Abstract
Peripheral nerve injuries lead to variable levels of functional loss depending on the extent of the injury. Despite the modern treatment methods, peripheral nerve regeneration is still a time-consuming process mainly because of the limited regeneration capacity of the nervous system. Unfortunately, attempts to increase the regeneration potential of the peripheral nervous system yielded a limited improvement. However, Tissue engineering emerged as a more promising tool to ease the traditionally laborious process of peripheral nerve regeneration. A tissue-engineered nerve is a combination of a biodegradable scaffold, a neurogenic cell line, and growth factors. The main focus of current research is to improve the cell–scaffold and scaffold–tissue interactions. Engineering a fully biocompatible and natural nerve-like nerve segment should be possible in the future with the improved understanding of biological mechanisms of nerve healing. This chapter provides a detailed look into the components of tissue-engineered nerve grafts along with a review of clinically relevant studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Peripheral nerves are prone to physical injuries due to their relatively superficial location in most parts of the body. The physical trauma usually comes in the form of transportation and construction accidents, natural disaster and war damage, and other trauma, as well as iatrogenic side effects of surgery. Approximately, 2.8 % of trauma patients suffer from an accompanying peripheral nerve injury, and the number of patients with upper extremity paralysis reaches up to 360,000 per annum in the USA. Therefore, peripheral nerve repair and regeneration have always been a popular and challenging topic of clinical research [1].
The first attempts to repair peripheral nerves by Galen date back to second century [2]. Sporadic descriptions of nerve coaptation sutures were reported later by Paul von Aegina in the seventh century and Rahzes and Avicenna in the ninth century [3]. The treatment techniques have evolved to a great extent over the following centuries; however, even with the most advanced techniques, peripheral nerve injuries often result in residual sensory and functional losses [3–5].
2 Classification of Peripheral Nerve Injuries
Peripheral nerve injuries are classified according to the Sunderland’s scale [6] which includes five degrees with an increasing severity of the injury: First-degree injury (also called neuropraxia) defines the injuries that cause a block in the action potential conduction, but axonal continuity is preserved. Complete healing is expected. Second-degree injury is associated with a loss in axonal continuity without damage to the surrounding glial and connective structures. Complete healing is expected since the regenerating axons can be properly oriented under the guidance of the original glial tubules in the distal nerve stump. Third-degree injuries include the endoneurial structures, and thus, although nerve continuity is maintained, the orientation of the regenerating axons to the proper target can be poorer than in second-degree lesions. Fourth-degree injuries refer to nerve injuries which cause the disruption of all nerve fibers and supporting structures except the epineurium. Regeneration can occur spontaneously; however, complete recovery is unlikely due to scar formation and improper orientation of regenerated axons. Finally, in fifth-degree injuries, complete nerve transection occurs. Healing is impossible unless nerve continuity is reconstructed surgically.
3 Conventional Treatment of Peripheral Nerve Injuries
Treatment for the simple cuts is direct cooptation, but severe injuries resulting in a wide gap require an autologous nerve graft or a nerve conduit to bridge the gap [7]. After the implantation of nerve graft, the axons within the graft are removed by phagocytes and Schwann cells with initial phagocytosis [4, 8]. In the next step, Schwann cells, which are responsible for the synthesis of the myelin sheath in the peripheral nerve tissue, proliferate and develop bands of Büngner. These are columns of cells lining the endoneurial tubes, eventually helping the regenerating axons to progress in the direction of denervated targets [5, 9].
Even though the autologous nerve grafting is currently the gold standard treatment for nerve defects, the drawbacks of the technique are as follows: limited donor tissue and donor site morbidity that may present as numbness and neuroma pain in the donor site [3, 4, 8]. Due to the limited regeneration capacity of human peripheral nerve tissue, the completion of the axonal regeneration is a time-consuming process even under ideal conditions, and a long period of rehabilitation is essential to obtain the maximum functional recovery [4, 5]. Functional recovery rates typically approach only 80 % for nerve injuries treated by autologous nerve grafts [1].
4 Tissue Engineering of Peripheral Nerves
Discovery of multipotent stem cells and the advancements in biomaterial engineering have enabled engineering of peripheral nerves in the laboratory medium. Tissue-engineered nerve grafts have attracted a large volume of interest as an alternative to autologous nerve grafting for the treatment for peripheral nerve defects considering the above-mentioned drawbacks of the latter technique. Similarly, to the other fields of regenerative medicine, peripheral nerve tissue engineering has raised great expectations within the general public, as well as in the scientific community, regarding its potential clinical application in the treatment of damaged nerves. However, in spite of the significant scientific advancements, clinical application of tissue-engineered nerve grafts is still very limited. To optimize the engineering strategy and accelerate the process of clinical translation, we should bring together the main pillars of tissue engineering which are as follows: scaffolds; growth factors, genes, and drugs; and support cells [8].
4.1 Nerve Scaffolds
4.1.1 Structure of Nerve Scaffolds
As mentioned above, the axons in the autologous nerve grafts do not integrate into the structure of the regenerated nerves, but they are degraded in the early steps of nerve regeneration, subsequently leaving a hallow lumen through which the regenerating axons progress distally. Therefore, using hallow nerve scaffolds instead of nerve grafts for bridging wide nerve gaps may help to decrease the donor site morbidity related with autologous nerve grafting [4, 8]. Figure 5.1 gives a summary of the structure of nerve scaffolds and the way that they can be used for peripheral nerve tissue engineering.
The types of neural scaffolds are shown on the left-hand side of the diagram, and the components of tissue-engineered nerve grafts are shown on the right-hand side. Adapted with permission from [1]
Scaffolds should be non-cytotoxic, non-immunogenic, non-allergenic, and non-carcinogenic as well as being sufficiently porous to allow the diffusion of nutrients while inhibiting the invasion of scar tissue [5, 10]. The fabrication technique (cutting holes on the wall, rolling of meshes, fiber spinning, adding a pore-forming agent, or injection molding followed by solvent evaporation) affects the permeability by altering the porous structure of neural scaffolds [11, 12]. Permeability of neural scaffold is also affected by the hydrophilic property of the scaffold material [1]. For a routine clinical application, the neural scaffold has to be easily fabricated, sterilized, and moreover has to satisfy many biomechanical and biological requirements such as biocompatibility and biodegradability [1]. On biomechanical side, a neural scaffold must be flexible to allow bending without kinking; too stiff scaffolds are easily dislocated, while too flexible scaffolds fail to provide sufficient mechanical support for axonal regeneration [1]. On biocompatibility side, the surface properties (including pH and surface charge) are the determining factors in terms of interactions between the neural scaffolds and nerve cells and the ability of the scaffold to blend in the implantation site and promote cell–substrate interaction [1, 10]. For example, the longitudinally oriented surface texture of the neural scaffold has been shown to help directional outgrowth of axons and uniform alignment of Schwann cells in vitro, resulting in improved nerve regeneration [1]. Similarly, multichannel neural scaffolds have greater surface areas for cell attachment and local release of growth factors, thus theoretically can support the nerve regeneration across a larger nerve gap. The data published thus far support the hypothesis that multichannel neural scaffolds reduce the dispersion of regenerating axons through the scaffold lumen; however, they displayed no significant benefit over single-lumen scaffolds [1, 13] probably because a complex multilayer internal structure may at the same time interfere with the permeability and flexibility of the neural scaffold [13].
