Abstract
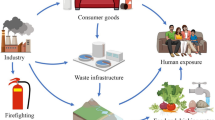
Perfluorinated compounds (PFCs) are ubiquitous environmental contaminants, which persist and may bioaccumulate through the food chain (Haukås et al. 2007; Martin et al. 2004b; Taniyasu et al. 2003). As a consequence, several PFCs have been detected in different biota worldwide. In recent years, an increasing number of papers report high levels of PFCs in blood, tissues, and breast milk from both occupationally and non-occupationally exposed human populations (Kannan et al. 2004; Kärrman et al. 2007; Olsen et al. 2007). The most important exposure pathways of perfluorinated compounds for humans are thought to be intake of drinking water and food and inhalation of dust (Björklund et al. 2009; Ericson et al. 2008a). Due to the widespread distribution, environmental degradation, and metabolism of the PFCs released into the environment, a very complex exposure situation exists (Fromme et al. 2007a). As a result, the relative contribution to human exposure from different routes or from a single source (e.g., diet) is not yet known. More specifically, it is currently unknown as to what extent exposure to drinking water, food, or dust contributes to the PFCs measured in human breast milk and blood. Moreover, data on levels of PFCs in the human diet are rather scarce (Kärrman et al. 2009; Tittlemier et al. 2006, 2007). Only PFC levels in fish appear to be well documented (Houde et al. 2006). Few studies, however, report the levels of PFCs in drinking water or human food such as vegetables, meat, and eggs (FSA 2006; US EPA 2001). Food processing such as cooking (boiling, baking, or grilling) could alter the concentration of PFCs in food and as a consequence affect the risk for humans.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Perfluorinated compounds (PFCs) are ubiquitous environmental contaminants, which persist and may bioaccumulate through the food chain (Haukås et al. 2007; Martin et al. 2004b; Taniyasu et al. 2003). As a consequence, several PFCs have been detected in different biota worldwide. In recent years, an increasing number of papers report high levels of PFCs in blood, tissues, and breast milk from both occupationally and non-occupationally exposed human populations (Kannan et al. 2004; Kärrman et al. 2007; Olsen et al. 2007). The most important exposure pathways of perfluorinated compounds for humans are thought to be intake of drinking water and food and inhalation of dust (Björklund et al. 2009; Ericson et al. 2008a). Due to the widespread distribution, environmental degradation, and metabolism of the PFCs released into the environment, a very complex exposure situation exists (Fromme et al. 2007a). As a result, the relative contribution to human exposure from different routes or from a single source (e.g., diet) is not yet known. More specifically, it is currently unknown as to what extent exposure to drinking water, food, or dust contributes to the PFCs measured in human breast milk and blood. Moreover, data on levels of PFCs in the human diet are rather scarce (Kärrman et al. 2009; Tittlemier et al. 2006, 2007). Only PFC levels in fish appear to be well documented (Houde et al. 2006). Few studies, however, report the levels of PFCs in drinking water or human food such as vegetables, meat, and eggs (FSA 2006; United States Environmental Protection Agency (US EPA) 2001). Food processing such as cooking (boiling, baking, or grilling) could alter the concentration of PFCs in food and as a consequence affect the risk for humans.
In this review, we summarize the existing data on the levels of perfluorinated compounds that appear in the human diet and in drinking water. Additionally, an overview will be given on what data are available in the literature on PFCs in indoor (house) dust. These overviews are designed to provide a current picture of the contributions PFCs make to human residue burdens by major routes of exposure.
Data were mainly obtained from peer-reviewed articles published up to March 2009. The literature search was performed through use of the web databases ISI Web of Knowledge and Science Direct. Furthermore, data from reports (FSA 2006; US EPA 2001; Schrap et al. 2004; Van den Heuvel-Greve et al. 2006) are included in the review. The collected data consisted mainly of PFC levels expressed as mean concentrations or data ranges.
This chapter will be divided into four parts. In the first part, concentrations of PFCs in edible aquatic species are listed. In the second part the data on PFCs in composite food samples, vegetables, meat, and other food are reviewed. Part three gives the data on levels in drinking water, and finally, in part four the levels in indoor dust are reported.
2 PFCs in Edible Fish and Seafood
It has been well documented that perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) may bioaccumulate in fish and that accumulation of PFCs with chain lengths of C8–C15 in fish tends to increase with increasing chain length (Martin et al. 2003b, 2004b; de Vos et al. 2008). Therefore, fish are potentially an important dietary source of PFCs for humans. In general, muscle is the main part of the fish body that is consumed by humans. Considering the fact that humans prefer muscle tissue, we mainly focus in this chapter on levels of perfluorinated compounds in such fish tissue. A summary of perfluorinated compounds, especially PFOS, PFOA, perfluorononanoic acid (PFNA) and perfluorooctanesulfonamide (PFOSA), that have been found in fish muscle and/or in whole fish homogenates of edible species is illustrated in Table 1. Among PFC fish contaminants, PFOS is the most crucial and prominent compound found. Reports suggest no considerable differences in PFC concentrations among freshwater and marine fish species. However, the highest mean PFOS concentration (170 ng/g wet weight (wwt)) was detected in lake trout, collected from Lake Ontario (Martin et al. 2004b). Other PFOS concentrations in lake trout from the Great Lakes ranged from 16 (Lake Michigan) to 121 ng/g wwt (Lake Erie) (Furdui et al. 2007).
The PFOS concentration in lake trout from Lake Ontario increased significantly from 43 to 180 ng/g wwt in the period 1980–2001 (Martin et al. 2004b). This temporal trend was not confirmed by the study of Furdui et al. (2007), wherein an average PFOS concentration of 46 ng/g wwt was measured in lake trout collected from Lake Ontario, in 2001.
Hitherto, only limited data were available from the same species collected at the same site in different periods; therefore, it is very difficult to draw conclusions on temporal changes of PFC contaminants in fish tissue.
PFOA is the second most perfluorinated compound that is most often subjected to analysis in fish. It has been shown that PFOA is detected at much lower concentrations than is PFOS. As a consequence, in most studies the concentrations of PFOA recorded in fish tissue remained below the detection limit. However, quantifiable concentrations were detected in lake trout (Martin et al. 2004b; Furdui et al. 2007), rainbow smelt (Martin et al. 2004b), and alewife, with concentrations ranging from 0.16 to 6.8 ng/g wwt. The difference between the observed PFOS and PFOA concentrations in fish suggests a lower potential of PFOA to bioaccumulate in fish as compared to PFOS. This observation was further confirmed by laboratory experiments, which revealed a 1,000-fold lower bioconcentration factor for PFOA compared to PFOS (Martin et al. 2003b; Gruber et al. 2007).
A restricted number of studies also reported perfluorohexane sulfonate (PFHxS), perfluoroundecanoic acid (PFUnA), perfluorodecanoic acid (PFDA), perfluoroheptanoic acid (PFHpA), and perfluorohexanoic acid (PFHxA) in fish muscle (Giesy and Kannan 2001; Kannan et al. 2005; Gulkowska et al. 2006; Nakata et al. 2006; Furdui et al. 2007; Ye et al. 2008), but these compounds were found above the detection limit in only a few cases. For example, Ye et al. (2008) detected PFHxS at a maximum concentration of 1.89 ng/g wwt in a mixture of whole fish in the Missouri River, USA. Concentrations of the other PFCs analyzed in this study were all generally at least one order of magnitude lower than PFOS levels and had an overall median concentration of 3.71 (PFHxA), 0.82 (PFDA), and 0.36 ng/g (PFHxS) wwt. Notably, high concentrations of the other PFCs in fish were also broadly dispersed among the rivers investigated, including PFHxS at 8.14 ng/g (Missouri), PFUnA at 48.0 ng/g (Mississippi), and PFDA at 9.01 ng/g and PFHxA at 18.4 ng/g wwt, both from the Ohio River (Ye et al. 2008). In whole body homogenates of lake trout collected from the Great Lakes in 2001, the highest values found were 4.9 ng/g wwt for PFDA, 3.5 ng/g wwt each for PFUnA and perfluorotridecanoic acid (PFTrA), and 9.8 ng/g wwt for perfluorodecane sulfonate (PFDS). An overview of analyses from long-chain perfluorinated acids (= C >10) in fish is given in Table 1.
Martin et al. (2004b) detected relatively high mean concentrations of the longer-chain PFCs in fish collected from Lake Ontario, Canada. The highest concentration of these PFCs was 8.3 ng/g wwt for PFUnA. These authors concluded that individual PFCs were generally detected at lower concentrations than were PFOS, and total PFOS equivalents (PFOS and PFOSA) exceeded the sum of all PFCs by a factor of between 1.8 and 12 within each species analyzed. This pattern was similar to the relative abundance of both fluorochemical classes in Arctic animals (Martin et al. 2004a) and in lake trout collected from the Great Lakes (Furdui et al. 2007).
Tomy et al. (2004b) detected a relatively high mean concentration (92.8 ng/g wwt) of N-ethyl perfluorooctane sulfonamide (N-EtPFOSA) in Arctic cod, ranging between 9.6 and 144.6 ng/g wwt. Since transformation of N-EtPFOSA to PFOS and PFOSA by rainbow trout microsomes has been reported (Tomy et al. 2004a), N-EtPFOSA is an important compound to measure in biota and in human samples. However, up to now, the available data on N-EtPFOSA levels in living biota have been limited.
Although several authorities recommend not eating fish liver because of the risk associated with high intake of persistent organic pollutants (POPs), fish liver (and oil) is still consumed in a limited number of countries (e.g., Norway, Japan, and Iceland). Therefore, it is interesting to note that PFC levels in liver are at least two orders of magnitude higher than exists in muscle tissue (Nania et al. 2009). In liver of tuna from the Mediterranean Sea, PFOS concentrations up to 87 ng/g wwt were detected (Kannan et al. 2002). In Japan, concentrations of total PFCs in skipjack tuna livers ranged from <1 to 83 ng/g wwt (Hart et al. 2008). PFOS and PFUnA were the prominent compounds found.
