Abstract
Although R&D is at the core of knowledge-intensive industries like Pharma, outsourcing parts of its activities hold considerable efficiency and effectiveness potentials. That means managers must understand, which R&D activities can be outsourced and which need to stay in-house in order to ensure competitiveness. Nevertheless, systematic approaches for understanding the finer details of the decision-making process on R&D outsourcing are lacking. To address this gap, we present a framework developed in the context of a multinational company, Bayer. The combination of literature studies and the study of the decision process in the pharmaceutical division at Bayer HealthCare allows us to unfold an outsourcing process model—the filter approach—that includes appropriate decision phases and proper tools. The underlying logic of the model is that outsourcing decisions are rather a learning process with different stages than a rational one-off decision.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Toward a Flexible Breathing Organization: R&D Outsourcing at Bayer
The pharmaceutical industry is a research-intensive industry with long development cycles, and in recent decades, pharmaceutical research and development (R&D) has faced major challenges. Most of the easily approachable indications or targets have already been addressed, and it is becoming increasingly difficult to find new targets or develop medications offering additional benefits. At the same time, regulatory authorities are paying more attention to the safety of new medications, which raises the approval hurdles. An average drug costs about USD 1 billion in R&D, and the average time to market is 10–15 years (DataMonitor 2011; Masia 2006; Adams and Branter 2009). This order of magnitude is further aggravated by current trends: R&D costs are increasing steadily and at a faster rate than sales (Weiss et al. 2009; CMR International 2009; Evaluate Pharma 2009). Although there have been efforts to lower costs, particularly in light of the recent financial crisis, productivity is lagging (Evaluate Pharma 2009; David et al. 2009; Morgan Stanley Research Europe 2010). Given the pressure from financial markets and the high intra-industry competition, pharmaceutical companies have had to find ways to address this situation. In fact, improved productivity in R&D has become a prerequisite for corporate survival. Moreover, the financial crisis has highlighted the importance of flexible capacity. In companies where 100 % capacity is held in-house, it will typically take longer time to react to fluctuations in demand.
Outsourcing is one possible way to increase productivity as well as increasing flexibility in R&D. Therefore, the extent of outsourcing of R&D activities has increased significantly in recent years, which has improved the ability to respond to demand fluctuations (Howells et al. 2008). It is mainly the more standardized part of the R&D value chain that has been outsourced to external providers, which have allowed companies to leverage cost-efficiencies and scale benefits, use internal capacities more effectively and focus on core activities. Few pharma companies can afford to ignore the cost savings that can be gained from outsourcing of more standardized R&D activities. A pressing question for pharmaceutical companies is how to best organize the R&D activities in order to improve productivity and flexibility: Which activities to keep in-house and which to outsource? These considerations require a thorough understanding of the R&D value chain activities and how these activities are interlinked. However, what is the appropriate process to apply for companies in order to decide on the proper organization of the R&D value chain activities? What is best practice in decision-making process on R&D outsourcing?
In the following, we will first discuss insights gained from the literature outlining the proper stages in the outsourcing decision process, and then, we will scrutinize and unfold a specific case of outsourcing decision. The specific case is the pharmaceutical division of Bayer HealthCare, structured decision-making process on outsourcing of preclinical development activities. When Bayer set out to optimize its set-up within its preclinical development in 2008, everything was still done in-house. However, it then started a journey that led to the development of a structured approach to making R&D outsourcing decisions. What is highlighted here is that in practice, the make-or-buy decision cannot be boiled down to one calculative choice, but does entail a number of steps or gates that needs to be passed in order to make an informed decision on the R&D outsourcing.
2 Decision-Making Process of R&D Outsourcing
Within R&D, one main method of accessing external sources during the clinical and preclinical phases of R&D is through outsourcing. The basic idea is that the firm should be able to better leverage core competences—the firm’s core internal skills and available resources—if it outsources non-core activities for which it does not have sufficient in-house expertise (Sen 2009). Basically, pharma companies are moving from a model of in-house handling and full ownership of R&D activities toward a model in which the focus is on the orchestration and combination of internal and external R&D inputs.
