Abstract
Trichloroethylene (trichloroethene, TCE) is a widely used organic solvent and a common environmental and occupational contaminant. Apart from diseases like cancer and heart defects, TCE exposure has also been implicated in the development of various autoimmune diseases (ADs), such as systemic lupus erythematosus (SLE), systemic sclerosis and fasciitis, both from occupational and environmental exposures. Experimental studies using MRL+/+ mice as an animal model also support an association between TCE exposure and autoimmunity. Increasing evidence suggests that free radical-mediated reactions could play a potential role in the pathogenesis of ADs, and TCE exposure is known to cause oxidative stress both in vivo and in vitro. Recent studies have contributed to the understanding of the role of oxidatively modified proteins, especially lipid peroxidation-derived aldehyde (LPDA)-modified proteins in TCE-induced autoimmune response. These studies support that oxidative modification of endogenous proteins leads to structural alterations, resulting in the formation of neoantigens which elicit autoimmune responses by stimulating T and/or B lymphocytes, particularly Th1 and Th17 lymphocytes. More detailed studies to understand the distinct pathways by which oxidative stress contributes to autoimmunity, especially mapping of gene expression, analyzing proteome, blocking/inhibiting specific signal transduction pathways will also unravel critical mechanisms in TCE-mediated autoimmunity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Trichloroethylene (trichloroethene, TCE) is a widely used organic solvent and a common environmental and occupational contaminant (Diot et al. 2002; Hardin et al. 2005; Bakke et al. 2007; Moran et al. 2007; ATSDR 2010; Purdue et al. 2011). About 3.5 million people are occupationally exposed to TCE in the United States mainly through its use in degreasing operation, but also through dry cleaning, textile scouring, and in handling adhesives, drugs, paints, leather and other products (Wu and Schaum 2000; Bakke et al. 2007; ATSDR 2010).
Environmental exposure to TCE occurs through air, contaminated ground water and drinking water. Most TCE used in the United States is released to the atmosphere from vapor degreasing operations, and its release to air also occurs at sewage treatment and disposal facilities, water treatment facilities, and landfills (Wu and Schaum 2000; Bakke et al. 2007; ATSDR 2010). TCE has been detected in the air throughout the United States, and the 1998 air levels across all 115 monitors ranged between 0.01–3.9 μg/m3 with a mean of 0.88 μg/m3 (Wu and Schaum 2000; Bakke et al. 2007; ATSDR 2010). TCE was detected in 28 % of 9,295 surface water reporting stations nationwide, and it is the most frequently reported organic contaminant in groundwater with up to 34 % of the drinking water supplies in USA contaminated with TCE (IARC 1995; Wu and Schaum 2000; ATSDR 2010).
TCE has also been identified in 72 food items in the US Food and Drug Administration’s Total Diet Study, including fruits, beverages and many foods prepared with oils and fats (Wu and Schaum 2000; ATSDR 2010). Because of its widespread commercial use and improper disposal, TCE has become a major occupational and environmental toxicant, and is one of the most abundant organic contaminants (NTP 1990; Bourg et al. 1992; Ashley et al. 1994; Hardin et al. 2005; Moran et al. 2007; ATSDR 2010). Therefore, there is clearly a need to extensively study the potential adverse health effect of TCE.
4.2 TCE Exposure and Autoimmune Response: Human Studies
TCE exposure has been associated with a variety of human diseases. Apart from diseases like cancer and heart defects (Boyer et al. 2000; Rhomberg 2000; Caldwell and Keshava 2006; Drake et al. 2006; Purdue et al. 2011), TCE has also been implicated in the development of various autoimmune diseases (ADs), such as systemic lupus erythematosus (SLE), systemic sclerosis and fasciitis, both from occupational (Phoon et al. 1984; Flindt-Hansen and Isager 1987; Lockey et al. 1987; Yáñez Díaz et al. 1992; Waller et al. 1994; Nietert et al. 1998; Cooper et al. 2009) and environmental exposures (Haustein and Ziegler 1985; Byers et al. 1988; Kilburn and Warshaw 1992; Hayashi et al. 2000 ; Albert et al. 2005 ; Cooper et al. 2009). The involvement of TCE exposure in ADs was first reported as early as in 1957 (Reinl 1957), and in recent years an increasing number of reports have further implicated TCE in the development of various ADs. Kilburn and Warshaw (1992) examined the prevalence of connective tissue disease symptoms and ANA, by comparing 362 residents of Tucson to 158 residents of another area of Southwest Arizona. The prevalence of some self-reported symptoms (malar rash, arthritis/arthalgias, Raynaud syndrome, skin lesions, and seizure or convulsion) and ANA levels were higher in Tucson residents (Kilburn and Warshaw 1992). Reports have shown that occupational TCE exposure is also associated with scleroderma (Flindt-Hansen and Isager 1987; Lockey et al. 1987; Yáñez Díaz et al. 1992; Nietert et al. 1998; Diot et al. 2002; Pralong et al. 2009) and fasciitis (Waller et al. 1994). Some case-control studies provided data specifically about TCE exposure, based on industrial hygienist review of job history data. Three of these studies are of scleroderma (Nietert et al. 1998; Diot et al. 2002; Garabrant et al. 2003), one is of undifferentiated connective tissue disease (Lacey et al. 1999), and one is of small vessel vasculitis involving anti-neutrophil cytoplasmic autoantibodies (Beaudreuil et al. 2005). Occupational TCE-induced Stevens-Johnson syndrome and other skin disorders have also drawn attention (Phoon et al. 1984; Huang et al. 2006; Kamijima et al. 2008; Jia et al. 2012).
4.3 TCE Exposure and Autoimmune Response: In Vivo Studies
Khan and his colleagues were first to propose and use MRL+/+ mice as an animal model to provide direct evidence of an association between TCE exposure and autoimmunity (Khan et al. 1995). This association was further substantiated by their subsequent studies and reports from other laboratories using MRL+/+ mice (Gilbert et al. 1999; Griffin et al. 2000a; Khan et al. 2001; Wang et al. 2007a, b, 2008, 2009, 2012a; Cai et al. 2008). MRL+/+ mice, therefore, have been the most often used animal models in experimental studies of TCE exposure.
