Abstract
Epicardial mapping and ablation of cardiac arrhythmias has been an important development in interventional electrophysiology. The fibrous and serous pericardia are separated by a space that permits introduction of a guide wire using a subxiphoid percutaneous approach. There are two major sinuses in the pericardial space. A sheath advanced over the guide wire provides a mean to introduce one or more catheters or probes to perform mapping and ablation epicardially. Atrial arrhythmias that require epicardial mapping and ablation include unusual types of atrial tachycardia or accessory pathways. In patients with atrial fibrillation or atypical atrial flutter, epicardial mapping and ablation may be required to obtain isolation or elimination of atrial substrate. In patients with ischemic heart disease and left ventricular dysfunction, as well as in patients with dilated cardiomyopathy, or with infiltrative or unusual forms of heart disease, ventricular tachycardia may originate epicardially or require epicardial mapping prior to performing endocardial ablation. There are numerous considerations requiring safety and management of patients undergoing epicardial mapping and ablation. Newer surgical and hybrid procedures, from the collaboration of electrophysiology and surgery, may also provide new approaches to performing interventional electrophysiology procedures that may require epicardial mapping and ablation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
In patients with the Wolff-Parkinson-White syndrome, surgical ablation of accessory pathways was shown to be successful both endocardially and epicardially [1, 2]. The development of catheter ablation allowed for percutaneous endocardial mapping and ablation of cardiac arrhythmias [3], although percutaneous epicardial delivery of low-energy DC in the coronary sinus [4] was initially shown to be safe and effective at eliminating left-sided accessory pathways, followed later by use of irrigated radiofrequency current [5]. Epicardial mapping and ablation of ventricular tachycardia using percutaneous access to the pericardial space was reported in 1996 [6], in patients with cardiomyopathy associated with Chagas disease.
Anatomic Features and Access to the Pericardial Space
The parietal (outer) or fibrous pericardium encloses the heart, and this most outer layer merges and is continuous with the great vessels. The serous (inner) pericardium consists of a double-layered membrane; the most inner aspect is the epicardium, which also merges and is continuous with the great vessels, while the outer aspect is embedded with the fibrous pericardium [7]. There are extensions of the pericardial cavity called sinuses and recesses [7, 8]. Most importantly are the oblique sinus and transverse sinus (Fig. 42.1). The latter allows access to the right atrium and region of the sinus node, while the former could be seen as the box created or within the four pulmonary veins [9].
Fluoroscopic image in an AP view of an epicardial multielectrode electroanatomic mapping catheter positioned in the oblique sinus. The catheter has been inserted from a subxiphoid approach, and a guide wire can be seen in the pericardial space, that may be used as backup for access to the pericardial space. Also shown are other catheters, including a 6 F decapolar catheter positioned in the coronary sinus, an octopolar catheter positioned in the RA, and the endocardial quadripolar electroanatomic mapping catheter advanced through a transseptal sheath to the LA
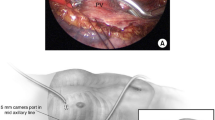
Percutaneous access to the pericardial space for cardiac mapping and ablation has generally consisted of the technique outlined by Sosa et al. [6]. Using a subxiphoid approach, a Tuohy needle with a blunt and curved tip is introduced, freely until feeling the resistance from the diaphragmatic muscle, followed by the pulsating heart against the needle. Advancing carefully the needle while applying negative pressure, the pericardial space is entered, removing a few ml of clear fluid and then injecting contrast seen under fluoroscopy to engage the pericardial space. A guide wire is introduced and can be seen on fluoroscopy on the outer aspect of the heart. Inadvertent puncture into the right ventricle would demonstrate during dye injection immediate washout rather than persistence of staining that outlines the heart shadow, while advancing a guide wire into the right ventricle would result in further advancement into the pulmonary artery.
Once access to the pericardial space has been effectively obtained, a sheath is introduced over the guide wire, often requiring dilation in view of the musculature of the diaphragm. If needed, more than one guide wire may be introduced through the sheath, followed by removal of that sheath to insert two or more sheaths into the pericardial space, to allow for introduction of the mapping and ablation catheter, or a dedicated sheath for fluid drainage or even an instrument such as balloon catheter or an endoscope [10–13].