Similar to fish, PFOS is the dominant PFC found in edible aquatic invertebrates such as shrimp, mussels, clams, and oysters (Table 1). Extremely high values were measured in shrimp collected in 2001 from the North Sea and the Western Scheldt (Belgium), which had mean PFOS concentrations of 139 and 215 ng/g wwt, respectively (Van de Vijver et al. 2003). However, in 2005, in samples collected from the same sites, the mean PFOS concentration had decreased to 29 ng/g wwt in shrimp from the Western Scheldt and was below the detection limit in shrimp from the North Sea (Schrap et al. 2004; Van den Heuvel-Greve et al. 2006). It is worth noting that, in the latter study, peeled rather than unpeeled shrimp were analyzed; however, it seems unlikely that this is the reason for the observed difference. Levels of PFOS in shrimp from the Western Scheldt were much higher compared to levels in shrimp collected from the Arctic Ocean and in China (i.e., 0.35 and 7.42 ng/g wwt, respectively (Tomy et al. 2004b; Gulkowska et al. 2006)).
A few papers report on PFC levels in bivalves. Kannan et al. (2002) measured PFOS concentrations up to 1,225 ng/g dry weight (dwt) in oysters collected in the Gulf of Mexico and in the Chesapeake Bay in the USA. Other studies on oysters reported much lower concentrations (6.0 and <1 ng/g wwt) from the Ariake Sea (Nakata et al. 2006) and China (Gulkowska et al. 2006), respectively. Cunha et al. (2005) measured PFOS in mussels from several estuaries in the North of Portugal. They detected high concentrations in all the investigated estuaries; the minimum level found was 36.8 and the maximum was 126.0 ng/g wwt. With the exception of the PFOS concentration measured in Portugal (Cunha et al. 2005), the average concentration (0.54 ng/g wwt) in mussels from this location was similar to that found in oysters collected in China and Japan. The PFOA values in mussels were generally in the same range as was PFOS, except for the mussels from the Ariake Sea in Japan, in which the PFOA concentration was 10 times higher (9.5 ng/g wwt; Nakata et al. 2006). In the Nania et al. (2009) study, higher PFOA than PFOS levels were found in clam but comparable levels were found in mussels, which was attributed to differences in habitat and feeding behavior. In contrast, Gulkowska et al. (2006) reported lower PFOA than PFOS concentrations in clams (Venus sp.).
Generally, the concentrations in the other molluscs follow the same trend as observed for mussels and oysters, i.e., PFC concentrations are lower than 1 ng/g wwt. This is consistent with the observations made in China, Belgium, Japan, and in the Artic Ocean. Only in Venus sp., collected from the Mediterranean Sea, was the mean PFOA concentration higher (15 ng/g wwt). In squid and cuttlefish, the measured PFOS and PFOA concentrations were slightly higher than those observed in bivalves; the observed range was between 0.31 and 3 ng/g wwt (Gulkowska et al. 2006; Nania et al. 2009).
Unfortunately, data on other PFCs in edible invertebrates are rare. Gulkowska et al. (2006) did find the following PFC components: PFHxS, PFUnA, PFDA, PFHpA, and PFHxA. Among perfluorocarboxylic acids, residues of PFOA and PFUnA were the most prominent compounds found in seafood. Quantifiable concentrations of PFOA were found in all crab and mollusc tissues and two shrimp species at a mean concentration of 0.48 ng/g wwt. PFUnA was found at a slightly greater mean concentration of 0.52 ng/g wwt (Gulkowska et al. 2006). This trend was in accord with the bioaccumulation potential (BCP) of perfluoroalkyl carboxylates (PFCAs), whereby bioaccumulation increases with increasing perfluoroalkyl chain length (Martin et al. 2003a, b). The widespread occurrence of long-chain PFCAs has been examined in the Canadian Artic (Martin et al. 2004a). The contamination profile for fish was similar to that observed by Gulkowska et al. (2006); the dominant PFCA in all fish was PFUnA, but lower concentrations were recorded for both longer- and shorter-chain homologs. Tomy et al. (2004b) detected relatively high mean concentrations of N-EtPFOSA in clams and in shrimp of, respectively, 20.1 and 10.4 ng/g wwt.
In general, concentrations of PFCs are expected to increase with increasing trophic level. This trend has been observed in the Great Lakes food chain (Kannan et al. 2005). However, higher concentrations of perfluoroalkyl contaminants were reported in lower trophic levels in seafood from China (Gulkowska et al. 2006) and in invertebrate species from Lake Ontario (Martin et al. 2004b). Several literature findings suggest that benthic organisms do not biomagnify PFCs to the extent that pelagic ones do, but so far, the overall picture is still inconclusive. Martin et al. (2004b) observed strong associations between PFC levels in pelagic species (Mysis, alewife, smelt, and lake trout) and trophic level, if benthic species (Diporeia and sculpin) were excluded from the regression analysis. Kidd et al. (2001) previously demonstrated that there is a divergence between pelagic and benthic-source biomagnification for persistent organochlorines due to the higher carbon turnover rate of benthic species. Martin et al. (2004b) observed statistically significant trends in the pelagic food web, whereby PFOS, PFDA, PFUnA, and PFTrA concentrations increased with increasing trophic level, suggesting that biomagnification occurred. In the benthic macroinvertebrate Diporeia, however, which occupied the lowest trophic level of all the organisms analyzed, the highest mean concentration of each perfluorinated contaminant was detected. This suggests that the major source of PFCs was the sediment, not the water (Martin et al. 2004b). This may be a result of the sorption of perfluorinated acids or neutral perfluoroalkyl substances to organic matter, followed by sedimentation and subsequent uptake by benthic invertebrates. Sorption coefficients of PFCs are relatively low for C4–C8-carboxylic acids and increase with increasing chain length (de Voogt et al. 2006). On the other hand, Tomy et al. (2004b) suggested that exposure concentrations are greater in the water column, because they observed higher concentrations in zooplankton compared to benthic invertebrates in an Arctic marine food web.
Biomagnification of PFOS in the estuarine food chain of the Western Scheldt estuary was observed by de Vos et al. (2008). The magnification ratios increase in primary and primary–secondary carnivores (carnivores that feed on both herbi-detritivores and primary carnivores), with the exception of primary–secondary carnivores to Sterna hirundo.
It is not clear if there is a difference between the concentrations of PFCs in edible fish from remote versus highly industrialized or urbanized areas or not. However, Gulkowska et al. (2006) observed slightly higher PFOS concentrations in fish from the highly urbanized and industrialized Guangzhou region compared to the concentration found in the more remote Zhoushan region.
In a few studies, positive correlations were found between PFC body burdens and self-reported fish consumption. In Poland, blood samples from 45 donors living near the Baltic Sea were analyzed in 2004 (Falandysz et al. 2006). Groups of people with a high consumption of regionally captured fish showed statistically higher PFC blood levels than the groups who consumed less regionally captured fish. The authors concluded that the consumption of seafood was an important determinant for internal PFC exposure. Results of another study, carried out in North Rhine-Westphalia area, also indicated a positive association between PFOS concentrations in plasma and consumption of locally caught fish, indicating that fish intake can be an important pathway for internal PFC exposures (Hölzer et al. 2008).
In recognition of the potential for human exposure to PFCs via fish consumption, the Minnesota Department of Health has recently issued fish consumption advisories for contaminated sections of the Mississippi River (Minnesota Department of Health 2007). This advisory suggests that people limit their intake of fish to no more than one meal a week, if PFOS levels in fillet exceed 38 ng/g. It is therefore interesting to note that 78.6% of the whole fish homogenates given in Table 1 had PFOS levels that exceeded that threshold. Of the muscle samples, at least five mean concentration values exceeded 38 ng/g. The relationship between measurements made with homogenates and fillets has not been examined, but the portion of all the analyzed samples that exceeded the advisory limit indicates that consumption of fish may be a route of PFC exposure that needs further evaluation.
Assuming a daily intake for fish of 50 g/d with a PFOS concentration at the advisory limit of 38 ng/g, we can calculate that humans would obtain a daily intake of 1.9 μg/d just for fish. The provisional tolerable daily intake (TDI) values proposed by the European Food Safety Authority (EFSA 2008) and Health Protection Agency (HPA 2009) amount to 150 ng/kg body weight (bwt)/d and 300 ng/kg bwt/d, respectively. For a person of 60 kg weight, this would mean a TDI of 9 and 18 μg/d, which is almost 10 times more than the estimated PFOS intake of fish (1.9 μg/d), based on the advisory limit. As this estimation is only based on fish, it is clear that the daily intake will be higher if other food and beverages are taken into account. Furthermore, the intake via non-dietary routes (e.g., dust) should also be included and would add to the total intake value.
3 Contamination of Food
Very few reports in the literature focus on concentrations of PFCs in food items. Nevertheless, it is likely that diet is an important source of human exposure to PFCs. The widespread occurrence of PFOS and PFOA in children and adults suggests that exposure results from a common source for all age groups. A wide variety of industrial and consumer applications for PFCs exist and lead to numerous possibilities for release into the environment, with subsequent human exposure via environmental routes. However, there are also more direct routes, e.g., dietary exposure, by which humans may be exposed to perfluorinated compounds (Tittlemier et al. 2007; Vestergren et al. 2008; Fromme et al. 2009; Nania et al. 2009). Vestergren et al. (2008) concluded that consumption of contaminated food and drinking water constitutes the major pathway for humans.
