Abstract
Scientific interest in edible and/or biodegradable films has increased greatly in recent decades. Of the macromolecules used to produce these materials, proteins have been extensively used due to their excellent functional properties. Moreover, proteins are a raw material normally obtained from renewable sources. In particular, gelatin is one of the most highly studied proteins, since it can be produced in abundance at relatively low cost. Gelatin films are practically colorless, and thus coloring may enhance their attractiveness. The objective of this chapter is to present and discuss the physical properties (including light-barrier properties) of artificially colored edible films, with an emphasis on gelatin films with the addition of chlorophyllide as a function of pigment concentration and film thickness. The films were produced by casting a film-forming solution (FFS) made of gelatin, sorbitol (plasticizer), and chlorophyllide in an aqueous solution (10%). The effect of concentration of this pigment on moisture content, water solubility, water vapor permeability, mechanical properties, gloss, light-barrier properties, opacity, and color of films was studied. Also, the effect of thickness of the colored films on color parameters was studied more extensively. Overall, increasing the chlorophyllide concentration significantly increased the color of the films without necessarily affecting the other physical properties studied. All color parameters were strongly affected by film thickness. The use of natural pigments may be valuable for edible film technology. In choosing the pigment, one should consider not only the intended color, but also the overall effect on film properties.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The question of the environmental impact of synthetic packaging has generally favored research on edible films and, in particular, biodegradable films, which are flexible materials elaborated with biological macromolecules capable of forming a continuous matrix (Gontard and Guilbert 1996; Krochta and De-Mulder-Johnston 1997; Van de Velde and Kiekens 2002; Tharanathan 2003). The main biopolymers of agricultural origin used in the elaboration of films are polysaccharides (Nisperos-Carriedo 1994) and proteins (Gennadios et al. 1994; Torres 1994). Synthetic biopolymers have also been studied in depth in recent decades (Van de Velde and Kiekens 2002).
Among the proteins, gelatin has stimulated much interest due to its excellent filmogenic properties (Arvanitoyannis 2002). Gelatin was one of the first macromolecules used in the production of films, and has continued to be widely studied for this purpose due to its functional properties, large scale production, and competitive prices (Arvanitoyannis 2002).
The properties of the macromolecules directly influence the final characteristics of the edible films. The properties of protein-based films are determined by the protein-protein and protein-water interactions, which can be controlled by the preparation conditions of the FFS and by the addition of plasticizers (Gennadios et al. 1994; Torres 1994; Gontard and Guilbert 1996; Arvanitoyannis 2002). Plasticizers are low molecular weight molecules and therefore can penetrate the biopolymeric matrix, increasing the free space between the macromolecular chains, causing a decrease in the intermolecular forces throughout the matrix (Banker 1966; Krochta and De-Mulder-Johnston 1997).
In practical terms, the final characteristics of edible and/or biodegradable films are the result of numerous parameters, such as the characteristics and concentrations of the macromolecule and other constituents (solvent, plasticizer, etc.); pH; denaturation conditions (in the case of proteins); type of support used; drying conditions; and environmental conditions (temperature and humidity).
Protein-based films, including those from gelatin, generally present excellent gas-barrier properties and good mechanical properties. In addition, they can serve as a support for active substances such as antioxidants, antimicrobial agents, etc. (Gómez-Guillén et al. 2007; López-Carballo et al. 2008; Gómez-Estaca et al. 2009). On another side, two papers on biopolymer-based films containing natural pigments can be found, in which the pigments in both cases are derived from chlorophyll (Corat et al. 2007; López-Carballo et al. 2008).
Since chlorophyll is a pigment, it can confer a green color on the material, which can be an added attraction. However, the properties of the material, notably the light-barrier properties, may be affected by the presence of this pigment (Corat et al. 2007). Thus, this chapter will present and discuss the effects of adding chlorophyll to the formulation of gelatin-based edible films, which would also permit the production of more attractive packaging with improved light-barrier properties.
2 Gelatin and Edible Films
Gelatin is a protein of animal origin resulting from the acidic or basic hydrolysis of the collagen obtained from bones, bovine or swine hides, or connective tissue (Gennadios et al. 1994). Configuration of the peptide chain in gelatin is normally controlled by interactions between the solvent and the amino acids in the main chain, and by certain preferential orientations of the peptide bonds, such that the amino acid sequence is responsible for the characteristics of the gelatin (Veis 1964). In general, glycine, proline, and hydroxyproline constitute one-third of the total amino acids in gelatin (Veis 1964; Gennadios et al. 1994).
The molecular weight of gelatin depends on the type of raw material and process conditions used, varying from 3,000 to 200,000 Da (Gennadios et al. 1994). According to the type of pretreatment used to remove impurities and to start the hydrolysis of the raw material, the gelatin is classified as Type A when an acid pretreatment is used, resulting in an isoelectric point between 7.0 and 9.4; or Type B, when a basic pretreatment is used, resulting in an isoelectric point between 4.5 and 5.3 (Veis 1964).
