Abstract
During the last 15–20 years, a new knowledge accumulated about NREM slow wave oscillations that have become the key issue of homeostatic regulation. A frequency-based classification of slow waves has been developed, differentiating between 0.1–1- and 1–4-Hz groups. The cortical <1-Hz slow activity during the so-called up states (surface-positive half wave), even ripple-like (50–200 Hz) fast activity, and down state (surface-negative half wave), an interruption of synaptic and neural activity, have been described. The alternation of these two microstates ensures a unique double working mode for the cortex, providing continuity for the contact and information processing with the environment during the up states even in deep sleep and providing a separation for trophotropic functions for further cognitive demands during the down states.
With progressive development of neuroimaging, source modeling studies on sleep slow waves by new neuroimaging tools have confirmed that the cortical areas are differentially involved in slow wave production and showed that sleep slow waves can be locally – mainly frontally – regulated. They are traveling along an anterior-posterior axis largely mediated by the so-called cingulate highway. Studies in this field emphasized that those areas with maximal involvement in slow waves’ production also show considerable overlap with the default network, paradoxically implicated in monitoring the external environment, and can be altered by sleep deprivation.
Ontogenetic studies revealed that the delta oscillation associated with rapid spindling is the agent of plastic changes of the cortex. Reactive (input-dependent) delta activity seems to be an essential element of plastic changes as early as during the neonatal development. Before the fetal brain might receive elaborated sensory inputs from the external word, spontaneous fetal movements provide sensory stimulation and drive delta-brush oscillation, contributing to the formation of cortical body maps.
The spectral power of sleep slow wave activity and the steepness of the slopes of sleep slow waves were shown to correlate positively with the gray matter volume of several cortical areas in children and adolescents between 8 and 19 years of age. When the production of cortical synapses is more efficient than their elimination (from birth until the prepubertal age), slow wave activity is high and increasing; while in adulthood, when the elimination of synapses exceeds their production, the amount of sleep slow wave activity decreases.
Discussing phylogenetic relations of slow wave activity during different vigilance states and state-dependent reactions to sensory inputs, we try to interpret some paradoxical observations on reptiles. We are proposing that the reason why reptiles are in a continuous NREM sleep like condition during behavioral waking state is the lack or underdevelopment of their cholinergic arousal system. Therefore, sensory stimulation elicits K-complex-like slow wave responses. In the waking state, reptiles apparently have sleep EEG and sleep-like EEG activity during behavioral activation. Our proposal incorporates an explanation for the lack of long-term homeostatic sleep regulation in reptiles, having at the same time short-term homeostatic slow wave supplementation in response to sensory stimulation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Slow oscillation
- Delta activity
- Infraslow oscillation
- Up states
- Down states
- Neuroimaging
- Ontogenesis of sleep
- Brain development
- Phylogenesis of sleep
- Reptilian wakefulness
7.1 A Short History of Slow Waves
The main focus of our present work is the phenomenon of slow waves, considered as a quintessence of sleep. Slow waves are also known as delta waves according to the classical EEG terminology, originating from William Gray Walter (1910–1977) in the 1930s (Walter 1936). He has performed their topographic mapping and recognized the link between brain lesions and local delta activity (meaning disease, degeneration, and death). The strong link between slow waves and sleep was first reported, also in the 1930s, by Alfred Lee Loomis (1935). He managed to make all-night EEG records of human sleep in his laboratory. This was an unbelievable technical achievement at those times as even Harvard researchers were unable to perform such records. Loomis characterized the EEG stages of sleep: the stage characterized by slow waves was called random, as the waves were considered as such in his view. He also described the increase in the amplitude of delta waves during the course of wake-sleep transition until reaching deep sleep, and most importantly he also recognized the reactivity of the slow waves and made a correct description on K-complexes evoked by knocking on the sleepers’ door (K-knock).
Thus, slow waves were considered mainly as signs of low arousal level (Loomis et al. 1935) or brain lesion (Walter 1936). This idea was present in the work of Giuseppe Moruzzi (1910–1986) and Horace Winchell Magoun (1907–1991) also who described the ascending reticular activating system in the brainstem and characterized it as follows: “The evidence given above points to the presence in the brain stem of a system of ascending reticular relays whose direct stimulation activates or desynchronizes the EEG, replacing high-voltage slow waves with low-voltage fast activity. This effect is exerted generally upon the cortex and is mediated, in part, at least, by the diffuse thalamic projection system” (Moruzzi and Magoun 1949). High-voltage slow waves were already considered as signs of sleep or low arousal in these works. This kind of “negative” view on slow waves (i.e., being signs of lack of something) persisted and was even more emphasized during and after the discovery of REM sleep in humans (Aserinsky and Kleitman 1953) or paradoxical sleep in cats (Jouvet et al. 1959). It was the appearance of fast waves instead of slow ones during sleep that seemed interesting in those times.
However, scientific interest has changed during the next few years. The search for the molecular sleep factor (S-factor) by the Pappenheimer group stimulated the search for some reliable physiological measure of sleep deprivation-induced sleep rebound. It was recognized that the best measure is sleep EEG slow wave activity. They wanted to introduce into these studies the time spent in those sleep phases characterized by EEG slow waves as well as the increase in their amplitude, instead of focusing on low-voltage fast activity (Pappenheimer et al. 1975). Similar approaches characterized the research on humoral sleep regulation (Schoenenberger et al. 1977).
Although the homeostatic regulation of slow wave sleep was already known in those studies, it was Wilse B. Webb who explicitly stated that the amount of stage 4 sleep was related to the amount of pre-sleep wakefulness (Webb and Agnew 1971). Hints on the potential recuperative role of slow wave sleep together with its precise homeostatic regulation changed the view of sleep researchers and have led to the generally accepted theories: slow waves are not merely passive expressions of reduced cortical activity due to brain lesions or low arousal; rather, they represent active self-organizational forms of neuronal activity, serving recuperative and off-line information processing functions.
7.2 Is There a Frequency-Based Typology of Slow Waves?
7.2.1 The Controversy of Below or Above 1 Hz
Mircea Steriade and his co-workers published a series of papers (Steriade et al. 1993a, b, c) reporting that:
-
1.
Cellular processes contributing to delta waves (<4 Hz) are inhomogeneous.
-
2.
The 0.1–1-Hz component of the delta (<4 Hz) activity has distinct cellular substrates and is of utmost importance in sleep rhythm generation.
-
3.
The 0.1–1-Hz waves are reflections of large-scale, rhythmic hyperpolarizations followed by widespread depolarization.
-
4.
The hyperpolarization-depolarization sequences originate from cortical neurons and are synchronized by corticocortical connections.
-
5.
The 0.1–1-Hz waves envelope delta and sigma (spindle) oscillations, resulting in complex, coalescent wave sequences (Fig. 7.1).
Fig. 7.1 Infraslow (0.01–0.1 Hz) oscillation (ISO), slow (<1 Hz) oscillation (SO), and delta (1–4 Hz) activity are coexisting and coalescent during slow wave sleep in humans. Negative peaks of the ISO, SO, and delta waves are shown by arrows. SO waves and delta waves emerge during the positive going phases (up states) of the ISO and SO, respectively
This novel cortical rhythm was called the slow oscillation, and the “<1-Hz” symbol was usually added in parentheses in order to make the distinction clear between the slow and the delta rhythms. According to this view, the 1–4-Hz waves are reflections of the thalamic clock-like delta activity and the cortical delta activity, while the <1-Hz component is of cortical origin reflecting different physiological processes (Amzica and Steriade 1998). A detailed scenario of sleep rhythm generation in the thalamocortical system was also given. This scenario was based on the hypothesis that the hyperpolarization-rebound sequences of thalamocortical feedback loops generating the spindle and the delta waves are triggered and grouped by the depolarization phases (up states) of the slow oscillation (Steriade et al. 1993c). The distinction between the slow oscillation and the delta waves was confirmed by the sleep records performed in animal model with the lack of T-type Ca2+ channels. The lack of this channel resulted in a significant decrease in delta and spindle oscillations, but not the slow (<1 Hz) oscillation, which remained unaffected (Lee et al. 2004). Other molecular evidences for the differentiation between the slow oscillation and the delta waves have come from pharmaco-EEG studies of sleep. Benzodiazepine hypnotics decrease NREM sleep EEG power in the delta range (>1 Hz), but may significantly increase the slower (<1 Hz) frequency components (Trachsel et al. 1990). Moreover, only the EEG frequencies <1.5 Hz, but not the higher delta bins, are affected by noradrenaline depletion in sleep-deprived rats: neurotoxic lesions with DSP-4 reduce the 0.5–1.5-Hz activity in recovery sleep, while >1.5-Hz activity remains unchanged (Cirelli et al. 2005).
