Abstract
We are dealing with the relationship between macrostructural (slope of the sleep cycles) and the microstructural dynamics. Descending (“D”) and ascending (“A”) slopes of cycles show opposite trends: “D” slope shows sleep promoting, while “A” slope shows wake/REM-promoting tendencies. Arousal-like phasic changes (PAT and CAP A2–3 phases) turned out to be overwhelming during the “A” slopes of sleep cycles, and their amount is increased from evening to morning. Within the “A” slopes their frequency increases before REM sleep periods. Sleep-like phasic changes have a different course. They are prevalent during the “D” slopes of the first sleep cycles and follow the homeostatic decay of slow wave oscillation. Accordingly, the elicitability of the two types of phasic events has opposite tendencies during night sleep from evening to morning: Arousal-like responses are prevalent during the “A” slopes and their responsivity increases, while sleep-like responses are prevalent during the “D” slopes and their elicitability decreases. Lastly, we point out the double nature of the forces behind tonic and phasic regulation: There are alternating chemical influences behind the tonic one and faster reciprocal sleep- and wake-promoting inputs behind the phasic regulation. Due to the intermingled phasic and tonic regulation, the sleeper is connected with the surrounding world and is able to execute two seemingly contradictory tasks: (1) separation for trophotropic (use dependent homeostatic) functions and (2) reactivity in form of state-dependent responses.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sleep cycles
- Descending slopes
- Ascending slopes
- Microstructural dynamics
- State-dependent reactivity
- Tonic- and phasic-regulation of sleep
4.1 Opposite Trends Across Descending and Ascending Slopes of Sleep Cycles
Since the beginning of the contemporary sleep studies, the stereotype sequences of sleep stages during sleep cycles have become well-known. The majority of studies have engaged in the characteristics of NREM-REM alternations, and the rules governing the stage sequences within the NREM cycle have received less attention. In the 1970s, researchers started to study how the sequence of NREM sleep stages influences the NREM-REM cyclicity (Brezinová 1974). One of the most important trends was to analyze the process-like behavior of the cyclic events (Caille and Bassano 1976), for example, how the preceding events or sequences determine the consecutive stages. These studies have found evidence supporting the “semi-Markov” nature of sleep stage sequences where the appearance of a certain stage depends partially on the preceding stages and partially on timing within the sleep process. Along these studies, it has become obvious that the sleep process is not only a continuous repetition of certain sequence of stages, but other long term processes from evening to morning also take place: The deepness of the cycles (the amount of slow wave sleep) decreases, while the length and density of REM periods grow from evening to morning.
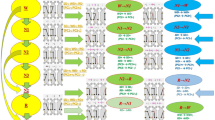
However, very few studies have been devoted to look for the mechanism behind the increasing-decreasing course of sleep depth during a sleep cycle. Blake and Gerard (1937) were the first who mentioned the increasing and decreasing states and EEG differences between them. They have emphasized that decreasing and increasing states are not like mirror images. Williams et al. (1964) analyzing stage sequences in sleep of young adult persons showed that on the deepening part of sleep cycles, the stages follow an order according to their depth, while on the A part of the cycles, some stages are skipped, and this part is usually shorter compared to the D one. Sinha et al. (1972) used the asymmetry (differences in steepness) of cycle slopes to measure the progress in the sleep process and forecast the time of awakening. In an early work (Halász 1982), we studied the order of sleep stages in two human sleep experiments according to their distribution along the “D” or “A” part of the cycle. The first two cycles of six whole night sleep records of one young healthy volunteer and in a second experiment whole night sleep records of eight other volunteers were studied. Part “D” was defined as a period of sleep between the waking state/REM sleep level and the first deepest phase of the given cycle, while part “A” as those periods of sleep between the last deepest phase end and the next highest level stage in the cycle. Our results are summarized in Fig. 4.1. The sequence of stages clearly showed the expected downward and upward order of stages along the “D” and “A” part of cycles, respectively. This was supported in 94.3 and 98.5 % of stage transitions in the 6 sleep records of the same subject and in the group of eight subjects, respectively. As a next step then, we measured the duration of the “D” and “A” slope of cycles in the same sleeps. A significant difference between the duration of “D” and A slopes has been found in the six sleeps of our young volunteers. The average duration of slope “D” was 37.7 min, while the duration of the slope A was only 21.9 min (p < 0.01). The same kind of asymmetry has been found in the other eight volunteers. The number of stage shifts was also measured in the two groups. It proved to be significantly higher on the “D” slope, compared with the “A” slope. Looking for the stage jumps, when the transition skipped a stage in the depth-order of stages, the “A” slope contained more of them, and the A slopes were found to be more steep just because of the higher number of these “jumps.”