One implication of this shift is that pharma companies need to develop new competences related to the coordination and integration of knowledge and research stemming from different individuals and groups (Teece et al. 1997). Competition previously centered on the quality of internal R&D, but this focus has shifted to the best ways in which to appropriate value when combining internal R&D with external R&D inputs from outsourcing partners. Therefore, the drawing of the boundaries of the firm and decisions regarding which R&D activities to keep internally and which to outsource have become pertinent issues for all pharma companies.
Existing literature on R&D outsourcing is mainly derived from the transaction cost theory and the resource-based perspective and such studies tend to adopt, either consciously or unwittingly, a calculative approach to outsourcing decisions (Ulset 1996; Mol 2005). Many possible determinants of R&D outsourcing decisions have been identified in this literature, including the characteristics of the tasks, the characteristics of the outsourcing companies and relationships with the external partner.
The studies in this line of research seem to indicate that the change from internal sourcing of R&D activities to outsourcing is a rather simple, calculated, strategic decision (Howells et al. 2008). The view is based on an economic approach, and its fundamental assumptions are that firms have full information and are quasi-rational in their choices. The implication is that once the cost and benefits of specific outsourcing projects are known, there is little room for managerial discretion.
This rational calculative viewpoint is contested by more longitudinal in-depth studies of how companies make outsourcing decisions in practice. These studies indicate that such decisions are not necessarily one-offs and that they, in actuality, encompass a learning process and several phases (McIvor 2005; Piachaud 2004). An outsourcing decision is not just the result of a simple cost-benefit calculation. It reflects more often a decision process, which is treated more like an innovation process with different “gates” at which risks, capabilities, costs and benefits are assessed (similar to a Stage-Gate model for technical innovations).
3 A Model for Outsourcing Decisions
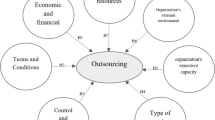
Based on studies of outsourcing decision, Van de Water and Van Peet have proposed a model developed for the manufacturing context that highlights the main phases of the outsourcing decision-making process (Van de Water and Van Peet 2006; Platts et al. 2002; Probert 1996). This model (Fig. 12.1) emphasizes that the decision process includes three distinct phases: (1) determining the performance objectives, (2) determining the relevant capabilities and value chain activities and (3) determining the type of relationship with the supplier(s). In the following, we scrutinize the three phases and the specific considerations related to each phase, as they provide the background and inspiration for our subsequent study of Bayer’s outsourcing decision model.
A three-phase model for the make-or-buy decision (Van de Water and Van Peet 2006). (this figure is reproduced with kind permission of Elsevier © August 11, 2012)
3.1 Phase 1: Determining the Performance Objectives
The first phase centers on determining the performance objectives. The following five main performance objectives of outsourcing are emphasized: cost, quality, speed, flexibility and reliability.
Cost considerations: One important factor in outsourcing decisions is costs. Depending on the R&D value chain activity in question, significant cost differentials can exist among different locations.
Quality, reliability, flexibility and speed: There may be benefits to outsourcing apart from those related to costs that have not been sufficiently examined from a theoretical perspective. Such benefits may include access to new markets, brand exposure, or access to untapped talent pools, as well as the ability to flexibly respond to changing markets (Gupta et al. 2007; Tate et al. 2009; Farrell 2005). In the context of R&D, in particular, the time factor has a significant impact on the potential revenue generation.
3.2 Phase 2: Determining Capabilities and Activities
Phase 2 focuses on the assessment of the company’s own capabilities relative to the potential benefits identified in Phase 1.
In the R&D setting, the task of global strategy increasingly “is to determine the optimal level of disaggregation of the firm’s operations over its entire value chain, to then determine the optimal global allocation of each piece” (Contractor et al. 2010). Activity analysis implies that the companies learn about their operations and processes (while assessing possibilities for standardization, new ways of bundling and potential for improved scale) in order to specify interfaces and coordination mechanisms. Through this process, true core activities (distinctive and crucial to competitive advantage) and essential activities (advanced activities that are complementary and important for competitive advantage) can be differentiated. Notably, firms are increasingly micro-analyzing their activities and dissecting their value chains into finer slices. This trend is now also evident in R&D, which has traditionally been viewed as a key activity that was previously located close to the heart of the company (Contractor et al. 2010).