Several studies in MRL+/+ mice have reported autoimmunity-related effects following exposure to TCE via drinking water (Blossom et al. 2004, 2007; Cai et al. 2008; Gilbert et al. 1999; Griffin et al. 2000a, b, c; Wang et al. 2007a) or ip injection (Cai et al. 2006; Khan et al. 1995; Wang et al. 2007b, 2008, 2009, 2012a). The initial drinking water studies used relatively high TCE concentrations of 2.5 and 5 mg/mL, with serologic measurements of ANA and IgG levels and assays for the activation of CD4+ T cells from spleen (Gilbert et al. 1999; Griffin et al. 2000a). Subsequent studies focused on examining TCE effects at lower exposure levels (0.1, 0.5, and 2.5 mg/mL) (Griffin et al. 2000b; Wang et al. 2007a, 2012a; Cai et al. 2008), also showed an accelerated autoimmune response. The effects observed by Griffin et al. (2000a) with respect to formation of TCE-protein adducts and CD4+ T cell activation was blocked by inhibiting CYP2E1 metabolic pathway (Griffin et al. 2000b), suggesting the role of TCE activation and its metabolites. In another chronic exposure study (0.5 mg/mL TCE in drinking water), Cai et al. (2008) found evidence of systemic inflammation as determined by serum cytokines measured after 36–48 weeks of exposure.
Some chronic oral exposure studies in the MRL+/+ mice, with exposure periods of 32–48 weeks, reported the presence of distinct clinical effects in exposed mice. One of these effects was characterized as an autoimmune hepatitis (Griffin et al. 2000b; Cai et al. 2008). Griffin et al. (2000b) found an inflammatory focal areas in the 0.5- and 2.5-mg/mL TCE-treated mice, with a dose-related effect on severity hepatic infiltrate in the portal tracts and lobular scores seen at 32 weeks. Cai et al. (2008) found similar liver lymphocytic infiltrates at 36 and 48 weeks in a study using 0.5 mg/mL TCE exposure through drinking water, and also infiltrates in the pancreas, lungs, and kidneys at 48 weeks. Wang et al. (2007a) observed increased autoantibodies in another study using 0.5 mg/mL TCE via drinking water for 48 weeks. In a 40-week study using trichloroacetaldehyde hydrate, Blossom et al. (2007) reported diffuse alopecia and skin inflammation and ulceration. Studies with other animal models also demonstrated the potential of TCE in inducing autoimmune response. For example, a chronic (26-week) drinking water exposure study in NZB × NZW mice reported increased level of proteinuria and prevalence of renal pathology with TCE exposure of 10,000 ppb via drinking water (Gilkeson et al. 2004). Production of anti-dsDNA and other antibodies was increased following 1,400 ppb TCE exposure for 19 weeks.
Several studies also evaluated the involvement of one or more metabolites of TCE in the induction of an autoimmune response observed in MRL+/+ mice. These include studies of dichloroacetyl chloride (Khan et al. 1995, 2001; Cai et al. 2006), trichloroacetaldehyde hydrate (Blossom et al. 2004, 2007; Blossom and Gilbert 2006; Gilbert et al. 2006), and trichloroacetic acid (Blossom et al. 2004). Effects were similar to those found with TCE in terms of accelerated autoantibody expression, T cell activation, and secretion of inflammatory cytokines.
4.4 Role of Oxidative Stress in Autoimmune Diseases
ADs such as SLE, rheumatoid arthritis and scleroderma are chronic and life-threatening disorders that affect ~3 % of United States population, and contribute disproportionately to morbidity and mortality among young to middle-aged women (Jacobson et al. 1997; Walsh and Rau 2000). Despite high prevalence of these diseases, molecular mechanisms underlying systemic autoimmune response remain largely unknown. In recent years, increasing evidence suggests that free radical-mediated reactions could play a potential role in the pathogenesis of ADs (Khan et al. 2001; Hadjigogos 2003; Frostegard et al. 2005; Kurien and Scofield 2008; Wang et al. 2008, 2010a; Vasanthi et al. 2009; Iuchi et al. 2010). Indeed increased oxidative stress is reported in various ADs (Grune et al. 1997; Frostegard et al. 2005; Tam et al. 2005; Morgan et al. 2009; Vasanthi et al. 2009; Shah et al. 2010; Wang et al. 2010a; Al-Shobaili and Rasheed 2012; Al-Shobaili et al. 2013).
Reactive oxygen species (ROS) including superoxide anion (O2 .−), hydrogen peroxide (H2O2), hydroxyl radical (.OH) and other reactive molecules containing oxygen formed during aerobic metabolism in cells as well as due to phagocyte and neutrophil activation during inflammation, have potential to initiate cellular damage to lipids, proteins and DNA (Biemond et al. 1984; Halliwell and Gutteridge 1984; Finkel 2011). A variety of ROS-mediated modifications of proteins have been reported in ADs and aging (Stadtman and Berlett 1998; Oates et al. 1999; Beal 2002; Morgan et al. 2005; Sheikh et al. 2007; Wang et al. 2010a; Al-Shobaili and Rasheed 2012; Al-Shobaili et al. 2013). Sheikh et al. (2007) observed increased protein carbonyls and recognition of ROS-modified human serum albumin by circulating SLE autoantibodies in SLE patients. Recently, Al-Shobaili et al. (2013) reported higher level of anti-oxidized-catalase (CAT)-antibodies in SLE patients with varying levels of disease activity according to SLE Disease Activity Index (SLEDAI). These antibodies showed strong relation with the SLEDAI, disease induction and progression (Al-Shobaili and Rasheed 2012; Al-Shobaili et al. 2013), suggesting oxidized protein may be a useful biomarker in evaluating the progression of SLE and in elucidating the mechanisms of disease pathogenesis.
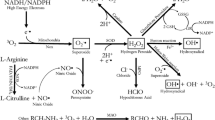
Reactive nitrogen species (RNS) are nitrogen-containing molecules, i.e., nitric oxide (.NO), peroxynitrite (ONOO−) and nitroxyl anion (HNO−) (Hill et al. 2010). Like ROS, RNS could also play a significant role in the pathogenesis of SLE and other ADs, and have drawn considerable attention in recent years. .NO, generated by the enzyme inducible nitric oxide synthase (iNOS), is one of the most important and widely studied RNS. The potential of .NO in disease pathogenesis lies largely to the extent of its production and generation of O2 .−, leading to formation of peroxynitrite (ONOO−). ONOO− is a potent nitrating and oxidizing agent which can react with tyrosine residues to form nitrotyrosine (NT; Weinberg et al. 1994; Xia and Zweier 1997; Khan et al. 2003). In addition, ONOO−-mediated modifications of endogenous proteins and DNA may enhance their immunogenicity, leading to a break in immune tolerance (Khan et al. 2003; Ohmori and Kanayama 2005; Kurien et al. 2006). Accumulating evidence in murine lupus shows increasing iNOS activity with the development and progression of ADs, and studies using competitive inhibitors suggest that iNOS could play a pathogenic role in murine ADs (Weinberg et al. 1994; Xia and Zweier 1997; Karpuzoglu and Ahmed 2006; Wang et al. 2009). Also elevated presence of nitrated proteins, particular NT, a stable end product of increased RNS production, has been found in many diseases including ADs (Oates et al. 1999; Morgan et al. 2005; Khan et al. 2006; Ohmori and Kanayama 2005). Growing observational data in humans also suggest that overexpression of iNOS and increased production of ONOO− may contribute to glomerular and vascular pathology and in the pathogenesis of many other ADs (Wanchu et al. 1998; Nagy et al. 2007a; Morgan et al. 2009). There is appreciable evidence that NT and other markers of protein oxidation are enhanced in diabetes and many other ADs, and may contribute to the pathogenesis of these diseases (Stadtman and Berlett 1998; Oates et al. 1999; Martín-Gallán et al. 2003; Morgan et al. 2005; Ohmori and Kanayama 2005; Khan et al. 2006; Khan and Ali 2006; Renke et al. 2007; Wang et al. 2010a).