Distribution of stage transitions during the descending and ascending slopes of sleep cycle in sleeps of eight healthy young volunteers. The thickness of the arrows indicates the occurrence (in percentage) of the indicated kind of transition. The large arrow shows that both on descending and ascending slopes of the cycle the overwhelming majority of stage transitions were going during the descending slope downward while during the ascending slope upward (Modified from Halász (1982))
Therefore, we can say that the two slopes of a certain sleep cycle reflect two different directions (downward vs. upward) of the sleep process. It seems probable that while the working mode of the “D” slope is “sleep promotion,” it is “wake/REM promotion” for the “A” slope. From a dynamic point of view, they deeply differ from each other. The consequence of this consideration means that the seemingly homogenous stage levels along different slopes of a cycle are not homogenous, for example, descending stage 2 is probably different from ascending stage 2 in several functional aspects reflecting the actual balance between sleep- and wake-promoting forces.
Another approach was taken by Merica and Fortune (1997) following the dynamic changes within a NREM cycle by power spectrum analysis. Spectral analysis provided a quantitative measure and showed how much the different oscillations (slow waves, spindles, fast frequency oscillations, etc.) coexist at all times in sleep. Their ratio defines stage assignment within the cycles. There are two important aspects in this work. One is the clear demonstration that transition from waking to sleep and further stage transitions within NREM sleep, NREM-REM transitions as well as the awakening process are all not sudden events but step by step progressive procedures, each involving transitional periods. Importantly, they also tried to trace back the EEG changes to the reciprocal inhibitory dynamics on the cellular activity level of the sleep- and wake-promoting structures. This analysis clearly demonstrates that sleep is never a static state during none of the stages, either on the “D” or the “A” slopes of the cycles. They measured the simultaneous power-time courses during the cycles and demonstrated the characteristic composition pattern of delta waves, sigma spindles, and beta activity shaping the less steep “D” and steeper “A” slopes of cycles. This time course of the different frequencies may be the result of the progressive decrease in the firing rate of wake-promoting neurons. It results in diminished excitatory input to the thalamus and a consequent, gradual hyperpolarization of the thalamic relay cells, building up the burst-firing pattern, leading first to spindling and later to develop delta activity. Interestingly, during the “D” slope of cycles they have found multiple delta peaks, instead of one monotonic rise of deltas, and they assumed a correlation of this phenomenon with CAP events, otherwise not taken into consideration in their work.
The structure of sleep cycles, at least that of the first three ones is obviously shaped by an asymmetric (longer) “D” and (shorter) “A” slope. The “turning point” from deep sleep during the first cycles is very characteristic and frequently marked by a sudden movement. (This period is “abused” by pathological arousal events in children with pavor nocturnus and adults with night terror). The dynamics of this period and subsequent uprising course of sleep leading to REM sleep need further explanation.
4.2 Distribution of Phasic Events and Micro-arousals Across the Sleep Process
4.2.1 The Distribution of PAT-Like Micro-arousals Across the Sleep Process
The distribution of PAT is not homogeneous across the sleep cycles: They are more frequent during the A slopes of the cycles compared with the D slopes, and their frequency increases from evening to morning (Schieber et al. 1971). These early findings were confirmed by Halász (1982) and also later after coining the American Sleep Disorders Association (ASDA) criteria of arousal (ASDA 1992; Terzano et al. 2002). The distribution of arousals consistent with ASDA criteria has followed similar distribution during night sleep: increasing from evening to morning (Terzano et al. 2002).