Internal and external interfaces. Internal and external customer relationships must be considered. This applies equally to internal and external customer interfaces, where external interfaces are particularly vulnerable because they are, ultimately, the source of revenue generation. Internal interfaces can arise when business processes, such as parts of accounting or HR, are outsourced. In this case, the provider will have multiple interfaces with the internal employees that they serve. External relationships refer to customers buying the focal company’s own products and services.
Internal relationships are particularly relevant in terms of the way in which globally dispersed R&D operations are organized and steered. If a global hub approach or a decentralized approach to R&D is in place, outsourcing R&D will affect a multitude of internal interfaces. However, if R&D is relatively centralized, there will be a central interface with the external providers. In addition, the effects of increasing R&D collaboration and networked operations have to be taken into account, which puts more emphasis on the ability to manage a network of relationships and to incorporate outsourced R&D activities into the overall web of activities in a meaningful, value-accretive manner.
Mobility. In terms of mobility, a global scope implies greater complexity in the evaluation of alternatives than the complexity associated with decisions made in an onshore or near-shore setting. In the global consideration of alternatives, a service may be outsourced to a provider operating in several locations or it may be allocated among various providers in different locations. These possibilities give rise to additional organization and communication challenges.
Again, the focus on human potential in service provision highlights the challenges: service providers must produce comparable outputs, and they have to be managed and connected within a global service delivery network. While physical assets can be moved (albeit at a cost), some services may be immobile, especially when specific knowledge is involved. However, suppliers of commodity services can be switched relatively easily. Therefore, the ability to integrate service providers into the R&D process in question becomes crucial.
3.3 Phase 3: Determining the Type of Relationship with the Supplier(s)
The relationship with supplier(s) is determined by considering the type of activity that is a candidate for outsourcing, while simultaneously considering the value discipline of the customer. In our context, these two factors relate to the type of R&D service in question.
The number of participants in a company’s supply chain, as well as their level of diversity, increases with decisions to outsource. Furthermore, a decision to engage in cross-border engagements with suppliers implies a need to consider various issues, such as how to cooperate efficiently across different (e.g., cultural) boundaries, how to create and transfer knowledge produced in the relationship, how to protect critical proprietary intellectual rights and how to ensure steady service provision, especially in situations of growing dependence on a highly customized supplier. These challenges are aggravated in the services context, as knowledge and innovations may be more tacit than they are in a goods context. This creates specific challenges related to knowledge diffusion and retention.
These considerations are further amplified in the R&D setting, as intellectual property (IP) protection is of paramount importance in protecting revenue generation and compensating for R&D costs. Therefore, the identification of activities that can be outsourced without IP threats, as well as the selection of trustworthy, reliable suppliers or partners, becomes critical success factors. In addition, a careful analysis of the IP environment in which the respective suppliers operate is necessary for an accurate assessment.
The need to find appropriate suppliers relates to the issue of industrial clusters. The management of dispersed networks and industrial clusters requires new capabilities. This makes it necessary to be continuously aware of developments in such clusters in order to fully grasp the implications of such a shift.
In the R&D context, this is reflected in the need to continuously monitor the global R&D landscape to record the manifestation and growth of new company clusters and academia-industry networks (such as those seen in Singapore). Furthermore, knowledge of talent pools within the R&D landscape (such as in India or in China) must be gathered and maintained.
The three phases in the outsourcing model by Van de Water and Van Peet are pointing at the main phases and headlines of the decision-making model, and in that sense, it is a very powerful model. However, the model is still short of specific tools on how to conduct the proper investigations on each stage, so decisions can be made on the different stages. In order to flesh out some of the relevant tools on the different stages of the outsourcing decision, we turn our attention to how the decision process is conducted and used in practice in Bayer. The intention is to gain more insights into those details of the model that are discussed more superficially in the literature. More specifically, we examine Bayer’s outsourcing decision process and explore how the company has determined its objectives, identified suitable activities and assessed its internal capabilities and external partners. In doing this, we will be able to specify the decision model further in the context of knowledge-intensive firms.
