Abstract
There are two interrelated concepts in food analysis that deal with acidity: pH and titratable acidity. Each of these quantities is analytically determined in separate ways and each has its own particular impact on food quality. Titratable acidity deals with measurement of the total acid concentration contained within a food (also called total acidity). This quantity is determined by exhaustive titration of intrinsic acids with a standard base. Titratable acidity is a better predictor of acid’s impact on flavor than pH.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
There are two interrelated concepts in food analysis that deal with acidity: pH and titratable acidity. Each of these quantities is analytically determined in separate ways and each has its own particular impact on food quality. Titratable acidity deals with measurement of the total acid concentration contained within a food (also called total acidity). This quantity is determined by exhaustive titration of intrinsic acids with a standard base. Titratable acidity is a better predictor of acid’s impact on flavor than pH.
Total acidity does not tell the full story, however. Foods establish elaborate buffering systems that dictate how hydrogen ions (H+ ), the fundamental unit of acidity, are expressed. Even in the absence of buffering, less than 3% of any food acid is ionized into H+ and its anionic parent species (its conjugate base). This percentage is further suppressed by buffering. In aqueous solution, hydrogen ions combine with water to form hydronium ions, H3O+. The ability of a microorganism to grow in a specific food is an important example of a process that is more dependent on hydronium ion concentration than on titratable acidity. The need to quantify only the free H3O+ concentration leads to the second major concept of acidity, that of pH (also called active acidity). In nature, the H3O+ concentration can span a range of 14 orders of magnitudes. The term pH is a mathematical shorthand for expressing this broad continuum of H3O+ concentration in a concise and convenient notation. In contemporary food analysis, pH is usually determined instrumentally with a pH meter; however, chemical pH indicators also exist.
For general and food-specific information on measuring pH and titratable acidity, see references (1–15). For the actual pH and titratable acidity of select foods, see reference (8).
2 Calculation and Conversion for Neutralization Reactions
2.1 Concentration Units
This chapter deals with the theory and practical application of titratable acidity calculation and pH determination. To quantitatively measure components of foods, solutions must be prepared to accurate concentrations and diluted into the desired working range.
The terms used for concentration in food analysis should be reviewed. The most common concentration terms are given in Table 13-1. Molarity and normality are the most common SI (International Scientific) terms used in food analysis, but solutions also can be expressed as percentages. It is important that the analyst be able to convert between both systems.
Molarity (M) is a concentration unit representing the number of moles of the solute per liter of solution. Normality (N) is a concentration unit representing the number of equivalents (Eq) of a solute per liter of solution. In acid and base solutions, the normality represents the concentration or moles of H+ or OH− per liter that will be exchanged in a neutralization reaction when taken to completion. For oxidation–reduction reagents, the normality represents the concentration or moles of electrons per liter to be exchanged when the reaction is taken to completion. The following are some examples of molarity vs. normality (equivalents):
Acid–Base Reactions
- 1 M H2SO4 = 2 N H2SO4 :
-
2 equivalents of H+ per mol of acid
- 1 M NaOH = 1 N NaOH:
-
1 equivalent of OH− per mol of base
- 1 M CH3COOH = 1 N acetic acid:
-
1 equivalent of H+ per mol of acid
- 1 M H2C4H4O5 = 2 N malic acid:
-
2 equivalents of H+ per mol of acid
Oxidation–Reduction Reactions
For example, \({\mathrm{HSO}}_{3}^{-} +{ \mathrm{I}}_{2} + \mathrm{{H}_{2}O} \rightleftarrows {{\mathrm{SO}}_{4}}^{2} +{ \mathrm{2I}}^{-} +{ \mathrm{3H}}^{+}\)
- 1 M I2 = 2 N iodine:
-
2 equivalents of electrons gained per mol of I2
- 1 \(M\,{{\mathrm{HSO}}_{3}}^{-} = 2\,N\) bisulfite:
-
2 equivalents of electrons lost per mol of bisulfate
Many analytical determinations in food analysis use the concept of equivalents to measure the amount of an unknown. Perhaps the most familiar of these are acid–base reactions in which hydrogen ions are exchanged and can be quantified through stoichiometric neutralization with a standard base. Acid–base reactions are used to determine nitrogen in the Kjeldahl protein determination (see Chap. 9), benzoic acid in sodas, and in determining percent titratable acidity. The concept of equivalents also is used in oxidation–reduction problems to quantify unknown analytes that are capable of direct electron transfer.
Equivalent weight can be defined as the molecular weight divided by the number of equivalents in the reactions. For example, the molecular weight of H2SO4 is 98.08 g. Since there are 2 equivalents per mole of H2SO4, the equivalent weight of H2SO4 is 49.04 g. Table 13-2 provides a list of molecular and equivalent weights for acids important in food analysis. In working with normality and milliliters, the term milliequivalents (mEq) is usually preferred. Milliequivalent weight is the equivalent weight divided by 1000.
Percentage concentrations are the mass amount of solute or analyte per 100 ml or 100 g of material. Percentage can be expressed for solutions or for solids and can be on a volume basis or mass basis. When the percentage becomes a number less than 1% parts per million (ppm), parts per billion (ppb) and even parts per trillion (ppt) usually are preferred. If percentage is defined as the mass of the solute or analyte per mass (or volume) of sample ×100, then ppm is simply the same ratio of mass of solute per mass of sample ×1, 000, 000.
2.2 Equation for Neutralization and Dilution
There are some general rules in evaluating equilibrium reactions that are helpful in most situations. At full neutralization the millequivalents (mEq) of one reactant in the neutralization equal the milliequivalents of the other reactant. This can be expressed mathematically as follows:
Equation [1] also can be used to solve dilution problems where X represents the stock solution and Y represents the working solution. When Equation [1] is used for dilution problems, any value of concentration (grams, moles, ppm, etc.) can be substituted for N. Units should be recorded with each number. Cancellation of units provides a quick check on proper setup of the problem. (See Practice Problems 1–8 at the end of Chap. 13.)
3 pH
3.1 Acid–Base Equilibria
The Brønsted-Lowry theory of neutralization is based upon the following definitions for acid and base:
- Acid::
-
A substance capable of donating protons. In food systems the only significant proton donor is the hydrogen ion.
- Base::
-
A substance capable of accepting protons.
- Neutralization :
-
is the reaction of an acid with a base to form a salt as shown below:
$$ {\rm HCl} {\rm + } {\rm NaOH} \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\rightarrow\over {\smash{\leftarrow}\vphantom{_{\vbox to.5ex{\vss}}}}$}} {\rm NaC1} {\rm + } {\rm H}_{\rm 2} O $$(2)
Acids form hydrated protons called hydronium ions (H3O+ ) and bases form hydroxide ions (OH− ) in aqueous solutions:
At any temperature, the product of the molar concentrations (moles/liter) of H3O+ and OH− is a constant referred to as the ion product constant for water (K w):
K w varies with the temperature. For example, at \(2{5\,}^{\circ }\mathrm{C},\ {K}_{\mathrm{w}} = 1.04 \times 1{0}^{-14}\) but at \(10{0\,}^{\circ }\mathrm{C},\ {K}_{\mathrm{w}} = 58.2 \times 1{0}^{-14}\).
The above concept of K w leads to the question of what the concentrations of [H3O+ ] and [OH− ] are in pure water. Experimentation has revealed that the concentration of [H3O+ ] is approximately 1. 0 × 10−7 M, as is that of the [OH− ] at 25 ∘C. Because the concentrations of these ions are equal, pure water is referred to as being neutral.
Suppose that a drop of acid is added to pure water. The [H3O+ ] concentration would increase. However, K w would remain constant (1. 0 × 10−14), revealing a decrease in the [OH− ] concentration. Conversely, if a drop of base is added to pure water, the [H3O+ ] would decrease while the [OH− ] would increase, maintaining the K w at 1. 0 × 10−14 at 25 ∘C.