4.2.2 The Distribution and Dynamics of K-Complexes Across the Sleep Process
Regardless of the level of sleep or sleep stage, the number of K-complexes seems to correlate with the actual direction of sleep process. In our earlier studies (Halász 1982), the number of K-complexes during the stage 2 sleep periods was rather variable. When the “D” types (preceded by a more superficial sleep stage and followed by a deeper one) and the A type ones (preceded by deeper one and followed by a more superficial one) were compared, a significant difference was found in the number of K-complexes. “A” type contained more K-complexes than the “D” type. K-complex number proved to be in positive correlation with the deepness of the actual cycle. K-complex frequency decreased across the night sleep from evening to morning, according the serial order of cycles, parallel with the well-known course of the S-process of Borbély. Under the influence of psychostimulant drug, this effect ceased together with the regular course of slow wave decay (Fig. 4.2). Unlike types D, a shift in the frequency of K-complexes was very characteristic for type A stage 2.
Regression lines for average K-complex densities as functions of sleep cycle order and different treatment conditions in eight healthy adult subjects. K-complex values have shown decreasing tendencies from evening to morning in all the studied conditions except under influence of psychostimulant (parallel with the missing of the regular slow wave decay). The highest value of K-complexes was observed under acoustic stimulation (Acoustic stimulation consisted of pseudo-random 200 Hz tone clicks with durations of 10 ms and an average inter-stimulus interval of 40 s) (Modified from Halász et al. (1985))
Continuously or periodically applied random acoustic stimulation increased the number of all registered K-complexes but simultaneously decreased the number of the spontaneous ones. This change is similar to the behavior of PAT, as seen before in Chap. 3. An analysis of the K-complex number distribution during stimulation nights showed that the increase of K-complexes could mainly be attributed to an elevation of the number of type A stage 2 periods. Comparing the “A” and “D” slopes of sleep cycles, K-complex rates along proved to change more on “A” slopes and less on “D” slopes (Fig. 4.3).
Also, these studies showed again very clearly the double nature of K-complex: behaving like an arousal pattern that can be triggered by acoustic stimuli and at the same time changing parallel with the delta activity that means dampening along the sleep process, and their rate is related to the depth of the actual sleep cycle. So we can say that K-complex formation is partially associated with phasic sensorial inputs and partially with the degree of sleep synchronization, for example, depth of sleep.
Furthermore, these data show that K-complex is an indicator of the clash between antagonistic sleep and wake promoting forces with the result of maintaining (protection) of sleep.
The distribution of K-complexes in different parts and orders of cycles as well as the differences in their elicitability associated to dynamic phasic moments of NREM sleep throws light again to the basic instability of sleep. It unmasks the ever changing balance of sleep- and wake-promoting forces shaping the course of sleep.
Since K-complex formation is a part of slow wave activity during sleep its behavior nicely shows that the slow wave cloud during our NREM sleep is partially reactive. Later, we will see that this applies also for CAP A1 sequences representing a much higher level beyond the K-complexes, behaving similarly to reactive sleep slow waves.
4.2.3 How Does CAP Behave Across the Cycles and During the Different Slopes of Cycles?
On the “D” slopes and especially during the first cycles, CAP A phases are less frequent than on the “A” slopes. Furthermore, the D slopes of the first cycles are characterized by the preponderance of the synchronization type, sleep-like answers (A1 type), associated with mild autonomic perturbations (Halász 1982; Halász et al. 1985; Terzano and Parrino 2000). During the “A” slopes, phasic events and micro-arousals are more frequent, and both the EEG morphology and the associated autonomic changes are more similar to the conventional desynchronization type arousal (A2 and A3 type). Expressing this in terms of CAP A subtypes, the distribution of the different phase A subtypes proved to be different across the sleep cycles. Subtype A1 occurs most frequently in the first cycles of sleep and during “D” slopes, while subtypes A2 and A3 are more frequent during later part of sleep across A slopes of the cycles (Parrino et al. 2012) (Fig. 4.4).