Gelatin is soluble in water at temperatures above 30°C. When submitted to temperatures above its melting point, the gelatin swells, dissolves, and forms heat-reversible gels when the temperature returns to room temperature (Slade and Levine 1987). At the molecular level, the formation of a gelatin gel involves restructuring of the proteins, implying transformation from a disordered state to an ordered state, formed by triple-helix structures characteristic of collagen in the active state, the structure and physical properties of these gels being a result of the formation of microcrystalline links (Slade and Levine 1987; Achet and He 1995; Ziegler and Foegeding 1990).
Various studies involving the formation of edible and/or biodegradable films have been carried out with mammalian (Arvanitoyannis et al. 1997, 1998a, b; Lim et al. 1999; Menegalli et al. 1999; Sobral 1999; Sobral et al. 2001; Carvalho and Grosso 2004; Bertan et al. 2005; Thomazine et al. 2005; Vanin et al. 2005; Carvalho and Grosso 2006; Carvalho et al. 2008a) and fish (Jongjareonrak et al. 2006a, b; Gómez-Guillén et al. 2007; Zhang et al. 2007; Carvalho et al. 2008b) gelatin. In general, gelatin-based films show good mechanical resistance but reduced efficiency as a water vapor barrier. On the other hand, due to the hydrophilic characteristics of gelatin, these films show an elevated susceptibility to environmental conditions, making the films difficult to use as packaging materials. Various alternatives have been studied in an attempt to improve these characteristics, such as enzymatic modifications (Lim et al. 1999; Carvalho and Grosso 2004), chemical modifications (Bigi et al. 2001; Carvalho and Grosso 2004, 2006; Cao et al. 2007; Carvalho et al. 2008a), the incorporation of different types of plasticizers (Vanin et al. 2005), the use of plasticizer blends (Thomazine et al. 2005), and lipid incorporation (Bertan et al. 2005), among others. In general, no significant improvements were observed with respect to the water vapor barrier and mechanical properties.
Another alternative used in an attempt to improve the mechanical resistance of these materials was the mixture of the biopolymers with synthetic polymers (Tharanathan 2003). One very interesting synthetic polymer for this type of study is the polyvinyl alcohol (PVA), which, despite being synthetic, is hydrophilic and biodegradable (Matsumura et al. 1999). Some films based on blends of PVA with gelatin have been developed (Chiellini et al. 2001; Bergo et al. 2006; Mendieta-Taboada et al. 2008; Maria et al. 2008; Silva et al. 2008; Carvalho et al. 2009). However, all these films were colorless, that is, the inclusion of pigments to make them more attractive was not studied.
3 Chlorophylls
Chlorophylls are the most abundant natural pigments in plants. They occur in the chloroplasts of leaves and other vegetable tissues, being very common in fruits and vegetables (Streit et al. 2005; Humphrey 2004). Precursors and derivatives of the chlorophylls are used in photodynamic medical treatments (Schoefs 2002). In addition, chlorophyllian pigments are currently of great industrial importance, since they can be used both as pigments and as antioxidants, although there is controversy in the research studies with respect to the latter property (Lanfer-Marquez 2003).
Due to their color and physical properties, the chlorophylls are also used as colorants for food products (Humphrey 2004; Chattopadhyay et al. 2008). Most chlorophyll colorants exist in a water-soluble form and are used in dairy products, soups, oils, sugar confections, drinks, and cosmetics (Francis 2002).
In plants, the chlorophyll molecules are associated with proteins, carotenoids, and tocopherols by way of noncovalent interactions, which give them certain stability (Delgado-Vargas and Paredes-López 2002). After their extraction from the vegetable tissues, these pigments are chemically unstable and can easily be altered or destroyed, modifying the perception and quality of the products. In general, the chlorophylls are relatively unstable and sensitive to light, heating, oxygen, and chemical degradation (Kidmose et al. 2002; Schoefs 2002).
Chlorophylls are molecules formed from complex porphyrin derivatives, constituting the most important subgroup of pigments within the tetrapyrrole group (Fig. 28.1) (Chattopadhyay et al. 2008). Structurally, they are formed by four pyrrolic rings and a fifth isocyclic ring located at the side of the third pyrrolic ring, bonded to each other via methylenic bridges and containing one magnesium atom on their inside. A molecule of propionic acid esterified to a long chain acyclic alcohol, usually a phytol, can be found on the fourth pyrrolic ring, conferring a hydrophobic character to the chlorophyll (Delgado-Vargas and Paredes-López 2002; Francis 2002; Lanfer-Marquez 2003; Humphrey 2004; Chattopadhyay et al. 2008). Porphyrin is a stable ring-shaped molecule around which electrons are free to migrate. Because the electrons move freely, the ring has the potential to gain or lose electrons easily, and thus to provide energized electrons to other molecules. This is the fundamental process by which chlorophyll captures the energy of sunlight (Wilska-Jeszka 2007).