3.1 Indirect Contamination of PFCs in Food Items
PFCs are widely used in food-packaging coatings. Some formulations are utilized in food packaging to form grease- and water-repellent coatings for papers and paperboards. In such coatings, the mixtures of perfluorooctylsulfonyl phosphate esters used include heptadecafluoro-1-octanesulfonamide (PFOSA), N-ethyl-heptadecafluoro-1-octanesulfonamide (N-EtPFOSA) and N,N-diethyl-heptadecafluoro-1-octanesulfonamide (N,N-Et2PFOSA). Perfluorooctylsulfonyl compounds may be present as manufacturing residuals in coatings and may migrate into food upon contact. Laboratory studies also indicate that some perfluorooctylsulfonyl compounds can be metabolized to PFOS (Tomy et al. 2004a; Xu et al. 2004).
Begley et al. (2005) investigated several potential sources of migration from food packaging. The most recognizable products to consumers are the uses of perfluorochemicals in non-stick coatings (polytetrafluoroethylene or PTFE) for cookware and also their use in paper coatings for oil and moisture resistance. PFOS is a residual impurity in some paper coatings used for food contact and PFOA is a processing aid in the manufacture of PTFE. Results from these authors showed that the largest potential source of PFCs from food contact materials appears to be paper with fluorochemical coatings/additives. Migration of 4,000 mg/kg to food oil was observed from popcorn bags. This amount of migration was hundreds of times more than the amount of fluorochemical that was calculated to migrate at 175°C from PTFE cookware.
Tittlemier et al. (2006) measured the concentrations of N-EtPFOSA, PFOSA; N,N-Et2PFOSA; N-methylperfluorooctane sulfonamide (N-MePFOSA), and N,N-dimethyl perfluorooctane sulfonamide (N,N-Me2PFOSA) in food items collected over a 12-year period (1992–2004) through the Canadian Total Diet Study to estimate dietary exposure of Canadians over 12 years of age. The most frequently detected analyte was N-EtPFOSA, found in 78 out of 151 composites, followed by N-MePFOSA (in 25 of 51). The highest concentrations and frequency of detection of analytes occurred in the fast food composites, particularly French fries, egg breakfast sandwiches, and pizza. Maximum concentrations of total perfluorooctane sulfonamides analyzed in fast foods ranged from 9.7 for French fries to 27.3 ng/g for pizza. Relatively high concentrations were also detected in cookies, Danish pastries, microwave popcorn, and wieners. Data generated from the study of Tittlemier et al. (2006) suggest that migration from food packaging had occurred.
One should realize, of course, that perfluorooctane sulfonamides can enter human food as a result of exposure of food-producing animals or plants to these compounds via environmental routes, such as inhalation or adsorption from air or intake of contaminated food. Perfluorooctane sulfonamides have indeed been detected in air (Martin et al. 2002; Stock et al. 2004; Shoeib et al. 2005) and water (Boulanger et al. 2005).
3.2 Direct Contamination of PFCs in Commercial Food Items
Tittlemier et al. (2007) analyzed 54 solid food composite samples for perfluorocarboxylates and perfluorooctane sulfonate. Forty-nine composite food samples originated from the Canadian Total Diet Study (TDS). Just over one half of the composites were from the 2004 TDS, the remaining composites were collected as the TDS was organized from 1992 to 2001. The latter ones were selected for analysis because they consisted of meat or other animal-derived food items or could have been stored in packaging treated with grease-resistant coatings. The food items originated from four different grocery stores and fast food restaurants. The food items were prepared for consumption and replicate food items from the various grocery stores or restaurants are combined and homogenized to form a composite sample. The composite items were analyzed for PFHpA, PFOA, PFNA, PFOS, PFDA, PFUnA, perfluorododecanoic acid (PFDoA), and perfluorotetradecanoic acid (PFTA). PFCs were detected in 9 out of 54 composites analyzed. PFOS was the dominant compound, followed by PFOA. PFHpA and PFNA were also detected. All the other investigated PFCs were below the detection limit, which varied between 0.4 and 5 ng/g wwt. Detected PFC concentrations ranged from 0.5 to 6 ng/g wwt. The highest PFOS concentration of 2.7 ng/g wwt was detected in beef steak, followed by a concentration of 2.6 ng/g wwt measured in a marine fish composite sample. The other PFOS concentrations ranged between 0.5 and 2.1 ng/g wwt, detected in ground beef, freshwater fish, microwave popcorn, and luncheon meats (cold cuts). Microwave popcorn contained the highest PFOA concentration of 3.6 ng/g wwt, followed by roast beef (2.6 ng/g wwt). Traceable PFOA levels were found in pizza. Only one composite contained a quantifiable PFNA concentration of 4.5 ng/g wwt. This concentration value was detected in beef steak and was the highest PFC concentration measured in this study. In two composites (pizza and microwave popcorn), low concentrations of PFHpA were found.
Researchers, in a study carried out by the UK Food Standards Agency (FSA 2006), analyzed 20 composites from the 2004 TDS for PFCs. Detectable levels of PFOS were found in canned vegetables, eggs and sugars, and in preserved food groups. PFOA was only detected in the potatoes group which included potato chips, French fries, instant mash, and other potato products. These results may have been due to analytical artifacts (Mortimer DN, personal communication). Other fluorinated chemicals PFOSA, perfluorobutane sulfonate (PFBS), PFHxS, PFHxA, PFNA, PFDA, PFUnA, PFDoA, perfluorotetradecanoic acid (PFTA) were detected only occasionally, although 10 different fluorinated compounds were found in the potatoes food group. PFHpA, perfluorohexadecanoic acid (PFHdA), and perfluorooctadecanoic acid (PFOdA) were not found. Bread, cereals, carcass meats, offal, meat products, poultry, fish, green vegetables, fresh fruit, beverages, milk, and nuts did not contain quantifiable concentrations of PFCs.
In a study conducted for 3 M Environmental Technology and Safety Services by the Battelle Memorial Institute (Columbus, OH, USA) (US EPA 2001), preliminary data about the presence of fluorochemicals in foods and in drinking water were collected and analyzed on PFOS, PFOA, and perfluorooctane sulfonamide (PFOSA). Each of three cities having manufacturing or commercial use of fluorochemical products (test cities) were paired with three cities that did not (control cities). A total of 12 samples were found to contain levels of PFCs above the limit of quantification. Of the 12 samples with measurable fluorochemical residue levels, 8 were samples collected in test cities. Measurable quantities of PFOS were found in five samples: four whole milk samples (three from test cities) and a ground beef sample (test city). PFOS residues found in the food samples ranged from non-quantifiable levels to 0.852 ng/g wwt. Measurable quantities of PFOA were found in seven samples: two ground beef samples (neither from test cities); two bread samples (one from a test city); two apple samples (both from test cities); and one green bean sample (from a test city). PFOA residue levels ranged from non-quantifiable levels to 2.35 ng/g wwt. A value of 14.7 ng/g wwt was found for PFOA in a bread sample from a control city, but was considered “suspect.” An important remark on these results is that only for the ground beef sample were these concentrations found in the replicates. The remaining values above the quantification limit were not detected in the duplicates. In general, the distributions of the PFOS, PFOA, and PFOSA residue data by food and city category reveal similar patterns of residue concentrations in the control and test cities for each type of food.
Ericson et al. (2008a) determined PFC levels in 36 composite samples of foodstuffs randomly purchased in various locations to determine the dietary intake of PFCs by the population of Tarragona County (Spain). PFOS, PFOA, and PFHpA were the only detected PFCs in the composite food samples. Fish, followed by dairy products and meats, were the main contributors to PFOS intake. The authors estimated the exposure to PFCs through the diet for various age and gender groups. Their results did not justify dietary intake as being the main route of exposure governing blood concentrations of other PFCs (Ericson et al. 2008a).
Fromme et al. (2007b) quantified the dietary intake of PFOS, PFOA, PFHxS, PFHxA, and PFOSA using 214 duplicate diet samples for an adult study population in Germany. The participants collected daily duplicate diet samples over seven consecutive days in 2005.
Overall, they detected PFOS, PFOA, PFHxS, and PFHxA in 33, 45, 3, and 9% of the 214 single duplicate samples, with concentrations ranging from <LOD (limit of detection) to 1.03 ng/g wwt, <LOD to 118.3 ng/g wwt, <LOD to 3.03 ng/g wwt, and <LOD to 3.2 ng/g wwt, respectively. PFOSA could not be detected above the LOD. Including only values above the detection limit (n = 47), a significant correlation was observed between PFOS and PFOA concentrations. The calculated median daily intake of PFOS and PFOA was 1.4 ng/kg bwt and 2.9 ng/kg, respectively. The median daily intake of PFHxS and PFHxA was 2.0 and 4.3 ng/kg bwt, respectively. The authors remarked that for the interpretation of this data, it has to be kept in mind that these analytes were detected only in few samples. In this study, gender differences were not observed for the target analytes.
Kärrman et al. (2009) investigated the relationship between dietary exposure and serum PFC levels in two Japanese cities. Therefore, 1-day composite diet samples from 20 women in Japan were collected in 2004 and were analyzed for PFBS, PFHxS, PFOS, PFHxA, PFHpA, PFOA, PFNA, PFDA, and PFUnA. This was done in a remote area and in an urban area so that the possible influence of industrialization could be investigated. Only PFOS and PFOA could be detected in the diet samples (within a range of 0.008–0.087 and 0.008–0.040 ng/g fresh wt, respectively). The levels of PFOA were relatively close to the method detection limit. PFOS and PFOA were detected in all diet samples and no difference was observed between the remote and the urban area. However, the importance of the diet for the body burden seems to vary between regions. The authors concluded that the dietary intake of PFOS and PFOA accounted for 22.4 and 23.7% of serum levels in females from the urban area and, in contrast, for 92.5 and 110.6% in females from the non-industrialized area, respectively.