A differential distribution of A1 versus A2/A3 events over the course of sleep. Distribution of A1 phases shows a gradual decay during each descending slope of cycles and a global decrease from evening to morning, according to the slow wave homeostatic decay. Contrasting the distribution of A2–3 phases have shown a peak during the ascending slopes of each cycle near before REM sleep, moving together more with the arousal process instead of the homeostatic sleep process (Based on Terzano and Parrino 2000)
The distribution of CAP sequences across different slopes of the sleep cycles is very much similar to the distribution showed for PATs and K-complexes. The distribution of CAP A1 (and less the A2) phase shows similar distribution to that of PATs, whereas the distribution of A1 (less A2) is similar to the distribution of K-complexes. This is not surprising hence A1 and A2 contains K-complexes, and the second part of A2 and mainly A3 is identical with PATs since CAP is a global framework and overlaps with the previously described isolated phasic events and micro-arousals.
4.3 Tonic and Phasic Regulation in NREM Sleep: Relationship of the Macro- and Microstructure of Sleep Cycles
Tonic and phasic regulation works simultaneously. During “D” slope the sleep-promoting system probably has a gradually increasing predominance inhibiting the wake-promoting cell groups. Accordingly, the phasic activation is rare and results in antiarousal (sleep-promoting) response. In contrast, during slope “A” the wake-promoting system becomes gradually more dominant (Fig. 2.1). Consequently, the phasic input is more frequent and becomes more variable (antiarousal responses are present together with desynchronizational responses). Some of the micro-arousals become effective promoting state transitions as well. Therefore, during the “D” slope, the phasic input serves the avalanching sleep process. During the “A” slope, however antiarousal answers (at least in the form of K-complexes) are more frequent: reflecting the progress of the arousal process, leading to REM sleep. So the phasic input, due to the changing tonic influence, has state-dependently different effect on the “D” and “A” slopes of sleep cycles.
The tonic regulation has endogenous neuromodulatory, chemical origin, serving the biological clock and showing little flexibility to external influences. It may have NREM-promoting (during the D slope) or REM-promoting (during the A slope) nature.
On the contrary – according to our concept – phasic regulation is more related to those neural mechanisms that serve the sensory connection with the environment, characterized by faster synaptic procedures. It is more variable and more dependent on external factors.
So the “D” slopes of the cycles are more determined by the factors of the internal biochemical clock, while the “A” slopes, for example, the second part of the cycles, depend more on the connection with the external world.
Recently, we have recognized several examples of the two types of regulation working simultaneously and together. One of them provides a central (usually paired reciprocal) level setting tonic effect, while the other works following the reflex principle ensuring fast answers to the stimuli of the external world. The central regulation has a level setting tonic influence determining the state, while the other reflex-like regulations provide a flexible reactivity for the system and promote the state dynamic.Footnote 1 The two systems interact. Dynamic microstructural oscillations governed by input-dependent phasic regulation (CAP system) modulate the larger scale chemically driven oscillation (sleep cyclicity), and in the same time their elicitability and reaction type (A1 or A2–3) are state dependently determined by the larger scale shifts of the tonic regulation. The same sensory stimulus may have sleep-promoting effect on the “D” slope and wake/REM-promoting effect on the “A” slope. Conversely, phasic activity shapes the course of tonic states providing them flexibility, promoting the state shifts.
The simultaneous tonic and phasic regulation of sleep ensures a flexible connection between the sleeper and the surrounding world and ensures the reversibility and self-regulatory nature of sleep (that differentiates sleep from comatose state).
