Abstract
Mitral valve disease comprises a variety of pathologies that lead to valvular regurgitation or stenosis. In the developed world, clinicians most often encounter patients with mitral regurgitation (MR). Advances in echocardiography, particularly 3D, have substantially improved the diagnosis of mitral valve pathology and quantification of lesion severity. Currently no medical therapy directed at primary MR improves the natural history of the disease, but significant progress has been made in the surgical management of primary MR where better repair techniques have decreased operative mortality and improved durability.
Clinical management of patients with secondary MR remains a challenge, including whether, when, and how to intervene to improve patient-centered outcomes and importantly current guidelines consider this a separate disease from primary MR.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mitral regurgitation
- Mitral stenosis
- Degenerative mitral valve disease
- Mitral valve prolapse
- Secondary mitral regurgitation
- Valvular heart disease
- Echocardiography
- Hemodynamics
- Mitral valve repair
- Mitral valve replacement
- Heart failure
Introduction
Primary mitral valve disease encompasses those entities where disease of the valve itself causes the pathophysiology leading to clinical impairment. Primary mitral valve disease comprises a variety of pathologies that lead to valvular regurgitation or stenosis. In the developed world, clinicians most often encounter patients with mitral regurgitation (MR) . Advances in echocardiography, particularly 3D, have substantially improved the diagnosis of mitral valve pathology and quantification of lesion severity. Currently no medical therapy directed at primary MR improves the natural history of the disease, but significant progress has been made in the surgical management of primary MR where better repair techniques have decreased operative mortality and improved durability.
Clinical Presentation
Mitral Regurgitation
Acute MR is relatively uncommon, but may occur due to disruption of various parts of the mitral apparatus, including a perforated leaflet (infective endocarditis), torn chord (myxomatous disease), or ruptured papillary muscle (myocardial infarction). In the context of a left atrium (LA) and left ventricle (LV) that have not undergone adaptive changes to volume overload, this sudden substantial increase in volume is associated with a rapid increase in LV end-diastolic and LA pressure leading to pulmonary edema and respiratory distress. Reflex tachycardia helps maintain forward flow, but cardiac output is often inadequate and associated with hypotension and shock. As such, patients with acute MR can present suddenly in a severely decompensated state requiring urgent supportive care and intervention.
The key to the diagnosis of all valvular disease is the auscultation of a typical murmur on physical examination. However because of the rapid rise in LA pressure in acute MR, the typical holosystolic murmur of MR is often shortened, occurring only in early systole and also may be relatively low in intensity.
Myxomatous degeneration of the mitral valve is the leading cause of primary MR in developed countries and often is first noted on physical exam as a mid-systolic click (as the redundant valve tissue and chordae snap as the valve closes) followed by a late systolic murmur as the leaflets extend past their coaptation point. Maneuvers that decrease LV volume, i.e., Valsalva, becoming upright, etc., cause the click to occur earlier in systole and the murmur to become louder and more holosystolic. The opposite occurs with maneuvers that increase LV volume such as squatting. As the disease progresses, the murmur becomes progressively more holosystolic and when regurgitation is severe may be followed by an S3 owing to the rapid filling of the large volume of blood stored in the LA during systole.
Chronic primary MR , however, can progress slowly over many years and symptoms may develop insidiously [1]. The development of symptoms is due to a number of factors including the regurgitant volume, compensation of the LV in terms of size and systolic function, and whether there is associated pulmonary hypertension or an atrial arrhythmia. Commonly, the initial symptom is dyspnea with considerable exertion that becomes more limiting over time with less exercise. These early symptoms may be missed in older patients who are more sedentary or dismissed as attributable to aging.
Mitral Stenosis
The most common cause of symptomatic mitral stenosis (MS) is rheumatic carditis, which has decreased in prevalence markedly in the developed world with the widespread availability of antibiotics [2, 3]. However this decrease began prior to antibiotic usage implicating improved socioeconomic factors in the decline in incidence. The process of inflammation, fibrosis, and calcification that occurs after rheumatic fever causes a progressive narrowing of the mitral orifice over decades. Symptom onset is insidious and related to the severity of orifice obstruction, severity of pulmonary vascular remodeling and hypertension, and patient activity level. Patients generally present with decreased exercise capacity, dyspnea on exertion, and fatigue. Other symptoms may include orthopnea, palpitations (from associated atrial arrhythmias), hemoptysis, and chest pain [3]. A systemic embolism may be the first presenting sign. Physiologic states that either increase transvalvular flow or decrease diastolic filling time (e.g., exercise, pregnancy, fever, hyperthyroidism, atrial arrhythmia) can precipitate or worsen symptoms due to increased LA pressure and transvalvular gradient. Extreme left atrial enlargement may impinge upon the left recurrent laryngeal nerve causing hoarseness (Ortners syndrome) or may cause dysphagia from compression of the esophagus.
The typical murmur of MS is a soft diastolic rumble heard best with the patient in the left lateral decubitus position. It may be accompanied by a diastolic thrill. S1 is typically loud as the valve, held open by the transmitral gradient throughout diastole, closes from the fully open (albeit stenotic) position. In very severe disease valve movement may be so limited that S1 is soft instead of loud. The diastolic rumble is usually preceded by an opening snap (OS). The interval between S2 and OS is a good guide to MS severity. In severe disease, high LA pressure opens the valve sooner than in mild disease producing a narrow S2–OS interval. If pulmonary hypertension has intervened, the pulmonic component of S2 has increased intensity and an RV lift may be palpated.
Disease Staging
The 2014 AHA/ACC guidelines on valvular heart disease shifted away from classifying the severity of valve disease into categories of mild, moderate, and severe [4, 5]. The emphasis, instead, is on a more integrative assessment of the stages of the valve disease that are more patient-centered with implications for the timing of intervention. These stages incorporate qualitative and quantitative hemodynamic assessment of the valve lesion, the response of the left and/or right ventricle to the volume or pressure overload, the effect of the valve lesion on the pulmonary circulation and heart rhythm, and patient symptoms [5]. With respect to mitral valve disease, stage A represents patients with risk factors for mitral valve disease (a history of rheumatic fever for instance); stage B represents patients with progressive mitral valve disease that is asymptomatic and not yet severe; stage C1 represents patients with severe mitral valve disease but compensated right and left ventricles, no or minimal pulmonary hypertension and no symptoms; stage C2 may be characterized by progressive ventricular enlargement and/or systolic impairment, and/or increasing pulmonary pressure, but still no symptoms; and stage D is characterized by severe mitral valve disease accompanied by symptoms and usually some combination of atrial/ventricular enlargement, impaired systolic function, pulmonary hypertension, and atrial arrhythmia [5]. Table 6.1 shows the integration of these factors for the stages of primary MR. Similarly, Table 6.2 shows the stages of MS; these characteristics are focused on rheumatic MS and, compared to prior guidelines, reflect emphasis on the hemodynamics usually associated with symptom onset.
Etiology and Classification
Mitral Regurgitation
There is a fundamental distinction between primary and secondary MR . Primary MR was often referred to as organic MR, whereas secondary MR (addressed in Chap. 7) was commonly described as functional MR. The two diseases have different etiologies, pathophysiology, natural history, and a different response to and indications for medical, transcatheter, and surgical therapies. Primary MR is a disease of the valve, including the leaflets, chordae tendineae, papillary muscles, or annulus. Because valve abnormalities and the regurgitation they cause are the disease, treating the valve can be curative. The most common cause of primary MR, and the most common etiology leading to surgery, is mitral valve prolapse (MVP) [1, 6]. Abnormal elongation and redundancy of the leaflets accompanied by elongation of chordae and dilatation of the annulus leads to prolapse of the valve past its coaptation point causing MR. Primary MR presents as a spectrum of lesions [7]. On one end are older patients with fibroelastic deficiency , inadequate tissue and localized pathology of a single scallop or a single chord (Fig. 6.1). At the other end of the spectrum are younger patients with excess tissue, extensive, diffuse, and marked myxomatous changes of the leaflets and chordal apparatus associated with billowing leaflets, often referred to as Barlow’s disease (Fig. 6.1).
Secondary MR is a disease of the LV that has led to abnormal shape/structure and function that cause displacement of one or both papillary muscles, leaflet tethering and inadequate coaptation, and often annular dilatation [8, 9]. Secondary MR may result from ischemic or nonischemic ventricular disease. Because the mitral valve itself is not the origin of the disease, therapy directed only at MR may reduce regurgitation but cannot cure the basic underlying pathology.
The Carpentier classification of the types of mitral valve pathologies is commonly used today (Fig. 6.2) [10]. Type I exhibits normal leaflet motion but annular dilation or leaflet perforation. Type II is leaflet prolapse. Type III describes leaflet restriction. Type III is further divided into IIIa (restricted opening) and IIIb (restricted closing).
