Abstract
Background: Sufficient calcium intake is essential for the maintenance of bone health in older people. However, the effect of dietary protein on bone mass of older women has been controversial. To the best of our knowledge, there has been no clinical trial evaluating the effect of protein supplementation on bone mass in older Chinese women.
Objective: To evaluate the effect of 1-year protein and calcium supplementation on bone mass in older Chinese women compared to calcium supplementation alone.
Design: A 1-year randomized controlled trial was conducted in 283 Chinese postmenopausal women aged 68.1 ± 0.5 years (range 60–86 years). Study participants were randomized to receive either protein powder containing 30 g soybean protein and 1,000 mg calcium as calcium carbonate (Pro + Ca group, n = 142) or only 1,000 mg calcium per day (Ca group, n = 141). Measurements performed include dietary intakes by 1-year food-frequency questionnaire, physical activity by International Physical Activity Questionnaire (IPAQ)-Short Form, and areal bone mineral density (aBMD) at hip, lumbar spine (L2–L4), and total body by DXA at baseline and 1 year later.
Results: There were no significant differences between the two groups in baseline characteristics. With supplementation, both groups had significantly higher calcium intake compared to the baseline (1,647 ± 53 mg/day vs. 879 ± 30 mg/day, P = 0.01), and the average dietary protein intake was significantly higher in the Pro + Ca group compared to the Ca group (107.8 ± 4.6 g/day vs. 75.7 ± 3.1 g/day, P < 0.001).
After 1-year supplementation, there was a slight but significant increase in aBMD at total body, femoral neck, trochanter, and total hip in both groups after adjusting for baseline age, BMI, calcium intake, physical activity level, and serum 25(OH)D level (time effect, all P < 0.05). There were no significant time effects on lumbar spine aBMD in either group.
The Pro + Ca group had significantly greater increase in total-body aBMD (9.5 mg/cm2) compared to the Ca group (0.4 mg/cm2) after 1 year of supplementation before adjustment for covariates (time × group interaction, P < 0.05). There were no significant effects of protein supplementation on aBMD of other sites.
Conclusion: Higher intake of dietary protein might have a positive effect on total-body bone mass in Chinese postmenopausal women when calcium intake is sufficient.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The prevalence of osteoporosis has been increasing in recent years because of increases in life expectancy and the number of older individuals in most countries, especially in China. For example, in 2004, osteoporosis and low bone density affected about 12 % of the total population in China [1].
Balanced diet is a basic strategy for the prevention of osteoporosis. Protein is a major component of bone matrix. However, data on the effect of dietary protein on bone mass has been controversial, especially in elderly. A few earlier studies indicated a negative effect of high protein intakes on bone mass [2], whereas most observation studies in elderly population have shown that relatively high protein intakes could reduce bone loss [3–5] and reduce the risk of hip fracture in elderly women [6].
Moreover, it was observed that the effect of protein on bone health could be related to calcium intake. A cross-sectional study showed that the highest quartile of protein intake (mean intake = 72 g/day) was associated with higher BMD in elderly women only when the calcium intake exceeded 408 mg/day [7].
Most studies evaluating the effect of protein intake on bone mass were carried out in western populations. It is well known that calcium intakes and bone structure are different to some extent between Chinese and westerners. To the best of our knowledge, there has been no published clinical trial with protein supplementation in Chinese older women. Thus, the objective of this study was to evaluate the effect of protein supplementation on bone mineral density in Chinese postmenopausal women who also received 1,000 mg calcium per day.
Subjects and Methods
Study Design
A 1-year, randomized, placebo-controlled trial of calcium and calcium plus protein in Chinese older women.
Participants
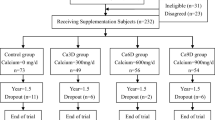
The study subjects were 283 Chinese women aged over 65 years. They were recruited by mail or poster in community in Beijing, China. The inclusion criteria were (A) Chinese women over the age of 60 years and (B) living in Beijing during intervention period. The exclusion criteria were (A) fracture within 6 months of screening; (B) patient with bone disease in the last 12 months; (C) previous osteoporosis treatments in the last 12 months except for calcium supplementation; (D) patient with liver or kidney disorder or other endocrinosis, such as diabetes; (E) significant prior neuromuscular disorders that impair balance; (F) oral corticosteroids in the last year; (G) patients with serious, uncontrolled disease likely to interfere with the study and/or likely to cause death within the study duration; and (H) participation in another clinical trial during the last half year. The investigator explained benefits and risks of participation in the study to each subject during the interview. A written informed consent form was signed by each subject prior to the initiation of nonroutine tests at baseline survey. The study was approved by the ethics committees of the Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention.