Some years after the first description of the slow oscillation in animals, it was shown that its role in human sleep homeostasis is different from that of delta activity. The fast Fourier transformation (FFT) power of the <1-Hz component does not significantly decrease between the first and the second sleep cycles, while the power of the 1–4-Hz delta component does (Achermann and Borbély 1997). This was interpreted as a sign for the lack of the homeostatic regulation of the slow oscillation. The idea on missing homeostatic regulation of the slow oscillation was supported by early studies revealing a decrease in NREM sleep EEG power >1 Hz after prolonged periods of wakefulness or sleep deprivation in humans and rats (Borbély et al. 1981; Tobler and Borbély 1990). However, this conclusion was a premature one. Although the time course of delta activity is characterized by a steeper decline over the sleep cycles (NREM periods) than the time course of the slow oscillation, in physiological conditions, both seem to be under precise homeostatic regulation. This was proven by examining the effects of intervening naps in studies performed in young good sleepers (Campbell et al. 2009). Based on their different time courses, Campbell et al. (2009) attributed a permissive role to the slow oscillation in sleep homeostasis: that is, the slow waves permit the expression of the actual sleep need, measurable by the actual level of delta, embedded in sleep cycles (Fig. 7.1). The slow oscillation provides the neural and metabolic conditions for homeostatic processes. The explanation of the peculiar decrease of the <1-Hz NREM sleep EEG in recovery sleep after sleep deprivation (Borbély et al. 1981; Tobler and Borbély 1990) was given by the studies examining the frequency and the slopes of the slow waves under different levels of sleep pressure. These studies have revealed the acceleration in the alternation of up and down states, thus an increase in the frequency of the slow waves as well as an increase in the steepness of slow waves in conditions of increased sleep pressure (Riedner et al. 2007; Bersagliere and Achermann 2010; Fig. 7.2). Several results suggest that the original frequency border of <1 Hz for the slow oscillation is too narrow, and in certain conditions, frequencies higher than 1 Hz may contribute to the phenomenon. Moreover, reports of the decreased <1-Hz activity during recovery sleep find their reason in a frequency shift or acceleration of the slow oscillation. The apparent dissociation between the frequencies >1 Hz and the remaining slow wave activity may simply reflect homeostatic changes in the slopes of slow waves (Hanlon et al. 2011).
General morphology, up and down states, as well as sleep pressure dependence of the slow (<1 Hz) oscillation in human slow wave sleep EEG. Down states are characterized by surface negativity and relative lack of superimposed higher-frequency oscillations, whereas up states are surface positive and superimposed with spindling (sigma activity). Higher sleep pressure is characterized by faster alternation of the up and the down states, steeper slopes, higher amplitude, as well as a relative lack of the well-formed multipeaks. The multipeak phenomenon during the lower sleep pressure is shown by an asterisk (*). Calibration marks: 100 μV and 1 s. EEG recordings come from stage 3 sleep of the first (high sleep pressure) and the third (low sleep pressure) sleep cycles of a young healthy subject (derivation F4-A1)
The cortical origin and corticocortical synchronization of the slow oscillation have led researchers to find the cognitive correlates of this sleep EEG rhythm. Several evidences were found for the correlation between the spectral measures of the slow oscillation and performance in different cognitive tasks. The performances were found to correlate with the sleep slow oscillation and were related to visuospatial memory as measured by the Rey-Osterrieth Complex Figure Test (Bódizs et al. 2002) and different forms of executive functions like nonverbal planning or word generation (Anderson and Horne 2003), as well as the overnight improvement in motor sequence learning (Moroni et al. 2008). Verbal learning was shown to increase EEG coherence in the up states of the slow oscillation during post-sleep learning (Mölle et al. 2004). Last, but not the least, the artificial boosting of the slow oscillation by applying transcranial direct current stimulation of 0.75-Hz frequency has significantly increased the overnight retention of paired associate verbal material (Marshall et al. 2006). It seems that there are two main cognitive features of the slow oscillation which were unraveled by the above investigations. One is a link between some type of cognitive performances and the baseline individual level of the slow oscillation. This could reflect a trait-like relationship revealing the commonalities in or reciprocal relationships between the neural background of wakeful cognitive performances and of NREM sleep cortical slow oscillation (Bódizs et al. 2002; Anderson and Horne 2003). The other type of cognitive aspect of the sleep slow oscillation is related to the sleep-dependent mechanisms of memory consolidation. There is strong evidence for the involvement of the sleep slow oscillation in the consolidation of memory traces (Marshall et al. 2006; Moroni et al. 2008).
7.2.2 Below 0.1 Hz: Infraslow Oscillation in Light of the Full-Band EEG Recordings
Direct current (DC)-EEG recordings of human sleep revealed a prominent infraslow activity roughly associated with 0.01–0.1-Hz frequency fluctuations (Vanhatalo et al. 2004; Monto et al. 2008; Picchioni et al. 2011; Hughes et al. 2011). Although the exact frequency limits of the infraslow oscillation are arbitrary and vary from study-to-study, the main issue unraveled was the evidence showing that it modulates neural excitability and interictal epileptic activity during sleep (Vanhatalo et al. 2004). Frequencies higher than 0.5 Hz were shown to be phase-coupled with (grouped by) the infraslow oscillation during both wakefulness (Monto et al. 2008) and NREM sleep (Vanhatalo et al. 2004; Fig. 7.1). The prominent very slow oscillatory patterns of preterm infants disclosed by DC-EEG during sleep were shown to take the form of long-lasting (1–5 s) occipital negative transients (200–700 μV), embedding the typical delta bursts seen in the conventional EEG (Vanhatalo et al. 2002). The power of the infraslow oscillation during sleep correlates positively with the BOLD signal intensity measured by fMRI in many subcortical regions (cerebellum, thalamus, basal ganglia) as well as some lateral cortical areas and the hippocampi. In contrast, the BOLD signal intensity of the paramedian heteromodal cortices correlated negatively with the power of infraslow activity (Picchioni et al. 2011). This was interpreted as the organization of the broad dissociation of activity between cortical and subcortical regions proven by the infraslow EEG oscillation during sleep. In vivo and in vitro data suggest that the thalamus could be a source of the infraslow oscillation (Lörincz et al. 2009; Hughes et al. 2011).
A particularly wide range of neurobehavioral phenomena fluctuate at frequencies corresponding roughly to the 0.01–0.1-Hz range. These fluctuations of autonomic nervous system activity (Lambertz and Langhorst 1998) had remained largely unmentioned in previous publications. Accumulating evidence suggests that processes providing the infraslow oscillation are the cellular substrates of some large-scale fluctuations which can appear as the CAP phenomenon in some parts of sleep.
We believe that the periodicity of the micro-arousals embedded in the CAP sequences is in fact due to the infraslow oscillation, shaping gross measures of neuronal excitability and being present in many neurobehavioral phenomena analyzed at this time scale (Table 7.1). The parallelism between these infraslow oscillations is striking when one considers that the periodic fluctuations in arousal (CAP) probably reflect the periodic fluctuations in neural excitability (infraslow oscillation). Thus, the periodicity in arousal could be gated by the periodicity in neural excitability. CAP could be a kind of an enhancement or phase resetting of infraslow activity due to internal or external sources of arousal or other forms of sleep instability. The possible link between the infraslow activity and the CAP phenomenon was suggested by other authors earlier (Lörincz et al. 2009).
7.3 Up and Down States of Slow Waves
We are witnessing that the hitherto very poorly understood sleep EEG graphoelements partially described earlier come step-by-step to life and fill up by meaning. They behave as an organic system with biological role, like the pieces of wood transformed to Pinocchio, a living child with full conscience in the hands of master Geppetto, in the famous tail.
We learned from the Steriade group that different rhythms (spindles, delta, and <1-Hz slow waves on one hand and fast frequencies on the other) prevail in coalescence during slow wave sleep.
Within the cortical <1-Hz slow activity during the so-called up states (surface-positive half wave) even ripple-like (50–200 Hz) fast activity and during the “down states” (surface-negative half wave), an interruption of synaptic and neural activity alternates (Fig. 7.2). “The entire cortical network can swing rhythmically between extremely different microstates, ranging from wakefulness-like network activation to functional disconnection in the space of a few milliseconds” (Massimini et al. 2003). The “black-hole”-like down state may provide a rest phase and restoration for the most vulnerable and recuperation demanding frontal cognitive activity.