4.1.2 Surface Properties of Nerve Scaffolds
In in vivo, the cells are located in three-dimensional microenvironments where they are surrounded by other cells and by the extracellular matrix, whose components, such as collagen, elastin, and laminin, are organized in nanostructures (i.e., fibers, triple helixes, etc.). This complex tissue network regulates the morphology, migration, and proliferation of Schwann cells, stimulates the release of nerve growth factors from Schwann cells, and also provides binding sites and directionality to the growing axons [14]. It is therefore essential to develop scaffolds that create synthetic microenvironments, providing 3D support, so as to control and direct the cellular behavior and to promote specific cell interactions [5].
Attempts to imitate the natural extracellular matrix by adding macromolecules (proteoglycans, collagen, elastin, laminin, fibronectin) to the internal environment of nerve scaffolds experienced a limited success [14–17]. However, nanomaterials provide a new dimension of interaction with biological systems that takes place on a subcellular level with a high degree of specificity [5, 18, 19]. For example, nanodiamond monolayers provide an excellent growth surface on various materials for functional neuronal networks and bypass the necessity of protein coating [20]. Furthermore, carbon nanotubes enhance nerve regeneration by rendering the scaffold more conductive [89].
The source of the scaffold is another aspect that effects the cell–scaffold and tissue–scaffold interactions. A scaffold may be derived from natural or synthetic materials [21].
4.1.3 Natural Nerve Scaffolds
Natural scaffolds are made from tissues that already exist in human body or from materials that exist naturally outside human body [5]. Natural biomaterials are attractive sources for nerve tissue engineering since they constitute cell-friendly matrices that stimulate adhesion, migration, growth, and proliferation of neurogenic cells. These materials also exhibit similar properties to the soft tissues that they are replacing, and they do not elicit foreign body reaction upon implantation and therefore have an excellent biocompatibility [1, 22]. The concerns about natural materials are as follows: the necessity of a careful purification process and inconsistent homogeneity of products between the lots [22].
-
Acellular Allogeneic or Xenogeneic Tissues
Acellular tissues from same or different species can be obtained by various physical, chemical, and enzymatic decellularization methods [23–25]. These methods aim to remove the immunogenic components and preserve the extracellular matrix components that are essential for the mechanical integrity of the tissue. Acellular tissues are especially useful in the repair of non-critical peripheral nerve gaps with a small length and diameter [1].
Acellular allogeneic and xenogeneic nerve tissues [25–32], tendon [33], and vein and muscle [34, 35] have been used by different groups in order to repair peripheral nerve defects. Avance1 (AxoGen Inc. Alachua, FL) is a commercially available acellular allogeneic nerve graft product which is fabricated from a donated cadaveric nerve through decellularization. Preliminary clinical applications of this product yielded favorable results [28]. However, the major concern about the use of acellular nerve scaffolds is the lack of axonal growth-stimulating bioactive components just like any other synthetic nerve scaffold. Recently, an increasing amount of effort has been directed toward incorporation of support cells or growth factors into the acellular allogeneic or xenogeneic-based neural scaffolds [30–32, 36]. The resultant tissue-engineered nerve grafts allow a better nerve regeneration than the acellular neural scaffolds alone.
-
Extracellular Matrix Components
Extracellular matrix is a network of proteins and fibers surrounding the cells that together form a complex 3-D structure crucial for proper biological functioning of the cells. Three main components of extracellular matrix, collagen, laminin, and fibronectin, have important roles in nerve regeneration.
Collagen has been extensively investigated as a potential scaffold for neural tissue engineering (Fig. 5.2) [37–41]. The properties of collagen scaffolds can be varied by using different concentrations of collagen or it can be denatured to gelatin that has also been used as a scaffold material [42]. The source of the collagen can be mammals such as rats, bovines, and humans; therefore, there is a risk of immune response if xenogeneic transplantation is used.
SEM views of a collagen nerve scaffold (Revolnerv® tube) with a smooth internal and external texture. Scale bars, 1 mm (a) and 100 μm (b). Adapted with permission from [37]
-
Chitin, Chitosan, and Other Natural Polysaccharides
Chitin is a natural polysaccharide that is commonly found in the outer shells of crustaceans, insect exoskeletons, and fungal cell walls. It is extensively used in biomedical applications. Chitosan is closely related to chitin and can be obtained from chitin via deactylation [22]. The molecular structure of chitosan has got a strong resemblance with the molecular structure of glycosaminoglycan; therefore, it can interact with the extracellular matrix molecules in a similar way. The favorable biological properties of chitin and chitosan made them useful materials for nerve tissue engineering [1, 43–46].
-
Silk Fibroin and Other Natural Molecules
Silk fibroin, keratins, and other matrix proteins extracted from human hair, wool, nail, and feather have been used as natural materials for the production of nerve scaffolds [47–49].
4.1.4 Synthetic Nerve Scaffolds
Synthetic scaffolds can be made from non-degradable materials such as silicone [50, 51] and poly-2-hydroxyethyl methacrylate-co-methyl methacrylate (PHEMA-MMA) [52] or from biodegradable polymers such as poly-3-hydroxybutyrate [53, 54], polyglycolic acid (PGA) [55, 56], poly L-lactic acid (PLA) [57, 58], poly-lactide-co-glycolide (PLGA) (Fig. 5.3) [59–61], and poly-lactide-co-caprolactone (PLC) [62, 63].