Reactive lipid species (RLS) are usually derived from unsaturated lipids, including lipid peroxidation-derived aldehydes (LPDAs) and reactive prostaglandins of A- and J-series (Higdon et al. 2012). LPDAs such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE) are highly reactive and can bind covalently to proteins resulting in their structural modifications and may elicit an autoimmune response and contribute to disease pathogenesis (Khan et al. 1997, 1999; Januszewski et al. 2005; Reed et al. 2009; Wang et al. 2010a; Ben Mansour et al. 2010). Indeed higher levels of MDA-/HNE-modified proteins have been observed in AD patients (Grune et al. 1997; Kurien and Scofield 2003; Frostegard et al. 2005; D’souza et al. 2008; Ben Mansour et al. 2010; Wang et al. 2010a), suggesting a potential role for these oxidatively modified proteins in ADs. Wang et al. (2010a) analyzed the sera from 72 SLE patients with varying levels of disease activity (according to the SLEDAI) and 36 age- and gender-matched healthy controls for oxidative stress markers and showed significantly higher levels of both MDA/HNE protein adducts and anti-MDA/anti-HNE protein adduct antibodies in SLE patients compared with healthy controls. Interestingly, not only was there an increased number of subjects positive for anti-MDA or anti-HNE antibodies, but also the levels of both of these antibodies were significantly higher among SLE patients whose SLEDAI scores were ≥6 as compared with SLE patients with lower SLEDAI scores (<6). In addition, a significant correlation was observed between the levels of anti-MDA or anti-HNE antibodies and the SLEDAI score, suggesting a possible causal relationship between these antibodies and SLE. The stronger response observed in serum samples from patients with higher SLEDAI scores suggests that markers of oxidative stress may be useful in evaluating the progression of SLE and in elucidating the mechanisms of disease pathogenesis. Recently, Wang et al. (2012b) observed age-related increases in the formation of MDA-/HNE-protein adducts, their corresponding antibodies and MDA-/HNE-specific immune complexes in MRL/lpr mice, the widely used model for SLE. Interestingly, HNE-MSA adducts mimic nuclear antigens and cause significant inhibition in ANA binding to nuclear antigens (Fig. 4.1; Wang et al. 2012b), suggesting that LPDA-modified proteins could be important sources of autoantibodies and CICs in these mice, and thus contribute to autoimmune disease pathogenesis.
4.5 TCE Exposure and Oxidative Stress
Free radical-mediated reactions have drawn increasing attention as the potential mechanism in the pathogenesis of ADs and other diseases (Khan et al. 2001; Hadjigogos 2003; Karpuzoglu and Ahmed 2006; Kurien et al. 2006; Cuzzocrea 2006; Nagy et al. 2007b). TCE has been shown to generate free radicals and induce oxidative stress both in vivo and in vitro (Ogino et al. 1991; Channel et al. 1998; Khan et al. 2001; Zhu et al. 2005; Wang et al. 2007a, b, 2008, 2012a). A list of studies leading to TCE-induced oxidative stress are summarized in Table 4.1. Several studies have reported an association between TCE exposure and increased oxidative stress, especially lipid peroxidation (Ogino et al. 1991; Channel et al. 1998; Khan et al. 2001; Zhu et al. 2005; Wang et al. 2007a, b, 2008, 2012a). Most of earlier studies (Cojocel et al. 1989; Ogino et al. 1991; Channel et al. 1998; Toraason et al. 1999) used high doses of TCE (125–2,000 mg/kg) to demonstrate TCE-induced lipid peroxidation which contributes to toxic response in the livers or kidneys. Wang et al. (2007b, 2008) reported that TCE exposure for 6 or 12 weeks led to significantly increased formation of MDA-/HNE-protein adducts in the livers of TCE-treated female MRL +/+ mice at both 6 and 12 weeks, but with greater response at 12 weeks. Further characterization of these adducts in liver microsomes showed increased formation of MDA-protein adducts with molecular masses of 86, 65, 56, 44, and 32 kD, and of HNE-protein adducts with molecular masses of 87, 79, 46, and 17 kD in TCE-treated mice (Wang et al. 2007b). In addition, significant induction of anti-MDA- and anti-HNE-protein adduct-specific antibodies was observed in the sera of TCE-treated mice, and showed a pattern similar to MDA- or HNE-protein adducts. TCE-induced formation of MDA-/HNE-protein adducts and their respective antibodies were also observed in mice exposed to a relatively lower dose of TCE (Wang et al. 2007a, 2012a ).
The potential of TCE in inducing nitrosative stress has also drawn attention recently (Wang et al. 2007a, 2009; Blossom et al. 2012). TCE exposure resulted in increased formation of NT and induction of iNOS in the serum of female MRL +/+ mice. TCE treatment also led to greater NT formation, and iNOS protein and mRNA expression in the livers and kidneys (Wang et al. 2009). TCE-induced formation of NT was also observed at relatively lower dosages of TCE (Wang et al. 2007a; Blossom et al. 2012), which could potentially contribute to TCE-induced autoimmune response.
The potential of TCE exposure leading to carbonylation of proteins has also been examined. TCE exposure (10 mmol/kg, i.p., every fourth day) in female MRL+/+ mice resulted in increased (~3 fold) serum protein carbonyls (a marker of protein oxidation) at both 6 and 12 weeks. Increased protein carbonyls were also observed in the livers and kidneys (2.1 and 1.3 fold, respectively) at 6 weeks, and to a greater extent at 12 weeks (3.5 and 2.1 fold, respectively) following TCE treatment (Wang et al. 2009). Increased protein carbonyls were also observed in ovaries, oocytes, sperms and kidneys if rats or mice exposed to TCE via drinking water (DuTeaux et al. 2004; Wu and Berger 2007; Fan et al. 2012). Fan et al. (2012) analyzed and characterized the carbonylated proteins by using two-dimensional (2D) gel electrophoresis, Western blot along with matrix-assisted laser-desorption ionization time-of-flight mass spectrometry (MALDI TOF/TOF MS/MS) in the kidneys following TCE exposure in female MRL+/+ mice (2 mg/ml via drinking water) for 36 weeks. TCE treatment led to significantly increased protein carbonyls in the kidney protein extracts. Interestingly, among 18 identified carbonylated proteins, 10 were found only in the kidneys of TCE-treated mice, whereas other eight were present in the kidneys of both control and TCE-treated mice. The identified carbonylated proteins represent skeletal proteins, chaperones, stress proteins, enzymes, plasma protein, and proteins involved in signaling pathways. Huang et al. (2012) examined the serum proteome in the TCE-induced hypersensitivity dermatitis patients via 2D gel coupled with MALDI-TOF-TOF/MAS and also found that inflammatory responses and oxidative stress might contribute to TCE-induced hypersensitivity dermatitis.