In patients who have had prior surgical interventions, or who have had pericarditis, there may be significant restriction to catheter movement in the pericardial space; Soejima et al. [14] reported in 2004 of a combined surgical approach of creating a small window in the subxiphoid space to allow for manual lysis of adhesions and introduction of surgical tools or sheaths for creating large access and ease of manipulation of a sheath and catheter. In six patients who had prior cardiac surgery and who failed percutaneous pericardial access, this technique allowed in four patients catheter manipulation in the pericardial space in the region of the anterior and lateral walls, resulting in successful mapping and ablation [14].
More recently, Scanavacca et al. expanded on percutaneous access to the pericardial space endocardially from the right atrial appendage [15, 16]. In a swine model, percutaneous transatrial access to the pericardial space was accomplished by advancing a long transseptal sheath to the right atrial appendage in 16 animals and through the left atrial appendage in one animal [16]. A guide wire and dilator were advanced and perforation was obtained by firmly pressing against the tissue, followed by advancing the guide wire to view it on the outer portion of the heart. Following atrial ablation procedures in these animals, a pigtail catheter was introduced to aspirate blood or fluid. In 13/16 animals, the sheath was removed, while in three animals, patent foramen closure device closed the pericardial access site. The animals were immediately sacrificed. In the latter group, there was one inadvertent placement of the occlusion device in the RV, while in the former group, two of the animals had cardiac tamponade [16].
Physiologic Studies of Endocardial and Epicardial Activation in the Atrium and Ventricle
Durrer et al. [17] studied endocardial and epicardial activation on isolated human hearts from seven individuals who died from cerebral conditions. Up to 870 intramural terminals were able to map excitation of the left and right ventricle. In two hearts, atrial activation was also evaluated. An earlier study by Puech et al. [18] in dogs described normal atrial activation using epicardial electrodes.
With the introduction of 3D non-fluoroscopic mapping systems, Lemery et al. [9] performed an endocardial and epicardial mapping study of the RA and LA is sinus rhythm. Following sinus node onset, there is early epicardial activation of the RA, followed by preferential conduction over Bachmann’s bundle [19] with the earliest left atrial activation being endocardial and anterior. The earliest epicardial LA activation occurs more than half way following onset of the P wave in sinus rhythm, whereas the terminal portion of the P wave is due to late activation of all components of atrial activation, including the RA, LA, coronary sinus, transverse sinus, and oblique sinus [9].
Epicardial Mapping and Ablation of Supraventricular Arrhythmias
Infrequently, percutaneous epicardial access to the pericardial space is required for mapping and ablation of accessory pathways or atrial tachycardia [20–25]. In patients with manifest or concealed accessory pathways [20–24], ineffective ablation or instability of catheter positioning against the tricuspid valve annulus for right free wall bypass tracts may be overcome by epicardial mapping and ablation. In rare cases of accessory pathways localized in the right atrial appendage [24], use of epicardial mapping can also assist in eliminating conduction over the accessory pathway. Most posteroseptal accessory pathways that are closer to the epicardium than the endocardium are ablated from the coronary sinus, although epicardial mapping and ablation may be required [20–23]. Atrial tachycardia requiring percutaneous epicardial mapping and ablation has also been reported; most patients have a focal atrial tachycardia arising from the region of the left atrial appendage [25].
Atrial fibrillation involves important physiologic mechanisms that are located on the pericardium. The ganglionated plexuses are mostly distributed in the left atrium, and these autonomic sites of innervation can be localized during endocardial or epicardial mapping [26–34]. The Marshall vein and bundle can be targeted endocardially or epicardially and may be important in initiating or maintaining atrial fibrillation [35–37]. Complex fractionated atrial electrograms can be mapped endocardially and epicardially (Fig. 42.2). The transseptal approach to perform circumferential endocardial ablation of the pulmonary veins in patients with atrial fibrillation requires delivery of energy to regions of the left atrium that are associated with greater recurrences of conduction (ridge, carina), that may require epicardial mapping and ablation [38–40].
