Abstract
Whether nutritional control can retard senescence of immune function and decrease mortality from infectious diseases has not yet been established; the difficulty of establishing a model has made this a challenging topic to investigate. Caenorhabditis elegans has been extensively used as an experimental system for biological studies. Particularly for aging studies, the worm has the advantage of a short and reproducible life span. The organism has also been recognized as an alternative to mammalian models of infection with bacterial pathogens in this decade. Hence we have studied whether the worms could be a model host in the fields of immunosenescence and immunonutrition. Feeding nematodes lactic acid bacteria (LAB) resulted in increases in average life span of the nematodes compared to those fed Escherichia coli strain OP50, a standard food bacteria. The 7-day-old nematodes fed LAN from age 3 days were clearly endurable to subsequent salmonella infection compared with nematodes fed OP50 before the salmonella infection. The worm could be a unique model to study effects of food factors on longevity and host defense, so-called immunonutrition. Then we attempted to establish an immunosenescence model using C. elegans. We focused on the effects of worm age on the Legionella infection and the prevention by immunonutrition. No significant differences in survival were seen between 3-day-old worms fed OP50 and 3-day-old worms infected with virulent Legionella strains. However, when the worms were infected from 7.5 days after hatching, the virulent Legionella strains were obviously nematocidal for the worms’ immunosenescence. In contrast, nematodes fed with bifidobacteria prior to Legionella infection were resistant to Legionella. C. elegans could act as a unique alternative host for immunosenescence and resultant opportunistic infection, and immunonutrition researches.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Caenorhabditis elegans is a small free-living soil nematode that feeds on bacteria; it has been extensively used as an experimental system for biological studies because of its simplicity, transparency, ease of cultivation, and suitability for genetic analysis (Riddle et al. 1997). Particularly for aging studies, the worm has the advantage of a short and reproducible life span (Finch and Ruvkun 2001). Recently, after Ausubel et al. reported infection due to Pseudomonas aeruginosa (Kurz and Tan 2004; Nicholas and Hodgkin 2004; Tan et al. 1999), the organism has also been recognized as an alternative to mammalian models of infection with bacterial pathogens. In the field of innate immunity research, C. elegans is becoming one of the most important experimental animals, similar to the fruit fly Drosophila (Kurz and Tan 2004; Nicholas and Hodgkin 2004; Schulenburg et al. 2004).
Age at infection is one of the most important determinants of disease morbidity and mortality (Miller and Gay 1997). Because aging is accompanied by functional and metabolic alterations in cells and tissues, senescence of the immune system results in an age-related increase of infections, malignancy, and autoimmunity (Grubeck-Loebenstein 1997; Moulias et al. 1985). Elderly humans have increased mortality from many different types of infections (Bradley and Kauffman 1990).
Whether nutritional control can retard senescence of immune function and decrease mortality from infectious diseases has not yet been established; the difficulty of establishing a model has made this a challenging topic to investigate. Although some studies have shown successful improvement of biomarkers relating to immunological functions (Bogden and Louria 2004), few reports have shown a beneficial influence of nutrition on immunity and the resultant outcome of experimental infection (Hayek et al. 1997; Effros et al. 1991). Hence we have studied whether C. elegans is a useful model host in the fields of immunosenescence and immunonutrition.
C. elegans as a Model for Immunonutrition
Probiotic bacteria are defined as living microorganisms that exert beneficial effects on human health when ingested in sufficient numbers (Naidu et al. 1999). Lactic acid bacteria (LAB) have been used in various fermented foods since antiquity. Metchnikoff, who first proposed the concept of probiotic bacteria in 1907, hypothesized that lactobacilli were important for human health and longevity (Metchnikoff 1907). LAB are the most commonly used probiotic microorganisms. LAB have been found to have a variety of physiological influences on their hosts, including antimicrobial effects, microbial interference, supplementary effects of nutrition, anti-tumor effects, reduction of serum cholesterol and lipids, and immunomodulatory effects. However, there have been no reports concerning the influence of LAB on longevity and immunosenescence.
First, we evaluated whether LAB could contribute to host defenses and prolong the lifetime of C. elegans (Ikeda et al. 2007). Lactobacilli and bifidobacteria were fed to worms, and their life span and resistance to Salmonella enterica were compared with those of worms fed Escherichia coli OP50, an international standard food for C. elegans. The worms were generally infected with inocula on conventional nematode growth medium, which contains peptone, raising the possibility that the inoculated pathogen would have proliferated regardless of whether it could successfully infect the nematodes and derive nutrition from the hosts. Garsin et al. showed that nutrition available in agar plates does influence the virulence of pathogens on the media (Garsin et al. 2001). Furthermore, some pathogens produce toxic metabolites on nutrient medium in situ (Anyanful et al. 2005). To avoid such a condition, our experiments were performed on modified nematode growth medium (mNGM) containing no peptone as we reported before (Hoshino et al. 2008). Worms fed heat-killed OP50 reportedly live longer than those fed alive bacteria on nutrient NGM, however this difference was not observed on modified NGM.
