Abstract
Bacterial biosorption can be used for the removal of pollutants from waters contaminated with pollutants that are not easily biodegradable, such as metals and dyes. A variety of biomaterials are known to bind these pollutants including bacteria, fungi, algae, and certain industrial and agricultural wastes. Biosorbents are less costly and more effective alternatives for the removal of metallic elements, especially heavy metals, from aqueous solution. In this chapter, the sorption abilities of bacterial biomass toward metal ions are emphasized. The appropriate conditions for immobilizing bacteria for maximum biosorption and the mechanism(s) involved are highlighted. The properties of cell wall constituents, such as peptidoglycan, and the role of functional groups, such as carboxyl, amine, and phosphonate, are discussed on the basis of their biosorption potentials. Binding mechanisms as well as the parameters influencing passive uptake of pollutants are analyzed. A detailed description of isotherm and kinetic models and the importance of mechanistic modeling are presented. To enhance biosorption capacity, biomass modifications through chemical methods and genetic engineering are needed for the effective removal of metal. For continuous treatment of effluents, a packed column configuration is suggested and the factors influencing its performance are discussed. The chapter also highlights the necessity for examination of biosorbents within real-world situations, as competition between solutes and water quality may affect biosorption performance. Thus, this chapter reviews the achievements and current status of biosorption technology and provides insights into this research frontier.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Enormous quantities of toxic metals are released into the environment annually as a result of human activities. In some cases, these releases are deliberate and well-regulated, like industrial emissions, while in other cases they are accidental and include chemical spills or improper land disposal (Lloyd 2002). Toxic metals of concern include lead, chromium, mercury, uranium, selenium, zinc, arsenic, cadmium, gold, silver, copper, and nickel. These pollutants are derived from mining, metallurgical, electronic, electroplating, chrome tanning, textiles, metal finishing, fertilizer manufacture, and steel and automobile industries. Quantities of heavy metals released into the environment have increased due to rapid industrialization and technological development, posing significant threats to ecosystems and public health because of their toxicity, accumulation in food chains, and persistence in nature (Sharma et al. 2006; Tuzen et al. 2008). Following the fate of toxic metal species after they enter the ecosystem becomes difficult; furthermore, they inflict damage as they move from one ecological trophic layer to another. Controlling heavy metal discharges and removing toxic heavy metals from water bodies has become a challenge for the twenty-first century.
Methods used for heavy metal removal from industrial effluents can be classified as physical, chemical, and biological. Physicochemical methods such as precipitation, ion exchange, filtration, membrane and electrochemical technologies, reverse osmosis, electrodialysis, adsorption on activated carbon, etc. require high capital and operating costs and may also be associated with the generation of secondary wastes which cause treatment problems. Therefore, recent attention has been drawn toward the development of alternative methodologies known as bioremediation processes. These technologies include, among other processes, biosorption. Biosorption or bioadsorption involves passive immobilization of metals by living biomass. Biosorption can be defined as the ability of biological materials to accumulate heavy metals from wastewaters through metabolically mediated or physiochemical pathways of uptake. Biosorbents for the removal of metals mainly come under the following categories: bacteria, fungi, algae, industrial wastes, agricultural wastes, and various polysaccharide materials. These biosorbents can effectively sequester dissolved metal ions from dilute complex solutions. The use of biological material in the removal of heavy metals from industrial effluents has gained importance during recent years because of the high efficiency, minimization of chemical/biological sludge, low operating cost, regeneration of biosorbents, and possibility of metal recovery.
12.2 Bacterial Biosorbents
Bacteria are the most abundant and versatile of microorganisms and constitute a significant fraction of the entire living terrestrial biomass, whose mass is estimated as ∼1018 g (Mann 1990). In the early 1980s, certain microorganisms were found to accumulate metallic elements at a high capacity (Vijayaraghavan and Yun 2008a, b). Biosorbents derived from bacterial biomass have since become popular because of their small size, ability to grow under controlled conditions, and their resilience to a wide range of environmental situations; furthermore, inexpensive nutrient sources are readily available for microbes. Potent metal biosorbents among bacteria include genera Bacillus, Pseudomonas, Streptomyces, Micrococcus, and Escherichia coli. Table 12.1 summarizes basic information regarding the use of bacterial biomass for metal biosorption.
Metal ions in solution are adsorbed on to bacterial surfaces through interactions with chemical functional groups such as carboxylate, amine, amide, imidazole, phosphate, thioether, hydroxyl, and other functional groups found in cell wall biopolymers. Biosorption includes a combination of several mechanisms such as electrostatic attraction, complexation, ion exchange, covalent binding, van der Waal’s forces, adsorption, and microprecipitation. The extent of biosorption not only depends on the type of metal ions, but also on the bacterial genus, due to variations in cellular constituents. Very short contact times are generally sufficient to attain a metal-bacterial biomass steady state. This is because biomass is used in the form of either fine powder or wet cells, where mass transfer resistances are usually negligible. The rapid kinetics observed with bacterial biomass represents an advantageous aspect for the design of wastewater treatment systems.
12.2.1 Bacterial Structure
The diameter of typical bacterial cells range from 0.5 to 1.0 μm; however, some are wider than 50 μm. Bacteria have simple morphology; the most common bacteria are present in three basic shapes: spherical or ovoid (coccus), rod (bacillus, with a cylindrical shape), and spiral (spirillum), although there is a great variety of shapes due to differences in genetics and ecology. The small size of bacteria ensures rapid metabolic processes. A “typical” bacterial cell (e.g., E. coli) contains a cell wall, cell membrane, and the cytoplasmic matrix which consists of several constituents that are not membrane enclosed (inclusion bodies, ribosomes, and the nucleoid with its genetic material). Bacteria are classified as either Gram-positive or Gram-negative as distinguished by the Gram stain (Beveridge 2001). This classification divides bacteria into two main groups that differ in their cell wall characteristics (Beveridge 1989; Sleytr and Beveridge 1999). Both cell wall types encompass a peptidoglycan layer that is rich in carboxylate groups and completely surrounds the cell (Beveridge 1989; Langley and Beveridge 1999). The peptidoglycan layer in the Gram-positive cell wall is ca. 25 nm thick, whereas the Gram-negative peptidoglycan layer is much thinner (ca. 7.5 nm). The walls of Gram-positive bacteria consist of three primary components: cytoplasm mixed with peptidoglycan, to which teichoic acids are covalently bound. The envelope of Gram-negative bacteria is more complex than that of Gram-positive bacteria. It consists of two membrane bilayers (the outer and plasma membrane) that are chemically and functionally distinct from one another and sandwich a thin peptidoglycan layer between them. Teichoic acids give the Gram-positive cell wall an overall negative charge, due to the presence of phosphodiester bonds between teichoic acid monomers. The highly charged nature of lipopolysaccharides confers an overall negative charge on the Gram-negative cell wall. The anionic functional groups present in the peptidoglycan, teichoic acids, and teichuronic acids of Gram-positive bacteria, and the peptidoglycan, phospholipids, and lipopolysaccharides of Gram-negative bacteria are the components primarily responsible for the anionic character and metal-binding capability of the cell wall (Moat et al. 2002; Prescott et al. 2002). Extracellular polysaccharides (EPSs) are also capable of binding metals; however, their availability depends on the bacterial species and growth conditions, and they can easily be removed by simple mechanical disruption or chemical washing (Yee and Fein 2001).
The cell walls of bacteria contain a large number of surface functional groups, in which carboxyl is generally the most acidic group in the bacteria. At low pH values, cell wall ligands are protonated and compete significantly with metals for binding. With increasing pH, more ligands, such as amino and carboxyl groups, could be exposed, leading to attraction between these negative charges and the metals, and hence increase biosorption onto the cell surface. Some bacteria have special structures, such as flagella and the S-layer. The S-layer is a surface and paracrystalline envelope present in several groups of bacteria and archaea. This layer is formed by protein or glycoprotein monomers that can self-assemble in two-dimensional structures (Sleytr et al. 2003). S-layers are associated with lipopolysaccharides (LPSs) of Gram-negative bacteria or peptidoglycan of a Gram-positive cell (Urrutia 1997; Madigan et al. 2000). Porosity is between 30 and 70% and the diameter of the pore between 2 and 8 nm. This characteristic can be exploited for metal binding. An important characteristic of this protein is its capacity to reassemble once isolated from the cell (Pollmann et al. 2006). Due to this effect, it can be used for bioremediation. S-layer proteins might execute a trapping role of metallic ions in both living and dead cells, being a potential alternative for bioremediation of heavy metals in the field.
Some bacterial cells can produce a capsule outside the bacterial cell wall. They are highly hydrated and loosely arranged polymers of carbohydrates and proteins. Capsules are composed of polysaccharides and a few consist of proteins or polymers of amino acids called polypeptides (often formed from the d- rather than the l-isomer of an amino acid). Bacillus anthracis, the anthrax bacillus, can produce polypeptide capsules composed of d-glutamic acid subunits. Capsules may be thick or thin, rigid or flexible, depending on specific organism. Several different terms can be found to describe the capsule layer, such as slime layer, glycocalyx, and EPS. Capsule polymers are usually acidic in nature although capsules can consist of neutral polysaccharide, charged polysaccharide, or charged polypeptide. Capsule arrangement is important to metal binding (Madigan et al. 2000; Moat et al. 2002). The composition of bacterial EPS is complex, depending on the strain and its culture conditions. EPS synthesis is also reported for several pseudomonads, Zoogloea ramigera, Rhizobium sp., Klebsiella sp., and Bacillus sp. Typical constituents of EPS are mainly polysaccharides and proteins, often accompanied by nucleic acids, lipids, or humic substances (Flemming and Wingender 2001; van Hullebusch et al. 2003). Generally, EPSs have a high molecular weight with an abundance of negatively charged functional groups (ligands), e.g., carboxyl, hydroxyl, and uronic acids (Sobeck and Higgins 2002; Yan et al. 2008). These ligands make it possible for EPS to capture metal ions through electrostatic interactions, forming multiple complexes (Pulsawat et al. 2003). Hence, EPSs have been recommended as a metal absorbent because of their extensive complexing capacity for heavy metals (Gutnick and Bach 2000). Recent studies from Yan et al. (2008) showed that the polymer from Bacillus sp. 19 possessed an affinity for copper.
12.3 Mechanisms of Biosorption
Localizing the metal deposition site within the biosorption biomass and understanding the metal sequestering mechanism, in combination with elucidation of the relevant metal solution chemistry and chemical structure of the metal deposition site, are all crucial aspects of the quest for an efficient biosorption process which should feature high metal selectivity and uptake. The attractive feature of biosorption is a certain specificity of the biosorbent for divalent and multivalent heavy metal cations. Metal uptake may vary widely for different genera and even for different mutant strains within a species. The nutrient status of the organism, its physiological state, the age of cells, and availability of micronutrients during growth, as well as environmental conditions during the biosorption process (e.g., pH, temperature, and presence of other metal ions), are all important parameters affecting the performance of a biosorbent. Solution chemistry of the metal also plays an important role in biosorption.
Biosorption is caused by a number of different physicochemical mechanisms, depending on a number of external environmental factors as well as on the metal, its ionic form in solution, and on the type of a particular active binding site responsible for sequestering the metal. Biosorption consists of several mechanisms, mainly ion exchange, chelating, adsorption, and diffusion through cell walls and membranes, which differ depending on the species used, the origin and processing of the biomass, and solution chemistry.
Research is in progress to establish biosorption as a commercially viable technique to trap and accumulate metals. Biosorption can serve as a tool for the recovery of precious metals (e.g., from processing solutions or seawater) and for the elimination of toxic metals (particularly from industrial wastewaters). The driving force of ion exchange is primarily the attraction of the biosorbent for the sorbate (metal). Metals can be bound electrostatically or by complexation. Interactions between the solute (metal) and the solvent play a role insofar as less hydrophilic molecules have a lower affinity for the liquid phase and are therefore sorbed more easily. Adsorption and microprecipitation involve binding of electrically neutral metals without the release of a stoichiometric amount of previously bound ions. In microprecipitation, the driving force is interaction between the solute and the solvent, whereas in adsorption affinity between sorbent and sorbate is the driving force. Microprecipitation does not necessarily involve a bond between biomass and metal.