4 The Bayer Case: Need for Flexibility
Bayer HealthCare is a sub-group of Bayer AG, and it is one of the world’s leading innovators in the field of pharmaceutical and medical products. Bayer HealthCare encompasses research, development, manufacturing and marketing activities related to innovative products that improve the health of people and animals. Bayer HealthCare has four operating divisions: Pharma, which focuses on prescription medicines; Consumer Care, which focuses on over-the-counter medicines and dietary supplements; Medical Care, which deals with blood glucose monitoring devices and contrast agent injection systems; and Animal Health, which focuses on veterinary medicines and grooming products.
Bayer HealthCare offers a suitable setting in which to address the research questions for several reasons. First, as a company dedicated to research and innovation, Bayer HealthCare is an appropriate environment for the study of decision making as it relates to the high-value/core activities of an R&D-intensive life science company. Second, the company recently made outsourcing decisions in the areas of preclinical drug metabolism, and pharmacokinetics and toxicology, which allows us to directly observe decision making in an R&D context.
In the following, we will unfold the decision-making process of outsourcing within preclinical development as we believe the decision process model at Bayer HealthCare provides a lot of insights that go beyond the case itself. In particularly, we examine the decision-making process in more details for the two areas of drug metabolism and pharmacokinetics (DMPK) and toxicology.
Bayer HealthCare’s R&D outsourcing approach was jointly developed by the department for preclinical DMPK, the department of toxicology and R&D experts from Bayer’s in-house consultancy unit “Business Consulting.” DMPK and toxicology have been full-service providers in the past. However, the company recognized that it would need to become more flexible in the long run. Outsourcing was viewed as an option that could compensate for fluctuating activity peaks.
Bayer HealthCare’s outsourcing decisions for R&D were formed and conducted with experienced specialists and managers from the R&D function, as well as experts from the in-house consultancy. The approach and material were later analyzed by researchers for this article. Bayer’s R&D outsourcing decision process model was developed in several phases that correspond to the project phases: a set of workshops to assess current practice and company experience, comparison with other outsourcing frameworks from the literature, the development of a research-based framework, refinement and final testing. Throughout this development process, the main reiterations revolved around the number of filters and the criteria. The final model’s three-filter approach is depicted in Fig. 12.2. Outsourcing frameworks in the manufacturing area typically apply only two filters. However, in this the research setting, three filters were considered more appropriate given the desire to obtain a more detailed view of the individual activities in order to assess the practicality of outsourcing before external offers are sought. The functional experts of Bayer HealthCare agreed with this approach.
The following statements illustrate how participants view the model. In terms of the need to differentiate between core and non-core activities, it became clear early in the process that activities needed to be rethought and then re-bundled:
We needed to be precise about what we considered to be core activities. “The criterion “of strategic relevance to the company” was not enough—that needed to be detailed. The criteria developed in the framework helped us to be fully transparent on this point” (Project Manager, Bayer Business Consulting) and “The system behind the model is simple: a company should plan its resources based on core activities. With few exceptions, everything else can be handled by third parties. Given fluctuations in the workload of core activities, outsourced activities offer a major benefit: in times of low core workload, outsourced activities can be performed internally. This ensures that our resources are always used in the most efficient way” (Principal, Bayer Business Consulting).
In terms of the need to define short- versus long-term capabilities, one major issue was the desire to retain as much essential human talent as possible. Although the application of the filter analysis was very critical on the activity dimension, one repeatedly mentioned focus was the need to keep as many internal capabilities embedded in the personnel as possible in order to fulfill future requirements:
We considered the expected future increase in the workload when we assessed our personnel resource requirements. To do otherwise would have been unfair to our employees, and we would have dismissed core competencies that we would have needed to hire back in a few years (Department Head, DMPK).
When comparing internal and external performance, participants showed a clear understanding of current strengths and weaknesses early on in the development of the model, as benchmarking had been performed previously. These benchmarks indicated that the primary goal of outsourcing would be related to flexibility rather than costs:
Our total costs were already below pharmaceutical industry benchmarks. When we compared contract research organization (CRO) study costs for non-core studies with our internal study costs, we found ourselves in a comparable and often more favorable cost position, especially if one considers the usual additional monitoring efforts required for externally conducted studies. In other words, outsourcing was not cheaper in itself but it made us more flexible (Head of Global Early Development).