Protein Supplements
A researcher who was not part of the study group was in charge of randomization. The randomization used block size of ten using random numbers. The 283 subjects were randomized into two groups:
-
Calcium group, n = 142, supplied with three calcium carbonate tablets, containing total of 1,000 mg calcium per day
-
Calcium + protein group, n = 141, supplied with two packets of powder containing total of 30 g soybean protein and 1,000 mg calcium per day
All supplementation were produced and packaged by the same factory. A researcher outside of the study group dispensed calcium tablets in bottles or protein and calcium powder in satchels to study participants every 3 months. The supplement was instructed to be taken with meal. A record form for taking supplementation was also delivered to all subjects with supplementation. During the supplementation period, the study staff contacted each subject every month to record the number of supplements taken and to promote compliance. The remaining bottles and satchels were collected and counted at the end of the study to calculate compliance.
Bone Measurements
Bone mineral density of spine (L2–L4), proximal femur (femoral neck, trochanter, and total hip), and total body were measured by dual-energy X-ray absorptiometry, using a Norland XR-46 densitometer in pencil-beam mode (Norland Medical Systems, Inc., Fort Atkinson, WI, USA) with software version 3.94 at baseline and the end of the study. Two experienced technicians performed the measurements throughout the study. The DXA had a variation in precision of <1.0 % for the measured bone sites at standard speed. A daily quality assurance test was performed with a manufacturer-supplied hydroxyapatite phantom, and the accuracy error was <1.0 %.
Biochemistry
Serum 25-hydroxyvitamin D [25(OH)D] concentration in fasting blood samples was determined by radioimmunoassay (RIA, DiaSorin Inc., Stillwater, MN, USA) in duplicates. All samples were measured in one laboratory. The interassay coefficient was 7.80 % at 18.9 ng/ml, with interassay and intra-assay coefficients of variation (CVs) of 11.1 and 8.8 %, respectively.
Dietary Intakes
Dietary intakes were assessed by using a 51-item food-frequency questionnaire, which was adopted from the questionnaire used in the 2002 China National Nutrition and Health Survey [8]. Nutrient intakes were calculated using the Chinese Food Composition Tables published in 2002 [9] and 2004 [10].
Other Assessments
Anthropometry measurements, including height and weight, were performed with subjects in light clothes and without shoes. Physical activity (PA) level was estimated using the Chinese version of the International Physical Activity Questionnaire (IPAQ)-Short Form. Total physical activity level (METs/week) was calculated and divided as high (score = 3), moderate (score = 2), and low level (score = 1).
Statistical Analysis
The repeated measurement for variables was analyzed by Mixed Linear Model for continuous variables (such as bone mineral density) in SAS program, version 9.0, with or without adjustment for body mass index (BMI), calcium intake, serum 25(OH)D, age, and other potential confounders. The supplement × time interaction was tested, and significant interaction indicates significant effect of supplementation. P value less than 0.05 was considered as statistically significant difference.
Results
In 283 subjects who participated in the protein intervention trial, 190 subjects were reexamined at the end of the intervention. No significant difference in dropout rates was observed between the calcium plus protein group and the calcium group (36.9 and 28.9 %, respectively, P = 0.40). At baseline, there were no significant differences in age, height, BMI, protein and other nutrients intakes, and bone measures between the subjects who completed the study and those who did not (data were not shown).
Baseline characteristics are given in Table 11.1 and were not significantly different between the protein plus calcium and the calcium group (P > 0.1 for all). Their mean calcium intake (879 ± 30 mg/day) was below the recommended intake level of 1,000 mg/day, and protein intake (72.0 ± 2.2 g/day) was slightly higher than the recommended intake level of 65 g/day published by Chinese Nutrition Society [11]. With supplementation, both groups had significantly higher calcium intake compared to the baseline (1,647 ± 53 mg/day, P = 0.01), and the average dietary protein intake was significantly higher in the Pro + Ca group compared to the Ca group (107.8 ± 4.6 g/day vs. 75.7 ± 3.1 g/day, P < 0.001).
There were no significant differences between groups in the bone mineral density (BMD) of total body, femoral neck, trochanter, total hip, and lumbar spine at baseline (P > 0.05 for all). After 1-year supplementation, aBMD at total body, femoral neck, trochanter, and total hip increased slightly but significantly in both groups after adjusting for baseline age, BMI, calcium intake, physical activity level, and serum 25(OH)D level (time effect, all P < 0.05). There were no significant time effects on lumbar spine aBMD before and after adjustment (Table 11.2).
The Pro + Ca group had significantly greater increase in total-body aBMD (9.5 mg/cm2) compared to the Ca group (0.4 mg/cm2) after 1 year of supplementation before adjustment and still after adjustment for covariates (time × group interaction, all P < 0.05). There were no significant effects of protein supplementation on aBMD of other sites (Table 11.2).
Discussion
The present study showed that after protein and calcium supplementation for 1 year, protein supplementation with calcium led to a significant increase in total-body BMD, compared to subjects who consumed only calcium supplement. These results suggest that at similar high level of calcium intake, higher protein intake contributed to a higher total-body BMD.