Dietary exposure to PFCs has also been indirectly examined by Falandysz et al. (2006), who observed a correlation between PFC concentrations in blood sampled from adults in Poland and self-reported consumption of Baltic fish. However, no estimation of dietary exposure to PFCs could be made from this study.
Concentrations observed in all the studies conducted to date were in the sub- to low-ng/g range. It is worth noting that concentrations of PFCs in individual food items used to prepare the composite samples will be higher than those reported for the composite ones, since PFC-free food items in the same composite can effectively dilute PFC concentrations in individual food items (Tittlemier et al. 2007). Cooking practices may also lead to additional contamination of the prepared meal, e.g., by transfer of PFCs from frying pans or from contaminated water used for cooking (Begley et al. 2005). On the other hand, Del Gobbo et al. (2008) suggested that cooking methods (baking, boiling, and frying) can reduce PFC concentrations in fish.
The PFC levels found in food reflect both the environmental exposure and the food-packaging sources of entry into prepared foods, since both animal-derived and vegetable-based foods were found to contain PFCs.
4 PFCs in Drinking Water
In this section, an overview of existing data on PFCs in drinking or tap water is given. To the best of our knowledge, studies on other drinks like soda, coffee, tea, or juices have not been reported in the literature.
Results for PFCs found in drinking water are summarized in Table 1. Fromme et al. (2009) summarized existing data on PFCs in drinking water. In potable tap water of Japan, PFOS concentrations between 0.1 and 51 ng/L were detected. The majority of the results for individual samples did not exceed 4 ng/L (Harada et al. 2003). Saito et al. (2004) reported concentrations of PFCs in drinking water from areas with known PFC sources; their results ranged between 5.4 and 40.0 ng/L for PFOS. For PFOA, the concentrations ranged between 1.1 and 1.6 ng/L, while in areas with no known sources, concentrations ranged from <0.1 to 0.2 ng/L for PFOS and from 0.1 to 0.7 ng/L for PFOA.
In a study in which tap water that originated from Italy’s Lake Maggiore was examined (Loos et al. 2007), all investigated PFCs (PFHpA, PFOA, PFOS, PFNA, PFDA, PFUnA, and PFDoA) were detected, with concentrations ranging from 0.1 to 9.7 ng/L. The highest values were 9.7 ng/L for PFOS, 2.9 ng/L for PFOA, and 2.8 ng/L for PFDoA.
Skutlarek et al. (2006) analyzed drinking water samples in a contaminated area of the Ruhr and Moehne catchment (Germany). The highest values found were at Neheim, and the PFOA levels found were 519 ng/L, followed by PFHpA (23 ng/L) and PFHxA (22 ng/L). Measured PFC levels in the tap water (∑PFC = 767 ng/L) were comparable to the levels in water from the Moehne river (∑PFC = 598 ng/L) that served as a water supply. The sum of PFCs in drinking water from Ruhr waterway supplies ranged between 54 and 301 ng/L. In drinking water from the northern part of Duisburg, all the PFCs were below the LOD. In the southern district of Duisburg, however, PFBS was determined at 26 ng/L. The observed variation in concentrations in Duisburg indicates that samples were collected from different water supplies. Perfluorinated chemicals at other selected drinking water sampling sites outside the Ruhr and Moehne were sporadically detected, with the highest observed concentration of PFBS in Koblenz (20 ng/L). The sum of PFCs for those sites varied between non-detected and 27 ng/L (Skutlarek et al. 2006).
Ericson et al. (2008b) analyzed 14 PFCs in drinking water (tap and bottled) from Tarragona Province (Catalonia, Spain). In 2007, municipal drinking (tap) water was obtained in public fountains of the three most populated towns in Tarragona Province. The bottled water samples from four commercial companies, whose water spring has different origins, were purchased from a supermarket. This is, to the best of our knowledge, the first study in which bottled water was analyzed. The PFC levels in tap water varied among the four places. In the Valls sample, the highest PFC levels were found; PFHpA (3.02 ng/L), PFOS (0.44 ng/L), and PFOA (6.28 ng/L). In the sample of tap water from Reus, PFHxS (0.28 ng/L), PFOA (0.98 ng/L), PFNA (0.52 ng/L), and PFOS (0.73 ng/L) were detected, while in that of Tarragona, PFHpA (0.64 ng/L) PFHxS (0.28 ng/L), PFOA (0.98 ng/L), PFNA (0.22 ng/L), and PFOS (0.87 ng/L) were found. The lowest contamination in tap water corresponded to the sample collected in Tortosa, in which only PFOA (0.32 ng/L) and PFOS (0.39 ng/L) were detected. The samples of bottled water contained some PFCs at levels that corresponded to the lowest values observed in tap water. In one sample (Veri) all the PFCs were below the respective limits of detections, whereas in those of Cardo and Caprabo only PFNA (0.13 ng/L) and PFOA (0.67 ng/L) could be detected. In the fourth sample (the Font Vella) PFHpA (0.40 ng/L), PFOA (0.34 ng/L), and PFNA (0.20 ng/L) were detected. Overall, the PFC levels were notably lower in bottled water, whereas PFOS could not be detected in any sample. The authors also determined the levels of daily intake of PFOS through tap water, assuming an intake of 2 L tap water/d for the four different sampling towns. The result varied between 0.78 and 1.74 ng/d (absolute). Ericson et al. (2008b) estimated, in a previous study, a dietary intake of PFOS at 62.5 or 74.2 ng/d (assuming ND = 0 or ND = ½ LOD, respectively) for an adult of 70 kg bwt living in the Tarragona Province.
In 2006–2007, tap water was collected from 14 water treatment plants in Osaka, one of the largest industrial cities of Japan. PFOS was detected in 25 tap water samples and PFOA was detected in all of the tap water samples analyzed. Concentrations of PFOS and PFOA in tap water were 0.16–22 and 2.3–84 ng/L, respectively (Takagi et al. 2008).
Dutch drinking water resources are known to contain PFCs, from non-detect to 43 ng/L (Mons et al. 2007), and little is known about the behavior of these compounds in the Dutch drinking water preparation cycle.
The Bureau of Safe Drinking Water (BSDW) initiated a preliminary occurrence study in July 2006 to determine whether PFOS and PFOA could be found in detectable concentrations in raw and treated public water systems throughout the state of New Jersey (USA). Five out of 23 public water samples showed non-detectable levels of PFOA. Detected and quantifiable levels were found in 15 samples with values ranged from 4.5 to 39 ng/L. Additionally, three samples showed levels of PFOA that were detected but not quantified (<4 ng/L). Ten out of 23 water samples collected from public water systems showed non-detectable levels of PFOS. In seven samples PFOS concentration could be quantified and varied between 4.2 and 19 ng/L. Six samples showed levels of PFOS but could not be quantified (<4 ng/L) (New Jersey Department of Environmental Protection Division of Water Supply 2007).
Hölzer et al. (2008) analyzed drinking water (tap) to investigate the correlation between the PFC concentration in water and serum of the local people in Arnsberg (Germany). As previously mentioned, Skutlarek et al. (2006) observed remarkably high PFC concentrations in surface waters in this area (Moehne and Ruhr catchment). Tap water samples were collected from the kitchen in the homes of all residents. Of the various analyzed PFCs, PFOA was the main compound found in drinking water (500–640 ng/L). In the drinking water samples from reference areas, Siegen and Brilon, PFOS and PFOA were not detected. After installation of activated charcoal filters in the waterworks, PFOA concentrations in Arnsberg were significantly reduced. However, during the study period, filtration performance declined and PFOA concentrations in tap water samples increased from below the LOD to 71 ng/L. As the detected blood concentrations of residents living in Arnsberg were 4.5–8.3 times higher than those for the reference populations, the authors concluded that the consumption of tap water at home was a significant predictor of PFOA blood concentrations in Arnsberg.
5 Safety Limits and Tolerable Daily Intakes
It must be noted that the international regulatory organizations (World Health Organization (WHO), European Union (EU)/EFSA, US EPA, etc.) have not established safety limits yet for PFCs in drinking water. However, for guidance purposes, the 3 M Company developed a lifetime Drinking Water Health Advisory (DWHA), which was estimated to be 0.1 μg/PFOS/L (assuming a consumption of 2 L/day of contaminated water; 3 M 2001). Recently, Schriks et al. (2010) derived provisional drinking water guideline values for PFOS and PFOA of 0.5 and 5.3 μg/L, respectively, on the basis of the TDI values proposed by EFSA (2008).
At a PFC works facility, near Washington, West Virginia, the action level of PFOA in drinking water agreed to between the US EPA and E.I Dupont de Nemours was 0.50 μg/L. If the PFOA level in water supplies reaches 0.5 μg/L the local producer of PFCs must take certain actions (such as providing residents with bottled water). When the PFOA level in drinking water is measured at 150 μg/L, the producer has to install water treatment equipment (http://www.epa.gov/region03//enforcement/dupont_factsheet.html). In 2006, EPA and the eight major PFC manufacturing companies in the industry launched the 2010/15 PFOA Stewardship Program, in which companies committed to reduce global facility emissions and product content of PFOA and related chemicals by 95% by 2010 and to work toward eliminating emissions and product content by 2015 (http://www.epa.gov/oppt/pfoa/pubs/stewardship/index.html).
Recently, in New Jersey, the Department of Environmental Protection developed preliminary health-based drinking water guidance for PFOA of 40 ng/L (http://www.defendinscience.org/case_studies/upload/pfoa_dwguidance.pdf).