In vitro studies (Chen et al. 2002; Zhu et al. 2005; Shen et al. 2007; Hu et al. 2008) using a variety of human cell lines such as human epidermal keratinocytes, human lung cancer H460 and p54-null H1299 cells have shown that TCE can induce oxidative stress, particularly lipid peroxidation in a time- and concentration-dependent pattern, and GSH, an intracellular antioxidant, provided protection against TCE-induced oxidative damage.
4.6 TCE-Induced Oxidative Stress and Induction of Autoimmunity
The role of oxidative stress in the TCE-induced autoimmune response was first proposed by Khan and his colleagues (Khan et al. 2001). That led to a series of studies examining the contributions of oxidative stress, especially the LPDAs, in TCE-induced autoimmune response by his research group (Wang et al. 2007a, b, 2008, 2009, 2012a; Fan et al. 2012). Their observations led them to hypothesize (Fig. 4.2) that TCE-induced oxidative stress leads to a variety of RONS-mediated structural modifications of the endogenous proteins, such as increased formation of LPDA-protein adducts (e.g. MDA-/HNA-protein adducts), carbonylation and nitration of proteins, which could potentially lead to generation of neoantigens. After antigen processing, these neoantigens could elicit autoimmune response by stimulating T and B lymphocytes, especially Th1 and Th17 cells (Wang et al. 2008, 2012a).
Wang et al. (2007b) detected increased formation of MDA- and HNE-protein adducts in the livers of MRL+/+ mice treated with TCE (10 mmol/kg, i.p., every fourth day) for 6 and 12 weeks. Significant induction of anti-MDA- and anti-HNE-protein adduct specific antibodies was also observed in the sera of TCE-treated mice, which showed a pattern similar to MDA-/HNE-protein adducts. More importantly, the increases in anti-MDA- and anti-HNE-protein adduct antibodies were associated with significant elevation in serum anti-nuclear (ANA)-, anti-ssDNA- and anti-dsDNA-antibodies at 6 weeks and, to a greater extent at 12 weeks. Those studies, even though at a relatively high dose, served as the basis for further evaluation of TCE-induced oxidative modification of proteins at occupationally relevant doses. Wang et al. (2007a, 2012a) observed that occupationally-relevant doses of TCE (0.5, 1.0 or 2.0 mg/ml in drinking water for 12, 24, 36, 48 weeks) led to dose- and time-related increases in MDA-/HNE-protein adducts and their corresponding antibodies in the sera. Furthermore, strong relationship between the increases in MDA-/HNE-protein adducts and significant elevation in serum ANA and anti-ssDNA-antibodies, suggested an association between TCE-induced oxidative stress and autoimmune response. Interestingly, stimulation of cultured splenic lymphocytes from both control and TCE-treated female MRL +/+ mice (10 mmol/kg, i.p., every fourth day for 4 weeks) with MDA-adducted mouse serum albumin (MDA-MSA) or HNE-MSA for 72 h showed significant proliferation of CD4+ T cells in TCE-treated mice as analyzed by flow cytometry. Also, splenic lymphocytes from TCE-treated mice released more IFN-γ and IL-2 into cultures when stimulated with MDA-MSA or HNE-MSA, suggesting a Th1 cell activation (Wang et al. 2008). Similarly, after female MRL +/+ mice were orally exposed to TCE (0.5, 1.0 or 2.0 mg/ml in drinking water) for 12, 24, 36 weeks, the splenocytes from mice treated with TCE for 24 weeks secreted significantly higher levels of IL-17 and IL-21 than did splenocytes from controls after stimulation with MDA-MSA or HNE-MSA adducts. The increased release of these cytokines was dose-dependent and more pronounced in mice treated with TCE for 36 weeks (Wang et al. 2012a; Fig. 4.3). These studies provide evidence that MDA- and or HNE-modified proteins contribute to TCE-mediated autoimmunity, which may be via activation of Th1, Th17 cells (Wang et al. 2008, 2012a). Recent studies in groups of female MRL +/+ mice treated with TCE, NAC or TCE plus NAC for 6 weeks (TCE, 10 mmol/kg, i.p., every fourth day; NAC, 250 mg/kg/day through drinking water), showed that NAC supplementation not only attenuated the TCE-induced formation of anti-MDA/-HNE-protein adduct antibodies and increased carbonylation of serum proteins, but also increases in serum levels of ANA, anti-Sm- and anti-dsDNA-antibodies, evidenced by their reduced levels in the sera of TCE plus NAC treated mice, further supporting a role of oxidatively modified proteins in TCE-induced autoimmune response (Wang et al. 2010b).
IL-17 release in the culture supernatants of splenocytes from control and TCE-treated mice (0.5, 1.0, 2.0 mg/ml of TCE in drinking water for 36 weeks). Splenocytes were stimulated with MSA alone, HNE-MSA, MDA-MSA or anti-CD3 antibody for 72 h. US un-stimulated cells. *p < 0.05 vs. US; #p < 0.05 vs. stimulated control group; +p < 0.05 vs. stimulated lower dose groups (0.5 and 1 mg/ml) (Adapted from Elsevier)
Recent studies have also explored the contribution of protein oxidation (carbonylation and nitration) in the induction of TCE-induced autoimmune response (Wang et al. 2007a, 2009, 2010b, 2013). TCE exposure (10 mmol/kg, i.p., every fourth day) in female MRL +/+ mice for 6 or 12 weeks (10 mmol/kg, i.p., every fourth day), led to time-dependent increases in carbonylation and nitration of proteins with enhanced iNOS activity. More importantly, the increases in TCE-induced protein oxidation (carbonylation and nitration) were associated with significant increases in Th1-specific cytokine (IL-2, IFN-γ) release into splenocyte cultures (Wang et al. 2009). These data along with the evidence that TCE induces autoimmune response (Khan et al. 1995; Wang et al. 2007a, 2008), suggest an association between oxidative modification of proteins and autoimmunity. The modification of proteins, such as nitration or carbonylation, may alter immunogenicity of self-antigens (converting them to neoantigens), and may lead to an autoimmune response by stimulating T cells (especially activation of Th1 cells; Wang et al. 2009). Lower dose of TCE exposure (0.5 mg/ml via drinking water) also led to significant increases of serum NT along with elevation of ANA and anti-dsDNA antibodies (Wang et al. 2007a). Interestingly, TCE treatment in iNOS-null female MRL+/+ mice even though still led to increases in serum ANA and anti-dsDNA, but the increases in these autoantibodies induced by TCE were significantly less pronounced compared to that in MRL+/+ mice (Wang et al. 2013). These results suggest an association between protein oxidation and induction/exacerbation of autoimmune response, and present a potential mechanism by which oxidatively modified proteins could contribute to TCE-induced autoimmune response (Wang et al. 2007a, 2009, 2010b, 2013).