4.4 The Nature of State Transitions and Defense Mechanisms Against Transitions (Providing Stability by Permitting Lability)
As we have shown in the previous chapters, sleep has several dynamic aspects. The sleep process contains cycles and each cycle (at least the first three to four) are built up by a “D” and an “A” slope which contains (according to the Rechtschaffen-Kales rules) sleep stages differing according to their situation on the “D” or “A” slopes of a given cycle. Stage borders are artificial, and sleep stages do not represent homogenous conditions, as was nicely shown by Merica and Fortune (1997). The stages reflect several signs of continuous deepening or lightening tendencies along the slopes of the cycles. Therefore, the transitions from one to another sleep stage seem to be stepwise: the onset of a stage bears the features of the previous one and its last part shows those of the next stage. However, under the seemingly smooth surface of sleep stages (which are merely artificially constructed conditions), there are continuous fluctuations in the form of phasic events systematized by the Parma school as a system of cyclic alternating pattern (CAP), moved by the actual balance of antagonistic sleep-promoting and arousal forces.
Contrasting to these small fluctuations, the switch from waking to sleep state and the turn from deep sleep at the through of a cycle to the “A” slope, furthermore switches to REM sleep and from REM to NREM, are larger events of the sleep/wake continuum.
As described in Chap. 1 both the wake- and sleep-promoting neuronal assemblies in the hypothalamus, like the REM-on and REM-off networks in the brain stem appear to be mutually inhibitory. Saper et al. (2001) proposed that this mutually antagonistic relationship would be similar to that seen with a so-called flip-flop electrical switch. This kind of switches ensures rapid and complete state transitions, since when either side of the antagonistic populations gains a small advantage, the inhibitory action of the other will rapidly lose its strength causing collapse of activity and an avalanche-like dominance of the other.
This kind of switch would ensure the after-switch stability both of waking or sleep and REM or NREM state. Saper et al. (2010) have arguments for the biological advantage of state stabilization saying that it may prevent an individual from falling asleep during boring activity or waking up during the night with every small sound of the house. However, in the reality we frequently fall asleep reading a not much interesting book, or wake up to noises with different significance. Sleep is differentiated from coma by arousability, which is a flexible possibility to wake up if the arousal stimulus has enough significance. Let us take the example of a prey animal. This kind of continuously tilting bilabile state ensures for the animal organism two seemingly controversial requirements: to maintain sleep separated from the environment and preserve floating attention to monitor potentially dangerous environmental events.
We have seen in the previous chapters that NREM sleep is an ever oscillating flexible state that contributes to preserve continuous contact with the environment and keeps the states in an ever changeable unsteady condition. This kind of regulation seems not realized by “bistable” states. Better to say that this regulation provides stability by permitting lability. The tilting microstates with alternating arousal or sleep-promoting oscillations ensure a higher level of stability and at the same time provide continuous possibilities for changes due to environmental demands. Therefore, in between the more deterministic state switches at the main macrostate steps, like wake to NREM and NREM to REM during a sleep cycle, the NREM states seem to behave not according to the rules of bistability predicted by the Saper model.
4.5 Promoting Forces Behind (Alternating Chemical Influences and Faster Phasic Reciprocal Sleep- and Wake-Promoting Inputs)
One of the factors determining reactivity of the organism to stimulation is the state in which the input arrives. We may speak about “state-dependent” reactivity. It is different in REM and NREM sleep and within NREM according to the “D” and “A” slope of sleep cycles (Halász 1982). A further question is the following: What are the forces underlying reciprocal tendencies during “D” and “A” slopes of the cycles and underlying shorter scale similar reciprocal tendencies reflected in phasic events and micro-arousals? Both the structure of cycles and nature of synchronizational and desynchronizational micro-arousals or in other words A1 versus A2–3 CAP types may be designated as either “sleep promoting” or “wake promoting.”