Mitral Stenosis
Mitral stenosis most commonly occurs as a consequence of rheumatic fever, a history of which is noted in approximately 60% of patients with pure MS [3, 5]. Significant annular calcification causing calcific MS is the next most common cause, but relatively infrequently leads to obstruction severe enough to warrant valve replacement.
Diagnosis
Imaging Assessment
Mitral Regurgitation
Two-Dimensional Transthoracic Echocardiography
Transthoracic echocardiography (TTE) is readily available and usually the initial diagnostic test to evaluate MR. The mitral valve apparatus includes the leaflets, the chordae tendineae, annulus, papillary muscles, and the insertions into the LV wall [11]. Because the diseases are so different, it is important to distinguish between primary and secondary MR, a distinction that can usually be made from two-dimensional (2D) TTE. In primary MR the diagnosis of prolapse is made on the TTE parasternal long view where the mitral leaflets are displaced >2 mm into the left atrium during systole [12]. Often, TTE can identify pathology of the specific scallops responsible for the leak. The six scallops of the mitral valve can be identified in the parasternal short axis view and color Doppler can then be used to confirm the origin of the MR jet and the scallops involved. In the parasternal long view, the A2 and P2 scallops can be visualized. The apical four chamber view allows for visualization of the A3, A2, and P1 scallops; while the TTE two chamber view displays P3, A2, P1 (Fig. 6.3). Unlike in primary MR where distinct valvular pathology is found, in secondary MR the valve itself is usually normal. Tethering of the valve by distorted LV geometry prevents leaflet coaptation causing a centrally directed jet.
TTE can provide guidance as to the feasibility of mitral valve repair by providing assessment of scallop anatomy or leaflet tethering and demonstrates the extent of mitral annular calcification. In addition to an assessment of the etiology and anatomy of the MR, TTE provides an accurate assessment of LA and LV size, LV function, and pulmonary artery pressures, all of which are important in clinical management.
Two-Dimensional Transesophageal Echocardiography
2D transesophageal echocardiography (TEE) can be used to evaluate the mitral valve apparatus and often adds important anatomical information in the assessment of mitral valve pathology in cases of moderate to severe MR. The echocardiographer should be familiar with mapping of the mitral valve using 2D TEE [13]. In the midesophageal four chamber view, typically at 0°, the tips of the leaflets are the A2 and P2 scallops. When the transducer is withdrawn slightly from the esophagus with the LV outflow tract in view, the A1 and P1 scallops are visualized. When the transducer is advanced further past the midesophageal view, the A3 and P3 scallops are then visualized. At the 60° view, also termed the bicommissural view, the scallops visualized are the P1, A2, and P3 scallops with the P1 scallop being closest to the left atrial appendage (LAA) and the P3 scallop the furthest from the LAA. Finally, at the 120° view, the A2 and P2 scallops are visualized (Fig. 6.4). Using this strategy the entire mitral valve can be visualized for the presence of prolapse and/or flail segments.
Transesophageal echocardiogram (TEE) demonstrating the different mitral scallops. Panel a: at 0°, A1 and P1 scallops are seen in the high esophageal view with the left ventricular outflow tract in view. Panel b: at 90°, the P3 scallops is seen which appears to prolapse. Panel c: at 120°, A2 and P2 scallops are seen. P2 scallop is flail
Three-Dimensional (3D) Echocardiography: Advantages and Pitfalls over 2D
Three-dimensional (3D) echocardiography has significantly improved the assessment of mitral valve disease [14]. With multiplane 2D TEE, the echocardiographer has to be familiar with the different mitral scallops and construct a map of the mitral valve to convey the valve pathology to surgeons or interventionalists. 3D TEE enables better communication between the surgeon and the echocardiographer by providing “the surgeon’s view” of the mitral valve. In this view, the mitral valve is visualized from the LA perspective with the aortic valve at the 12 o’clock position (Fig. 6.5). Advances in 3D echocardiography allow for excellent spatial and temporal resolution so that a very accurate assessment of mitral valve structure, function, and dynamic changes can be recorded [15]. 3D echocardiography allows for better characterization of leaflet pathology compared to 2D TEE. Due to the saddle shape of the mitral annulus, distortion in the mitral anatomy can lead to misinterpretation of scallops with 2D TEE (Fig. 6.6). In addition, 3D echocardiography allows for better characterization of lesions such as mitral clefts and commissural scallops which are not easily identifiable by 2D echocardiography alone (Fig. 6.7). Moreover 3D echo with color can identify the exact location of the regurgitant jet (Fig. 6.8).
Transesophageal echocardiogram (TEE) demonstrating that 2D TEE by itself may lead to misrepresentation of scallops. In panel a at 120°, the P2 scallop appears to be flail. However in panel b when 3D echocardiogram is utilized, it is actually the P3 scallop (which is very large) that prolapses rather than the P2 scallop
3D echocardiography can be utilized to identify mitral clefts, which would not be seen on 2D TEE. Panel a demonstrates 3D of the mitral valve showing indentations between the mitral scallops (red arrows) that extend from the mitral leaflet tips to the annulus. Panel b demonstrates the clefts as seen from the ventricular aspect
However, 3D echocardiography has its limitations [15, 16]. It requires that the echocardiographer be familiar with image acquisition and manipulation. Errors in manipulation of the image can lead to misinterpretation of the lesions. The echocardiographer should routinely position the aortic valve at the 12 o’clock position relative to the mitral valve when using 3D TEE in mitral valve assessment. 3D echocardiography frequently involves combining several volumes of image sectors, so any patient movement or irregular heart rhythm can produce what is termed “stitch artifact ” (Fig. 6.9). Finally, the echocardiographer has to be aware of image dropout, which may once again lead to misinterpretation of lesions (Fig. 6.10).
Computed Tomography and Magnetic Resonance Imaging in Assessment of Mitral Regurgitation
Computed tomography (CT) and magnetic resonance imaging (MRI) are infrequently utilized in the evaluation of the mitral valve, but can provide important, complementary information to echocardiography. CT can be utilized for diagnosis of mitral valve prolapse [17]. An advantage of CT over echocardiography is that it not only can help in the diagnosis of mitral valve prolapse, but also can concomitantly assess coronary anatomy [18], LV function [19, 20], and the presence of left atrial appendage thrombus [21]. CT can also be used to evaluate the extent of mitral annular calcification, which may help plan surgery and assess the feasibility of mitral repair. MRI is also useful for the assessment of MR, particularly in patients with poor echocardiographic views. MRI provides an assessment of mitral valve anatomy, quantification of MR severity, and accurate assessment of LV size and function [22].
Left Ventriculography in Assessment of Mitral Regurgitation
Left ventriculography can qualitatively assess severity of mitral regurgitation [23]. With regurgitation that is classified as mild (1+), contrast clears from the LA with one beat, and does not opacify the LA; 2+ regurgitation is classified as moderate, and contrast does not clear with one beat and faintly opacifies the entire LA; 3+ regurgitation is classified as moderate to severe, contrast opacifies the entire LA in one beat; 4+ regurgitation is classified as severe, and contrast densely opacifies the entire LA into the pulmonary veins (Fig. 6.11). The regurgitant volume can also be calculated by subtracting the stroke volume obtained by the Fick or thermodilution method from the difference between end diastolic volume and end systolic volume. Common pitfalls in the use of ventriculography in assessing MR are (1) induction of ventricular extrasystoles that can cause factitious MR and (2) injecting too little contrast to opacify both LA and LV (at least 50 cc should be injected), thereby underestimating MR severity.
Doppler Quantitation of Mitral Regurgitation
Qualitative and quantitative indices of MR severity are shown in Table 6.1 [5, 12, 24]. One of the more commonly used qualitative parameters is regurgitant jet area. Large jets represent a greater severity of MR than smaller jets but this method has several pitfalls. The Nyquist limit is often set too low, which (falsely) increases the jet area. Jet area may be deceptively underrepresented in acute severe MR where there is rapid rise in left atrial pressure diminishing the transvalvular driving gradient and confining MR to early systole. In addition, central jets usually appear larger than eccentric jets due to entrainment of red blood cells on either side of the jet. Thus the echocardiographer must use caution in interpreting highly eccentric jets when using color Doppler and jet area alone (Fig. 6.12).
Highly eccentric jets may lead to underestimation of the degree of mitral regurgitation not only by color Doppler but also by the PISA method. In this figure, there is a flail posterior leaflet leading to a highly eccentric jet of severe mitral regurgitation. However, color Doppler appears to underestimate the severity of MR
Color Doppler can also be used to assess the size of the vena contracta, which is the narrowest portion or neck of the regurgitant jet as it crosses the mitral annular plane into the LA and reflects MR severity by implying the size of the regurgitant orifice (Fig. 6.13). One advantage of the vena contracta method is that it can be used in eccentric jets. The cutoff values for the different degrees of MR are listed in Table 6.1.
