Abstract
Amphotericin B (AmB) has been the cornerstone of antifungal therapy for almost 50 years. Discovered in the late 1950s, it was approved for human use as an antifungal agent in 1960. Initial formulations of AmB were plagued with impurities. Allergic responses, presumably secondary to these impurities, and endotoxin-like infusion-related reactions were common. Although improvements in purification and fermentation over the last 30 years have enhanced tolerability, infusion-related reactions and renal dysfunction are still commonplace with the use of the deoxycholate solubilized formulation. Formulations using a lipid carrier have significantly improved tolerability. Safety aside, AmB remains the most effective, broad-spectrum, fungicidal agent with the greatest experience for the treatment of systemic mycoses. Both intrinsic and acquired resistance are limited. The treatment failures seen with AmB are multifaceted. These can be attributed to delays in diagnosis of invasive mycoses, the immune compromised state of the patient being treated, the unique pharmacokinetic/pharmacodynamic properties of the different formulations, and dose limitations related to toxicity. In an effort to enhance antifungal efficacy and reduce toxicity, AmB has been combined with other antifungals and new nonlipid formulations are being evaluated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Invasive Aspergillosis
- Cryptococcal Meningitis
- Lipid Formulation
- Systemic Fungal Infection
- Febrile Neutropenic Patient
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Amphotericin B (AmB) has been the cornerstone of antifungal therapy for almost 50 years. Discovered in the late 1950s, it was approved for human use as an antifungal agent in 1960. Initial formulations of AmB were plagued with impurities. Allergic responses, presumably secondary to these impurities, and endotoxin-like infusion-related reactions were common. Although improvements in purification and fermentation over the last 30 years have enhanced tolerability, infusion-related reactions and renal dysfunction are still commonplace with the use of the deoxycholate solubilized formulation. Formulations using a lipid carrier have significantly improved tolerability. Safety aside, AmB remains the most effective, broad-spectrum, fungicidal agent with the greatest experience for the treatment of systemic mycoses. Both intrinsic and acquired resistance are limited. The treatment failures seen with AmB are multifaceted. These can be attributed to delays in diagnosis of invasive mycoses, the immune compromised state of the patient being treated, the unique pharmacokinetic/pharmacodynamic properties of the different formulations, and dose limitations related to toxicity. In an effort to enhance antifungal efficacy and reduce toxicity, AmB has been combined with other antifungals and new nonlipid formulations are being evaluated.
Alternative formulations of AmB have been devised in an effort to improve the therapeutic index of this agent. A water-soluble methyl ester preparation showed promise; unfortunately, several patients developed leukoencephalopathy in clinical trials and the product was abandoned [1]. Nanoparticle science has led to investigation of novel orally absorbed AmB and intravenous products [2, 3]. In addition, highly purified AmB and biosynthesis of deoxyamphotericins have been reported in the literature [4]. Nebulized AmB lipid formuations are currently being investigated for prophylaxis in lung transplant receipients [5].
This chapter discusses FDA-approved AmB deoxycholate (AmB-d) and the lipid preparations of amphotericin B separately. Where appropriate, we compare and contrast these different formulations with an emphasis on unique pharmacologic properties or clinically relevant differences in toxicity or outcome that favor one preparation over another.
Amphotericin B Deoxycholate
Chemistry
AmB is one of several polyene antifungals produced by the soil actinomycete Streptomyces nodosus. The AmB molecule is a heptaene macrolide consisting of seven conjugated double bonds within the main ring, a connecting mycosamine through a glycoside side chain, and a connecting free carboxyl group (Fig. 1). AmB is relatively insoluble in water and derives its name from its amphoteric property to form methanol soluble salts under both basic and acidic conditions [6]. AmB is available as an intravenous preparation formulated by combination with sodium deoxycholate (AmB-d), which results in formation of a micellar dispersion upon reconstitution in 5% dextrose in water [6].
Mechanisms of Action
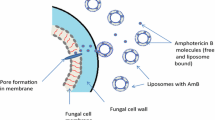
The primary antifungal activity of AmB is mediated by its preferential binding to fungal cell membrane ergosterol. This interaction results in the formation of pores consisting of eight AmB molecules in the membrane, allowing leakage of cellular components, such as potassium, that ultimately leads to cell death [7]. Although AmB has a greater affinity for the fungal ergosterol, it still has some affinity for binding to the cholesterol of mammalian cell membranes. The latter probably plays an important role in its associated toxicity [8].
There is also evidence suggesting that cell death may be due in part to the oxidizing properties of the drug that results in the production of reactive oxygen species and lipid peroxidation of fungal cell membranes [7]. In support of oxidative cell injury, Sokol-Anderson and colleagues have shown that AmB-mediated lysis of Candida albicans protoplasts and whole cells is reduced, independent of potassium leakage, in the absence of oxygen and in the presence of exogenous catalase and superoxide dismutase [9]. The presence of seven conjugated double bonds in the chemical structure of AmB renders it prone to auto-oxidation [9], leading some investigators to speculate that AmB may also act as an antioxidant, although clinical data to support this hypothesis are lacking. Finally, AmB also has been shown to inhibit the respiration of actively metabolizing Aspergillus fumigatus [10].
AmB may indirectly modulate antifungal efficacy by its ability to alter immune function. The immunomodulatory effects of AmB have been found to be diverse, and research results are contradictory. The reported differences in AmB-induced immunomodulation may be the result of a number of factors, including antifungal concentration, the in vitro conditions, and the animal model used. AmB has been shown to act as an immunoadjuvant by stimulating cell proliferation and cell-mediated immunity in murine models [11]. AmB has also been shown to enhance the phagocytic, tumoricidal, and antibacterial activity of macrophages along with increasing colony-stimulating factor concentrations in mice [12]. AmB induces production of multiple inflammatory cytokines (i.e., IL-1β, TNF-α, and IL-1RA) and increases nitric oxide synthesis in vitro while increasing immune modulators (i.e., IL-12 and IFN-γ) in mice [13–15].
In contrast, AmB has been shown to inhibit chemotactic responsiveness, phagocytic capacity, and killing by human neutrophils [16]. Inhibition of both spontaneous and antigen-induced transformation, as well as antibody-dependent cellular toxicity of human lymphocytes, has been reported with AmB [17]. It has also been reported to diminish human peripheral blood mononuclear cell along with T-cell responses to phytohemagglutinin [18] and to impair NK cell activity [17].
Taken collectively, these data suggest that AmB exerts its direct antifungal activity through three mechanisms of action: pore formation, oxidative damage, and inhibition of metabolic activity. While the direct antifungal activity of AmB has been extensively validated, the in vivo role of its immunomodulatory properties has not been sufficiently defined.
Spectrum of Activity
AmB is active against most of the common yeasts, moulds, and dimorphic fungi causing human infection including: Candida species, Cryptococcus neoformans, Blastomyces dermatitidis, Histoplasma capsulatum, Coccidioides species, Paracoccidioides brasiliensis, Sporothrix schenckii, Aspergillus species, and the zygomycetes. This polyene also has some degree of activity against the protozoa Leishmania brasiliensis, Trypanosoma species, and Naegleria fowleri [6].
Relatively few organisms manifest intrinsic resistance to AmB. Scedosporium apiospermum (Pseudallescheria boydii), Candida lusitaniae, Candida guilliermondii, Scopulariopsis species, Aspergillus terreus and Fusarium species generally are considered intrinsically resistant to AmB [19]. Acquired resistance to AmB, whether through selective laboratory techniques or after clinical usage, appears to be uncommon. Recently, however, resistant isolates of C. albicans, C. glabrata, C. tropicalis, and C. neoformans have been isolated from patients with AIDS [20–23].
Studies of resistant clinical isolates of C. albicans and C. neoformans suggest that resistance occurs through alterations of the genes encoding α8,7-isomerase or α5,6-desaturase within the sterol biosynthesis pathway. These isolates accumulate alternative sterols, allowing the organism to evade the activity of AmB [21, 22, 24]. Others have suggested that resistance to AmB in yeasts may occur through increased catalase activity, impairing AmB-induced oxidative damage [24, 25].