How did the term pH derive from the above considerations? In approaching the answer to this question, one must observe the concentrations of [H3O+ ] and [OH− ] in various foods, as shown in Table 13-3. The numerical values found in Table 13-3 for [H3O+ ] and [OH− ] are bulky and led a Swedish chemist, S.L.P. Sørensen, to develop the pH system in 1909.
pH is defined as the logarithm of the reciprocal of the hydrogen ion concentration. It also may be defined as the negative logarithm of the molar concentration of hydrogen ions. Thus, a [H3O+ ] concentration of 1 × 10−6 is expressed simply as pH 6. The [OH− ] concentration is expressed as pOH and would be pOH 8 in this case, as shown in Table 13-4.
While the use of pH notation is simpler from the numerical standpoint, it is a confusing concept in the minds of many students. One must remember that it is a logarithmic value and that a change in one pH unit is actually a tenfold change in the concentration of [H3O+ ]. (See Practice Problems 9–12 at the end of Chap. 13.)
It is important to understand that pH and titratable acidity are not the same. Strong acids such as hydrochloric, sulfuric, and nitric acids are almost fully dissociated at pH 1. Only a small percentage of food acid molecules (citric, malic, acetic, tartaric, etc.) dissociate in solution. This point may be illustrated by comparing the pH of 0. 1 N solutions of hydrochloric and acetic acids.
The HCl fully dissociates in solution to produce a pH of 1.02 at 25 ∘C. By contrast, only about 1% of CH3COOH is ionized at 25 ∘C, producing a significantly higher pH of 2.89. The calculation and significance of partial dissociation on pH is presented in more detail in Sect. 13.4.2.1.
3.2 pH Meter
3.2.1 Activity vs. Concentration
In using pH electrodes, the concept of activity vs. concentration must be considered. Activity is a measure of expressed chemical reactivity, while concentration is a measure of all forms (free and bound) of ions in solution. Because of the interactions of ions between themselves and with the solvent, the effective concentration or activity is, in general, lower than the actual concentration, although activity and concentration tend to approach each other at infinite dilution. Activity and concentration are related by the following equation:
where:
The activity coefficient is a function of ionic strength. Ionic strength is a function of the concentration of, and the charge on, all ions in solution. Activity issues can become significant for hydronium ions below pH 1 and for hydroxyl ions at pH 13 and above.
3.2.2 General Principles
The pH meter is a good example of a potentiometer (a device that measures voltage at infinitesimal current flow). The basic principle of potentiometry (an electrochemical method of voltammetry at zero current) involves the use of an electrolytic cell composed of two electrodes dipped into a test solution. A voltage develops, which is related to the ionic concentration of the solution. Since the presence of current could alter the concentration of surrounding ions or produce irreversible reactions, this voltage is measured under conditions such that infinitesimal current ( 10−12 amperes or less) is drawn.
Four major parts of the pH system are needed: (1) reference electrode, (2) indicator electrode (pH sensitive), (3) voltmeter or amplifier that is capable of measuring small voltage differences in a circuit of very high resistance, and (4) the sample being analyzed (Fig. 13-1).
The measuring circuit of the potentiometric system. E a: contact potential between Ag:AgCl electrode and inner liquid. E a is independent of pH of the test solution but is temperature dependent. E b: potential developed at the pH-sensitive glass membrane. E b varies with the pH of the test solution and also with temperature. In addition to this potential the glass electrode also develops an asymmetry potential, which depends upon the composition and shape of the glass membrane. It also changes as the electrode ages. E c: diffusion potential between saturated KCl solution and test sample. E c is essentially independent of the solution under test. E d: contact potential between calomel portion of electrode and KCl salt bridge. E d is independent of the solution under test but is temperature dependent. [From (3), used with permission.]
One notes that there are two electrodes involved in the measurement. Each of these electrodes is designed carefully to produce a constant, reproducible potential. Therefore, in the absence of other ions, the potential difference between the two electrodes is fixed and easily calculated. However, H3O+ ions in solution contribute a new potential across an ion-selective glass membrane built into the indicating electrode. This alters the potential difference between the two electrodes in a way that is proportional to H3O+ concentration. The new potential resulting from the combination of all individual potentials is called the electrode potential and is readily convertible into pH readings.
Hydrogen ion concentration (or more accurately, activity) is determined by the voltage that develops between the two electrodes. The Nernst equation relates the electrode response to the activity where:
where:
For monovalent ions (such as the hydronium ion) at 25 ∘C, the relationship of 2.303 RT/F is calculated to be 0.0591, as follows:
Thus, voltage produced by the electrode system is a linear function of the pH, the electrode potential being essentially + 59 mV (0.059 V) for each change of one pH unit. At neutrality (pH 7), the electrode potential is 0 mV. At pH 6, the electrode potential is + 60 mV, while at pH 4, the electrode potential is + 180 mV. Conversely, at pH 8, the electrode potential is − 60 mV.
It must be emphasized that the above relationship between millivolts and pH exists only at 25 ∘C, and changes in temperature will erroneously alter the pH reading. For example, at 0 ∘C, the electrode potential is 54 mV, while at 100 ∘C it is 70 mV. Modern pH meters have a sensitive attenuator (temperature compensator) built into them in order to account for this effect of temperature.
3.2.3 Reference Electrode
The reference electrode is needed to complete the circuit in the pH system. This half cell is one of the most troublesome parts of the pH meter. Problems in obtaining pH measurements are often traced to a faulty reference electrode.
The saturated calomel electrode (Fig. 13-1) is the most common reference electrode. It is based upon the following reversible reaction:
The \({E}_{0,2{5\,}^{\circ }\mathrm{C}}\) for the saturated KCl salt bridge is + 0. 2444 V vs. a standard hydrogen electrode; the Nernst equation for the reaction is as follows:
Thus, one observes that the potential is dependent upon the chloride ion concentration, which is easily regulated by the use of saturated KCl solution in the electrode.
A calomel reference electrode has three principal parts: (1) a platinum wire covered with a mixture of calomel (Hg2Cl2), (2) a filling solution (saturated KCl), and (3) a permeable junction through which the filling solution slowly migrates into the sample being measured. Junctions are made of ceramic or fibrous material. These junctions tend to clog up, causing a slow, unstable response and inaccurate results.
A less widely used reference electrode is the silver–silver chloride electrode. Because the calomel electrode is unstable at high temperatures (80 ∘C) or in strongly basic samples (pH > 9), a silver–silver chloride electrode must be used for such application. It is a very reproducible electrode based upon the following reaction:
The internal element is a silver-coated platinum wire, the surface silver being converted to silver chloride by hydrolysis in hydrochloric acid. The filling solution is a mixture of 4 M KCl, saturated with AgCl that is used to prevent the AgCl surface of the internal element from dissolving. The permeable junction is usually of the porous ceramic type. Because of the relative insolubility of AgCl, this electrode tends to clog more readily than the calomel reference electrode. However, it is possible to obtain a double-junction electrode in which a separate inner body holds the Ag/AgCl internal element electrolyte and ceramic junction. An outer body containing a second electrolyte and junction isolates the inner body from the sample.
3.2.4 Indicator Electrode
The indicator electrode most commonly used in measuring pH today is referred to as the glass electrode. Prior to its development, the hydrogen electrode and the quinhydrone electrode were used.
The history of the glass electrode goes back to 1875, when it was suggested by Lord Kelvin that glass was an electrical conductor. Cremer discovered the glass electrode potential 30 years later when he observed that a thin glass membrane placed between two aqueous solutions exhibited an electrical potential sensitive to changes in acidity. Subsequently, the reaction was shown to be dependent upon the hydrogen ion concentration. These observations were of great importance in the development of the pH meter.