PLGA conduits (a) and structure of the scaffold under scanning electron microscopy (SEM) (b). Scale bar, 100 μm. Adapted with permission from [61]
The main advantage of synthetic nerve scaffolds is their tunable chemical and physical properties. However, their incompatibility with cell adhesion and tissue integration poses a challenge to nerve tissue engineering. Therefore, synthetic materials are often chemically modified to render them more biocompatible [1].
-
Non-degradable Synthetic Materials
The early neural scaffolds were made of non-degradable synthetic materials such as silicone, rubber, acrylic polymer, polyethylene, elastomer, etc. Even though they achieved variable levels of success, their clinical use is currently limited because of the long-term complications such as chronic nerve compression, chronic foreign body reaction, and the necessity of a second surgical procedure to remove the scaffold [64, 65].
-
Biodegradable Synthetic Materials
As the concerns rose over the use of non-degradable synthetic materials as nerve scaffolds, biodegradable synthetic scaffolds, which degrade within a reasonable time span after implantation, were developed. Ideally, the neural scaffolds should remain intact throughout the axon regeneration and then gradually degrade with minimal swelling and foreign body reaction [1]. In case of a 10-mm gap of the rat sciatic nerve, the minimum time period that a biodegradable nerve scaffold must remain intact for sufficient nerve regeneration is 3 months [5]. Both the premature and delayed degradation of nerve scaffold might result in increased scar formation that further delays nerve regeneration [5, 66].
Further chemical modifications can be used to increase the biocompatibility of degradable scaffolds via adding sites for cell or extracellular matrix adhesion to allow cells to infiltrate into the scaffold lumen (Fig. 5.4) [19, 67, 68].
SEM image shows Schwann cells on PCL/gelatin nanofibrous scaffolds after 12 days of coculture. Nanofibers encourage the attachment of Schwann cells. Scale bar, 200 μm. Adapted with permission from [19]
4.2 Growth Factors
Growth factors act in coordination with extracellular matrix to control the survival, proliferation, migration, and differentiation of various cell types involved in the nerve regeneration [69]. Incorporation of growth factors, such as nerve growth factor, glial cell line-derived neurotrophic factor, and brain-derived neurotrophic factor (BDNF), into the tissue-engineered nerve grafts has been actively attempted in order to avoid the possible side effects and morbidity associated with cellular therapy [70–76]. However, growth factor therapy has fallen short of expectations because of the unpredictable side effects, unknown optimal dosage, and short half-life of the growth factors [5, 74, 75, 77].
4.3 Cell Sources for Peripheral Nerve Tissue Engineering
Due to the in vivo inefficiency of growth factors, some researchers implanted cells into the lumen of neural scaffolds in an attempt to provide a continuous supply of growth factors to the nerve defect area [35, 78–81]. Moreover, engineering a complete peripheral nerve is not possible without the in vitro culture and seeding of cells onto the nerve scaffolds. The major cell types used were as follows: Schwann cells, embryonic stem cells (ESCs), neural stem cells (NSCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs) (Table 5.1).
These cell lines could be implanted into the scaffolds via direct injection or coculturing methods before or after in vitro differentiation [33, 82]. The markers for successful neural differentiation are as follows: GFAP, p75NGFR, and S-100 for Schwann cells [83–86] and nestin and NeuN [84, 87] for neural progenitor cells.
-
Schwann Cells
Schwann cells can be obtained from allogeneic, syngeneic, or autologous sources. Their function is to create a suitable environment for axonal growth by expressing cell adhesion molecules, secreting nerve growth factors, and forming an endoneurial myelin sheath that acts as a guide for the regenerating axons [3, 34]. This central role of Schwann cells in peripheral nerve regeneration made them the most commonly used cell type for experimental nerve tissue engineering applications. Schwann cell-enriched nerve scaffolds improved both the quality and rate of axonal regeneration in rat sciatic nerve defect model [88, 89]. However, among the obstacles in front of their clinical use are; suboptimal attachment of Schwann cells to the nerve scaffolds and the difficulties in obtaining and in vitro expansion of autologous Schwann cells [1, 81].
-
ESCs
As an alternative to Schwann cells, ESCs can easily be expanded and have a great potential to proliferate and differentiate into neurons under various protocols (Fig. 5.5) [87, 90]. However, the ethical concerns on the use of ESCs for clinical applications limit their use.
Phenotype characterization of ESCs differentiated into neural progenitors. Confocal fluorescence microscopy imaging shows the cell phenotype 3 days after differentiation induction. Images demonstrate double staining of NeuN (red) (a) and S-100 (green) (b) in neural progenitor cells differentiated from ESCs. Merged image demonstrates the positivity of some of the cells for both NeuN and S-100, implying that these neural progenitors can further differentiate into either neuronal or glia/Schwann cells (c). A close-up view is seen in pane (d). Scale bars, 40 μm (a, b) and 20 μm (c, d). Adapted with permission from [87]
-
NSCs
Neural stem cells, just like ESCs, are multipotent, highly mobile, and can easily be isolated and cultured in vitro. These properties make NSCs an attractive alternative source of support cells for nerve tissue engineering [91–93].
-
MSCs
MSCs from various adult tissues (bone marrow, adipose tissue, etc.) became the subject of interest because of various advantages over the other cell lines. It is relatively easily to obtain MSCs through minimally invasive procedures such as the aspiration of the bone marrow or liposuction. MSCs can easily be expanded in a large scale by in vitro culture [94, 95].
Even though they do not possess the wide range of differentiation of ESCs, adult MSCs are multipotent, secrete growth factors and other soluble mediators, and moreover can serve as a vehicle for drug delivery and gene therapy [96]. Several in vitro studies have shown that MSCs can be induced to differentiate into neural lineages including Schwann cell-like cells (Fig. 5.6) [85, 97–101]; however, MSCs promote nerve regeneration not only by direct differentiation but also via spontaneous fusion with host cells and possibly by secreting growth factors [102, 103].