4.7 Conclusions and Future Direction
Recent studies have contributed to the understanding of the role of oxidatively modified proteins, especially LPDAs modified proteins, in TCE-induced autoimmune response. These studies support that oxidative modification of proteins (e.g., MDA-/HNE-protein adducts, nitration and carbonylation of proteins) cause structural alterations to endogenous proteins, resulting in the formation of neoantigens which elicit autoimmune responses by stimulating T and/or B lymphocytes, particularly Th1 and Th17 lymphocytes (Khan et al. 2001; Wang et al. 2007a, b, 2008, 2009, 2012a). These studies not only demonstrated that TCE exposure leads to increased formation of MDA-/HNE-protein adducts, nitration and carbonylation of proteins, and formation of anti-MDA-/HNE-protein adduct antibodies, but more importantly, observed a significant association between formation of these modified proteins, corresponding antibodies and increased autoantibodies. Furthermore, MDA-/HNE-MSA stimulated greater release of IFN-γ, IL-2, IL-17 and IL-21, suggesting the contribution of oxidatively modified proteins in TCE-mediated autoimmune responses. Further detailed studies to unravel the distinct pathways by which oxidative stress contributes to autoimmunity, especially mapping of gene expression, analyzing proteome, blocking/inhibiting specific signal transduction pathways, knocking out/down target genes and exploring the epigenetic involvement will also provide critical mechanisms in TCE-induced autoimmunity.
References
Albert DA, Albert AN, Vernace M, Sebastian JK, Hsia EC (2005) Analysis of a cluster of cases of Wegener granulomatosis. J Clin Rheumatol 11:188–193
Ali F, Sultana S (2012) Repeated short-term stress synergizes the ROS signaling through up regulation of NFkB and iNOS expression induced due to combined exposure of trichloroethylene and UVB rays. Mol Cell Biochem 360:133–145
Al-Shobaili HA, Rasheed Z (2012) Immunological studies of oxidized superoxide dismutase in patients with systemic lupus erythematosus. Correlation with disease induction and progression. Saudi Med J 33:1177–1184
Al-Shobaili HA, Robaee AA, Alzolibani AA, Rasheed Z (2013) Immunological studies of reactive oxygen species damaged catalase in patients with systemic lupus erythematosus: correlation with disease activity index. Immunol Invest 42:191–203
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Wooten JV (1994) Blood concentrations of volatile organic compounds in a nonoccupationally exposed US population and in groups with suspected exposure. Clin Chem 40(7 Pt 2):1401–1404
ATSDR (Agency for Toxic Substances and Disease Registry, Division of Toxicology) (2010) Public Health Statement for trichloroethylene. Toxicological Profile for Trichloroethylene (TCE), 1997. Available at http://www.atsdr.cdc.gov
Bakke B, Stewart PA, Waters MA (2007) Uses of and exposure to trichloroethylene in U.S. industry: a systematic literature review. J Occup Environ Hyg 4:375–390
Beal MF (2002) Oxidatively modified proteins in aging and disease. Free Radic Biol Med 32:797–803
Beaudreuil S, Lasfargues G, Lauériere L, El Ghoul Z, Fourquet F, Longuet C, Halimi JM, Nivet H, Büchler M (2005) Occupational exposure in ANCA-positive patients: a case-control study. Kidney Int 67:1961–1966
Ben Mansour R, Lassoued S, Elgaied A, Haddouk S, Marzouk S, Bahloul Z, Masmoudi H, Attia H, Aïfa MS, Fakhfakh F (2010) Enhanced reactivity to malondialdehyde-modified proteins by systemic lupus erythematosus autoantibodies. Scand J Rheumatol 39:247–253
Biemond P, Swaak AJ, Koster JF (1984) Protective factors against oxygen free radicals and hydrogen peroxide in rheumatoid arthritis synovial fluid. Arthritis Rheum 27:760–765
Blossom SJ, Gilbert KM (2006) Exposure to a metabolite of the environmental toxicant, trichloroethylene, attenuates CD4+ T cell activation-induced cell death by metalloproteinase-dependent FasL shedding. Toxicol Sci 92:103–114
Blossom SJ, Pumford NR, Gilbert KM (2004) Activation and attenuation of apoptosis of CD4+ T cells following in vivo exposure to two common environmental toxicants, trichloroacetaldehyde hydrate and trichloroacetic acid. J Autoimmun 23:211–220
Blossom SJ, Doss JC, Gilbert KM (2007) Chronic exposure to a trichloroethylene metabolite in autoimmune-prone MRL+/+ mice promotes immune modulation and alopecia. Toxicol Sci 95:401–411
Blossom SJ, Doss JC, Hennings LJ, Jernigan S, Melnyk S, James SJ (2008) Developmental exposure to trichloroethylene promotes CD4+ T cell differentiation and hyperactivity in association with oxidative stress and neurobehavioral deficits in MRL+/+ mice. Toxicol Appl Pharmacol 231:344–353
Blossom SJ, Melnyk S, Cooney CA, Gilbert KM, James SJ (2012) Postnatal exposure to trichloroethylene alters glutathione redox homeostasis, methylation potential, and neurotrophin expression in the mouse hippocampus. Neurotoxicology 33:1518–1527
Bourg ACM, Mouvet C, Lemer DN (1992) A review of the attenuation of trichloroethylene in soils and aquifers. Q J Eng Geol Hydrogeol 25:359–370
Boyer AS, Finch WT, Runyan RB (2000) Trichloroethylene inhibits development of embryonic heart valve precursors in vitro. Toxicol Sci 53:109–117
Byers VS, Levin AS, Ozonoff DM, Baldwin RW (1988) Association between clinical symptoms and lymphocyte abnormalities in a population with chronic domestic exposure to industrial solvent-contaminated domestic water supply and a high incidence of leukaemia. Cancer Immunol Immunother 27:77–81
Cai P, König R, Khan MF, Qiu S, Kaphalia BS, Ansari GA (2006) Autoimmune response in MRL+/+ mice following treatment with dichloroacetyl chloride or dichloroacetic anhydride. Toxicol Appl Pharmacol 216:248–255
Cai P, König R, Boor PJ, Kondraganti S, Kaphalia BS, Khan MF, Ansari GA (2008) Chronic exposure to trichloroethene causes early onset of SLE-like disease in female MRL +/+ mice. Toxicol Appl Pharmacol 228:68–75