What is the design of the glass electrode? This electrode (Fig. 13-1) also has three principal parts: (1) a silver–silver chloride electrode with a mercury connection that is needed as a lead to the potentiometer; (2) a buffer solution consisting of 0. 01 N HCl, 0. 09 N KCl, and acetate buffer used to maintain a constant pH (E a); and (3) a small pH-sensitive glass membrane for which the potential (E o) varies with the pH of the test solution. In using the glass electrode as an indicator electrode in pH measurements, the measured potential (measured against the calomel electrode) is directly proportional to the pH as discussed earlier, E = E 0 − 0. 059 pH.
Conventional glass electrodes are suitable for measuring pH in the range of pH 1–9. However, this electrode is sensitive to higher pH, especially in the presence of sodium ions. Thus, equipment manufacturers have developed modern glass electrodes that are usable over the entire pH range of 0–14 and feature a very low sodium ion error, such as < 0. 01 pH at 25 ∘C.
3.2.5 Combination Electrodes
Today, most food analysis laboratories use combination electrodes that combine both the pH and reference electrodes along with the temperature sensing probe in a single unit or probe. These combination electrodes are available in many sizes and shapes from very small microprobes to flat surface probes, from all glass to plastic, and from exposed electrode tip to jacketed electrode tips to prevent glass tip breakage. Microprobes may be used to measure pH of very small systems such as inside a cell or a solution on a microscope slide. Flat surface electrode probes can be used to measure pH of semisolid and high-viscosity substances such as meat, cheese, and agar plates and small volumes as low as 10 μl.
3.2.6 Guidelines for Use of pH Meter
It is very important that the pH meter be operated and maintained properly. One should always follow the specific instructions provided by the manufacturer. For maximum accuracy, the meter should be standardized using two buffers (two-point calibration). Select two buffers of pH values about 3 pH units apart, bracketing that of the anticipated sample pH. The three standardization buffers used most widely in laboratories are a pH 4.0 buffer, a pH 7.0 buffer, and a pH 9.0 buffer (at 25 ∘C). These are the typical pink, yellow, and blue solutions found adjacent to pH meters in many laboratories.
When standardizing the pH electrode, follow manufacturer’s instructions for one-point calibration; rinse thoroughly with distilled water and blot dry. Immerse electrode in the second buffer (e.g., pH 4) and perform a second standardization. This time, the pH meter slope control is used to adjust the reading to the correct value of the second buffer. Repeat these two steps, if necessary, until a value within 0.1 pH unit of the correct value of the second buffer is displayed. If this cannot be achieved, the instrument is not in good working condition. Electrodes should be checked, remembering that the reference electrode is more likely in need of attention. One should always follow the electrode manufacturer’s specific directions for storage of a pH electrode. In this way, the pH meter is always ready to be used and the life of the electrodes is prolonged. One precaution that should be followed pertains to a calomel reference electrode. The storage solution level always should be at least 2 cm below the saturated KCl solution level in the electrode to prevent diffusion of storage solution into the electrode (Fig. 13-2).
Correct and incorrect depth of calomel electrodes in solutions. [Reprinted with permission from (12). Copyright 1971 American Chemical Society.]
4 Titratable Acidity
4.1 Overview and Principle
The titratable acidity measures the total acid concentration in a food. Food acids are usually organic acids, with citric, malic, lactic, tartaric, and acetic acids being the most common. However, inorganic acids such as phosphoric and carbonic (arising from carbon dioxide in solution) acids often play an important and even predominant role in food acidulation. The organic acids present in foods influence the flavor (i.e., tartness), color (though their impact on anthocyanin and other pH-influenced pigments), microbial stability (via inherent pH-sensitive characteristics of organisms), and keeping quality (arising from varying chemical sensitivities of food components to pH). The titratable acidity of fruits is used, along with sugar content, as an indicator of maturity (Sect. 13.4.6). While organic acids may be naturally present in the food, they also may be formed through fermentation or they may be added as part of a specific food formulation.
Titratable acidity is determined by neutralizing the acid present in a known quantity (weight or volume) of food sample using a standard base. The endpoint for titration is usually either a target pH or the color change of a pH-sensitive dye, typically phenolphthalein. The volume of titrant used, along with the normality of the base and the volume (or weight) of sample, is used to calculate the titratable acidity, expressed in terms of the predominant organic acid.
4.2 General Considerations
Many food properties correlate better with pH than with acid concentration. The pH is also used to determine the endpoint of an acid–base titration. The pH determination can be achieved directly with a pH meter, but more commonly using a pH-sensitive dye. In some cases, the way pH changes during titration can lead to subtle problems. Some background in acid theory is necessary to fully understand titration and to appreciate the occasional problems that might arise.
4.2.1 Buffering
Although pH can hypothetically range from − 1 to 14, pH readings below 1 are difficult to obtain due to incomplete dissociation of hydrogen ions at high acid concentrations. At 0. 1 N, strong acids are assumed to be fully disassociated. Therefore, for titrations involving strong acids, fully dissociated acid is present at all titrant concentrations; the pH at any point in the titration is equal to the hydrogen ion concentration of the remaining acid (Fig. 13-3).
By contrast, all food acids are weak acids. Less than 3% of their ionizable hydrogens are dissociated from the parent molecule. When free hydrogen ions are removed through titration, new hydrogen ions can arise from other previously undissociated parent molecules. This tends to cushion the solution from abrupt changes in pH. This property of a solution to resist change in pH is termed buffering. Buffering occurs in foods whenever a weak acid and its salt are present in the same medium. Because of buffering, a graph of pH vs. titrant concentration is more complex for weak acids than for strong acids. However, this relationship can be estimated by the Henderson–Hasselbalch equation.
[HA] represents the concentration of undissociated acid. [A− ] represents the concentration of its salt, also known as the conjugated base. The conjugated base is equal in concentration to the conjugated acid [H3O+ ]. The pK a is the pH at which equal quantities of undissociated acid and conjugated base are present. The equation indicates that maximum buffering capacity will exist when the pH equals the pK a. A graph showing the titration of 0. 1 N acetic acid with 0. 1 N NaOH illustrates this point (Fig. 13-4).
Di- and triprotic acids will have two and three buffering regions, respectively. A pH vs. titrant graph of citric acid is given in Fig. 13-5. If the pK a steps in polyprotic acids differ by three or more pK a units, then the Henderson–Hasselbalch equation can predict the plateau corresponding to each step. However, the transition region between steps is complicated by the presence of protons and conjugate bases arising from other disassociation state(s). Consequently, the Henderson–Hasselbalch equation breaks down near the equivalence point between two pK a steps. However, the pH at the equivalence point is easily calculated. The pH is simply \((\mathrm{p}{K}_{\mathrm{a}}1 + \mathrm{p}{K}_{\mathrm{a}}2)/2\). Table 13-5 lists pK a values of acids important in food analysis.
Titration of a weak polyprotic acid with a strong base. Buffering regions are established around each pK a. The Henderson–Hasselbalch equation can predict the pH for each pK a value if pK a steps are separated by more than three units. However, complex transition mixtures between pK a steps make simple calculations of transition pH values impossible.
Precise prediction of pH by the Henderson–Hasselbalch equation requires that all components form ideal solutions. An approximation to ideal solutions occurs at infinite dilution for all active components. However, real solutions may not behave ideally. For such solutions, the Henderson–Hasselbalch equation may only provide a good estimate of pH.
4.2.2 Potentiometric Titration
At the equivalence point in a titration, the number of acid equivalents exactly equals the number of base equivalents, and total acid neutralization is achieved. As the equivalence point is approached, the denominator [HA] in the Henderson–Hasselbalch equation becomes insignificantly small and the quotient [A− ] ∕ [HA] increases exponentially. As a result, the solution pH rapidly increases and ultimately approaches the pH of the titrant. The exact equivalent point is the halfway mark on this slope of abrupt pH increase. The use of a pH meter to identify the endpoint is called the potentiometric method for determining titratable acidity. The advantage of determining the equivalence point potentiometrically is that the precise equivalence point is identified. Since a rapid change in pH (and not some final pH value per se) signals the end of titration, accurate calibration of the pH meter is not even essential. However, in order to identify the equivalence point, a careful record of pH vs. titrant must be kept. This and the physical constraints of pH probes and slow response with some electrodes make the potentiometric approach somewhat cumbersome.