In the work of Csercsa et al. (2010) intracortical laminar local field potential gradient, multiple-unit and single-unit activities were recorded during slow wave sleep, related to simultaneous electrocorticography, and were analyzed by current source density and spectral methods. We found that slow wave activity in humans reflects a rhythmic oscillation between widespread cortical activation and silence. Cortical activation was demonstrated as increased wideband (0.3–200 Hz) spectral power including virtually all bands of cortical oscillations, increased multiple- and single-unit activities, as well as powerful inward transmembrane currents, mainly localized to the supragranular layers. Neuronal firing in the “up state” was sparse, and the average discharge rate of single cells was less than expected from animal studies. Synchronization patterns of action potentials across all cortical layers suggested that any layer could initiate firing at the onset of up states.
These findings provide strong direct experimental evidence that slow wave activity in humans is characterized by hyperpolarizing currents associated with suppressed cell firing, alternating with high levels of oscillatory synaptic/transmembrane activity associated with increased cell firing. Our results emphasize the major involvement of supragranular layers in the genesis of slow wave activity.
The recurrent cortical disfacilitation during down states together with high-level cortical activity during up states has a serious consequence for sensory transmission. The main one is that the thalamocortical sensory processing during deep slow wave sleep is not steady (Massimini et al. 2003). This could be shown by experiments where the averaging of sensory evoked potentials was grouped according to the up and down states of <1-Hz slow wave oscillation in deep sleep of animals. By this method, the amplitudes of cortical somatosensory evoked responses were found to be different: high, although not reaching wakefulness-like levels, during the up states (confirming the possibility of detecting meaningful events even in deep sleep) and low during the disfacilitation of the down state. This lack of stationarity may impair sensory integration during deep slow wave sleep.
7.4 Imaging of Slow Waves in Sleep
The activation of certain brain structures in slow wave sleep has been recorded by high-tech neuroimaging methods recently.
The advent of functional neuroimaging allowed the registration of regional cerebral blood flow (SPECT) and metabolic (PET scanning) and later neuronal activity BOLD (fMRI) changes also for sleep studies.
PET studies using/18F/-fluorodeoxyglucose as a tracer showed that global cerebral glucose metabolism in humans is lowest in stages 2–3 in NREM sleep, in contrast to REM sleep in which it is similar to the waking state (Maquet et al. 1990). Later, the resolution of PET methods substantially improved both in time and in space, and a more reliable determination of the regional differences during sleep has become possible (Braun et al. 1997; Hofle et al. 1997; Nofzinger et al. 1997; Maquet and Phillips 1998; Maquet 2000). A significant decrease (greater than in the rest of the brain) in rCBF has been shown during NREM sleep in the brainstem (dorsal pons and mesencephalon), thalamus, basal ganglia, basal forebrain, and hypothalamus and in the orbitofrontal and anterior cingulate cortex, as well as the precuneus.
The fMRI studies offer sophisticated knowledge about BOLD activation patterns related to sleep (Czisch et al. 2002, 2004, 2009; Kaufmann et al. 2006; Schabus et al. 2007; Dang-Vu et al. 2008). Several brain regions’ activities were found to be reduced during each NREM sleep stage. Most of these regions are located in the frontal lobes, but some regions of the thalamus, limbic system (anterior and posterior cingulum), as well as wider areas of the temporal, parietal, and occipital lobes, and the midbrain, hypothalamus, and mamillary bodies have also been shown to have decreased activity (Kaufmann et al. 2006). Certain patterns of activation sequences were described along the process of falling asleep, like loss of alertness during expectation of environmental stimuli as well as during deepening sleep. The decrease of insular activity gained significance related to interoceptive awareness. Special attention was paid to the crucial hypothalamic region showing globally decreased activity reflecting to the decreased firing of wake-promoting neurons. It is interesting that “some pronounced synchronous BOLD activity changes” were observed in the hypothalamus in NREM that correlated with a simultaneous activity in wider cortical areas resembling to the activity in the pathways of the ascending arousal system during NREM sleep. Kaufmann et al. (2006) emphasize the role of a hypothalamic-driven network regulation both in the initiation and maintenance of sleep. They observed a decreased activity of the hypothalamic regions throughout NREM sleep stages, reflecting the reduced activity of wake-promoting neurons. Due to limited spatial resolution, differentiating hypothalamic subregions was not possible. However, “some pronounced synchronous BOLD activity changes that correlated with the hypothalamus occurred: limbic structures, regions of the frontal and parietal cortex, the basal forebrain and brainstem showed a similar time course of activation as hypothalamic regions did.” Kaufman et al. interpreted this pattern of activation as resembling to the pathway of the ascending arousal system which sends projections from the brainstem and posterior hypothalamus throughout the forebrain. These findings are congruent with the role of arousal-driven phasic activity, as described in this book. Phasic increases were observed in regional brain activity in relation to discrete events as slow waves or delta waves, compared to baseline sleep activity (Dang-Vu et al. 2008). Delta waves (>1 Hz) were associated with frontal responses. Significant increases in activity were associated with the <1-Hz waves in several cortical areas, including the inferior frontal, medial prefrontal, and precuneus and posterior cingulate areas. The <1-Hz slow waves are associated with significant activity in the parahippocampal gyrus, cerebellum, and brainstem, whereas delta waves are related to frontal regions compared to baseline activity. No decrease of activity (negative BOLD signal) was observed. A partial overlap was seen between the default network of wakefulness and responses associated with slow wave oscillation. They unexpectedly found significant responses associated with slow oscillations in the midbrain and pontine tegmentum, especially in regions of cholinergic and aminergic nuclei. This particular finding again points to traces of arousal activity during NREM sleep, the importance of which was highlighted in Chap. 6
Using high-density EEG and source modeling methods, sleep slow waves occurring spontaneously and evoked by transcranial magnetic stimulation were studied (Murphy et al. 2009). It was found that some areas of the cortex are differentially involved in slow waves and that sleep slow waves can be locally regulated. Slow waves as a group were associated with large currents in the medial, middle, and inferior frontal gyri, the anterior cingular, the precuneus, and posterior cingular regions. This study again emphasized that areas showing maximal involvement in slow waves also show considerable overlap with the default network which is paradoxically implicated in monitoring the external environment and can be altered by sleep deprivation.
Further understanding of the role of slow wave oscillation and particularly of the up and down states in plastic changes during sleep would be crucial; however, spatial resolution of fMRI does not allow us to reveal the time and space structure of cortical up and down states during NREM sleep changes.
7.5 Relation of K-Complexes and Sleep Slow Waves
We have shown that K-complexes express a double nature (Janus-faced): they show responsivity to sensory (mainly acoustic) stimuli and behave as slow waves following the homeostatic decay increasing after sleep deprivation and being related to the deepness of the actual sleep cycle. Furthermore, the studies of Amzica and Steriade (1998) and later Cash et al. (2009) proved that the main surface-negative large N550 component of K-complexes is characterized by an important decrease of unit discharges and synaptic activity in the frontal cortex similar to the “down state” of the frontal slow oscillation during NREM sleep (Fig. 3.4).
According to the fMRI studies of Riedner et al. (2011), even the spontaneous K-complexes wear, at their initial segment, the traces of multisensory processing. Therefore, we may consider K-complexes as the most characteristic prototypes of the “reactive slow wave activity.” Although K-complexes can be well obtained by the averaging also in deep slow wave sleep (Fig. 3.3), they are more frequent in stage 2.
K-complexes are essential constituents of CAP sequences, mainly in the A1 phase events. At the same time A1 events occur much more frequently during the descending slopes of the first cycles, but both spontaneous and elicited K-complexes have higher frequency during the “A” slope. This difference in distribution is even more pronounced if we consider the behavior of evoked K-complexes under continuous random sensory stimulation (Halász 1982). While less reactive slow activity can be elicited in the form of CAP A1 phases during the “A” slopes of the first cycles, the responsivity in the form of singular K-complexes is more preserved compared to the “D” slopes.
Thus, K-complex occurs more frequently during stage 2 sleep and during “A” slopes of cycles and seems to be more linked to the arousal compared to other sleep slow waves. If we involve into the group of reactive sleep graphoelements the classical vertex sharp transients, a continuum of these graphoelements can be outlined more clearly. In superficial sleep (stage 1–2), sensory (mainly acoustic) stimuli evoke the vertex sharp wave, which can be considered as an exaggerated form of acoustic evoked potential, without any important slow wave constituent. Later, in stage 2, the stimulation evokes K-complexes instead of vertex waves, with recognizable remnants of the acoustic evoked potential, but the essential part is the slow wave complex (N350-N550-P900, see Fig. 3.3). Beginning from stage 2, in deeper NREM sleep, the evoked response becomes more complex in the form of CAP A1, which incorporates K-complexes, slow waves, and spindles, while the original evoked potential compartment is hardly visible. The response to sensory stimulation shifts from a multimodal evoked potential-type response to a nonspecific bilateral, frontally dominant slow wave response.