Guidelines have also been developed in Europe, more specifically in Germany. The occurrence of PFCs in surface and drinking waters of the Ruhr and Moehne area (Skutlarek et al. 2006) caused considerable concern, in view of the possible effects on humans and the ecosphere. Therefore, German authorities have recommended guide values for PFOA and PFOS in drinking water. Immediately after the increased PFOA levels were observed, German Drinking Water Commission (DWC) of the German Ministry of Health at the Federal Environment Agency established guide values for human health protection. Precautionary actions for infants will be taken if PFOA and PFOS concentrations in drinking water reach 0.5 μg/L. If additional PFCs are present, the health-based precautionary value (long-term minimum quality goal) becomes 0.1 μg/L. As a result, local health authorities recommended that residents in parts of Arnsberg to not use the drinking water for preparation of baby food and advised pregnant women to avoid regular intake of such water (http://www.umweltbundesamt.de/uba-info-presse-e/hintergrund/pft-in-drinking-water.pdf).
Most monitoring studies have focused only on PFOS and PFOA, but a few also reported on other PFCs that appear at rather high concentrations in potable water such as PFBS, PFDoA, perfluoropentanoic acid (PFPeA), and PFHxA (Skutlarek et al. 2006; Loos et al. 2007, Ericson et al. 2008b). Therefore, it is important to increase monitoring efforts with a view to setting more comprehensive safety limits for PFCs in potable water.
The relatively high concentrations of PFCs that have been observed in drinking water samples indicate that the common water treatment steps used do not effectively eliminate perfluorinated compounds. It should be noted that the washing of food samples with tap water may introduce an additional source of PFCs (Ericson et al. 2008b).
Recently, several scientific institutions have derived TDIs from toxicological end points by applying an uncertainty factor. The Scientific Panel on Contaminants in the Food Chain (CONTAM) established a TDI for PFOS of 150 ng/kg bwt/d and for PFOA of 1.5 μg/kg bwt/d (EFSA 2008). The UK Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) proposed a TDI for PFOS and PFOA of, respectively, 300 and 3,000 ng/kg bwt/d (COT 2006a, b). Furthermore, the German Federal Institute for Risk Assessment proposed a TDI of 100 ng/kg bwt/d for both PFOS and PFOA (BfR 2006).
These intake values are notably higher than those derived from actual human exposure via diet and beverages. For example, Fromme et al. (2007b) estimated a median dietary intake of 1.4 ng PFOS/kg bwt/d and of 2.9 ng/PFOA kg bwt/d. The results were similar to those calculated in the study of Kärrman et al. (2009) for two regions in Japan. They calculated a median daily intake of PFOS and PFOA, respectively, from 1.1 to 1.5 ng/kg bwt/d and from 0.72 to 1.3 ng/g bwt/d. In other studies, estimates of the daily intake were derived through market-basket surveys of food items instead of duplicate diet samples. In Canada, the average dietary intake of total PFCs and PFOS for individuals between the ages of 12 and those over 65 was estimated to be 250 ng/d (Tittlemier et al. 2007). This estimate was based upon results from 25 food composite samples, which did not represent the whole diet. These results represented only food that could have been environmentally exposed through bioaccumulation of PFCs or may have come into contact with food packaging treated with PFCs. The UK Food Standards Agency calculates average dietary intakes from the whole diet; these calculations give intake values of 100 ng/kg bwt/d for PFOS and 70 ng/kg bwt/d for PFOA. The UK dietary intake estimate is notably higher than that derived from other studies, probably due to the relatively high concentrations found in the potato composite samples. Finally, a food market study conducted in Spain (Tarragona County) resulted in a PFOS daily intake estimate of 1.07 ng/kg bwt/d for adult men (Ericson et al. 2008a). The EFSA (2008) study estimates indicated actual daily intakes of 60 ng/kg bwt/d of PFOS and 2 ng/kg bwt/d of PFOA.
Although diet and drinking water are thought to be two of the major exposure sources for humans, one should remember that all of these daily intake values, including the (provisional) TDIs cited above, are derived solely from food and/or beverage PFC levels (mainly PFOS and PFOA). Non-dietary exposure routes were not included and neither were the contributions of precursor compounds. As mentioned before, the calculated daily intake values are lower than the TDIs proposed. Nevertheless, these estimates were calculated for adults and the intake for infants, toddlers, and children may be much higher. As Trudel et al. (2008) have shown, the youngest among the consumer groups tend to experience higher total intake doses (on a body weight basis) than do teenagers and adults; this higher uptake results from the higher relative intake via food consumption and also hand-to-mouth transfer of PFCs from treated carpets and ingestion of dust.
6 Perfluorinated Compounds in House Dust and Air
In a few studies, the presence of perfluorinated compounds has been documented to occur in the indoor environment (Table 1). Given the wide range of consumer and residential products that contain or have been treated with PFCs, it is reasonable to hypothesize that as these products age and degrade their debris will accumulate indoors. Perhaps the most obvious potential source of PFCs in indoor dust would be the anti-stain agents used on carpets and upholstery (Strynar and Lindstorm 2008).
Moriwaki et al. (2003) analyzed dust for PFOS and PFOA in 16 Japanese houses. They collected bags from vacuum cleaners that were voluntarily donated. Hair and plastic garbage were removed from the samples using forceps and a loupe. In each sample, both analytes were found at levels above the limit of determination. The concentrations ranged from 11 to 2,500 ng/g for PFOS and from 69 to 3,700 ng/g for PFOA. In one sample, extremely high concentrations of 2,500 (PFOS) and 3,700 (PFOA) ng/g were found. Without this sample, the maximum concentrations were 120 ng/g for PFOS and 380 ng/g for PFOA. For all the dust samples analyzed in this study, the concentrations of PFOA were higher than those for PFOS (Moriwaki et al. 2003).
In dust samples (particle size of 75 μm to 1 mm) from seven Japanese houses, PFOA and PFOS were detected; their levels ranged from 18 to 89 ng/g and 7 to 41 ng/g, respectively (Nakata et al. 2007).
In Canada, dust samples were investigated for PFCs from 67 randomly selected homes during the winter of 2002/2003 (Kubwabo et al. 2005). The dust was collected from vacuum cleaners and sieved (opening 1.18 mm) before analysis of the samples for PFBS, PFHxS, PFOA, PFOS, and PFOSA. Results indicate that PFOA, PFHxS, and PFOS had much higher detection frequencies (63, 85, and 67%, respectively) than did PFOSA, which was detected in 10% of the samples. PFBS was not detected in any of the samples, possibly because this compound had just been introduced to the market at the time of these analyses. The concentrations of PFOA, PFOS, and PFHxS varied between 1.2 and 1,234, between 2.4 and 5,065, and between 2.3 and 4,305 ng/g, respectively.
In 2000–2001, dust samples from 102 homes and 10 day-care centers in North Carolina and Ohio were collected (Strynar and Lindstrom 2008). The samples were taken from vacuum cleaning bags and sieved to remove particles of diameters >150 μm. Quantifiable levels of PFOA, PFOS, and PFHxA were detected in 96.4; 94.6, and 92.9% of the samples, respectively. This is, to our knowledge, the first study in which PFHxA, PFHpA, PFNA, PFDA, PFUnA, and PFDoA were analyzed in dust samples. These analytes were, respectively, detected in 93, 74, 43, 30, 37, and 19% of the analyzed samples. PFBS was detected in 33% of the samples. The highest concentration observed among the samples analyzed amounted to 35,700 ng/g for PFOS. Generally, the fluorotelomer alcohols or FTOHs (6:2, 8:2, and 10:2 FTOHs) were detected in 50% of the samples and had median concentrations that ranged between 24 and 33 ng/g (Strynar and Lindstrom 2008).
Additionally, in a pilot study in Germany, 12 dust samples were collected using a vacuum cleaner. Median (range) PFOS and PFOA concentrations in the sieved fraction (particles < 630 μm) were 0.016 and 0.011 μg/g, respectively. Significantly lower median concentrations were observed in the unsieved samples, indicating that PFCs were mainly associated with smaller particles (Fromme et al. 2008).
Shoeib et al. (2005) were the first to analyze N-methylperfluorooctane sulfonamidoethanol (MeFOSE), N-ethylperfluorooctane sulfonamidoethanol (EtFOSE), N-ethylperfluorooctane sulfonamide (EtFOSA), and N-methylperfluorooctane sulfonamidethylacrylate (MeFOSEA) in indoor dust samples. During the winter of 2002/2003, dust was collected from 66 randomly selected homes in the city of Ottawa, Canada. The dust used for analysis came from the occupants’ vacuum bags or central vacuum containers. MeFOSEA was detected in ∼30% of dust samples and had a geometric mean value of ∼8 ng/g. The highest concentrations in dust were observed for MeFOSE and EtFOSE, which had geometric means of 113 and 138 ng/g, respectively (Shoeib et al. 2005).
During the winter of 2007 and spring of 2008, dust samples were collected from primary school and nursery classrooms in the West Midlands of the UK using a portable vacuum cleaner to which a sock with a 25-μm mesh size was inserted. After filtration through a 500-μm mesh the samples were analyzed for PFOA and PFOS. The concentrations of PFOA and PFOS found varied between 42 and 640 and between 85 and 3,700 ng/g, respectively (Goosey et al. 2008). Recently, a study was published in which the dust samples collected in Stockholm city (Sweden) from 10 houses, 38 apartments, 10 day-care centers, 10 offices, and 5 cars were investigated (Björklund et al. 2009). The dust samples were collected during 2006/2007 on pre-weighed cellulose filters in styrene–acrylonitrile holders that were inserted into a polypropylene nozzle; the nozzle was then attached to the intake nozzle of an industrial strength vacuum cleaner. Sampling of surfaces was performed at least one meter above the floor; sampled surfaces included bookshelves, moldings, counters, and lampshades. For cars, plastic surfaces and seat covers were vacuumed. Analysis of the samples from the 38 apartments disclosed PFOS and PFOA residues in 79 and 100% of the samples, respectively. PFOS was detected in 3 out of 5 car samples, whereas PFOA was detected in all of the car samples. PFOA concentrations were higher than those of PFOS in 60% of all the samples. Although offices had the highest median PFOS concentrations, the highest individual concentrations were found in apartments. The highest individual PFOA concentrations were also found in some apartments. The highest variation for both compounds was seen in apartments. Houses and day-care centers had much lower concentration variability. The median concentrations found in the different microenvironments were within one order of magnitude of each other. The highest median PFOS concentrations were seen in offices (110 ng/g dwt); similar but lower concentrations were seen in apartments (85 ng/g dwt), houses (39 ng/g dwt), and day-care centers (31 ng/g dwt); and lowest concentrations were seen in cars (12 ng/g dwt). For PFOA, the concentrations were more similar than was PFOS, between different microenvironments, with highest median concentrations found in apartments (93 ng/g dwt) and offices (70 ng/g dwt). Offices had higher median PFOS than PFOA concentrations, whereas the opposite was found for the other microenvironments (Björklund et al. 2009).