In the case of physicochemical interaction based on physical adsorption, ion exchange, and complexation between metal and functional groups of the cell surface, metal uptake does not depend on cellular metabolism. The mechanism by which a metal binds onto the cell surface most likely includes electrostatic interactions, van der Waals forces, covalent bonding, or some combination of these processes. Negatively charged groups such as carboxyl, hydroxyl, and phosphoryl groups of the bacterial cell wall adsorb metal cations by electrostatic forces. Tunali et al. (2006) indicate that the biosorption of lead and copper by Bacillus sp. (ATS-1) involve an ion-exchange mechanism. Since the main mechanism involved in biosorption is ion exchange, protons compete with metal cations for the binding sites and for this reason pH is the operational condition which influences the process most strongly (Schiewer and Volesky 2000). pH determines protonation/deprotonation of metal ion binding sites and thus influences the availability of site to the sorbate. By lowering pH, it is also possible to release metal ions from the binding site. This property is used for the recovery of metal cations and regeneration of biosorbent.
12.4 Techniques Used in Metal Biosorption Studies
Carboxyl groups are negatively charged and abundantly available, actively participate in binding of metal cations. Mishra and Doble (2008) indicated that carboxyl and amino groups were responsible for the binding of chromate. Kang et al. (2007) observed that amine groups protonated at pH 3 and attracted negatively charged chromate ions via electrostatic interaction. Potentiometric titrations can provide information on type and number of binding sites. Kang et al. (2007) titrated Pseudomonas aeruginosa and determined the pKa values of available binding sites. Jian-hua et al. (2007) successfully correlated the quantity of acidic groups present on Bacillus cereus biomass, determined via potentiometric titrations, with the metal uptake capacity.
The nature of the binding sites and their involvement during biosorption can be approximately evaluated using FTIR. Loukidou et al. (2004) analyzed the FTIR spectra of Cd2+ loaded and unloaded Aeromonas caviae. Several band transformations allowed the authors to predict the possible involvement of amino, carbonyl, carboxyl, and phosphate groups in the biosorption of Cd2+. Cayllahua et al. (2009) used FTIR spectra to confirm the presence of amide, carboxyl, and phosphate groups in Rhodococcus sp. biomass. Energy dispersive X-ray (EDX) can provide information regarding the chemical and elemental characteristics of biomass. Tunali et al. (2006) analyzed both Pb2+ and Cu2+ loaded Bacillus sp. using EDX, and confirmed the involvement of an ion-exchange mechanism during biosorption. In order to elucidate the chemical nature of bacterial cell-bound lanthanum, Kazy et al. (2006) employed X-ray diffraction (XRD) analysis and confirmed the involvement of cellular carboxyl and phosphate groups in the binding of lanthanum by Pseudomonas biomass. SEM micrographs have aided researchers in analyzing cell surface morphology before and after biosorption. Tunali et al. (2006) visualized the surface of metal-loaded Bacillus sp.
12.5 Factors Affecting Heavy Metal Biosorption
12.5.1 pH
Since the main mechanism involved in biosorption is ion exchange, protons compete with metal cations for the binding sites and for this reason pH is the operational condition which influences the process most strongly (Schiewer and Volesky 2000). The different chemical species of a metal occurring at different pH values will have variable charges and adsorbability at solid–liquid interfaces. In many instances, biosorption experiments conducted at alkaline pH values have been reported to complicate the evaluation of biosorbent potential as a result of metal precipitation (Selatnia et al. 2004c; Iqbal et al. 2007). pH determines the speciation and solubility of toxic metal ions and also affects the properties of the biomass (Chen et al. 2008). Many metals occur as free hydrated species at lower pH, hydroxides are formed with increasing pH and eventually precipitation may occur. pH influences the magnitude of negative charge on the surface of the material by either protonation or deprotonation of metal-binding sites. The different pH sorption profiles for various heavy metal ions may be related to the nature of chemical interactions of each metal with biomass (Kiran et al. 2005; Bueno et al. 2008). For different biosorption systems of metal ions, the optimal pH will differ. Both cations and anions show different patterns of sorption on sorbent in the same pH range. Ma and Tobin (2004) reviewed that uptake of anions is favored at low pH with typical maximum biosorption in the range of 1–2 while cation biosorption is maximal at a higher pH range. Solution pH primarily affects the surface properties of the biomass (Antizar-Ladislao and Galil 2004). It is worth noting that the capability of microorganism biomass to adsorb or chelate metal ions is due to the presence of several chemical groups on the biomass surface which are polar or anionic in nature such as carboxyl, phosphate, amine, amino, hydroxyl, and sulfhydryl. Such groups will contribute to the electrokinetic potential (zeta potential) of the surface (Zouboulis et al. 1999). Different isoelectric points (i.e., pH value when net surface charge is zero) are exhibited by different microorganisms due to the differing chemical compositions of the cell wall. At pH lower than the isoelectric point, the overall charge of the biomass surface will become positive, whereas at pH higher than the isoelectric point, the overall surface charge will become negative (Zouboulis et al. 2004). In general, increasing pH increases the negative charge on the cell surface until all relevant functional groups are deprotonated, which favors electrochemical attraction and adsorption of cations. Furthermore, the increase in metal uptake with an increase in pH may be the result of more efficient competition of cations with H+ for binding sites on bacteria (Ziagova et al. 2007; Green-Ruiz et al. 2008; Zamil et al. 2009). Anions would be expected to interact more strongly with cells with an increasing concentration of positive charges, due to the protonation of functional groups at lower pH values. Many papers discuss the effect of this factor on biosorption performance (Uslu and Tanyol 2006; Bueno et al. 2008; Gabr et al. 2008) by, e.g., determination of zeta potential, electrostatic attraction, and contribution of ion-exchange mechanisms (Xu et al. 2006).
Metal ions in solution undergo hydrolysis as the pH increases. The extent of hydrolysis at different pH values differs with each metal, but the usual sequence of hydrolysis involves the formation of hydroxylated monomeric species followed by the formation of polymeric species, and subsequently the formation of crystalline oxide precipitates after aging (Ziagova et al. 2007; Hasan and Srivastava 2009). The different chemical species of a metal that occur with pH changes vary in charge and adsorbability at solid–liquid interfaces. Therefore, adsorption of metals on interfaces is highly pH-dependent, and there is a critical pH range, usually of less than one pH unit, for each metal wherein the amount of metal adsorbed increases significantly.
12.5.2 Temperature
Biosorption by nonliving biomass is not significantly affected by the temperature. In contrast, the metabolism of living cells is temperature dependent, and hence change in this parameter will strongly affect the biosorption processes. Adsorption and ion exchange are exothermic in nature and hence the rate of these processes will increase with an increase in the temperature. However, at high temperatures, cell walls may be permanently damaged and for this reason a reduction in metal uptake is observed. Most of the increase in uptake has been reported in the temperature range of 4–23°C, whereas only a marginal increase is observed between 23 and 40°C. Metal uptake is reduced significantly when temperature is increased beyond this value. It is always desirable to conduct/evaluate biosorption at room temperature, as this condition is easy to replicate.
12.5.3 Initial Metal Ion Concentration
Initial solute concentration appears to have an impact on biosorption, with a higher concentration resulting in a high solute uptake (Öztürk 2007; Bueno et al. 2008; Uzel and Ozdemir 2009). This occurs because at lower initial solute concentrations, the ratio of the initial moles of solute to the available surface area is low; subsequently, the fractional sorption becomes independent of the initial concentration. However, at higher concentrations, the sites available for sorption become fewer compared with the moles of solute present and, hence, the removal of solute is strongly dependent upon initial solute concentration.
12.5.4 Initial Concentration of Biosorbent
The dosage of a biosorbent strongly influences the extent of biosorption. An increase in biomass concentration generally increases the amount of solute biosorbed, due to the increased surface area of the biosorbent, which in turn increases the number of binding sites (Ziagova et al. 2007; Bueno et al. 2008). Conversely, the quantity of biosorbed solute per unit weight of biosorbent decreases with an increasing biosorbent dosage, which may be due to the complex interaction of several factors. An important factor at high sorbent dosages is that the available solute is insufficient to completely cover the available exchangeable sites on the biosorbent, usually resulting in low solute uptake. The interference between binding sites due to increased biosorbent dosages cannot be overruled, as this will result in low specific uptake.
12.5.5 Presence of Competing Ions
Wastewaters usually contain multiple metals. The presence of more than one metal in wastewater is expected to cause interactive effects as a function of many factors, such as the number of metals competing for binding sites, metal concentration, and biosorbent dose. Many biosorption studies have been conducted with single-metal ion species in aqueous solutions. Metal uptake is significantly affected by the presence of other co-ions, as they will also compete for binding sites because many of the functional groups present on the cell wall and membrane are nonspecific. Therefore, metal uptake from mixed solutions is often found to be lower than those in a single-species system. Generally, metal uptake increases as the ionic radius of metal cation increases, with metals having higher ionic charge showing greater binding to biomass. Furthermore, the extent of reduction in metal uptake in the presence of other cations is found to be dependent on concentrations of the other cations. In particular, as the concentration of other cations increases, uptake of the metal further decreases. Bueno et al. (2008) reported that the presence of co-ions, whether in binary or ternary combinations, decreased the metal uptake when compared with the single-metal system. They observed that the presence of copper ions resulted in inhibition of lead uptake, which was greater than inhibition measured in the presence of chromium and copper ions together. In the presence of other metal ions in solution, chemical interactions between these species as well as with biomass may take place, resulting in competition for adsorption sites on the surface. As a consequence, the first component has a smaller “parking space” and its uptake is decreased (Akar et al. 2005).
Among the factors that affect biosorption preferences of a sorbent, binding of metal ions on biomaterials largely depends on physicochemical properties of the metallic species. It has been reported that the metal removal increases with the increase in ionic radius (Sag et al. 2002), which follows the order Pb(II) > Cu(II) > Cr(III). The differences in sorption affinities may also be attributed to differences in the electronegativity of the atoms, which also follows the order Pb(II) > Cu(II) > Cr(III). The greater the electronegativity or ionic radius, the greater the affinity, which also explains the significant suppression of lead uptake in the presence of copper and the moderate effect of chromium on lead biosorption. Uslu and Tanyol (2006) observed that the competitive biosorption capacities of Pseudomonas putida for Pb and Cu ions were lower than that under noncompetitive conditions.
Low atomic weight metal ions, such as Ca2+, Na+, and K+, occur in industrial wastewater. The experimental data has shown that these metal ions have little effect on heavy metal biosorption, indicating low biomass affinity for the lighter ions. The presence of anions also affects biosorption of metal ions. Kapoor and Viraraghavan (1997) reported that biosorption capacity decreased in the presence of ethylenediamine tetraacetate (EDTA), sulfate, chloride, phosphate, carbonate, glutamate, citrate, and pyrophosphate. The anions in solution may form a complex with the metal ions, which would significantly reduce metal biosorption capacity. In general, biosorption is reduced at increased ligand concentrations.
12.6 Development of Bacterial Biosorbents
Feasible approaches leading to improvement of heavy-metal biosorption efficiency include the development of more powerful biosorbents and the design of more efficient biosorption processes. Biosorbent development could be achieved by either isolating organisms with high capacity or high specificity to heavy metals or by tailoring genetically modified organisms abundant in high-affinity metal-binding proteins or polypeptides (Bae et al. 2002; Pazirandeh et al. 1998; Huang et al. 2003). Bae et al. (2003) reported that the metalloregulatory protein, MerR, which exhibits high affinity and selectivity toward mercury, was exploited for the construction of microbial biosorbents specific for mercury removal. Expression of mer operon genes encoding for cysteine-containing mercuric ion transport proteins (such as periplasmic protein MerP or inner membrane protein MerT) (Huang et al. 2003, Zhao et al. 2005) on E. coli is very effective biosorbents for heavy metal removal. In addition, several other metal-binding proteins, such as metallothioneins (MTs) (Kao et al. 2006), phytochelatins (PCs) (Grill 1987), and metal-binding peptides (Huang et al. 2003) were also expressed on E. coli to create powerful biosorbents. The MerP protein is a target for the development of genetically engineered biosorbents (Chen et al. 1998). Kao et al. (2008) used recombinant E. coli biosorbents with overexpression of MerP proteins for the biosorption of copper, nickel, and zinc from aqueous solutions. Deng et al. (2008) demonstrated biosorption by immobilized recombinant cells expressing human metallothionein proteins. Samuelson et al. (2000) generated recombinant Staphylococcus xylosus and Staphylococcus carnosus strains with surface-exposed chimeric proteins containing polyhistidyl peptides. Both strains of staphylococci gained improved nickel-binding capacities due to the introduction of H1 or H2 peptide into their surface proteins.