Human MSCs can be differentiated into Schwann cell-like cells. This picture shows immunofluorescence staining for S100, P0, p75NGFR, GFAP, L1, O4 in human MSCs (a–f), and human MSC-derived Schwann cells (g–l). The untreated human MSCs slightly expressed S100 (a) but were negative for other Schwann cell markers before induction (b–f). However, after the induction, cells became positive for all the other Schwann cell markers and exhibited an increased immunoreactivity for S100 (g). Macroscopic view of the tissue within the nerve scaffold seeded with MSC-derived Schwann 3 weeks after transplantation of the scaffold into a rat sciatic nerve defect (black arrows) (m). Neurofilament-positive nerve fibers (red) observed in the newly formed tissue in the scaffold, white arrows mark the coaptation sites (n). Scale bar, 100 μm. Adapted with permission from [101]
-
iPSCs
The latest and maybe the most significant advancement in stem cell field is the reprogramming of adult somatic cells (e.g., skin fibroblasts) into pluripotent stem cells by the introduction of genes Oct3/4, Sox2, c-Myc, and KLF4 [104]. These cells are named iPSCs, and since they are derived from somatic cells, they bypass the immune system of the host. Similar to ESCs, iPSCs possess an unlimited expansion potential, and they can be differentiated into almost every tissue in human body yet without any of the ethical concerns surrounding ESCs (Fig. 5.7). However, iPSCs require a significantly more genetic manipulation than any other stem cell type during the induction process that subsequently leads to some safety concerns in clinical application [105].
In vitro differentiation of iPSC into Schwann cells. Differentiated cells are positive for GFAP (a) and S100b (b). Scale bar, 100 μm. Adapted with permission from [105]
4.4 Toward Clinical Applications
The repair of critical-sized rat sciatic nerve defects by tissue-engineered nerve grafts has been the experiment model that supplied the major percentage of preclinical data on in vivo application of peripheral nerve tissue engineering. Studies with different cell lines and scaffolds yielded similar results [34, 36, 80, 81, 84, 86, 106, 107]. Even though it constitutes a strong background for clinical translation, the differences between the biology’s of small animals and human beings should always be considered when interpreting the preclinical data.
Wakao et al. used a cynomolgus monkey peripheral nervous system injury model to examine the safety and efficacy of bone marrow MSCs differentiated into Schwann cells as a cell source for peripheral nerve tissue engineering [108]. Differentiated MSCs were seeded onto a collagen sponge at a concentration of 2 × 106 cells, and collagen sponge was placed into the lumen of a PLC scaffold to repair a 20-mm median nerve defect. For the evaluation, in addition to other techniques, they have performed 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) scanning for in vivo tracking of the injected cells. No abnormal accumulation of radioactivity except in regions with expected physiological accumulation was observed excluding a possible neoplastic transformation of the injected cells.
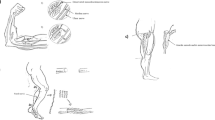
Hu et al. used rhesus monkey for two different experiments. In the first experiment, acellular allogeneic nerve segments were seeded either with autologous bone marrow MSCs or autologous Schwann cells to repair a 40 mm defect in the ulnar nerve of rhesus monkeys [30]. The concentration of the cells was 2 × 107 cells per graft, and the recovery with the MSC-seeded allografts was similar to that observed with Schwann cell-seeded allografts and autologous nerve grafts. This study demonstrated that MSCs could be a solid alternative to Schwann cells as a cell source for peripheral nerve tissue engineering given the difficulties in purifying sufficient quantities of Schwann cells for peripheral nerve regeneration. In the second study, they used chitosan/PLGA-based, autologous marrow MSC-containing tissue-engineered nerve grafts for bridging a 50 mm long median nerve defect in rhesus monkeys (Fig. 5.8) [30]. They injected 1 × 108 cells per ml of suspension. At 12 months after grafting, locomotive activity observation, electrophysiological assessments, and gold retrograde tracing tests were carried out, and the recovery of nerve function by tissue-engineered nerve was found to be more efficient than the one by scaffold alone. In addition, the authors performed a safety evaluation of MSC-based therapies. Blood test and histopathological examination demonstrated that tissue-engineered nerve graft could be safely used in the primate body. These two studies were milestone studies toward the clinical application, considering the striking similarity in the anatomy and function of forearm nerves between human and monkey hands.
Intra-operative views of the repair of a median nerve defect in a monkey forearm. A 5-cm median nerve segment was excised following the dissection of the nerve (a). The defect was bridged by a chitosan/PLGA scaffold injected with an autologous BMSC suspension (b). Macroscopic view of the regenerated nerve at 12 months after the operation. The white arrows mark the proximal and distal coaptation sites, respectively (c). Histological examination confirmed the nerve regeneration at 12 months: Meyer trichrome staining of transverse and longitudinal nerve sections depicts the myelinated axons within the regenerated nerve (myelin seen in red) (d, e); double immunostaining with anti-neurofilament 200 (green color) and anti-S100 (red color) of transverse nerve segments shows neurofilament-200-positive axons encircled by S-100-positive myelin (f). Scale bars, 20 μm. Transmission electron micrographs show myelin around the axons as dense black circles (g). Scale bar, 5 μm. Adapted with permission from [78]
Bone marrow MSCs seeded on a PLGA scaffold was used to repair a dog sciatic nerve defect in two different studies [107, 109]. Both of these studies demonstrated that repair of the peripheral nerve defects with tissue-engineered nerve grafts yielded similar results with autologous nerve graft repair.
The data obtained from the experiments using bigger animal models are more clinically relevant. The results of these experiments will definitely help to determine the guidelines of human applications of tissue-engineered nerve grafts. Nevertheless, more studies are warranted to find the appropriate cell type and number of cells for peripheral nerve tissue engineering and possible side effects of cellular treatment.
Among the preclinical studies, another one that deserves some emphasis is a recent study that reported in vivo transplantation of differentiated adipose-derived stem cells on a three-dimensional nerve scaffold composed of fibroblasts. This is an important study in terms of omitting the peripheral nerve tissue engineering problems related to scaffolds such as cell adhesion and biocompatibility [83].
4.5 Conclusion and Future Prospects
Unpredictable outcomes and morbidity associated with the traditional methods of treatment of nerve defects encouraged surgeons to consider alternative methods for peripheral nerve repair. Tissue engineering has recently emerged as a useful tool for the treatment for a variety of diseases that were previously known to be untreatable. The combination of bioengineered scaffolds and multipotent/pluripotent stem cells hold a great potential toward peripheral nerve tissue engineering. However, the clinical translation of peripheral nerve tissue engineering is a delicate process that should proceed toward realistic expectations following a set of carefully determined guidelines. Further refinement of the available techniques is required for routine, safe, and efficient clinical application.
Even though the volume of the experimental data is encouraging, the clinical success of peripheral nerve tissue engineering depends on the controlled regulation of cell behavior and tissue progression in synthetic nerve scaffolds. To achieve this goal, 3D imitation of the extracellular matrix structure and a sophisticated cell–extracellular matrix interaction are crucial. In this regard, incorporation of nanofibers into the scaffold had greatly enhanced the biocompatibility of scaffolds. Altogether, addition of cells and growth factors into the lumens of scaffolds and surface modifications should help the scaffolds to mimic the natural conditions and improve the outcomes of surgical repair of peripheral nerve defects.