In Norway, PFOS levels were found in May 2005 in the particulate phase of one indoor air location. The concentrations were below the limit of determination (= 0.0474 ng/m3). For PFOA levels ranged between 0.0034 and 0.0069 ng/m3 (Barber et al. 2007).
Concentrations of PFOS and PFOA in the particulate phase of European air were also reported (Barber et al. 2007). Air samples were collected in 2005 at two locations in the UK and two locations in Norway. PFOS levels in an urban area ranged from 0.041 to 0.051 ng/m3 in the UK in March and from 0.0009 to 0.0071 ng/m3 in the UK in November. In southern Norway, PFOS levels ranged from 0.0009 to 0.0011 ng/m3 in November. The levels of PFOS in the particulate phase of air in the UK were the highest reported anywhere to date. Levels of PFOA in the particulate phase of UK air varied from 0.226 to 0.828 ng/m3 in March 2005, and from 0.006 to 0.222 ng/m3 in November. Differences in PFOA levels between the rural and the urban site in the UK were less clear than for PFOS. The levels of PFOA at the rural Norwegian site were significantly lower than those found in the UK. In southern Norway (data from November), levels varied between 0.0014 and 0.0017 ng/m3.
7 Correlation Between PFCs
In some studies, the correlation between the analyzed PFCs was investigated to determine any potential associations. A significant positive relationship between the concentrations of PFOS and PFOA was observed in dust from Japanese houses (Moriwaki et al. 2003). This relationship was confirmed by Kubwabo et al. (2005), Goosey et al. (2008), and Björklund et al. (2009). The significant correlation between PFOS and PFOA, observed in these studies, suggests that these compounds may be from a common source or may originate from the same precursor compound. Kubwabo et al. (2005) observed a significant and positive relation between PFOS, PFOA, and PFHxS in the dust samples of homes in Ottawa. A strong correlation between PFOS and PFHxS (Spearman’s rank-order correlation coefficient (r s) of 0.868, p < 0.0001) suggests that these compounds originated from the same sources in house dust (Kubwabo et al. 2005).
In addition, Strynar and Lindstorm (2008) observed that, generally, almost all of the investigated compounds (PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA, PFDoA, PFOS, and PFHxS) had significant correlations with each other except PFBS. The FTOHs, detected in the dust samples, were highly correlated with each other (r s > 0.82, p < 0.0001) suggesting a common source of these compounds (Strynar and Lindstorm 2008). No significant correlation between the perfluorinated sulfonamides, MeFOSE, and EtFOSE was observed (Shoeib et al. 2005). Kubwabo et al. (2005) also investigated the relationship between the analyzed PFCs and the characteristics of the house. Their results indicated that lower levels of PFOA and PFOS were found in older homes. Furthermore, PFOA, PFOS, and PFHxS and the total of these PFCs were significantly and positively correlated with the percentage of carpeting found in the house. The authors noticed that in addition to carpet, other multiple sources of perfluorinated compounds in homes may exist (Kubwabo et al. 2005).
Generally, in the Japanese studies, the PFOA concentrations were higher than those for PFOS (Moriwaki et al. 2003; Nakata et al. 2007). A similar trend was observed in Sweden, except for the levels found in offices (Björklund et al. 2009). The opposite was observed for the studies carried out in the USA (Strynar and Lindstorm 2008), Canada (Kubwabo et al. 2005), UK, and Germany (Fromme et al. 2008), where the PFOS concentrations were higher than PFOA. This observation could suggest a difference in PFC sources. The median PFOS concentration measured in the American study were 5–12 times higher than in the other studies. The median PFOA concentration in the USA was roughly comparable with one study in Japan and also 7–13 times higher than the median concentration found in the other Japanese study. Nevertheless, the ranges were similar between the studies except for the studies from Nakata et al. (2007) and Fromme et al. (2008), who measured much lower maximum values. A possible reason for the observed differences is that Strynar and Lindstorm (2008) included dust from day-care centers, where possibly, more PFC-containing waxes and cleaning products were used. Possibly, this can also explain the high median concentrations of PFOS (1,200 ng/g) and PFOA (220 ng/g) that were detected in the primary schools and nursery classrooms in the UK. More data are necessary to draw conclusions on the possible differences between geographic regions. Further research is also required to understand the different parameters contributing to the presence of perfluorinated compounds in house dust and in the indoor environment, in general (Strynar and Lindstorm 2008).
The concentrations summarized in Table 1 indicate that dust is a potential reservoir of different PFCs in homes, and these may be available for human exposures. Given that human exposure routes remain poorly characterized, the potential role of house dust needs to be more completely evaluated (Strynar and Lindstorm 2008). Additional studies are also needed to address the correlation between the levels of PFCs in blood of humans occupying the house being tested and the concentration of PFCs in house dust (Kubwabo et al. 2005).
8 Outlook
From the discussion above it is obvious that it is imperative to further assess the origin of PFCs in the human diet and the diet’s contribution to total human PFC exposure. Several initiatives have been taken recently to that end; these include the development of more robust and reliable analytical tools for the determination of PFCs, which will enable the qualification and quantification of PFCs in our diet, and the understanding of how PFCs are transferred from the environment into dietary items. In Europe, several recently launched scientific projects (e.g., EU projects Perfood; Confidence; Safefoodera; Norman network, and some national programs, e.g., in Norway, UK, and Belgium) deal with or emphasize the human exposure to PFCs and related compounds. These projects involve method development, source apportionment, exposure modeling, risk assessment, migration from packaging materials and food preparation methods, etc. Newly gained knowledge from these completed projects will enable us to evaluate the possible routes, including their relative importance, of human exposure to PFCs via our diet, to assess the role of the technosphere in the contamination of our food, help identify ways to reduce PFC contamination of dietary articles, and will help to establish relevant safe guideline values for dietary items.
Meanwhile, the steady, almost exponential increase of literature published illustrates the huge efforts taken to elucidate PFOS levels in the environment and the possible effects of PFCs and their mechanisms of action. The awareness of the current PFC issue by all stakeholders is reflected in joint efforts, such as the Stewardship Program in the USA, cited above, and joint initiatives in Europe between academic researchers and industry (e.g., PERFORCE 1 and 2 projects (see http://www.science.uva.nl/perforce)). The European Union, USA, Canada, Japan, UK, Norway, Sweden, and Australia, as well as several international groups, such as the OECD, are developing strategies to reduce PFC emissions and find safer alternatives to their use. Bans on use of PFOS in consumer products have been issued (EU 2006) and PFOS has been listed as a POP under the Stockholm Convention. The next decade of investigations will tell us whether or not the initiatives taken will lead to a substantial decrease in environmental and dietary exposure levels and if the recent reports of decreasing levels observed in human blood and in wildlife are a sign that the measures taken are indeed effective.
9 Summary
The widespread distribution and the degradation of PFCs in the environment results in a very complex exposure pattern, which makes it difficult to define the relative contribution to human exposure from different exposure pathways. The present review is designed to provide an overview of the existing data on levels of PFCs measured in the human diet and in drinking water. Data on levels of PFCs in the human diet are rather scarce, but the levels in fish appear to be well documented. Among PFCs, PFOS and PFOA are the best studied compounds in fish from both experimental and monitoring studies. Recently, the number of publications that address other PFCs has increased, but the total number available is still limited. In general, we discovered that care should be exercised when using the reviewed data, because, in the majority of the publications, quality control and/or details on analyses are, at least partly, lacking.
It has been well documented that PFOA and PFOS have the potential to accumulate in fish and concentrations up to 7 and 170 ng/g wwt, respectively, in edible fish species have been found. PFOS is the most crucial and prominent compound identified, followed by PFOA. Also, in aquatic invertebrates such as shrimps, mussels, clams, and oysters, high PFOS values have been reported (up to 387 ng/g wwt). However, in most publications PFC levels reported in molluscs were less than 1 ng/g wwt. Positive correlations were found between PFC body burdens and self-reported fish consumption. In recognition of the potential for human exposure to PFCs via fish consumption, the Minnesota Department of Health has recently issued fish consumption advisories for contaminated sections of the Mississippi River. It is interesting to note that 79% of the reviewed publications on PFCs in whole fish homogenates exceed that threshold. Moreover, five of the PFC concentrations reported in muscle tissue exceeded the advisory level of 38 ng/g wwt. Even though several authors concluded that consumption of contaminated food and drinking water constitutes the major exposure pathway for humans, only a few reports on PFCs in composite food exist. Food can be contaminated in an indirect way, because PFCs are widely used in food-packaging coatings and cooking materials. On the other hand, PFCs can also enter food organisms via environmental routes such as inhalation or adsorption from air. In a few studies, composite samples, duplicate diet samples, or other food items were analyzed for several PFCs. PFOS, PFOA, PFHpA, PFHxA, and PFHxS were measured and displayed concentrations ranging from non-detected up to 15 ng/g wwt. In one study, a very high PFOA concentration of 118 ng/g wwt was reported, but overall, PFC levels are below 10 ng/g wwt. It is important to note that, among all studies reviewed, PFCs were found in a maximum of 50% of the analyzed samples and generally only in 10% or less of samples analyzed.