4.2.3 Indicators
For simplicity in routine work, an indicator solution is often used to approximate the equivalence point. This approach tends to overshoot the equivalence point by a small amount. When indicators are used, the term endpoint or colorimetric endpoint is substituted for equivalence point. This emphasizes that the resulting values are approximate and dependent on the specific indicator. Phenolphthalein is the most common indicator for food use. It changes from clear to red in the pH region 8.0–9.6. Significant color change is usually present by pH 8.2. This pH is termed the phenolphthalein endpoint.
A review of pK a values in Table 13-5 indicates that naturally occurring food acids do not buffer in the region of the phenolphthalein endpoint. However, phosphoric acid (used as an acidulant in some soft drinks) and carbonic acid (carbon dioxide in aqueous solution) do buffer at this pH. Consequently, taking the solution from the true equivalence point to the endpoint may require a large amount of titrant when quantifying these acids. Indistinct endpoints and erroneously large titration values may result. When these acids are titrated, potentiometric analysis is usually preferred. Interference by CO2 can be removed by boiling the sample and titrating the remaining acidity to a phenolphthalein endpoint.
Deeply colored samples also present a problem for endpoint indicators. When colored solutions obscure the endpoint, a potentiometric method is normally used. For routine work, pH vs. titrant data are not collected. Samples are simply titrated to pH 8.2 (the phenolphthalein endpoint). Even though this is a potentiometric method, the resulting value is an endpoint and not the true equivalence point, since it simply reflects the pH value for the phenolphthalein endpoint.
A pH of 7 may seem to be a better target for a potentiometric endpoint than 8.2. This pH, after all, marks the point of true neutrality on the pH scale. However, once all acid has been neutralized, the conjugate base remains. As a result, the pH at the equivalence point is slightly greater than 7. Confusion also might arise if pH 7 was the target for colored samples and pH 8.2 was the target for noncolored samples.
Dilute acid solutions (e.g., vegetable extracts) require dilute solutions of standard base for optimal accuracy in titration. However, a significant volume of dilute alkali may be required to take a titration from the equivalence point to pH 8.2. Bromothymol blue is sometimes used as an alternative indicator in low-acid situations. It changes from yellow to blue in the pH range 6.0–7.6. The endpoint is usually a distinct green. However, endpoint identification is somewhat more subjective than the phenolphthalein endpoint.
Indicator solutions rarely contain over a few tenths percent dye (wt/vol). All indicators are either weak acids or weak bases that tend to buffer in the region of their color change. If added too liberally, they can influence the titration by conferring their own acid/base character to the sample under analysis. Therefore, indicator solutions should be held to the minimum amount necessary to impart effective color. Typically, two to three drops of indicator are added to the solution to be titrated. The lower the indicator concentration, the sharper will be the endpoint.
4.3 Preparation of Reagents
4.3.1 Standard Alkali
Sodium hydroxide (NaOH) is the most commonly used base in titratable acidity determinations. In some ways, it appears to be a poor candidate for a standard base. Reagent grade NaOH is very hygroscopic and often contains significant quantities of insoluble sodium carbonate (Na2CO3). Consequently, the normality of working solutions is not precise, so each new batch of NaOH must be standardized against an acid of known normality. However, economy, availability, and long tradition of use for NaOH outweigh these shortcomings. Working solutions are normally made from a stock solution containing 50% sodium hydroxide in water (wt/vol). Sodium carbonate is essentially insoluble in concentrated alkali and gradually precipitates out of solution over the first 10 days of storage.
The NaOH can react with dissolved and atmospheric CO2 to produce new Na2CO3. This reduces alkalinity and sets up a carbonate buffer that can obscure the true endpoint of a titration. Even just CO2 and water react to form buffering compounds and generate hydrogen ions, as shown in the following equations:
Therefore, CO2 should be removed from water prior to making the stock solution. This can be achieved by purging water with CO2-free gas for 24 h or by boiling distilled water for 20 min and allowing it to cool before use. During cooling and long-term storage, air (with accompanying CO2) will be drawn back into the container. Carbon dioxide can be stripped from reentering air with a soda lime (20% NaOH, 65% CaO, 15% H2O) or ascarite trap (NaOH-coated silica base). Air passed through these traps also can be used as purge gas to produce CO2-free water.
Stock alkali solution of 50% in water is approximately 18 N. A working solution is made by diluting stock solution with CO 2 -free water. There is no ideal container for strong alkali solutions. Glass and plastic are both used, but each has its drawbacks. If a glass container is used it should be closed with a rubber or thick plastic closure. Glass closures should be avoided since, over time, strong alkali dissolves glass, resulting in permanent fusion of the contact surfaces. Reaction with glass also lowers the normality of the alkali. These liabilities also are relevant to long-term storage of alkali in burettes. NaOH has a low surface tension. This predisposes to leakage around the stopcock. Stopcock leakage during titration will produce erroneously high acid values. Slow evaporation of titrating solution from the stopcock valve during long periods of nonuse also creates a localized region of high pH with ensuing opportunities for fusion between the stopcock and burette body. After periods of nonuse, burettes should be emptied, cleaned, and refilled with fresh working solution.
Long-term storage of alkali in plastic containers also requires special vigilance because CO2 permeates freely through most common plastics. Despite this shortcoming, plastic containers are usually preferred for long-term storage of stock alkali solutions. Whether glass or plastic is used for storage, working solutions should be restandardized weekly to correct for alkalinity losses arising from interactions with glass and CO2.
4.3.2 Standard Acid
The impurities and hygroscopic nature of NaOH make it unsuitable as a primary standard. Therefore, NaOH titrating solutions must be standardized against a standard acid. Potassium acid phthalate (KHP) is commonly used for this purpose.
KHP’s single ionizable hydrogen (pK a = 5. 4) provides very little buffering at pH 8.2. It can be manufactured in very pure form, it is relatively nonhygroscopic, and it can be dried at 120 ∘C without decomposition or volatilization. Its high molecular weight also favors accurate weighing.
KHP should be dried for 2 h at 120 ∘C and allowed to cool to room temperature in a desiccator immediately prior to use. An accurately measured quantity of KHP solution is titrated with a base of unknown normality. The base is always the titrant. CO2 is relatively insoluble in acidic solutions. Consequently, stirring an acid sample to assist in mixing will not significantly alter the accuracy of the titration.
4.4 Sample Analysis
A number of official methods exist for determining titratable acidity in various foods (1). However, determining titratable acidity on most samples is relatively routine, and various procedures share many common steps. An aliquot of sample (often 10 ml) is titrated with a standard alkali solution (often 0. 1 N NaOH) to a phenolphthalein endpoint. Potentiometric endpoint determination is used when sample pigment makes use of a color indicator impractical.
Typical titration setups are illustrated in Fig. 13-6 for potentiometric and colorimetric endpoints. Erlenmeyer flasks are usually preferred for samples when endpoint indicators are used. A magnetic stirring bar may be used; but mixing the sample with hand swirling is usually adequate. When hand mixing is used the sample flask is swirled with the right hand. The stopcock is positioned on the right side. Four fingers on the left hand are placed behind the stopcock valve and the thumb is placed on the front of the valve. Titrant is dispensed at a slow, uniform rate until the endpoint is approached and then added dropwise until the endpoint does not fade after standing for some predetermined period of time, usually 5–10 s.