As the biosorption process is involved in mainly cell surface sequestration, modification of the cell wall can greatly alter the binding of metal ions. A number of methods have been employed for cell wall modification of microbial cells in order to enhance the metal-binding capacity of biomass and to elucidate the mechanism of biosorption. Physical treatments include heating/boiling, freezing/thawing, drying, and lyophilization. The various chemical treatments used for biomass modification include washing biomass with detergents, cross-linking with organic solvents, and alkali or acid treatment. Pretreatments could modify the surface characteristics/groups either by removing or masking the groups or by exposing more metal-binding sites (Vijayaraghavan and Yun 2008a, b). For example, grafting of long polymer chains onto the biomass surface through direct grafting or polymerization of a monomer could introduce functional groups onto the surface of biomass.
12.7 Biosorption and Equilibrium Studies of Heavy Metals
The type of process governs the rate of biosorption, which is considered as a rapid physical/chemical process. Biosorption can also be defined as a collective term for a number of passive accumulation processes, which may include ion exchange, coordination, complexation, chelation, adsorption, and microprecipitation. In equilibrium, a certain relationship prevails between solute concentration in solution and adsorbed state (i.e., the amount of solute adsorbed per unit mass of adsorbent). The equilibrium concentrations are a function of temperature; therefore, the adsorption equilibrium relationship at a given temperature is referred to as an adsorption isotherm. Several adsorption isotherms originally used for gas-phase adsorption are available and have been adopted to correlate adsorption equilibria in heavy metals biosorption. Some of the common equilibria are Freundlich, Langmuir, Redlich–Paterson, and the Sips equation. Freundlich and Langmuir equations are the most widely used. These isotherms for the removal of heavy metals from water and wastewater by biosorbents are discussed below.
12.7.1 Freundlich Isotherm
The Freundlich isotherm is an empirical equation and the most widely used isotherm for the description of adsorption equilibrium. It describes the adsorption of organic and inorganic compounds on a wide variety of adsorbents including biosorbents. The equation is written as:
where q e is the amount adsorbed, K F the characteristic constant related to the adsorption capacity, C e the equilibrium concentration, and n the characteristic constant related to adsorption intensity or degree of favorability of adsorption.
Equation (12.1) can also be expressed in the linearized logarithmic form:
The plot of log q e versus log C e has a slope with the value of 1/n and an intercept magnitude of log K F. Log K F is equivalent to log q e when C e equals unity. However, in other cases when 1/n = 1, the value of K F depends on the units upon which q e and C e are expressed. A Freundlich constant n between 1 and 10 indicates favorable adsorption. A larger value of n (smaller value of 1/n) implies stronger interaction between biosorbent and heavy metal while 1/n equal to 1 indicates linear adsorption leading to identical adsorption energies for all sites (Site 2001). The Freundlich isotherm has the ability to fit nearly all experimental adsorption–desorption data, and is excellent for fitting data from highly heterogeneous sorbent systems.
A 1/n value higher than unity (n < 1) suggests the presence of a curved upward isotherm, sometimes termed as a solvent-affinity type isotherm (Site 2001). Within this type of isotherm, the marginal sorption energy increases with increasing surface concentration. Sorption of solute on any sorbent can occur either by physical bonding, ion exchange, complexation, chelation or through a combination of these interactions. In the first case of physical bonding, as the solute is loosely bound, it can easily be desorbed using distilled water. Given the fact that miscellaneous functional groups such as hydroxyl, carbonyl, carboxyl, sulfhydryl, thioether, sulfonate, amine, imine, amide, imidazole, phosphonate, and phosphodiester groups can be present within the structure of the biosorbent, the mechanism of adsorption will not be restricted to physical bonding (Dursun 2006; Hanif et al. 2007; Wang et al. 2006a; Agarwal et al. 2006; Al-Rub 2006; Amarasinghe and Williams 2007; Basha et al. 2008; Liu et al. 2007; Parvathi et al. 2007; Popuri et al. 2007; Baral et al. 2007; Gokhale et al. 2008; Vijaya et al. 2008; Calfa and Torem 2008; Schiewer and Patil 2008; Kumar et al. 2008). Different mechanisms can be involved as the interaction between sorbent and solute molecules is expected to be strong.
Adsorption capacity is the most important characteristic of an adsorbent. It is defined as the amount of adsorbate taken up by the adsorbent per unit mass of adsorbent. This variable is governed by a series of properties, such as pore and particle size distribution, specific surface area, cation exchange capacity, pH, surface functional groups, and temperature.
As a precautionary note, the Freundlich equation is unable to predict the adsorption equilibria data at extreme concentrations. Furthermore, this equation is not reduced to linear adsorption expression at very low concentrations. However, researchers rarely face this problem, as moderate concentrations are frequently used in most biosorption studies.
12.7.2 Langmuir Isotherm
Another popular model for describing heavy metal sorption to biosorbents is the Langmuir model. The Langmuir equation relates the coverage of molecules on a solid surface to concentration of a medium above the solid surface at a fixed temperature. This isotherm is based on three assumptions, namely, adsorption is limited to monolayer coverage, all surface sites are alike and can only accommodate one adsorbed atom, and the ability of a molecule to be adsorbed on a given site is independent of its neighboring site’s occupancy. By applying these assumptions, and a kinetic principle (rate of adsorption and desorption from the surface is equal), the Langmuir equation can be written in the following form:
where q e is the amount adsorbed, C e the equilibrium concentration, q max the saturated monolayer adsorption capacity, and K L the sorption equilibrium constant.
This equation is often written in different linear forms (Ho 2006, Ho and Ofomaja 2006):
In biosorption process, the saturation limit of certain biomass is affected by several factors such as the number of sites in the biosorbent material, accessibility of the sites, chemical state of the sites (i.e., availability), and affinity between site and metal (i.e., binding strength). In the case of covalent metal binding, supposing that an occupied site is theoretically available, the extent to which the site is to be occupied by a given metal depends further on its binding strength and concentration when compared with the metals already occupying the site.
The decrease of K L value with an increase in temperature signifies the exothermicity of the adsorption process (physical adsorption) (Ho 2006; Padmavathy 2008; Djeribi and Hamdaoui 2008; Shaker 2007), while the opposite trend indicates that the process needs thermal energy (endothermic), leading to chemisorption (Dursun 2006; Ho 2006; Malkoc and Nuhoglu 2005; Wang et al. 2006b; Deng et al. 2006; Dundar et al. 2008; Aydin et al. 2008; Gupta and Rastogi 2008; Green-Ruiz et al. 2008; Vilar et al. 2008). During physical adsorption, the bonding between heavy metals and active sites of the biosorbent weakens at higher temperatures in contrast to chemisorption bonding, which becomes stronger. The exothermicity or endothermicity of the biosorption process can be determined via the heat of adsorption. This thermodynamic property is commonly obtained through an integrated Van’t Hoff equation, which relates the Langmuir constant, K L, to the temperature:
where K o is the adsorption equilibrium constant, E a the activation energy of adsorption/heat of adsorption, R the gas constant (0.0083 kJ/(mol K)), and T the absolute temperature (K).
12.7.3 Temkin Isotherm
The derivation of Temkin isotherm is based on the assumption that the decline of heat of sorption as a function of temperature is linear rather than logarithmic, as implied in the Freundlich equation (Basha et al. 2008; Isik 2008). The Temkin isotherm has the form:
where b is the Temkin constant in relation to heat of sorption (kJ/mol) and a the Temkin isotherm constant (L/g).
Several experimental studies in chemisorption systems are correlated using this equation (Mondal et al. 2008; Isik 2008; Kiran and Kaushik 2008). Mondal et al. (2008) studied the biosorption of As, Fe, Mn, Cu, and Zn on Ralstonia eutropha. For several systems such as biosorption of Ni(II) by ureolytic mixed culture (Isik 2008) and biosorption of Cr(VI) by Lyngbya putealis exopolysaccharides (Kiran and Kaushik 2008), Temkin isotherms are incapable of predicting biosorption equilibria. Since the basis of derivation for the Temkin equation involves simple assumptions, the complex phenomenon involved in liquid-phase adsorption is not taken into account by this equation. As a result, this equation is often not suitable for the representation of experimental data in complex systems.
12.7.4 Dubinin–Radushkevich Equation
The Dubinin–Radushkevich (DR) equation is excellent for interpreting sorption equilibria for organic compounds (in gas-phase condition) in porous solids. The DR equation is rarely applied onto liquid-phase adsorption due to the complexities associated with other factors such as pH and ionic equilibria inherent in these systems. In addition, the solute–solvent interactions often render the bulk solution nonideal. The mathematical expression for the DR equation in the liquid-phase system is given below:
where β is a constant (proportional to the liquid molar volume) and E 0 the solid characteristic energy toward a reference compound.
By taking into account the energetically nonuniform surface, this equation is capable of describing biosorption data as well (Igwe and Abia 2007; Cabuk et al. 2007; Vijayaraghavan et al. 2006; Apiratikul and Pavasant 2008; Kiran and Kaushik 2008). One of the best features of the DR equation lies in the fact that it is temperature dependent. If the adsorption data at different temperatures are plotted as the logarithm of the amount adsorbed against the square of potential energy, all suitable data shall, in general, lie on the same curve, termed the characteristic curve. This curve can later be utilized as an initial “tool” to measure the applicability of the DR equation in expressing adsorption equilibria data.
12.7.5 Brunauer–Emmer–Teller (BET) Model
In the Langmuir model, it was assumed that adsorption only occurs on the unoccupied adsorption sites. In the BET model, this restriction is removed. Supposing that the initial adsorbed layer can act as a substrate for further adsorption, then the isotherm, instead of leveling off to some saturated value at high concentrations, is able to rise indefinitely. The same kinetics concept proposed by Langmuir is applied to this multiple layering process, i.e., the rate of adsorption on any layer is equal to the rate of desorption from that layer. The simplified form of BET equation can be written in the following form:
where B is a constant related to the energy of adsorption and \( {C}_{\text{s}}^{*}\)the saturation concentration of solute (mg/L).
Kiran and Kaushik (2008) showed a superb applicability of this model for Cr(VI) biosorption using L. putealis exopolysaccharides. They claimed that multilayer adsorption occurred in this system. As a note, other ideal assumptions within this model, namely, all sites are energetically identical along with no horizontal interaction between adsorbed molecules, may be correct for heterogeneous material and simple nonpolar gases but not for complex systems involving heterogeneous adsorbent such as biosorbents and metals. For that reason, this equation is unpopular in the interpretation of liquid-phase adsorption data for complex solids.
12.7.6 Redlich–Paterson Isotherm
Redlich–Paterson is another empirical equation, designated as the “three parameter equation,” which is capable of representing adsorption equilibria over a wide concentration range. This equation has the following form:
where a RP, K RP, and β are Redlich–Paterson’s parameters.
Equation (12.12) reduces to a linear isotherm at low surface coverage and to the Langmuir isotherm when β is equal to 1. This equation is quite popular for the prediction of heavy metal biosorption equilibria data (Dursun 2006; Basha et al. 2008; Ho and Ofomaja 2006; Ho 2006; Preetha and Viruthagiri 2007; Padmavathy 2008; Vijayaraghavan et al. 2006). Redlich and Paterson incorporated the characteristics of Langmuir and Freundlich isotherms into a single equation. Two limiting behaviors exist, i.e., the Langmuir form for β = 1 and Henry’s law form for β = 0.