Susceptibility Testing
The recent efforts of the Clinical Laboratory Standards Institute (CLSI) have been instrumental in the development of standardized methodology for antifungal susceptibility testing for yeasts and moulds [26–28]. Despite these improvements, the routine use of susceptibility testing of clinical isolates to AmB is not recommended. Susceptibility testing of clinical isolates may be helpful for patients who are failing therapy. For example, clinical failure using AmB-d to treat serious candidal and cryptococcal infections has been associated with minimum inhibitory concentrations (MICs) of >1.0 μg/mL [20, 29].It should be noted, however, that clinical failure in AmB-treated patients is not necessarily indicative of fungal resistance, but is often related to the underlying immunodeficiency of the patient. Susceptibility testing may also be clinically useful in guiding treatment of rare pathogens for which resistance is likely or unpredictable. Interpretive break points that correlate in vitro activity with clinical outcomes are limited for moulds.
Pharmacology and Pharmacokinetics
Polyenes are poorly absorbed through mammalian membranes (less than 5%), hence the requirement of an intravenous formulation for the treatment of systemic mycoses. Following an intravenous infusion, AmB-d is bound primarily to lipoproteins, cholesterol, and erythrocytes. Peak serum concentrations of approximately 1–3 μg/mL are achieved during the first hour following a 4- to 6-h AmB-d infusion at a dose of 0.6 mg/kg. Serum concentrations rapidly fall to a prolonged plateau phase with measured concentrations of 0.2–0.5 μg/mL. Following an initial half-life of 24–48 h, there is a terminal elimination half-life of approximately 15 days. This terminal elimination phase most likely represents the slow release of AmB from the tissues (Table 1).
AmB-d is distributed to many tissues, including the lungs, spleen, liver, and kidneys [30]. The volume of distribution is 4 L/kg and appears to follow a three-compartment model of distribution. AmB-d, however, does not distribute into adipose tissue, supporting the premise that dosage should be based on lean body mass. Unfortunately, the measurement of lean body mass is not always practical. Hence dosing of AmB-d in obese patients should be based on calculated ideal body weight. AmB-d is bound extensively in tissues and can be detected in the liver, spleen, and kidney for months after treatment has been terminated [31]. Despite this extensive and prolonged tissue binding, the relationship of serum versus tissue concentration and clinical efficacy or toxicity has not been clearly established.
AmB-d concentrations in peritoneal, pleural, and synovial fluids are less than half of the simultaneous serum concentrations [32]. Concentrations in the vitreous body in noninflamed eyes are not measurable. Although clinical efficacy of AmB-d has been repeatedly documented for the treatment of central nervous system fungal infections, such as cryptococcal meningitis, cerebrospinal fluid levels are low, usually less than 5% even in the presence of inflamed meninges. This enhanced clinical efficacy may reflect higher levels of AmB-d in the meninges as compared to the cerebrospinal fluid, as has been documented in animal models of meningitis [6].
Despite almost 50 years of clinical experience, little is known about the metabolism of AmB-d. No metabolites have yet been identified. Less than 5% of the administered dose is excreted in the urine and bile. Serum concentrations, as such, are not changed and accumulation of AmB-d does not occur in patients with hepatic or renal failure. Likewise, hemodialysis or peritoneal dialysis does not influence serum concentrations [6, 33–35].
Several pharmacokinetic parameters of AmB-d are different in children than in adults [36, 37]. For instance, children have a smaller volume of distribution and a larger clearance compared to adults. When equivalent weight-based doses of AmB-d are administered, peak serum concentrations in children are approximately one-half of those obtained in adults. The increased clearance of AmB-d in children may, in part, explain the clinical finding that higher doses are better tolerated in children as compared to adults. Cerebrospinal fluid concentrations of AmB-d treated neonates are higher than those noted in adults.
Pharmacodynamics
Pharmacodynamics involves the integration of several pharmacologic measurements made in vitro (e.g., susceptibility studies, time-kill studies, dynamic models, viability, postantifungal effect [PAFE], etc.) and in vivo (drug concentrations, toxicity, efficacy, etc.). For antibacterial agents. several variables have been assigned quantitative limits that are predictive of therapeutic success and include the time that the serum drug concentration exceeds MIC [T > MIC]; the ratio of maximum serum drug concentration to MIC [C max:MIC]; and the ratio of the area under the concentration–time curve during a 24-h dosing period to MIC [AUC0–24:MIC] [38]. These parameters have proven useful in classifying antibiotics as either concentration-dependent or time-dependent in their bactericidal activity and have also been instrumental in selecting the optimal antibacterial treatment regimens.
Pharmacodynamic parameters are less clearly defined for the antifungal drugs. AmB has traditionally been portrayed as a concentration-dependent antifungal agent. Concentration-dependence is characterized by a long PAFE and therapeutic success when the C max:MIC ratio is high. Determination of C max:MIC ratios of AmB and their relationship to clinical outcome in human infections is incomplete. Additional studies are required to evaluate the predictive value and clinical usefulness of these pharmacodynamic parameters in optimizing therapy of human infections.
Initial studies evaluating AmB pharmacodynamic models in vitro and in vivo have been contradictory. For example, the PAFE of AmB-d for Candida species was prolonged when studied in vivo. In a study of neutropenic mice infected with Candida, the antifungal effects of AmB-d were observed for 23–30 h [39]. In contrast, several in vitro studies have shown a shorter duration of antifungal effect (0–10.6 h) depending on the MIC and the length of drug exposure [40, 41]. The longer PAFE noted in vivo might be due to the immunomodulatory properties and/or the slow release of AmB-d from tissue. Also confounding pharmacodynamic studies on drug concentration at the site of infection, the MIC of the organism, and the density of organisms at the site of infection impact the composite sum of these factors. Data on these important parameters affecting antifungal pharmacodynamics and clinical outcome have not been adequately defined.
A few studies have attempted to define clinically relevant pharmacodynamic parameters of AmB-d that affect clinical outcome. Drutz and colleagues reported improved clinical outcomes when AmB-d serum concentrations were maintained greater than twice the fungal MIC [42]. Animal models of infection have further demonstrated that high peaks relative to the MIC are correlated with improved survival and decreased fungal burden, as defined by CFU per gram of tissue in a variety of organs [39, 43]. When studied in a neutropenic mouse model of infection, a serum C max: MIC ratio of 10:1 was associated with the greatest decrease in kidney fungal burden. Additionally, using nonlinear regression, a strong relationship was also found for the length of time the serum concentration remained above the MIC. This latter pharmacodynamic property is characteristic of a non-concentration-dependent, that is time-dependent antifungal agent [39, 43]. A reasonable hypothesis in reconciling these results involves the enhanced tissue binding of AmB-d. Specifically, the enhanced tissue storage and long elimination rates of AmB-d confound traditional dynamic estimates, and the release of free drug from tissue sites is difficult to discriminate from the residual effects of inhibitory antifungal concentrations.
Adverse Effects
The utility of AmB-d is hindered by significant toxicity. Although AmB-d has a greater affinity for ergosterol, its affinity for cholesterol in the mammalian cell membrane likely plays a role in its toxicity [8]. The resulting nonselective disruption of mammalian cells is believed to be the underlying cause of most of the adverse effects associated with this drug [44–46].