References
Gu X, Ding F, Yang Y, Liu J (2011) Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol 93(2):204–230. doi:10.1016/j.pneurobio.2010.11.002
Terzis JK, Sun DD, Thanos PK (1997) Historical and basic science review: past, present, and future of nerve repair. J Reconstr Microsurg 13(3):215–225. doi:10.1055/s-2007-1006407
Raimondo S, Fornaro M, Tos P, Battiston B, Giacobini-Robecchi MG, Geuna S (2011) Perspectives in regeneration and tissue engineering of peripheral nerves. Ann Anat 193(4):334–340. doi:10.1016/j.aanat.2011.03.001
Sinis N, Kraus A, Drakotos D, Doser M, Schlosshauer B, Muller HW, Skouras E, Bruck JC, Werdin F (2011) Bioartificial reconstruction of peripheral nerves using the rat median nerve model. Ann Anat 193(4):341–346. doi:10.1016/j.aanat.2011.02.018
Sedaghati T, Yang SY, Mosahebi A, Alavijeh MS, Seifalian AM (2011) Nerve regeneration with aid of nanotechnology and cellular engineering. Biotechnol Appl Biochem 58(5):288–300. doi:10.1002/bab.51
Sunderland S (1951) A classification of peripheral nerve injuries producing loss of function. Brain 74(4):491–516
Millesi H, Meissl G, Berger A (1972) The interfascicular nerve-grafting of the median and ulnar nerves. J Bone Joint Surg Am 54(4):727–750
Battiston B, Raimondo S, Tos P, Gaidano V, Audisio C, Scevola A, Perroteau I, Geuna S (2009) Chapter 11: tissue engineering of peripheral nerves. Int Rev Neurobiol 87:227–249. doi:10.1016/s0074-7742(09)87011-6
Geuna S, Raimondo S, Ronchi G, Di Scipio F, Tos P, Czaja K, Fornaro M (2009) Chapter 3: histology of the peripheral nerve and changes occurring during nerve regeneration. Int Rev Neurobiol 87:27–46. doi:10.1016/s0074-7742(09)87003-7
Cunha C, Panseri S, Antonini S (2011) Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomedicine 7(1):50–59. doi:10.1016/j.nano.2010.07.004
Dellon AL, Mackinnon SE (1988) An alternative to the classical nerve graft for the management of the short nerve gap. Plast Reconstr Surg 82(5):849–856
Rodriguez FJ, Gomez N, Perego G, Navarro X (1999) Highly permeable polylactide-caprolactone nerve guides enhance peripheral nerve regeneration through long gaps. Biomaterials 20(16):1489–1500
de Ruiter GC, Spinner RJ, Malessy MJ, Moore MJ, Sorenson EJ, Currier BL, Yaszemski MJ, Windebank AJ (2008) Accuracy of motor axon regeneration across autograft, single-lumen, and multichannel poly (lactic-co-glycolic acid) nerve tubes. Neurosurgery 63(1):144–153. doi:10.1227/01.NEU.0000335081.47352.78 (discussion 153–145)
Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ (2007) ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng 13(12):2863–2870. doi:10.1089/ten.2007.0055
Whitworth IH, Brown RA, Dore C, Green CJ, Terenghi G (1995) Orientated mats of fibronectin as a conduit material for use in peripheral nerve repair. J Hand Surg Br 20(4):429–436
Plow EF, Haas TA, Zhang L, Loftus J, Smith JW (2000) Ligand binding to integrins. J Biol Chem 275(29):21785–21788. doi:10.1074/jbc.R000003200
Massia SP, Hubbell JA (1991) An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J Cell Biol 114(5):1089–1100
Behan BL, DeWitt DG, Bogdanowicz DR, Koppes AN, Bale SS, Thompson DM (2011) Single-walled carbon nanotubes alter Schwann cell behavior differentially within 2D and 3D environments. J Biomed Mater Res A 96(1):46–57. doi:10.1002/jbm.a.32939
Gupta D, Venugopal J, Prabhakaran MP, Dev VR, Low S, Choon AT, Ramakrishna S (2009) Aligned and random nanofibrous substrate for the in vitro culture of Schwann cells for neural tissue engineering. Acta Biomater 5(7):2560–2569. doi:10.1016/j.actbio.2009.01.039
Thalhammer A, Edgington RJ, Cingolani LA, Schoepfer R, Jackman RB (2010) The use of nanodiamond monolayer coatings to promote the formation of functional neuronal networks. Biomaterials 31(8):2097–2104. doi:10.1016/j.biomaterials.2009.11.109
Siemionow M, Bozkurt M, Zor F (2010) Regeneration and repair of peripheral nerves with different biomaterials: review. Microsurgery 30(7):574–588. doi:10.1002/micr.20799
Willerth SM, Sakiyama-Elbert SE (2007) Approaches to neural tissue engineering using scaffolds for drug delivery. Adv Drug Deliv Rev 59(4–5):325–338. doi:10.1016/j.addr.2007.03.014
Gilbert TW, Sellaro TL, Badylak SF (2006) Decellularization of tissues and organs. Biomaterials 27(19):3675–3683. doi:10.1016/j.biomaterials.2006.02.014
Hiles RW (1972) Freeze dried irradiated nerve homograft: a preliminary report. Hand 4(1):79–84
Sondell M, Lundborg G, Kanje M (1998) Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res 795(1–2):44–54
Yang LM, Liu XL, Zhu QT, Zhang Y, Xi TF, Hu J, He CF, Jiang L (2011) Human peripheral nerve-derived scaffold for tissue-engineered nerve grafts: histology and biocompatibility analysis. J Biomed Mater Res B Appl Biomater 96(1):25–33. doi:10.1002/jbm.b.31719
Wang D, Liu XL, Zhu JK, Jiang L, Hu J, Zhang Y, Yang LM, Wang HG, Yi JH (2008) Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res 1188:44–53. doi:10.1016/j.brainres.2007.09.098
Karabekmez FE, Duymaz A, Moran SL (2009) Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y) 4(3):245–249. doi:10.1007/s11552-009-9195-6
Jia H, Wang Y, Tong XJ, Liu GB, Li Q, Zhang LX, Sun XH (2012) Sciatic nerve repair by acellular nerve xenografts implanted with BMSCs in rats xenograft combined with BMSCs. Synapse 66(3):256–269. doi:10.1002/syn.21508
Hu J, Zhu QT, Liu XL, Xu YB, Zhu JK (2007) Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol 204(2):658–666. doi:10.1016/j.expneurol.2006.11.018