12.7.7 Multicomponent Heavy Metals Biosorption
The majority of the studies on biosorption of heavy metal ions by various biosorbents have focused on single-metal uptake. However, contrary to this, various metals are present in wastewater. The equilibrium modeling of multimetal biosorption, which is essential in the design of treatment systems, is often neglected. The effects of binary metal ions in various combinations seem to be more representative than single-metal studies (Aksu et al. 2002).
One of the major concerns arising from the adsorption of heavy metals from wastewater is the simultaneous presence of miscellaneous metals in wastewater. The interference and competition among different metals, metals and solvents, as well as metals and adsorption sites are significantly enough to be taken into account, leading to a more complex mathematical formulation of the equilibrium. Given the adsorption of heavy metals in real systems involving more than one component, adsorption equilibria involving competition between molecules of different types are warranted for better understanding of the system and design purposes. Only a few isotherms were developed to describe equilibrium in such systems. These models range from simple equations associated only with the individual isotherm parameters (nonmodified adsorption models) to more complex models exploiting the individual isotherm parameters along with correction factors (modified adsorption models) (Aksu et al. 2002).
Multicomponent adsorption models such as the multicomponent Langmuir model including its modification as well as the multicomponent Freundlich model have become popular. The multicomponent Langmuir model is expressed in the following form:
12.8 Kinetics of Heavy Metal Biosorption
Adsorption equilibria studies are important for determining the efficacy of metal adsorption. In addition, it is necessary to identify the adsorption mechanism type in a given system. For the purpose of investigating the mechanism of biosorption and its potential rate-controlling steps that include mass transfer and chemical reaction processes, kinetic models have been used to test the experimental data. In addition, information on the kinetics of metal uptake is required to select the optimum conditions for full-scale batch metal removal processes.
Adsorption kinetics is expressed as the solute removal rate that controls the residence time of the sorbate in the solid–solution interface. Several adsorption kinetic models have been described for the adsorption kinetics and rate-limiting step. These include pseudo-first and -second-order rate models, the Weber and Morris sorption kinetic model, the Adam–Bohart–Thomas relation (Djeribi and Hamdaoui 2008), the first-order reversible reaction model (Baral et al. 2006), the external mass transfer model (Apiratikul and Pavasant 2008), the first-order equation of Bhattacharya and Venkobachar (Sag and Aktay 2002), and Elovich’s model and Ritchie’s equation. The pseudo-first and -second-order kinetic models are the most widely used models for biosorption kinetics of heavy metals and quantify the extent of uptake in biosorption kinetics.
12.8.1 Pseudo-First-Order Kinetics
The Lagergren first-order rate expression based on solid capacity is generally expressed as follows:
where q is the amount adsorbed at time t and k 1 the rate constant of first-order adsorption.
Integration of (12.14) with the boundary conditions as t = 0, q = 0, and at t = t, q = q, gives:
Equation (12.15) can be written in the nonlinear form:
Hypothetically, to ascertain the rate constants and equilibrium metal uptake, the straight-line plots of log(q e − q) against t of (12.15) were made at different initial metal concentrations (Ho and McKay 2002). The q e value acquired by this method is then compared with the experimental value. If large discrepancies are posed, the reaction cannot be classified as first-order although this plot has a high correlation coefficient from the fitting process. Nonlinear fitting of (12.16) is another way to achieve the predicted value of q e and k 1, although this is not a common exercise. The trend shows that the predicted q e values seem to be lower than the experimental values. A time lag, probably caused by the presence of a boundary layer or external resistance controlling the beginning of the sorption process, was argued to be the responsible factor behind the discrepancy (Vijayaraghavan et al. 2006).
12.8.2 Pseudo-Second-Order Kinetics
The pseudo-second-order model is derived on the basis of the sorption capacity of the solid phase, expressed as:
where k 2 is the rate constant for pseudo-second-order model. Integration of (12.17) with the boundary conditions t = 0, q = 0, and at t = t, q = q, results in:
Equation (12.18) can be stated in the linear form as:
The pseudo-second-order rate constants were determined experimentally by plotting t/q against t. Ho (2006) conducted an evaluation using linear and nonlinear methods to determine the pseudo-second-order kinetic parameters. He chose cadmium as the heavy metal and tree fern as the biosorbent. As-acquired kinetic parameters from four kinetic linear equations using the linear method have discrepancies among themselves. Further, for the linear method, the pseudo-second-order model as written in (12.19) has the highest coefficient of determination. In contrast to the linear model, the resulting kinetic parameters from the nonlinear model were almost identical among each other. To that end, the nonlinear method is considered as a better way to ascertain the desired parameters. Still, most of the biosorption studies in the literatures utilize (12.19).
As such, in comparison to pseudo-first-order kinetics, this model is considered more appropriate to represent the kinetic data in biosorption systems. This tendency comes as an indication that the rate-limiting step in biosorption of heavy metals are chemisorption involving valence forces through the sharing or exchange of electrons between sorbent and sorbate (Javed et al. 2007; Ofomaja and Ho 2007; Nasreen et al. 2008; Namasivayam and Sureshkumar 2008; Dundar et al. 2008; Yu et al. 2007; Rahaman et al. 2008; Mack et al. 2008; Pamukoglu and Kargi 2007; Miretzky et al. 2008; Guo et al. 2008), complexation, coordination, and/or chelation (Yu et al. 2007; Baral et al. 2007; Lu and Gibb 2008).
12.8.3 The Weber and Morris Sorption Kinetic Model
The Weber and Morris (WM) sorption kinetic model was initially employed by Pasavant et al. (2006) to describe their biosorption experimental data. This model has the following form:
where K WM is the Weber and Morris intraparticle diffusion rate.
In their investigation, the sorption process by biomass for Cu(II), Cd(II), Pb(II), and Zn(II) was regulated by two main mechanisms, i.e., intraparticle diffusion and external mass transfer. The intraparticle diffusion can be estimated with the following equation:
where d p is the mean particle diameter.
The external mass transfer process was determined by:
where \(K{{^\prime}}_{\text{L}}\) is the liquid–solid mass transfer coefficient, A the specific surface area of biomass, C the liquid-phase concentration of sorbate in the bulk solution at t, and \( {C}_{\text{s}}^{i}\) the concentration of sorbate in the inner pore of sorbent.
They observed that the external mass transfer coefficients can be ordered from high to low values as Cu(II) > Pb(II) > Zn(II) > Cd(II) while the intraparticle diffusion coefficients (also in the decline sequence) as Cd(II) > Zn(II) > Cu(II) > Pb(II).
12.8.4 First-Order Reversible Reaction Model
To derive this model, the sorption of metal on biosorbent is assumed to be a first-order reversible reaction, as expressed by the following reaction mechanism (Baral et al. 2006):
In turn, the rate equation for the reaction is expressed as:
where C B is the concentration of metal in sorbent at time t, C A the concentration of metal in solution at time t, \( {k}_{1}^{0}\text{and}{k}_{2}^{0}\)the first-order rate constants, C A0 the initial concentration of adsorbate, C B0 the initial concentration of adsorbent, C Be the equilibrium concentration of metal in adsorbent, and C Ae the equilibrium concentration of metal in adsorbate.
At equilibrium conditions:
Integrating (12.24) and applying the equilibrium condition gives:
Baral et al. (2006) tried several equations to represent the Cr6+ biosorption experimental data, and one among these equations was first-order reversible reaction model. This equation fits well for their experimental data. The reduced rate constants and increasing equilibrium constant with the rise in temperature signifies that the biosorption of Cr6+ onto treated sawdust has exothermic nature. These observations, however, suggesting a complication as a careful examination onto the rate constant parameters revealed an existing violation toward Le Chatelier’s principle. Since the adsorption process is exothermic as a rule, the rate constant value of \( {k}_{1}^{0}\) should decrease with increasing temperature. Based on the Le Chatelier’s principle, if the adsorption is exothermic, desorption would be endothermic. Therefore, the rate constant value of \( {k}_{2}^{0}\) should be enhanced in parallel with the rise in temperature. As mentioned previously, sorption of heavy metals on any biosorbent takes place by either physical bonding, ion exchange, complexation, coordination/chelation or a combination of these. By being restricted to a reversible chemical reaction assumption, this model fails to capture any other possible complex mechanism involved.
12.9 Immobilization of Bacteria
In addition to the high biosorption yield obtained by bacteria, the heavy metal bioremediation process requires microorganisms to be attached to a solid surface. Surface fixation and cell entrapment are the two methods of immobilization. Different matrices were tested for cell immobilization (Beolchini et al., 2003; Xiangliang et al., 2005). Support matrices suitable for biomass immobilization include alginate, polyacrylamide, polyvinyl alcohol, polysulfone, silica gel, cellulose, and glutaraldehyde (Wang 2002; Vijayaraghavan and Yun 2008a, b). The polymeric matrix determines the mechanical strength and chemical resistance of the final biosorbent particle to be utilized for successive sorption–desorption cycles, so it is important to choose the correct immobilization matrix. Akar et al. (2009) measured the biosorption of 100 mg L−1 of nickel at pH 6.5 to be 33.83 and 7.50 mg g−1 for silica gel and Proteus vulgaris, respectively, whereas the immobilized biosorbent had a biosorption capacity of 45.48 mg g−1 under the same conditions. Maximum biosorption obtained using immobilized biomass provides promise for immobilized cells in a column reactor for the remediation of heavy metals. At pH 5.0, the Cd2+ biosorption capacity of E. coli biomass-free PVA beads was 1.30 mg g−1, which was significantly lower than the adsorption capacity of PVA-immobilized cells, displaying a capacity of 2.18 and 4.41 mg/g for biomass loading of 8.42 and 19.5 wt%, respectively (Kao et al. 2009).
Although cell entrapment imparts mechanical strength and resistance to chemical and microbial degradation upon the biosorbent, the costs of immobilizing agent cannot be ignored. Free cells are not suitable for use in a column, due to their low density and size they tend to plug the bed, resulting in marked declines in pressure. For industrial applications of biosorption, it is important to utilize an appropriate immobilization technique to prepare commercial biosorbents which retain the ability of microbial biomass to adsorb metal(s) during the continuous treatment process. The immobilization of biomass in solid structures would create a biosorbent material with the right size, mechanical strength, rigidity, and porosity necessary for use in practical processes. The immobilized materials can be used in a manner similar to ion-exchange resins and activated carbon such as adsorption–desorption cycles (i.e., recovery of the adsorbed metal, reactivated and reuse of the biomass) (Veglio and Beolchini 1997).
In different matrices, tested surface fixation was chosen as the immobilization methodology instead of cell entrapment. Cell immobilization has successfully been achieved mostly in calcium alginate beads, but this matrix also has a high affinity for heavy metals. Metal retention kinetics studies with calcium alginate confirmed that almost 100% of the metal assayed was retained by the beads (Vullo et al. 2003) and that it is pointless to try to improve heavy metal retention by bacterial cell entrapment in calcium alginate beads (Arica et al. 2001; Davis et al. 2003; Vullo et al. 2003; Arica et al. 2004). Although calcium alginate is useful for entrapping cells in its gel structure, its advantage resides mostly in the re-utilization of the entrapped cells. However, the high heavy metal affinity of alginate makes it unusable for the development of continuous industrial processes, as the recovery of the alginic acid would increase the final costs of effluent treatment. Successful bacterial immobilization was achieved on inert surfaces such as Teflon membranes, silicone rubber, and polyurethane foams. Best results of surface fixation were obtained with Pseudomonas veronii 2E, which was able to grow on all three surfaces. This organism developed a film over the matrix surfaces, and also formed aggregates and adhered to glass during batch culture work. The development of other bacteria on the same surfaces was barely observed.
