Abstract
A main part of fundamental knowledge about sleep regulation roots in the recognition of reciprocally antagonistic sleep-wake-promoting influences originating in brainstem/hypothalamic neuronal networks and in their dynamic interplay. All the presently known concepts and models of sleep regulation are essentially based on the anatomy and function of these subcortical networks. From the beginning of this century, the Harvard School of sleep research and the Lyon sleep research group have introduced important concepts about the working of the flip-flop model of “sleep switch” governing sleep/wake alternations and tried to connect these views with the earlier ideas explaining sleep cyclicity. These results have suggested that the subcortical neuronal assemblies have indeed a governing role in the EEG and behavioral changes during the sleep/wake cycle. However, within the NREM sleep period, robust dynamical changes take place, about which the flip-flop model has nothing to say about. NREM sleep cannot be taken as a “stable” state. Growing evidences about the “microstructure” of NREM sleep present sleep as an ever-changing fluctuating state intermingled with microarousals, strongly contradicting to this simple model.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hypothalamus
- Sleep regulation
- Sleep switch
- Flip-flop model
- NREM sleep
- Wake-sleep transition
- Sleep microstructure
The first important step in understanding wake/sleep regulatory system in the brain was the discovery of the Viennese neurologist Baron Constantin von Economo in 1930. Based on clinicopathologic studies on victims of pandemic encephalitis lethargica, he observed that the prolonged sleepiness of one part of the victims is due to posterior hypothalamus and rostral midbrain injury. He has also observed that those affected people who suffered prolonged insomnia had lesions of the preoptic area and basal forebrain. Based on his observations, Economo assumed that the region of the hypothalamus near to the optic chiasm should contain sleep-promoting, while the posterior hypothalamus wake-promoting neurons (von Economo 1930). The key role of these two regions in sleep/wake regulation has been confirmed by animal experiments (Nauta 1946; Swett and Hobson 1968; McGinty and Sterman 1968).
Almost 20 years later, Moruzzi and Magoun (1949) discovered the ascending reticular arousal system (RAS), originating in the upper brainstem, fuelled by the sensory brainstem input pathways. During the subsequent years, the different chemical transmission branches of RAS system were explored in detail (Jones 2003).
The question how this powerful system of wakefulness is switched of when we fall asleep remained enigmatic for a long time. At the turn of the twentieth/twenty-first century, Saper et al. (2001) and Gallopin et al. (2000) showed clearly that the ventrolateral preoptic area (VLPO and extended VPLO) sends GABA-ergic and galaninergic inhibitory impulses to all the brainstem nuclei harboring the ascending pathways of the arousal systems and keeps firing throughout the whole NREM sleep, providing the substrate of the “sleep system” having opposite function to the “wake system.” Later, it turned out that the arousal systems also exert inhibitory effect on the “sleep-promoting” preoptic neurons (Saper et al. 2010).Footnote 1
The discovery of the orexinergic system as lastly recognized special member of the arousal system provided further evidences for the mutually antagonistic system governing the actual balance between wakefulness and sleep (Nishino 2011). The area of basal forebrain has been thoroughly explored looking for sleep-promoting neuronal groups. It has been established by the c-Fos method that the ventrolateral preoptic nucleus (VLPO), a small circumscript area, exerts enduring neuronal activity during animal sleep. Further studies showed that in the preoptic region, a diffusely situated area (MnPN) has sleep-promoting activity too. Additional confirmation of the sleep-promoting role of this area came from neurotoxic destruction experiments of the VLPO and MnPN neurons (McGinty et al. 2004).
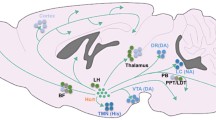
It has also been demonstrated that VLPO and the suprachiasmatic nucleus have synchronized activity, and both of them receive input from the retinal ganglionic cells. Therefore, circadian and visual information may modulate the VLPO activity that would be the substrate of circadian timing for the sleep process. Interesting tract-tracing and stimulation studies confirmed the presence of reciprocal inhibitory connection between the VLPO/MnPN neurons and the neurons of the ascending arousal system. These data serve as the substrate of the “sleep switch model” (Saper et al. 2001, 2010) based on the mutual inhibition between VLPO and the major arousal systems (Fig. 1.1).
A flip-flop switch model for wake-sleep regulation. Preoptic sleep-promoting neurons inhibit the arousal systems during sleep and are inhibited by the arousal systems during wake. As a result, the two mutually inhibitory systems form a flip-flop switch that is stable in either state but switches rapidly between them. Red arrows sleep promotion, green arrows wake promotion, VLPO ventrolateral preoptic area, LDT/PPT laterodorsal/pedunculopontine tegmental cells, LC locus coeruleus, TMN tuberomammillary nucleus (Modified from Saper et al. 2001)
When VLPO neurons fire during sleep they would inhibit the arousal system cell groups thus disinhibiting and reinforcing their own firing. Similarly when arousal neurons fire at high rate during wakefulness, they would inhibit the VLPO, thereby disinhibiting their own firing. This reciprocal relationship is similar to a type of circuit that electrical engineers call ‘flip-flop.’ The two halves of a flip-flop circuit, by each strongly inhibit the other; create a feedback loop that is bistable, with two possible stable patterns of firing and a tendency to avoid intermediate states (Saper et al. 2001).