Quantification of mitral regurgitation requires identification of the zone of flow convergence. One should identify the narrowest portion of the jet as demonstrated by the yellow arrow (vena contracta). Also, one should identify the PISA radius (white arrow) to allow for calculation of the effective regurgitant orifice area (EROA) using the PISA method. The echocardiographer should also obtain a continuous wave Doppler of the mitral regurgitation since the peak velocity and the velocity time integral are also used to calculate the EROA and the regurgitant volume
The proximal isovelocity surface area (PISA) or flow convergence method is utilized in the quantitative assessment of the severity of MR (Fig. 6.13). This method is based on the premise that as blood approaches a regurgitant orifice it forms hemispheric shells of increasing velocity and decreasing surface area [25]. If the Nyquist limit is known, then the area of the hemisphere, which provides an effective regurgitant orifice area (EROA) , can be calculated using the formula [24, 25]:
where r represents the radius of the hemisphere, V a represents the velocity of the Nyquist limit, and PkV reg represents the peak MR velocity obtained by continuous wave Doppler. A limitation of the PISA method is the need to use a correction factor if the base of the hemisphere is not a flat surface. In addition, in highly eccentric jets, 2D EROA may underestimate the severity of MR; thus, this method is more applicable to central jets. Because the true shape of the EROA may not actually be a hemisphere, 3D EROA maybe more useful in the true quantitative assessment of MR severity (Fig. 6.14) [26]. In addition, any error made in measurement of the PISA radius will lead to a substantial error in the EROA calculation because the radius is squared in the flow convergence equation.
Pulsed wave Doppler is another method to qualitatively assess MR severity [24]. In patients with severe MR, the E wave velocity is usually greater than 1.2 m/s; the presence of an A wave dominant mitral inflow pattern virtually excludes the presence of severe MR [27]. Pulsed wave Doppler can also be used to calculate the MR regurgitant volume and fraction using the continuity equation [25]. This method is useful when the MR jet is highly eccentric. However, since the annular measurement is a key component of the analysis, any error in its measurement can produce large errors in the calculation of the regurgitant volume and fraction.
Lastly, pulsed wave Doppler can be used to assess pulmonary vein flow. In patients with severe MR, forward systolic pulmonary vein flow can be reversed or blunted (Fig. 6.15). Caution should be used when using this method as the sole criteria for assessing MR severity since elevations in LA pressure and atrial fibrillation may also cause the systolic flow in the pulmonary vein to be blunted [25].
Common pitfalls in the echocardiographic assessment of MR severity are shown in Table 6.3.
Echocardiographic Evaluation of Mitral Stenosis
It is important to distinguish between calcific and rheumatic MS because it has implications for treatment strategies wherein balloon valvuloplasty is ineffective in calcific disease. Calcific MS mainly involves annular calcification and is seen in elderly patients, those with renal disease, hypertension, and atherosclerotic disease [28]. The commissures are usually spared in this disease and valve thickening, if present, predominates at the base of the leaflets while the leaflet tips are usually spared (Fig. 6.16a). This is in contrast to rheumatic MS where there is commissural fusion, chordal calcification, leaflet thickening, and calcification that predominates at the leaflet tips (Fig. 6.16b) [3].
Differentiating calcific mitral stenosis (MS) from rheumatic MS. Panel a demonstrates a patient with calcific MS. The arrow demonstrates the calcium at the mitral annulus which is classic for calcific MS where the leaflets are generally spared. Panel b demonstrates a patient with rheumatic MS with the calcification most prominent at the leaflet tips. The calcification may also extend into the subvalvular apparatus
Transthoracic Echocardiographic Evaluation of Mitral Stenosis
TTE is not only used to assess the etiology of MS (calcific versus rheumatic) but is also used to determine the severity and hemodynamic consequences of MS [25]. Continuous wave Doppler (CWD) should be used to assess peak and mean mitral gradients (Fig. 6.17). In addition, the heart rate at which the gradients are measured should be noted as tachycardia can increase the gradient. Anemia, hyperthyroidism, fever, pregnancy, or significant MR can also increase the transvalvular gradient due to increased flow.
The mitral valve area is commonly calculated from the pressure half-time (T 1/2), which is defined as the time required for the maximum pressure gradient to decrease by half of its original value. The pressure half-time is determined by measuring the slope of E wave obtained by CWD (Fig. 6.18). Mitral valve area can then be calculated by the formula [29]:
where T 1/2 represents the pressure half-time. However, pressure half-time can be affected by changes in LV compliance and moderate or severe aortic regurgitation. Thus, pressure half-time should not be the sole criterion used to assess the severity of MS. In rheumatic MS, mitral valve area can also be determined by planimetry [25, 30], performed in the parasternal short axis view at the level of the mitral valve, taking care to measure at the leaflet tips (Fig. 6.19). The transmitral pressure gradient is determined by the modified Bernoulli equation and valve area can be determined using the continuity equation. This method is most accurate in the absence of mitral regurgitation. TTE is also used to assess pulmonary artery pressures since pulmonary hypertension is a known complication of significant MS.
Transesophageal Echocardiography in Assessment of Mitral Stenosis
TEE is usually employed prior to balloon valvuloplasty to confirm the absence of an LA appendage thrombus, assess the degree of concomitant mitral regurgitation, better assess the degree of leaflet and subvalvular calcification and thickening [31], and develop the Wilkins score for predicting the outcome of percutaneous balloon mitral valvuloplasty [32]. In this evaluation, one to four points each is given according to ascending severity for leaflet mobility, leaflet calcification, leaflet thickening and subvalvular disease yielding a score ranging from 4–16. A score of 8 or less is predicts a suitable percutaneous valvuloplasty. In contrast, a valve with a score of 12 or more portends an unfavorable outcome. Contraindications for percutaneous balloon mitral valvuloplasty include the presence of moderate or more mitral regurgitation and the presence of a left atrial appendage thrombus. 3D TEE can also be used to assess the mitral orifice area and is more accurate than 2D planimetric measurements.
Strengths and limitations of various echocardiographic modalities for assessing MR and MS are shown in Table 6.4.
Hemodynamic Assessment
Invasive hemodynamics has been the cornerstone of our understanding of valvular heart disease [33] and has served as the gold standard for validation of modern noninvasive techniques [34]. However, given the advances in noninvasive imaging of the mitral valve and LV, hemodynamic assessment of mitral disease is performed infrequently today. Doppler echocardiography and 2D and 3D TTE and TEE are excellent for the evaluation of mitral valve pathologies and are currently considered the diagnostic tools of choice [5]. Nonetheless, invasive hemodynamic assessment of mitral valve pathologies should still be considered in patients when the clinical history and symptoms don’t correlate with noninvasive assessments or when the various noninvasive modalities don’t agree with each other. Invasive hemodynamics may also be of help when imaging is suboptimal or when the noninvasive hemodynamic estimates don’t correlate with the visual interpretation of the echocardiographic images. Invasive hemodynamics help clarify the clinical significance of mitral valve pathology in a patient with shortness of breath and concomitant lung disease. Elevated LA and LV filling pressures support a cardiac cause of the patient’s dyspnea while normal LA and LV filling pressures together with increased pulmonary artery pressure suggest a pulmonary cause. Additionally pharmacologic agents can be administered to assess reversibility of pulmonary hypertension in a patient with mitral stenosis . Because dyspnea usually occurs with exercise but not at rest, exercise hemodynamic studies can provide additional information regarding symptom causation, information that may be difficult to evaluate with noninvasive modalities. Lastly invasive studies are helpful immediately post procedure because noninvasive evaluation, especially Doppler pressure half-time derived mitral valve area, is inaccurate immediately post mitral balloon valvuloplasty [35].