Two chlorophylls, chlorophyll A and chlorophyll B, are currently important as food dyes (Chattopadhyay et al. 2008). These pigments are obtained from plants and only differ with respect to the position of the functional groups –CH3 and –CHO, respectively, on carbon 3 (Kidmose et al. 2002; Humphrey 2004). These differences in the structure of the chlorophyll are sufficient to produce different wavelength absorptions and hence a variety of different green hues. The colors vary from greenish-yellow to greenish-blue, and the derivatives of these chlorophylls would be probable producers of orange hues or, under drastic chemical conditions, a red color (Delgado-Vargas and Paredes-López 2002).
Moreover, chlorophyllide is a green pigment obtained by removal of the phytyl group of chlorophyll through hydrolysis in dilute alkali or the action of chlorophyllase, and is thus water soluble (Kidmose et al. 2002; Lanfer-Marquez 2003). These chlorophylls are green in color because they absorb strongly in the red and blue regions of the visible spectrum (Kidmose et al. 2002).
4 Experimental Considerations
The films were produced from FFSs containing 2 g gelatin/100 g FFS and 25 g sorbitol/100 g gelatin. Pigskin gelatin was used, provided by Gelita South America (São Paulo, Brazil). To prepare these solutions, the gelatin was first hydrated for 30 min. at room temperature, followed by dissolution at 55°C in a water bath (TE 184-Tecnal) (Sobral et al. 2001). After dissolution the plasticizer previously dissolved in water and the chlorophyll pigment were added. A water soluble form of chlorophyll, the chlorophyllide (10% solution of chlorophyll 70008) provided by Germinal aditivos para alimentos (Cabreúva, Brazil), was used for practical reasons. To test the effect of the pigment concentrations, 0, 2, 4, 6, 8, and 10 g of chlorophyll solution/100 g gelatin were added to the FFS.
After drying in an oven with air circulation (MA 037-TECNAL) at 30°C for about 24 h, easily handled films were obtained with thicknesses between 0.075 and 0.081 mm, determined using a Mitutoyo digital micrometer (±0.001 mm). The films were then characterized after preconditioning in desiccators containing NaBr (relative humidity 58%) at 25°C for at least 7 days. The film characteristics (mechanical properties, solubility, moisture content, water vapor, and optical barrier properties) were determined in an acclimatized room with a temperature of about 22°C and relative humidity between 55% and 65%.
The moisture content of the films was determined in an oven at 105°C to constant weight. The water solubility of the films was determined after 24 h of immersion, according to Gontard et al. (1993), and expressed in terms of dissolved dry mass. Water vapor permeability was determined using the method proposed by Gontard et al. (1993). The films were fixed in cells containing silica gel, which were then placed in desiccators containing distilled water and maintained at 25°C in an oven (BOD TE 390 TECNAL) (±0.2°C). Weight gain of the system was determined at 24 h intervals, over a period of 120 h. Water vapor permeability (WVP) was calculated from (28.1) (Gontard et al. 1993).
where x is the mean film thickness (mm), A is the permeation area (cm2), ΔP is the partial vapor pressure difference between the inside of the cell (silica gel, P 1 = 0 Pa) and the distilled water (P 2 = 3,166 Pa), and the term w/t corresponds to the angular coefficient of the linear regression of the graph of mass versus time (g/h).
The mechanical properties (stress at break, elongation at break, and elastic modulus) of the films were determined by a tensile stress test using the TA.XT2i texturometer (Stable Micro Systems) with the tensile grip probe, moving at 0.9 mm/s, according to Thomazine et al. (2005). The color parameters (a*, b*, and L*), the total color difference (ΔE*), and the opacity were determined according to Sobral (1999) using the Miniscan XE (HunterLab) colorimeter. The parameters a*, b*, and L* were determined by the superimposition of the films on a white standard and the total difference in color according to (28.2) (Gennadios et al. 1996).
where ΔL* = L*standard − L*sample, Δa* = a*standard − a*sample, Δb* = b*standard − b*sample.
Opacity was determined according to Sobral (2000) with the same apparatus and computer program used to measure the color, and was calculated as the ratio between the opacity of the film superimposed on the black standard (Y b ) and that on the white standard (Y w ) (Y = Y b /Y w ). The gloss of the films was determined using the Rhopoint NGL 20/60 glossimeter at an angle of 20° according to Villalobos et al. (2005). This angle was sufficient because gelatin-based films have high gloss values (Maria et al. 2008; Silva et al. 2008). The UV and visible light barrier properties were measured at wavelengths varying from 190 to 800 nm, using a UV-Visible spectrophotometer (Biochrom, Libra S22), as described by Fang et al. (2002). The Duncan test was used to compare the means of these results (α = 5%) using the SAS computer program (version 6.8, SAS Inc., Carry, NC, USA).