Hess JR, Brenner MJ, Fox IK, Nichols CM, Myckatyn TM, Hunter DA, Rickman SR, Mackinnon SE (2007) Use of cold-preserved allografts seeded with autologous Schwann cells in the treatment of a long-gap peripheral nerve injury. Plast Reconstr Surg 119(1):246–259. doi:10.1097/01.prs.0000245341.71666.97
Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, Xu J, Yang W, Fan X, Du B, Gao P, Hu G, Jin Y (2010) A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials 31(20):5312–5324. doi:10.1016/j.biomaterials.2010.03.029
Nishiura Y, Brandt J, Nilsson A, Kanje M, Dahlin LB (2004) Addition of cultured Schwann cells to tendon autografts and freeze-thawed muscle grafts improves peripheral nerve regeneration. Tissue Eng 10(1–2):157–164. doi:10.1089/107632704322791808
Fansa H, Keilhoff G, Wolf G, Schneider W (2001) Tissue engineering of peripheral nerves: a comparison of venous and acellular muscle grafts with cultured Schwann cells. Plast Reconstr Surg 107(2):485–494 (discussion 495–486)
Hassan NH, Sulong AF, Ng MH, Htwe O, Idrus RB, Roohi S, Naicker AS, Abdullah S (2012) Neural-differentiated mesenchymal stem cells incorporated into muscle stuffed vein scaffold forms a stable living nerve conduit. J Orthop Res 30(10):1674–1681. doi:10.1002/jor.22102
Frerichs O, Fansa H, Schicht C, Wolf G, Schneider W, Keilhoff G (2002) Reconstruction of peripheral nerves using acellular nerve grafts with implanted cultured Schwann cells. Microsurgery 22(7):311–315. doi:10.1002/micr.10056
Alluin O, Wittmann C, Marqueste T, Chabas JF, Garcia S, Lavaut MN, Guinard D, Feron F, Decherchi P (2009) Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials 30(3):363–373. doi:10.1016/j.biomaterials.2008.09.043
Archibald SJ, Shefner J, Krarup C, Madison RD (1995) Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J Neurosci 15(5 Pt 2):4109–4123
Farole A, Jamal BT (2008) A bioabsorbable collagen nerve cuff (NeuraGen) for repair of lingual and inferior alveolar nerve injuries: a case series. J Oral Maxillofac Surg 66(10):2058–2062. doi:10.1016/j.joms.2008.06.017
Goto E, Mukozawa M, Mori H, Hara M (2010) A rolled sheet of collagen gel with cultured Schwann cells: model of nerve conduit to enhance neurite growth. J Biosci Bioeng 109(5):512–518. doi:10.1016/j.jbiosc.2009.11.002
Suri S, Schmidt CE (2010) Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng Part A 16(5):1703–1716. doi:10.1089/ten.tea.2009.0381
Gamez E, Goto Y, Nagata K, Iwaki T, Sasaki T, Matsuda T (2004) Photofabricated gelatin-based nerve conduits: nerve tissue regeneration potentials. Cell Transplant 13(5):549–564
Freier T, Montenegro R, Shan Koh H, Shoichet MS (2005) Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 26(22):4624–4632. doi:10.1016/j.biomaterials.2004.11.040
He Q, Zhang T, Yang Y, Ding F (2009) In vitro biocompatibility of chitosan-based materials to primary culture of hippocampal neurons. J Mater Sci Mater Med 20(7):1457–1466. doi:10.1007/s10856-009-3702-8
Itoh S, Suzuki M, Yamaguchi I, Takakuda K, Kobayashi H, Shinomiya K, Tanaka J (2003) Development of a nerve scaffold using a tendon chitosan tube. Artif Organs 27(12):1079–1088
Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS (2008) Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26(1):1–21. doi:10.1016/j.biotechadv.2007.07.009
Tang X, Ding F, Yang Y, Hu N, Wu H, Gu X (2009) Evaluation on in vitro biocompatibility of silk fibroin-based biomaterials with primarily cultured hippocampal neurons. J Biomed Mater Res A 91(1):166–174. doi:10.1002/jbm.a.32212
Yang Y, Chen X, Ding F, Zhang P, Liu J, Gu X (2007) Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 28(9):1643–1652. doi:10.1016/j.biomaterials.2006.12.004
Apel PJ, Garrett JP, Sierpinski P, Ma J, Atala A, Smith TL, Koman LA, Van Dyke ME (2008) Peripheral nerve regeneration using a keratin-based scaffold: long-term functional and histological outcomes in a mouse model. J Hand Surg Am 33(9):1541–1547. doi:10.1016/j.jhsa.2008.05.034
Kim SM, Lee SK, Lee JH (2007) Peripheral nerve regeneration using a three dimensionally cultured schwann cell conduit. J Craniofac Surg 18(3):475–488. doi:10.1097/01.scs.0000249362.41170.f3
Lundborg G, Dahlin LB, Danielsen N (1991) Ulnar nerve repair by the silicone chamber technique. Case report. Scand J Plast Reconstr Surg Hand Surg 25(1):79–82
Belkas JS, Munro CA, Shoichet MS, Johnston M, Midha R (2005) Long-term in vivo biomechanical properties and biocompatibility of poly (2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials 26(14):1741–1749. doi:10.1016/j.biomaterials.2004.05.031
Kalbermatten DF, Pettersson J, Kingham PJ, Pierer G, Wiberg M, Terenghi G (2009) New fibrin conduit for peripheral nerve repair. J Reconstr Microsurg 25(1):27–33. doi:10.1055/s-0028-1090619
Mosahebi A, Wiberg M, Terenghi G (2003) Addition of fibronectin to alginate matrix improves peripheral nerve regeneration in tissue-engineered conduits. Tissue Eng 9(2):209–218. doi:10.1089/107632703764664684
Rosson GD, Williams EH, Dellon AL (2009) Motor nerve regeneration across a conduit. Microsurgery 29(2):107–114. doi:10.1002/micr.20580
Waitayawinyu T, Parisi DM, Miller B, Luria S, Morton HJ, Chin SH, Trumble TE (2007) A comparison of polyglycolic acid versus type 1 collagen bioabsorbable nerve conduits in a rat model: an alternative to autografting. J Hand Surg Am 32(10):1521–1529. doi:10.1016/j.jhsa.2007.07.015
Pierucci A, Duek EA, de Oliveira AL (2009) Expression of basal lamina components by Schwann cells cultured on poly (lactic acid) (PLLA) and poly (caprolactone) (PCL) membranes. J Mater Sci Mater Med 20(2):489–495. doi:10.1007/s10856-008-3614-z