When discussing the overall intention of outsourcing, participants stated that the goal was to create an organization with more flexibility and permeable boundaries (a “breathing organization”):
We recognized that we cannot do everything in-house. We needed to become more flexible and create a “breathing organization” (Head of Global Early Development).
4.1 Toward a Systematic Outsourcing Filter Approach
Each activity is put through the filters in the R&D decision process model. In this process, the first filter assesses the strategic value of the activities to the company from an internal perspective. Actions with strategic value are considered to be core activities and are kept in-house. The second filter is used to assess non-core activities in terms of whether they are, by their nature, suitable for outsourcing. Non-core activities that do not have inherent outsourcing potential should be kept in-house. Activities with outsourcing potential are then assessed in the third step. This third filter takes all important external framework conditions, including costs, quality and risk, into consideration. Only activities that pass through all three filters should be outsourced. The filters are shown in Fig. 12.2.
If an organization does not have the necessary resources to perform strategically valuable activities in-house, outsourcing must also be considered for those activities. In the long run, however, each company should focus on those activities that provide it with a real strategic advantage.
4.1.1 Filter 1: Strategic Value
Several important criteria are used in the assessment of strategic value. The first is competitive advantage. Some activities might be rare on the market, and keeping of these in-house might provide a competitive advantage, perhaps in terms of knowledge development or speed. The second criterion is IP criticality. Activities that involve vital intellectual property rights should not be outsourced. The third criterion is the ability to develop knowledge important for future projects. When testing compounds, method expertise or knowledge on new substance classes might be created that could be important for future projects.
Each criterion was assessed by the internal company experts on the basis of a three-level scale (high, medium and low). Based on the assessment of these criteria, the experts made an overall assessment of the strategic value of each activity, which was also based on a scale ranging from high to low. In this process, the assessment criteria were applied to the individual activities with different weights. Generally, “competitive advantage” and “IP criticality” were assigned a higher weight in the overall strategic assessment than the “development of knowledge.”
One important consideration related to IP criticality was need to ensure exact, high-quality study results, regardless of whether those results were obtained through in-house or outsourced studies. As regulatory authorities might ask for additional data (beyond the usual contents of a study report issued by a third-party provider), failure to ensure that this requirement has been met when working with a third-party provider can result in significant delays or even project cancellation if the information is no longer retrievable (a situation that might be caused by a change in personnel at the provider).
The individual rating and weighting of each criterion, however, depend to a great extent on the company and the department under examination. Activities with a high overall strategic value should be kept in-house. Activities with a medium or low strategic value pass on to the second filter. The following examples illustrate the application of this filter at Bayer HealthCare.
In the first filter, a set of long-term animal DMPK bioanalytics studies were found to provide no real competitive advantage and the related IP situation was assessed as non-critical. Both criteria were therefore rated “low.” The development of knowledge on the compound through the performance of studies in-house was assessed as “medium.” Based on all three criteria, the overall strategic value of these DMPK studies was rated “low” and this activity was passed on to the second filter.
In toxicology, a mouse lymphoma assay was assessed as having a low competitive advantage and medium IP criticality. The development of additional knowledge was assessed as high. Overall, the strategic value of this study was assessed as “low,” which was mainly based on the competitive advantage assessment. This study was also passed on to the second filter.
4.1.2 Filter 2: Outsourcing Potential
The second filter assesses the outsourcing potential of non-core activities, regardless of the activity’s availability on the market, the related cost or the resulting quality.
Four criteria are used for assessment in this filter. The first is the level of complexity. The more complex an activity and/or the higher the necessity of making adaptations based on the findings, the more expertise is required to run a study properly. The second is time criticality. The shorter the given timeframe, the higher the inherent risk of time delays resulting from use of an external provider. For example, additional time must be spent with external providers to handle the initial briefing and to review the results. The third criterion is the number of interfaces with other functions. The lower the number of interfaces with other functions when planning and performing a study and interpreting the results (aside from standard study initiations and data handovers), the smoother and quicker a study can be performed in-house. The final criterion is average study duration per compound. Very short studies, for example, those lasting only a few days, might not be worth the outsourcing effort and can often be more easily handled in-house.