Mitral Stenosis
Mitral stenosis is probably the most common mitral valve pathology for which a clinician might pursue invasive hemodynamic assessment. Severe MS produces an LA/LV diastolic gradient without diastasis. The pulmonary capillary wedge pressure (PCWP) is commonly used as a surrogate for left atrial pressure and a pigtail catheter is used to cross the aortic valve to assess LV pressure (Fig. 6.20). These standard measurements provide the diastolic gradient from PCWP to LV and help judge stenosis severity with reasonable accuracy [36]. The gradient measurement from the LA to LV is critical to the calculation of the mitral valve area but using the PCWP as a surrogate for direct LA pressure measurement has pitfalls. PCWP waveforms are delayed by 40–120 ms relative to the LA waveforms and proper gradient assessment requires phase shifting the PCWP waveform back such that the V wave intersects the downslope of the LV pressure tracing (Fig. 6.20) [37]. Operators should also obtain a blood aspirate demonstrating highly oxygenated LA blood from the catheter to prove that the catheter is truly wedged [36]. PCWP should not be used as a surrogate for LA pressure in cases of veno-occlusive disease or when there is any doubt about the quality of the PCWP tracing. In such cases the transvalvular gradient is evaluated by simultaneous measurement of the LV and LA via trans-septal puncture. Modern computer software computes the area delineated by the diastolic PCWP and LV waveforms to obtain the mean gradient; 5 cardiac cycles should be averaged for patients in sinus rhythm (Fig. 6.21). Atrial fibrillation is common in patients with MS and the variability in diastolic filling time greatly affects the mean gradient. Thus 10 cycles should be averaged in patients with atrial fibrillation (Fig. 6.22).
Simultaneous LV (yellow) and PCWP (green) pressure tracings at 100 mm/s recording speed in a patient being evaluated for mitral stenosis. The PCWP pressure tracing has been phase shifted such that the V wave intersects the downslope of the LV pressure tracing. Note the absence of diastasis in the LV tracing and the significant gradient during diastole between the PCWP and the LV such that the PCWP is always higher than the LV pressure; both features of significant mitral stenosis
Simultaneous LV (red) and LA (yellow) pressure tracings at 25 mm/s recording speed in a patient after balloon mitral valvuloplasty and in atrial fibrillation. Notice the significant difference in area for each traced diastole. All shaded diastolic areas were averaged and the valve area was calculated according to the Gorlin formula as shown
Traditionally, the Gorlin formula has been used to derive the mitral valve area and requires measurement of the diastolic filling period and the cardiac output (Table 6.5) [38, 39]. Hakki proposed a simpler formula to determine the area of a stenotic valve and showed an excellent correlation to mitral valve area calculated by the Gorlin formula (Table 6.5) [40]. Both formulas still rely on the planimetric assessment of the gradient between the LA and LV during diastole, which can be cumbersome without the aid of software programs. Cui proposed a simpler determination of the mean transvalvular gradient that estimates mean LV diastolic pressure as left ventricular end diastolic pressure/2 and showed excellent correlation with the gradient determined by the standard planimetric approach [41]. Additionally, this method allows for determination of mitral valve area by simple pullback from the LV to LA, potentially making arterial puncture unnecessary when trans-septal catheterization is employed. Importantly, the Cui method requires measurement of mean LA pressure and it should be used with caution in patients with tachycardia as this may violate the assumption of LV waveform morphology underlying Cui’s simplification. However since invasive hemodynamics are usually employed only when the diagnosis is unclear and because an error in gradient calculation of as little as 3 mmHg might change management, all effort should be made to determine the actual LA-LV gradient.
Mitral Regurgitation
Although more common than MS, MR is not commonly evaluated with invasive hemodynamic measures. When noninvasive imaging of MR severity is not clear, left ventriculography in addition to hemodynamic assessments can be performed. Severe MR should be considered when a V wave noted in the PCWP or LA pressure tracing is two times the higher than the mean PCWP while a V wave three times the mean PCWP is specific for severe MR [42]. Ventricular afterload, LA compliance and LV dysfunction can all affect the size of the V wave independent of mitral valve pathology and, as such, the absence of a V wave should not be used to rule out significant MR [43]. The ratio of the V wave to the total LV diastolic area may also be helpful in grading mitral regurgitation but is largely unavailable outside the catheterization laboratory [44]. Figure 6.23 shows an LA pressure tracing before and after a MitraClip (Abbott Vascular) procedure where a prominent V wave nearly disappears after the MR is corrected. Although somewhat rare, a prominent V wave that subsides spontaneously should trigger an evaluation for ischemic papillary muscle dysfunction as transient MR may not be evident on noninvasive imaging unless ischemia is provoked. Finally, invasive hemodynamic evaluation can provide some insight into the chronicity of MR. Acute or decompensated MR usually produces a very prominent V wave and steep Y descent signaling a very high LA pressure. In contrast, chronic compensated MR may demonstrate elevated filling pressure but with a relatively small V wave as atrial compliance has had time to increase, compensating for the chronic volume load.
Medical Therapy
Mitral Regurgitation
Acute severe MR generally requires urgent/emergent surgical therapy. As a temporizing measure, intravenous vasodilator therapy with nitroprusside or hydralazine may reduce the regurgitant volume (thus, reducing pulmonary edema) and improving forward flow [45,46,47]. Temporary percutaneous mechanical support with intra-aortic balloon counterpulsation or the Impella (Abiomed) catheter may also be considered to stabilize the patient prior to definitive treatment. For those with suspected infective endocarditis as the cause of the acute MR, antibiotics should be administered immediately after blood cultures have been obtained.
For chronic primary MR with preserved LV function, there is currently no indication for medical therapy directed at the MR to improve clinical outcomes. While it would seem reasonable that vasodilator therapy would lessen LV afterload resulting in a decreased MR, less LV volume overload, and presumably a more favorable natural history, small studies testing this hypothesis have shown little or no benefit [48,49,50]. Accordingly, vasodilator therapy is not indicated for normotensive asymptomatic patients with primary MR [5]. An early phase clinical study suggested potential benefits for LV function in patients with chronic MR treated with ß1-receptor blockade and a preclinical study raised the possibility of benefit from the phosphodiesterase type 5 inhibitor sildenafil [51, 52]. Further studies are needed to identify if medical therapy will slow the natural history and improve patient outcomes for those with significant primary MR. Despite the lack of a role for medical therapy for normotensive patients with chronic primary MR, hypertension should be treated per guideline recommendations given the known morbidity and mortality associated with uncontrolled hypertension.
Chronic secondary MR is characterized by LV dysfunction either due to myocardial infarction or nonischemic cardiomyopathy. As such, it is appropriate to treat such patients with guideline-directed medical therapy for heart failure , including ß-blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and aldosterone antagonists [5].
Mitral Stenosis
Secondary prevention of rheumatic fever is indicated for patients with rheumatic MS (see Chap. 2 for details) [5]. Atrial arrhythmia occurs in 30–40% of patients with severe MS [3, 5]. Because of significant LA enlargement and fibrosis that can occur with severe MS, rhythm control is often ineffective and therapy is directed toward rate control. Atrial fibrillation with a rapid ventricular response can be particularly problematic for patients with MS as the decreased diastolic filling time increases LA pressure and causes or exacerbates shortness of breath. Negative dromotropic agents can be used to slow the ventricular response. Because of great stroke risk, anticoagulation with vitamin K antagonists is indicated for patients with MS and (1) an atrial arrhythmia (regardless of whether it is paroxysmal, persistent, or permanent); (2) prior embolic stroke; or (3) a LA thrombus [5].
Surgical Therapy
Indications for Mitral Valve Surgery
Mitral Regurgitation
As surgical outcome has improved over time, indications for surgery have become progressively more liberal. In the past when operative mortality for mitral valve replacement was high, patients were treated medically even for symptomatic MR. However today’s mortality rates for patients with preserved LV function who undergo mitral valve repair for MR is less than 1% with 5-year mortality from cardiac causes less than 5% [53]. The AHA/ACC recommendations for surgical intervention for primary MR are shown in Table 6.6 [5]. Mitral valve surgery is indicated for symptomatic patients with chronic severe primary MR and LV ejection fraction >30%. Mitral valve surgery is recommended for asymptomatic patients with chronic severe primary MR and LV dysfunction or dilation (LV ejection fraction 30–60% and/or LV end-systolic dimension ≥40 mm). The guidelines emphasize the importance of valve repair over replacement for primary MR, recognizing that repair is much less satisfactory for rheumatic valvulopathy. They also emphasize the performance of mitral valve repair at centers of excellence by high volume surgeons who have a high successful valve repair rate at a low mortality rate. The skill and experience of the surgeon and center is particularly important for more complicated valve pathology (e.g., anterior or bileaflet prolapse). In excellent hands mitral repair returns survival to that of normal subjects [54]. However variability in repair rate can vary from 0% to 90% for individual surgeons [55].