Surface ECG leads I, II, and III V1 and V6 in a patient with epicardial and endocardial mapping of atrial fibrillation. The catheters record bipolar electrograms and are positioned in the coronary sinus (CS, distal to proximal 1–2 to 9–10), oblique sinus (Epi, 1–2 to 7–8), high right atrium (HRA 3–4), and His bundle region (His 1–2 and 3–4). The ablation catheter (ABL 1–2) is positioned in the antral region of the left superior pulmonary vein (left panel) and left inferior pulmonary vein (right panel). Complex fractionated atrial electrograms are observed at different time intervals, but distinct isoelectric periods are also observed
The esophageal position in the posterior left atrium may result in the inability to deliver high enough energies to obtain pulmonary vein isolation. Percutaneous techniques to protect the esophagus by use of a balloon catheter or use of extra pericardial fluid have been described [12, 13, 39]. Combined endocardial-epicardial percutaneous mapping and ablation procedures in patients with atrial fibrillation have been reported [39]. However, development of hybrid techniques involving surgical access to the pericardial space using minimally invasive techniques, combined with electrophysiological mapping and ablation during or following surgical ablation, may provide more comprehensive end points, especially in patients with persistent atrial fibrillation requiring a maze-like procedure [41, 42].
Epicardial Mapping and Ablation of Ventricular Arrhythmias
Ventricular arrhythmias associated with cardiomyopathies were initially reported as having an increased incidence of epicardial foci. In the original report a direct subxiphoid percutaneous approach by Sosa et al. [6], ventricular tachycardia was associated with Chagas cardiomyopathy. In this trypanosomiasis, cardiac involvement often results in a basal inferolateral scar, associated with ventricular tachycardia that can be reproducibly induced during programmed electrical stimulation. Subepicardial reentry may result in unsuccessful ablation from the endocardium; in a series of 257 consecutive patients with VT, epicardial VT occurred in 37 % with Chagas disease, as compared with 28 % in patients with ischemic VT, and 24 % in patients with idiopathic dilated cardiomyopathy [43]. Although significant reduction in shocks from an implantable defibrillator in patients with Chagas disease has been shown following combined endocardial-epicardial VT ablation procedures, recurrence rates remain elevated during long-term follow-up [43].
Patients with ischemic [44, 45] or idiopathic cardiomyopathies showing a predominant left ventricular involvement or patients with arrhythmogenic right ventricular cardiomyopathy may also require epicardial mapping and ablation [46–49]. In a recent report of 22 patients with epicardial VT associated with idiopathic dilated cardiomyopathy [47], the epicardial regions showing low voltages were significantly larger and more adjacent than those seen on the endocardium. In approximately half of patients with an epicardial origin of VT, low-voltage regions were mapped in close proximity to the aortic or mitral valve [47]. In patients with arrhythmogenic right ventricular cardiomyopathy, epicardial mapping has demonstrated significant scarring, beyond the endocardial regions of abnormal low voltage [48, 49]. In 13 patients with arrhythmogenic right ventricular cardiomyopathy who failed endocardial VT ablation [48], epicardial mapping and ablation was associated with no recurrence of VT in 10/13 patients (77 %) during a mean follow-up of 18 months (range 5–41).
Patients with nonischemic VT occurring in patients without overt heart disease typically have VT occurring from the outflow tract in the RV or LV. Infrequently, these patients may have an epicardial origin of their VT from the anterior interventricular vein or distal great cardiac vein or other unusual left ventricular myocardial sites [50]. When the venous anatomy does not allow for adequate positioning of the mapping and ablation catheter, a percutaneous approach to the epicardium may result in successful mapping and ablation of VT [50].
In patients with VT, an epicardial focus is typically associated with specific electrocardiographic features [50–52]. These include a pseudo delta wave of 34 ms or greater, an intrinsicoid deflection of 85 ms or greater, and a precordial RS complex duration of 121 ms or greater. In addition, the paced QRS complex has been shown to be significantly longer from paced epicardial sites as compared with endocardial sites of pacing [50–52].
Mapping and ablation in patients with an epicardial origin of VT requires attention to numerous details [53, 54]. Most patients have significant LV dysfunction; use of an intra-aortic balloon catheter or other methods of LV assistance may be required. An underlying ICD is frequently present, and specific coronary anatomic features can be delineated during pre-procedure coronary angiography or CT angiography. Figure 42.3 shows a Carto map view of the epicardium.
Energy Sources
The pericardial space has several limitations to energy delivery during ablation [55, 56]. Anatomic considerations such as coronary anatomy, phrenic nerve, or esophagus may limit the site of energy delivery [56–59], while epicardial fat may be associated with ineffective energy delivery [60]. The closed space of the pericardium with absence of circulating blood flow may result in electrode temperature increase, requiring low-power applications. The latter may limit effective energy delivery, resulting in need for irrigated radiofrequency energy delivery [55]. Regular drainage of pericardial fluid throughout the mapping and ablation procedure is necessary.