The recent functional neuroimaging results seem to confirm this continuum. Stern et al. (2011), investigating vertex sharp wave generation by fMRI, have shown that BOLD positive activations of regions principally encompass the primary sensorimotor cortical regions for vision, hearing, and touch. Earlier in this book, we detailed the findings of Riedner et al. (2011) who had highlighted the initial evoked potential compartments before the slow wave components of K-complexes. The fMRI counterparts of sleep slow waves were discussed in detail in Sect. 6.2.
The special situation of K-complex in sensory information workup and in fulfilling trophotropic and plastic functions of NREM sleep is nicely supported by the findings of Jahnke et al. (2012). In their fMRI study, they found that K-complex is associated with positive BOLD signal changes in subcortical (brainstem and thalamus), cerebellar, sensory (auditory, visual, and sensorimotor) motor, and midline (anterior and midcingular gyri, precuneus) areas and regions which form part of the so-called default mode network (DMN) (prefrontal cortex, inferior parietal lobule, precuneus). Negative BOLD signal changes were observed in bilateral insular cortices. The primary auditory cortex was the first cortical region to be influenced during K-complex.
These findings confirm “the central role of the thalamus in the mediation of cortically generated K-complex leading to sleep spindles and slow waves” and also that “K-complex permits a low level information processing in one hand and a sleep protective counteraction on the other.” The DMN-type activation is interpreted in line with the “sentinel hypothesis,” describing DMN activity “in the context of a broad low level focus of attention when one – like a sentinel – monitoring the external world for unexpected events.” This version of the Hamletian question (“to wake or not to wake”) is very near to our interpretation (Halász 2005).
Summing up, within the continuum of reactive sleep graphoelements, K-complex seems to be situated between the evoked potential type and reactive slow wave type responses (Table 7.2). The functional significance of this Janus-faced character is that during K-complex, the brain decides “not to wake up” and compensates the disturbing effect of the incoming stimulus by producing slow wave activity.
7.6 Ontogenetic Aspects of Slow Wave Activity in Sleep
The EEG shows recurrent delta waves joined by 8–25-Hz spindle-like oscillatory bursts, called “delta brushes,” during the third trimester of gestation. The phenomenon has been known for long time seen in classical descriptions of the neonatal EEG. Studied in preterm infants, these patterns nowadays have obtained functional significance recently. Khazipov et al. (2004) noticed that hand and foot movements of the human neonates heralded the appearance of delta brushes in the corresponding areas of the cortex. Direct hand and foot stimulation also evoked delta brushes over the same areas.
These results highlighted the role of sensory feedback from spontaneous fetal movements, triggering delta-brush oscillations in consistent somatotopic regions of the central cortex. They assumed that before the fetal brain has received elaborated sensory input from the environment, spontaneous movements provide sensory stimulation and drive delta-brush oscillation, contributing to the formation of cortical body maps.
Similar “slow activity transients” like the delta brushes were observed in the rat visual cortex during the second postnatal week. Colonnese and Khazipov (2010) have shown that rhythmic beta oscillations nested by infraslow oscillations sharing several characteristics of delta brushes are driven by certain retinal waves transferred through the lateral geniculate nucleus to cortex. The visual input proved to be transformed to beta oscillations by the thalamocortical system.
Thus, slow activity associated with beta oscillations of rat visual cortex during postnatal weeks appears to be rather the cortical response to visual input than an internally generated spontaneous cortical activity. Taking into account that the synchronized oscillatory network is believed to be essential for the generation of neuronal circuits, both in the visual and somatosensory cortex, the afferent input seems to play an important role in this process.
Here, we see the first example how delta oscillation associated with rapid spindling is the agent of plastic changes of the cortex, both in the neonatal period of rodents and human neonates.
Observations showing that early sensory deprivation in animal life reduces sleep slow wave activity (Miyamoto et al. 2003) seem to be congruent with the role of sensorial input in the development of cortical functions: for example, input-dependent delta activity seems to be an essential element of plastic changes, as early as in the neonatal period of development.
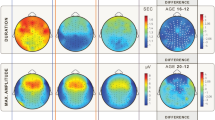
Delta and/or slow EEG activities are of special interest for the studies examining the ontogeny of neuronal systems during childhood and adolescence. A striking parallelism between changes in the amplitude of sleep slow wave activity, cortical metabolism, and synaptic density was evident since the observations of Feinberg et al. (1990). Sleep slow waves (amplitude and/or power spectral density) were shown to increase from birth to the prepubertal ages. Thereafter, a steep decline in sleep slow wave activity is evident in all studies focusing on the age dependency of these measures. The decline was shown to be maximal during adolescence and appeared earlier in girls than in boys. This is congruent with the earlier biological maturation of girls. The spectral power of sleep slow wave activity was shown to correlate positively with the gray matter volume of several cortical areas in children and adolescents between 8 and 19 years of age. The associations were particularly evident in those regions characterized by an age-dependent decrease in gray matter volume (Buchmann et al. 2011). Besides amplitude and/or spectral power of sleep slow wave activity, the steepness of the slopes of sleep slow waves were shown to be different in the age groups: prepubertal children were shown to be characterized by steeper slopes than mature adolescents, both during baseline and after sleep deprivation. This difference is still present after the control of overall amplitude differences (Kurth et al. 2010a). The pattern of anteroposterior differences in sleep slow waves is particularly informative from the point of view of cortical maturation. That is, the frontal predominance of slow wave activity during sleep is gradually achieved during development. The pattern of anteroposterior difference is parallel with the anteroposterior gradient in cortical maturation (Kurth et al. 2010b). The differences between the production and the elimination of cortical synapses were related to the age dependency of sleep slow wave activity. During adolescence, elimination dominates production. This is associated with a decline in slow wave activity during NREM sleep. When the production of cortical synapses is more efficient than their elimination (from birth until the prepubertal age), slow wave activity is high and increasing, and elimination is associated with decreasing amounts of sleep slow wave activity in adults (Ringli and Huber 2011). Moreover, computer simulations of the thalamocortical system suggest that a greater density or greater efficacy of cortical synapses or both are the potential basis of sleep slow waves with steeper slopes. Last, but not the least, aging, and especially pathological aging, is associated with a decrease in efficient synaptic strength and decrease in cells/synapses. This is parallel with the decreased slow wave sleep and slow wave activity in aged and especially pathologically aged humans.
If we consider reactive slow elements separately, the same maturational features emerge. CAP A1 subtype is preponderant in the peripubertal period, thereafter showing a gradual decrease (Parrino et al. 2012, see Fig. 3.8). An age-dependent decrease in the amplitude of the K-complexes is a similar phenomenon (Colrain 2005).
7.7 Phylogenetic Aspects of Slow Wave Activity
7.7.1 Peculiarities in the EEG Amplitude of Poikilotherms
We have seen that the amplitude of the EEG is mainly determined by its low-frequency components, at least during physiological conditions. This is related to the power-law relationship between EEG amplitude and frequency (Feinberg et al. 1984) or to the exponential decay (of the 1/fα-type) of spectral power along the frequency axis (Pereda et al. 1998; Freeman et al. 2007). It was repeatedly shown that several nonhuman or even nonmammalian species possess an ongoing neuronal population activity (we will call it EEG) with similar amplitude-frequency relationship. This was evidently outlined for several mammalian, reptilian, and amphibian species, as well as for fishes (Bullock and Basar 1988). The states of sleep/behavioral rest and waking/behavioral alertness seem to be almost universal in the animal kingdom. Based on the similarities between the amplitude-frequency relationship of the reptilian and mammalian EEG, we would expect larger EEG amplitudes in sleeping or resting reptiles when compared to the behaviorally active ones. At least this is what we usually see in mammals. However, this is not the case in poikilotherm vertebrates, namely, fishes, amphibians, and reptiles. For example, the EEG power of the lizard Gallotia galloti was shown to be higher during active waking than during passive waking. Both were higher than the EEG power of the state of nocturnal rest (Fig. 7.3). As regards sensory stimulation, post-stimulation amplitudes of the EEG significantly exceeded the amplitude of brain electrical activity recorded during the pre-stimulation period (Nicolau et al. 2000; Rial et al. 2010; Fig. 7.4). The above results seem to be a general feature of the brain physiology of reptiles, amphibians, and fishes. This thesis is supported by 14 early reports, published between 1964 and 1988, all highlighting higher amplitude of brain electrical activity during states of alert waking and a lower one during rest (Nicolau et al. 2000). Thus, unlike in mammals, states of higher activity and arousal are associated with higher amplitude of brain electrical activity in poikilotherm vertebrates. The higher EEG amplitude during/after stimulation or behavioral arousal is reminiscent for the reactive delta activity or K-complexes during mammalian slow wave sleep. But, are these high-amplitude waves slow waves or some other frequency components of the EEG?