Significant debate surrounds the question of whether to offer surgery to asymptomatic patients with severe primary MR and preserved LV function versus close observation and surgery when symptoms or early LV dysfunction is detected [56,57,58,59]. In this regard, the AHA/ACC guidelines indicate that performance of mitral repair is reasonable in the asymptomatic patient with severe primary MR and preserved LV function especially when there is new onset atrial fibrillation or pulmonary hypertension and when there is a high likelihood of successful and durable repair. The triggers for surgery connoting LV dysfunction are an ejection fraction of ≤60% and or an LV end systolic dimension of ≥40 mm [5]. However the 2017 ACC/AHA focused update of the valve guidelines indicates that it is not necessary to wait until these thresholds are reached to opt for surgery, especially if longitudinal studies indicate progression toward those triggers [60]. In the absence of new onset atrial fibrillation or pulmonary hypertension, the guidelines indicate that it is reasonable to perform mitral repair in the asymptomatic patient with severe primary MR when the likelihood of a successful and durable repair without residual MR is >95% with an expected mortality rate of <1% and when it is performed at a valve center of excellence [5, 60]. It must be emphasized that the guideline does not mean the center (or surgeon) must repair 95% of all mitral valves operated. It means that there is a 95% chance of repair of the particular valve in question by that specific surgeon, a highly likely outcome, for instance for P2 prolapse.
Mitral Stenosis
The first recorded surgery for an intra-cardiac correction was for MS, a relatively common condition in the late 1800s and early 1900s following rheumatic fever. Sir Thomas Lauder Brunton from Scotland, opined in 1902 that the mitral valve might be surgically repaired, and Dr. Elliott Cutler at Harvard Medical School in Boston accomplished the first recorded mitral operation in 1923 by using a neurosurgical tenotomy knife through a ventricular incision to open the fused commissures [61]. A few years later, in 1925, Henry Souttar, a British surgeon, successfully fractured the stenotic mitral valve using his finger through a hole in the LA appendage and relieved the stenosis of a young woman. His contemporary physicians did not think the procedure safe and he received no further referrals, despite the survival of his patient [62]. Subsequent attempts by the group at Harvard had only a single survivor (the first out of a sequence of 10) and the procedure was abandoned. It was not until 1948 when several surgeons independently employed the same or similar techniques to relieve mitral stenosis that the surgical world was transformed and cardiac surgery became a reality [63,64,65,66].
Mitral valve intervention is generally recommended for patients with severe MS (mitral valve area [MVA] ≤1.5 cm2) and symptoms or, in the absence of symptoms, when cardiac surgery will be performed for another indication [5]. In the asymptomatic patient with MVA ≤1.5 cm2, intervention would be reasonable when there is very severe MS (MVA ≤1.0 cm2) or a concomitant atrial arrhythmia or significant pulmonary hypertension [5].
Today, for patients with rheumatic MS, standard corrective treatment follows the same principles as the earlier surgical commissurotomy although the approach is less invasive: percutaneous balloon valvuloplasty is now the primary mode of intervention for these patients when pathology is suitable for this approach (see Chap. 8) [5, 32, 67, 68]. When anatomy is not suitable for the percutaneous approach, or if a patient must undergo surgery for another cardiac pathology, open mitral valve commissurotomy or, more commonly, valve replacement is performed [5].
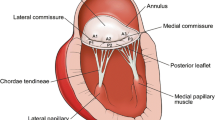
Mitral Valve Anatomy for the Surgeon
The two leaflet valve is a complex structure, and disturbance of any part of the apparatus can result in regurgitation. The components of the valve are: the two leaflets (anterior, posterior), the annulus, the chordae, the two papillary muscles, and the LV wall. The leaflets are scalloped into thirds, conventionally labeled A1, A2, and A3 for the anterior leaflet and P1, P2, and P3 for the posterior leaflet (Fig. 6.24), and these designations are used for operative planning and intervention. The fibrous trigone is a continuation of tissue of the anterior leaflet of the mitral valve and is contiguous with the aortic valve (including the left and non-coronary leaflets) and contributes importantly to the fibrous skeleton of the heart. Because of this fibrous construction, it has been widely held that this portion of the annulus is preserved from dilatation in pathologic states, which allows for successful annuloplasty with a partial ring or band. There is limited data which suggests that some dilatation may occur [69]. The left and right fibrous trigones are used to anchor sutures for annuloplasty or valve replacement superiorly. Approximately 40% of the annulus is accounted for by these trigones (contiguous with the mitral valve anterior leaflet) while the remaining 60% of the annulus is along the posterior leaflet attachment.
The chordae tendineae, string-like fibrous connective tissue which attach the leaflets to the ventricular wall, are important supporting structures consisting of both primary chordae (attached to the edge of the leaflet) and secondary chordae (attached to the underside of the leaflets between the edge and the annulus). Each of the papillary muscles (anterolateral and posteromedial) has chordae which attach to both leaflets. The surgeon can make use of the intact chordae from one leaflet to support a flail portion of the other by transferring a section of the leaflet with intact chordae (chordal transfer, almost exclusively from posterior leaflet to anterior leaflet). The posterior leaflet also has tertiary chordae which attach directly from the underside of the valve to the ventricular wall. The blood supply to the anterolateral papillary muscle typically comes from the left anterior descending and the circumflex arteries, while the posteromedial papillary muscle is usually supplied by only a posterior descending artery or branch of the circumflex, which makes it more susceptible to ischemia, infarction, and rupture.
Leaflet redundancy can cause increased leaflet stress and increased systolic movement into the atrium (prolapse) of the affected leaflet leading to decreased coaptation and regurgitation. The increased systolic stress on abnormal leaflets can cause rupture of weakened chordae worsening regurgitation. Any of these pathologic states may be present, requiring surgical attention to annular dilatation (usually addressed by annuloplasty), excess leaflet tissue (resection), and/or ruptured chordae (resection or chordal repair/replacement). Today, the mainstay of surgical intervention of myxomatous disease is repair (as opposed to replacement), as excellent outcomes have been achieved with advanced techniques. Even in valves with extensive myxomatous changes, or Barlow’s valves [70, 71], good results from repair with resection of redundant valve tissue and chordal replacement can be achieved [72].
Important structures to be aware of (and avoided) for the surgeon also include the atrioventricular node superiomedially and aortic valve leaflets and root superiorly. Although not a part of the valve per se, the coronary sinus runs adjacent to the posterior annulus and the circumflex artery courses near A1, P1, and P2 (Fig. 6.24).
Mitral Valve Repair
As repair of the mitral valve should be accomplished in the vast majority of cases, the surgeon’s understanding of the techniques and limitations is imperative. One of the most common indications for surgery in a patient with a myxomatous valve is isolated prolapse of the middle scallop of the posterior leaflet (P2) and a dilated annulus and the AHA/ACC guidelines note that planned mitral valve replacement of simple P2 prolapse is no longer acceptable therapy [5]. Quadrangular resection of the prolapsing segment (often found with a ruptured chordae on the segment) with ring or band annuloplasty has been the mainstay of treatment [73]. Simplification of the resection by substitution of a triangular resection (instead of quadrangular resection which requires leaflet advancement) is effective and eliminates the need for disconnection of the leaflet. In the majority of cases, this simple repair technique, coupled with an annuloplasty, will eliminate the mitral regurgitation (Fig. 6.25).
Ensuring that the coaptation area along the leaflets after repair is sufficient is key in eliminating MR; typically five millimeters will ensure an adequate area of support for the valve during systole. The surgeon must be facile with testing methods in the operating room after completion of the repair to ensure adequacy of repair and to help prevent unnecessary additional cross-clamp time for revision.
As annuloplasty became increasingly important in the repair of mitral valves, several devices became available to facilitate the procedure. Multiple choices exist but the most common include rigid or flexible full circumferential rings (buttressing the entire annulus) and rigid or flexible partial bands (buttressing the posterior annulus, leaving the fibrous trigone section untethered). Sizing of the ring is accomplished by measurement of the height or area of the anterior leaflet. Excellent results have been reported with both full and partial rings. The decision to implant a flexible versus rigid or full ring versus partial band is generally left up to the surgeon based on familiarity with the device and assessment of the fibrous trigone to predict the possibility of future annular enlargement.
Complex Repairs: Chordal Replacement or Transfer
Replacement of chordae can be accomplished by creation of neochordae (typically using polytetrafluoroethylene, or PTFE sutures) or by chordal transfers. These methods are generally reserved for ruptured chordae other than P2 or elongated chordae. Measurement of adjacent chordae can be used to re-create the neo-chord optimal length or pre-measured lengths of suture material can be used. Both anterior and posterior chordae can be replaced. Likewise chordal transfer is a useful technique in instances where adjacent leaflet tissue can be resected (where it would not compromise valve function) and a chordae transferred to the pathologic leaflet. In this manner, the natural chordal length is ensured. The most common and effective chordal transfer involves moving an intact P2 chordae to an area of ruptured chordae on A2. Chordal shortening techniques have also been employed when chordae are intact but the leaflet edge is prolapsed, although most surgeons have gravitated toward chordal replacement in this setting based on the perception of improved durability. A typical P3 rupture can be repaired with or without resection of the posterior flail portion of the leaflet and closure of the portion of the commissure closest to the annulus with inversion of leaflet edges, followed by annuloplasty (Fig. 6.26).
