular leakage of aortic mechanical valve.
Paravalvular leak (PVL) is a regurgitant jet that originates between the outer margin of the prosthetic ring and the native tissue around the valve. It is a common complication of aortic valve replacement that has been found to occur in about 10% of cases. The majority of patients remain asymptomatic, however, 1–5% 0f them present with congestive symptoms [1], hemolytic complications, or both. The congestive heart failure symptoms are related to the defect size, numbers, and LV volume overload degree, whereas hemolytic complications have no correlation with defect size and even occur in small defects. Surgery is the gold standard treatment for operable patients with intractable symptoms and severe PVL (Class I), while in those with high-risk surgery, intractable symptoms and anatomically suitable for catheter-based closure in hands of expert operators is reasonable.
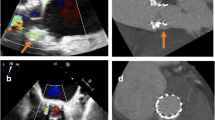
For the aortic valve, the LCC is located between 11 and 3 o’clock, the RCC between 3 and 7 o’clock and the NCC between 7 and 11 o’clock. The aortic PLVs are most often between 7 and 11 o’clock (46%) and also between 11 and 3 o’clock (36%) [2] (Figs. 76.1 and 76.2).
Diagnostic Work-Up
TTE is the first modality for evaluation of LV, RV size and functions, mechanical valve evaluation, the severity of trans-valvular or paravalvular leaks, any clots, pannus formation, or vegetation. The 3-D TEE can precisely demonstrate the location, shape, size, depth, and number of the defects, dehiscence, surrounding structures, and differentiated PVL from trans-valvular regurgitations. Another modalities like CT Angiography and CMR can also give more information for better evaluation of the defects.
Procedural Technique
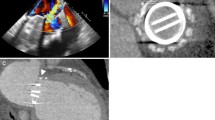
Under general anesthesia, after insertion of right femoral arterial sheath (6F), aortic root injections was done in LAO and RAO projections to localized the site, size, and severity of the paravalvular leakage (Fig. 76.3a). Then under TEE guidance and fluoroscopy, by retrograde approach, in LAO view, the defect (at 10 o’clock) crossed with straight hydrophilic wire and the JR catheter was passed over it (Fig. 76.3b). After that, the wire was exchanged with Extra-support wire (Lunderquist) over that, a hydrophilic delivery sheath (Epsylar 7F) was advanced to the LV. Based on the TEE defect size (6 mm) and shape, the Muscular VSD 8 mm (Occlutech) device was chosen. After loading the device and connecting to the delivery cable, the device was passed over the delivery sheath. First distal disk (DD) was partially extruded in the LV then by retracting the whole system toward the defect, the DD was totally deployed in the ventricular side and anchored within the defect (Fig. 76.4a), then after rechecking by TEE and angiography, the waist and the proximal disk (PD) was deployed (Fig. 76.4b). Prior to the release the device, the most attention (by TEE and fluoroscopy) must be paid to the prosthetic valve to be sure that there is no interference with valve function (free moving leaflets) and no coverage of coronary ostia (Fig. 76.5). Final injection showed mild residual leakage, no interference with leaflets, and stable position of the device. In case of device implantation in the area of NCC, anterior mitral valve leaflet should be check.
(a, b) Based on the TEE defect size (6 mm) and defect shape, Muscular VSD 8 mm (Occlutech) device was chosen. First distal disk (DD) was partially extruded in the LV then by retracting the whole system toward the defect, the DD was totally deployed in the ventricular side and anchored within the defect, then after rechecking by TEE and angiography, the waist and the proximal disk (PD) was deployed
Conclusion
Experience with percutaneous PVL closure is still limited. The procedural success rate varies from 63–95% based on the location of PVL and available devices. After successful closure, the congestive symptoms improve within the weeks, on the other hand, the hemolysis may continue or even worsen for the first few months until device endothelialization [3].
References
Ionescu A, Fraser AG, Butchart EG. Prevalence and clinical significance of incidental paraprosthetic valvular regurgitation: a prospective study using transoesophageal echocardiography. Heart. 2003;89:1316–21.
Hammermeister K, Sethi GK, Henderson WG, et al. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–8.
Pate GE, Al Zubaidi A, Chandavimol M, et al. Percutaneous closure of prosthetic paravalvular leaks: case series and review. Catheter Cardiovasc Interv. 2006;68:528–33.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer-Verlag London Ltd., part of Springer Nature
About this chapter
Cite this chapter
Firouzi, A., Hosseini, Z. (2021). Percutaneous Closure of Aortic Paravalvular Leakage. In: Maleki, M., Alizadehasl, A. (eds) Case-Based Clinical Cardiology. Springer, London. https://doi.org/10.1007/978-1-4471-7496-7_76
Download citation
DOI: https://doi.org/10.1007/978-1-4471-7496-7_76
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-7495-0
Online ISBN: 978-1-4471-7496-7
eBook Packages: MedicineMedicine (R0)