The color parameters were also evaluated in relation to the variation in film thickness. This study was carried out by superimposing the films from each formulation and of known thickness, one on top of the other. A colorimeter reading was made after the addition of each film. The regression analyses were carried out using the software Statistica 8.0 (StatSoft, Inc.).
5 Physical Properties of Gelatin-Based Films Colored with Chlorophyllide
In general the chlorophyllide concentration in the FFS affected the main physical properties studied, although with no logical sequence, that is, one could not affirm that the properties presented in Table 28.1 increased or decreased as a function of increase in chlorophyllide concentration, despite observing significant differences (P < 0.05) between the means in some cases. The moisture content of the films colored with chlorophyll were similar to those determined by Sobral et al. (2001) and Thomazine et al. (2005) for gelatin-based films plasticized with sorbitol and conditioned in the same way.
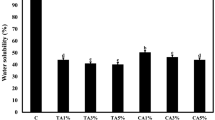
The addition of chlorophyllide did not significantly (P > 0.05) affect the water solubility of the films, independent of the concentration added. These water solubility result values were slightly higher than those observed by Carvalho and Grosso (2004, 2006) for gelatin-based (25–30 g/100 g of film) films that were modified chemically and enzymatically and plasticized with glycerol.
The water vapor permeability oscillated between 3 and 7 × 108 g mm/h cm2 Pa, with no clear behavior as a function of increase in chlorophyllide concentration in the film. Despite some significant differences (P < 0.05) observed between some of the mean values, such variation did not constitute an important effect, that is, all the films continued to be highly permeable to water vapor.
The addition of chlorophyllide to the film did affect the mechanical resistance, although there was no direct relationship between the pigment concentration and the stress at break. The observed stresses at break values were similar to those obtained by Thomazine et al. (2005) for gelatin-based films plasticized with sorbitol (25 g sorbitol/100 g of gelatin). On the other hand, it was observed that an increase in chlorophyllide concentration caused a greater variation in elongation at break. Independent of the addition of chlorophyllide, the elongation at break values was higher than that observed by Thomazine et al. (2005). With respect to the elastic modulus, the property that indicates the rigidity of the material, an increase in chlorophyllide concentration did not cause significant variations in this property. Apparently, the incorporation of chlorophyll can affect the mobility of the polymeric matrix due to its structure and possible interaction with protein, a behavior typical of plasticizing agents. In fact, such behavior can also be observed with synthetic materials. According to Durston (2006), there are limits to the amount of colored pigment that can be added to polyethylene, without affecting the mechanical properties of the film.
High gloss samples are better differentiated using measurements at smaller angles (Villalobos et al. 2005). Thus, only the results obtained at 20° were presented in Table 28.1. In general, the addition of chlorophyllide caused an increase in gloss on the film surface, indicating that the incorporation of pigment favored the morphology of the polymeric matrix, that is, decreased surface roughness as compared to pure gelatin. The films developed in the present study presented greater gloss than whey protein isolate-based films dried in a microwave dryer, with gloss values between 87 and 96 (Kaya and Kaya 2000), or hydroxypropyl methylcellulose-based films with gloss values below 100 (Villalobos et al. 2005). The opposite behavior is normally observed with synthetic materials, for which a specific coloring process should be used to prevent it from adversely affecting the surface gloss of the films (Durston 2006).
Opacity was also not significantly (P > 0.05) affected by increases in the chlorophyllide concentration in the gelatin films. Thus, it could be suggested that the inclusion of chlorophyllide in the film formulation, at the concentrations studied, did not affect the translucence of the films, which continued similar to that of pure gelatin films, which are extremely transparent (very low opacity) (Sobral 1999; Vanin et al. 2005). Since many packaging applications require the contents to be visible, film transparency is of considerable interest (Hanlon et al. 1998).
In addition, this result for opacity suggests that the chlorophyllide was completely dissolved in the polymeric matrix, since it is known that the presence of a dispersed, nonmiscible phase promotes opacity as a function of the differences in refractive indexes of the phases and the concentration and particle size of the dispersed phase (Villalobos et al. 2005).
6 UV and Light Barrier Properties of Gelatin-Based Films Colored with Chlorophyllide
Although gelatin constitutes an excellent UV light barrier (Fig. 28.2) the presence of chlorophyllide in the gelatin-based films contributed to an increase in this property, since the films showed no transmittance at 200 nm, and between 2.6 and 6.0 at 280 nm. The chlorophyllide absorbed UV radiation, having a protective effect against the degradation of the material (Hanlon et al. 1998) and the packaged foodstuff (Coltro et al. 2003). Thus, films colored with chlorophyllide show excellent UV barrier properties, suggesting that this type of material could be used to package foods rich in lipids and/or oxygen-susceptible vitamins (Bekbolet 1990; Fang et al. 2002; Artharn et al. 2007). According to Saffert et al. (2009), light is known to have a damaging effect on several foodstuffs, such as milk and other dairy products, due to the presence of light-sensitive vitamins like vitamins A and B2. The most pronounced effect of light catalyzed reactions is observed with light in the lower wavelengths of the visible spectrum and in the UV spectrum (Bekbolet 1990).