It is clinically useful to classify AmB-d-associated reactions as infusion-related, dose-related, or idiosyncratic reactions. Infusion-related reactions include a symptom complex of fever, chills, nausea, vomiting, headache, and hypotension. Infusion-related fever and chills are observed in over half the patients receiving AmB-d. Our clinical experience is that patients having severe infusion reactions often have undiagnosed adrenal insufficiency (especially those with disseminated histoplasmosis); consequently, adrenal function should be evaluated in these individuals. These infusion-related effects are believed to be due to the production of proinflammatory mediators by monocytes and macrophages in response to AmB-d [46–48]. AmB-d has been shown to up-regulate a number of genes encoding pro-inflammatory proteins such as IL-1α, IL-1β, TNFα, IL-8, MIP-1α, MIP-1β, and MCP-1 [14, 47, 48]. Production of these respective gene products, along with release of PGE2 from endothelial cells, likely mediates the infusion-related toxicity. The patient-to-patient variability of AmB-d infusion-related toxicity may correlate with quantitative differences in cytokine production in vivo. Other adverse effects that may be related to the cytokine mechanism include thrombophlebitis, nausea, vomiting, headaches, myalgias, and arthralgias.
Less frequently, cardiac arrhythmias have been reported. Arrhythmias may occur when high concentrations are rapidly infused, especially in patients with heart disease, patients with renal failure, and those receiving an accidental drug overdosage [49]. Caution is also recommended for patients receiving the drug by a central venous catheter.
Dose-related reactions occur with longer courses of treatment and are related to total dose. AmB-induced nephrotoxicity includes decreased glomerular filtration, decreased renal blood flow, and renal tubular acidosis. Secondary consequences, such as hypokalemia and hypomagnesemia are common. Additionally, normochromic, normocytic anemia is frequently observed, likely in response to decreased erythropoietin production [50]. Calcium deposits have been found in the renal tubule lumen, tubule cells, and interstitium upon histopathologic examination of renal tissue specimens obtained from patients treated with AmB-d [51, 52]. Reversible renal impairment occurs within 2 weeks of therapy in more than 80% of AmB-d treated patients [53]. Onset of nephrotoxicity may occur before laboratory or clinical signs and symptoms are evident. With the onset of nephrotoxicity, the action taken ranges from AmB-d discontinuation or dosage reduction, stopping concurrent nephrotoxic drugs, changing to an alternate day infusion schedule, or pretreating patients with normal saline. There are no clinical trials that identify the optimal therapeutic option.
The mechanism of AmB-d-induced nephrotoxicity is multifaceted. Animal studies have demonstrated the vasoconstrictive properties of AmB-d, particularly with regard to the afferent arteriole [54]. Increased tubule permeability has also been demonstrated [55]. Other studies suggest that AmB-d inhibits sodium-potassium ATPases and affects proton exchange, which could contribute to renal tubular acidosis. Conversely, damage to the medullary thick ascending limb was ameliorated by ouabain in a rat kidney model, suggesting an alternative role for this pump in AmB-d-induced nephrotoxicity. Others have suggested a role for AmB-d-induced release of prostaglandins and leukotrienes as well as oxidative injury in this process [52].
The tubuloglomerular feedback mechanism normally involved in renal homeostasis also plays a prominent role in the pathogenesis of AmB-d-induced nephrotoxicity [56]. This feedback process is believed to be activated by transport of sodium chloride across the macula densa cells into the distal nephron, resulting in constriction of the afferent arteriole, possibly mediated by adenosine, and subsequent impairment of glomerular filtration [51]. Dehydration and sodium depletion accentuate this response and exacerbate AmB-d related renal failure. Sodium loading with intravenous administration of 500–1,000 mL of normal saline prior to initiation of AmB-d, when tolerated by the patient, is recommended in order to decrease the likelihood of renal toxicity [57].
Idiosyncratic reactions are rare, unpredictable, and include anaphylaxis, liver failure, hypertension, and respiratory failure.
Drug Interactions
Corticosteroids and nonsteroidal antiinflammatory drugs (NSAIDs) are the agents most frequently used to prevent infusion-related toxicities [58]. Controversy exists concerning the risk:benefit ratio of corticosteroids for prevention of infusion-related reactions. Clinical experience overwhelmingly supports the therapeutic benefit of administering hydrocortisone to patients suffering infusion-related reactions. However, circumstantial evidence suggests that administration of this immunosuppressant could be detrimental to the therapeutic success of AmB-d [59, 60]. Although further investigation of this therapeutic issue is required, it seems prudent to limit the dose and duration of corticosteroids by a therapeutic taper once infusion-related reactions are ameliorated. Likewise, routine use of NSAIDs for premedication should be avoided owing to their potential to enhance AmB-d related renal insufficiency. Intravenous meperidine has proven useful in abrogating infusion-related rigors [61].
Enhanced nephrotoxicity associated with AmB-d administration has been observed with cyclosporine or tacrolimus, diuretics, NSAIDs, pentamidine [62], and other nephrotoxic agents, such as aminoglycosides or radio-opaque dyes. Diligent monitoring of renal function is warranted in patients treated concurrently with these nephrotoxic agents.
A variety of other therapeutic agents may result in AmB-d associated adverse events that require diligent monitoring. Pulmonary leukostasis and respiratory failure associated with concomitant leukocyte transfusions or indium-labeled leukocyte scanning can be life-threatening [63, 64]. However, the incidence of this reaction has markedly decreased with less frequent use of leukocyte infusions. Skeletal muscle relaxants and neuromuscular blocking agents have been reported to enhance curariform effects related to hypokalemia. AmB-d-induced hypokalemia can also enhance the cardiac effects of digitalis glycosides. In these cases, patients suffered cardiac dysfunction that would be difficult to differentiate from the direct effects of AmB-d on the myocardial tissue [46]. Amiloride has been suggested for concomitant administration to decrease the hypokalemia in patients receiving digitalis glycosides. However, the effect is difficult to predict and requires further study. Cyclophosphamide and doxorubicin appear to penetrate cells more effectively when administered with AmB-d and this results in enhanced toxicity [65].
Drug interactions also encompass incompatibilities of pharmaceuticals in solution. AmB-d, and the AmB lipid formulations are incompatible in solutions with high saline content, including lactated Ringer’s or normal (0.9%) sodium chloride. In addition, the infusion of AmB formulations concomitantly with other antiinfectives (amikacin, ampicillin, aztreonam, carbenicillin, clindamycin, cotrimoxazole, fluconazole, gentamicin, linezolid, nitrofurantoin, penicillin G, and piperacillin) may induce precipitation of either agent.
Combination Therapy
One approach to improving the activity and/or toxicity profile of AmB-d is its administration in combination with another antifungal or pharmacologic agent. Animal data and anecdotal experience suggest that colony-stimulating factors, rifampin, or tetracyclines may be effective adjuvants [66, 67]. A more traditional approach would be to use another antifungal in combination with AmB-d. While many in vitro studies of antifungal combinations with AmB-d have been performed, the results of these have not been consistent. For example, pretreatment with an imidazole prior to the administration of AmB-d has been reported to be antagonistic [68]. Other studies, however, have documented additive or synergistic activity when triazoles were combined with AmB-d [69]. Owing to these differing results, the routine use of an azole with AmB-d has not been recommended. However, one randomized blinded clinical trial showed no antagonism and actually improvement in clearing candidemia when fluconazole was combined with AmB-d [70]. In contrast to studies with the azoles, the clinical benefit of using AmB-d in combination with flucytosine for the treatment of cryptococcal meningitis has been clearly documented in both AIDS and non-AIDS patients [71–73]. In addition, smaller cohorts of patients with candidemia and other serious candidal infections have been treated successfully with AmB-d combined with flucytosine. Unfortunately, clinical studies evaluating the efficacy of other antifungal combinations are relatively few [74].
Administration
There are no well-controlled trials that delineate the optimal dosing regimen for AmB-d. The daily dose has traditionally ranged from 0.3 mg/kg up to 1.5 mg/kg depending on the specific mycosis and severity of disease. The duration of therapy for most systemic fungal infections has varied from 4 to 12 weeks, although courses of many months have been reported. The availability of the triazoles, however, has resulted in AmB-d being used for shorter treatment courses, usually until clinical improvement is evident, before step-down therapy is initiated with a less toxic azole.