Let us concentrate first to the alternating tendencies observed during sleep cycles. The alternation of NREM and REM sleep was formulated by McCarley and Hobson as early as 1975 in a “reciprocal interaction” model at the cellular level between cholinergic and aminergic neuronal populations located in the brain stem (McCarley and Hobson 1975). The model was based on the contemporary findings showing firing aminergic REM-off neurons located to the locus coeruleus and dorsal raphe nucleus with an activity in waking, gradual decrease of firing during NREM, and a firing of REM-on cholinergic neuron population in the mesopontine (laterodorsalis tegmental and pedunculopontine tegmental nucleus) region during REM. A mathematical model using the Lotka-Volterra equation (originally worked out for the predator/prey interrelationship) seemed to be satisfactory explaining the detected firing relations in the aminergic and cholinergic brain stem nuclei and also to explain the state alternation on the EEG and behavioral level. Later the model was revised and adapted according to new neuromodulatory data and incorporating the influence of external noise by McCarley and Massaquoi (1992).
However, the relevance of the McCarley-Hobson model has been challenged by studies showing that lesions damaging the cholinergic and aminergic neuron populations had little effect on REM sleep (Webster and Jones 1988; Shouse and Siegel 1992; Lu et al. 2006; Blanco-Centurion et al. 2007). These data suggest only a modulator role for the cholinergic and aminergic systems in NREM/REM sleep switch. Subsequent studies revealed the key role of sublaterodorsal, pre-coeruleus and medial parabrachial nuclei in the mesopontin region. This model predicts a gradual transition to prepare the start of the REM period which may provide explanation for the changes experienced on the A slope of the NREM cycle. Accordingly, the disinhibition of the cholinergic nuclei starts at the turning point of the cycle, and the rising cholinergic activity brings the REM period closer.
Therefore, the “A” slope of the NREM cycle belongs more to the preparation phase to REM and arousal (ergotroph) activity, and the “D” slope can be considered as a slow wave sleep-promoting endophylactic process.
We have seen that each sleep stage both during the “D” and “A” slopes of the cycles is shaped by microstructural oscillations consistent with the CAP phenomena with alternative sleep- and wake-promoting micro-changes. Therefore, it seems logical to conclude that the microstructural oscillations are somehow related to sleep- and wake-promoting subcortical hypothalamic networks described by von Economo and later by the Boston (Saper and coworkers) and Lyon (Lupi and coworkers) research groups.
We may explain the wake-promoting phasic influences by periodic short-term (micro) arousals fuelled by internal and/or external sleep-perturbing impulses transitorily breaking through VLPO exerted inhibition of the RAS system. There are several proofs for the existence of such events even in neuroimaging studies (Riedner et al. 2011; Kaufmann et al. 2006; Dang-Vu et al. 2008).
The mechanism of sleep-promoting (antiarousal) phasic events (K-complex, A1 phase) seems to be more complicated. The question is how phasic activation of the VLPO system is transferred to cortical slow wave group or K-complex? This transformation from arousing to sleep-promoting effect of a sleep-disturbing input seems to be clearly connected with the overwhelming domination of the burst-firing working mode of the thalamocortical system, after the shutting down of RAS system. The wake-promoting ascending arousal systems and the sleep-promoting VLPO system are in a reciprocal antagonistic relationship (Saper et al. 2001). When VLPO systems exert GABA- and galanin-ergic inhibitory influence and keep firing in a certain rate during sleep the inhibitory influence of the arousal system dissipates. When a phasic arousal influence is arriving, the VLPO system transitorily becomes weakly inhibited, but if the arousal influence stops, the VLPO system becomes liberated again fuelling the back inhibition of the arousal system, producing (quasi rebound) sleep phenomena by switching on the thalamocortical system (CAP A1 phase). This holds especially true when on the “D” slopes of the first cycles the VLPO system is prevailing and the arousal system is weak. During the third part of sleep on the “A” slopes of the cycles, the situation is different: The VLPO system is probable less active in inhibiting the arousal system; therefore, the arousal impulses easily activate the cortex and the phasic arousals achieve more prominent arousals in the form of CAP A2 and A3 responses driving the sleeper toward more superficial vigilance states.
We will see in the next chapter how the so-called homeostatic regulation modulates sleep. The homeostatic pressure is the force which determines the course of the D slope. It keeps high the firing rate of VLPO at the beginning of D slopes, inhibiting the wake-promoting system and therefore liberating the burst-firing thalamic machine of slow wave sleep, entraining sensory stimuli as fuel for slow wave sleep by the instant homeostatic delta injections of CAP A1 phases.