7.7.2 Peculiarities in the Slow Waves of Poikilotherms: Vigilance Level and Responsivity
The direct recordings from the cortical surface of the lizard Gallotia galloti were shown to contain well-formed slow waves and groups of waves with spindle-like morphology (De Vera et al. 1994; Nicolau et al. 2000). Just as the general amplitude of brain electrical activity, these slow waves were shown to be characterized by higher amplitude during periods of behavioral and sensory arousal (Fig. 7.5) and by higher amplitude during higher ambient temperatures in the poikilotherm vertebrates. Again, it is important to note that high body temperatures induced by passive heating or physical activity can induce significant increases in sleep slow wave activity of humans. That is, high temperatures increase slow wave activity during behaviorally active periods of reptiles and during slow wave sleep of mammals.
Reported slow waves in the lizard Gallotia galloti are of various frequencies. A low-frequency spectral peak between 0.5 and 4 Hz is present at different body temperatures (De Vera et al. 1994). Nicolau et al. (2000) reported occasional slow waves in the posterior cortex mainly, which were shown to be independent of breathing and cortical or olfactory spindles. They also presented evidences for well-formed slow waves appearing during continuous sensory stimulation, that is, the effects of lights. In the paper of Rial et al. (2010), the same group presented continuous slow wave activity mixed with high-frequency waves in behaviorally active reptiles. Based on the figures and reported frequency bands of several papers, it seems that these slow waves cover the slow oscillation, as well as the delta and theta waves. It has to be noted, however, that there is no unequivocal evidence for the categorization of different types of slow waves in poikilotherm vertebrates (Table 7.3).
Indirect evidence for the specific dynamism and reactivity of reptilian slow wave activity comes from the studies showing an increased relative predominance of 0.3–7.5-Hz band power of the Gallotia galloti lizards’ brain electrical activity during states of eyes open compared to the states with eyes closed. Moreover, a specific interaction between temperature and eyes open/closed states was shown to shape the slow waves of the same species. Namely, a temperature-dependent increase of harmonic power is invariantly observed in the higher frequencies (8–30 Hz). However, the harmonic power of the slower waves (0.3–7.5 Hz) is shaped by both the ambient temperature and sensory arousal: these waves are temperature-dependently increased in the states with open eyes (sensory stimulation), but not in the states with eyes closed. Thus, the slow waves are the ones reflecting more directly and specifically the sensory/behavioral arousal. Based on the above findings, it is tempting to say that slow wave activity is reactive in nature being an activation-related phenomenon in reptiles.
Last, but not the least, there are reported similarities between reptilian brain electrical activity and human slow wave sleep EEG. These similarities are evident for the Gallotia galloti lizard at 25 grades of Celsius and are defined by a main spectral peak with a central frequency of ∼1 Hz, with the presence of nonlinear structure and the lack of correlation between the correlation dimension and the fractal exponent derived from coarse graining spectral analysis.
It is important to note that some influential studies deny the existence of slow waves in reptiles and other species lacking a well-developed pallium (Rattenborg 2006). This is an especially controversial issue because the lack of some kind of brain electrical activity cannot be concluded if one uses the test of a visual inspection only. For a mammalian example we could invoke the case of slow waves during wakefulness in humans and monkeys. It is clear the slow waves are not particularly striking components of the mammalian EEG during wakefulness, yet these relatively low-amplitude waves were shown to participate in attention and stimulus detection (Lakatos et al. 2008). As we have seen, the 1/fα-type spectra of reptilian brain electrical activity imply the existence of some kind of slow activity. The amplitude of this activity is clearly lower than the amplitude of the slow waves in mammalian and avian sleep. But, the question of the amplitude of ongoing brain electrical activity is class- and species-specific and is related to the level of telencephalization; hence, the differences in amplitude are rather a natural consequence of brain evolution. The question is whether the specific dynamic nature of this slow wave activity reflects some specific rules, which were uncovered in other species – especially mammalian ones – before.
Effects of external stimuli are usually studied by the evoked potential technique in mammals. This implies the use of several repetitions of the same stimuli and the averaging of the post-stimulation EEG records in order to filter out the so-called noise and retain the stimuli-dependent activities. It is worth noting that such techniques are seldom used in nonmammalian species. This could be caused by the strikingly visible effects of external stimuli on the ongoing brain electrical activity of poikilotherms. A common feature of this evoked activity is a large-amplitude slow component and a change in frequency of the background activity that follows it. Earlier reports are common in not even mentioning the former and focusing on the latter aspect only.
External stimuli seem to interrupt the ongoing brain electrical activity of lizards Gekko gecko by high-amplitude, sharp-slow complexes, which remain uncommented, but clearly visible in the figures reported by Gaztelu et al. (1991). These complexes are followed by an increase in faster activities thereafter. Similar events are observed in the lizard Sceloporus olivaceus. An air puff may induce a large sharp-slow complex followed by a change in background activity (Hunsaker and Lansing 1962). Weak electrical stimulation of elephantnose fishes (Gnathonemus petersii) induces a large-amplitude slow component termed delta F wave by Prechtl et al. (1998). Delta refers to the change and not to the slow nature of these potentials here: that is, a change in frequency (fastening) follows these potentials. But the potentials are of large amplitude and slow indeed, thus the term delta derived from the EEG nomenclature could be coherent with the nature of these waves too. The appearance of a stripe (moving visual stimuli) induces oscillatory events in the turtle Pseudemys scripta, which are superimposed on a slow potential (Bullock et al. 1993). Last, but not the least, flash evoked potentials were characterized by slow triphasic components with superimposed spindles in Gallotia galloti lizards. These wave complexes were similar to the K-complexes of human sleep (De Vera et al. 1994).
The large slow waves following external stimuli are characteristic features of mammalian slow wave sleep. Humans were shown to produce K-complexes after their sensory stimulation during NREM sleep (Loomis et al. 1938). The slow component of the K-complex seems to be the EEG outcome of an isolated cortical down state (Cash et al. 2009). These isolated down states are preceded in some cases by a localized sharp component corresponding to a stimulation-induced localized up state. Spontaneous K-complexes appear with a frequency of around 1/35 s during NREM sleep (Halász 2005). These K-complexes are similar to the spontaneous slow potentials appearing in the ongoing brain electrical activity of different poikilotherm vertebrate species.
7.7.3 Peculiarities in the Chemical Background of Arousal in Poikilotherms
Neuropharmacological studies undoubtedly revealed the relationship between cortical acetylcholine (Ach) and brain electrical potentials. Ach seems to be the most powerful inducer of high-frequency activity in mammalian brain (Jones 2005). The direct relationship between Ach and the suppression of slow wave activity was evidenced in the studies showing that unihemispheric slow waves did negatively correlated with Ach levels in the ipsilateral hemisphere of marine mammals. There was no relationship between unihemispheric slow wave activity and contralateral Ach level. Moreover, the muscarinic antagonist atropine is known to induce slow waves in mammals (Dringenberg and Vanderwolf 1998), including humans (Czopf et al. 1977), whereas during atropine-induced coma, the evoked potentials consist of large-amplitude, biphasic slow wave reminiscent of K-complexes. The amplitude of these evoked slow waves is positively correlated with the deepness of the coma (Czopf et al. 1977).
A major change in the views around the issue of the phylogeny of sleep-waking states was provoked by the reconsideration of the physiology of the platypus and of other monotremes. These primitive mammals were thought to miss the state of REM sleep. However, later studies revealed that these animals are indeed champions of REM sleep time, but that these REM sleep periods are characterized by slow cortical waves instead of the well-known fast activity seen in other mammals. The lack of a significant decrease in slow waves during full-blown REM states was attributed to peculiarities of the cholinergic system of the platypus. The cholinergic nuclei of the hypothalamus were shown to be missing in this species, which might contribute to a decreased cortical cholinergic tone during REM sleep. It is unclear whether the lack of an efficient Ach system is related to the lack of the classical arousal reaction in the poikilotherm vertebrates. Earlier reports suggested the lack of cholinergic interneurons in the reptilian brain. This could be suggestive, but not a very strong evidence yet. As we previously mentioned, direct effects of atropine on mammals are known to be characterized by the increase in slow wave activity. Such effects of atropine are lacking in the lizard Gallotia galloti. Thus, muscarinic receptors are not participating in the process of initiation/suppression of slow waves in these reptiles. Other reported pharmacological probes include the α1-adrenergic antagonist prazosin and the β-blocker propranolol. Both reduced significantly the slow wave activity of lizards. Moreover, both prazosin and propranolol were shown to reduce slow wave sleep or slow wave activity in mammals (Hilakivi et al. 1978, 1987). Thus, the adrenergic system seems to play similar roles in shaping the slow wave activity of reptiles and mammals.