Specific administration and dosing recommendations are as variable as the number of institutions that utilize this antifungal polyene. Selection of dosing regimens, including premedications, is often based on clinicians’ concerns for toxicity, rather than achievement of efficacy. Dosing recommendations that have been approved by the Food and Drug Administration are outlined in Table 1. Specific recommendations for the administration of AmB-d, based on the authors’ clinical experiences, are outlined in Table 2.
The practice of administering a 1 mg test dose of AmB prior to the initial dose, while recommended by the manufacturer, is controversial among clinicians [75]. The dose is administered as 1 mg AmB-d in 50 mL of D5W administered over 30–60 min. In most instances, however, the test dose is given as part of the initial dose, which in turn is then given in full if no adverse effects are observed. The test dose is designed to identify patients who will experience immediate type hypersensitivity reactions or pronounced infusion-related reactions. While evidence supporting this practice is sparse, many experienced clinicians continue to use this approach. Others argue that immediate type hypersensitivity would be observed with the test dose, whereas the AmB-d concentration provided by a test dose would be insufficient to produce the proinflammatory response responsible for fever and chills. Also of concern is the potential delay in therapy that may occur with the use of an initial test dose [75]. The authors do not recommend a test dose.
A second controversy centers around the length of infusions, e.g., short versus long. Several studies have explored toxicity and tolerability of standard infusion times (4–6 h) versus more rapid infusions of 2–4 h and even less than an hour [76]. The results of these studies indicate that rapid infusion times are equally well tolerated and have similar rates of adverse events as compared to infusions given over 4–6 h. Due to the risk of cardiac arrhythmias, rapid infusions should not be used in patients with renal failure, heart disease, and history of cardiac arrhythmias, and in those receiving AmB-d through a central venous catheter [77, 78]. A recent study reported less toxicity, including nephrotoxicity, in febrile neutropenic patients treated with continuous-infusion AmB-d when compared to patients treated with 4-h infusions [79]. However, clinical experience with continuous infusion of AmB-d is limited, and there is concern about achieving adequate serum concentrations for maximum efficacy of AmB. Until safety and efficacy are better documented, continuous infusion cannot be recommended at this time.
Other routes of administration for AmB-d are used when therapeutic goals are not or cannot be achieved with intravenous dosing [6, 69]. Topical preparations (3% lotion, creams, or ointments, 10-mg lozenges or oral suspension, 100 mg/mL) may be compounded as needed for the treatment of superficial or cutaneous yeast infections. Intrathecal or intraventricular routes have been used in refractory cases of fungal meningitis, most frequently coccidioidal meningitis [6, 69, 80]. Intrathecal administration is problematic due to the poor distribution and the development of arachnoiditis at the injection site. Intraventricular administration is preferred. Long-term administration should be performed using a subcutaneous Ommaya or Rickham reservoir. AmB-d should be mixed with sterile water to a final concentration of 250 μg/mL. Initial dosing (10–25 μg) can be escalated slowly up to a dose of 250 μg/day to as high as 500 μg/day day, depending on the mycosis being treated and patient’s tolerance of this therapy. This route of administration is often limited by local reactions (radicular pain, headache, vomiting, and arachnoiditis). More severe neurologic complications include ventricular hemorrhage and bacterial superinfection [80].
Ocular administration of AmB-d is frequently used for the treatment of fungal eye infections [6, 81]. Topical ophthalmic application (0.25–1.5% solution) or subconjunctival injection (100–200 μg/0.5 mL) is appropriate for most superficial infections. However, little medication penetrates into the vitreous body, and intravitreal injection of 5–10 μg/0.1 mL is often used for vitritis [81].
The therapeutic benefit and optimal dose of nonparenteral routes of administration are not well established, and local inflammatory responses specific to the sites of administration are common and are frequently dose limiting [6, 69]. Intraperitoneal administration for the treatment of peritoneal dialysis-associated Candida infections can be achieved by administering AmB-d within the dialysate or intraperitoneally, but this is extremely irritating and is no longer recommended [82]. Intraarticular doses (5–15 mg) administered for fungal arthritis are rarely indicated. Bladder instillation/irrigation with an AmB-d solution (50 mg/L) by continuous infusion through a triple-lumen catheter for 5 days has been used for candidal cystitis and candiduria [83] AmB-d (10 mg) in 5 ml has been administered twice a day via nebulization for prevention of pulmonary aspergillosis in neutropenic patients [84, 85]. Specific adverse reactions with aerosolized AmB-d include dyguesia, gastrointestinal distress, dyspnea, and cough. Less frequently, intracavitary irrigation has been used for treatment of pulmonary aspergilloma.
Use in Pregnancy
AmB is the antifungal agent with which there has been the most experience in pregnancy [86, 87]. Both the deoxycholate and lipid-based formulations are assigned to risk category B by their manufacturers. While the pharmacokinetics of AmB in pregnancy have not been studied, the drug appears to cross the placenta and enter the fetal circulation [85]. Among case reports of AmB use in pregnancy, azotemia was the most common maternal adverse drug reaction reported, followed by anemia, hypokalemia, acute nephrotoxicity, fever, chills, headache, nausea, and vomiting. Individual cases of possible fetal toxicity include transient acidosis with azotemia, anemia, transient maculopapular rash, and respiratory failure requiring mechanical ventilation. Only a single case of congenital malformation (microcephaly with a pilonidal dimple) has been associated with AmB-d [86]. To date there have been no reports of animal teratogenesis attributed to AmB [86, 87].
Lipid Preparations of Amphotericin B
Three lipid-based products are currently available in the United States: AmB colloidal dispersion (ABCD), liposomal AmB (L-AmB), and AmB lipid complex (ABLC) (Fig. 2). In addition to these commercial formulations, lipid-based preparations have been admixed by individual institutions by combining AmB deoxycholate and 20% lipid emulsion [88, 89]. While AmB lipid emulsion is attractive from the standpoint of cost, several concerns have been raised, encompassing the stability of the emulsion, the need for filtration, and the possibility of fat overload syndrome. One pharmaceutical company pursued development of this formulation for several years, but a stable suspension was not achieved. Administration of this formulation is, therefore, not recommended [90].
Chemistry
The commercial lipid formulations are distinct as regards their phospholipid content, particle size and shape, electrostatic charge, and bilayer rigidity [91]. Liposomal AmB is formulated as a unilamellar spherical vesicle with a single lipid bilayer comprised of hydrogenated phosphatidylcholine, cholesterol, and distearoyl phosphatidylglycerol in a 2:1:0.8 ratio. Amphotericin is located on the inside and outside of the vesicle. L-AmB has the smallest particle size. ABCD was developed by complexing AmB with cholesteryl sulfate in a 1:1 molar ratio. These complexes form tetramers that have a hydrophobic and a hydrophilic portion. The tetramers aggregate to form disk-like structures that are larger in size than L-AmB. ABLC consists of nonliposomal AmB-complexed ribbon structures and was originally derived from multilaminar liposomes prepared by mixing two phospholipids, dimyristoyl phosphatidylcholine (DMPC) and dimyristoyl phosphatidylglycerol (DMPG) in a 7:3 molar ratio. ABLC is much larger than the other two formulations [91–93] (Table 1).
Proposed Mechanisms for Enhanced Therapeutic Index
Although the lipid formulations have been shown to have an improved therapeutic index as compared to AmB-d, the mechanism(s) by which this occurs has not been adequately defined. Several mechanisms have been proposed. The unifying concept in all of these proposals involves the ability of lipid formulations to prevent binding to the kidney and the selective distribution of lipid-bound AmB to other tissues [91].