In contrast to what is observed in fish and other food items, PFOA levels in drinking water (ND – 50 ng/L) usually exceed levels of PFOS (ND – 51 ng/L) and other PFCs (1–3 ng/L). In one study, extremely high values (519 ng/L) were measured in drinking water of a contaminated area in the Ruhr region. In Spain, bottled water was analyzed and four PFCs (PFOA, PFNA, PFDA and PFHpA) were found at low levels (<1 ng/L). Because of higher levels found in drinking water at several locations, some provisional drinking water guideline values for PFOS and PFOA have already been established, e.g., in the UK, Bavaria, and Minnesota. Since PFCs are present both in food and drinking water, Tolerable Daily Intake values for PFOS and PFOA have also been proposed by several institutes in Europe and in the USA.
The ingestion of dust through hand-to-mouth transfer from indoor house dust can also be a potential source of PFC exposure, especially for toddlers and children. In publications on PFCs in indoor dust, the mean PFOS and PFOA levels varied between 39 and 1,200 ng/g and between 11 and 220 ng/g, respectively.
Overall, it is clear that there is still a lack of PFC exposure data for food and beverages, which renders the assessment of the contribution of the diet to total human PFC exposure uncertain. It is, therefore, appropriate that several scientific projects have recently been launched that addresses the assessment of human exposure to PFCs and related compounds from dietary sources.
References
3 M (2001) Environmental monitoring—multi-city study, water, sludge, sediment, POTW effluent and landfill leachate samples. Executive summary, 3 M Environmental Laboratory, St Paul, MN, June 25, 2001. Available from http://www.ewg.org/files/multicity_full.pdf
Barber JL, Berger U, Chaemfa C, Huber S, Jahnke A, Temme C, Jones KC (2007) Analysis of per- and polyfluorinated alkyl substances in air samples from Northwest Europe. J Environ Monit 9:530–541
Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA (2005) Perfluorochemicals: potential sources of and migration from food packing. Food Add Contam 22:1023–1031
BfR (Bundesinstitut für Risikobewertung) (2006) Hohe Gehalte an perfluorierten organischen Tensiden (PFT) in Fischen sind gesundheitlich nicht unbedenklich Stellungnahme Nr.035/2006 des BfR vom 27. Juli 2006. In German only. Available at http://www.bfr.bund.de/cm/208/hohe_gehalte_an_perfluorierten_organischen_tensiden_in_fischen_sind_gesundheitlich_nicht_unbedenklich.pdf
Björklund JA, Thuresson K, de Wit CA (2009) Perfluoroalkyl compounds (PFCs) in indoor dust: concentrations, human exposure estimates, and sources. Environ Sci Technol 43:2276–2281
Boulanger B, Peck AM, Schnoor JL, Hornbuckle KC (2005) Mass budget of perfluorooctane surfactants in Lake Ontario. Environ Sci Technol 39:74–79
COT (Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment) (2006a) COT statement on the tolerable daily intake for perfluorooctane sulfonate. COT statement 2006/09, October 2006. Available online at http://www.food.gov.uk/multimedia /pdfs/cotstatementpfos200609.pdf
COT (Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment) (2006b) COT statement on the tolerable daily intake for perfluorooctanoic acid. COT statement 2006/10, October 2006 available online http://www.food.gov.uk/multimedia /pdfs/cotstatementpfoa200610.pdf
Cunha I, Hoff P, Van de Vijver K, Guilhermino L, Esmans E, De Coen W (2005) Baseline study of perfluorooctane sulfonate occurrence in mussels, Mytilus galloprovincialis, from north-central Portuguese estuaries. Mar Pollut Bull 50:1121–1145
de Voogt P, Berger U, de Coen W, de Wolf W, Heimstad E, Mclachlan M, van Leeuwen S, van Roon A (2006) PERFORCE: perfluorinated organic compounds in the European environment. Final report to the European Commission, project NEST-508967, University of Amsterdam, Amsterdam, pp 1–126
de Vos MG, Huijbregts MAJ, van den Heuvel-Greve MJ, Vethaak AD, Van de Vijver KI, Leonards PEG, van Leeuwen SPJ, Voogt P, Hendriks AJ (2008) Accumulation of perfluorooctane sulfonate (PFOS) in the food chain of the Western Scheldt estuary. Comparing field measurements with kinetic modeling. Chemosphere 70:1766–1773
Del Gobbo L, Tittlemier S, Diamond M, Pepper K, Tague B, Yeudall F, Vanderlinden L (2008) Cooking decreases observed perfluorinated compound concentrations in fish. J Agric Food Chem 56:7551–7559
EFSA (European Food Safety Authority) (2008) Opinion of the scientific panel on contaminants in the food chain on perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts, EFSA, Parma. EFSA J 653:1–131
Ericson I, Marti-Cid R, Nadal M, van Bavel B, Lindstrom G, Domingo JL (2008a) Human exposure to perfluorinated chemicals through the diet: intake of perfluorinated compounds in foods from the Catalan (Spain) Market. J Agric Food Chem 56:1787–1794
Ericson I, Nadal M, van Bavel B, Lindström G, Domingo JL (2008b) Levels of perfluorochemicals in water samples from Catalonia, Spain: is drinking water a significant contribution to human exposure? Environ Sci Pollut Res 15:614–619
EU (European Union) (2006) Directive 2006/122/EC of the European Parliament and of the Council. Official Journal of the European Union L372, 32–34, 27-12-2006
Falandysz J, Taniyasu S, Gulkowska A, Yamashita N, Schulte-Oehlmann U (2006) Is fish a major source of fluorinated surfactants and repellents in humans living on the Baltic Coast? Environ Sci Technol 40:748–751
Fromme H, Albrecht M, Angere J, Drexler H, Gruber L, Schlummer M, Parlar H, Körner W, Wanner A, Heitmann D, Roscher E, Bolte G (2007a) Integrated Exposure Assessment Survey (INES) exposure to persistent and bioaccumulative chemicals in Bavaria, Germany. Int J Hyg Environ Health 210:345–349
Fromme H, Nitschke L, Kiranoglu M, Albrecht M, Völkel W (2008) Perfluorinated substances in house dust in Bavaria, Germany. Organohalogen Compd (70:001048–001050)
Fromme H, Schlummer M, Möller A, Gruber L, Wolz G, Ungewiß J, Böhmer S, Dekant W, Mayer R, Liebl B, Twardella D (2007b) Exposure of an adult population to perfluorinated substances using duplicate diet portions and biomonitoring data. Environ Sci Technol 41:7928–7933
Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D (2009) Perfluorinated compounds – exposure assessment for the general population in Western countries. Int J Hyg Environ Health 212:239–270
FSA (Food Standards Agency) (2006) Fluorinated chemicals: UK dietary intakes. Food Survey Information Sheet 11/06, London, UK
Furdui VI, Stock NL, Ellis DA, Butt CM, Whittle DM, Crozier PW, Reiner EJ, Muir DCG, Mabury SA (2007) Spatial distribution of perfluoroalkyl contaminants in lake trout from The Great Lakes. Environ Sci Technol 41:1554–1559
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35:1339–1342
Goosey E, Abou-Elwafa AM, Harrad S (2008) Dust from primary school and nursery classrooms in the UK: its significance as a pathway to exposure to PFOS, PFOA, HBCDs and TBBP-A. Organohalogen Compd 70:855–858
Gruber L, Schlummer M, Ungewiss J, Wolz G, Moeller A, Weise N, Sengl M, Frey S, Gerst M, Schwaiger J (2007) Tissue distribution of perfluorooctanesulfonate (PFOS) and perfluorooctanoic acid (PFOA) in fish. Organohalogen Compd 69:3
Gulkowska A, Jiang Q, So MK, Taniyasu S, Lam PKS, Yamashita N (2006) Persistent perfluorinated acids in seafood collected from two cities of China. Environ Sci Technol 40:3736–3741
Harada K, Saito N, Sasaki K, Inoue K, Koizumi A (2003) Perfluorooctane sulfonate contamination of drinking water in the Tama River, Japan: estimated effects on resident serum levels. Bull Environ Contam Toxicol 71:31–36
Hart K, Kannan K, Tao L, Takahashi S, Tanabe S (2008) Skipjack tuna as a bioindicator of contamination by perfluorinated compounds in the ocean. Sci Total Environ 403:215–221
Haukås M, Berger U, Hop H, Gulliksen B, Gabrielsen GW (2007) Bioaccumulation of per- and polyfluorinated alkyl substances in selected species from the Barents Sea food web. Environ Pollut 148:360–371
Hoff PT, Van de Vijver KI, Van Dongen W, Esmans EL, Blust R, De Coen W (2003) Perfluorooctane sulfonic acid (PFOS) in bib (Trisopterus luscus) and plaice (Pleuronectes platessa) from the Western Scheldt and the Belgian North Sea: distribution and effect. Environ Toxicol Chem 22:608–614
Hölzer J, Midasch O, Rauchfuss K, Kraft M, Reupert R, Angerer J, Kleeschulte P, Marschall N, Wilhelm M (2008) Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate (PFOA)-contaminated drinking water. Environ Health Perspect 116:651–657
Houde M, Bujas TAD, Small J, Wells RS, Fair PA, Bossart GD, Solomon KR, Muir DCG (2006) Biomagnification of perfluoroalkyl compounds in the bottlenose dolphin (Tursiops truncatus) food web. Environ Sci Technol 40:4138–4144
HPA (Health Protection Agency) (2009) Maximum acceptable concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in drinking water. Available online http://www.hpa.nhs.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1195733828490?p=1158313435037
Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Van Wouwe N, Yang JH, Aldous KM (2004) Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol 38:4489–4495
Kannan K, Hansen JH, Wade TL, Giesy GP (2002) Perfluorooctane sulfonate in oysters, Crassostrea virginica, from the Gulf of Mexico and the Chesapeake Bay, USA. Arch Environ Contam Toxicol 42:313–318
Kannan K, Tao L, Sinclair E, Pastva SD, Jude DJ, Giesy JP (2005) Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch Environ Contam Toxicol 48:559–566
Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S, Lindström G (2007) Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect 115:226–230
Kärrman A, Harada KH, Inoue K, Takasuga T, Ohi E, Koizumi A (2009) Relationship between dietary exposure and serum perfluorochemical (PFC) levels – A case study. Environ Int 35:712–717
Kidd KA, Bootsma HA, Hesslein RH (2001) Biomagnification of DDT through the benthic and pelagic food webs of Lake Malawi, East Africa: importance of trophic level and carbon source. Environ Sci Technol 35:14–20
Kubwabo C, Stewart B, Zhu J, Marro L (2005) Occurrence of perfluorosulfonates and other perfluorochemicals in dust from selected homes in the city of Ottawa, Canada. J Environ Monit 7:1074–1078
Loos R, Wollgast J, Huber T, Hanke G (2007) Polar herbicides, pharmaceutical products, perflurooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and nonylphenol and its carboxylates and ethoxylates in surface and tap waters around lake Maggiore in Northern Italy. Anal Bioanal Chem 387:1469–1478
Martin JW, Mabury SA, Solomon K, Muir DCG (2003a) Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 22:196–204
Martin JW, Mabury SA, Solomon K, Muir DCG (2003b) Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 22:189–195
Martin JW, Muir DCG, Moody CA, Ellis DA, Kwan WC, Solomon KR, Mabury SA (2002) Collection of airborne fluorinated organics and analysis by gas chromatography/chemical ionization mass spectrometry. Anal Chem 74:584–590
Martin JW, Smithwick MM, Braune BM, Hoekstra PF, Muir DCG, Mabury SA (2004a) Identification of long-chain perfluorinated acids in biota from the Canadian Arctic. Environ Sci Technol 38:373–380
Martin JW, Whittle DM, Muir DCG, Mabury SA (2004b) Perfluoroalkyl contaminants in a food web from Lake Ontario. Environ Sci Technol 38:5379–5385
Minnesota Department of Health (2007) Fish consumption advisory program meal advice categories based on levels of PFOS in fish. http://wwwhelathstatmnus/divs/eh/fish/eating/mealadivcetable4pdf
Mons M, van Roon A, de Voogt P (2007) Perfluoroalkylated substances in Dutch drinking water sources, KIWA water research, BTO 07.048, Nieuwegein, 2007
Moriwaki H, Takata Y, Arakawa R (2003) Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoic (PFOA) in vacuum cleaner dust collected in Japanese homes. J Environ Monit 5:753–757
Nakata H, Kannan K, Nasu T, Cho H-S, Sinclair E, Takemura A (2006) Perfluorinated contaminants in sediments and aquatic organisms collected from shallow water and tidal flat areas of the Ariake Sea, Japan: environmental fate of perfluorooctane sulfonate in aquatic ecosystems. Environ Sci Technol 40:4916–4921
Nakata A, Katsumata T, Iwasaki Y, Ito R, Saito K, Izumi S, Makino T, Kishi R, Nakazawa H (2007) Measurement of perfluorinated compounds in human milk and house dust. Organohalogen Compd 69:2844–2846
Nania V, Pellegrini GE, Fabrizi L, Sesta G, De Sanctis P, Lucchetti D, Di Pasquale M, Coni E (2009) Monitoring of perfluorinated compounds in edible fish from the Mediterranean Sea. Food Chem 115:951–957
New Jersey Department of Environmental Protection Division of Water Supply (2007) Determination of perfluorooctanoic acid (PFOA) in aqueous samples, January 2007
Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR (2007) Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115:1298–1305
Saito N, Harada K, Inoue K, Sasaki Y, Yoshinaga T, Koizumi A (2004) Perfluorooctanoate and perfluorooctane sulfonate concentrations in surface water in Japan. J Occup Health 46:49–59
Schrap SM, Pijnenburg AMCM, Geerdink RB (2004) Geperfluoreerde verbindingen in Nederlands oppervlaktewater; een screening in 2003 van PFOS en PFOA RIKZ rapport 2004037 Rijkswaterstaat/Rijksinstituut voor Kust en Zee, Den Haag (in Dutch only)
Schriks M, Heringa MC, van der Kooi MME, de Voogt P, van Wezel AP (2010) Toxicological relevance of emerging contaminants for drinking water quality. Water Res 44:461–476
Shoeib M, Harner T, Wilford BH, Jones KC, Zhu J (2005) Perfluorinated sulfonamides in indoor and outdoor air and indoor dust: occurrence, partitioning, and human exposure. Environ Sci Technol 39:6599–6606
Skutlarek D, Exner M, Färber H (2006) Perfluorinated surfactants in surface and drinking waters. Environ Sci Pollut Res 13:299–307
Stock NL, Lau FK, Ellis DA, Martin JW, Muir DCG, Marbury SA (2004) Polyfluorinated telomere alcohols and sulfonamides in the North American troposphere. Environ Sci Technol 38:991–996
Strynar MJ, Lindstrom AB (2008) Perfluorinated compounds in house dust from Ohio and North Carolina, USA. Environ Sci Technol 42:3751–3756
Takagi S, Adachi F, Miyana K, Koizumi Y, Tanaka H, Mimura M, Watanabe I, Tanabe S, Kannan K (2008) Perfluorooctanesulfonate and perfluorooctanoate in raw and treated tap water from Osaka, Japan. Chemosphere 72:1409–1412
Taniyasu S, Kannan K, Horii Y, Horri Y, Hanari N, Yamashita N (2003) A survey of perfluorooctane sulfonate and related perfluorinated organic compounds in water, fish, birds, and humans from Japan. Environ Sci Technol 37:2634–2639
Tittlemier SA, Pepper K, Edwards L (2006) Concentrations of perfluorooctanesulfonamides in Canadian Total Diet Study composite food samples collected between 1992–2004. J Agric Food Chem 54:8385–8389
Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao X-L, Dabeka RW (2007) Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and fast food items prepared in their packaging. J Agric Food Chem 55:3203–3210
Tomy GT, Budakowski W, Halldorson T, Helm PA, Stern GA, Friesen K, Pepper K, Tittlemier SA, Fisk AT (2004b) Fluorinated organic compounds in an Eastern Arctic marine food web. Environ Sci Technol 38:6475–6481
Tomy GT, Tittlemier SA, Palace VP, Budakowski WR, Brarkevelt E, Brinkworth L, Friesen (2004a) Biotransformation of N-ethyl perfluorooctanesulfonamide by rainbow trout (Oncorhynchus mykiss) liver microsomes. Environ Sci Technol 38:758–762
Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbuehler K (2008) Estimating consumer exposure to PFOS and PFOA. Risk Anal 40:251–269
US EPA (Environmental Protection Agency) (2001) Analysis of PFOS, FOSA, and PFOA from various food matrices using HPLC electrospray/mass spectrometry. 3 M study conducted by Centre Analytical Laboratories, Inc. http://www.ewg.org/files/multicity_-full.pdf. Accessed 4 Jan 2008
Van de Vijver IK, Hoff PT, Van Dongen W, Esmans E, Blust R, De Coen W (2003) Exposure patterns of perfluorooctane sulfonate in aquatic invertebrates from the Western Scheldt estuary and the southern North Sea. Environ Toxicol Chem 22:2037–2041
Van den Heuvel-Greve M, Leonards P, Vethaak D (2006) Dioxin research Westerschelde: measuring percentages of dioxins, dioxin-like substances and other possible problematic substances in fishery products, sediment and food chains of the Westerschelde [Dioxineonderzoek Westerschelde: meting van gehalten aan dioxinen, dioxineachtige stoffen en andere mogelijke probleemstoffen in visserijproducten, sediment en voedselketens van de Westerschelde] Rapport RIKZ, 2006011 RIKZ: Middelburg, The Netherlands 80 pp
Vestergren R, Cousins IT, Trudel D, Wormuth M, Shseringer M (2008) Estimating the contribution of precursor compounds in consumer exposure to PFOS and PFOA. Chemosphere 73:1617–1624
Xu L, Krenitsky DM, Seacat AM, Butenhoff JL, Anders MW (2004) Biotransformation of N-ethyl-N-(2-hydroxyethyl)perfluorooctanesulfonamide by rat liver microsomes, cytosol, and slices and by expressed rat and human cytochromes P450. Chem Res Toxicol 17:767–775
Ye X, Strynar MJ, Nakayama SF, Varns J, Helfant L, Lazorchak J, Lindstrom AB (2008) Perfluorinated compounds in whole fish homogenates from the Ohio, Missouri, and Upper Mississippi rivers, USA. Environ Pollut 156:1227–1232
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
D’Hollander, W., de Voogt, P., De Coen, W., Bervoets, L. (2010). Perfluorinated Substances in Human Food and Other Sources of Human Exposure. In: De Voogt, P. (eds) Reviews of Environmental Contamination and Toxicology Volume 208. Reviews of Environmental Contamination and Toxicology, vol 208. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-6880-7_4
Download citation
DOI: https://doi.org/10.1007/978-1-4419-6880-7_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-6879-1
Online ISBN: 978-1-4419-6880-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)