There are other endogenous neurochemical agents characterized by a prominent role in sleep-wake and arousal regulations. The neuropeptide orexin localized in the lateral hypothalamus was shown to be released during wakefulness, stimulate the major arousal systems of the brain, and suppress the activity of the hypothalamic VLPO GABA-ergic and galaninergic sleep-inducing neurons. Orexin was shown to be present in the brain of several nonmammalian species, but there is evidence for the fact that unlike in mammals, orexinergic neurons of the goldfish (Carassius auratus) do not form regular synapses with neurons of the arousal-inducing aminergic nuclei (Huesa et al. 2005). Although this view is not shared by many comparative neuroanatomists, who present data on the close vicinity of orexinergic fibers with noradrenergic and serotoninergic cells in fishes and lizards (Panula 2010; Domínguez et al. 2010), neither of these authors specifically addresses the question put by Huesa et al. (2005). Do orexinergic fibers form synapses with the somata or proximal dendrites of the aminergic cells? If the answer to the above question is no, or is just less than in mammals, this could be suggestive for the lack of the flip-flop circuitry-stabilizing arousal in these nonmammalian species. Such a lack may result in the lack of the long-term homeostasis of slow waves which is well documented in mammals (Borbély 2001). The short-term dynamical regulation or the instant homeostasis is present indeed. Stimuli induce a parallel increase in low- and high- frequency activity, which we hypothesize to be a kind of balanced cortical reaction providing the stabilization of the network by a so-called delta injection.
The above hypothesis is congruent with the view that reptilian wakefulness is some kind of NREM sleep-like state. According to this view mammalian wakefulness is a phylogenetically new behavioral state, which has no antecedents in the pre-mammalian species. The old type of wakefulness, which was characterized by the reactive slow wave type of functioning mode, was indeed suppressed, its daily emergence being limited to the periods of behavioral rest/sleep (Nicolau et al. 2000; Rial et al. 2010). This sequence of phylogenetic adaptation could lead to the appearance of the long-term homeostasis of slow waves (accumulation of the need for slow waves during wakefulness and their recuperation during sleep), which are indeed largely suppressed during the new mammalian type of wakefulness. There are at least two additional issues supporting this hypothesis. One is the parallelism between the positive neuroanatomical correlates of sleep slow waves in the human brain (larger brain associated with higher sleep slow wave activity) and a similar correlation between telencephalization and EEG amplitude of different species. The latter is evidenced by several sources and scientific reports. First, it was Bullock and Basar (1988), who outlined that the EEG amplitude is increasing in the fish-amphibian-reptile series. These classes form an unquestionable series in telencephalization (fishes < amphibians < reptiles), which seems to be parallel with their increasing EEG amplitude. Secondly, there is a characteristic species-specific increase in sleep EEG amplitude of several mammalian species directly compared by Kleinlogel (1983). The species investigated so far were the human, the rat, the Syrian hamster (Mesocricetus auratus), the guinea pig (Cavia porcellus), and the Vietnamese potbellied pig (Sus scrofa f. domestica) (Kleinlogel 1983). The largest slow waves (relative to the EEG of the wakeful state) were recorded in humans. The precise order was the following: human > Vietnamese pig > rat > guinea pig > hamster. This result is coherent with the view that the slow waves are parts of some reorganization in large-scale neuronal assemblies which are especially needed in beings with high levels of telencephalization.
The predominance of reactive slow waves in the EEG of poikilotherm vertebrates and the peculiar parallelism between the dynamics of mammalian sleep slow waves and reptilian activity-dependent slow waves are changing the views on the ontogeny of sleep. The delta brushes presented before are in fact activation-induced reactive slow wave groups in early (preterm) infancy which are congruent with the Haeckel’s law claiming that ontogeny repeats phylogeny. Similar signs for this kind of pre-mammalian physiology are the non-separability of NREM and REM sleep in early infancy as well as the temporal coincidence of eye movements and slow waves during sleep.
7.8 The Slow Waves in REM Sleep
Although slow waves are considered as hallmarks of NREM sleep, some forms of slow wave EEG activity are measurable in REM sleep too. It is worth noting that the level of objectively measured slow wave activity in REM sleep is usually intermediate between NREM sleep and wakefulness (NREM > REM > wakefulness). Theories are very restrictive in explaining the nature and origin of slow wave activity in REM sleep. Here we provide some clues aiming to focus the attention of the scientific community on some of these potentially important aspects of REM sleep.
The slow wave activity in the human EEG has state-unspecific aspects, as increases in frontal 1–7-Hz power after sleep deprivation were evidenced for both NREM and REM phases of recovery sleep. Moreover, the sleep deprivation-induced increases in NREM and REM sleep 2–7-Hz activity correlated positively across the subjects (Marzano et al. 2010). Similar observations were made for wakefulness, but for this state, the sleep deprivation-induced increase was largest in (but not limited to) the theta band (Tinguely et al. 2006). Thus, the long-term homeostasis of the slow waves is evidently present in all three behavioral states. However, we have no evidence for the instant delta homeostasis or delta injection (reactive delta) in REM sleep or wakefulness. That is, the bursting mode of the thalamocortical system is a necessary prerequisite of reactive slow wave EEG activity, while the slowing of the background EEG can occur in the tonic firing mode also. The phenomenon of local sleep could be a physiological framework of this latter case.
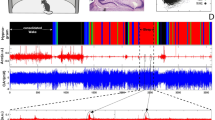
Hippocampal theta waves or hippocampal rhythmic slow wave activity was shown to be a main characteristic of explorative behavior and REM sleep in many mammalian species (Grastyan and Karmos 1961; Vanderwolf 1969; Arnolds et al. 1979; Robinson 1980). When aiming to describe and characterize this phenomenon during human REM sleep, we relied on foramen ovale records of epileptic patients undergoing presurgical evaluation. These electrodes are inserted in the ambient cistern, are parallel with the long axis of the hippocampus, and record parahippocampal activity. We recorded rhythmic waves of 1.5–3-Hz frequency specifically associated with the states of REM sleep (Bódizs et al. 2001; Fig. 7.6). The waves were continuous during the whole REM periods, and no other frequency had this high rhythmicity. We concluded that this oscillation is the counterpart of the hippocampal theta of mammalian REM sleep and that the 1.5–3-Hz delta EEG activity is a basic neurophysiologic feature of human REM sleep. The REM sleep delta waves recorded in this clinical situation were grouping high-frequency activities, including gamma activity, just as it was observed in the case of hippocampal theta waves in rodents (Clemens et al. 2009). Further evidence for REM sleep-related rhythmic hippocampal delta activity (2–4 Hz) of human subjects was presented by Moroni et al. (2011) in a recent study. Given these evidences, it is tempting to say that the hippocampal RSA of humans is in fact a delta frequency activity, contributing to the temporal and perhaps posterior delta power measured during REM sleep (Bódizs et al. 2001, 2005). Moreover, these findings fit well with the allometric theory of the frequency of hippocampal RSA, suggesting that larger brains display slower hippocampal RSA (Blumberg 1989). The phase coupling of hippocampal delta with gamma in human REM sleep is reminiscent for the phase coupling between the slow oscillation and fast EEG activities during NREM sleep (Steriade 2006). Although we have no data on this issue, given the nature of the hippocampal RSA, it is improbable that REM sleep hippocampal delta activity is homeostatically regulated, and we do not know whether it could be triggered or evoked by external stimuli.
Characteristic REM sleep delta (∼2 Hz) activity near the parahippocampal gyrus in an adult human subject implanted with foramen ovale electrodes (fo1–8). Insertion is a part of the presurgical examination of epilepsy. Top: Scalp EEG shows low-amplitude EEG activity typical for REM sleep, with occasional eye movement artifacts. The contralateral mastoid is used as EEG reference. Calibration marks: 100 μV and 1 s. Bottom: State dependency of the relative power of foramen ovale recorded EEG in wakefulness-eyes open (W-EO), wakefulness-eyes closed (W-EC), light slow wave sleep (LSWS), deep slow wave sleep (DSWS), REM sleep tonic phases (REM-TO), and REM sleep phasic phases (REM-PH). Frequency bands are the following: LD (low delta), MD (mid-delta), HD (high delta), LT (low theta), (MT) mid theta, (HT) and high theta
Another source of (posterior) slow wave activity in human REM sleep was reported by Magnin et al. (2004). The authors recorded thalamic activity in epileptic patients implanted with intracranial electrodes and found a prominent delta (1.5–4 Hz) activity in the medial pulvinar nucleus during REM sleep. This was interpreted as a lack of activation of the human medial pulvinar nucleus during REM sleep hypothesized to contribute to the cognitive peculiarities of dreams. Again, we have no data for the homeostasis of REM sleep medial pulvinar delta activity or on its reactivity.