The first mechanism involves the rapid endocytic uptake of lipid-associated AmB by macrophages in tissues, often at the sites of infection. Following this targeted delivery, AmB is then slowly released into the tissues and the circulation. In addition to this selective tissue targeting, macrophage uptake of AmB also limits the amount of free drug, and presumably also LDL-bound drug, in the circulation capable of binding to human cells. The second mechanism involves the selective transfer of AmB from the lipid carrier to the fungal cell membrane. In this instance, AmB has a stronger affinity for the lipid carrier than for the cholesterol in mammalian cells. On the other hand, the affinity of AmB for ergosterol in the fungal cell is stronger than its affinity for either the lipid carrier or cholesterol. A third mechanism proposes that the lipid-based formulations are less nephrotoxic by limiting the amount of free drug in the blood and by preventing AmB binding to circulating LDL. Free AmB and LDL-bound AmB are considered to be more nephrotoxic than either HDL or other lipid-bound AmB. The fourth mechanism proposes that the lipid-based formulations elicit reduced cytokines, such as TNF-α or IL-1 from human cells as compared with AmB-d. These proinflammatory cytokines are putative mediators for infusion-related reactions and nephrotoxicity. This fourth hypothesis is supported primarily by in vitro data. The final purported mechanism involves the action of extracellular phospholipases produced by yeasts and moulds in releasing the lipid-bound AmB at the site of infection. As such, more AmB is released in the infected tissues. The phospholipid carrier of ABLC is especially susceptible to these fungal phospholipases.
Therapeutic Indications
In general, all three lipid formulations are indicated for the treatment of systemic fungal infection in patients refractory to or intolerant of therapy with AmB-d (Table 1) [91, 93]. L-AmB has also been approved for the empiric therapy of presumed fungal infection in febrile neutropenic patients [94]. In routine clinical practice, however, lipid formulations are frequently used as primary therapy for patients with baseline renal insufficiency and in patients at high risk for renal failure, including transplant recipients and patients receiving concurrent treatment with other nephrotoxic agents. Lipid preparations, however, should not be used as primary therapy for dialysis-dependent patients unless they fail therapy with AmB-d. Some authors consider the lipid formulations, due to their high concentrations in the liver and spleen, to be ideal for the treatment of patients with chronic disseminated candidiasis [95]. Finally, many infectious disease physicians consider lipid formulations of AmB to be superior to AmB-d for the treatment of patients with aggressive mould infections, such as invasive aspergillosis and zygomycosis. The data supporting this use are anecdotal, and comparative trials documenting the superiority of lipid formulations for these infections are lacking at present.
Pharmacology and Pharmacokinetics
The lipid agents’ biochemical/biophysical properties have a profound effect on the pharmacology of these lipid formulations (Table 1). For example, L-AmB is not as readily taken up by macrophages, and L-AmB achieves higher serum concentrations and a greater area under the curve (AUC) in blood compared to AmB-d or the other lipid preparations. On the other hand, the larger lipid formulation, ABLC, is more readily taken up by the tissues and has the greatest volume of distribution. Comparative data on the pharmacokinetic parameters of the lipid formulations, either compared to each other or to AmB-d, are limited. However, profound differences in some of the parameters have been documented and have led to unique therapeutic options (Table 1) [92, 96]. In amimals, ABLC lung penetration far exceeds (70- to 375-fold) the penetration by other formulations [97]. This tissue saturation also results in increased drug clearance from the serum. Whether any of these pharmacologic differences significantly affect clinical outcome or toxicity has not been studied adequately.
Pharmacodynamics
Owing to a variety of confounding variables, the pharmacodynamic information obtained with AmB-d cannot be directly extrapolated to the lipid formulations. In general, studies utilizing AmB lipid formulations have revealed a poor correlation between pharmacodynamic parameters and outcome [43]. Measurement of free AmB has been hypothesized to potentially resolve these discrepancies. However, the ability to accurately measure or predict free AmB is difficult [95].
As mentioned earlier, L-AmB achieves serum concentrations many-fold higher than the other lipid formulations of AmB, leading to a tremendously increased AUC versus time curve that in turn impacts all pharmacodynamic calculations. Using traditional calculations, L-AmB would not be predicted to be an effective therapy for central nervous system infections. To the contrary, L-AmB proved effective in animal studies [43] and a clinical trial of patients with AIDS-associated cryptococcal meningitis [98]. Although cerebrospinal fluid levels were low or undetectable, brain tissue concentrations exceeded expectations and, in the animal studies, were higher than those found with either AmB-d or the other lipid formulations. Brain tissue concentrations in patients receiving L-AmB were not as high as those documented in the animal studies [43, 98].
After 7 days of parenteral treatment of rabbits, mean AmB concentrations in inflamed eyes were significantly higher in the aqueous humor for L-AmB (0.73 μg/mL) compared with ABLC (0.03 μg/mL) and AmB-d (0.13 μg/mL). Levels in the vitreous body were also higher for L-AmB (0.47 μg/mL) than for ABLC (0.27 μg/mL) and AmB-d (0.16 μg/mL). Little, if any AmB-d can be detected in noninflamed eyes [99, 100].
Disproportionate distribution into the reticuloendothelial system has been observed for two lipid formulations, ABLC and L-AmB. As a result, very high tissue concentrations of these agents are detected in the liver and spleen relative to serum. These high tissue concentrations have been hypothesized to be a therapeutic advantage for these agents in treating patients with chronic disseminated candidiasis [95]. In support of this theory, clearance of C. albicans from the liver was superior in mice treated with L-AmB (1.5 mg/kg) compared to mice treated with AmB-d at equal doses [101]. In contrast, clearance of yeasts from lung was not enhanced in L-AmB-treated mice, but high concentrations of ABLC were detected in lung tissue, suggesting that this fomulation may be optimal for the treatment of pulmonary mycoses.
Adverse Events
All three lipid-based preparations currently available in the United States exhibit less nephrotoxicity than AmB-d [91, 102]. However, infusion-related toxicities similar to AmB-d are still observed [103, 104]. Several studies have demonstrated significantly fewer infusion-related adverse events associated with L-AmB when compared with AmB-d or with ABLC [91, 94, 102].
Although uncommon, acute respiratory events have been associated with administration of AmB and are typically characterized by tachypnea, dyspnea, and wheezing. Recently, there have also been reports of chest discomfort and altered pulmonary function associated with the lipid-based preparations of AmB [105, 106]. In fact, a triad of symptoms including: (1) chest pain, dyspnea, and hypoxia; (2) severe abdominal, flank, and leg pain; and (3) flushing and urticaria, has been reported with L-AmB [107]. These reactions appear in approximately 20% of patients, start within 5 min of infusion, and respond to antihistamines (diphenydramine).
The mechanisms causing these “uncommon” reactions are unclear, but may be related to the ability of AmB to elicit chemokine production from monocytes [14, 48]. The ability of IL-8 to recruit neutrophils could then mediate the pulmonary toxicity occasionally observed during administration of this agent. The lipid formulations of AmB deliver higher amounts of drug to the pulmonary tissue [105]. Thus, it is conceivable that enhanced pulmonary neutrophil recruitment in response to elevated local concentrations of IL-8 could lead to pulmonary leukostasis and thereby explain in part the pulmonary toxicity associated with AmB preparations. Indeed, studies in animal models have demonstrated that AmB pulmonary toxicity involves neutrophil recruitment to the lungs [108, 109]. Another possibility is that the lipid component of these preparations may itself contribute to these physiologic effects. Irrespective of the cause of the pulmonary toxicity, it seems prudent to administer the initial dose of any of the AmB lipid formulations under close observation and to reduce the rate of infusion in instances in which these effects are observed [105, 106].
Other adverse events reported with the lipid preparations include headache, hypotension, hypertension, diarrhea, nausea, vomiting, and rashes. Laboratory abnormalities reported include hypokalemia, hypomagnesemia, hypocalcemia, elevated liver function tests, and thrombocytopenia [91]. Regarding frequency of infusion-related adverse events of available AmB preparations, data suggest the following rank order by greatest to least frequency: AmB-d > ABCD > ABLC > L-AmB.