Feeding nematodes bifidobacteria or lactobacilli resulted in increases in average life span of the nematodes compared to those fed OP50 (Fig. 3.1). To examine whether or not the beneficial effects of LAB were brought about by their harmless nature compared to OP50, survival was compared with that of nematodes fed on heat-killed OP50. Heat-killed OP50 did not prolong the worms’ longevity as much as LAB did (Fig. 3.2).
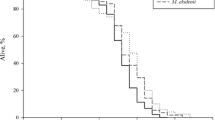
Effects of lactic acid bacteria on the life span of C. elegans. Adult worms fed a diet of E. coli strain OP50 for 3 days after hatching were transferred to diets of bifidobacteria. The bifidobacteria used were B. infantis (BI) or B. longum (BL). The life spans of nematodes fed bifidobacteria were significantly extended (***p < 0.001). The mean life spans (in days) of worms fed B. infantis or B. longum were 15.1 ± 0.40 (29%) and 13.6 ± 0.50 (17%), respectively: numbers in parentheses are percentage differences in the mean relative to controls fed OP50
Salmonella killed about 40% of the nematodes in 5 days after the worms were transferred to the lawn of this pathogen at age 7 days, while 80% of the worms fed OP50 remained alive after 5 days. The 3-day-old worms were not killed in 5 days when fed either OP50 or Salmonella. The 3-day-old worms were clearly more resistant to Salmonella compared to the 7-day-old nematodes (Fig. 3.3); the initial number of Salmonella recovered from those worms in which infection started at age 3 days was smaller than the number recovered from worms infected from age 7 days (Fig. 3.4).
Importantly, 7-day-old nematodes fed bifidobacteria or lactobacilli from age 3 days were clearly more tolerant to subsequent Salmonella infection compared with nematodes fed OP50 before the Salmonella infection (Fig. 3.5). LAB seem to make the worms tolerant rather than resistant to Salmonella infection; the number of Salmonella recovered from worms fed LAB was the same as that recovered from worms grown on OP50.
Effects of lactic acid bacteria on resistance of C. elegans to Salmonella. Adult worms fed a diet of E. coli strain OP50 for 3 days after hatching were transferred to a diet of lactobacilli. The lactobacilli used were L. helveticus (LH), L. plantarum (LP), or L. rhamnosus (LR). Four days later the nematodes were transferred to Salmonella plates, and survival curves were determined. Nematodes fed each type of lactobacilli were significantly more resistant than controls to the pathogen (***p < 0.001). Mean days of survival of worms fed LH, LP, LR before the salmonella infection were 7.1 ± 0.25 (46%), 6.6 ± 0.28 (35%), and 6.6 ± 0.27 (35%), respectively; numbers in parentheses are percentage differences in the mean relative to controls fed OP50
The mechanisms how LAB brought the worms longevity effects and made them tolerant have not been elucidated. However, if the increased longevity was due to enhancement of host defenses as one of the probiotic effects, the worm could be a unique model to study effects of not only LAB but other nutrients on host defense, so-called immunonutrition. C. elegans is useful for studying the relationship between innate immunity and pathogens because the nematode lacks an adaptive immune system. Although C. elegans does not have phagocytes specialized for innate host defense, it produces a variety of humoral antibiotic substances such as lysozymes, caenopores, lipase, lectins and C3-like thioester-containing proteins, and defensin-like antibiotic peptides. These substances in the bacteriophagous nematodes might be considered to be digestive enzymes; the worm’s intestine could be considered analogous to a phagosome. Bacteria resistant to these antibacterial substances are more likely to be nematocidal. Consequently, C. elegans may be most suitable to study anti-innate immunity properties of pathogens since the organisms have to contend with the humoral defense factors produced by the host.
C. elegans as a Model for Immunosenescence
In a second step, we attempted to establish an immunosenescence model using C. elegans. Although Salmonella enterica serovar Enteritidis killed old nematodes more quickly after the infection than young worms as we described above, we wanted to develop a model capable of testing whether opportunistic infections increase due to immunosenescence. Legionella pneumophila, an environmental bacterium naturally found in fresh water, is the major causative agent of Legionnaires’ disease (Fields et al. 2002). Fresh water amoeba, a natural host of Legionella, has been used as an infection model to study invasion of Legionella into human macrophages and subsequent intracellular growth (Jules and Buchrieser 2007). However, analyses using these protozoa have inevitably concentrated on the intracellular lifestyle of L. pneumophila. The fate of Legionella organisms in non-mammalian metazoans had not been reported (Hilbi et al. 2007) until a very recent report by Brassinga et al. (Brassinga et al. 2010). Since Legionella is prone to infect elderly people, we focused on the effects of worm age on Legionella infection and the prevention of infection by immunonutrition (Komura et al. 2010). Infections in young and old nematodes were compared. Furthermore, survival curves were compared between worms fed with OP50 and those fed bifidobacteria prior to infection with Legionella organisms, since lactic acid bacteria exert beneficial effects on human and animal health (Naidu et al. 1999).