The above findings suggest that there are slow waves with specific origin and function being characteristic for REM instead of NREM sleep. Although their detailed investigation waits for further research, it seems that they are of different sources and have different functions compared to the NREM sleep slow waves.
References
Achermann P, Borbély AA. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81(1):213–22.
Albrecht D, Royl G, Kaneoke Y. Very slow oscillatory activities in lateral geniculate neurons of freely moving and anesthetized rats. Neurosci Res. 1998;32(3):209–20.
Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998;82(3):671–86.
Anderson C, Horne JA. Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40(3):349–57.
Arnolds DE, Aitink JW, Boeyinga P, Lopes da Silva FH. Hippocampal EEG in dog, cat and man (author’s transl). Rev Electroencephalogr Neurophysiol Clin. 1979;9(4):326–32.
Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118(3062):273–4.
Bersagliere A, Achermann P. Slow oscillations in human non-rapid eye movement sleep electroencephalogram: effects of increased sleep pressure. J Sleep Res. 2010;19(1 Pt 2):228–37.
Blumberg MS. An allometric analysis of the frequency of hippocampal theta: the significance of brain metabolic rate. Brain Behav Evol. 1989;34(6):351–6.
Bódizs R, Kántor S, Szabó G, Szűcs A, Erőss L, Halász P. Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus. 2001;11:747–53.
Bódizs R, Békésy M, Szűcs A, Barsi P, Halász P. Sleep-dependent hippocampal slow activity correlates with waking memory performance in humans. Neurobiol Learn Mem. 2002;78(2):441–57.
Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51(5):483–95.
Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–97.
Buchmann A, Kurth S, Ringli M, Geiger A, Jenni OG, Huber R. Anatomical markers of sleep slow wave activity derived from structural magnetic resonance images. J Sleep Res. 2011;20(4):506–13.
Bullock TH, Basar E. Comparison of ongoing compound field potentials in the brains of invertebrates and vertebrates. Brain Res. 1988;472(1):57–75.
Bullock, TH and Achimowicz JZ. A comparative survey of oscillatory brain activity, especially gamma-band rhythms. In: Symposium on “oscillatory event related brain dynamics”, Tecklenburg/Münsterland, 1993.
Cirelli C, Huber R, Gopalakrishnan A, Southard TL, Tononi G. Locus ceruleus control of slow-wave homeostasis. J Neurosci. 2005;25(18):4503–11.
Clemens Z, Weiss B, Szucs A, Eross L, Rásonyi G, Halász P. Phase coupling between rhythmic slow activity and gamma characterizes mesiotemporal rapid-eye-movement sleep in humans. Neuroscience. 2009;163(1):388–96.
Colonnese MT, Khazipov R. “Slow activity transients” in infant rat visual cortex: a spreading synchronous oscillation patterned by retinal waves. J Neurosci. 2010;30(12):4325–37.
Csercsa R, Dombovári B, Fabó D, Wittner L, Eross L, Entz L, Sólyom A, Rásonyi G, Szucs A, Kelemen A, Jakus R, Juhos V, Grand L, Magony A, Halász P, Freund TF, Maglóczky Z, Cash SS, Papp L, Karmos G, Halgren E, Ulbert I. Laminar analysis of slow wave activity in humans. Brain. 2010;133(9):2814–29.
Czisch M, Wetter TC, Kaufmann C, Pollmächer T, Holsboer F, Auer DP. Altered processing of acoustic stimuli during sleep: reduced auditory activation and visual deactivation detected by a combined fMRI/EEG study. Neuroimage. 2002;16(1):251–8.
Czisch M, Wehrle R, Kaufmann C, Wetter TC, Holsboer F, Pollmächer T, Auer DP. Functional MRI during sleep: BOLD signal decreases and their electrophysiological correlates. Eur J Neurosci. 2004;20(2):566–74.
Czisch M, Wehrle R, Stiegler A, Peters H, Andrade K, Holsboer F, Sämann PG. Acoustic oddball during NREM sleep: a combined EEG/fMRI study. PLoS One. 2009;4(8):e6749.
Czopf J, Karmos G, Gaszner P, Kelényi L. A vizuális kiváltott válasz változása terápiás atropin coma alatt. Ideggyogy Sz. 1977;30:81–9.
Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, Carrier J, Moonen G, Balteau E, Degueldre C, Luxen A, Phillips C, Maquet P. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci USA. 2008;105(39):15160–5.
De Vera L, González J, Rial RV. Reptilian waking EEG: slow waves, spindles and evoked potentials. Electroencephalogr Clin Neurophysiol. 1994;90(4):298–303.
Depootere H, Granger P, Leonardon J, Terzano MG. Evaluation of cyclic alternating pattern in rats by automatic analysis of sleep amplitude variations. Effect of zolpidem. In: Terzano MG, Halász P, Declerck AC, editors. Phasic events and dynamic organization of sleep. New York: Raven Press; 1991. p. 17–33.
Domínguez L, Morona R, Joven A, González A, López JM. Immunohistochemical localization of orexins (hypocretins) in the brain of reptiles and its relation to monoaminergic systems. J Chem Neuroanat. 2010;39(1):20–34.
Dringenberg HC, Vanderwolf CH. Involvement of direct and indirect pathways in electrocorticographic activation. Neurosci Biobehav Rev. 1998;22(2):243–57.
Ehlers CL, Foote SL. Ultradian periodicities in EEG and behavior in the squirrel monkey (Saimiri sciureus). Am J Primatol. 1984;7:381–9.
Feinberg I, Thode Jr HC, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142(2):149–61.
Fischgold H, Mathis P. Obnubilations, comas et stupeurs. Etudes electroenceph. Paris: Masson et Cie; 1959.
Gaztelu JM, García-Austt E, Bullock TH. Electrocorticograms of hippocampal and dorsal cortex of two reptiles: comparison with possible mammalian homologs. Brain Behav Evol. 1991;37(3):144–60.
Grastyan E, Karmos G. A study of a possible “dreaming” mechanism in the cat. Acta Physiol Acad Sci Hung. 1961;20:41–50.
Halász P. K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment. Sleep Med Rev. 2005;9(5):391–412.
Halász P. The role of the non-specific sensory activation in sleep regulation and in the pathomechanism of generalized epilepsy with generalized spike-wave discharge. Doctoral thesis, The Hungarian Academy of Sciences, Budapest; 1982.
Hanlon EC, Vyazovskiy VV, Faraguna U, Tononi G, Cirelli C. Synaptic potentiation and sleep need: clues from molecular and electrophysiological studies. Curr Top Med Chem. 2011;11(19):2472–82.
Hilakivi I, Mäkelä J, Leppävuori A, Putkonen PT. Effects of two adrenergic beta-receptor blockers on the sleep cycle of the cat. Med Biol. 1978;56(3):138–43.
Hilakivi I, Kovala T, Leppävuori A, Shvaloff A. Effects of serotonin and noradrenaline uptake blockers on wakefulness and sleep in cats. Pharmacol Toxicol. 1987;60(3):161–6.
Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans AC, Jones BE. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. J Neurosci. 1997;17(12):4800–8.
Huesa G, van den Pol AN, Finger TE. Differential distribution of hypocretin (orexin) and melanin-concentrating hormone in the goldfish brain. J Comp Neurol. 2005;488(4):476–91.
Hughes SW, Lorincz ML, Parri HR, Crunelli V. Infraslow (<0.1 Hz) oscillations in thalamic relay nuclei basic mechanisms and significance to health and disease states. Prog Brain Res. 2011;193:145–62.
Hunsaker 2nd D, Lansing RW. Electroencephalographic studies of reptiles. J Exp Zool. 1962;149:21–32.
Jahnke K, von Wegner F, Morzelewski A, Borisov S, Maischein M, Steinmetz H, Laufs H. To wake or not to wake? The two-sided nature of the human K-complex. Neuroimage. 2012;59(2):1631–8.
Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26(11):578–86.
Jouvet M, Michel F, Courjon J. On a stage of rapid cerebral electrical activity in the course of physiological sleep. C R Seances Soc Biol Fil. 1959;153:1024–8.
Kaufmann C, Wehrle R, Wetter TC, Holsboer F, Auer DP, Pollmächer T, Czisch M. Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: an EEG/fMRI study. Brain. 2006;129(Pt 3):655–67.
Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432(7018):758–61.
Kleinlogel H. Sleep in various species of laboratory animals. Neuropsychobiology. 1983;9(2–3):174–7.