Contrarily, Shiku et al. (2004) working on films based on fish sarcoplasmic proteins, and Artharn et al. (2007), working on films based on fish muscle proteins, determined no transmittance between 200 and 280 nm. It is highly possible that this excellent capacity as a UV barrier was caused by the presence of nondissolved proteins, constituting physical barriers (Villalobos et al. 2005; Monedero et al. 2009). However, Fang et al. (2002), in working with whey-based films, obtained values for transmittance between 6 and 7 also between 200 and 280 nm. The great barrier property of gelatin-based films could be explained by the presence of amino acids containing aromatic rings in their residues, as can be seen in the aminogram determined by Sobral et al. (2001).
7 Color Characteristics of Gelatin-Based Films Colored with Chlorophyllide
The addition of chlorophyllide to the gelatin films caused significant alterations in the parameters a* and b* (Fig. 28.3), but did not affect parameter L*, remaining practically constant at about 91, which is almost equivalent to the parameter of the white standard used as the support.
The parameter a*, which varies from green (−) to red (+) decreased linearly with the pigment concentration (C p ) (28.3), indicating, effectively, that the material became greener with increase in concentration of the pigment as expected. On the other hand, the parameter b*, which varies from blue (−) to yellow (+), increased linearly with increase in the pigment concentration (28.4), suggesting that the films presented a yellowy green coloration.
The values of the color parameters determined in the present study were similar to those obtained by López-Carballo et al. (2008), who characterized edible films prepared with 10 g of gelatin/100 mL, 2.5 g glycerol/100 mL and 80 μg of chlorophyllin E-140 or E-141/mL: a*= −3.2 and −4.4, b* = 7.2 and 4.6, and L* = 85 and 87, respectively. According to these authors, the films had a vivid green-yellow color as compared to the gelatin films.
An increase in chlorophyllide concentration also caused a linear increase (28.5) in the total color difference (Fig. 28.4), signifying that the films containing additive evidently became more colored with greater pigment concentration.
Nevertheless, the values for ΔE* were not as high as those that would have been expected from the presence of pigment in the formulation. Although the gelatin films with added chlorophyll were more colored than films based on various other proteins without the addition of pigments, such as gelatin (Vanin et al. 2005) and ovoalbumins (Gennadios et al. 1996), they were apparently less colored than other films, also those containing no pigments such as films based on Nile Tilapia myofibrillar proteins (Sobral 2000) and soy proteins (Kunte et al. 1997) (Table 28.2).
However, despite the behavior of increasing coloration with increasing chlorophyll concentration in the film formulation, the coloration was practically imperceptible to the eye. According to Nassau (1996), the appearance of color as detected by the eye and interpreted by the brain depends significantly on the exact viewing circumstances, including sample transparency. In fact, the color of a pigmented medium, such as a plastic, depends on both transmittance and reflectance, which in turn are different if the material is opaque or transparent (Saunderson 1942). Moreover, according to Hanlon et al. (1998), in some plastics the thickness of translucent material has a direct bearing on the chroma {C* = [(a*)2 + (b*)2]0.5}.
Thus, considering that, although transparent, the opacity of gelatin films increases with increase in thickness (Sobral 1999), the perception of the color of the material would be different depending on the thickness of the films. Thus, analyses of the color of superimposed films, simulating films with increasing thickness, allowed for the confirmation that the thickness effectively influenced film coloration.
Figures 28.5 and 28.6 show that the parameters a*, b*, L* and the total color difference (ΔE*) varied linearly with the thickness (x) of the films, with different slopes, depending on the chlorophyllide concentrations (C p ) in the film formulation. Thus, these data were submitted to a multilinear regression analysis to observe both effects of x and C p (28.6)–(28.9):
The quality of fitting can be also observed in Fig. 28.7, where it is possible to observe the capacity of all models to predict the experimental data. The data are closely banded around the straight line with a slope of 45°, which indicates the effectiveness of the (28.6)–(28.9) in describing the effect of pigment concentration and thickness on color parameters.
The linear behavior between the total color difference and thickness of biopolymer-based films was verified by Sobral (1999, 2000) working with gelatin and Tilapia myofibrillar protein films (respectively). However, no paper was found in the specialized literature on the effect of the concentration of a pigment on the actual effect of the thickness.
Moreover, it can be noted now that the thicker films (x→0.400 mm) presented very high color difference values (ΔE* ≅ 23), much higher than those observed for colorless films (Table 28.2).