Wang HB, Mullins ME, Cregg JM, Hurtado A, Oudega M, Trombley MT, Gilbert RJ (2009) Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J Neural Eng 6(1):016001. doi:10.1088/1741-2560/6/1/016001
Bryan DJ, Tang JB, Holway AH, Rieger-Christ KM, Trantolo DJ, Wise DL, Summerhayes IC (2003) Enhanced peripheral nerve regeneration elicited by cell-mediated events delivered via a bioresorbable PLGA guide. J Reconstr Microsurg 19(2):125–134. doi:10.1055/s-2003-37820
Bini TB, Gao S, Xu X, Wang S, Ramakrishna S, Leong KW (2004) Peripheral nerve regeneration by microbraided poly (L-lactide-co-glycolide) biodegradable polymer fibers. J Biomed Mater Res A 68(2):286–295. doi:10.1002/jbm.a.20050
Li BC, Jiao SS, Xu C, You H, Chen JM (2010) PLGA conduit seeded with olfactory ensheathing cells for bridging sciatic nerve defect of rats. J Biomed Mater Res A 94(3):769–780. doi:10.1002/jbm.a.32727
Meek MF, Jansen K (2009) Two years after in vivo implantation of poly (DL-lactide-epsilon-caprolactone) nerve guides: has the material finally resorbed? J Biomed Mater Res A 89(3):734–738. doi:10.1002/jbm.a.32024
Mligiliche NL, Tabata Y, Kitada M, Endoh K, Okamato K, Fujimoto E, Ide C (2003) Poly lactic acid–caprolactone copolymer tube with a denatured skeletal muscle segment inside as a guide for peripheral nerve regeneration: a morphological and electrophysiological evaluation of the regenerated nerves. Anat Sci Int 78(3):156–161. doi:10.1046/j.0022-7722.2003.00056.x
Deng M, Chen G, Burkley D, Zhou J, Jamiolkowski D, Xu Y, Vetrecin R (2008) A study on in vitro degradation behavior of a poly (glycolide-co-L-lactide) monofilament. Acta Biomater 4(5):1382–1391. doi:10.1016/j.actbio.2008.03.011
Braga-Silva J (1999) The use of silicone tubing in the late repair of the median and ulnar nerves in the forearm. J Hand Surg Br 24(6):703–706. doi:10.1054/jhsb.1999.0276
den Dunnen WF, van der Lei B, Robinson PH, Holwerda A, Pennings AJ, Schakenraad JM (1995) Biological performance of a degradable poly (lactic acid-epsilon-caprolactone) nerve guide: influence of tube dimensions. J Biomed Mater Res 29(6):757–766. doi:10.1002/jbm.820290612
Elbert DL, Hubbell JA (2001) Conjugate addition reactions combined with free-radical cross-linking for the design of materials for tissue engineering. Biomacromolecules 2(2):430–441
Groll J, Fiedler J, Engelhard E, Ameringer T, Tugulu S, Klok HA, Brenner RE, Moeller M (2005) A novel star PEG-derived surface coating for specific cell adhesion. J Biomed Mater Res A 74(4):607–617. doi:10.1002/jbm.a.30335
Terenghi G (1999) Peripheral nerve regeneration and neurotrophic factors. J Anat 194(Pt 1):1–14
Ho PR, Coan GM, Cheng ET, Niell C, Tarn DM, Zhou H, Sierra D, Terris DJ (1998) Repair with collagen tubules linked with brain-derived neurotrophic factor and ciliary neurotrophic factor in a rat sciatic nerve injury model. Arch Otolaryngol Head Neck Surg 124(7):761–766
Kokai LE, Bourbeau D, Weber D, McAtee J, Marra KG (2011) Sustained growth factor delivery promotes axonal regeneration in long gap peripheral nerve repair. Tissue Eng Part A 17(9–10):1263–1275. doi:10.1089/ten.TEA.2010.0507
Sun H, Xu F, Guo D, Yu H (2012) Preparation and evaluation of NGF-microsphere conduits for regeneration of defective nerves. Neurol Res 34(5):491–497. doi:10.1179/1743132812y.0000000037
Madduri S, di Summa P, Papaloizos M, Kalbermatten D, Gander B (2010) Effect of controlled co-delivery of synergistic neurotrophic factors on early nerve regeneration in rats. Biomaterials 31(32):8402–8409. doi:10.1016/j.biomaterials.2010.07.052
Lee AC, Yu VM, Lowe JB 3rd, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE (2003) Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol 184(1):295–303
Lin YC, Ramadan M, Hronik-Tupaj M, Kaplan DL, Philips BJ, Sivak W, Rubin JP, Marra KG (2011) Spatially controlled delivery of neurotrophic factors in silk fibroin-based nerve conduits for peripheral nerve repair. Ann Plast Surg 67(2):147–155. doi:10.1097/SAP.0b013e3182240346
Fu KY, Dai LG, Chiu IM, Chen JR, Hsu SH (2011) Sciatic nerve regeneration by microporous nerve conduits seeded with glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor gene transfected neural stem cells. Artif Organs 35(4):363–372. doi:10.1111/j.1525-1594.2010.01105.x
Madduri S, Gander B (2012) Growth factor delivery systems and repair strategies for damaged peripheral nerves. J Control Release 161(2):274–282. doi:10.1016/j.jconrel.2011.11.036
Hu N, Wu H, Xue C, Gong Y, Wu J, Xiao Z, Yang Y, Ding F, Gu X (2013) Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials 34(1):100–111. doi:10.1016/j.biomaterials.2012.09.020
Hou SY, Zhang HY, Quan DP, Liu XL, Zhu JK (2006) Tissue-engineered peripheral nerve grafting by differentiated bone marrow stromal cells. Neuroscience 140(1):101–110. doi:10.1016/j.neuroscience.2006.01.066
Erba P, Mantovani C, Kalbermatten DF, Pierer G, Terenghi G, Kingham PJ (2010) Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg 63(12):e811–e817. doi:10.1016/j.bjps.2010.08.013
Fansa H, Dodic T, Wolf G, Schneider W, Keilhoff G (2003) Tissue engineering of peripheral nerves: epineurial grafts with application of cultured Schwann cells. Microsurgery 23(1):72–77. doi:10.1002/micr.10081
Komiyama T, Nakao Y, Toyama Y, Vacanti CA, Vacanti MP, Ignotz RA (2004) Novel technique for peripheral nerve reconstruction in the absence of an artificial conduit. J Neurosci Methods 134(2):133–140. doi:10.1016/j.jneumeth.2003.11.020
