Abstract
Normal structure and maturity of sperm chromatin is essential for the fertilizing ability of spermatozoa in vivo. It is a relatively independent measure of semen quality that yields additional prognostic information complementary to standard sperm parameters – concentration, motility, and morphology. Several methods are used to assess sperm chromatin status. At present, indirect methods for sperm DNA fragmentation assessment are routinely used in andrological workup. However, several simple and efficient tests for chromatin maturation status are also available. The normality ranges and predictive thresholds for male fertility potential for these assays still need to be established or clarified
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chytochemical tests for sperm chromatin maturity
- Sperm chromatin maturity
- Sperm chromatin, structural probes
- Planar ionic dyes in sperm chromatin
Infertility is a major medical problem that affects approximately 15% of couples trying to conceive, and a male cause is believed to be a contributing factor in approximately half of these cases [1]. In andrological practice, visual light microscopic examination of semen quality plays principal role in male fertility potential evaluation. This consists of measuring seminal volume, pH, sperm concentration, motility, morphology, and vitality. However, often a diagnosis of male fertility cannot be made as a result of basic semen analysis. This is caused by a significant overlap in the values of sperm concentration, motility, and morphology between fertile and infertile men, as it has been demonstrated by several studies [2]. In addition, quality control introduction within and between laboratories has highlighted the subjectivity and variability of traditional semen parameters.
It has been demonstrated that abnormalities in the male genome, characterized by distur-bed chromatin packaging and damaged sperm deoxyribonucleic acid (DNA), may be a cause for male infertility regardless of routine semen parameters [3, 4]. Sperm chromatin abnormalities have been studied extensively in the past several years as a cause of male infertility [5]. Focus on the chromatin integrity and maturity of the male gamete has been especially intensified by the growing concern about transmission of damaged DNA through assisted reproductive techniques (ARTs) such as intracytoplasmic sperm injection (ICSI). Accumulating evidence suggests a negative relationship between disorganization of the chromatin material in sperm nuclei and the fertility potential of spermatozoa both in vivo and in vitro [4–12].
Abnormalities in the sperm chromatin organization, characterized both by damaged DNA and incompletely remodeled chromatin in mature sperm cells, may be indicative of male infertility regardless of normal semen parameters [3, 13]. Evaluation of sperm chromatin structure is an independent measure of sperm quality that provides good diagnostic and prognostic capabilities. Therefore, it may be considered a reliable predictor of a couple’s inability to conceive [14, 15]. Sperm chromatin quality correlates with pregnancy outcome in in vitro fertilization (IVF) [14–18].
Many techniques have been described for evaluation of the chromatin status and maturity. In andrological practice, the most popular are indirect methods for estimation of DNA integrity in sperm chromatin. These methods are based on the ability of some stains to test the conformation of sperm chromatin, which in turn depends on DNA strand breaks and DNA interaction with proteins [19–22]. However, since some studies had demonstrated that spermatozoa with abnormal nuclear chromatin packaging are more frequent in infertile men than in fertile men, a number of techniques have been developed to test sperm chromatin maturation status. These techniques help to evaluate male reproductive status and might be also useful for ART outcome prediction [23, 24]. These assays, often referred as “cytochemical,” include acidic aniline blue (AAB), Chromomycin A3, and Toluidine Blue (TB) tests.
Cytochemical Properties of Human Sperm Chromatin and Basis of its Testing by Planar Ionic Dyes
In many mammals, spermatozoa nuclei are highly homogenous and compact. This allows mature sperm nuclei to adopt a volume 40 times less than that of normal somatic nuclei [25]. This highly compact packaging of the primary sperm DNA filament is produced by DNA–protamine complexes [26]. Human sperm nuclei, on the contrary, contain considerably fewer protamines (around 85%) than sperm nuclei of several other mammals (such as bull, stallion, hamster, and mouse) [27, 28], and therefore, they are less regularly compacted and frequently contains DNA strand breaks [29, 30]. Sperm DNA is packed in specific toroids, each containing 50–60 kilobases of DNA. Individual toroids represent the DNA loop-domains, highly condensed by protamines and fixed at the nuclear matrix. Toroids are cross-linked by disulfide bonds formed by oxidation of sulfhydryl groups of cysteine present in the protamines [25, 26, 31]. Such condensed, insoluble, and highly organized structure of sperm chromatin is necessary to protect the genetic integrity during transport of the paternal genome through the male and female reproductive tracts [32–34].