8 Conclusion
The use of a water-soluble pigment such as chlorophyll in the form of chlorophyllide is perfectly viable in edible film technology. The addition of this pigment does not affect the principal physical or functional properties of the material as compared to pure gelatin. However, perception of the green coloration is difficult, since the material is transparent. An increase in film thickness could contribute to a better perception of the color provided by the pigment.
References
Achet DÇ, He XW (1995) Determination of the renaturation level in gelatin films. Polymer 36(4):787–791
Artharn A, Benjakul S, Prodpran T, Tanaka M (2007) Properties of a protein-based film from round scad (Decapterus maruadsi) as affected by muscle types and washing. Food Chem 103:867–874
Arvanitoyannis IS (2002) Formation and properties of collagen and gelatin films and coatings. In: Gennadios A (ed) Protein-based films and coatings. CRC Press, Boca Raton, pp 275–304
Arvanitoyannis I, Psomiadou E, Nakayama A, Aiba S, Yamamoto N (1997) Edible films made from gelatin, soluble starch and polyols, Part 3. Food Chem 60(4):593–604
Arvanitoyannis I, Nakayama A, Aiba S (1998a) Edible films made from hydroxypropyl starch and gelatin and plasticized by polyols and water. Carbohydr Polym 36:105–119
Arvanitoyannis I, Nakayama A, Aiba S (1998b) Chitosan and gelatin-based edible films: state diagrams, mechanical and permeation properties. Carbohydr Polym 37:371–382
Banker GS (1966) Film coating, theory and practice. J Pharmacol Sci 55:81–89
Bekbolet M (1990) Light effects on food. J Food Prot 53:430–440
Bergo PV, Carvalho RA, Sobral PJA, Bevilacqua FRS, Pinto JKC, Souza JP (2006) Microwave insertion loss measurements in gelatin-based films. Meas Sci Technol 17:3261–3264
Bertan LC, Tanada-Palmu PS, Siani ACC, Grosso RF (2005) Effect of fatty acids and ‘Brazilian elemi’ on composite films based on gelatin. Food Hydrocolloids 19(1):73–82
Bigi A, Cojazzi G, Panzavolta S, Rubini K, Roveri N (2001) Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 22(7):763–768
Bourtoom T, Chinnan MS, Jantawat P, Sanguandeeku R (2006) Effect of select parameters on the properties of edible film from water-soluble fish proteins in surimi wash-water. Lebensmittel Wiss Technol 39:405–418
Cao N, Fu Y, He J (2007) Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocolloids 21(4):575–584
Carvalho RA, Grosso CRF (2004) Characterization of gelatin based films modified with transglutaminase, glyoxal and formaldehyde. Food Hydrocolloids 18:717–726
Carvalho RA, Grosso CRF (2006) Properties of chemically modified gelatin films. Braz J Chem Eng 23(1):45–53
Carvalho RA, Grosso CRF, Sobral PJA (2008a) Effect of chemical treatment on the mechanical properties, water vapour permeability and sorption isotherms of gelatin-based films. Packag Technol Sci 21:165–169
Carvalho RA, Sobral PJA, Thomazine M, Habitante AMQB, Giménez B, Guillen CG, Montero P (2008b) Development of edible films based on differently processed Atlantic halibut (Hippoglossus hippoglossus) skin gelatin. Food Hydrocolloids 22:1117–1123
Carvalho RA, Moraes ICF, Bergo PVA, Kamimura ES, Habitante AMQB, Sobral PJA (2009) Study of some physical properties of biodegradable films based on blends of gelatin and poly(vinyl alcohol) using a response-surface methodology. Mater Sci Eng, C 29:485–491
Chattopadhyay P, Chatterjee S, Sen SK (2008) Biotechnological potential of natural food grade biocolorants. Afr J Biotechnol 7(17):2972–2985
Chiellini E, Cinelli P, Fernandes EG, Kenawy ES, Lazzeri A (2001) Gelatin-based blends and composites. Morphological and thermal mechanical characterization. Biomacromolecules 2:806–811
Coltro L, Padula M, Saron ES, Borghetti J, Buratin AEP (2003) Evaluation of a UV absorber added to PET bottles for edible oil packaging. Packag Technol Sci 16(1):15–20
Corat M, Carvalho RA, Trindade CSF, Sobral PJA (2007) Produção e caracterização de filmes a base de gelatina coloridos com clorofila. Alimentos: Ciencia e Ingeniería 16:94–96
Delgado-Vargas F, Paredes-López O (2002) Natural colorants for food and nutraceutical uses. CRC Press, Boca Raton
Durston J (2006) Flexible package closures and sealing systems. In: Theobald N, Winder B (eds) Package closures and sealing systems. CRC Press, Boca Raton, pp 204–230
Fang Y, Tung MA, Britt IJ, Yada S, Dalgleish DG (2002) Tensile and barrier properties of edible films made from whey proteins. J Food Sci 67:188–193