12.10 Desorption of Heavy Metals
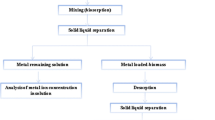
Biosorption is a process of treating pollutant-bearing solutions to render it contaminant-free. However, it is also necessary to be able to regenerate the biosorbent. This is possible only with the aid of appropriate elutants which usually results in a concentrated pollutant solution. Therefore, the overall achievement of a biosorption process is to concentrate the solute, i.e., sorption followed by desorption. Desorption is of utmost importance when biomass preparation/generation is costly, as it is possible to decrease process cost and the dependency of the process on a continuous supply of biosorbent. A successful desorption process requires the proper selection of elutants which strongly depends on the type of biosorbent and the mechanism of biosorption. In addition, the elutant must be (1) nondamaging to the biomass, (2) less costly, (3) environmental friendly, and (4) effective. Several investigators have conducted exhaustive screening experiments to identify appropriate elutants for this process. Kuyucak and Volesky (1989) examined several chemical agents to desorb Co2+ from cobalt-laden Ascophyllum nodosum, and identified CaCl2 in the presence of HCl as a suitable elutant.
The performance of an elutant also strongly depends on the type of mechanism responsible for biosorption. For instance, electrostatic attraction was found to be the primary mechanism responsible for biosorption of negatively charged dye anions to a positively charged cell surface (O’Mahony et al. 2002). Therefore, it would be logical to make the cell surface negative using alkaline solutions to repel the negatively charged reactive dyes (Won and Yun 2008). Elution is also influenced by the volume of elutant, which should be as low as practically possible to obtain the maximum solute concentration in the smallest possible volume (Volesky 2001). At the same time, the volume of the solution should be sufficient to provide maximum solubility for the desorbed solute. Also, one has to realize that the desorbed sorbate stays in solution and a new equilibrium is established between that and the one (remaining) still fixed on the biosorbent. This leads to the concept of a “desorption isotherm” where the equilibrium is strongly shifted toward the sorbate dissolved in the solution (Yang and Volesky 1996). Thus, it is necessary to evaluate the suitable elutant volume, which can be performed using experiments with different solid-to-liquid ratios. The solid-to-liquid ratio is defined as the mass of solute-laden biosorbent to the volume of elutant. Davis et al. (2000) observed that solid-to-liquid ratio affected copper elution efficiency of CaCl2 solutions, while it was nearly independent in the case of 0.1 M HCl. The purpose of desorption is to unbind a contaminant from a biosorbent, so both the recovered solute and biosorbent can be reused. After desorption, the biosorbent should be close to its original form, both morphologically and effectually. Also, during the desorption process, removal of all bound sorbate from biosorbent should be assured. If this does not occur, a diminished uptake should be expected in the next cycle. Puranik and Paknikar (1999) regenerated and reused a polysulfone-immobilized Citrobacter strain over three cycles for the biosorption of lead, cadmium, and zinc, using 0.1 M HCl and 0.1 M EDTA as elutants, but only with limited success, and emphasized the need for further screening work. Beolchini et al. (2003) immobilized Sphaerotilus natans into a polysulfone matrix for the biosorption of copper, and with the aid of 0.05 M CaCl2 regenerated and reused the beads over ten cycles with satisfactory results.
12.11 Biosorption and Its Column Performance
Continuous biosorption studies are of utmost importance to evaluate the technical feasibility of a process for real applications. Among the different column configurations, packed bed columns have been established as an effective, economical, and most convenient setup for biosorption processes (Zhao et al. 1999; Saeed and Iqbal 2003; Volesky et al. 2003; Chu 2004). These authors make best use of the concentration difference, which is known to be the driving force for sorption, and allow more efficient utilization of the sorbent capacity, resulting in better effluent quality (Aksu and Gönen 2004). Also, packed bed sorption has a number of process engineering merits, including a high operational yield and the relative ease of scaling-up procedures (Aksu 2005). Other column contactors, such as fluidized and continuous stirred tank reactors, are rarely used for biosorption (Prakasham et al. 1999; Solisio et al. 2000). Continuous stirred tank reactors are useful when the biosorbent is in the form of a powder (Cossich et al. 2004); however, they suffer from high capital and operating costs (Volesky 1987). Fluidized bed systems, which operate continuously, require high flow rates to keep the biosorbent particles in suspension (Muraleedharan et al. 1991). Various parameters can be used to characterize the performance of packed bed biosorption, including the length of the sorption zone, uptake, removal efficiency, and slope of the breakthrough curve (Volesky et al. 2003; Vijayaraghavan et al. 2004). A mass transfer zone will develop between the gradually saturated section of the column and the fresh biosorbent section (Naja and Volesky 2006). The length of this zone is important practically, which can be calculated from:
where Z denotes bed depth (cm), and t b and t e the column breakthrough and exhaustion times (h), respectively. The uptake is an important parameter often used to characterize the performance of a biosorbent in a packed column. The column uptake (Q col) can be calculated by dividing the total mass of biosorbed sorbate (m ad) by that of the biosorbent (M). The mass of biosorbed sorbate is calculated from the area above the breakthrough curve (C vs. t) multiplied by the flow rate. The removal efficiency (%) can be calculated, from the ratio of the sorbate mass biosorbed to the total mass of sorbate sent to the column, as follows:
where C 0 and F are the inlet solute concentration (mg/L) and flow rate (L/h), respectively. It is important to note that the removal efficiency is independent of the biosorbent mass, but solely dependent on the flow volume. Therefore, it is necessary to consider both the uptake and removal efficiency when evaluating biosorbent potential.
The slope of the breakthrough curve from t b to t e (dc/dt) is often used to characterize the shape of the curve (Volesky et al. 2003). It is preferential to have an extended breakthrough curve with a steep slope, as it is usually the result of a shorter mass transfer zone, which implies a longer column service time and greater utilization of the sorbent portion inside the column (Kratochvil and Volesky 1998). Thus, for effective biosorbents, a delayed breakthrough, earlier exhaustion, shortened mass transfer zone, high uptake, steep breakthrough curve, and high removal efficiency would be expected.
12.11.1 Column Regeneration
Regeneration of biosorbent is relatively easier in a packed column arrangement, with the aid of an appropriate elutant. When the column becomes saturated, the contaminant solution flow should be switched to the elutant flow. In general, an elution process is usually rapid compared with that of sorption. Thus, a high contaminant concentration in a small elutant volume would be expected under optimized process conditions. In addition, it is desirable to limit the contact of the elutant with the sorbent. This is because extreme process conditions such as highly alkaline or acidic solutions are often employed for elution; thus, morphological damage to the biosorbent can be expected. Therefore, the optimal flow rate for the elution should be identified for successful reuse of the biosorbent over multiple cycles. In a typical elution curve, a sharp concentration increase is expected at the beginning, followed by a gradual decrease (Volesky et al. 2003; Vijayaraghavan et al. 2005).
Even with the successful optimization of an elution process, several investigators have observed a decrease in biosorption performance over subsequent cycles (Saeed and Iqbal 2003; Volesky et al. 2003; Vijayaraghavan et al. 2004). A loss of sorption performance during long-term use may occur for a variety of reasons, e.g., changes in the chemistry and structure of the biosorbent as well as flow and mass transport conditions within the column.
12.11.2 Sorption Column Model
The Bohart–Adams sorption model (Jansson-Charrier et al. 1996; Muraleedharan et al. 1994), developed primarily for carbon sorption, has often been used in studies of biosorption column performance; however, it is not an appropriate model that would reflect the uptake mechanism of ion exchange. The most complete column model taking into account dominant intraparticle mass transfer was developed for ion exchange by Tan and Spinner (1994). In principle, this mass transfer model can predict breakthrough curves for all species removed by the biosorbent and also the elution curves obtained during sorbent regeneration.
To predict biosorption in fixed-bed columns, the model based on the work of Kratochvil and Volesky (2000) is used. Adaptation of this model was necessary in order to study binary systems as well as ternary and quaternary systems. Its transformation allowed testing the modeling approach for the case of multicomponent biosorption systems. The adapted approach consisted of numerically solving a mixed system of partial differential, ordinary differential, and algebraic equations describing the dynamics of multicomponent ion exchange in a flow-through fixed bed. Assuming isothermal conditions and constant physical properties for the feed solution, the differential molar balance for a sorbate species M is:
The sorption rate equation can be written as (12.30), assuming a linear driving force for the sorption process and a combined film and intraparticle mass transfer resistance:
With
where t is the time (h), ρb the packing density of dry biomass in the packed bed (g L−1), Q the concentration of binding sites in the biosorbent (meq g−1), C 0 the normality of the column feed (meq L−1), ε the column void fraction, L 0 the length of the column (cm), ν the interstitial fluid velocity (cm min−1), D Z the dispersion coefficient in the liquid phase (cm2 min−1), ShM the rate constant for ion exchange (min−1), C M the concentration of species M in the liquid phase (meq L−1), q M the uptake of species M by the biosorbent (meq g−1), \( {q}_{\text{M}}^{*}\) the dimensionless equilibrium uptake of species M at C M, D gM the solute distribution parameter, K fM the overall mass transfer coefficient of species M (min−1), and P ec is the column Peclet number.
12.12 Conclusion
Biosorption offers an economically feasible technology for efficient removal and recovery of metal(s) from aqueous solution. The process of biosorption has many attractive features including selective removal of metals over a broad range of pH and temperature, rapid kinetics of adsorption and desorption, and low capital and operational costs. Biosorbents can easily be produced using inexpensive growth media or obtained as a by-product from industry. The use of immobilized biomass rather than native biomass has been recommended for large-scale application, but various immobilization techniques have yet to be thoroughly investigated for ease, efficacy, and cost effectiveness. When designing a reactor for water treatment, it is important to achieve optimal conditions for metal retention at the lowest cost. Also, for an ex situ bioremediation process, costs will be lower when there is no need to include nutrients. The use of fixed or fluidized bed reactors is preferred because of easier recovery of the treated effluent, so successful bacterial immobilization on different matrices is required. Experiments in complexing capacity evaluation in industrial effluents are necessary to verify metal bioavailability and improve the efficiency of the process. Although continuous processes of immobilized cells have been realized at laboratory scale, there is still a long way to go for biosorption commercialization. Selection of quality, inexpensive support materials for biomaterial immobilization, improvement of reuse methods, and enhancement of properties of immobilized biosorbents such as pore ratio, mechanical intensity, and chemical stability are also important factors for application (Hu and Wang 2003).
Biosorption processes are applicable to effluents containing low concentrations of heavy metals for an extended period. This aspect makes it even more attractive for the treatment of dilute effluent that originates either from an industrial plant or from a primary wastewater treatment facility. Thus, biomass-based technologies need not necessarily replace conventional treatment routes but may complement them. To provide an economically viable treatment, the appropriate choice of biomass and proper operational conditions must be identified.
Critical analysis reveals that not all metal-polluted wastewater-generating industries have the interest or the capability to treat effluents and most industries opt only for basic treatment techniques simply to comply with regulations. To attract greater usage of biosorption, strategies must be formulated to centralize facilities for accepting the used biosorbent where its processing can be carried out to either regenerate the biomass or convert the recovered metal into a usable form. This will further require an interdisciplinary approach with the integration of metallurgical skills along with sorption and wastewater treatment to apply biosorption technology for combating heavy metal pollution in aqueous systems.
12.13 Future Prospects
For the future of biosorption, there are two trends of development for the removal of metals. One is to use hybrid technology for pollutant removal, especially using living cells. A second trend requires the improvement of biomaterials immobilization, as well as optimization of the parameters of the biosorption process and physicochemical conditions, including reuse and recycling. Market factors for successful application of biosorption should be considered. The mechanisms involved in biosorption or metal–microbe interactions should be further studied. Molecular biotechnology, a powerful tool to elucidate mechanisms at the molecular level, should be considered more in the future to construct an engineered organism with higher sorption capacity and specificity for target metal ions.
References
Agarwal, G. S., Bhuptawat, H. K., and Chaudhari, S. 2006. Biosorption of aqueous chromium(VI) by Tamarindus indica seeds. Bioresour. Technol. 97:949–956.
Akar, T., Tunali, S., and Kiran, I. 2005. Botrytis cinerea as a new fungal biosorbent for removal of Pb(II) from aqueous solutions. Biochem. Eng. J. 25:227–235.