The bulkiness of the pH electrode usually demands that beakers be used instead of Erlenmeyer flasks when samples are analyzed potentiometrically. Mixing is almost always achieved through magnetic stirring, and loss of sample through splashing is more likely with beakers than with Erlenmeyer flasks. Otherwise, titration practices are identical to those described previously for colorimetric endpoint titrations.
Problems may arise when concentrates, gels, or particulate-containing samples are titrated. These matrices prevent rapid diffusion of acid from densely packed portions of sample material. This slow diffusion process results in a fading endpoint. Concentrates can simply be diluted with CO2-free water. Titration then is performed, and the original acid content is calculated from dilution data. Starch and similar weak gels often can be mixed with CO2-free water, stirred vigorously, and titrated in a manner similar to concentrates. However, some pectin and food gum gels require mixing in a blender to adequately disrupt the gel matrix. Thick foams are occasionally formed in mixing. Antifoam or vacuum can be used to break the foams.
Immediately following processing, the pH values of particulate samples often vary from one particulate piece to another. Acid equilibration throughout the entire mass may require several months. As a result, particulate-containing foods should be liquefied in a blender before titrating. The comminuting process may incorporate large quantities of air. Air entrapment makes the accuracy of volumetric measurements questionable. Aliquots often are weighed when air incorporation may be a problem.
4.5 Calculation of Titratable Acidity
In general chemistry, acid strength is frequently reported in normality (equivalents per liter) and can be calculated using the equation N titrant ×V titrant = N sample ×V sample, where N is normality and V is volume (often in milliliters). However, food acids are usually reported as percent of total sample weight. Thus, the equation for titratable acidity is as follows:
where:
Note that the normality of the titrant is expressed in milliequivalents (mEq) per ml, which is a typical way of reporting normality for small volumes. This value is numerically the same as equivalents/liter. Also note that it is easier to report sample mass in grams instead of milligrams, so multiplying sample mass by the factor of 1000 mg/g allows units to cancel.
For routine titration of fruit juices, milliliters can be substituted for sample weight in grams, as shown in Equations [15] and [16]. Depending on the soluble solids content of the juice, the resulting acid values will be high by 1–6%. However, this is common practice.
or
where:
For example, if it takes 17.5 ml of 0. 085 N NaOH to titrate a 15-ml sample of a juice, the total titratable acidity of that juice, expressed as percent citric acid (molecular weight = 192; equivalent weight = 64), would be 0.635%, wt/vol, citric acid:
Notice that the equivalent weight of anhydrous (vs. hydrous) citric acid always is used in calculating and reporting the results of titration.
4.6 Acid Content in Food
Most foods are as chemically complex as life itself. As such, they contain the full complement of Krebs cycle acids (and their derivatives), fatty acids, and amino acids. Theoretically, all of these contribute to titratable acidity. Routine titration cannot differentiate between individual acids. Therefore, titratable acidity is usually stated in terms of the predominant acid. For most foods this is unambiguous. In some cases, two acids are present in large concentrations, and the predominant acid may change with maturity. In grapes, malic acid often predominates prior to maturity while tartaric acid typically predominates in the ripe fruit. A similar phenomenon is observed with malic and citric acids in pears. Fortunately, the equivalent weights of common food acids are fairly similar. Therefore, percent titratable acidity is not substantially affected by mixed predominance or incorrect selection of the predominant acid.
The range of acid concentrations in foods is very broad. Acids can exist at levels below detection limits or they can be the preeminent substance present in the food. The contribution of acids to food flavor and quality is not told by acid content alone. The tartness of acids is reduced by sugars. Consequently, the Brix/acid ratio (often simply called ratio) is usually a better predictor of an acid’s flavor impact than Brix or acid alone. Acids tend to decrease with the maturity of fruit while sugar content increases. Therefore, the Brix/acid ratio often is often an index of fruit maturity. For mature fruit, this ratio can also be affected by climate, variety, and horticultural practices. Table 13-6 gives typical acid composition and sugar levels for many commercially important fruits at maturity. Citric and malic acids are the most common acids in fruits and most vegetables; however, leafy vegetables also may contain significant quantities of oxalic acid. Lactic acid is the most important acid in dairy foods for which titratable acidity is commonly used to monitor the progress of lactic acid fermentations in cheese and yogurt production (15).
Organic acids contribute to the refractometer reading of soluble solids. When foods are sold on the basis of pound solids, Brix readings are sometimes corrected for acid content. For citric acid, 0. 20 ∘Brix is added for each percent titratable acidity.
4.7 Volatile Acidity
In acetic acid fermentations, it is sometimes desirable to know how much acidity comes from the acetic acid and how much is contributed naturally by other acids in the product. This can be achieved by first performing an initial titration to measure titratable acidity as an indicator of total acidity. The acetic acid is then boiled off, the solution is allowed to cool, and a second titration is performed to determine the fixed acidity. The difference between fixed and total acidity is the volatile acidity. A similar practice is used sometimes in the brewing industry to separate acidity due to dissolved CO2 from fixed acids. Fixed acids are titrated after CO2 is removed by low heat (40 ∘C) and gentle agitation.
4.8 Other Methods
High-performance liquid chromatography (HPLC) and electrochemistry both have been used to measure acids in food samples. Both methods allow identification of specific acids. HPLC uses refractive index, ultraviolet, or for some acids electrochemical detection. Ascorbic acid has a strong electrochemical signature and significant absorbance at 265 nm. Significant absorbance of other prominent acids does not occur until 200 nm or below.
Many acids can be measured with such electrochemical techniques as voltammetry and polarography. In ideal cases, the sensitivity and selectivity of electrochemical methods are exceptional. However, interfering compounds often reduce the practicality of electrochemical approaches.
Unlike titration, chromatographic and electrochemical techniques do not differentiate between an acid and its conjugate base. Both species inevitably exist side by side as part of the inherent food-buffer system. As a result, acids determined by instrumental methods may be 50% higher than values determined by titration. It follows that Brix/acid ratios can be based only on acid values determined by titration.
5 Summary
Organic acids have a pronounced impact on food flavor and quality. Unlike strong acids that are fully dissociated, food acids are only partially ionized. Some properties of foods are affected only by this ionized fraction of acid molecules while other properties are affected by the total acid content. It is impractical to quantify only free hydronium ions in solution by chemical methods. Once the free ions are removed by chemical reaction, others arise from previously undissociated molecules. Indicator dyes, which change color depending on the hydronium ion environment, exist but they only identify when a certain pH threshold has been achieved and do not stoichiometrically quantify free hydronium ions. The best that can be done is to identify the secondary effect of the hydronium ion environment on some property of the system such as the color of the indicator dyes or the electrochemical potential of the medium. The pH meter measures the change in electrochemical potential established by the hydronium ion across a semipermeable glass membrane on an indicator electrode. The shift in the indicator electrode potential is indexed against the potential of a reference electrode. The difference in millivolt reading between the two electrodes can be converted into pH using the Nernst equation. The hydronium ion concentration can be back-calculated from pH using the original definition of pH as the negative log of hydrogen ion concentration. Buffer solutions of any pH can be created using the Henderson–Hasselbalch equation. However, the predictions of all these equations are somewhat approximate unless the activity of acids and conjugate bases is taken into account.
Titratable acidity provides a simple estimate of the total acid content of a food. In most cases, it is only an estimate since foods often contain many acids that cannot be differentiated through titration. Titratable acidity is not a good predictor of pH, since pH is a combined function of titratable acid and conjugate base. Instrumental methods such as HPLC and electrochemical approaches measure acids and their conjugate bases as a single compound and, therefore, tend to produce acid contents that are higher than those determined by titration. Titratable acidity, somewhat curiously, is a better predictor of tartness than the concentration of free hydronium ions as reflected by pH. The perception of tartness is strongly influenced by the presence of sugars. Indicator dyes are used commonly to identify the endpoint of acidity titrations, although pH meters can be used in critical work or when sample color makes indicators impractical.