No significant differences in survival were seen between 3-day-old worms fed OP50 and 3-day-old worms infected with virulent Legionella strains. However, when the worms were infected from 7.5 days after hatching, the virulent Legionella strains were obviously nematocidal (Fig. 3.6). These data show that L. pneumophila is virulent even on peptone-free mNGM if the targets are elderly worms. Our previous study showed that Salmonella is clearly virulent to both older and younger worms, although more so in elderly worms (Ikeda et al. 2007). These findings appear to be similar to the epidemiological characteristics of both pathogens in humans: Legionella tends to infect older people in an opportunistic manner, while Salmonella can cause enteritis irrespective of host age.
As with the case of many other pathogens in the C. elegans model, Legionella mutants that are less virulent in the lungs of guinea pigs (Miyamoto et al. 2003) or in human macrophages (Sadosky et al. 1993), are also less virulent in C. elegans. Interestingly, the attenuated mutant LELA 1718, which is reportedly cytolethal compared to the other attenuated mutants in a cytotoxicity assay with HL-60-derived human macrophages (Sadosky et al. 1993), showed modest virulence in the nematode compared to other avirulent mutants. The pathogenicity of L. pneumophila in C. elegans seems to correlate well with that in macrophages, and the nematode could serve as a unique host of Legionella spp.
Nematodes fed with bifidobacteria prior to Legionella infection were resistant to Legionella (Fig. 3.7). The number of Legionella recovered from the worms showed no significant difference between groups fed with bifidobacteria or OP50. This phenomenon is similar to the tolerance that we observed when nematodes were fed lactic acid bacteria prior to Salmonella infection (Ikeda et al. 2007).
Development of a Method for Oral Administration to Nematodes
Despite increased use of C. elegans in a variety of studies, there is no efficient method to administer chemicals orally. When chemicals need to be administered to nematodes, they are either dissolved in the NGM or the solution is poured onto the OP50 lawn. C. elegans ingests relatively large particles such as bacteria, that are suspended in water, and then spits out much of the liquid, while retaining the particles (Avery and Thomas 1997). This feeding behavior is likely to be inefficient for ingestion of solutions.
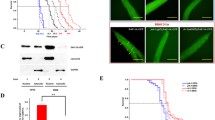
In the third step of our studies, we aimed to develop methods for oral administration that is essential for developing a biologically relevant C. elegans immunonutrition model. We hypothesized that nematodes would be able to take up liposomes, similar to their ingestion of bacteria. We used liposomes loaded with the hydrophilic fluorescent reagent uranin to test oral administration of water-soluble substances to C. elegans, and compared the efficiency of liposome-mediated delivery with conventional methods (Shibamura et al. 2009).
Dietary supplements of antioxidants were previously reported to have positive effects on longevity, while other studies reported controversial results. Water-soluble antioxidants were administered using both our newly developed liposome method and conventional methods to compare the effect on lifespan of nematodes and on host defense against Salmonella infection. Using our the liposome method, we showed marked longevity effects of antioxidants on the lifespan of C. elegans (Fig. 3.8). Oral administration was more than 200 times as efficient as the conventional method in dose response tests. We expect that this method could open new phase of C. elegans research as a model host.
Survival curves of nematodes supplemented with 25 μL of liposomes containing 180 μg reduced glutathione. After hatching, nematodes were grown on E. coli OP50 for 3 days, and then the adult worms were divided into groups that were supplemented with chemicals. Water-containing liposomes were administered to control worms and those maintained on mNGM containing 180 μg of glutathione. ***p < 0.001, compared to the control
Conclusion
Due to increasing ethical considerations as well as economic reasons, the use of mammalian hosts is decreasing in popularity. We showed that C. elegans could act as a unique alternative host for immunosenescence and resultant opportunistic infection and immunonutrition experiments. Compared with murine infection models, it is not easy to extrapolate whether the nematocidal activity of a particular pathogen would be reflected in virulence in human pathogenesis. However, for simplicity, transparency, ease of cultivation, and suitability for genetic analysis, C. elegans is a uniquely useful model. Particularly for studies on aging of host defense, the worm has the great advantage of a short and reproducible life span.