Kurth S, Jenni OG, Riedner BA, Tononi G, Carskadon MA, Huber R. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010a;33(4):475–80.
Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010b;30(40):13211–9.
Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320(5872):110–3.
Lambertz M, Langhorst P. Simultaneous changes of rhythmic organization in brainstem neurons, respiration, cardiovascular system and EEG between 0.05 Hz and 0.5 Hz. J Auton Nerv Syst. 1998;68(1–2):58–77.
Lee J, Kim D, Shin HS. Lack of delta waves and sleep disturbances during non-rapid eye movement sleep in mice lacking alpha1G-subunit of T-type calcium channels. Proc Natl Acad Sci USA. 2004;101(52):18195–9.
Loomis AL, Harvey EN, Hobart G. Further observations on the potential rhythms of the cerebral cortex during sleep. Science. 1935;82(2122):198–200.
Loomis AL, Harvey EN, Hobart GA. Distribution of disturbance-patterns in the human electroencephalogram, with special reference to sleep. J Neurophysiol. 1938;1(5):413–30.
Lörincz ML, Geall F, Bao Y, Crunelli V, Hughes SW. ATP-dependent infra-slow (<0.1 Hz) oscillations in thalamic networks. PLoS One. 2009;4(2):e4447.
Magnin M, Bastuji H, Garcia-Larrea L, Mauguière F. Human thalamic medial pulvinar nucleus is not activated during paradoxical sleep. Cereb Cortex. 2004;14(8):858–62.
Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res. 2000;9(3):207–31.
Maquet P, Phillips C. Functional brain imaging of human sleep. J Sleep Res. 1998;7 Suppl 1:42–7.
Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–3.
Marzano C, Ferrara M, Curcio G, De Gennaro L. The effects of sleep deprivation in humans: topographical electroencephalogram changes in non-rapid eye movement (NREM) sleep versus REM sleep. J Sleep Res. 2010;19(2):260–8.
Massimini M, Rosanova M, Mariotti M. EEG slow (approximately 1 Hz) waves are associated with nonstationarity of thalamo-cortical sensory processing in the sleeping human. J Neurophysiol. 2003;89(3):1205–13.
McKeown MJ, Humphries C, Achermann P, Borbély AA, Sejnowski TJ. A new method for detecting state changes in the EEG: exploratory application to sleep data. J Sleep Res. 1998;7:48–56.
Miyamoto H, Katagiri H, Hensch T. Experience-dependent slow-wave sleep development. Nat Neurosci. 2003;6(6):553–4.
Mölle M, Marshall L, Gais S, Born J. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci USA. 2004;101(38):13963–8.
Monto S, Palva S, Voipio J, Palva JM. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J Neurosci. 2008;28(33):8268–72.
Moroni F, Nobili L, De Carli F, Massimini M, Francione S, Marzano C, Proserpio P, Cipolli C, De Gennaro L, Ferrara M. Slow EEG rhythms and inter-hemispheric synchronization across sleep and wakefulness in the human hippocampus. Neuroimage. 2011;60(1):497–504.
Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1(4):455–73.
Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci USA. 2009;106(5):1608–13.
Nicolau MC, Akaârir M, Gamundí A, González J, Rial RV. Why we sleep: the evolutionary pathway to the mammalian sleep. Prog Neurobiol. 2000;62(4):379–406.
Nofzinger EA, Mintun MA, Wiseman M, Kupfer DJ, Moore RY. Forebrain activation in REM sleep: an FDG PET study. Brain Res. 1997;770(1–2):192–201.
Novak P, Lepicovska V. Slow modulation of EEG. Neuroreport. 1992;3(2):189–92.
Panula P. Hypocretin/orexin in fish physiology with emphasis on zebrafish. Acta Physiol (Oxf). 2010;198(3):381–6.
Pappenheimer JR, Koski G, Fencl V, Karnovsky ML, Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975;38(6):1299–311.
Penttonen M, Nurminen N, Mietinnen R, Sirviö J, Henze DA, Csicsvári J, Buzsáki G. Ultra-slow oscillation (0.025 Hz) triggers hippocampal afterdischarges in Wistar rats. Neuroscience. 1999;94:735–43.
Pereda E, Gamundi A, Rial R, González J. Non-linear behaviour of human EEG: fractal exponent versus correlation dimension in awake and sleep stages. Neurosci Lett. 1998;250(2):91–4.
Picchioni D, Horovitz SG, Fukunaga M, Carr WS, Meltzer JA, Balkin TJ, Duyn JH, Braun AR. Infraslow EEG oscillations organize large-scale cortical-subcortical interactions during sleep: a combined EEG/fMRI study. Brain Res. 2011;1374:63–72.
Prechtl JC, von der Emde G, Wolfart J, Karamürsel S, Akoev GN, Andrianov YN, Bullock TH. Sensory processing in the pallium of a mormyrid fish. J Neurosci. 1998;18(18):7381–93.
Rattenborg NC. Evolution of slow-wave sleep and palliopallial connectivity in mammals and birds: a hypothesis. Brain Res Bull. 2006;69(1):20–9.
Rial RV, Akaârir M, Gamundí A, Nicolau C, Garau C, Aparicio S, Tejada S, Gené L, González J, De Vera LM, Coenen AM, Barceló P, Esteban S. Evolution of wakefulness, sleep and hibernation: from reptiles to mammals. Neurosci Biobehav Rev. 2010;34(8):1144–60.
Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, Tononi G. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30(12):1643–57.
Riedner BA, Hulse BK, Murphy MJ, Ferrarelli F, Tononi G. Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves. Prog Brain Res. 2011;193:201–18.
Ringli M, Huber R. Developmental aspects of sleep slow waves: linking sleep, brain maturation and behavior.Prog Brain Res. 2011;193:63–82.
Robinson TE. Hippocampal rhythmic slow activity (RSA; theta): a critical analysis of selected studies and discussion of possible species-differences. Brain Res. 1980;203(1):69–101.
Ruskin DN, Bergstrom DA, Kaneoke Y, Patel BN, Twery MJ, Walters JR. Multisecond oscillations in firing rate in the basal ganglia: robust modulation by dopamine receptor activation and anesthesia. J Neurophysiol. 1999;81(5):2046–55.
Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, Phillips C, Rauchs G, Schnakers C, Sterpenich V, Vandewalle G, Luxen A, Maquet P. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2007;104(32):13164–9.
Schoenenberger GA, Maier PF, Tobler JH, Monnier M. A naturally occurring delta-EEG enhancing nonapeptide in rabbits. X. Final isolation, characterization and activity test. Pflugers Arch. 1977;369(2):99–109.
Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137(4):1087–106.
Steriade M, Contreras D, Curró Dossi R, Nuñez A. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993a;13(8):3284–99.
Steriade M, Nuñez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993b;13(8):3266–83.
Steriade M, Nuñez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993c;13(8):3252–65.
Stern JM, Caporro M, Haneef Z, Yeh HJ, Buttinelli C, Lenartowicz A, Mumford JA, Parvizi J, Poldrack R. A functional imaging of sleep vertex sharp transients. Clin Neurophysiol. 2011;122(7):1382–6.
Tinguely G, Finelli LA, Landolt HP, Borbély AA, Achermann P. Functional EEG topography in sleep and waking: state-dependent and state-independent features. Neuroimage. 2006;32(1):283–92.
Tobler I, Borbély AA. The effect of 3-h and 6-h sleep deprivation on sleep and EEG spectra of the rat. Behav Brain Res. 1990;36(1–2):73–8.
Trachsel L, Dijk DJ, Brunner DP, Klene C, Borbély AA. Effect of zopiclone and midazolam on sleep and EEG spectra in a phase-advanced sleep schedule. Neuropsychopharmacology. 1990;3(1):11–8.
Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26(4):407–18.
Vanhatalo S, Tallgren P, Andersson S, Sainio K, Voipio J, Kaila K. DC-EEG discloses prominent, very slow activity patterns during sleep in preterm infants. Clin Neurophysiol. 2002;113(11):1822–5.
Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci USA. 2004;101(14):5053–7.
Walter WG. The location of cerebral tumours by electro-encephalography. Lancet. 1936;228(5893):305–8.
Webb WB, Agnew Jr HW. Stage 4 sleep: influence of time course variables. Science. 1971;174(4016):1354–6.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Halász, P., Bódizs, R. (2013). Slow Wave Activity as Substrate of Homeostatic Regulation. In: Dynamic Structure of NREM Sleep. Springer, London. https://doi.org/10.1007/978-1-4471-4333-8_7
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4333-8_7
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4332-1
Online ISBN: 978-1-4471-4333-8
eBook Packages: MedicineMedicine (R0)