Complications and Post-procedure Management
Access to the pericardial space may result in inadvertent puncture of the left lobe of the liver or of the abdominal cavity [54, 61]. In patients with a megacolon with underlying Chagas disease [43] or with previous abdominal surgery, inadvertent puncture through the bowel may result in air under the diaphragm, or abdominal hemorrhage may occur. These patients may be managed conservatively, but abdominal interventions may be required. Access to the pericardial space may be associated with inadvertent puncture of the RV. Generally, if a guide wire has been introduced in the RV, removal and continuation of the procedure can generally be done. Insertion of a sheath in the RV may require surgical intervention for closure of the hole. A case of epicardial laceration of a coronary artery has also been described [62].
Collateral damage from mapping and ablation in the epicardial space includes injury to the coronary arteries, phrenic nerve, lungs, esophagus, and vagus nerve [56–59, 61, 62]. Post-procedure pericarditis usually responds to nonsteroidal anti-inflammatories. Intrapericardial use of 0.5–1.0 mg/kg of methylprednisolone may reduce the risks of post-procedure pericarditis or the development of significant pericardial effusions [63].
Future Perspectives and Hybrid Surgical-Interventional Electrophysiological Collaboration
Percutaneous epicardial mapping and ablation for the treatment of selected cardiac arrhythmias will likely grow in importance over the next years. Specific approaches and tools for working in the pericardial space will need to be developed. Remote magnetic navigation applications to epicardial mapping and ablation may facilitate catheter navigation and provide favorable vector orientations for catheter energy delivery [64]. New hybrid surgical-electrophysiological procedures may also result in improved patient outcomes [42, 65].
References
Sealy WC, Gallagher JJ, Wallace AG. The surgical treatment of Wolff-Parkinson-White syndrome: evolution of improved methods for identification and interruption of the Kent bundle. Ann Thorac Surg. 1976;22:443.
Guiraudon GM, Klein GJ, Gulamhusein S, Jones DL, Yee R, Perkins DG, Jarvis E. Sureical reDair of Wolff-Parkinson-White syndrome: a new closed-heart technique. Ann Thorac Surg. 1984;37:67.
Jackman WM, Wang X, Friday KJ, Roman CA, Moulton KP, Beckman KJ, McClelland JH, Twidale N, Hazlitt A, Pior MI, Margolis D, Calame JD, Overholt ED, Lazarra R. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med. 1991;324:1605–11.
Lemery R, Talajic M, Roy D, Coutu B, Lavoie L, Lavallee E, Cartier R. Success, safety, and late electrophysiological outcome of low-energy direct-current ablation in patients with the Wolff-Parkinson-White syndrome. Circulation. 1992;85:957–62.
Yamane T, Jais P, Shah DC, Hocini M, Peng JT, Deisenhofer I, Clementy J, Haissaguerre M. Efficacy and safety of an irrigated-tip catheter for the ablation of accessory pathways resistant to conventional radiofrequency ablation. Circulation. 2000;102:2565–8.
Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysical laboratory. J Cardiovasc Electrophyisol. 1996;7:537–8.
McAlpine WA. Heart and coronary arteries: an anatomical atlas for clinical diagnosis, radiological investigation, and surgical treatment. Berlin/New York: Springer; 1975.
D’Avila A, Scanacca M, Sosa E, Ruskin JE, Reddy VY. Pericardial anatomy for the interventional electrophysiologist. J Cardiovasc Electrophysiol. 2003;14:422–30.
Lemery R, Birnie D, Tang A, Green M, Gollob M, Hendry M, Lau E. Normal atrial activation and voltage during sinus rhythm in the human heart. J Cardiovasc Electrophysiol. 2007;18:402–8.
Nazarian S, Kantsevoy SV, Zviman MM, Matsen FA, Calkins H, Berger RD, Halperin HR. Feasibility of endoscopic guidance for nonsurgical transthoracic atrial and ventricular epicardial ablation. Heart Rhythm. 2008;5(8):1115–9.