Francis FJ (2002) Food colorings. In: MacDougall DB (ed) Colour in food. CRC Press, New York, pp 307–340
Gennadios A, McHugh TH, Weller CL, Krochta JM (1994) Edible coating and films based on proteins. In: Krochta JM, Baldwin EA, Nisperos-Carriedo MO (eds) Edible coatings and to improve food quality. Technomic Publishing Company, Lancaster, pp 201–277
Gennadios A, Weller CL, Handa MA, Froning GW (1996) Mechanical properties of egg albumen films. J Food Sci 61(3):585–589
Gómez-Estaca J, Giménez B, Montero P, Gómez-Guillén MC (2009) Incorporation of antioxidant borage extract into edible films based on sole skin gelatin or a commercial fish gelatin. J Food Eng 92(1):78–85
Gómez-Guillén MC, Ihl M, Bifani V, Silva A, Montero P (2007) Edible films made from tuna-fish gelatin with antioxidant extracts of two different murta ecotypes leaves (Ugni molinae Turcz). Food Hydrocolloids 21:1133–1143
Gontard N, Guilbert S (1996) Bio-packaging: technology and properties of edible and/or biodegradable material of agricultural origin. Boletim da SBCTA 30(1):3–15
Gontard N, Guilbert S, Cuq J-L (1993) Water and glycerol as plasticizers effect mechanical and water vapor barrier properties of an edible wheat gluten film. J Agr Food Chem 58:206–211
Hanlon JF, Kelsey RJ, Forcinio HE (1998) Handbook of package engineering, 3rd edn. Technomic Publishing Company Inc., Lancaster
Humphrey AM (2004) Chlorophyll as a color and functional ingredient. J Food Sci 69(5):C422–C425
Jongjareonrak A, Benjakul S, Visessanguan W, Prodpran T, Tanaka M (2006a) Characterization of edible films from skin gelatin of brownstripe red snapper and bigeye snapper. Food Hydrocolloids 20(4):492–501
Jongjareonrak A, Benjakul S, Visessanguan W, Tanaka M (2006b) Effects of plasticizers on the properties of edible films from skin gelatin of bigeye snapper and brownstripe red snapper. Eur Food Res Technol 222:229–235
Kaya S, Kaya A (2000) Microwave drying effects on properties of whey protein isolate edible. J Food Eng 43:91–96
Kidmose U, Edelenbos M, Nørbæk R, Christensen LP (2002) Colour stability in vegetables. In: MacDougall DB (ed) Colour in food. CRC Press, New York, pp 189–242
Krochta JM, De-Mulder-Johnston CD (1997) Edible and biodegradable polymer films: challenges and opportunities. Food Technol 51:61–74
Kunte LA, Gennadios A, Cuppett SL, Hanna MA, Weller CL (1997) Cast films from soy protein isolates and fractions. Cereal Chem 74(2):115–118
Lanfer-Marquez UM (2003) O papel da clorofila na alimentação: uma revisão. Braz J Pharm Sci 39(3):227–242
Lim LT, Mine Y, Tung A (1999) Barrier and tensile properties of transglutaminase cross-linked gelatin films as affect by relative humidity, temperature, and glycerol content. J Food Sci 64(4):616–622
López-Carballo G, Hernández-Muñoz P, Gavara R, Ocio MJ (2008) Photoactivated chlorophyllin-based gelatin films and coatings to prevent microbial contamination of food products. Int J Food Microbiol 126:65–70
Maria TMC, Carvalho RA, Sobral PJA, Habitante AMBQ, Solorza-Feria JS (2008) The effect of the degree of hydrolysis of the PVA and the plasticizer concentration on the color, opacity, and thermal and mechanical properties of films based on PVA and gelatin blends. J Food Eng 87:191–199
Matsumura S, Tomizawa N, Toki A, Nishikawa K, Toshima K (1999) Novel poly(vinyl alcohol)-degrading enzyme and the degradation mechanism. Macromolecules 32:7753–7761
Mendieta-Taboada OWM, Sobral PJA, Carvalho RA, Habitante AMBQ (2008) Thermomechanical properties of biodegradable films based on blends of gelatin and poly(vinyl alcohol). Food Hydrocolloids 22:1485–1492
Menegalli FC, Sobral PJA, Roques M, Laurent S (1999) Characteristics of gelatin biofilms in relation to drying process conditions near melting. Drying Technol 17:1697–1706
Micard V, Balamri R, Morel M-H, Guilbert S (2000) Properties of chemically and physically treated wheat gluten films. J Agric Food Chem 48(7):2948–2953
Monedero FM, Fabra MJ, Talens P, Chiralt A (2009) Effect of oleic acid-beeswax mixtures on mechanical, optical and water barrier properties of soy protein isolate based films. J Food Eng 91(4):509–515
Nassau K (1996) Fundamentals of color science. In: Nassau K (ed) Color for science, art and technology. North Holland, Lebanon, pp 1–30