Edge-to-Edge Apposition or “Alfieri” Stitch
The “Alfieri stitch” or “edge-to-edge” apposition stitch that sutures the bellies of the two leaflets together can be used when other methods fail [74]. This stitch creates a double orifice valve. Good results of this technique when coupled with annuloplasty have been reported, although long-term results of the technique without annuloplasty have been unsatisfactory [75, 76]. Concern for creating a non-physiologic state without maintenance of the normal bileaflet anatomy has precluded widespread use, and currently this procedure is limited to application in high-risk patients or as a rescue procedure when standard techniques fail. The Alfieri principle is the basis of percutaneous edge-to-edge repair [77, 78].
Intraoperative Echocardiography
Intraoperative echocardiography is essential for successful mitral surgery by defining the valve pathoanatomy, planning the operation and confirming the achievement of good results or the need for repair revision after native circulation has returned. TEE is indicated in every case unless contraindicated for esophageal anatomic reasons [5]. The decision to operate on the mitral valve is based upon preoperative assessment of MR under normal resting conditions. Because anesthesia greatly alters LV loading and MR severity, assessment of MR severity from intraoperative TEE should never override the preoperative decision that had been based on conscious pathophysiology.
Mitral Valve Replacement
Although mitral valve repair should supplant replacement in most cases of myxomatous disease, there are situations in which a valve replacement becomes necessary, especially in treating rheumatic valvulopathy . Replacement can be achieved by resection of the diseased leaflet tissue with preservation of the leaflet edge and primary chordal attachments, and incorporation of the leaflet tissue into the trans-annular sutures, which allows support of the papillary muscles and ventricular wall after the procedure. Maintenance of papillary-annular continuity is associated with improved LV function following mitral valve replacement [79,80,81,82]. Superior long-term outcomes in patients who had chord-sparing procedures versus those without has made it the standard of care in cases where replacement is necessary [83]. With severe rheumatic involvement, often the chordae are too foreshortened and thickened to permit preservation. In these circumstances, PTFE neochordae (as described above) can be inserted to maintain annular continuity to both papillary muscles.
Bioprosthetic vs. Mechanical
Mechanical valves have superior longevity but require anticoagulation, typically with warfarin. Bioprosthetic valves can be used to avoid the need for anticoagulation with warfarin (aspirin or another antiplatelet oral medication is sufficient) but can deteriorate and may require subsequent valve replacement. Age, ability to comply with long-term anticoagulation, risks of bleeding, and other patient factors including preference must be considered in the selection of valve type.
Special Considerations
Papillary Muscle Rupture
In cases of papillary muscle rupture that causes torrential MR, surgery should be undertaken emergently, as this condition is poorly tolerated. Even if there is a partial papillary muscle rupture with hemodynamic stability, urgent surgery is indicated because it may progress to complete papillary muscle rupture. In cases of ruptured chordae tendineae, mitral repair is usually feasible and preferred over replacement but with papillary muscle rupture, expedient replacement is generally preferred. The patient’s overall condition must be considered and timing of surgery balanced with patient comorbidities and the possibility of optimization of concurrent problems prior to surgery (e.g., renal failure, sepsis, heart failure ).
Atrial Fibrillation
As atrial fibrillation is a common sequela of mitral valve disease, correction of this arrhythmia in tandem with mitral surgery is usually warranted. Excellent results of the Cox-Maze IV procedure using bipolar radiofrequency ablation and cryoablation have been reported by several groups, with freedom from atrial fibrillation >90% after 1 year. Addition of the lesion set as described by Cox, Boineau, Schuessler et al. in the late 1980s yields the highest rates of freedom from atrial fibrillation [84]. Most of the incisions can be replaced with ablations (either bipolar radiofrequency or cryothermy) that create transmural lesions with similar excellent long-term outcomes (Fig. 6.27) [85]. In high-risk cases or in patients undergoing second or subsequent surgeries for mitral disease, a more limited set of lesions such as pulmonary vein isolation and an isthmus lesion may be warranted. These sub-sets of lesions uniformly have lower rates of success and patients should be made aware of the tradeoff of risks and benefits of a full Cox-Maze procedure versus a subset of equivalent lesions [86].
Cox-Maze lesions in the left atrium (right atrial lesions not shown) for atrial fibrillation at the time of mitral valve repair. Standard left atriotomy with mitral valve visualized after repair. Ablations (green dashed lines) can be carried out with bipolar radiofrequency clamp or with cryo. Lesions encircling the pulmonary veins are connected along the back of the left atrium to isolate the entire posterior left atrium. An ablation is carried out from the inferior edge of the atriotomy to the mitral valve annulus. When this lesion is completed with bipolar radiofrequency, an additional cryo lesion on the valve annulus is necessary (green) along with an additional lesion on the exterior of the heart to ablate the coronary sinus (not shown). An additional lesion connecting the left superior pulmonary vein with the resected left atrial appendage is completed
Systolic Anterior Motion
Systolic anterior motion (SAM) of the mitral valve occurs when the anterior leaflet is captured in the left ventricular outflow tract during systole, which can precipitate outflow obstruction, hemodynamic compromise with hypotension, tachycardia, and death. In patients with hypertrophic cardiomyopathy, the risk increases for SAM after mitral repair. Several risk factors have been described, but elongated/redundant valve tissue with displacement of the coaptation point toward the left ventricular outflow tract (LVOT) clearly predisposes to SAM. Patients who undergo repair with annuloplasty are at risk, as leaflet tissue and the coaptation point may be moved posteriorly, and tissue leaflet may get caught up in the LVOT during systole. Initial therapy for intraoperative SAM include: (1) cessation of inotropes, (2) intravascular volume expansion, (3) beta blockade, and (4) augmentation of LV afterload. Surgical revision is rarely necessary [87].
Complications
Mitral Annular Calcification
Valves with extensive mitral annular calcification (often referred to as “MAC”) may require debridement of the annular tissue near the posterior leaflet for both relief of stenosis and/or to create an area of suitability to place annular stitches to support a ring or new valve. In this condition, risk of a postoperative atrioventricular groove disruption can occur as pressurized blood from the ventricle can intercalate into the newly debrided area and create a highly morbid hematoma formation between the left atrium and ventricle. This condition usually progresses to hemorrhage and carries a very high mortality. Repair is often only achievable by emergent redo surgery with removal (and subsequent re-replacement) of the valve or ring and creation of a supporting patch over the area using ventricular muscle and atrial layers to support the patch and then the patch to support the replacement valve.
Coronary Artery Occlusion
Coronary artery occlusion can occur if care is not taken in the sutures placed in the annulus at A1, P1, and P2, as the circumflex artery courses near the annulus in this area (Fig. 6.24). Aortic valve injury can occur with stitches placed too deeply in one of the fibrous trigones of the anterior annulus.
Paravalvular Regurgitation
Paravalvular regurgitation or ring dehiscence can be seen if inadequate sutures are placed at the time of surgery, or if the tissue holding the suture is weakened by subsequent infection or other pathology. Severity of the regurgitation, heart failure symptoms, and the presence of hemolysis will guide the decision of whether a subsequent procedure to correct the problem is necessary.
Summary
Surgical techniques for mitral valve disease have improved dramatically over the last three decades. Current surgical techniques allow for the repair of the majority of degenerative valves with excellent results and very low operative mortality rates. Patients with mitral valve disease should be referred to a center of excellence with high volume mitral valve surgery to ensure optimal outcomes for patients.
Understanding and treatment of mitral valve disease continues to evolve. It is now recognized that primary and secondary MR are virtually two different diseases with different etiologies, pathophysiologies, therapies, and outcomes. The advances in echocardiography and mitral repair for primary MR allow for earlier and safer surgery without exposing the patient to the risks of prosthetic valve replacement. The biggest challenge in management is in the therapy for secondary MR where there is still no treatment for the advanced LV damage that usually accompanies the condition.
For the treatment of mitral stenosis, a disease becoming rare in developed countries because rheumatic fever has become rare, percutaneous balloon valvuloplasty has replaced surgery in most cases.
References
Otto CM. Clinical practice. Evaluation and management of chronic mitral regurgitation. N Engl J Med. 2001;345(10):740–6.
Ralph AP, Carapetis JR. Group a streptococcal diseases and their global burden. Curr Top Microbiol Immunol. 2013;368:1–27.
Wood P. An appreciation of mitral stenosis. I. Clinical features. Br Med J. 1954;1(4870):1051–63.
Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(13):e1–142.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521–643.