Caldwell JC, Keshava N (2006) Key issues in the modes of action and effects of trichloroethylene metabolites for liver and kidney tumorigenesis. Environ Health Perspect 114:1457–1463
Channel SR, Latendresse JR, Kidneym JK, Grabaum JH, Lanem JW, Steel-Goodwinm L, Gothausm MC (1998) A subchronic exposure to trichloroethylene causes lipid peroxidation and hepatocellular proliferation in male B6C3F1 mouse liver. Toxicol Sci 43:145–154
Chen SJ, Wang JL, Chen JH, Huang RN (2002) Possible involvement of glutathione and p53 in trichloroethylene- and perchloroethylene-induced lipid peroxidation and apoptosis in human lung cancer cells. Free Radic Biol Med 33:464–472
Cojocel C, Beuter W, Muller W, Mayer D (1989) Lipid peroxidation: a possible mechanism of trichloroethylene-induced nephrotoxicity. Toxicology 55:131–141
Cooper GS, Makris SL, Nietert PJ, Jinot J (2009) Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect 117:696–702
Cuzzocrea S (2006) Role of nitric oxide and reactive oxygen species in arthritis. Curr Pharm Des 12:3551–3570
D’souza A, Kurien BT, Rodgers R, Shenoi J, Kurono S, Matsumoto H, Hensley K, Nath SK, Scofield RH (2008) Detection of catalase as a major protein target of the lipid peroxidation product 4-HNE and the lack of its genetic association as a risk factor in SLE. BMC Med Genet 9:62–69
Diot E, Lesire V, Guilmot JL, Metzger MD, Pilore R, Rogier S, Stadler M, Diot P, Lemarie E, Lasfargues G (2002) Systemic sclerosis and occupational risk factors: a case-control study. Occup Environ Med 59:545–549
Drake VJ, Koprowski SL, Hu N, Smith SM, Lough J (2006) Cardiogenic effects of trichloroethylene and trichloroacetic acid following exposure during heart specification of avian development. Toxicol Sci 94:153–162
DuTeaux SB, Berger T, Hess RA, Sartini BL, Miller MG (2004) Male reproductive toxicity of trichloroethylene: sperm protein oxidation and decreased fertilizing ability. Biol Reprod 70:1518–1526
Fan X, Wang G, English RD, Khan MF (2012) Proteomic analysis of carbonylated protein in the kidney of Trichloroethene-exposed MRL+/+ mice. The Toxicologist (2012 SOT Annual Meeting), p 252
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194:7–15
Flindt-Hansen H, Isager H (1987) Scleroderma after occupational exposure to trichlorethylene and trichlorethane. Acta Derm Venereol 67:263–264
Frostegard J, Svenungsson E, Wu R, Gunnarsson I, Lundberg IE, Klareskog L, Horkko S, Witztum JL (2005) Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum 52:192–200
Garabrant DH, Lacey JV Jr, Laing TJ, Gillespie BW, Mayes MD, Cooper BC, Schottenfeld D (2003) Scleroderma and solvent exposure among women. Am J Epidemiol 157:493–500
Gharib OA (2009) Effects of Kombucha on oxidative stress induced nephrotoxicity in rats. Chin Med 4:23
Gilbert KM, Griffin JM, Pumford NR (1999) Trichloroethylene activates CD4+ T cells: potential role in an autoimmune response. Drug Metab Rev 31:901–916
Gilbert KM, Pumford NR, Blossom SJ (2006) Environmental contaminant trichloroethylene promotes autoimmune disease and inhibits T-cell apoptosis in MRL(+/+) mice. J Immunotoxicol 3:263–267
Gilkeson GS, Keil D, Peden-Adams MM (2004) Immune effects of trichloroethylene on autoimmune disease in mice. In: Mohr LC, Hoel DG, Jollow D (eds) Trichloroethylene: the scientific basis of risk assessment. Medical University of South Carolina, Charleston, pp 87–98
Griffin JM, Blossom SJ, Jackson SK, Gilbert KM, Pumford NR (2000a) Trichloroethylene accelerates an autoimmune response by Th1 T cell activation in MRL +/+ mice. Immunopharmacology 46:123–137
Griffin JM, Gilbert KM, Lamps LW, Pumford NR (2000b) CD4+ T-cell activation and induction of autoimmune hepatitis following trichloroethylene treatment in MRL+/+ mice. Toxicol Sci 57:345–352
Griffin JM, Gilbert KM, Pumford NR (2000c) Inhibition of CYP2E1 reverses CD4+ T-cell alterations in trichloroethylene-treated MRL+/+ mice. Toxicol Sci 54:384–389
Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, Siems WG (1997) Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radic Biol Med 23:357–360
Hadjigogos K (2003) The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med 45:7–13
Halliwell B, Gutteridge JM (1984) Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet 1:1396–1397
Hardin BD, Kelman BJ, Brent RL (2005) Trichloroethylene and dichloroethylene: a critical review of teratogenicity. Birth Defects Res A Clin Mol Teratol 73:931–955
Haustein UF, Ziegler V (1985) Environmentally induced systemic sclerosis-like disorders. Int J Dermatol 24:147–151
Hayashi N, Igarashi A, Matsuyama T, Harada S (2000) Eosinophilic fasciitis following exposure to trichloroethylene: successful treatment with cyclosporin. Br J Dermatol 142:830–832
Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM (2012) Cell signaling by reactive lipid species: new concepts and molecular mechanisms. Biochem J 442:453–464
Hill BG, Dranka BP, Bailey SM, Lancaster JR Jr, Darley-Usmar VM (2010) What part of NO don’t you understand? Some answers to the cardinal questions in nitric oxide biology. J Biol Chem 285:19699–19704
Hu C, Jiang L, Geng C, Zhang X, Cao J, Zhong L (2008) Possible involvement of oxidative stress in trichloroethylene-induced genotoxicity in human HepG2 cells. Mutat Res 652:88–94
Huang H, Kamijima M, Wang H, Li S, Yoshikawa T, Lai G, Huang Z, Liu H, Chen J, Takeuchi Y, Nakajima T, Li L (2006) Human herpesvirus 6 reactivation in trichloroethylene-exposed workers suffering from generalized skin disorders accompanied by hepatic dysfunction. J Occup Health 48:417–423
Huang Z, Yue F, Yang X, Xia L, Chen C, Qiu X, Huang J, Li L, Kamijima M, Nakajima T, Huang H (2012) Upregulation of calprotectin and downregulation of retinol binding protein in the serum of workers with trichloroethylene-induced hypersensitivity dermatitis. J Occup Health 54:299–309
IARC (1995) IARC monographs on the evaluation of carcinogenic risks to humans. In: Dry cleaning. Some chlorinated solvents and other industrial chemicals, vol 63. International Agency for Research on Cancer, Lyon