The experimental evidence for the sleep switch model of Saper is mainly based on the work of Takahashi and coworkers (2006, 2009, 2010). The Japanese group systematically detected the neuronal firing pattern of different sleep- and wake-promoting hypothalamic neuronal populations during falling asleep, sleeping, and awakening. Transitions from wake to sleep were characterized by the decrease of the firing rate of neurons of the wake-promoting and rising in the sleep-promoting neuronal network, and during awakening, an opposite dynamics of firing pattern was detectable (Fig. 1.2). These results confirm that these subcortical neuronal assemblies indeed have governing role in the EEG and behavioral changes during the sleep/wake cycle. However, the dynamics of falling asleep showed that the initiation of sleep is caused by the decreased activity of the wake-promoting neurons (disfacilitation) and not by the activity of sleep-promoting neurons (see also Steriade 2001). These results need confirmation and further research.
Reciprocal firing patterns between sleep-promoting neurons in the preoptic area and wake-promoting neurons in the locus coeruleus (LC), tuberomammillary nucleus (TMN), and basal forebrain. The top panel shows changes in firing rate during the transition from NREM sleep to wake, and the bottom panel shows firing rates during the transition from wake into light NREM sleep. Note that the firing rates of some cell groups, such as the LC, begin to increase or decrease 1–2 s in advance of awakening or falling asleep, suggesting that they may help drive the transition. In contrast, neurons in the TMN begin to fire only after the transition to wake, suggesting these cells may play more of a role in maintenance of wakefulness. Recordings were made in unanesthetized, head-restrained mice (Adapted from Saper et al. (2010))
However, the course of sleep – depicted even roughly in the conventional hypnograms (Fig. 1.3) – shows us that sleep is not a big black hole which we fall in (“off switch”) at evening and from which we suddenly get out (“on switch”) at awakening in the morning. Sleep is organized in cycles where sleep goes deeper and deeper for a while, but after cc. 60 min, the direction of the process changes and stepwise became more and more superficial giving place to the first REM period. This sequence of event repeats in 4–6 times shaping the consecutive sleep cycles. In other words, within the NREM sleep period, robust dynamical changes take place, about which the flip-flop model has nothing to say about.
NREM sleep cannot be considered as a “stable” state, and as we will see in later parts of this book, indeed, growing evidences about the “microstructure” of NREM sleep (Terzano et al. 1985) present sleep as an ever-changing fluctuating state intermingled with microarousals, strongly contradicting this simple model (Halász et al. 2004).
Our guiding principle in this monography will be just the opposite view: sleep is essentially an unstable (multistable) state where the transitions from one state to another are not distinct, but gradual and the different levels of instability are maintained by continuous oscillation between sleep- and arousal-promoting influences. The macrotendencies prevail through oscillations: microstates build up macrostates. We will propose that input-dependent instability, expressed by phasic activation/deactivation, is an essential inbuilt feature of NREM sleep.
References
Détári L, Vanderwolf CH. Activity of identified cortically projecting and other basal forebrain neurones during large slow waves and cortical activation in anaesthetized rats. Brain Res. 1987;437(1):1–8.
Détári L, Juhász G, Kukorelli T. Firing properties of cat basal forebrain neurones during sleep-wakefulness cycle. Electroencephalogr Clin Neurophysiol. 1984;58(4):362–8.
Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, Audinat E, Mühlethaler M, Serafin M. Identification of sleep-promoting neurons in vitro. Nature. 2000;404(6781):992–5.
Halász P, Terzano M, Parrino L, Bódizs R. The nature of arousal in sleep. J Sleep Res. 2004;13(1):1–23.
Jones BE. Arousal systems. Front Biosci. 2003;8:s438–51.
McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160(833):1253–5.
McGinty D, Gong H, Suntsova N, Alam MN, Methippara M, Guzman-Marin R, Szymusiak R. Sleep-promoting functions of the hypothalamic median preoptic nucleus: inhibition of arousal systems. Arch Ital Biol. 2004;142(4):501–9.
Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1(4):455–73.
Nauta WJ. Hypothalamic regulation of sleep in rats; an experimental study. J Neurophysiol. 1946;9:285–316.
Nishino S. Hypothalamus, hypocretins/orexin, and vigilance control. Handb Clin Neurol. 2011;99:765–82.
Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726–31.
Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68(6):1023–42.
Steriade M. Active neocortical processes during quiescent sleep. Arch Ital Biol. 2001;139(1–2):37–51.
Swett CP, Hobson JA. The effects of posterior hypothalamic lesions on behavioral and electrographic manifestations of sleep and waking in cats. Arch Ital Biol. 1968;106(3):283–93.
Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370(1):82–92.
Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26(40):10292–8.
Takahashi K, Lin JS, Sakai K. Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience. 2009;161(1):269–92.
Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience. 2010;169(3):1115–26.
Terzano MG, Mancia D, Salati MR, Costani G, Decembrino A, Parrino L. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep. 1985;8(2):137–45.
von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71(3):1–5.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Halász, P., Bódizs, R. (2013). Development of the Concept of Sleep-Wake-Promoting Systems in the Brainstem and Hypothalamus. In: Dynamic Structure of NREM Sleep. Springer, London. https://doi.org/10.1007/978-1-4471-4333-8_1
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4333-8_1
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4332-1
Online ISBN: 978-1-4471-4333-8
eBook Packages: MedicineMedicine (R0)