Nisperos-Carriedo MO (1994) Edible coatings and films based on polysaccharides. In: Krochta JM, Baldwin EA, Nisperos-Carriedo M (eds) Edible coatings and films to improve food quality. Technomic Publishing Company, Lancaster, pp 305–336
Paschoalick TM, Garcia FT, Sobral PJA, Habitante AMQB (2003) Characterization of some functional properties of edible films based on muscle proteins of Nile Tilapia. Food Hydrocolloids 17:419–427
Saffert A, Pieper G, Jetten J (2009) Effect of package light transmittance on the vitamin content of milk, part 3: fortified UHT low-fat milk. Packag Technol Sci 22:31–37
Saunderson JL (1942) Calculation of the color of pigmented plastics. J Opt Soc Am 32(12):727–729
Schoefs B (2002) Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci Technol 13:361–371
Shiku Y, Hamaguchi PY, Benjakul S, Visessanguan W, Tanaka M (2004) Effect of surimi quality on properties of edible films based on Alaska Pollack. Food Chem 86:493–499
Silva GGD, Sobral PJA, Carvalho RA, Bergo PVA, Mendieta-Taboada OW, Habitante AMQB (2008) Biodegradable films based on blends of gelatin and poly (vinyl alcohol): effect of PVA type or concentration on some physical properties of films. J Polym Environ 16:276–285
Slade L, Levine H (1987) Polymer-chemical properties of gelatin in foods. In: Pearson AM, Dutson TR, Bailey AJ (eds) Advances in meat research, collagen as a food. Elsevier Applied Science, London, pp 251–266
Sobral PJA (1999) Propriedades funcionais de biofilmes de gelatina em função da espessura. Ciência & Engenharia 8:60–67
Sobral PJA (2000) Influência da espessura sobre certas propriedades de biofilmes à base de proteínas miofibrilares. Pesqui Agropecu Bras 35:1251–1259
Sobral PJA, Menegalli FC, Hubinguer MD, Roques MA (2001) Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocolloids 15:423–32
Streit MN, Canterle LP, Canto MW, Hecktheuer LHH (2005) As clorofilas. Ciência Rural 35:748–755
Tapia-BLácido DT, Mauri A, Menegalli FC, Sobral PJA, Añon MC (2007) Contribution of the starch, protein and lipid fractions to the physical, thermal and structural properties of amaranth (amaranthus caudatus) flour films. J Food Sci 72:E293–E300
Tharanathan RN (2003) Biodegradable films and composite coatings: past, present and future. Trends Food Sci Technol 14:71–78
Thomazine M, Carvalho RA, Sobral PJA (2005) Physical properties of gelatin films plasticized by blends of glycerol and sorbitol. J Food Sci 70:172–176
Torres JA (1994) Edible films and coatings from proteins. In: Hettiarachy NS, Ziegler GR (eds) Protein functionality in food systems. Marcel Dekker, New York, pp 467–507
Van de Velde K, Kiekens P (2002) Biopolymers: overview of several properties and consequences on their applications. Polym Test 21(4):433–442
Vanin FM, Sobral PJA, Menegalli FC, Carvalho RA, Habitante AMQB (2005) Effects of plasticizers and their concentrations on thermal and functional properties of gelatin based films. Food Hydrocolloids 70:172–176
Veis A (1964) The molecular characterization of gelatin. In: The macromolecular chemistry of gelatin. Academic, New York
Villalobos R, Chanona J, Hernández P, Gutiérrez G, Chiralt A (2005) Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocolloids 19:53–61
Wilska-Jeszka J (2007) Food Colorants. In: Sikorski ZE (ed) Chemical and functional properties of food components. CRC Press, Boca Raton, pp 245–274
Zhang S, Wang Y, Herring JL, Oh J-H (2007) Characterization of edible film fabricated with channel catfish (Ictalurus punctatus) gelatin extract using selected pretreatment methods. J Food Sci 72(9):C498–C503
Ziegler CR, Foegeding EA (1990) The gelation of proteins. Adv Food Nutr Res 34:203–298
Acknowledgements
to the Foundation for Research Support of the State of São Paulo (FAPESP) and the National Council for Scientific and Technological Development (CNPq) for their support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer New York
About this paper
Cite this paper
Sobral, P.J.A., Carvalho, R.A., Fávaro-Trindade, C.S. (2010). Physical Properties of Edible Gelatin Films Colored with Chlorophyllide. In: Aguilera, J., Simpson, R., Welti-Chanes, J., Bermudez-Aguirre, D., Barbosa-Canovas, G. (eds) Food Engineering Interfaces. Food Engineering Series. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-7475-4_28
Download citation
DOI: https://doi.org/10.1007/978-1-4419-7475-4_28
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-7474-7
Online ISBN: 978-1-4419-7475-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)