Akar, T., Kaynak, Z., Ulusoy, S., Yuvaci, D., Ozsari, G., and Akar S. T. 2009. Enhanced biosorption of nickel(II) ions by silica-gel-immobilized waste biomass: biosorption characteristics in batch and dynamic flow mode. J. Hazard. Mater. 163:1134–1141.
Aksu, Z. 2005. Application of biosorption for the removal of organic pollutants: a review. Process Biochem. 40:997–1026.
Aksu, Z., and Gönen, F. 2004. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: prediction of breakthrough curves. Process Biochem. 39:599–613.
Aksu, Z., Acikel, U., Kabasakal, E., and Tezer, S. 2002. Equilibrium modelling of individual and simultaneous biosorption of chromium(VI) and nickel(II) onto dried activated sludge. Water Res. 36:3063–3073.
Al-Rub, F. A. A. 2006. Biosorption of zinc on palm tree leaves: equilibrium, kinetics, and thermodynamics studies. Sep. Sci. Technol. 41:3499–3515.
Amarasinghe, B. M. W. P. K., and Williams, R. A. 2007. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 132:299–309.
Antizar-Ladislao, A., and Galil, N. I. (2004). Biosorption of phenol and chlorophenols by acclimated residential biomass under bioremediation conditions in a sandy aquifer. Water. Res. 38:267–276.
Apiratikul, R., and Pavasant, P. 2008. Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour. Technol. 99:2766–2777.
Arica, M. A., Kacar, Y., and Genc, O. 2001. Entrapment of white-rot fungus Trametes versicolor in Ca-alginate beads: preparation and biosorption kinetic analysis for cadmium removal from an aqueous solution. Bioresour. Technol. 80:121–129.
Arica, M. A., Bayremoglu, G., Yilmaz, M., Bektas, S., and Genc, O. 2004. Biosorption of Hg2+, Cd2+ and Zn2+ by Ca-alginate and immobilized wood-rotting fungus Funalia trogii. J. Hazard. Mater. B109:191–199.
Aydin, H., Bulut, Y., and Yerlikaya, C. 2008. Removal of copper (II) fromaqueous solution by adsorption onto low-cost adsorbents. J. Environ. Manage. 87:37–45.
Bae, W., Mulchandani, A., and Chen, W. 2002. Cell surface display of synthetic phytochelatins using ice nucleation protein for enhanced heavy metal bioaccumulation, J. Inorg. Biochem. 88:223–227.
Bae, W., Wu, C. H., Kostal, J., Mulchandani, A., and Chen, W. 2003. Enhanced mercury biosorption by bacterial cells with surface-displayed MerR. Appl. Environ. Microbiol. 69:3176–3180.
Baral, S. S., Das, S. N., and Rath, P. 2006. Hexavalent chromium removal fromaqueous solution by adsorption on treated sawdust. Biochem. Eng. J. 31:216–222.
Baral, S. S., Das, S. N., Rath, P., Roy Chaudhury, P. G., and Swamy, Y. V. 2007. Removal of Cr(VI) from aqueous solution using waste weed, Salvinia cucullata. Chem. Ecol. 23:105–117.
Basha, S., Murthy, Z. V. P., and Jha, B. 2008. Sorption of Hg(II) from aqueous solutions onto Carica papaya: application of isotherms. Ind. Eng. Chem. Res. 47:980–986.
Beolchini, F., Pagnanelli, F., Toro, L., and Vegliò, F. 2003. Biosorption of copper by Sphaerotilus natans immobilised in polysulfone matrix: equilibrium and kinetic analysis. Hydrometallurgy. 70:101–112.
Beolchini, F., Pagnanelli, F., Toro, L., and Vegliò, F. 2006. Ionic strength effect on copper biosorption by Sphaerotilus natans: equilibrium study and dynamic modelling in membrane reactor. Water. Res. 40:144–152.
Beveridge, T. J. 1989. Role of cellular design in bacterial metal accumulation and mineralization. Annu. Rev. Microbiol. 43:147–171.
Beveridge, T. J. 2001. Use of the Gram stain in microbiology. Biotech. Histochem. 76:111–8.
Bueno, B. Y. M., Torem, M. L., Molina, F., and de Mesquita. L. M. S. 2008. Biosorption of lead(II), chromium(III) and copper(II) by R. opacus: equilibrium and kinetic studies. Miner. Eng. 21:65–75.
Cabuk, A., Akar, T., Tunali, S., and Gedikli, S. 2007. Biosorption of Pb(II) by industrial strain of Saccharomyces cerevisiae immobilized on the biomatrix of cone biomass of Pinus nigra: equilibrium and mechanism analysis. Chem. Eng. J. 131:293–300.
Calfa, B. A., and Torem, M. L. 2008. On the fundamentals of Cr(III) removal from liquid streams by a bacterial strain. Miner. Eng. 21:48–54.
Cayllahua, J. E. B., de Carvalho, R. J., and Torem. M. L. 2009. Evaluation of equilibrium, kinetic and thermodynamic parameters for biosorption of nickel(II) ions onto bacteria strain, Rhodococcus opacus. Miner. Eng. 22:1318–1325.
Chen, S. L., Kim, E., Shuler, M. L., and Wilson, D. B. 1998. Hg removal by genetically engineered Escherichia coli in a hollow fiber bioreactor. Biotechnol. Prog. 14:667–671.
Chen, X. C.,Wang, Y. P., Lin, Q., Shi, J. Y., Wu, W. X., and Chen, Y. X. 2005. Biosorption of copper(II) and zinc(II) from aqueous solution by Pseudomonas putida CZ1. Colloid. Surf. B Physicochem. Eng. Aspect. 46:101–107.
Chen, G., Zeng, G., Tang, L., Du, C., Jiang, X., Huang, G., Liu, H., and Shen, G. 2008. Cadmium removal from simulated wastewater to biomass byproduct of Lentinus edodes. Bioresour. Technol. 99:7034–7040.
Choi, S. B., and Yun, Y. S. 2004. Lead biosorption by waste biomass of Corynebacterium glutamicum generated from lysine fermentation process. Biotechnol. Lett. 26:331–336.
Choudhary, S., and Sar, P. 2009. Characterization of a metal resistant Pseudomonas sp. isolated from uranium mine for its potential in heavy metal (Ni2+, Co2+, Cu2+, and Cd2+) sequestration. Bioresour. Technol. 100:2482–2492.
Chu, K. H. 2004. Improved fixed bed models for metal biosorption. Chem. Eng. J. 97:233–239.
Chubar, N., Behrends, T., and Cappellen, P. V. 2008. Gram-negative bacterium Shewanella putrefaciens. Colloid. Surf. B Physicochem. Eng. Aspect. 65:126–133
Cossich, E. S., da Silva, E. A., Tavares, C. R. G., Filho, L. C., and Ravagnani, T. M. K. 2004. Biosorption of chromium(III) by biomass of seaweed Sargassum sp. in a fixed-bed column. Adsorption 10:129–138.
Davis, T. A., Volesky, B., and Vieira, R. H. S. F. 2000. Sargassum seaweed as biosorbent for heavy metals. Water Res. 34:4270–4278.
Davis, T. A., Volesky, B., and Mucci, A. 2003. A review of the biochemistry heavy metal biosorption by brown algae. Water Res. 37:4311–4330.
de Vargas, I., Macaskie, L. E., and Guibal, E. 2004. Biosorption of palladium and platinum by sulfate-reducing bacteria. J. Chem. Technol. Biotechnol. 79:49–56.
Deng, L., Su, Y., Su, H., Wang, X., and Zhu, X. 2006. Biosorption of copper (II) and lead (II) fromaqueous solutions by nonliving green algae Cladophora fascicularis: equilibrium, kinetics and environmental effects. Adsorption 12:267–277.
Deng, X., Yi, X., and Liu, G. 2008. Cadmium removal from aqueous solution by gene-modified Escherichia coli JM109. J. Hazard. Mater. 139:340–344.
Djeribi, R., and Hamdaoui, O. 2008. Sorption of copper(II) from aqueous solutions by cedar sawdust and crushed brick. Desalination 225:95–112.
Dundar, M., Nuhoglu, C., and Nuhoglu, Y. 2008. Biosorption of Cu(II) ions onto the litter of natural trembling poplar forest. J. Hazard. Mater. 151:86–95.
Dursun, A. Y. 2006. A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper(II) and lead(II) ions onto pretreated Aspergillus niger. Biochem. Eng. J. 28:187–195.
Flemming, H. C., and Wingender, J. 2001. Relevance of microbial extracellular polymeric substances (EPSs) – Part I: structural and ecological aspects. Water. Sci. Technol. 43:1–8.
Gabr, R. M., Hassan, S. H. A., and Shoreit A. A. M. 2008. Biosorption of lead and nickel by living and non-living cells of Pseudomonas aeruginosa ASU 6a. Int. Biodeterior. Biodegradation 62:195–203.
Gokhale, S. V., Jyoti, K. K., and Lele, S. S. 2008. Kinetic and equilibrium modeling of chromium (VI) biosorption on fresh and spent Spirulina platensis/Chlorella vulgaris biomass. Bioresour. Technol. 99:3600–3608.
Green-Ruiz, C. 2006. Mercury(II) removal from aqueous solutions by nonviable Bacillus sp. from a tropical estuary. Bioresour. Technol. 97:1907–1911.
Green-Ruiz, C., Rodriguez-Tirado, V., and Gomez-Gil, B. 2008. Cadmium and zinc removal from aqueous solutions by Bacillus jeotgali: pH, salinity and temperature effects. Bioresour. Technol. 99:3864–3870.
Grill, E. 1987. Phytochelatins, the heavy metal binding peptides of plants: characterization and sequence determination. Experientia. Suppl. 52:317–322.
Guo, X. Y., Zhang, A. Z., and Shan, X. Q. 2008. Adsorption of metal ions on lignin. J. Hazard. Mater. 151:134–142.
Gupta, V. K., and Rastogi, A. 2008. Biosorption of lead from aqueous solutions by green algae Spirogyra species: kinetics and equilibrium studies. J. Hazard. Mater. 152:407–414.
Gutnick, D. L., and Bach. H. 2000. Engineering bacterial biopolymers for the biosorption of heavy metals; new products and novel formulations. Appl. Microbiol. Biotechnol. 54:451–461.
Hanif, M. A., Nadeem, R., Bhatti, H. N., Ahmad, N. R., and Ansari, T. M. 2007. Ni(II) biosorption by Cassia fistula (Golden Shower) biomass. J. Hazard. Mater. B139:345–355.
Hasan, S. H., and Srivastava, P. 2009. Batch and continuous biosorption of Cu2+ by immobilized biomass of Arthrobacter sp. J. Environ. Manage. 90:3313–3321.
Hasan, S. H., Srivastava, P., and Talat, M. 2009. Biosorption of Pb(II) from water using biomass of Aeromonas hydrophila: Central composite design for optimization of process variables. J. Hazard. Mater. 15:1155–1162.
Ho, Y. 2006. Isotherms for the sorption of lead onto peat: comparison of linear and non-linear methods. Pol. J. Environ Stud. 15:81–86.
Ho, Y. S., and McKay, G. 2002. Application of kinetic models to the sorption of copper(II) on to peat. Adsorp. Sci. Technol. 20:797–815.
Ho, Y., and Ofomaja, A. E. 2006. Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent. Biochem. Eng. J. 30:117–123.
Huang, C. C., Su, C. C., Hsieh, J. L., Tseng, C. P., Lin, P. L., and Chang, J. S. 2003. Polypeptides for heavy-metal biosorption: capacity and specificity of two heterogeneous MerP proteins. Enzyme Microb. Technol. 33:379–385.
Hu, H. T., and Wang, H. N. 2003. Heavy metal treatment in water by biosorption. Environ. Protect. Xinjiang. 25:22–25.
Igwe, J. C., and Abia, A. A. 2007. Equilibrium sorption isotherm studies of Cd(II), Pb(II) and Zn(II) ions detoxification from waste water using unmodified and EDTA modified maize husk. Electron. J. Biotechnol. 10:536–548.