6 Study Questions
-
1.
Explain the theory of potentiometry and the Nernst equation as they relate to being able to use a pH meter to measure H+ concentration.
-
2.
Explain the difference between a saturated calomel electrode and a silver–silver chloride electrode; describe the construction of a glass electrode and a combination electrode.
-
3.
You return from a 2-week vacation and ask your lab technician about the pH of the apple juice sample you gave him or her before you left. Having forgotten to do it before, the technician calibrates a pH meter with one standard buffer stored next to the meter and then reads the pH of the sample of unpasteurized apple juice immediately after removing it from the refrigerator (40 ∘F), where it has been stored for 2 weeks. Explain the reasons why this stated procedure could lead to inaccurate or misleading pH values.
-
4.
For each of the food products listed below, what acid should be used to express titratable acidity?
-
Orange juice
-
Yogurt
-
Apple juice
-
Grape juice
-
-
5.
What is a “Brix/acid ratio,” and why is it often used as an indicator of flavor quality for certain foods, rather than simply Brix or acid alone?
-
6.
How would you recommend determining the endpoint in the titration of tomato juice to determine the titratable acidity? Why?
-
7.
The titratable acidity was determined by titration to a phenolphthalein endpoint for a boiled and unboiled clear carbonated beverage. Which sample would you expect to have a higher calculated titratable acidity? Why? Would you expect one of the samples to have a fading endpoint? Why?
-
8.
Why and how is an ascarite trap used in the process of determining titratable acidity?
-
9.
Why is volatile acidity useful as a measure of quality for acetic acid fermentation products, and how is it determined?
-
10.
What factors make KHP a good choice as a standard acid for use in standardizing NaOH solutions to determine titratable acidity?
-
11.
Could a sample that is determined to contain 1.5% acetic acid also be described as containing 1.5% citric acid? Why or why not?
-
12.
An instructor was grading lab reports of students who had determined the titratable acidity of grape juice. One student had written that the percent titratable acidity was 7.6% citric acid. Give two reasons why the answer was marked wrong. What would have been a more reasonable answer?
7 Practice Problems
-
1.
How would you prepare 500 ml of 0.1 M NaH2PO4 starting with the solid salt?
-
2.
Starting with reagent grade sulfuric acid (36 N), how would you prepare 1 L of 2 M H2SO4? How many milliliters of 10 N NaOH would be required to neutralize this acid?
-
3.
How would you prepare 250 ml of 2 N HCl starting with reagent grade HCl (12 N)?
-
4.
How would you prepare 1 L of 0.04 M acetic acid starting with reagent grade HOAc (17 M)?
-
5.
How would you prepare 150 ml of 10% NaOH?
-
6.
If about 8.7 ml of saturated NaOH is required to prepare 1 L of 0. 1 N NaOH, how would you prepare 100 ml of 1 N NaOH?
-
7.
What is the normality of a (1 + 3) HCl solution?
-
8.
You are performing a titration on duplicate samples and duplicate blanks that require 4 ml of 1 N NaOH per titration sample. The lab has 10% NaOH and saturated NaOH. Choose one and describe how you would prepare the needed amount of NaOH solution.
-
9.
Is a 1% HOAc solution the same as a 0.1 M solution? Show calculations.
-
10.
Is a 10% NaOH solution the same as a 1 N solution? Show calculations.
-
11.
What is the normality of a 40% NaOH solution?
-
12.
You are performing duplicate titrations on five samples that require 15 ml of 6 N HCl each. How would you prepare the needed solution from reagent grade HCl?
-
13.
What is the pH of a 0.057 M HCl solution?
-
14.
Vinegar has a [H+ ] of 1. 77 × 10−3 M. What is the pH? What is the major acid found in vinegar, and what is its structure?
-
15.
Orange juice has a [H+ ] of 2. 09 × 10−4 M. What is the pH? What is the major acid found in orange juice and what is its structure?
-
16.
A sample of vanilla yogurt has a pH of 3.59. What is the [H+ ]? What is the major acid found in yogurt and what is its structure?
-
17.
An apple pectin gel has a pH of 3.30. What is the [H+ ]? What is the major acid found in apples, and what is its structure?
-
18.
How would you make 100 ml of a 0. 1 N solution of KHP?
-
19.
How would you make 100 ml of a citrate buffer that is 0. 1 N in both citric acid (anhydrous) and potassium citrate KH2C6H5O7 (MW 230.22)?
-
20.
What would be the pH of the 0. 1 N citrate buffer described in Problem 19?
-
21.
How would you make 1 L of 0. 1 N NaOH solution from an 18 N stock solution?
-
22.
A stock base solution assumed to be 18 N was diluted to 0. 1 N. KHP standardization indicated that the normality of the working solution was 0. 088 N. What was the actual normality of the solution?
-
23.
A 20-ml sample of juice requires 25 ml of 0. 1 N NaOH titrant. What would be the percent acid if the juice is (1) apple juice, (2) orange juice, (3) grape juice?
-
24.
A lab analyzes a large number of orange juice samples. All juice samples will be 10 ml. It is decided that 5 ml of titrant should equal 1% citric acid. What base normality should be used?
-
25.
A lab wishes to analyze apple juice. They would like each milliliter of titrant to equal 0.1% malic acid. Sample aliquots will all be 10 ml. What base normality should be used?
Answers
-
1.
The question asks for 500 ml of a 0.1 M NaH2PO4 solution. The molecular weight of this salt is 120 g/mol. You can use Equation [1] to solve this problem.
$$\begin{array}{rcl} & & \mbox{ (500\,ml of sodium phosphate)} \\ & & \quad \times \mbox{ (molarity of sodium phosphate)} \\ & & \qquad = \mbox{ millimoles of sodium phosphate} \\ & & \frac{(500\,\mathrm{ml})(0.1\,M)(120\,\mathrm{g}/\mathrm{mol})} {1000\,\mathrm{ml}/\mathrm{L}} = 6\,\mathrm{g}\,{\mathrm{NaH}}_{2}\,{\mathrm{PO}}_{4} \\ \end{array}$$ -
2.
-
1000 ml of 2 M H2SO4 is required. Reagent grade H2SO4 is 36 N and 18 M. Therefore,
$$\begin{array}{rcl} (\mathrm{18}\,\mathrm{M})(x\,\mathrm{ml})& =& (\mathrm{2}\,\mathrm{M})(\mathrm{1,}000\mathrm{ml}). \\ x\,\mathrm{ml}& =& \mathrm{111.1\,ml\ of\ conc.\ acid\ diluted\ to\ 1L.} \\ \end{array}$$(When diluting concentrated acids, always add concentrated acid to about one half the final volume of water to dilute and to dissipate the heat generated by mixing. Never add the water to the concentrated acid!)
-
$$\begin{array}{rcl} & & \\ & & (\mathrm{1}000\,\mathrm{ml}\,{\mathrm{H}}_{\mathrm{2}}{\mathrm{SO}}_{\mathrm{4}})(\mathrm{2}\,M\,{\mathrm{H}}_{\mathrm{2}}{\mathrm{SO}}_{\mathrm{4}})(\mathrm{2}\,N/\mathrm{1}\,M) \\ & & \quad = (x\,\mathrm{ml}\,\mathrm{NaOH})(\mathrm{1}0\,N\,\mathrm{NaOH}) \\ & & x\,\mathrm{ml} = \mathrm{4}00\,\mathrm{ml}\,\mathrm{NaOH} \\ \end{array}$$
-
-
3.
Using Equation [1]:
$$\begin{array}{rcl} (\mathrm{25}0\,\mathrm{ml})(\mathrm{2}\,N\,\mathrm{HCl})& =& (x\,\mathrm{ml})(\mathrm{12}\,N\,\mathrm{HCl}) \\ x\,\mathrm{ml}& =& \mathrm{41}.\mathrm{67}\,\mbox{ ml of conc. HCl} \\ & & \mbox{ diluted with water to 25}0\,\mathrm{ml} \\ \end{array}$$ -
4.