Criteria 1–3 are assessed on a scale ranging from high to low. The average duration per compound was assessed in units of real time. On the basis of these criteria, the experts made an overall assessment of outsourcing potential of each activity, which was also based on a scale ranging from high to low. Activities for which the overall outsourcing potential was rated “low” should be kept in-house, while activities with “medium” or “high” outsourcing potential pass on to the third filter.
The two examples presented in conjunction with the first filter were also rated against the second filter’s criteria. DMPK’s animal long-term bioanalytics studies’ level of complexity was ranked “low,” while time criticality was rated as “medium.” The average study duration per compound is one to three months, and the studies have a medium level of interfaces. Based on these criteria, these studies were found to have high outsourcing potential and they were passed on to the third filter.
In contrast, the mouse lymphoma assay in toxicology was rated as having a low level of complexity. Time criticality, however, was assessed as “high,” as this was one of the studies defining the critical path in toxicology. The average study duration was 13 weeks, and few interfaces with other functions within the company were necessary. The overall outsourcing potential was assessed as low, mainly due to the time criticality of the activity. In general, time criticality proved to be a major hurdle for activities in the second filter. In the case of the mouse lymphoma assay, the toxicology department decided to perform these studies in-house.
4.1.3 Filter 3: Outsourcing Attractiveness
The third filter, outsourcing attractiveness, takes market conditions into consideration. The assessment criteria for this filter cover four aspects. The first is availability. Activities can only be outsourced if the service is available externally. The second, cost, reflects the costs of the activity if services are sourced externally but does not include internal time spent for briefing and monitoring. The third criterion, monitoring effort as a percentage of total study capacity, focuses on the ratio of internal time that would be required for the study to the effort required to monitor the study if it is conducted externally. The final criterion, risk, includes all risk factors, including potential time delays, and insufficient quality of the research or the resulting data. The risk assessment must take any longer-term threats, such as potential delays in filing or safety issues once the product is on the market, into account. If the inherent risk of outsourcing is too high, studies should be performed in-house.
In terms of availability, the importance of good laboratory practices (GLP) must be mentioned as one criteria that will make supplier selection in some destinations (e.g., India and China) difficult given the risk to benefit ratio (i.e., the ratio of a risk-delayed regulatory approval—or no approval at all—to the benefit of lower costs). Therefore, this is also the criterion that directly corresponds to the location question. Any type of delay translates directly into high financial losses, as each day of lost exclusivity impairs a company’s ability to reap the payback from drug development. This was one of the main reasons that these destinations did not pass through the third filter.
The nature of DMPK’s long-term animal bioanalytics studies qualified them for outsourcing. However, whether outsourcing was attractive was to be clarified in the third filter. The CRO costs were moderate, while the associated risk was assessed as “low.” The additional internal monitoring effort that would be required was rated as “medium,” and the availability of external services for these studies was found to be “high.” Overall, animal long-term bioanalytics studies were assessed as having medium outsourcing attractiveness. In other words, these studies qualify for outsourcing.
After developing the final R&D outsourcing filter model, it was applied to the existing service landscape for all DMPK and non-clinical toxicology activities. The distribution of activities along the R&D value chain regarding the suitability for outsourcing is shown in Fig. 12.3.
Not surprisingly, most activities in the early research phases were considered core given their IP criticality, related opportunities to develop or gain important knowledge on the compounds, and time criticality, which imply that they should be handled in-house. The closer the activity was in the development chain to product launch, the more likely it was that the activity could be outsourced. As shown in Fig. 12.3, the likelihood that an activity will pass through the three filters depends on its placement within the R&D value chain. For research, many activities will be blocked from further consideration of outsourcing suitability at the first filter. However, at later stages of development, activities are more likely to pass through to the third filter, where the question is one of the outsourcing attractiveness—the availability of suitable external providers, the cost proposition and similar considerations.