Protein could provide amino acids as substrates for building bone matrix; therefore, adequate protein intake is important for the maintenance of bone mass in the elderly. A number of cross-sectional and longitudinal studies with older subjects have shown that relatively high protein intakes were associated with reduced bone loss [2, 3, 5]. For example, the Framingham Osteoporosis Study showed that participants in the two lowest quartiles of protein intake (<67 g/day) had greater bone loss at the femoral neck compared to those in the highest quartile (>84 g/day) [3]. In this intervention study in Chinese postmenopausal women, a higher protein intake with calcium had positive effect on total-body BMD after 1 year. Our results extend the findings of observational studies of postmenopausal women that suggest beneficial effects of higher protein intake on BMD.
In a recent study in older Western Australian women, 30 g extra protein per day did not affect change in bone density or strength over 2 years [12]. A possible reason for the lack of effect in older Western Australian women was their relatively high usual dietary protein intake, 1.1 g/kg body weight/day at baseline, which is well above the Australian EAR of 0.75 g/kg body weight/day for older women [13]. In the present study in Chinese older women, the mean protein intake at baseline was 1.1 g/kg body weight/day, which is slightly less than the RNI for Chinese older people of 1.27 g/kg body weight/day [11]. The protein RNI for Chinese is higher than that for western countries because Chinese consume more plant-based protein.
Several studies have indicated that the effect of protein intake on bone mass in the elderly could be influenced by calcium intake [7, 14]. A prospective study showed that higher protein intake was significantly associated with a favorable 3-year change in total-body BMD in the supplemented group with a mean calcium intake of 1,346 mg/day but not in the placebo group with a mean calcium intake of 871 mg/day [14]. Therefore, sufficient calcium intake is important for the beneficial effect of protein on bone mass.
A limitation of the present study was that the assessment of bone mass with dual-energy X-ray absorptiometry, which cannot evaluate the changes in bone geometry and volumetric density. Another limitation of the present study was that we did not have another control group with low calcium and high protein intake, so we cannot evaluate the effect of higher protein intake on bone health in Chinese older women with lower calcium intake.
In conclusion, the present study showed that 1-year protein supplementation with sufficient calcium intake would benefit total-body bone mineral density, but not other skeletal sites in Chinese postmenopausal women. Both calcium and protein nutrition are important for bone health in Chinese postmenopausal women.
References
Zhao D, Wu H, Liu Z. The epidemiology of osteoporosis in China. Chin J Osteoporos. 2004;10:614–8.
Pannemans D, Schaafsma G, Westerterp K. Calcium excretion, apparent calcium absorption and calcium balance in young and elderly subjects: influence of protein intake. Br J Nutr. 1997;77:721–9.
Hannan M, Tucker K, Dawson-Hughes B, Cupples L, Felson D, Kiel D. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504–12.
Promislow J, Goodman-Gruen D, Slymen D, Barrett-Connor E. Protein consumption and bone mineral density in the elderly: the Rancho Bernardo Study. Am J Epidemiol. 2002;155:636–44.
Devine A, Dick I, Islam A, Dhaliwal S, Prince R. Protein consumption is an important predictor of lower limb bone mass in elderly women. Am J Clin Nutr. 2005;81:1423–8.
Wengreen H, Munger R, Cutler D, Corcoran C, Zhang J, Sassano N. Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah. J Bone Miner Res. 2004;19:537–45.
Rapuri P, Gallagher J, Haynatzka V. Protein intake: effects on bone mineral density and the rate of bone loss in elderly women. Am J Clin Nutr. 2003;77:1517–25.
Wang DL. Report of China National Nutrition and Health Survey in 2002 I: total report. Beijing: Public Health; 2005.
Yang Y, Wang G, Pan X. China food composition 2002. Beijing: Peking University Medical Press; 2002.
Yang Y. China food composition 2004-book 2. Beijing: Peking University Medical Press; 2004.
Chinese Nutrition Society. Chinese DRIs. Beijing: Chinese Light Industry Press; 2000.
Zhu K, Meng X, Kerr D, Devine A, Solah V, Binns C, et al. The effects of a two-year randomised controlled trial of whey protein supplementation on bone structure, IGF-I and urinary calcium excretion in older postmenopausal women. J Bone Miner Res. 2011;26(9):2298–306.
National Health and Medical Research Council. Dietary guidelines for protein intake affects bone mass 1427. Canberra: Australian Government Publishing Service; 1991.
Dawson-Hughes B, Harris S. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr. 2002;75:773–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Zhang, Q. et al. (2013). The Effects of Protein Supplementation on Bone Mass in Chinese Postmenopausal Women. In: Burckhardt, P., Dawson-Hughes, B., Weaver, C. (eds) Nutritional Influences on Bone Health. Springer, London. https://doi.org/10.1007/978-1-4471-2769-7_11
Download citation
DOI: https://doi.org/10.1007/978-1-4471-2769-7_11
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-2768-0
Online ISBN: 978-1-4471-2769-7
eBook Packages: MedicineMedicine (R0)