However, in comparison to other species [35], human sperm chromatin packaging is exceptionally variable. This variability has been mostly attributed to its basic protein component. The retention of 15% histones, which are less basic than protamines, leads to the formation of a less-compact chromatin structure [28]. Moreover, human spermatozoa contain two types of protamines, P1 and P2, with a second type deficient in cysteine residues [36]. This results in diminished disulfide cross-linking if compared with species in which sperm contain only P1 group of proteins [37].
Chromatin structural probes using planar ionic dyes allow to analyze chromatin structure in terms of protein packaging correctness and disulfide cross-linking density. Their cytochemical background, however, is quite complex. Several factors influence the staining of chromatin by planar ionic dyes: (1) secondary structure of DNA, (2) regularity and density of chromatin packaging, and (3) binding of DNA to chromatin proteins, which influences its charge.
DNA Secondary Structure and Conformation – Fragmented DNA is easily denaturable [38]. However, even a single DNA strand break causes conformational transition of the DNA loop-domain from a supercoiled state to a relaxed state. Supercoiled DNA avidly takes up intercalating dyes (such as acridine orange [AO]) because this reduces the free energy of torsion stress. By contrast, the affinity for intercalation is low in relaxed DNA and is lost in fragmented DNA. In this case, an external mechanism of dye binding to DNA phosphate residues and dye polymerization (metachromasy) is favored [39, 40]. Nevertheless, fragmentation of DNA is not the only factor affecting the choice between metachromatic vs. orthochromatic staining. Chromatin packaging density also influences this balance.
Chromatin Packaging Density – in the regularly arranged and sufficiently densely packed sperm chromatin, coplanar dye polymerization providing metachromatic shift (change of color) is favored [41, 42]. However, in even more densely (as in normal sperm) packaged chromatin, the polymerization of the dye is hindered [43] and may even impair dye binding and coplanar polymerization. The latter is seen with aniline blue (AB) at low pH where it stains basic proteins loosely associated with DNA and is unable to bind to the chromatin of normal sperm, which is very densely packaged and uncharged. Substitution of histones to more cationic protamines occurring during spermiogenesis neutralizes DNA charge and decreases the accessibility of DNA-specific dyes. However, after removal of nuclear proteins, increase in sperm DNA stainability can vary depending on the chemical structure of the dye and the binding type which the dye forms with the DNA substrate [19, 44–46].
Chromatin Proteins affect the binding of DNA dyes in the way that they themselves bind differently to relaxed, fragmented, or supercoiled DNA. DNA supercoiling requires covalent binding of some nuclear matrix proteins and tighter ionic interactions between DNA and chromatin proteins to support negative supercoils [47]. Relaxed and fragmented DNA has looser ionic interactions with chromatin proteins, which can be easily displaced from the DNA, favoring external metachromatic binding of the dye to DNA phosphate groups. Both mechanisms of dye binding, external and intercalating, compete within each other within constraint loop-domain (toroid) depending on its conformational state.
Sperm Chromatin Structural Probes
Chromatin proteins in sperm nuclei with the impaired DNA appear to be more accessible to binding with the acidic dye, as found by the AB test [48]. An increase in the ability to stain sperm by acid AB indicates a looser chromatin packaging and increased accessibility of the basic groups of the nucleoproteins. This is due to the presence of residual histones [49], and correlates well with the AO test [50]. Chromomycin A3 (CMA3) is another staining technique that has been used as a measure of sperm chromatin condensation anomalies. CMA3 is a fluorochrome specific for GC-rich sequences and is believed to compete with protamines for binding to the minor groove of DNA. The extent of staining is, therefore, related to the degree of protamination of mature spermatozoa [51, 52]. In turn, phosphate residues of sperm DNA in nuclei with loosely packed chromatin and/or impaired DNA will be more liable to binding with basic dyes. Such conclusions were also deduced from the results of staining with basic dyes, such as TB, methyl green, and Giemsa stain [52, 53].
Acidic Aniline Blue
The AAB stain discriminates between lysine-rich histones and arginine/cysteine-rich protamines. This technique provides a specific positive reaction for lysine and reveals differences in the basic nuclear protein composition of human spermatozoa. Histone-rich nuclei of immature spermatozoa are rich in lysine and will consequently take up the blue stain. On the contrary, protamine-rich nuclei of mature spermatozoa are rich in arginine and cysteine and contain relatively low levels of lysine, which means they will not take up the stain [54].