Using Equation [1]:
$$\begin{array}{rcl} & (0.0\mathrm{4}\,M\,\mathrm{HOAc})(\mathrm{1}\,\mathrm{L})(\mathrm{1}000\,\mathrm{ml}/\mathrm{L}) = (x\,\mathrm{ml})(\mathrm{17}\,M\,\mathrm{HOAc}) & \\ & x\,\mathrm{ml} = \mathrm{2}.\mathrm{35}\,\mbox{ ml conc. acetic acid that is diluted to 1\,L}& \\ \end{array}$$ -
5.
Usually with a solid starting material like NaOH, the percent is a weight-to-volume percent (or percent wt/vol). Therefore, 10% NaOH = 10 g NaOH/100 ml of solution. Thus, 150 ml of 10% NaOH requires 15 g NaOH = 15 g NaOH/150 ml = 10% NaOH.
-
6.
If about 8.7 ml of saturated NaOH diluted to 1 L gives 0. 1 N, this equals (0. 1 N)(1000 ml) = 100 mEq. Since both solutions contain the same number of milliequivalents, they both must require the same volume of saturated NaOH, 8.7 ml.
-
7.
The convention (1 + 3) HCl, as used for some analytical food methods (e.g., AOAC Methods), means 1 part concentrated acid and 3 parts distilled water, or a 1-in-4 dilution. Starting with concentrated HCl at 12 N, a 1-in-4 dilution will yield \((1/4)(12\,N\ \mathrm{HCl}) = 3.00\,N\) HCl.
-
8.
Four titrations of 4 ml each will be performed requiring a total of about 16 ml of 1 N NaOH. For simplicity, 20 ml of 1 N NaOH can be prepared. If a 10% NaOH stock solution is used, then
$$\begin{array}{rcl} 10\,\mathrm{g\ NaOH/100\,ml}& =& 100\,\mathrm{g\ NaOH/L} = 2.5\,N\,\mathrm{NaOH} \\ (\mathrm{2}0\,\mathrm{ml})(\mathrm{1}\,N\,\mathrm{NaOH})& =& (x\,\mathrm{ml})(\mathrm{2}.\mathrm{5}\,N) \\ x\,\mathrm{ml}& =& \mathrm{8}\,\mbox{ ml of 10\% diluted to 20\,ml} \\ & & \mbox{ with distilled water} \\ \end{array}$$If saturated NaOH is used, remember from Problem 6 that approximately 8.7 ml of saturated NaOH diluted to 100 ml yields 1. 0 N. Therefore, 1.87 ml or 2 ml of saturated NaOH diluted to 20 ml with distilled water will yield about 1 N NaOH
-
9.
1% HOAc = 1 g HOAc/100 ml = (10 g HOAc/L)/(60.05 g/mol) = 0.17 mole/L = 0.17 M and
$$\begin{array}{rcl} & \mbox{ 0.1\,$M$ HOAc} = \mbox{ 0.1\,mol}\ \mathrm{HOAc/L} \times 60.05\,\mathrm{g/mol}& \\ & \mbox{ 6.005\,g HOAc/L} = 0.60\,\mathrm{g}/100\,\mathrm{ml} = 0.60\%\ \mathrm{HOAc}& \\ \end{array}$$Therefore, the two acetic acid solutions are not the same, differing by a factor of about 2.
-
10.
10% NaOH = 10 g NaOH/100 ml = 100 g NaOH/L100 g NaOH/(40 g/mol)/L = 2.5 M NaOH and
$$\begin{array}{rcl} \mbox{ 1\,$N$ NaOH} = \mbox{ 1\,mol NaOH/L}& =& \mbox{ 40\,g NaOH/L} \\ \mbox{ 4\,g NaOH/100\,ml}& =& \mbox{ 4\% NaOH} \\ \end{array}$$No, the solutions are not the same.
-
11.
40% NaOH = 40 g NaOH/100 ml = 400 g NaOH/L(400 g NaOH/L)/(40 g NaOH/mol) = 10 mol/L = 10 N
-
12.
A total of (5 samples)(2 duplicates)(15 ml) = 150 ml of 6 N HCl
$$\begin{array}{rcl} \mbox{ (150\,ml)(6\,$N$ HCl)}& =& \mbox{ (x ml)(12\,$N$ HCl)} \\ \mbox{ x ml}& =& \mbox{ 75\,ml concentrated HCl diluted} \\ & & \mbox{ with distilled water to 150\,ml} \\ \end{array}$$ -
13.
Since HCl is a strong acid, it will be completely dissociated. Therefore, the molar concentration of HCl is the molar concentration of H+ and of Cl−.
$$\begin{array}{rcl} ({\mathrm{H}}^{+})& & = 0.0\mathrm{57}\,N = \mathrm{5}.\mathrm{7} \times \mathrm{1}{0}^{-\mathrm{2}}M \\ \mathrm{pH}& & = -\mathrm{log(5}.\mathrm{7} \times \mathrm{1}{0}^{-\mathrm{2}}\mathrm{)}M \\ & & = (0.76 - 2) \\ & & = -(-1.24) \\ & & = (1.24) \\ \end{array}$$What is the pH of a 0.025 N NaOH solution?
$$\begin{array}{rcl} ({\mathrm{OH}}^{-})& & = 0.0\mathrm{25}\,M = \mathrm{2}.\mathrm{5} \times \mathrm{1}{0}^{-\mathrm{2}}M \\ \mathrm{pOH}& & = -\mathrm{log(2.5 \times \mathrm{1}{0}^{-\mathrm{2}})}M \\ & & = -(0.40 - 2) \\ & & = 1.6 \\ \mathrm{pH}& & = 14 - 1.6 \\ & & = 12.40 \end{array}$$How many grams of NaOH are required to make l00 ml of 0. 5 N NaOH?
$$\begin{array}{rcl} \mbox{ l00\,ml}\ \mathrm{NaOH} \times 0.5\,N = 50\,\mathrm{mEq}\ \mbox{ or}\ \mbox{ 0.050 Eq}& & \\ \end{array}$$Since NaOH has molecular weight of 40.0 g/mol and one equivalent per mole, the equivalent weight is 40.0 g per equivalent.
-
14.
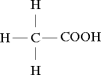
2.75; acetic acid; (Use the equation in Step 1 of Table 13-4, \(\mathrm{pH}\,=\, -\log [{\mathrm{H}}^{+}]\), to solve Problems 14–17.)
-
15.
3.68; citric acid;

-
16.
1. 1 × 10−4 M; lactic acid;

-
17.
5. 0 × 10−4 M; malic acid;

-
18.
From Table 13-2, the equivalent weight of KHP is 204.22 g/Eq. The weight of KHP required can be calculated from the equation.
$$\begin{array}{rcl} \mathrm{Acid\ wt}.& =& \frac{\mathrm{Desired\ volume}\,\mathrm{(ml)}} {\mathrm{1}000\,\mathrm{ml}/\mathrm{L}} \\ & & \times \mathrm{Eq\ wt}.\,(\mathrm{g}/\mathrm{Eq}) \\ & & \times \mathrm{desired}\,N\,(\mathrm{Eq}/\mathrm{L}) \\ \end{array}$$Therefore,
$$\begin{array}{rcl} \mathrm{KHP\ wt}& =& \frac{\mathrm{1}00\,\mathrm{ml}} {\mathrm{1}000\,\mathrm{ml}/\mathrm{L}} \times \mathrm{2}0\mathrm{4}\,\mathrm{g}/\mathrm{Eq} \times 0.\mathrm{1}\,\mathrm{Eq}/\mathrm{L} \\ & =& \mathrm{2}.0\mathrm{422}\,\mathrm{g} \\ \end{array}$$The solution can be made by weighing exactly 2.0422 g of cool, dry KHP into a 100-ml volumetric flask and diluting to volume.