Nagashima K, Watanabe I, Okumura Y, Ohkubo K, Kofune M, Ohya T, Kasamaki Y, Hirayama A. Efficacy and feasibility of pericardial endoscopy by subcutaneous approach. Europace. 2011;13:1501–3.
Buch E, Nakahara S, Shivkumar K. Intra-pericardial balloon retraction of the left atrium. A novel method to prevent esophageal injury during catheter ablation. Heart Rhythm. 2008;5(10):1473–5.
Nakahara S, Ramirez R, Buch E, Michowitz Y, Vaseshi M, de Diego C, Boyle N, Mahajan A, Shivkumar K. Intrapericardial balloon placement for prevention of collateral injury during catheter ablation of the left atrium in a porcine model. Heart Rhythm. 2010;7:81–7.
Soejima K, Couper G, Coopr JM, Sapp JL, Epstein LM, Stevenson WG. Subxiphoid surgical approach for epicardial catheter-based mapping and ablation in patients with prior cardiac surgery or difficult pericardial access. Circulation. 2004;110:1197–201.
Verner RL, Waxman S, Lovett EG, Moreno R. Transatrial access to the normal pericardial space: an approach for diagnostic sampling, pericardiocentesis, and therapeutic interventions. Circulation. 1998;98(21):2331–3.
Scanavacca M, Vanancio A, Pisani C, Lara S, Hachul D, Darrieux F, Hardy C, Paola E, Aiello V, Mhapatra S, Sosa E. Percutaneous transatrial access to the pericardial space for epicardial mapping and ablation. Circ Arrhytm Electrophysiol. 2011;4:331–6.
Durrer D, Van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher C. Total excitation of the isolated human heart. Circulation. 1970;41:899–912.
Puech P, Esclavissat M, Sodi-Pollares D, Cisneros F. Normal auricular activation in the dog’s heart. Am Heart J. 1954;47:174–91.
Lemery R, Guiraudon G, Veinot JP. Anatomic description of Bachmann’s bundle and its relation to the atrial septum. Am J Cardiol. 2003;91:1482–5.
Schweikert RA, Saliba WI, Tomassoni G, Marrouche NF, Cole CR, Dressing TJ, Tchou PJ, Bash D, Beheiry S, Lam C, Kanagarathan L, Natale A. Percutaneous pericardial instrumentation for endo-epicardial mapping of previously failed ablations. Circulation. 2003;108(11):1329–35.
Valderrabano M, Cessario DA, Ji S, Shannon K, Weiner I, Swerdlow CD, Oral H, Morady F, Shivkumar K. Percutaneous epicardial mapping during ablation of difficult accessory pathways as an alternative to cardiac surgery. Heart Rhythm. 2004;1(3):311–6.
Ho I, D’Avila A, Ruskins J, Mansour M. Images in cardiovascular medicine. Percutaneous epicardial mapping and ablation of a posteroseptal accessory pathway. Circulation. 2007;115:e418–21.
de Paloa AAV, Leite LR, Mesas CE. Nonsurgical transthoracic epicardial ablation for the treatment of a resistant posteroseptal accessory pathway. Pacing Clin Electrophysiol. 2004;27:259–61.
Lam C, Schweikert R, Kanagaratnam L, Natale A. Radiofrequency ablation of a right atrial appendage-ventricular accessory pathway by transcutaneous epicardial instrumentation. J Cardiovasc Electrophysiol. 2000;11:1170–3.
Phillips KP, Natale A, Sterba R, Saliba WI, Burkhardt JD, Wazni O, Liberman L, Schweikert RA. Percutaneous pericardial instrumentation for catheter ablation of focal atrial tachycardias arising from the left atrial appendage. J Cardiovasc Electrophysiol. 2008;19:430–3.
Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247(2):289–98.
Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13 Suppl 1:37–42.
Lemery R, Birnie D, Tang SL, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006;3:387–96.
Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, Trombetta I, Negrao CE, Sosa E. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–85.
Lemery R. How to perform ablation of the parasympathetic ganglia of the left atrium. Heart Rhythm. 2006;3(10):1237–9.
Scherlag BJ, Po S. The intrinsic cardiac nervous system and atrial fibrillation. Curr Opin Cardiol. 2006;21(1):51–4.
Pokushalov E, Romanov A, Shugayev P, Artymenko S, Shirokova N, Turov A, Katritsis DG. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2009;6(9):1257–64.