This is reflected in the input–output relation of the model. In total, 33 DMPK and 59 activity clusters were assessed. Of these, only 8 DMPK and 32 toxicology activity clusters passed through to the second filter. The rest were considered of high strategic relevance to the company. In the second filter, three DMPK and nine toxicology activity clusters were classified as having no or little outsourcing potential. The remaining 5 DMPK and 23 toxicology activity clusters passed through to the third filter, where 4 DMPK and all of the toxicology activity clusters were found to be attractive outsourcing candidates. The fifth DMPK study cluster was found to have high external study costs.
Bayer HealthCare found that the framework offered several benefits. First, it was intuitive to use. Second, it enabled management to easily determine which activities would be suitable for outsourcing.
In addition to the specific activities that the analysis indicated could be outsourced, several of Bayer HealthCare’s core activities were already outsourced, as the in-house knowledge necessary to handle those activities had not been sufficiently built up within the organization. Outsourcing was therefore chosen as an interim solution until these activities could be completely handled in-house.
4.2 The Framework in Action: Core is Not Core Anymore
In general, the application of the outsourcing framework allowed for the identification of concrete activities that could be sourced externally in the future. Such opportunities for outsourcing help to support a “breathing” organization that can balance workload highs and lows by highlighting areas in which external resources can be flexibly switched on and switched off on demand. Furthermore, activities that actually qualify as “core” may be viewed as candidates for outsourcing. This particularly applies when the necessary internal knowledge and resources are not available in the short term. Nevertheless, the importance of bringing in this knowledge and building up the resources necessary to handle these activities in-house needs to be emphasized. When core activities are kept in-house, strategically vital information and skills stay in the hands of the company. In that sense, Bayer HealthCare follows a resource-complementation strategy in its outsourcing rather than a purely non-core cost-efficiency strategy, therefore this resembles a case of strategic, rather than tactical, outsourcing (Jahns et al. 2006; Javalgi et al. 2009). Moreover, the case illustrates that core activities are not static, but change over time as markets change, too. Therefore, regular reviews to assess the outsourcing potential are important elements of readjusting strategically. Applying systematic frameworks, such as the one presented here in the research context help in making this process explicit and reproducible.
The model can be applied to other R&D settings as well. The basic filters would apply to many contexts, although the criteria would require some adaption, as this case focuses on the specific needs of the research setting, precisely speaking of DMPK and non-clinical toxicology. In order to be applied to other contexts such as the development setting, the criteria would have to be reassessed. The criteria that would most likely have to be interpreted differently include IP criticality, development of expert knowledge on the compound and study duration, as they have a different importance in development than in research. Other filter criteria, such as complexity and time criticality, as well as the entire third filter, can probably be used in a similar form.
5 The Core of the Future: Quo Vadis R&D?
The filter approach can practically help in the structuring of decision making in a setting that is not highly outsourcing intensive. As emphasized, outsourcing’s main benefits are related to time, costs and flexibility. The latter can be represented by the metaphor of a breathing organization that can balance capacity utilization with a focus on core competencies.
Moreover, the case presented here demonstrates that at times, it can make sense to deliberately outsource core activities for which there is not enough in-house knowledge or resources. The need to outsource such activities also highlights those areas in which more in-house expertise is needed in the mid- to long term. On the external side, what starts as an arm’s length relationship may actually develop into more hybrid setups, such as alliances or joint ventures. In this sense, the systematic approach provides an opportunity to think through the company’s own value proposition and priorities to foster strategic clarity and simultaneously achieve a joint understanding inside the company.
In terms of the desire for flexibility in adapting to demand fluctuations, the model proposed here can help firms identify those activities most suitable to create a “breathing organizations.” In such an organization, when demand drops, it is easier to protect internal core employees from layoffs by reducing or eliminating external support, and redistributing core, essential and non-core tasks internally until demand improves. This serves as a highly valuable mechanism for ensuring that core employees’ critical capabilities are retained.
This study also provides outsourcing-related insights into high-skill, knowledge-intensive research settings. R&D is an area in which outsourcing has long been controversial. The more generally accepted path has been to keep R&D in-house and close to headquarters. The case studied here illustrates that companies can move beyond that general perception to further develop their non-core and core activity portfolios via systematic analyses of outsourcing opportunities.