Comparative Trials
Comparative trials between lipid AmB preparations and AmB-d are enlightening. Empiric therapy for febrile neutropenic patients has received the most attention. In two different studies, ABCD (4 mg/kg/day) and L-AmB (3 mg/kg/day) were each compared to standard therapy with AmB-d (0.6–0.8 mg/kg/day) [94, 104]. In both studies, patients treated with the lipid preparations had a more rapid defervescence and lower death rate, although in neither study were these clinical differences statistically significant. In contrast, patients receiving either of the lipid preparations had statistically superior outcomes compared to patients treated with AmB-d for (1) the time to onset and rates of renal dysfunction; (2) rates of infusion-related reactions; and (3) prevention of breakthrough invasive fungal infections [94, 104]. Another study of therapy for febrile neutropenic patients compared two different doses of L-AmB (3 mg/kg/day and 5 mg/kg/day) to ABLC (5 mg/kg/day). Clinical outcomes were equivalent for all patient groups, except that the rates of renal dysfunction were significantly less for both doses of L-AmB compared to the ABLC formulation [102].
Comparative studies of the different AmB formulations in the treatment of documented infections have been primarily nonblinded and limited in number. ABCD (0.5–8 mg/kg/day), L-AmB (4 mg/kg/day) and ABLC (1.2–5 mg/kg/day) have been compared with AmB-d (0.1–1.5 mg/kg/day) for the treatment of invasive aspergillosis [103, 110] and cryptococcal meningitis [98, 111]. Patients with proven or probable aspergillosis who received ABCD experienced higher response rates (50%) compared to a historical control group treated with AmB-d [103]. However, in a randomized, double-blind trial, ABCD showed equal but no better efficacy than AmB-d as therapy for invasive aspergillosis (52% vs 51%) [110]. In two open label, randomized trials comparing a lipid formulation for the treatment of AIDS-associated cryptococcal meningitis, the clinical and microbiologic responses rates favored the lipid preparations [98, 111]. Of note, in these studies, significantly lower rates of nephrotoxicity were observed in patients treated with the lipid formulations.
A randomized, blinded treatment trial compared AmB-d, 0.7 mg/kg daily, with L-AmB, 3 mg/kg daily, for AIDS patients who had moderately severe to severe disseminated histoplasmosis. L-AmB was found to be superior in regard to efficacy and time to defervescence and there were fewer adverse reactions in the L-AmB arm [112].
Administration and Dosage
The approved daily dose and rate of administration are different for each lipid formulation. Other than for ABCD, the maximal daily dose that can be safely administered in humans has not been adequately defined. More interestingly, the equivalent doses of the individual lipid formulations that compare to the recommended dose of AmB-d for a particular fungal infection has not been established.
The recommended initial dose of L-AmB is 3 mg/kg/day for empiric therapy and 3–5 mg/kg/day for documented systemic fungal infections. The drug is usually infused over 2 h, but the infusion time can be decreased to 1 h if tolerated. The currently approved daily dose of ABLC is 5 mg/kg, and this is infused at a rate of 2.5 mg/kg/h. Daily doses of L-AmB and ABLC have been titrated considerably higher than the recommended daily doses and appear to be well tolerated in selected patients with refractory diseases. Treatment with ABCD should be initiated with a daily dose of 3–4 mg/kg. The dose can then be escalated to 6 mg/kg/day based on patient tolerance and clinical response. The recommended maximal daily dose is 7.5 mg/kg. Infusion-related toxicities with ABCD become more severe with doses of 8 mg/kg or greater.
Costs
A major consideration regarding the lipid-based formulations of AmB is their high cost in comparison to AmB-d. Data indicate that the lipid formulations range from 10- to 50-fold higher in acquisition cost per dose [113]. These agents are less nephrotoxic than AmB-d, and their overall therapeutic:toxic ratio is clearly improved over that of the parent drug. However, superiority in clinical efficacy has been definitively established in head-to-head comparative trials only in the case of disseminated histoplasmosis. Consequently, well-done pharmacoeconomic studies are needed to justify the higher cost of the lipid formulations.
References
Schmitt HJ. New methods of delivery of amphotericin B. Clin Infect Dis. 1993;17 Suppl 2:S501–6.
Wasan EK, Bartlett K, Gershkovich P, et al. Development and characterization of oral lipid-based amphotericin B formulations with enhanced drug solubility, stability and antifungal activity in rats infected with Aspergillus fumigatus or Candida albicans. Int J Pharm. 2009;372:76–84.
Fukui H, Koike T, Saheki A, Sonoke S, Tomii Y, Seki J. Evaluation of the efficacy and toxicity of amphotericin B incorporated in lipid nano-sphere (LNS). Int J Pharm. 2003;263:51–60.
Cleary JD, Chapman SW, Swiatlo E, Kramer R. High purity amphotericin B. J Antimicrob Chemother. 2007;60(6): 1331–40.
Monforte V, Ussetti P, López R, et al. Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: pharmacokinetics and safety. J Heart Lung Transpl. 2009; 28:170–5.
Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–29.
Brajtburg J, Powderly WG, Kobayashi GS, Medoff G. Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother. 1990;34:183–8.
Abu-Salah KM. Amphotericin B: An update. Br J Biomed Sci. 1996;53:122–33.
Sokol-Anderson ML, Brajtburg J, Medoff G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J Infect Dis. 1986;154:76–83.
Sandhu DK. Effect of amphotericin B on the metabolism of Aspergillus fumigatus. Mycopathologia. 1979;68:23–9.
Bistoni F, Vecchiarelli A, Mazzolla R, Puccetti P, Marconi P, Garaci E. Immunoadjuvant activity of amphotericin B as displayed in mice infected with Candida albicans. Antimicrob Agents Chemother. 1985;27:625–31.
Lin H, Medoff G, Kobayashi GS. Effects of amphotericin B on macrophages and their precursor cells. Antimicrob Agents Chemother. 1977;11:154–60.
Mozaffarian N, Berman JW, Casadevall A. Enhancement of nitric oxide synthesis by macrophages represents an additional mechanism of action for amphotericin B. Antimicrob Agents Chemother. 1997;41:1825–9.
Rogers PD, Jenkins JK, Chapman SW, Ndebele K, Chapman BA, Cleary JD. Amphotericin B activation of human genes encoding for cytokines. J Infect Dis. 1998;178:1726–33.
Cenci E, Mencacci A, Del Sero G, Bistoni F, Romani L. Induction of protective Th1 responses to Candida albicans by antifungal therapy alone or in combination with an interleukin-4 antagonist. J Infect Dis. 1997;176:217–26.
Marmer DJ, Fields BT, France GL, Steele RW. Ketoconazole, amphotericin B, and amphotericin B methyl ester: Comparative in vitro and in vivo toxicological effects on neutrophil function. Antimicrob Agents Chemother. 1981;20:660–5.
Nair MPN, Schwartz SA. Immunomodulatory effects of amphotericin-B on cellular cytotoxicity of normal human lymphocytes. Cell Immunol. 1982;70:287–300.
Stewart SJ, Spagnuolo PJ, Ellner JJ. Generation of suppressor T lymphocytes and monocytes by amphotericin B. J Immunol. 1981;127:135–9.
Speeleveld E, Gordts B, Van Landuyt HW, De Vroey C, Raes-Wuytack C. Susceptibility of clinical isolates of Fusarium to antifungal drugs. Mycoses. 1996;39:37–40.
Powderly WG, Keath EJ, Sokol-Anderson M, Robinson K, Kitzd, Little JR. Amphotericin B resistant Cryptococcus neoformans in a patient with AIDS. Infect Dis Clin Pract. 1992;1:314–6.
Le TP, Tuazoncu CU, Levine M, Borum M, Rollhauser C. Resistance to fluconazole and amphotericin B in patients with AIDS who are being treated for candidal esophagitis. Clin Infect Dis. 1996;23:649–50.
Kelly SL, Lamb DC, Kelly DE, et al. Resistance to fluconazole and cross resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta 5, 6-desaturation. FEBS Lett. 1997;400:80–2.
Lass-Flörl C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses. 2009;52:197–205.
Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic stratagies. Antimicrob Agents Chemother. 1996;40:279–91.
Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12:501–17.
Park BJ, Arthington-Skaggs BA, Hajjeh RA, et al. Evaluation of amphotericin B interpretive breakpoints for Candida bloodstream isolates by correlation with therapeutic outcome. Antimicrob Agents Chemother. 2006;50:1287–92.
Clinical Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition. Document M27-A3. Wayne: Clinical and Laboratory Standards Institute, 2008
Clinical Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard CLSI document M38-A. Wayne: Clinical Laboratory Standards Institute, 2001.
Larsen RA, Bauer M, Brouwer AE, et al. In vitro-clinical correlations for amphotericin B susceptibility in AIDS-associated cryptococcal meningitis. Antimicrob Agents Chemother. 2007;51:343–5.
Collette N, van der Auwera P, Lopez AP, Heymans C, Meunier F. Tissue concentrations and bioactivity of amphotericin B in cancer patients treated with amphotericin B-deoxycholate. Antimicrob Agents Chemother. 1989;33:362–8.
Christiansen KJ, Bernard EM, Gold JWM, Armstrong D. Distribution and activity of amphotericin B in humans. J Infect Dis. 1985;152:1037–43.
van der Voort PH, Boerma EC, Yska JP. Serum and intraperitoneal levels of amphotericin B and flucytosine during intravenous treatment of criticlly ill patients with Candida peritonitis. J Antimicrob Chemother. 2007;59:952–6.
Atkinson Jr AJ, Bennett JE. Amphotericin B pharmacokinetics in humans. Antimicrob Agents Chemother. 1978;13:271–6.
Daneshmend TK, Warnock DW. Clinical pharmacokinetics of systemic antifungal drugs. Clin Pharmacokinet. 1983;8:17–42.
Gussak HM, Rahman S, Bastani B. Administration and clearance of amphotericin B during high-efficiency or high-efficiency/high-flux dialysis. Am J Kidney Dis. 2001;37:E45.
Starke JR, Mason Jr EO, Kramer WG, Kaplan SL. Pharmacokinetics of amphotericin B in infants and children. J Infect Dis. 1987;155:766–74.
Benson JM, Nahata MC. Pharmacokinetics of amphotericin B in children. Antimicrob Agents Chemother. 1989;33:1989–93.
Gunderson BW, Ross GH, Ibrahim KH, Rotschafer JC. What do we really know about antibiotic pharmacodynamics? Pharmacotherapy. 2001;21:302S–18.
Andes D, Stamsted T, Conklin R. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob Agents Chemother. 2001;45(3):922–6.
Turnidge JD, Gudmondsson S, Vogelman B, Craig WA. The postantibiotic effect of antifungal agents against common pathogenic yeast. J Antimicrob Chemother. 1994;34:83–92.
Ernst E, Klepser ME, Pfaller MA. Post-antifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob Agents Chemother. 2000;44:1108–11.
Drutz DJ, Spickard A, Rogers DE, Koenig MG. Treatment of disseminated mycotic infections. A new approach to amphotericin B therapy. Am J Med. 1968;5:405–18.
Groll AH, Giri N, Petraitis V, et al. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis. 2000;182:274–82.
Andreoli TE. On the anatomy of amphotericin B-cholesterol pores in lipid bilayer membranes. Kidney Int. 1973;4:337–45.
Hsuchen CC, Feingold DS. Selective membrane toxicity of the polyene antibiotics: studies on natural membranes. Antimicrob Agents Chemother. 1973;4:316–9.
Cleary JD, Chapman SW, Nolan RL. Pharmacologic modulation of interleukin-1 expression by amphotericin B-stimulated human mononuclear cells. Antimicrob Agents Chemother. 1992;36:977–81.
Rogers PD, Stiles JK, Chapman SW, Cleary JD. Amphotericin B induces expression of genes encoding chemokines and cell adhesion molecules in the human monocytic cell line THP-1. J Infect Dis. 2000;182:1280–3.
Rogers PD, Perason MM, Cleary JD, Chapman SW, Sullivan DC. Differential expression of genes encoding for immunodulatory proteins in response to amphotericin B in the human monocytic cell line THP-1 identified by cDNA array analysis. J Antimicrob Chemother. 2002;50:811–7.
Cleary JD, Hayman J, Sherwood J, Lasala GP, Piazza-Hepp T. Amphotericin B overdose in pediatric patients with associated cardiac arrest. Ann Pharmacother. 1993;27:715–8.
Lin AC, Goldwasser E, Bernard EM, Chapman SW. Amphotericin B blunts erythropoietin response to anemia. J Infect Dis. 1990;161:348–51.
Sabra R, Branch RA. Amphotericin B nephrotoxicity. Drug Saf. 1990;5:94–108.
Carlson MA, Condon RE. Nephrotoxicity of amphotericin B. J Am Coll Surg. 1994;179:361–81.
Butler WT, Bennett JE, Alling DW, Wertlake PT, Utz JP, Hill G. Nephrotoxicity of amphotericin B. Early and late effects in 81 patients. Ann Intern Med. 1964;61:175–87.
Sawaya BP, Weihprech TH, Campbell WR, et al. Direct basal vasoconstriction as a possible cause for amphotericin B nephrotoxicity in rats. J Clin Inves. 1991;87:2097–107.
Cheng JT, Witty RT, Robinson RR, Yarger WE. Amphotericin B nephrotoxicity: increased renal resistance and tubule permeability. Kidney Int. 1982;22:626–33.
Branch RA, Jackson EK, Jacqz E, et al. Amphotericin B nephrotoxicity in humans decreased by sodium supplements with coadministration of ticarcillin or intravenous saline. Klin Wochenschr. 1987;65:500–6.
Branch RA. Prevention of amphotericin B-induced renal impairment: a review of the use of sodium supplementation. Arch Intern Med. 1988;148:2389–94.
Goodwin SD, Cleary JD, Walawander CA, Taylor JW, Grasela TH. Pretreatment regimens for adverse events related to infusion of amphotericin B. Clin Infect Dis. 1995;20:755–61.
North RJ. The action of cortisone acetate on cell-mediated immunity to infection: Suppression of host cell proliferation and alteration of cellular composition of infective foci. J Exp Med. 1971;134:1485–500.
Snyder DS, Unanue ER. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982;129:1803–5.
Burks LC, Aisner J, Fortner CL, Wiernik PH. Meperidine for the treatment of shaking chills and fever. Arch Intern Med. 1980;140:483–4.
Antoniskis D, Larsen RA. Acute, rapidly progressive renal failure with simultaneous use of amphotericin B and pentamidine. Antimicrob Agents Chemother. 1990;34:470–2.
Wright DG, Robichaud KJ, Pizzo PA, Deisseroth AB. Lethal pulmonary reactions associated with the combination use of amphotericin B and leukcocyte transfusions. N Engl J Med. 1981;304:1185–9.
Dutcher JP, Kendall J, Norris D, Schiffer C, Aisner J, Wiernik PH. Granulocyte transfusion therapy and amphotericin B adverse reactions. Am J Hematol. 1989;31:102–8.
Present CA, Klahr C, Santala R. Amphotericin B induction of sensitivity to adriamycin, 1, 3-bis (2-chloroethyl)-1 nitrosourea (BCNU) plus cyclophosphamide in human neoplasia. Ann Intern Med. 1977;86:47–9.
Medoff G. Controversial areas in antifungal chemotherapy: short-course and combination therapy with amphotericin B. Rev Infect Dis. 1987;9:403–7.
Stevens DA. Combination immunotherapy and antifungal chemotherapy. Clin Infect Dis. 1998;26:1266–9.
Sugar AM. Use of amphotericin B with azoles with antifungal drugs: what are we doing? Antimicrob Agents Chemother. 1995;39:1907–12.
Peacock Jr JE, Herrington DA, Cruz JM. Amphotericin B therapy: past, present, future. Infect Dis Clin Pract. 1993;2:81–93.