Pokushalov E, Romanov A, Artyomenko S, Shirokva N, Turov A, Karaskov S, Katritsis D, Po SS. Ganglionated plexi ablation directed by high-frequency stimulation and complex fractionated atrial electrograms for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2012;35:776–84.
Lemery R. Autonomics and ablation of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2012;35:773–5.
Doshi RN, Wu TJ, Yashima M, Kim YH, Ong JJ, Cao JM, Hwang C, Yashar P, Fishbein MC, Karagueuzian HS, Chen PS. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–83.
Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of Marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–5.
Valderrabano M, Liu X, Sasaridis C, Sidhu J, Little S, Khoury DS. Ethanol infusion in the vein of Marshall: adjunctive effects during ablation of atrial fibrillation. Heart Rhythm. 2009;6:1552–8.
Reddy VY, Neuzil P, Ruskin JN. Extra-ostial pulmonary venous isolation: use of epicardial ablation to eliminate a point of conduction breakthrough. J Cardiovasc Electrophysiol. 2003;14(6):663–6.
Pak HN, Hwang C, Lim HE, Kim JS, Kim YH. Hybrid epicardial and endocardial ablation of persistent or permanent atrial fibrillation: a new approach to difficult cases. J Cardiovasc Electrophysiol. 2007;18(9):917–23.
Reddy V, Neuzil P, d’Avila A, JN R. Isolating the posterior left atrium and pulmonary veins with a “box” lesion set: use of epicardial ablation to complete electrical isolation. J Cardiovasc Electrophysiol. 2008;19(3):326–9.
Shivkumar K. Percutaneous epicardial ablation of atrial fibrillation. Heart Rhythm. 2008;5:152–4.
Krul SPJ, Driessen AHG, van Boven WJ, Linnebank AC, Geuzebroek GSC, Jackman WM, Wilde AAM, de Bakker JMT, de Groot JR. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:262–70.
Scanavacca M, Sosa E. Epicardial ablation of ventricular tachycardia in Chagas heart disease. Cardiac Electrophysiol Clin. 2010;2:55–67.
Sosa E, Scanavacca M, d’Avila A, Oliveira F, Ramires JAF. Nonsurgical transthoracic epicardial ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol. 2000;35(6):1442–9.
Brugada J, Berruezo A, Cuesta A, Osca J, Chueca E, Fosch X, Waynar L, Mont L. Nonsurgical transthoracic epicardial radiofrequency ablation: an alternative in incessant ventricular tachycardia. J Am Coll Cardiol. 2003;41(11):2036–43.
Soejima E, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004;43:1834–42.
Cano O, Hutchinson M, Lin D, Garcia F, Zado E, Bala R, Riley M, Cooper J, Dixit S, Gerstenfeld E, Callans D, Marchlinski FE. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular non-ischemic cardiomyopathy. J Am Coll Cardiol. 2009;54(9):799–808.
Farcia FC, Bazan V, Zado ES, Ren JF, Marchlinksi FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120:366–75.
Berruezo A, Fernandez-Armenta J, Mont L, Zeliko H, Andreu D, Herczku C, Boussy T, Tolosana JM, Arbelo E, Brugada J. Combined endocardial and epicardial ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol. 2012;5:111–21.
Daniels DV, Lu YY, Morton JB, Santucci P, Akar JG, Green AI, Wilber DJ. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of valsalva electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation. 2006;113:1659–66.
Berruezo A, Mont L, Nava S, Cheuca E, Bartholomay E, Brugada J. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004;109:1842–7.
Bazan V, Gertsenfeld E, Garcia F, Bala R, Rivas N, Dixit S, Zado E, Callans D, Marchlinski E. Site-specific twelve-lead ECG features to identify an epicardial origin for left ventricular tachycardia in the absence of myocardial infarction. Heart Rhythm. 2007;4:1403–10.
Aliot EM, Stevenson WG, Almendral-Garrote JM, Bogun F, Calkins CH, Delacretaz E, Della Bella P, Hindricks G, Jails P, Josephson MF, Kautzner J, Kay GN, Kuck KH, Lerman BB, Marchlinski F, Reddy V, Schalij MJ, Schilling R, Soejima K, Wilber D. EHRA/ESC/HRS/ACC/AHA expert consensus on catheter ablation of ventricular arrhythmias. Heart Rhythm. 2009;6:886–933.