Iqbal, M., Saeed, A., and Zafar, S. I. 2007. Hybrid biosorbent: an innovative matrix to enhance the biosorption of Cd(II) from aqueous solution. J. Hazard. Mater. 148:47–55.
Isik, M. 2008. Biosorption of Ni(II) from aqueous solutions by living and non-living ureolytic mixed culture. Colloids Surf. B Biointerfaces 62:97–104.
Jansson-Charrier, M., Guibal, E., Roussy, J., Surjous, R., and LeCloirec, P. 1996. Dynamic removal of uranium by chitosan: influence of operating parameters. Water Sci. Technol. 34:169–177.
Javed, M. A., Bhatti, H. N., Hanif, M. A., and Nadeem, R. 2007. Kinetic, equilibrium modeling of Pb(II) and Co(II) sorption onto rose waste biomass. Sep. Sci. Technol. 42:3641–3656.
Jian-hua, P., Rui-xia, L., and Hong-xiao, T. 2007. Surface reaction of Bacillus cereus biomass and its biosorption for lead and copper ions. J. Environ. Sci. 19:403–408.
Kang, S. Y., Lee, J. U., and Kim, K. W. 2007. Biosorption of Cr(III) and Cr(VI) onto the cell surface of Pseudomonas aeruginosa. Biochem. Eng. J. 36:54–58.
Kao, W. C., Chiu, Y. P., Chang, C. C., and Chang, J. S. 2006. Localization effect on the metal biosorption capability of recombinant mammalian and fish metallothioneins in Escherichia coli. Biotechnol. Prog. 22:1256–1264.
Kao, W., Huang, C., and Chang, J. 2008. Biosorption of nickel, chromium and zinc by MerP-expressing recombinant Escherichia coli. J. Hazard. Mater. 158:100–106.
Kao, W. C., Wu, J. Y., Chang, C. C., and Chang J. S. 2009. Cadmium biosorption by polyvinyl alcohol immobilized recombinant Escherichia coli. J. Hazard. Mater. 169:651–658.
Kapoor, A., and Viraraghavan, T. 1997. Heavy metal biosorption sites in Aspergillus niger. Bioresour. Technol. 61:221–227.
Kazy, S. K., Das, S. K., and Sar, P. 2006. Lanthanum biosorption by a Pseudomonas sp.: equilibrium studies and chemical characterization. J. Ind. Microbiol. Biotech. 33:773–83.
Kiran, B., and Kaushik, A. 2008. Chromium binding capacity of Lyngbya putealis exopolysaccharides. Biochem. Eng. J. 38:47–54.
Kiran, I., Akar, T., and Tunali, S. 2005. Biosorption of Pb(II) and Cu(II) from aqueous solutions by pretreated biomass of Neurospora crassa. Process Biochem. 40:3550–3558.
Kratochvil, D., and Volesky, B. 1998. Advances in the biosorption of heavy metals. TIBTECH. 16:291–300.
Kratochvil, D., and Volesky, B. 2000. Multicomponent biosorption in fixed beds. Water Res. 34:3186–3196.
Kumar, R., Bishnoi, N. R., Garima, and Bishnoi, K. 2008. Biosorption of chromium(VI) from aqueous solution and electroplating wastewater using fungal biomass. Chem. Eng. J. 135:202–208.
Kuyucak, N., and Volesky, B. 1989. Desorption of cobalt-laden algal biosorbent. Biotechnol. Bioeng. 33:815–822.
Langley, S., and Beveridge, T. J. 1999. Effect of O-side-chainlipopolysaccharide chemistry on metal binding. Appl. Environ. Microbiol. 65:489–498.
Lin, C. C., and Lai, Y. T. 2006. Adsorption and recovery of lead(II) from aqueous solutions by immobilized Pseudomonas aeruginosa PU21 beads. J. Hazard. Mater. 137:99–105.
Liu, H. L., Chen, B. Y., Lan, Y. W., and Cheng, Y. C. 2004. Biosorption of Zn(II) and Cu(II) by the indigenous Thiobacillus thiooxidans. Chem. Eng. J. 97:195–201.
Liu, R., Ma, W., Jia, C., Wang, L., and Li, H. 2007. Effect of pH on biosorption of boron onto cotton cellulose. Desalination 207:257–267.
Lloyd, J. R. 2002. Bioremediation of metals: the application of microorganisms that make and break minerals. Microbiol. Today 29:67–69.
Loukidou, M. X., Karapantsios, T. D., Zouboulis, A. I., and Matis, K. A. 2004. Diffusion kinetic study of chromium (VI) biosorption by Aeromonas caviae. Ind. Eng. Chem. Res. 242:93–104.
Lu, S., and Gibb, S. W. 2008. Copper removal from wastewater using spent-grain as biosorbent. Bioresour. Technol. 99:1509–1517.
Lu, W. B., Shi, J. J., Wang, C. H., and Chang, J. S. 2006. Biosorption of lead, copper and cadmium by an indigenous isolate Enterobacter sp J1 possessing high heavy-metal resistance. J. Hazard. Mater. 134:80–86.
Ma, W., and Tobin, J. M. (2004). Determination and modeling of effects of pH on peat biosorption of chromium, copper and cadmium. Biochem. Eng. J. 18:33–40.
Mack, C. L., Wilhelmi, B., Duncan, J. R., and Burgess, J. E. 2008. A kinetic study of the recovery of platinum ions from an artificial aqueous solution by immobilized Saccharomyces cerevisiae biomass. Miner. Eng. 21:31–37.
Madigan, M. T., Martinko, J. M., and Parker, J. 2000. Brock biology of microorganisms. Upper Saddle River, NJ: Pearson Prentice Hall.
Malkoc, E., and Nuhoglu, Y. 2005. Investigations of nickel (II) removal from aqueous solutions using tea factory waste. J. Hazard. Mater. B127:120–128.
Mann, H. 1990. Removal and recovery of heavy metals by biosorption. In: Volesky B, ed. Biosorption of heavy metals, pp.93–137. Boca Raton: CRC press.
Miretzky, P., Munoz, C., and Carrillo-Chavez, A. 2008. Experimental binding of lead to a low cost on biosorbent: Nopal (Opuntia streptacantha). Bioresour. Technol. 99:1211–1217.
Mishra, S., and Doble, M. 2008. Novel chromium tolerant microorganisms: isolation, characterization and their biosorption capacity. Ecotoxicol. Environ. Saf. 71:874–879.
Moat, A. G., Foster, J. W., and Spector, M. P. 2002. Microbial physiology. New York: Wiley-Liss.
Mondal, P., Majumder, C.B., and Mohanty, B., 2008. Growth of three bacteria in arsenic solution and their application for arsenic removal from wastewater. J. Basic. Microbiol. 48:1–5.
Muraleedharan, T. R., Iyengar, L., and Venkobachar, C. 1991. Biosorption: an attractive alternative for metal removal and recovery. Curr. Sci. 61:379–385.
Muraleedharan, T., Philip, R., Iyengar, L. L., and Venkobachar, C. 1994. Application studies of biosorption for monazite processing industry effluents. Bioresour. Technol. 49:179–186.
Naja, G., and Volesky, B. 2006. Behavior of the mass transfer zone in a biosorption column. Environ. Sci. Technol. 40:3996–4003.
Nakajima, A., and Tsuruta, T. 2004. Competitive biosorption of thorium and uranium by Micrococcus luteus. J. Radioanal. Nucl. Chem. 260:13–18.
Namasivayam, C., and Sureshkumar, M. V. 2008. Removal of chromium(VI) from water and wastewater using surfactant modified coconut coirpith as biosorbent. Bioresour. Technol. 99:2218–2225.
Nasreen, K., Muhammad, I., Iqbal, Z. S., and Javed, I. 2008. Biosorption characteristics of unicellular green alga Chlorella sorokiniana immobilized in loofa sponge for removal of Cr(III). J. Environ. Sci. 20:231–239.
Ofomaja, A. E., and Ho, Y. 2007. Effect of pH on cadmium biosorption by coconut copra meal. J. Hazard. Mater. B139:356–362.
O’Mahony, T., Guibal, E., and Tobin, J. M. 2002. Reactive dye biosorption by Rhizopus arrhizus biomass. Enzyme Microb. Technol. 31:456–463.
Ozdemir, G., and Baysal, S. H. 2004. Chromium and aluminum biosorption on Chryseomonas luteola TEM05. Appl. Microbiol. Biotechnol. 64:599–603.
Ozdemir, S., Kilinc, E., Poli, A., Nicolaus, B., and Guvena, K. 2009. Biosorption of Cd, Cu, Ni, Mn and Zn from aqueous solutions by thermophilic bacteria, Geobacillus toebii sub.sp. decanicus and Geobacillus thermoleovorans sub.sp. stromboliensis: equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 152:195–206.
Öztürk, A. 2007. Removal of nickel from aqueous solution by the bacterium Bacillus thuringiensis. J. Hazard. Mater. 147:518–523.
Öztürk, A., Artan, T., and Ayar, A. 2004. Biosorption of nickel(II) and copper(II) ions from aqueous solution by Streptomyces coelicolor A3(2). Colloid. Surf. B Physicochem. Eng. Aspect. 34:105–111.
Padmavathy, V. 2008. Biosorption of nickel(II) ions by baker’s yeast: kinetic, thermodynamic and desorption studies. Bioresour. Technol. 99:3100–3109.
Pamukoglu, M. Y., and Kargi, F. 2007. Effects of operating parameters on kinetics of copper(II) ion biosorption onto pre-treated powdered waste sludge (PWS). Enzyme Microb. Technol. 42:76–82.
Parvathi, K., Nagendran, R., and Nareshkumar, R. 2007. Lead biosorption onto waste beer yeast by-product, a means to decontaminate effluent generated from battery manufacturing industry. Electron. J. Biotechnol. 10:1–14.
Pasavant, P., Apiratikul, R., Sungkhum, V., Suthiparinyanont, P., Wattanachira, S., and Marhaba, T. F. 2006. Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour. Technol. 97:2321–2329.
Pazirandeh, M., Wells, B. M., and Ryan, R. L. 1998. Development of bacterium-based heavy metal biosorbents: enhanced uptake of cadmium and mercury by Escherichia coli expressing a metal binding motif. Appl. Environ. Microbiol. 64:4068–4072.
Pollmann, K., Raff, J., Merroun, M., Fahmy, K., and Selenska-Pobell, S. 2006. Metal binding by bacteria from uranium mining waste piles and its technological applications. Biotechnol. Adv. 24:58–68.
Popuri, S. R., Jammala, A., Reddy, K. V. N. S., and Abburi, K. 2007. Biosorption of hexavalent chromium using tamarind (Tamarindus indica) fruit shell – a comparative study. Electron. J. Biotechnol. 10:358–367.
Prakasham, R. S., Merrie, J. S., Sheela, R., Saswathi, N., and Ramakrishna, S. V. 1999. Biosorption of chromium VI by free and immobilized Rhizopus arrhizus. Environ. Pollut. 104:421–427.
Preetha, B., and Viruthagiri, T. 2007. Batch and continuous biosorption of chromium(VI) by Rhizopus arrhizus. Separat. Purific. Technol. 57:126–133.
Prescott, L. M., Harley, J. P., and Klein, D. A. 2002. Microbiology. London: McGraw-Hill Science/Engineering/Math.
Pulsawat, W., Leksawasdi, N., Rogers, P. L., and Foster, L. J. R. 2003. Anions effects on biosorption of Mn(II) by extracellular polymeric substance (EPS) from Rhizobium etli. Biotech. Lett. 25(15):1267–1270.
Puranik, P. R., and Paknikar, K. M. 1999. Biosorption of lead, cadmium, and zinc by Citrobacter strain MCM B-181: characterization studies. Biotechnol. Prog. 15:228–237.
Quintelas, C., Zélia Rocha, Z., Silva, B., Fonseca, B., Figueiredo, H., and Tavares, T. 2009. Removal of Cd(II), Cr(VI), Fe(III) and Ni(II) from aqueous solutions by an E. coli biofilm supported on kaolin. Chem. Eng. J. 149:319–324.
Rahaman, M. S., Basu, A., and Islam, M. R. 2008. The removal of As(III) and As(V) from aqueous solutions by waste materials. Bioresour. Technol. 99:2815–2823.