Deloche A, Jebara VA, Relland JY, Chauvaud S, Fabiani JN, Perier P, Dreyfus G, Mihaileanu S, Carpentier A. Valve repair with Carpentier techniques. The second decade. J Thorac Cardiovasc Surg. 1990;99(6):990–1001; discussion 1001–2.
Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J. 2010;31(16):1958–66.
Di Salvo TG, Acker MA, Dec GW, Byrne JG. Mitral valve surgery in advanced heart failure. J Am Coll Cardiol. 2010;55(4):271–82.
Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112(5):745–58.
Carpentier A. Cardiac valve surgery—the “French correction”. J Thorac Cardiovasc Surg. 1983;86(3):323–37.
Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. London: Elsevier; 2011.
Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL, Scientific Document Committee of the European Association of Cardiovascular I. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14(7):611–44.
Foster GP, Isselbacher EM, Rose GA, Torchiana DF, Akins CW, Picard MH. Accurate localization of mitral regurgitant defects using multiplane transesophageal echocardiography. Ann Thorac Surg. 1998;65(4):1025–31.
O’Gara P, Sugeng L, Lang R, Sarano M, Hung J, Raman S, Fischer G, Carabello B, Adams D, Vannan M. The role of imaging in chronic degenerative mitral regurgitation. JACC Cardiovasc Imaging. 2008;1(2):221–37.
Tsang W, Lang RM. Three-dimensional echocardiography is essential for intraoperative assessment of mitral regurgitation. Circulation. 2013;128(6):643–52; discussion 652.
McCarthy PM. Three-dimensional echocardiography is not essential for intraoperative assessment of mitral regurgitation. Circulation. 2013;128(6):653–8; discussion 658.
Feuchtner GM, Alkadhi H, Karlo C, Sarwar A, Meier A, Dichtl W, Leschka S, Blankstein R, Gruenenfelder J, Stolzmann P, Cury RC. Cardiac CT angiography for the diagnosis of mitral valve prolapse: comparison with echocardiography1. Radiology. 2010;254(2):374–83.
Meijboom WB, Mollet NR, Van Mieghem CA, Kluin J, Weustink AC, Pugliese F, Vourvouri E, Cademartiri F, Bogers AJ, Krestin GP, de Feyter PJ. Pre-operative computed tomography coronary angiography to detect significant coronary artery disease in patients referred for cardiac valve surgery. J Am Coll Cardiol. 2006;48(8):1658–65.
Dewey M, Muller M, Eddicks S, Schnapauff D, Teige F, Rutsch W, Borges AC, Hamm B. Evaluation of global and regional left ventricular function with 16-slice computed tomography, biplane cineventriculography, and two-dimensional transthoracic echocardiography: comparison with magnetic resonance imaging. J Am Coll Cardiol. 2006;48(10):2034–44.
Wu YW, Tadamura E, Kanao S, Yamamuro M, Okayama S, Ozasa N, Toma M, Kimura T, Kita T, Marui A, Komeda M, Togashi K. Left ventricular functional analysis using 64-slice multidetector row computed tomography: comparison with left ventriculography and cardiovascular magnetic resonance. Cardiology. 2008;109(2):135–42.
Hur J, Kim YJ, Nam JE, Choe KO, Choi EY, Shim CY, Choi BW. Thrombus in the left atrial appendage in stroke patients: detection with cardiac CT angiography—a preliminary report. Radiology. 2008;249(1):81–7.
Cawley PJ, Maki JH, Otto CM. Cardiovascular magnetic resonance imaging for valvular heart disease: technique and validation. Circulation. 2009;119(3):468–78.
Moscucci M. Grossman and Baim’s cardiac catheterization, angiography, and intervention. 8th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2013.
Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802.
Armstrong WF. Feigenbaum’s echocardiography. 7th ed. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2009.
Yosefy C, Levine RA, Solis J, Vaturi M, Handschumacher MD, Hung J. Proximal flow convergence region as assessed by real-time 3-dimensional echocardiography: challenging the hemispheric assumption. J Am Soc Echocardiogr. 2007;20(4):389–96.
Thomas L, Foster E, Schiller NB. Peak mitral inflow velocity predicts mitral regurgitation severity. J Am Coll Cardiol. 1998;31(1):174–9.
Wunderlich NC, Beigel R, Siegel RJ. Management of mitral stenosis using 2D and 3D echo-Doppler imaging. JACC Cardiovasc Imaging. 2013;6(11):1191–205.
Thomas JD, Weyman AE. Doppler mitral pressure half-time: a clinical tool in search of theoretical justification. J Am Coll Cardiol. 1987;10(4):923–9.
Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10(1):1–25.
Carabello BA. Modern management of mitral stenosis. Circulation. 2005;112(3):432–7.
Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60(4):299–308.
Mueller RL, Sanborn TA. The history of interventional cardiology: cardiac catheterization, angioplasty, and related interventions. Am Heart J. 1995;129(1):146–72.
Nishimura RA, Carabello BA. Hemodynamics in the cardiac catheterization laboratory of the 21st century. Circulation. 2012;125(17):2138–50.
Thomas JD, Wilkins GT, Choong CY, Abascal VM, Palacios IF, Block PC, Weyman AE. Inaccuracy of mitral pressure half-time immediately after percutaneous mitral valvotomy. Dependence on transmitral gradient and left atrial and ventricular compliance. Circulation. 1988;78(4):980–93.
Lange RA, Moore DM Jr, Cigarroa RG, Hillis LD. Use of pulmonary capillary wedge pressure to assess severity of mitral stenosis: is true left atrial pressure needed in this condition? J Am Coll Cardiol. 1989;13(4):825–31.
Kern M, Lim M, Goldstein J. Hemodynamic rounds: interpretation of cardiac pathophysiology from pressure waveform analysis. New York: Wiley-Blackwell; 2009.
Gorlin R, Gorlin SG. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. I. Am Heart J. 1951;41(1):1–29.
Cohen MV, Gorlin R. Modified orifice equation for the calculation of mitral valve area. Am Heart J. 1972;84(6):839–40.
Hakki AH, Iskandrian AS, Bemis CE, Kimbiris D, Mintz GS, Segal BL, Brice C. A simplified valve formula for the calculation of stenotic cardiac valve areas. Circulation. 1981;63(5):1050–5.
Cui W, Dai R, Zhang G. A new simplified method for calculating mean mitral pressure gradient. Catheter Cardiovasc Interv. 2007;70(5):754–7.
Baim DS. Grossman’s cardiac catheterization, angiography, and intervention. 7th edition: Philadelphia, PA: Lippincott Williams & Wilkins; 2006; pp. 642–3.
Fuchs RM, Heuser RR, Yin FC, Brinker JA. Limitations of pulmonary wedge V waves in diagnosing mitral regurgitation. Am J Cardiol. 1982;49(4):849–54.
Freihage JH, Joyal D, Arab D, Dieter RS, Loeb HS, Steen L, Lewis B, Liu JC, Leya F. Invasive assessment of mitral regurgitation: comparison of hemodynamic parameters. Catheter Cardiovasc Interv. 2007;69(2):303–12.
Goodman DJ, Rossen RM, Holloway EL, Alderman EL, Harrison DC. Effect of nitroprusside on left ventricular dynamics in mitral regurgitation. Circulation. 1974;50(5):1025–32.
Greenberg BH, Massie BM, Brundage BH, Botvinick EH, Parmley WW, Chatterjee K. Beneficial effects of hydralazine in severe mitral regurgitation. Circulation. 1978;58(2):273–9.
Yoran C, Yellin EL, Becker RM, Gabbay S, Frater RW, Sonnenblick EH. Mechanism of reduction of mitral regurgitation with vasodilator therapy. Am J Cardiol. 1979;43(4):773–7.
Dujardin KS, Enriquez-Sarano M, Bailey KR, Seward JB, Tajik AJ. Effect of losartan on degree of mitral regurgitation quantified by echocardiography. Am J Cardiol. 2001;87(5):570–6.
Harris KM, Aeppli DM, Carey CF. Effects of angiotensin-converting enzyme inhibition on mitral regurgitation severity, left ventricular size, and functional capacity. Am Heart J. 2005;150(5):1106.
Wisenbaugh T, Sinovich V, Dullabh A, Sareli P. Six month pilot study of captopril for mildly symptomatic, severe isolated mitral and isolated aortic regurgitation. J Heart Valve Dis. 1994;3(2):197–204.
Ahmed MI, Aban I, Lloyd SG, Gupta H, Howard G, Inusah S, Peri K, Robinson J, Smith P, McGiffin DC, Schiros CG, Denney T Jr, Dell’Italia LJ. A randomized controlled phase IIb trial of beta(1)-receptor blockade for chronic degenerative mitral regurgitation. J Am Coll Cardiol. 2012;60(9):833–8.