Adams AM, Arruda EM, Larkin LM (2012) Use of adipose-derived stem cells to fabricate scaffoldless tissue-engineered neural conduits in vitro. Neuroscience 201:349–356. doi:10.1016/j.neuroscience.2011.11.004
Ao Q, Fung CK, Tsui AY, Cai S, Zuo HC, Chan YS, Shum DK (2011) The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials 32(3):787–796. doi:10.1016/j.biomaterials.2010.09.046
Chen X, Wang XD, Chen G, Lin WW, Yao J, Gu XS (2006) Study of in vivo differentiation of rat bone marrow stromal cells into schwann cell-like cells. Microsurgery 26(2):111–115. doi:10.1002/micr.20184
Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H (2001) Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci 14(11):1771–1776
Cui L, Jiang J, Wei L, Zhou X, Fraser JL, Snider BJ, Yu SP (2008) Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells 26(5):1356–1365. doi:10.1634/stemcells.2007-0333
Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP (2000) A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng 6(2):119–127. doi:10.1089/107632700320748
Mosahebi A, Woodward B, Wiberg M, Martin R, Terenghi G (2001) Retroviral labeling of Schwann cells: in vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia 34(1):8–17
Craff MN, Zeballos JL, Johnson TS, Ranka MP, Howard R, Motarjem P, Randolph MA, Winograd JM (2007) Embryonic stem cell-derived motor neurons preserve muscle after peripheral nerve injury. Plast Reconstr Surg 119(1):235–245. doi:10.1097/01.prs.0000244863.71080.f0
Alessandri G, Emanueli C, Madeddu P (2004) Genetically engineered stem cell therapy for tissue regeneration. Ann N Y Acad Sci 1015:271–284. doi:10.1196/annals.1302.023
Guo BF, Dong MM (2009) Application of neural stem cells in tissue-engineered artificial nerve. Otolaryngol Head Neck Surg 140(2):159–164. doi:10.1016/j.otohns.2008.10.039
Hsu SH, Su CH, Chiu IM (2009) A novel approach to align adult neural stem cells on micropatterned conduits for peripheral nerve regeneration: a feasibility study. Artif Organs 33(1):26–35. doi:10.1111/j.1525-1594.2008.00671.x
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295. doi:10.1091/mbc.E02-02-0105
Mosna F, Sensebe L, Krampera M (2010) Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev 19(10):1449–1470. doi:10.1089/scd.2010.0140
Horwitz EM, Dominici M (2008) How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy 10(8):771–774. doi:10.1080/14653240802618085
Lu J, Moochhala S, Moore XL, Ng KC, Tan MH, Lee LK, He B, Wong MC, Ling EA (2006) Adult bone marrow cells differentiate into neural phenotypes and improve functional recovery in rats following traumatic brain injury. Neurosci Lett 398(1–2):12–17. doi:10.1016/j.neulet.2005.12.053
Munoz-Elias G, Woodbury D, Black IB (2003) Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor functions. Stem Cells 21(4):437–448. doi:10.1634/stemcells.21-4-437
Suzuki H, Taguchi T, Tanaka H, Kataoka H, Li Z, Muramatsu K, Gondo T, Kawai S (2004) Neurospheres induced from bone marrow stromal cells are multipotent for differentiation into neuron, astrocyte, and oligodendrocyte phenotypes. Biochem Biophys Res Commun 322(3):918–922. doi:10.1016/j.bbrc.2004.07.201
Orbay H, Uysal AC, Hyakusoku H, Mizuno H (2012) Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg 65(5):657–664. doi:10.1016/j.bjps.2011.11.035
Shimizu S, Kitada M, Ishikawa H, Itokazu Y, Wakao S, Dezawa M (2007) Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Commun 359(4):915–920. doi:10.1016/j.bbrc.2007.05.212
Weimann JM, Charlton CA, Brazelton TR, Hackman RC, Blau HM (2003) Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc Natl Acad Sci U S A 100(4):2088–2093. doi:10.1073/pnas.0337659100
Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, Wang CC, Wang WY, Huang YS, Hsu SH (2007) Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol 204(1):443–453. doi:10.1016/j.expneurol.2006.12.004
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. doi:10.1016/j.cell.2007.11.019
Wang A, Tang Z, Park IH, Zhu Y, Patel S, Daley GQ, Li S (2011) Induced pluripotent stem cells for neural tissue engineering. Biomaterials 32(22):5023–5032. doi:10.1016/j.biomaterials.2011.03.070
Blais M, Grenier M, Berthod F (2009) Improvement of nerve regeneration in tissue-engineered skin enriched with schwann cells. J Invest Dermatol 129(12):2895–2900. doi:10.1038/jid.2009.159
Ding F, Wu J, Yang Y, Hu W, Zhu Q, Tang X, Liu J, Gu X (2010) Use of tissue-engineered nerve grafts consisting of a chitosan/poly (lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng Part A 16(12):3779–3790. doi:10.1089/ten.TEA.2010.0299
Wakao S, Hayashi T, Kitada M, Kohama M, Matsue D, Teramoto N, Ose T, Itokazu Y, Koshino K, Watabe H, Iida H, Takamoto T, Tabata Y, Dezawa M (2010) Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp Neurol 223(2):537–547. doi:10.1016/j.expneurol.2010.01.022
Xue C, Hu N, Gu Y, Yang Y, Liu Y, Liu J, Ding F, Gu X (2012) Joint use of a chitosan/PLGA scaffold and MSCs to bridge an extra large gap in dog sciatic nerve. Neurorehabil Neural Repair 26(1):96–106. doi:10.1177/1545968311420444
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Orbay, H., Cai, W. (2014). Tissue Engineering Applications for Peripheral Nerve Repair. In: Cai, W. (eds) Engineering in Translational Medicine. Springer, London. https://doi.org/10.1007/978-1-4471-4372-7_5
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4372-7_5
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4371-0
Online ISBN: 978-1-4471-4372-7
eBook Packages: EngineeringEngineering (R0)