Iuchi Y, Kibe N, Tsunoda S, Suzuki S, Mikami T, Okada F, Uchida K, Fujii J (2010) Implication of oxidative stress as a cause of autoimmune hemolytic anemia in NZB mice. Free Radic Biol Med 48:935–944
Jacobson DL, Gange SJ, Rose NR, Graham NM (1997) Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84:223–243
Januszewski AS, Alderson NL, Jenkins AJ, Thorpe SR, Baynes JW (2005) Chemical modification of proteins during peroxidation of phospholipids. J Lipid Res 46:1440–1449
Jia Q, Zang D, Yi J, Dong H, Niu Y, Zhai Q, Teng Y, Bin P, Zhou W, Huang X, Li H, Zheng Y, Dai Y (2012) Cytokine expression in trichloroethylene-induced hypersensitivity dermatitis: an in vivo and in vitro study. Toxicol Lett 215:31–39
Kamijima M, Wang H, Huang H, Li L, Shibata E, Lin B, Sakai K, Liu H, Tsuchiyama F, Chen J, Okamura A, Huang X, Hisanaga N, Huang Z, Ito Y, Takeuchi Y, Nakajima T (2008) Trichloroethylene causes generalized hypersensitivity skin disorders complicated by hepatitis. J Occup Health 50:328–338
Karpuzoglu E, Ahmed SA (2006) Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide 15:177–186
Khan F, Ali R (2006) Antibodies against nitric oxide damaged poly L-tyrosine and 3-nitrotyrosine levels in systemic lupus erythematosus. J Biochem Mol Biol 39:189–196
Khan MF, Kaphalia BS, Prabhakar BS, Kanz MF, Ansari GAS (1995) Trichloroethene-induced autoimmune response in female MRL +/+ mice. Toxicol Appl Pharmacol 134:155–160
Khan MF, Kaphalia BS, Ansari GAS (1997) Time-dependent autoimmune response of dichloroacetyl chloride in female MRL +/+ mice. Immunopharmacol Immunotoxicol 19:265–277
Khan MF, Wu X, Boor PJ, Ansari GAS (1999) Oxidative modification of lipids and proteins in aniline-induced splenic toxicity. Toxicol Sci 48:134–140
Khan MF, Wu X, Ansari GAS (2001) Anti-malondialdehyde antibodies in MRL+/+ mice treated with trichloroethene and dichloroacetyl chloride: possible role of lipid peroxidation in autoimmunity. Toxicol Appl Pharmacol 170:88–92
Khan MF, Wu X, Kaphalia BS, Boor PJ, Ansari GA (2003) Nitrotyrosine formation in splenic toxicity of aniline. Toxicology 194:95–102
Khan MF, Kannan S, Wang J (2006) Activation of transcription factor AP-1 and mitogen-activated protein kinases in aniline-induced splenic toxicity. Toxicol Appl Pharmacol 210:86–93
Khan S, Priyamvada S, Khan SA, Khan W, Farooq N, Khan F, Yusufi AN (2009) Effect of trichloroethylene (TCE) toxicity on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in kidney and other rat tissues. Food Chem Toxicol 47:1562–1568
Kilburn KH, Warshaw RH (1992) Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ Res 57:1–9
Kurien BT, Scofield RH (2003) Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Sci 73:1655–1666
Kurien BT, Scofield RH (2008) Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev 7:567–573
Kurien BT, Hensley K, Bachmann M, Scofield RH (2006) Oxidatively modified autoantigens in autoimmune diseases. Free Radic Biol Med 41:549–556
Lacey JV Jr, Garabrant DH, Laing TJ, Gillespie BW, Mayes MD, Cooper BC, Schottenfeld D (1999) Petroleum distillate solvents as risk factors for undifferentiated connective tissue disease (UCTD). Am J Epidemiol 149:761–770
Lockey JE, Kelly CR, Cannon GW, Colby TV, Aldrich V, Livingston GK (1987) Progressive systemic sclerosis associated with exposure to trichloroethylene. J Occup Med 29:493–496
Martín-Gallán P, Carrascosa A, Gussinyé M, Domínguez C (2003) Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med 34:1563–1574
Moran MJ, Zogorski JS, Squillace PJ (2007) Chlorinated solvents in groundwater of the United States. Environ Sci Technol 41:74–81
Morgan PE, Sturgess AD, Davies MJ (2005) Increased levels of serum protein oxidation and correlation with disease activity in systemic lupus erythematosus. Arthritis Rheum 52:2069–2079
Morgan PE, Sturgess AD, Davies MJ (2009) Evidence for chronically elevated serum protein oxidation in systemic lupus erythematosus patients. Free Radic Res 43:117–127
Nagy G, Koncz A, Fernandez D, Perl A (2007a) Nitric oxide, mitochondrial hyperpolarization, and T cell activation. Free Radic Biol Med 42:1625–1631
Nagy G, Clark JM, Buzás EI, Gorman CL, Cope AP (2007b) Nitric oxide, chronic inflammation and autoimmunity. Immunol Lett 111:1–5
Nietert PJ, Sutherland SE, Silver RM, Pandey JP, Knapp RG, Hoel DG, Dosemeci M (1998) Is occupational organic solvent exposure a risk factor for scleroderma? Arthritis Rheum 41:1111–1118
Oates JC, Christensen EF, Reilly CM, Self SE, Gilkeson GS (1999) Prospective measure of serum 3-nitrotyrosine levels in systemic lupus erythematosus: correlation with disease activity. Proc Assoc Am Physicians 111:611–621
Ogino K, Hobara T, Kobayashi H, Ishiyama H, Gotoh M, Imamura A, Egami N (1991) Lipid peroxidation induced by trichloroethylene in rat liver. Bull Environ Contam Toxicol 46:417–421
Ohmori H, Kanayama N (2005) Immunogenicity of an inflammation-associated product, tyrosine nitrated self-proteins. Autoimmun Rev 4:224–229
Phoon WH, Chan MO, Rajan VS, Tan K, Thirumoorthy T, Goh CL (1984) Stevens-Johnson syndrome associated with occupational exposure to trichloroethylene. Contact Dermatitis 10:270–276
Pralong P, Cavailhes A, Balme B, Cottin V, Skowron F (2009) Diffuse systemic sclerosis after occupational exposure to trichloroethylene and perchloroethylene. Ann Dermatol Venereol 136:713–717
Purdue MP, Bakke B, Stewart P, De Roos AJ, Schenk M, Lynch CF, Bernstein L, Morton LM, Cerhan JR, Severson RK, Cozen W, Davis S, Rothman N, Hartge P, Colt JS (2011) A case-control study of occupational exposure to trichloroethylene and non-Hodgkin lymphoma. Environ Health Perspect 119:232–238
Reed TT, Pierce WM, Markesbery WR, Butterfield DA (2009) Proteomic identification of HNE-bound proteins in early Alzheimer disease: insights into the role of lipid peroxidation in the progression of AD. Brain Res 1274:66–76
Reinl W (1957) Scleroderma caused by trichloroethylene in workers. Bull Hyg 32:678–679