Technique: slides are prepared by smearing 5 μL of either raw or washed semen sample. The slides are air-dried and fixed for 30 min in 3% glutaraldehyde in phosphate-buffered saline (PBS). The smear is dried and stained for 5 min in 5% aqueous AB solution (pH 3.5). Sperm heads containing immature nuclear chromatin stain blue and those with mature nuclei do not. The percentage of spermatozoa stained with AB is determined by counting 200 spermatozoa per slide under bright-field microscopy [55].
Results of AAB staining have shown a clear association between abnormal sperm chromatin and male infertility [56]. However, the correlation between the percentage of AB-stained spermatozoa and other sperm parameters remains controversial. Immature sperm chromatin may or may not correlate with asthenozoospermic samples and abnormal morphology patterns [55, 56]. Most important is the finding that chromatin condensation as visualized by AB staining is a good predictor for IVF outcome, although it cannot determine the fertilization potential and the cleavage and pregnancy rates following ICSI [57].
Toluidine Blue Stain Assay
TB is a basic planar nuclear dye used for metachromatic and orthochromatic staining of the chromatin. The phosphate residues of sperm DNA in nuclei with loosely packed chromatin and/or impaired DNA become more liable to binding with basic TB, providing a metachromatic shift due to dimerization of the dye molecules from light blue to purple–violet color [58]. This stain is a sensitive structural probe for DNA structure and packaging.
Technique: thin sperm smears are prepared on precleaned defatted slides and then air-dried for 30–60 min. Dried smears are fixed with freshly made 96% ethanol–acetone (1:1) at 4°C for 30 min to 12 h and air-dried. Hydrolysis is performed with 0.1 mol/L HCl at 4°C for 5 min followed by three changes of distilled water, 2 min each. TB (0.05% in 50% McIlvain’s citrate phosphate buffer at pH3.5, is applied for 5 min. The slides are rinsed briefly in distilled water, lightly blotted with filter paper, dehydrated in tertiary butanol at 37°C (2 and 3 min) and xylene at room temperature (2 and 3 min), and mounted with DPX.
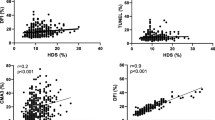
The results of the TB test are estimated using oil-immersion (10 and 100) light microscope. Sperm heads with good chromatin integrity stain light blue and those with diminished integrity stain violet (purple) [59]. The proportion of cells with violet heads (high optical density) are calculated based on 200 sperm cells examined per sample. Based on the different optical densities of cells stained by the TB, the image analysis cytometry test has been elaborated [60] (Figs. 12.1 and 12.2).
TB staining may be considered a fairly reliable method for assessing sperm chromatin. Abnormal nuclei (purple–violet sperm heads) have been shown to be correlated with counts of red–orange sperm heads as revealed by the AO method [58]. Also, correlations between the results of the TB, sperm chromatin structure assay (SCSA), and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) tests have been demonstrated. The proportion of sperm cells with abnormal DNA conformation, detected by the TB test (violet heads), correlated significantly with the proportion of spermatozoa containing denaturable DNA detected as SCSA percentage DFI (r = 0.84, P < 0.001) and with the fraction of spermatozoa with fragmented DNA in the FCM TUNEL test (r = 0.80, P < 0.001) [59]. Thresholds for the TB test between fertile and infertile men also were set. A threshold for proportion of cells with violet heads was set at 45%; it provides 92% specificity and 42% sensitivity for infertility detection [61].
TB staining is simple and inexpensive and has the advantage of providing permanent preparations for use with an ordinary microscope. The smears stained with the TB method can also be used for morphological assessment of the cells. However, these methods may have the inherent limits of repeatability dictated by a limited number of cells, which can be reasonably scored.
Chromomycin A3 Assay
Chromomycin A3 is a fluorochrome that specifically binds to guanine–cytosine DNA sequences. It reveals chromatin that is poorly packaged in human spermatozoa by visualization of protamine-deficient DNA. Chromomycin A3 and protamines compete for the same binding sites in the DNA. Therefore, high CMA3 fluorescence is a strong indicator of the low protamination state of spermatozoa chromatin [62].