When the homeostatic pressure gradually decreases along the course of the “D” slope, this gives way to the continuously incoming sensory impulses, which start to bring the sleeper along the “A” slope to REM sleep.
Notes
- 1.
Another example of similar cooperation between the tonic and phasic systems in a certain physiological functions is seen in the relationship of brain stem (descending) influence of muscular tone regulation versus phasic activity realized in spinal reflexes.
References
ASDA Report. EEG arousals: scoring, rules and examples. Sleep. 1992;15(2):173–84.
Blake H, Gerard RW. Brain potentials during sleep. Am J Physiol. 1937;119:692–703.
Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27(51):14041–8.
Brezinová V. Sleep cycle content and sleep cycle duration. Electroencephalogr Clin Neurophysiol. 1974;36(3):275–82.
Caille EJ, Bassano JL. Tonic structure and model of sleep distortion. Rev Electroencephalogr Neurophysiol Clin. 1976;6(2):330–4.
Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, et al. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci USA. 2008;105(39):15160–5.
Halász P. The role of the non-specific sensory activation in sleep regulation and in the pathomechanism of generalized epilepsy with generalized spike-wave discharge. Doctoral thesis, Budapest; 1982.
Halász P, Pál I, Rajna P. K-complex formation of the EEG in sleep. A survey and new examinations. Acta Physiol Hung. 1985;65(1):3–35.
Kaufmann C, Wehrle R, Wetter TC, Holsboer F, Auer DP, Pollmächer T, et al. Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: an EEG/fMRI study. Brain. 2006;129(Pt 3):655–67.
Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94.
McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975;189(4196):58–60.
McCarley RW, Massaquoi SG. Neurobiological structure of the revised limit cycle reciprocal interaction model of REM cycle control. J Sleep Res. 1992;1(2):132–7.
Merica H, Fortune RD. A neuronal transition probability model for the evolution of power in the sigma and delta frequency bands of sleep EEG. Physiol Behav. 1997;62(3):585–9.
Parrino L, Ferri R, Bruni O, Terzano MG. Cyclic alternating pattern (CAP): the marker of sleep instability. Sleep Med Rev. 2012;16:27–45.
Riedner BA, Hulse BK, Murphy MJ, Ferrarelli F, Tononi G. Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves. Prog Brain Res. 2011;193:201–18.
Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726–31.
Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68(6):1023–42.
Schieber JP, Muzet A, Ferriere PJ. Phases of spontaneous transitory activation during normal sleep in humans. Arch Sci Physiol (Paris). 1971;25(4):443–65.
Shouse MN, Siegel JM. Pontine regulation of REM sleep components in cats: integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571:50–63.
Sinha AK, Smythe H, Zarcone VP, Barchas JD, Dement WC. Human sleep-electroencephalogram: a damped oscillatory phenomenon. J Theor Biol. 1972;35(2):387–93.
Terzano MG, Parrino L, Boselli M, Smerieri A, Spaggiari MC.CAP components and EEG synchronization in the first 3 sleep cycles.Clin Neurophysiol. 2000 Feb;111 (2):283–90.
Terzano MG, Parrino L, Rosa A, Palomba V, Smerieri A. CAP and arousals in the structural development of sleep: an integrative perspective. Sleep Med. 2002;3(3):221–9.
Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302.
Williams RL, Agnew HW, Webb WB. Sleep patterns in young adults: an EEG study. Electroencephalogr Clin Neurophysiol. 1964;17:376–81.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Halász, P., Bódizs, R. (2013). The Cyclic Structure of Sleep: Relationship Between the Macrostructural Slopes of Cycles and Microstructural Dynamics. In: Dynamic Structure of NREM Sleep. Springer, London. https://doi.org/10.1007/978-1-4471-4333-8_4
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4333-8_4
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4332-1
Online ISBN: 978-1-4471-4333-8
eBook Packages: MedicineMedicine (R0)