Saeed, A., and Iqbal, M. 2003. Bioremoval of cadmium from aqueous solution by black gram husk (Cicer arientinum). Water Res. 37:3472–3480.
Sag, Y., and Aktay, Y. 2002. Kinetic studies on sorption of Cr(VI) and Cu(II) ions by chitin, chitosan, and Rhizopus arrhizus. Biochem. Eng. J. 12:143–153.
Sag, Y., Akeael, B., and Kutsal, T. 2002. Ternary biosorption equilibria of Cr(VI), Cu(II) and Cd(II) on Rhizopus arrhizus. Sep. Sci. Technol. 37(2):279–309.
Şahin, Y., and Öztürk, A. 2005. Biosorption of chromium(VI) ions from aqueous solution by the bacterium Bacillus thuringiensis. Process Biochem. 40:1895–1901.
Samuelson, P.,Wernérus, H., Svedberg, M., and Ståhl, S. 2000. Staphylococcal surface display of metal-binding polyhistidyl peptides. Appl. Environ. Microbiol. 66:1243–1248.
Schiewer, S., and Patil, S. B. 2008. Pectin-rich fruit wastes as biosorbents for heavy metal removal: equilibrium and kinetics. Bioresour. Technol. 99:1896–1903.
Schiewer, S., and Volesky, B. 2000. Biosorption Processes for Heavy Metal Removal. In: D. E. Lovley, ed. Environmental microbe–metal interactions, pp. 329–362. Washington, DC: ASM Press.
Selatnia, A., Bakhti, M. Z., Madani, A., Kertous, L., and Mansouri, Y. 2004a. Biosorption of Cd2+ from aqueous solution by a NaOH-treated bacterial dead Streptomyces rimosus biomass. Hydrometallurgy. 75:11–24.
Selatnia, A., Boukazoula, A., Kechid, N., Bakhti, M. Z., and Chergui, A. 2004b. Biosorption of Fe3+ from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Process Biochem. 39:1643–1651.
Selatnia, A., Boukazoula, A., Kechid, N., Bakhti, M. Z., Chergui, A., and Kerchich, Y. 2004c. Biosorption of lead (II) from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Biochem. Eng. J. 19:127–135.
Shaker, M. A. 2007. Thermodynamic profile of some heavy metal ions adsorption onto biomaterial surfaces. Am. J. Appl. Sci. 4:605–612.
Sharma, P., Kumari, P., Srivastava, M. M., and Srivastava, S. 2006. Romoval of cadmium from aqueous system by shelled Moringa oleifera Lam. Seed powder. Bioresour. Technol. 97:299–305.
Shevchuk, I. A., and Klimenko, N. I. 2009. Biological features of sorption of U (VII) and strontium ions by Bacillus polymyxa IMV 8910 cells. J. Water Chem. Technol. 31:324–328.
Silva, R. M. P., Rodríguez, A. A., Montes De Oca, J. M. G., and Moreno, D. C. 2009. Biosorption of chromium, copper, manganese and zinc by Pseudomonas aeruginosa AT18 isolated from a site contaminated with petroleum. Bioresour. Technol. 100:1533–1538
Site, A. D. 2001. Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants. A review. J. Phys. Chem. 30:187–439.
Sleytr, U., and Beveridge, T. 1999. Bacterial S-layers. Trends. Microbiol. 7:253–260.
Sleytr, U., Györvary, E., and Pum, D. 2003. Crystallization of S-layer protein lattices on surfaces and interfaces. Progr. Org. Coating. 47:279–287.
Sobeck, D. C., and Higgins, M. J. 2002. Examination of three theories for mechanisms of cation-induced bioflocculation. Water Res. 36(3):527–538.
Solisio, C., Lodi, A., Converti, A., and Borghi, M. D. 2000. The effect of acid pre-treatment on the biosorption of chromium(III) by Sphaerotilus natans from industrial wastewater. Water Res. 34:3171–3178.
Tan, H. K. S., and Spinner, I. H. 1994. Multicomponent ion exchange column dynamics. Can. J. Chem. Eng. 72:330–341.
Tunali, S., Çabuk, A., and Akar, T. 2006. Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem. Eng. J. 115:203–211.
Tuzen, M., Saygi, K. O., Usta, C., and Soylak, M. 2008. Pseudomonas aeruginosa immobilized multiwalled carbon nanotubes as biosorbent for heavy metal ions. Bioresour. Technol. 99:1563–1570.
Urrutia, M. M. 1997. General Bacterial Sorption Processes. In: J. Wase and C. Forster (eds). Biosorbents for metal ions, pp. 39–66. London: CRC Press.
Uslu, G., and Tanyol, M. 2006. Equilibrium and thermodynamic parameters of single and binary mixture biosorption of lead(II) and copper(II) ions onto Pseudomonas putida: effect of temperature. J. Hazard. Mater. 135:87–93.
Uzel, A., and Ozdemir, G. 2009. Metal biosorption capacity of the organic solvent tolerant Pseudomonas fluorescens TEM08. Bioresour. Technol. 100:542–548.
van Hullebusch, E. D., Zandvoort, M. H., and Lens, P. N. L. 2003. Metal immobilisation by biofilms: mechanisms and analytical tools. Rev. Environ. Sci. Biotechnol. 2:9–33.
Veglio, F., and Beolchini, F. 1997. Removal of metals by biosorption: a review. Hydrometallurgy. 44:301–316.
Vijaya, Y., Popuri, S. R., Boddu, V. M., and Krishnaiah, A. 2008. Modified chitosan and calcium. Carbohyd. Poly. 72:261–271.
Vijayaraghavan, K., and Yun, Y. S. 2008a. Bacterial biosorbents and biosorption. Biotechnol. Adv. 26:266–291.
Vijayaraghavan, K., and Yun, Y. S. 2008b. Biosorption of C.I. Reactive Black 5 from aqueous solution using acid-treated biomass of brown seaweed Laminaria sp. Dyes Pigm. 76:726–732.
Vijayaraghavan, K., Jegan, J., Palanivelu, K., and Velan, M. 2004. Removal of nickel(II) ions from aqueous solution using crab shell particles in a packed bed up-flow column. J. Hazard. Mater. 113:223–230.
Vijayaraghavan, K., Jegan, J., Palanivelu, K., and Velan, M. 2005. Batch and column removal of copper from aqueous solution using a brown marine alga Turbinaria ornata. Chem. Eng. J. 106:177–184.
Vijayaraghavan, K., Palanivelu, K., and Velan, M. 2006. Biosorption of copper(II) and cobalt(II) from aqueous solutions by crab shell particles. Bioresour. Technol. 97:1411–1419.
Vilar, V. J. P., Botelho, C. M. S., and Boaventura, R. A. R. 2008. Copper removal by algae Gelidium, agar extraction algal waste and granulated algal waste: kinetics and equilibrium. Bioresour. Technol. 99:750–762.
Volesky, B. 1987. Biosorbents for metal recovery. TIBTECH. 5:96–101.
Volesky, B. 2001. Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59:203–216.
Volesky, B., Weber, J., and Park, J. M. 2003. Continuous-flow metal biosorption in a regenerable Sargassum column. Water Res. 37:297–306.
Vullo, D. L., Ceretti, H. M., Ramírez, S., and Zalts, A. 2003. Metal retention in calcium alginate (Retencio´n de metales en gel de alginato de calcio). VI Society of Environmental Toxicology and Chemistry Latinoamerican Annual Meeting (SETAC), Buenos Aires, Argentina.
Vullo, D. L., Ceretti, H. M., Daniel, M. A., Ramirez, S. A. M., and Zalts, A. 2008. Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E. Bioresour. Technol. 99:5574–5581.
Wang, J. L. 2002. Microbial immobilization techniques and water pollution technology. Beijing: Science Press. 233–248.
Wang, X., Qin, Y., and Li, Z. 2006a. Biosorption of zinc from aqueous solutions by rice bran: kinetics and equilibrium studies. Sep. Sci. Technol. 41:747–756.
Wang, X., Xia, S., Chen, L., Zhao, J., Chovelon, J., and Nicole, J. 2006b. Biosorption of cadmium(I1) and lead(I1) ions from aqueous solutions onto dried activated sludge. J. Environ. Sci. 18:840–844.
Won, S. W., and Yun, Y. S. 2008. Biosorptive removal of Reactive Yellow 2 using waste biomass from lysine fermentation process. Dyes Pigm. 76:502–507.
Xiangliang, P., Jianlong, W., and Daoyong, Z. 2005. Biosorption of Pb(II) by Pleurotus ostreatus immobilized in calcium alginate gel. Process. Biochem. 40:2799–28803.
Xie, S., Yang, J., Chen, C., Zhang, X., Wang, Q., and Zhang, C. 2008. Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J. Environ. Radioact. 99:126–133.
Xu, H., Liu, Y., and Tay, J. H. 2006. Effect of pH on nickel biosorption by aerobic granular sludge. Bioresour. Technol. 97:359–363.
Yan, Z., Xuliang, F., Zhilong, Y., Yahong, L., and Weimin, C. 2008. Biosorption of Cu(II) on extracellular polymers from Bacillus sp. F19. J. Environ. Sci. 20:1288–1293.
Yang, J., and Volesky, B. 1996. Intraparticle diffusivity of Cd ions in a new biosorbent material. J. Chem. Technol. Biotechnol. 66:355–364.
Yee, N., and Fein, J. B. 2001. Cd adsorption onto bacterial surfaces: a universal adsorption edge? Geochim. Cosmochim. Acta. 65:2037–2042.
Yilmaz, E. I., and Ensari, N. Y. 2005. Cadmium biosorption by Bacillus circulans strain EB1. World J. Microbiol. Biotechnol. 21:777–779.
Yu, J., Tong, M. S., and Li, X. B. 2007. A simple method to prepare poly(amic acid)-modified biomass for enhancement of lead and cadmium adsorption. Biochem. Eng. J. 33:126–133.
Zamil, S., Ahmad, S. S., Choi, M. H., Park, J. Y., and Yoon S. C. 2009. Correlating metal ionic characteristics with biosorption capacity of Staphylococcus saprophyticus BMSZ711 using QICAR model. Bioresour. Technol. 100:1895–1902.
Zhao, M., Duncan, R., and Van Hille, R. P. 1999. Removal and recovery of zinc from solution and electroplating effluent using Azolla filiculoides. Water Res. 33:1516–1522.
Zhao, X. W., Zhou, M. H., Li, Q. B., Lu, Y. H., He, N., Sun, D. H., and Deng, X. 2005. Simultaneous mercury bioaccumulation and cell propagation by genetically engineered Escherichia coli. Process Biochem. 40:1611–1616.
Zhou, M., Liu, Y., Zeng, G., Li. X., Xu, W., and Fan, T. 2007. Kinetic and equilibrium studies of Cr (VI) biosorption by dead Bacillus licheniformis biomass. World J. Microbiol. Biotechnol. 23:43–48.
Ziagova, M., Dimitriadis, G., Aslanidou, D., Papaioannou, X., Tzannetaki, E. L., and Liakopoulou-Kyriakides, M. 2007. Comparative study of Cd(II) and Cr(VI) biosorption on Staphylococcus xylosus and Pseudomonas sp. in single and binary mixtures. Bioresour. Technol. 98:2859–2865.
Zouboulis, A. I., Rousou, E. G., Matis, K. A., and Hancock, I. C. 1999. Removal of toxic metals from aqueous mixtures: Part 1. Biosorption. J. Chem. Tech. Biotechnol. 74:429–436.
Zouboulis, A. I., Loukidou, M. X., and Matis, K. A. 2004. Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem. 39:909–916.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Ansari, M.I., Masood, F., Malik, A. (2011). Bacterial Biosorption: A Technique for Remediation of Heavy Metals. In: Ahmad, I., Ahmad, F., Pichtel, J. (eds) Microbes and Microbial Technology. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-7931-5_12
Download citation
DOI: https://doi.org/10.1007/978-1-4419-7931-5_12
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-7930-8
Online ISBN: 978-1-4419-7931-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)