Technique: for CMA3 staining, semen smears are first fixed in methanol–glacial acetic acid (3:1) at 4°C for 20 min and are then allowed to air-dry at room temperature for 20 min. The slides are treated for 20 min with 100 μL of CMA3 solution. The CMA3 solution consists of 0.25 mg/mL CMA3 in McIlvain’s buffer (pH 7.0) supplemented with 10 mmol/L MgCl2. The slides are rinsed in buffer and mounted with 1:1 v/v PBS-glycerol. The slides are then kept at 4°C overnight. Fluorescence is evaluated using a fluorescent microscope. A total of 200 spermatozoa are randomly evaluated on each slide. CMA3 staining is evaluated by distinguishing spermatozoa that stain bright yellow (CMA3 positive) from those that stain dull yellow (CMA3 negative) [62].
As a discriminator of IVF success (>50% oocytes fertilized), CMA3 staining has a sensitivity of 73% and specificity of 75%. Therefore, it can distinguish between IVF success and failure [63]. In cases of ICSI, percentage of CMA3 positivity does not indicate failure of fertilization entirely and suggested that poor chromatin packaging contributes to a failure in the decondensation process and probably reduced fertility [64]. It appears that semen samples with high CMA3 positivity (>30%) may have significantly lower fertilization rates if used for ICSI [65].
The CMA3 assay yields reliable results as it is strongly correlated with other assays used in the evaluation of sperm chromatin. In addition, the sensitivity and specificity of the CMA3 stain are comparable with those of the AAB stain (75 and 82%, 60 and 91%, respectively) if used to evaluate the chromatin status in infertile men [66]. However, the CMA3 assay is limited by observer subjectivity.
Conclusion
Normal structure and maturity of sperm chromatin is essential for the fertilizing ability of spermatozoa in vivo. It is a relatively independent measure of semen quality that yields additional prognostic information complementary to standard sperm parameters – concentration, motility, and morphology. Several methods are used to assess sperm chromatin status. At present, indirect methods for sperm DNA fragmentation assessment are routinely used in andrological workup. However, several simple and efficient tests for chromatin maturation status are also available. The normality ranges and predictive thresholds for male fertility potential for these assays still need to be established or clarified.
References
Oehninger S. Strategies for the infertile man. Semin Reprod Med. 2001;19:231–7.
Guzick DS, Overstreet JW, Factor-Litvak P, et al. National Cooperative Reproductive Medicine Network. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–93.
Sakkas D, Tomlinson M. Assessment of sperm competence. Semin Reprod Med. 2000;18:133–9.
Agarwal A, Allamaneni SSR. Sperm DNA damage assessment: a test whose time has come. Fertil Steril. 2005;84:850–3.
Agarwal A, Said Tamer M. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9:331–45.
Erenpreiss J, Spano M, Erenpreisa J, Bungum M, Giwercman A. Sperm chromatin structure and male fertility: biological and clinical aspects. Asian J Androl. 2006;8:11–29.
Evenson D, Regina W. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod Biomed Online. 2006;12:466–72.
Borini A, Tarozzi N, Bizzaro D, Bonu MA, Fava L, Flamigni C, et al. Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART. Hum Reprod. 2006;21:2876–81.
Aitken RJ, De Iuliis GN. Origins and consequences of DNA damage in male germ cells. Reprod Biomed Online. 2007;14:727–33.
Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–9.
Ozmen B, Koutlaki N, Youssry M, Diedrich K, Al-Hasani S. DNA damage of human spermatozoa in assisted reproduction: origins, diagnosis, impacts and safety. Reprod Biomed Online. 2007;14:384–95.
Tarozzi N, Bizzaro D, Flamigni C, Borini A. Clinical relevance of sperm DNA damage in assisted reproduction. Reprod Biomed Online. 2007;14:746–57.
Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod. 1998;13: 896–900.
Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14:1039–49.
Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod. 1997;56:602–7.
Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;17:3122–8.
Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod. 2000;15:1717–22.
De Jonge C. The clinical value of sperm nuclear DNA assessment. Hum Fert. 2002;5:51–3.
Evenson D, Darzynkiewicz Z, Jost L, Janca F, Ballachey B. Changes in accessibility of DNA to various fluorochromes during spermatogenesis. Cytometry. 1986;7:45–53.