Moreover, given our finding that some activities close to the core can be outsourced, we suggest that the distinction is not only one of “core” versus “non-core.” The core activities are shrinking in the sense that many “essential activities” previously considered as part of the “core” will be target for outsourcing and offshoring in the future.
References
Adams C, Branter V (2009) Spending on new drug development. Health Econ 19(2):130–141
CMR International (2009) Pharmaceutical R&D factbook. CMR International, New York
Contractor F, Kumar V, Kundu S, Torben P (2010) Reconceptualizing the firm in a world of outsourcing and offshoring: the organizational and geographical relocation of high-value company functions. J Purch Supply Manag 47(8):1417–1433
DataMonitor (2011) Pharmaceutical key trends 2010. Datamonitor, London
David E, Tramontin T, Zemmel R (2009) Pharmaceutical R&D: the road to positive returns. Nat Rev Drug Discov 8:609–610
Evaluate Pharma (2009) World preview 2014. EvaluatePharma, London
Farrell D (2005) Offshoring: value creation through economic change. J Manage Stud 42(3):675–683
Gupta A, Seshasai S, Mukherji S, Ganguly A (2007) Offshoring: the transition from economic drivers toward strategic global partnership and 24 hour knowledge factors. J Electron Commerce Organizations 5(2):1–23
Howells J, Gagliardi D, Mali K (2008) The growth and management of R&D outsourcing: evidence from UK pharmaceuticals. R&D Manage 38(2):205–219
Jahns C, Hartmann E, Bals L (2006) Offshoring: dimensions and diffusion of a new business concept. J Purch Supply Manag 12(4):218–231
Javalgi R, Dixit A, Scherer R (2009) Outsourcing to emerging markets: theoretical perspectives and policy implications. J Int Manag 15:156–168
Masia N (2006) The cost of developing a new drug. In: Bureau of international information programs focus on intellectual property rights. U.S. Department of state publication, Washington
McIvor R (2005) The outsourcing process. Cambridge University Press, Cambridge
Mol MJ (2005) Does being R&D intensive still discourage outsourcing? Evidence from Dutch manufacturing. Res Pol 34(4):571–582
Morgan Stanley Research Europe (2010) Pharmaceuticals: exit research and create value. Morgan Stanley, New York
Piachaud B (2004) Outsourcing R&D in the pharmaceutical industry. Palgrave Macmillan, Basingstoke
Platts K, Probert D, Canez L (2002) Make versus buy decisions: a process incorporating multi-attribute decision-making. Int J Product Econ 77(3):247–257
Probert D (1996) The practical development of a make-or-buy strategy: the issue of process positioning. Integ Manuf Syst 7(2):44–51
Sen AK (2009) Outsourcing of research and development activities: evidence from U.S. BioPharmaceutical Firms. GJBR 3/1:73–82
Tate W, Ellram L, Bals L, Hartmann E (2009) Offshore outsourcing of services: an evolutionary perspective. Int J Product Econ 120(2):512–524
Teece DJ, Pisano GP, Shuen A (1997) Dynamic capabilities and strategic management. Strateg Manage J 18:509–533
Ulset S (1996) R&D outsourcing and contractual governance: an empirical study of commercial R&D projects. J Econ Behav Organ 30(1):63–82
Van de Water H, Van Peet H (2006) A decision support model based on the analytic hierarchy process for the make or buy decision in manufacturing. J Purch Supply Manag 12(5):258–271
Weiss D, Naik P, Weiss R (2009) The ‘big pharma’ dilemma: develop new drugs or promote existing ones? Nat Rev Drug Discov 8:533–534
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Bals, L., Kneis, K.C., Lemke, C., Pedersen, T. (2013). Toward a Flexible Breathing Organization: R&D Outsourcing at Bayer . In: Pedersen, T., Bals, L., Ørberg Jensen, P., Larsen, M. (eds) The Offshoring Challenge. Springer, London. https://doi.org/10.1007/978-1-4471-4908-8_12
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4908-8_12
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4907-1
Online ISBN: 978-1-4471-4908-8
eBook Packages: EngineeringEngineering (R0)