Kim KH, Kim YJ, Ohn JH, Yang J, Lee SE, Lee SW, Kim HK, Seo JW, Sohn DW. Long-term effects of sildenafil in a rat model of chronic mitral regurgitation: benefits of ventricular remodeling and exercise capacity. Circulation. 2012;125(11):1390–401.
Bridgewater B, Hooper T, Munsch C, Hunter S, von Oppell U, Livesey S, Keogh B, Wells F, Patrick M, Kneeshaw J, Chambers J, Masani N, Ray S. Mitral repair best practice: proposed standards. Heart. 2006;92(7):939–44.
David TE, Armstrong S, McCrindle BW, Manlhiot C. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation. 2013;127(14):1485–92.
LaPar DJ, Ailawadi G, Isbell JM, et al. Mitral valve repair rates correlate with surgeon and institutional experience. J Thorac Cardiovasc Surg. 2014;148(3):995–1003.
Gillam LD, Schwartz A. Primum non nocere: the case for watchful waiting in asymptomatic “severe” degenerative mitral regurgitation. Circulation. 2010;121(6):813–21; discussion 821.
Suri RM, Vanoverschelde JL, Grigioni F, Schaff HV, Tribouilloy C, Avierinos JF, Barbieri A, Pasquet A, Huebner M, Rusinaru D, Russo A, Michelena HI, Enriquez-Sarano M. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310(6):609–16.
Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D, Schemper M, Maurer G, Baumgartner H. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. 2006;113(18):2238–2244.59.
Borer JS. Early surgery or watchful waiting for asymptomatic severe degenerative mitral regurgitation: is the answer now clear? J Am Coll Cardiol. 2014;63(22):2408–10.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252–89.
Elliott C, Cutler SAL. Cardiotomy and valvulotomy for mitral stenosis: experimental observations and clinical notes concerning an operated case with recovery. Boston Med Surg J. 1929;188:1023.
Souttar HS. The surgical treatment of mitral stenosis. Br Med J. 1925;2(3379):603–6.
Crawford FA Jr. Horace Smithy: pioneer heart surgeon. Ann Thorac Surg. 2010;89(6):2067–7.
Harken DE, Ellis LB, et al. The surgical treatment of mitral stenosis; valvuloplasty. N Engl J Med. 1948;239(22):801–809.65.
Bailey CP. The surgical treatment of mitral stenosis (mitral commissurotomy). Dis Chest. 1949;15(4):377–97.
Baker C, Brock RC, Campbell M. Valvulotomy for mitral stenosis; report of six successful cases. Br Med J. 1950;1(4665):1283–93.
Lock JE, Khalilullah M, Shrivastava S, Bahl V, Keane JF. Percutaneous catheter commissurotomy in rheumatic mitral stenosis. N Engl J Med. 1985;313(24):1515–8.
Reyes VP, Raju BS, Wynne J, Stephenson LW, Raju R, Fromm BS, Rajagopal P, Mehta P, Singh S, Rao DP, et al. Percutaneous balloon valvuloplasty compared with open surgical commissurotomy for mitral stenosis. N Engl J Med. 1994;331(15):961–7.
McCarthy PM. Does the intertrigonal distance dilate? Never say never. J Thorac Cardiovasc Surg. 2002;124(6):1078–9.
Barlow JB, Bosman CK, Pocock WA, Marchand P. Late systolic murmurs and non-ejection (“mid-late”) systolic clicks. An analysis of 90 patients. Br Heart J. 1968;30(2):203–18.
Barlow JB, Pocock WA. The significance of late systolic murmurs and mid-late systolic clicks. Md State Med J. 1963;12:76–7.
Jouan J, Berrebi A, Chauvaud S, Menasche P, Carpentier A, Fabiani JN. Mitral valve reconstruction in Barlow disease: long-term echographic results and implications for surgical management. J Thorac Cardiovasc Surg. 2012;143(4 Suppl):S17–20.
Perier P. Quadrangular resection for repair of posterior leaflet prolapse. Multimed Man Cardiothorac Surg. 2005;2005(1129):mmcts.2004.000893.
De Bonis M, Lapenna E, Buzzatti N, Taramasso M, Calabrese MC, Nisi T, Pappalardo F, Alfieri O. Can the edge-to-edge technique provide durable results when used to rescue patients with suboptimal conventional mitral repair? Eur J Cardiothorac Surg. 2013;43(6):e173–9.
De Bonis M, Lapenna E, Maisano F, Barili F, La Canna G, Buzzatti N, Pappalardo F, Calabrese M, Nisi T, Alfieri O. Long-term results (</=18 years) of the edge-to-edge mitral valve repair without annuloplasty in degenerative mitral regurgitation: implications for the percutaneous approach. Circulation. 2014;130(11 Suppl 1):S19–24.
De Bonis M, Lapenna E, Lorusso R, Buzzatti N, Gelsomino S, Taramasso M, Vizzardi E, Alfieri O. Very long-term results (up to 17 years) with the double-orifice mitral valve repair combined with ring annuloplasty for degenerative mitral regurgitation. J Thorac Cardiovasc Surg. 2012;144(5):1019–24.
Tommaso CL, Fullerton DA, Feldman T, Dean LS, Hijazi ZM, Horlick E, Weiner BH, Zahn E, Cigarroa JE, Ruiz CE, Bavaria J, Mack MJ, Cameron DE, Bolman RM 3rd, Miller DC, Moon MR, Mukherjee D, Trento A, Aldea GS, Bacha EA. SCAI/AATS/ACC/STS operator and institutional requirements for transcatheter valve repair and replacement. Part II. Mitral valve. J Thorac Cardiovasc Surg. 2014;148(2):387–400.
O’Gara PT, Calhoon JH, Moon MR, Tommaso CL. Transcatheter therapies for mitral regurgitation: a professional society overview from the American College of Cardiology, The American Association for Thoracic Surgery, Society for Cardiovascular Angiography and Interventions Foundation, and The Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 2014;147(3):837–49.
Moon MR, DeAnda A Jr, Daughters GT 2nd, Ingels NB Jr, Miller DC. Experimental evaluation of different chordal preservation methods during mitral valve replacement. Ann Thorac Surg. 1994;58(4):931–43; discussion 943–4.
Moon MR, DeAnda A Jr, Daughters GT 2nd, Ingels NB Jr, Miller DC. Effects of mitral valve replacement on regional left ventricular systolic strain. Ann Thorac Surg. 1999;68(3):894–902.
Moon MR, DeAnda A Jr, Daughters GT 2nd, Ingels NB, Miller DC. Effects of chordal disruption on regional left ventricular torsional deformation. Circulation. 1996;94(9 Suppl):II143–51.
Lillehei CW, Levy MJ, Bonnabeau RC Jr. Mitral valve replacement with preservation of papillary muscles and chordae tendineae. J Thorac Cardiovasc Surg. 1964;47:532–43.
Reardon MJ, David TE. Mitral valve replacement with preservation of the subvalvular apparatus. Curr Opin Cardiol. 1999;14(2):104–10.
Cox JL, Schuessler RB, D’Agostino HJ Jr, Stone CM, Chang BC, Cain ME, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101(4):569–83.
Melby SJ, Zierer A, Bailey MS, Cox JL, Lawton JS, Munfakh N, Crabtree TD, Moazami N, Huddleston CB, Moon MR, Damiano RJ Jr. A new era in the surgical treatment of atrial fibrillation: the impact of ablation technology and lesion set on procedural efficacy. Ann Surg. 2006;244(4):583–92.
Robertson JO, Lawrance CP, Maniar HS, Damiano RJ Jr. Surgical techniques used for the treatment of atrial fibrillation. Circ J. 2013;77(8):1941–51.
Ibrahim M, Rao C, Ashrafian H, Chaudhry U, Darzi A, Athanasiou T. Modern management of systolic anterior motion of the mitral valve. Eur J Cardiothorac Surg. 2012;41(6):1260–70.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer-Verlag London Ltd., part of Springer Nature
About this chapter
Cite this chapter
Lindman, B.R., Melby, S.J., Quader, N., Sintek, M.A., Moon, M.R. (2020). Comprehensive Assessment of Primary Mitral Valve Disease: Clinical Presentation, Diagnosis, Medical and Surgical Therapy. In: Carabello, B. (eds) Valvular Heart Disease. Cardiovascular Medicine. Springer, London. https://doi.org/10.1007/978-1-4471-2840-3_6
Download citation
DOI: https://doi.org/10.1007/978-1-4471-2840-3_6
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-2839-7
Online ISBN: 978-1-4471-2840-3
eBook Packages: MedicineMedicine (R0)