Erenpreiss J, Bars J, Lipatnikova V, Erenpreisa J, Zalkalns J. Comparative study of cytochemical tests for sperm chromatin integrity. J Androl. 2001;22: 45–53.
Gledhill BL, Gledhill MP, Rigler R, Ringertz NR. Atypical changes of deoxyribonucleo-protein during spermiogenesis associated with a case of infertility in the bull. J Reprod Fertil. 1966;12:575–8.
Gledhill BL, Gledhill MP, Rigler R, Ringertz NR. Changes in deoxyribonucleoprotein during spermiogenesis in the bull. Exp Cell Res. 1966;41:652–65.
Nijs M, Creemers E, Cox A, Franssen K, Janssen M, Vanheusden E, et al. Chromomycin A3 staining, sperm chromatin structure assay and hyaluronic acid binding assay as predictors for assisted reproductive outcome. Reprod Biomed Online. 2009;19(5):671–84.
Iranpour FG, Nasr-Esfahani MH, Valojerdi MR, al-Taraihi TM. Chromomycin A3 staining as a useful tool for evaluation of male fertility. J Assist Reprod Genet. 2000;17(1):60–6.
Ward WS, Coffey DS. DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells. Biol Reprod. 1991;44:569–74.
Fuentes-Mascorro G, Serrano H, Rosado A. Sperm chromatin. Arch Androl. 2000;45:215–25.
Gatewood JM, Cook GR, Balhorn R, et al. Sequence-specific packaging of DNA in human sperm chromatin. Science. 1987;236:962–4.
Bench GS, Friz AM, Corzett MH, et al. DNA and total protamine masses in individual sperm from fertile mammalian subjects. Cytometry. 1996;23: 263–71.
Sakkas D, Mariethoz E, Manicardi G, et al. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–7.
Irvine DS, Twigg JP, Gordon EL, et al. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21:33–44.
Ward WS. Deoxyribonucleic acid loop domain tertiary structure in mammalian spermatozoa. Biol Reprod. 1993;48:1193–201.
Solov’eva L, Svetlova M, Bodinski D, et al. Nature of telomere dimers and chromosome looping in human spermatozoa. Chromosome Res. 2004;12:817–23.
Ward WS, Zalensky AO. The unique, complex organization of the transcriptionally silent sperm chromatin. Crit Rev Eukaryot Gene Expr. 1996;6:139–47.
De Jonge CJ. Paternal contributions to embryo-genesis. Reprod Med Rev. 2000;8:203–14.
Lewis JD, Song Y, de Jong ME, et al. A walk through vertebrate and invertebrate protamines. Chromosoma. 1999;111:473–82.
Corzett M, Mazrimas J, Balhorn R. Protamine 1: protamine 2 stoichiometry in the sperm of eutherian mammals. Mol Reprod Dev. 2002;61:519–27.
Jager S. Sperm nuclear stability and male infertility. Arch Androl. 1990;25:253–9.
Darzynkiewicz Z. Acid-induced denaturation of DNA in situ as a probe of chromatin structure. Methods Cell Biol. 1994;41:527–41.
Erenpreisa EA, Zirne RA, Zaleskaia ND, S’iakste TG. Effect of single-stranded breaks on the ultrastructural organization and cytochemistry of the chromatin in tumor cells. Biull Eksp Biol Med. 1988;106:591–3. [Article in Russian].
Erenpreisa EA, Sondore O, Zirne RA. Conformational changes in the chromatin of tumor cells and the phenomenon of nuclear achromasia. Eksp Onkol. 1988;10:54–7. [Article in Russian].
Sculthore HH. Metachromasia. Med Lab Sci. 1978;35:365–70.
Erenpreisa J, Zaleskaya N. Effect of triton X-100 on cytochemical and ultrastructural pattern of chromatin. Acta Morphol Hung. 1983;31:387–93.
Erenpreisa J, Freivalds T, Selivanova G. Influence of chromatin condensation on the absorption spectra of nuclei stained with toluidine blue. Acta Morphol Hung. 1992;40:3–10.
Brewer LR, Corzett M, Balhorn R. Protamine-induced condensation and decondensation of the same DNA molecule. Science. 1999;286:120–3.
Brewer L, Corzett M, Balhorn R. Condensation of DNA by spermatid basic nuclear proteins. J Biol Chem. 2002;277:38895–900.