Rex JH, Pappas PG, Karchmer AW, et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis. 2003;36:1221–8.
Bennett JE, Dismukes WE, Duma RJ, et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N Engl J Med. 1979;301:126–31.
Dismukes WE, Cloud G, Gallis HA, et al. Treatment of cryptococcal meningitis with combination amphotericin B and flucytosine for four as compared with six weeks. N Engl J Med. 1987;317:334–41.
Van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337:15–21.
Lewis RE, Kontoyiannis P. Rationale for combination antifungal therapy. Pharmacotherapy. 2001;21:149S–64.
Griswold MW, Briceland LL, Stein DS. Is amphotericin B test dosing needed? Ann Pharmacother. 1998;32:475–7.
Cleary JD, Weisdorf D, Fletcher CV. Effect of infusion rate on amphotericin B-associated febrile reactions. Drug Intell Clin Pharm. 1988;22:769–72.
Craven PC, Gremillion DH. Risk factors for ventricular fibrillation during rapid amphotericin B infusion. Antimicrob Agents Chemother. 1985;27:868–71.
Bowler WA, Weiss PJ, Hill HE, et al. Risk of ventricular dysrythmias during one hour infusions of amphotericin B in patients with preserved renal function. Antimicrob Agents Chemother. 1992;36:2542–3.
Eriksson U, Seifert B, Schaffner A. Comparison of effects of amphotericin B deoxycholate infused over 4 or 24 hours: randomized controlled trial. Br Med J. 2001;322:579–82.
Wen DY, Bottini AG, Hall WA, Haines SJ. Infections in neurologic surgery. The intraventricular use of antibiotics. Neurosurg Clin N Am. 1992;3:343–54.
Lesar TS, Fiscella RG. Antimicrobial drug delivery to the eye. Drug Intell Clin Pharm. 1985;19:642–54.
Piraino B, Bailie GR, Bernardini J, et al. ISPD guidelines/recommendations. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005;25:107–31.
Fan-Havard P, Odonovan C, Smith SM, Oh J, Bamberger M, Eng RHK. Oral fluconazole versus amphotericin B bladder irrigation for treatment of candidal funguria. Clin Infect Dis. 1995; 21:960–5.
O’Riordan T, Faris M. Inhaled antimicrobial therapy. Respir Care Clin N Am. 1999;5:617–31.
Diot P, Dequin PF, Rivoire B, et al. Aerosols and anti-infectious agents. J Aerosol Med. 2001;14:55–64.
King CT, Rogers PD, Cleary JD, Chapman SW. Antifungal therapy during pregnancy. Clin Infect Dis. 1998;27:1151–60.
Sobel JD. Use of antifungal drugs in pregnancy: a focus on safety. Drug Saf. 2000;23:77–85.
Caillot D, Casasnovas O, Solary E, et al. Efficacy and tolerance of an amphotericin B lipid (Intralipid) emulsion in the treatment of candidemia in neutropenic patients. J Antimicrob Chemother. 1993;31:161–9.
Ayestaran A, Lopez RM, Montoro JB, et al. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob Agents Chemother. 1996;40:609–12.
Cleary JD. Amphotericin B formulated in a lipid emulsion. Ann Pharmacother. 1996;30:409–12.
Slain D. Lipid-based amphotericin B for the treatment of fungal infections. Pharmacotherapy. 1999;19:306–23.
Janknergt R, de Marie S, Bakker-Woudenberg IAJM, Crommelin DJA. Liposomal and lipid formulations of amphotericin B: clinical pharmacokinetics. Clin Pharmacokinet. 1992;23: 279–91.
Robinson RF, Nahata MC. A comparative review of conventional and lipid formulations of amphotericin B. J Clin Pharm Ther. 1999;24:249–57.
Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N Engl J Med. 1999;340:764–71.
Walsh TJ, Whitcomb P, Piscitelli S, et al. Safety, tolerance, and pharmacokinetics of amphotericin B lipid complex in children with hepatosplenic candidiasis. Antimicrob Agents Chemother. 1997;41:1944–8.
Villani R, Regazzi MB, Maserati R, Viale P, Alberici F, Giacchino R. Clinical and pharmacokinetic evaluation of a new lipid-based delivery system of amphotericin B in AIDS patients. Arzneimittelforschung. 1996;46:445–9.
Groll AH, Lyman CA, Petraitis V, Petraitiene R, Armstrong D, Mickiene D, et al. Compartmentalized intrapulmonary pharmacokinetics of amphotericin B and its lipid formulations. Antimicrob Agents Chemother. 2006;50:3418–23.
Leenders C, Reiss P, Portegies P, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. AIDS. 1997;11:1463–71.
Goldblum D, Rohrer K, Frueh BE, Theurillat R, Thormann W, Zimmerli S. Corneal concentrations following systemic administration of amphotericin B and its lipid preparations in a rabbit model. Ophthalmic Res. 2004;36:172–6.
Goldblum D, Rohrer K, Frueh BE, Theurillat R, Thormann W, Zimmerli S. Ocular distribution of intravenously administered lipid formulations of amphotericin B in a rabbit model. Antimicrob Agents Chemother. 2002;46:3719–23.
Kretschmar M, Nichterlein T, Hannak D, Hof H. Effects of amphotericin B incorporated into liposomes and in lipid suspensions in the treatment of murine candidiasis. Arzneimittelforschung. 1996;46:711–5.
Wingard JR, White MH, Anaissie E, Raffalli J, Goodman J, Arrieta AL Amph/ ABLC Collaborative Study Group. a randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. Clin Infect Dis. 2000;31:1155–63.
White MH, Anaissie EJ, Kusne S, et al. Amphotericin B colloidal dispersion vs. amphotericin B as therapy for invasive aspergillosis. Clin Infect Dis. 1997;24:635–42.
White MH, Bowden RA, Sandler ES, et al. Randomized, double-blind clinical trial of amphotericin B colloidal dispersion vs. amphotericin B in the empirical treatment of fever and neutropenia. Clin Infect Dis. 1998;27:296–302.
Johnson MD, Drew RH, Perfect JR. Chest discomfort associated with liposomal amphotericin B: report of three cases and review of the literature. Pharmacotherapy. 1998;18:1053–61.
Collazos J, Martinez E, Mayo J, Ibarra S. Pulmonary reactions during treatment with amphotericin B: review of published cases and guidelines for management. Clin Infect Dis. 2001;33:E75–82.
Roden MM, Nelson LD, Knudsen TA, et al. Triad of acute infusion-related reactions associated with liposomal amphotericin B: analysis of clinical and epidemiological characteristics. Clin Infect Dis. 2003;36:1213–20.
McDonnell TJ, Chang SW, Westcott JY, Voelkel NF. Role of oxidants, eicosanoids, and neutrophils in amphotericin B lung injury in rats. J Appl Physiol. 1988;65:2195–206.
Hardie WD, Wheeler AP, Wright PW, Swindell BB, Bernard GR. Effect of cyclooxygenase inhibition on amphotericin B-induced lung injury in awake sheep. J Infect Dis. 1992;166:134–8.
Bowden R, Chandrasekar P, White MH, et al. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion for treatment of invasive aspergillosis in immunocompromised patients. Clin Infect Dis. 2002;35:359–66.
Sharkey PK, Graybill JR, Johnson ES, et al. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1996;22:329–30.
Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med. 2002;137:105–9.
Rex JH, Walsh TJ. Editorial response: estimating the true cost of amphotericin B. Clin Infect Dis. 1999;29:1408–10.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Chapman, S.W., Cleary, J.D., Rogers, P.D. (2011). Amphotericin B. In: Kauffman, C., Pappas, P., Sobel, J., Dismukes, W. (eds) Essentials of Clinical Mycology. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-6640-7_3
Download citation
DOI: https://doi.org/10.1007/978-1-4419-6640-7_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-6639-1
Online ISBN: 978-1-4419-6640-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)