References
Anyanful A et al (2005) Paralysis and killing of Caenorhabditis elegans by enteropathogenic Escherichia coli requires the bacterial tryptophanase gene. Mol Microbiol 57:988–1007
Avery L, Thomas JH (1997) Feeding and defecation. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds) C. elegans II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 679–716
Bogden JD, Louria DB (2004) Nutrition and immunity in the elderly. In: Hughes DA, Gail Darlington J, Bendich A (eds) Diet and human immune function nutrition and health. Humana Press, Totowa, pp 79–101
Bradley SF, Kauffman CA (1990) Aging and the response to salmonella infection. Exp Gerontol 25:75–80
Brassinga AK et al (2010) Caenorhabditis is a metazoan host for Legionella. Cell Microbiol 12:343–361
Effros RB, Walford RL, Weindruch R, Mitcheltree C (1991) Influences of dietary restriction on immunity to influenza in aged mice. J Gerontol 46:B142–147
Fields BS, Benson RF, Besser RE (2002) Legionella and legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev 15:506–526
Finch CE, Ruvkun G (2001) The genetics of aging. Annu Rev Genomics Hum Genet 2:435–462
Garsin DA et al (2001) A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci USA 98:10892–10897
Grubeck-Loebenstein B (1997) Changes in the aging immune system. Biologicals 25:205–208
Hayek MG et al (1997) Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J Infect Dis 176:273–276
Hilbi H, Weber SS, Ragaz C, Nyfeler Y, Urwyler S (2007) Environmental predators as models for bacterial pathogenesis. Environ Microbiol 9:563–575
Hoshino K et al (2008) Evaluation of Caenorhabditis elegans as the host in an infection model for food-borne pathogens. Jpn J Food Microbiol 25:137–147
Ikeda T, Yasui C, Hoshino K, Arikawa K, Nishikawa Y (2007) Influence of lactic acid bacteria on longevity of Caenorhabditis elegans and host defense against Salmonella entetica serovar Enteritidis. Appl Environ Microbiol 73:6404–6409
Jules M, Buchrieser C (2007) Legionella pneumophila adaptation to intracellular life and the host response: clues from genomics and transcriptomics. FEBS Lett 581:2829–2838
Komura T, Yasui C, Miyamoto H, Nishikawa Y (2010) Caenorhabditis elegans as an alternative model host for Legionella pneumophila and the protective effects of Bifidobacterium infantis. Appl Environ Microbiol 76:4105–4108
Kurz CL, Tan MW (2004) Regulation of aging and innate immunity in C. elegans. Aging Cell 3:185–193
Metchnikoff E (1907) The prolongation of life. Heinemann, London
Miller E, Gay N (1997) Effect of age on outcome and epidemiology of infectious diseases. Biologicals 25:137–142
Miyamoto H, Yoshida S, Taniguchi H, Shuman HA (2003) Virulence conversion of Legionella pneumophila by conjugal transfer of chromosomal DNA. J Bacteriol 185:6712–6718
Moulias R et al (1985) Respective roles of immune and nutritional factors in the priming of the immune response in the elderly. Mech Ageing Dev 31:123–137
Naidu AS, Bidlack WR, Clemens RA (1999) Probiotic spectra of lactic acid bacteria (LAB). Crit Rev Food Sci Nutr 39:13–126
Nicholas HR, Hodgkin J (2004) Responses to infection and possible recognition strategies in the innate immune system of Caenorhabditis elegans. Mol Immunol 41:479–493
Riddle DL, Blumenthal T, Meyer BJ, Priess JR (1997) C. elegans II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sadosky AB, Wiater LA, Shuman HA (1993) Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun 61:5361–5373
Schulenburg H, Kurz CL, Ewbank JJ (2004) Evolution of the innate immune system: the worm perspective. Immunol Rev 198:36–58
Shibamura A, Ikeda T, Nishikawa Y (2009) A method for oral administration of hydrophilic substances to Caenorhabditis elegans: effects of oral supplementation with antioxidants on the nematode lifespan. Mech Ageing Dev 130:652–655
Tan MW, Mahajan-Miklos S, Ausubel FM (1999) Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA 96:715–720
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media, LLC
About this paper
Cite this paper
Komura, T., Ikeda, T., Hoshino, K., Shibamura, A., Nishikawa, Y. (2012). Caenorhabditis elegans as an Alternative Model to Study Senescence of Host Defense and the Prevention by Immunonutrition. In: Mylonakis, E., Ausubel, F., Gilmore, M., Casadevall, A. (eds) Recent Advances on Model Hosts. Advances in Experimental Medicine and Biology, vol 710. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-5638-5_3
Download citation
DOI: https://doi.org/10.1007/978-1-4419-5638-5_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-5637-8
Online ISBN: 978-1-4419-5638-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)