Brewer L, Corzett M, Lau EY, Balhorn R. Dynamics of protamine 1 binding to single DNA molecules. J Biol Chem. 2003;278:42403–8.
Benyajati C, Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976;9:393–407.
Terquem T, Dadoune JP. Aniline blue staining of human spermatozoa chromatin. Evaluation of nuclear maturation. In: Adre J, editor. The sperm cell. The Hague: Martinus Nijhoff Publishers; 1983. p. 249–52.
Liu DY, Baker HW. Sperm nuclear chromatin normality: relationship with sperm morphology, sperm-zona pellucida binding, and fertilization rates in vitro. Fertil Steril. 1992;58:1178–84.
Manicardi GC, Bianchi PG, Pantano S, Azzoni P, Bizzaro D, Bianchi U, et al. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod. 1995;52:864–7.
Bianchi PG, Manicardi GC, Bizzaro D, Bianchi U, Sakkas D. Effect of deoxyribonucleic acid protamination on fluorochrome staining and in situ nick-translation of murine and human mature spermatozoa. Biol Reprod. 1993;49:1083–8.
Mello ML. Induced metachromasia in bull spermatozoa. Histochemistry. 1982;74:387–92.
Andreetta AM, Stockert JC, Barrera C. A simple method to detect sperm chromatin abnormalities: cytochemical mechanism and possible value in predicting semen quality in assisted reproductive procedures. Int J Androl. 1995;18 Suppl 1:23–8.
Hammadeh ME, Zeginiadov T, Rosenbaum P, et al. Predictive value of sperm chromatin condensation (aniline blue staining) in the assessment of male fertility. Arch Androl. 2001;46:99–104.
Baker H, Liu D. Assessment of nuclear maturity. In: Acosta A, Kruger T, editors. Human spermatozoa in assissted reproduction. London: CRC Press; 1996. p. 193–203.
Foresta C, Zorzi M, Rossato M, et al. Sperm nuclear instability and staining with aniline blue: abnormal persistence of histones in spermatozoa in infertile men. Int J Androl. 1992;15:330–7.
Hammadeh ME, Stieber M, Haidl G, et al. Association between sperm cell chromatin condensation, morphology based on strict criteria, and fertilization, cleavage and pregnancy rates in an IVF program. Andrologia. 1998;30:29–35.
Erenpreiss J, Bars J, Lipatnikova V, et al. Comparative study of cytochemical tests for sperm chromatin integrity. J Androl. 2001;22:45–53.
Erenpreiss J, Jepson K, Giwercman A, et al. Toluidine blue cytometry test for sperm DNA conformation: comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum Reprod. 2004;19:2277–82.
Erenpreisa J, Erenpreiss J, Freivalds T, et al. Toluidine blue test for sperm DNA integrity and elaboration of image cytometry algorithm. Cytometry. 2003;52:19–27.
Tsarev I, Bungum M, Giwercman A, Erenpreisa J, Ebessen T, Ernst E, et al. Evaluation of male fertility potential by Toluidine Blue test for sperm chromatin structure assessment. Hum Reprod. 2009;24:1569–74.
Manicardi GC, Bianchi PG, Pantano S, et al. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod. 1995;52:864–7.
Esterhuizen AD, Franken DR, Lourens JG, et al. Sperm chromatin packaging as an indicator of in-vitro fertilization rates. Hum Reprod. 2000;15:657–61.
Sakkas D, Urner F, Bianchi PG, et al. Sperm chromatin anomalies can influence decondensation after intracytoplasmic sperm injection. Hum Reprod. 1996;11:837–43.
Sakkas D, Urner F, Bizzaro D, et al. Sperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo development. Hum Reprod. 1998;13:11–9.
Fernandez JL, Vazquez-Gundin F, Delgado A, et al. DNA breakage detection-FISH (DBD-FISH) in human spermatozoa: technical variants evidence different structural features. Mutat Res. 2000;453:77–82.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Tsarev, I., Erenpreiss, J. (2011). Cytochemical Tests for Sperm Chromatin Maturity. In: Zini, A., Agarwal, A. (eds) Sperm Chromatin. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-6857-9_12
Download citation
DOI: https://doi.org/10.1007/978-1-4419-6857-9_12
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-1781-2
Online ISBN: 978-1-4419-6857-9
eBook Packages: MedicineMedicine (R0)