Sacher F, Roberts-Thomson K, Maury P, Tedro U, Nault I, Steven D, Hocini M, Koplan B, Lerpux L, Derval N, Seiler J, Wright MJ, Epstein L, Haissaguerre M, Jais P, Stevenson WG. Epicardial ventricular tachycardia ablation of multicenter safety study. J Am Coll Cardiol. 2010;55:2366–72.
d’Avila A, Houghtaling C, Gutierrez P, Vragovic O, Ruskin JN, Josephson ME, Reddy VY. Catheter ablation of ventricular epicardial tissue. A comparison of standard and cooled-tip radiofrequency energy. Circulation. 2004;109:2363–9.
d’Avila A, Gutierrez P, Scanavacca M, Reddy V, Lustgarten BDL, Sosa E, Ramires JA. Effects of radiofrequency pulses delivered in the vicinity of the coronary arteries: implication for nonsurgical transthoracic epicardial catheter ablation to treat ventricular tachycardia. Pacing Clin Electrophysiol. 2002;25:1488–95.
Buch E, Vaseghi M, Cesario DA, Shivkumar K. A novel method for preventing phrenic nerve injury during catheter ablation. Heart Rhythm. 2007;4:95–8.
Di Biase L, Burkhardt JD, Pelargonio G, Dello Russo A, Casella M, Santarelli P, Horton R, Sanchez J, Gallinghouse JG, Al-Ahmad A, Wang P, Cummings JE, Schweikert RA, Natale A. Prevention of phrenic nerve injury during epicardial ablation: comparison of methods for separating the phrenic nerve from the epicardial surface. Heart Rhythm. 2009;6:957–61.
Sanchez-Quintana D, Cabrera JA, Climent V, Murillo M, Cabrera JA. Anatomic evaluation of the left phrenic nerve relevant to epicardial and endocardial catheter ablation: implications for phrenic nerve injury. Heart Rhythm. 2009;6:764–8.
Tung R, Nakahara S, Ramirez R, Lai C, Fishbein MC, Shivkumar K. Distinguishing epicardial fat from scar: analysis of electrograms using high-density electroanatomic mapping in a novel porcine infarct model. Heart Rhythm. 2010;7:389–95.
Koruth J, Aryana A, Dukkipati S, Pak HN, Kim YH, Sosa EA, Scanavacca M, Mahapatra S, Ailawadi G, Reddy V, d’Avila A. Unusual complications of percutaneous epicardial access and epicardial mapping and ablation of cardiac arrhythmias. Circ Arrhythm Electrophysiol. 2011;4:882–8.
Hsieh CH, Ross DL. Case of coronary perforation with epicardial access for ablation of ventricular tachycardia. Heart Rhythm. 2011;8:318–21.
D’Avila A, Neuzil P, Thaigalingam A, Gutierrez P, Aleong R, Ruskin J, Reddy V. Experimental efficacy of pericardial instillation of anti-inflammatory agents during percutaneous epicardial catheter ablation to prevent postprocedure pericarditis. J Cardiovasc Electrophysiol. 2007;18:1178–83.
Di Biase L, Santangeli P, Astudillo V, Conti S, Mohanty P, Sanchez J, Horton R, Thomas B, Burkhardt JD, Natale A. Endo-epicardial ablation of ventricular arrhythmias in the left ventricle with the Remote Magnetic Navigation System and the 3.5-mm open irrigated magnetic catheter: results from a large single-center case–control series. Heart Rhythm. 2010;7:1029–35.
Gersak B, Pernat A, Robic B, Sinkovec M. Prospective long-term outcomes after atrial fibrillation treatment using the convergent epicardial and endocardial ablation procedure. J Cardiovasc Electrophysiol. 2012;23(10):1059–66. doi:10.1111/j.1540-8167.2012.02355.x.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Lemery, R. (2014). Epicardial Mapping and Ablation of Cardiac Arrhythmias. In: Kibos, A., Knight, B., Essebag, V., Fishberger, S., Slevin, M., Țintoiu, I. (eds) Cardiac Arrhythmias. Springer, London. https://doi.org/10.1007/978-1-4471-5316-0_42
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5316-0_42
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5315-3
Online ISBN: 978-1-4471-5316-0
eBook Packages: MedicineMedicine (R0)