Renke J, Szlagatys A, Hansdorfer-Korzon R, Szumera M, Kamińska B, Knap N, Popadiuk S, Szarszewski A, Woźniak M (2007) Persistence of protein oxidation products and plasma antioxidants in juvenile idiopathic arthritis. A one-year follow-up study. Clin Exp Rheumatol 25:112–114
Rhomberg LR (2000) Dose-response analyses of the carcinogenic effects of trichloroethylene in experimental animals. Environ Health Perspect 108(Suppl 2):343–358
Shah D, Kiran R, Wanchu A, Bhatnagar A (2010) Oxidative stress in systemic lupus erythematosus: relationship to Th1 cytokine and disease activity. Immunol Lett 129:7–12
Sheikh Z, Ahmad R, Sheikh N, Ali R (2007) Enhanced recognition of reactive oxygen species damaged human serum albumin by circulating systemic lupus erythematosus autoantibodies. Autoimmunity 40:512–520
Shen T, Zhu QX, Yang S, Ding R, Ma T, Ye LP, Wang LJ, Liang ZZ, Zhang XJ (2007) Trichloroethylene induce nitric oxide production and nitric oxide synthase mRNA expression in cultured normal human epidermal keratinocytes. Toxicology 239:186–194
Shen T, Zhu QX, Yang S, Wu CH, Zhang HF, Zhou CF, Zhang XJ (2008) Trichloroethylene induced cutaneous irritation in BALB/c hairless mice: histopathological changes and oxidative damage. Toxicology 248:113–120
Stadtman ER, Berlett BS (1998) Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab Rev 30:225–243
Tabrez S, Ahmad M (2011) Some enzymatic/nonenzymatic antioxidants as potential stress biomarkers of trichloroethylene, heavy metal mixture, and ethyl alcohol in rat tissues. Environ Toxicol 26:207–216
Tam LS, Li EK, Leung VY, Griffith JF, Benzie IF, Lim PL, Whitney B, Lee VW, Lee KK, Thomas GN, Tomlinson B (2005) Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: a double blind, placebo controlled pilot study. J Rheumatol 32:275–282
Toraason M, Clark J, Dankovic D, Mathias P, Skaggs S, Walker C, Werren D (1999) Oxidative stress and DNA damage in Fischer rats following acute exposure to trichloroethylene or perchloroethylene. Toxicology 138:43–53
NTP (National Toxocology Program) (1990) Carcinogenesis studies of trichloroethylene (without epichlorohydrin) in F344/N rats and BC63F1 mice (gavage study), NTP Technical Report 243, CAS No. 79-01-06. NTP, Research Triangle Park
Vasanthi P, Nalini G, Rajasekhar G (2009) Status of oxidative stress in rheumatoid arthritis. Int J Rheum Dis 12:29–33
Waller PA, Clauw D, Cupps T, Metcalf JS, Silver RM, Leroy EC (1994) Fasciitis (not scleroderma) following prolonged exposure to an organic solvent (trichloroethylene). J Rheumatol 21:1567–1570
Walsh SJ, Rau LM (2000) Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am J Public Health 90:1463–1466
Wanchu A, Khullar M, Deodhar SD, Bambery P, Sud A (1998) Nitric oxide synthesis is increased in patients with systemic lupus erythematosus. Rheumatol Int 18:41–43
Wang G, Cai P, Ansari GA, Khan MF (2007a) Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response. Toxicology 229:186–193
Wang G, Ansari GA, Khan MF (2007b) Involvement of lipid peroxidation-derived aldehyde-protein adducts in autoimmunity mediated by trichloroethene. J Toxicol Environ Health A 70:1977–1985
Wang G, König R, Ansari GAS, Khan MF (2008) Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4+ T cells. Free Radic Biol Med 44:1475–1482
Wang G, Wang J, Ma H, Khan MF (2009) Increased nitration and carbonylation of proteins in MRL+/+ mice exposed to trichloroethene: potential role of protein oxidation in autoimmunity. Toxicol Appl Pharmacol 237:188–195
Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF (2010a) Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum 62:2064–2072
Wang G, Ma H, Fan X, Wang J, Khan MF (2010b) N-acetylcysteine supplementation protests against trichloroethene-induced autoimmunity: role of oxidative stress. The Toxicologist (Annual Meeting of Society of Toxicology), p 67
Wang G, Wang J, Fan X, Ansari GA, Khan MF (2012a) Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: dose- and time-response studies in female MRL+/+ mice. Toxicology 292:113–122
Wang G, Li H, Khan MF (2012b) Differential oxidative modification of proteins in MRL+/+ and MRL/lpr mice: increased formation of lipid peroxidation-derived aldehyde-protein adducts may contribute to accelerated onset of autoimmune response. Free Radic Res 46:1472–1481
Wang G, Wakamiya M, Wang J, Ansari GA, Khan MF (2013) Attenuation of trichloroethene-mediated autoimmune response in iNOS-null MRL+/+ mice. The Toxicologist (Annual Meeting of Society of Toxicology), p 306
Weinberg JB, Granger DL, Pisetsky DS, Seldin MF, Misukonis MA, Mason SN, Pippen AM, Ruiz P, Wood ER, Gilkeson GS (1994) The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med 179:651–660
Wu KL, Berger T (2007) Trichloroethylene metabolism in the rat ovary reduces oocyte fertilizability. Chem Biol Interact 170:20–30
Wu C, Schaum J (2000) Exposure assessment of trichloroethylene. Environ Health Perspect 108(Suppl 2):359–363
Xia Y, Zweier JL (1997) Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci U S A 94:6954–6958
Yáñez Díaz S, Morán M, Unamuno P, Armijo M (1992) Silica and trichloroethylene-induced progressive systemic sclerosis. Dermatology 184:98–102
Zhu QX, Shen T, Ding R, Liang ZZ, Zhang XJ (2005) Cytotoxicity of trichloroethylene and perchloroethylene on normal human epidermal keratinocytes and protective role of vitamin E. Toxicology 209:55–67
Acknowledgements
This work was supported by Grant ES016302 from the National Institute of Environmental Health Sciences (NIEHS), National Institute of Health (NIH), and it contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Khan, M.F., Wang, G. (2014). Trichloroethylene-Induced Oxidative Stress and Autoimmunity. In: Gilbert, K., Blossom, S. (eds) Trichloroethylene: Toxicity and Health Risks. Molecular and Integrative Toxicology. Springer, London. https://doi.org/10.1007/978-1-4471-6311-4_4
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6311-4_4
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6310-7
Online ISBN: 978-1-4471-6311-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)