-
19.
This problem is the same as Problem 18, except that two components are being added to 100 ml of solution. From Table 13-2, the equivalent weight of citric acid (anhydrous) is 64.04 g/Eq. Therefore, the weight of citric acid (CA) would be
$$\begin{array}{rcl} \mathrm{CA}\,\mathrm{wt}& =& \frac{\mathrm{1}00\,\mathrm{ml}} {\mathrm{1}000\,\mathrm{ml}/\mathrm{L}} \times \mathrm{64}.0\mathrm{4}\,\mathrm{g}/\mathrm{Eq} \times 0.\mathrm{1}\,\mathrm{Eq}/\mathrm{L} \\ & =& 0.6404\,\mathrm{g} \\ \end{array}$$Potassium citrate (PC) is citric acid with one of its three hydrogen ions removed. Consequently, it has one less equivalent per mole than CA. The equivalent weight of PC would be its molecular weight (230.22) divided by its two remaining hydrogen ions, or 115.11 g per equivalent. Therefore, the weight contribution of PC would be
$$\begin{array}{rcl} \mathrm{PC}\,\mathrm{wt}& =& \frac{\mathrm{1}00\,\mathrm{ml}} {\mathrm{1}000\,\mathrm{ml}} \\ & & \times \mathrm{115}.\mathrm{11}\,\mathrm{g}/\mathrm{Eq} \times 0.\mathrm{1}\,\mathrm{Eq}/\mathrm{L} = \mathrm{1}.\mathrm{511}\,\mathrm{g} \\ \end{array}$$ -
20.
The relationship between pH and conjugate acid/base pair concentrations is given by the Henderson–Hasselbalch equation.
$$\mathrm{pH} = \mathrm{p}{K}_{\mathrm{a}} + \mathrm{log}\frac{[{\mathrm{A}}^{-}]} {[\mathrm{HA}]}$$When acid and conjugate base concentrations are equal, \([{\mathrm{A}}^{-}]/[\mathrm{HA}] = 1\). Since the log of 1 is 0, the pH will equal the pK a of the acid. Because CA and PC are both 0. 1 N, the pH will equal the pK a1 of citric acid given in Table 13-5 (pH = 3. 2).
-
21.
Using Equation [1] and solving for volume of concentrate, we get
$$\begin{array}{rcl} \mathrm{ml}\,\mathrm{concentrated}\,\mathrm{solution}& =& \frac{\mathrm{final}\,N \times \mathrm{final}\,\mathrm{ml}} {\mathrm{beginning}\,N} \\ & =& \frac{0.\mathrm{1}\,N \times \mathrm{1}000\,\mathrm{ml}} {\mathrm{18}\,N} = \mathrm{5}.\mathrm{55}\,\mathrm{ml} \\ & & \\ \end{array}$$Consequently, 5.55 ml would be dispensed into a 1-L volumetric flask. The flask would then be filled to volume with distilled CO2-free water.
The normality of this solution will only be approximate since NaOH is not a primary standard. Standardization against a KHP solution or some other primary standard is essential. It is useful sometimes to back-calculate the true normality of the stock solution. Even under the best circumstances, the normality will decrease with time, but back-calculating will permit a closer approximation of the target normality the next time a working standard is prepared.
-
22.
This answer is a simple ratio.
$$\frac{0.088} {0.100} \times 18 = 15.85\,N$$ -
23.
Table 13-6 indicates that the principal acids in apple, orange, and grape juice are malic, citric, and tartaric acids, respectively. Table 13-2 indicates that the equivalent weight of these acids are malic (67.05), citric (64.04), and tartaric (75.05). The percent acid for each of these juices would be as follows:
$$\begin{array}{rcl} & & \mathrm{Malic\ acid} \\ & & \quad = \frac{0.\mathrm{1}\,\mathrm{mEq}/\mathrm{ml}\,\mathrm{NaOH} \times \mathrm{25}\,\mathrm{ml} \times \mathrm{67}.0\mathrm{5}\,\mathrm{mg/mEq}} {\mathrm{2}0\,\mathrm{ml(10)}} \\ & & \quad = \mathrm{}0.\mathrm{84}\% \\ & & \mathrm{Citric\ acid} \\ & & \quad = \frac{0.\mathrm{1\,mEq}/\mathrm{ml\,NaOH} \times \mathrm{25\,ml} \times \mathrm{64}.0\mathrm{4\,mg}/\mathrm{mEq}} {\mathrm{2}0\,\mathrm{ml(10)}} \\ & & \quad = 0.\mathrm{8}0\% \\ \end{array}$$$$\begin{array}{rcl} & & \\ & & \mathrm{Tartaric\ acid} \\ & & \quad = \frac{0.\mathrm{1}\,\mathrm{mEq}/\mathrm{ml}\,\mathrm{NaOH} \times \mathrm{25}\,\mathrm{ml} \times 75.05\,\mathrm{mg}/\mathrm{mEq}} {\mathrm{2}0\,\mathrm{ml(10)}} \\ & & \quad = 0.94\% \\ \end{array}$$ -
24.
Quality control laboratories often analyze a large number of samples having a specific type of acid. Speed and accuracy are increased if acid concentration can be read directly from the burette. It is possible to adjust the normality of the base to achieve this purpose. The proper base normality can be calculated from the equation:
$$N = \frac{\mathrm{1}0 \times A} {B \times C}$$where:
$$\begin{array}{rcl} A& =& \mbox{ weight (or volume) of the sample to be titrated} \\ B& =& \mbox{ volume (ml) of titrant you want to equal 1\% acid} \\ C& =& \mbox{ equivalent weight of the acid} \\ \end{array}$$$$N = \frac{\mathrm{1}0 \times \mathrm{1}0} {\mathrm{5} \times \mathrm{64}.0\mathrm{4}} = 0.\mathrm{3123}\,N$$In actuality, the standard alkali solution used universally by the Florida citrus industry is 0. 3123 N.
-
25.
Since each milliliter will equal 0.1% malic acid, 1% malic acid will equal 10 ml. Therefore,
$$N = \frac{\mathrm{1}0 \times \mathrm{1}0} {\mathrm{5} \times \mathrm{67}.05} = 0.\mathrm{1491}\,N$$
References
AOAC International (2007) Official methods of analysis, 18th edn., 2005; Current through revision 2, 2007 (On-line). AOAC International, Gaithersburg, MD
Beckman Instruments (1995) The Beckman handbook of applied electrochemistry. Bulletin No. BR-7739B. Fullerton, CA
Dicker DH (1969) The laboratory pH meter. American Laboratory, February
Efiok BJS, Eduok EE (2000) Basic calculations for chemical and biological analysis, 2nd edn. AOAC International, Gaithersburg, MD
Fisher Scientific (1996) Fisher electrode handbook, 7th edn. Bulletin No. 120P. Pittsburgh, PA
Gardner WH (1996) Food acidulants. Allied Chemical Co., New York
Harris DC (2002) Quantitative chemical analysis, 6th edn. Macmillan, New York
Joslyn MA (1970) pH and buffer capacity, ch. 12, and acidimetry, ch. 13. In: Methods in food analysis, Academic, New York
Kenkel J (1988) Analytical chemistry for technicians. Lewis, Chelsea, MI
Mohan C (1995) Buffers. Calbiochem – Novabiochem International, La Jolla, CA
Nelson PE, Tressler DK (1980) Fruit and vegetable juice process technology, 3rd edn. AVI, Westport, CT
Pecsok RL, Chapman K, Ponder WH (1971) Modern chemical technology, vol 3, revised edn. American Chemical Society, Washington, DC
Pomeranz Y, Meloan CE (1994) Food analysis: theory and practice, 3rd edn. Chapman & Hall, New York
Skogg DA, West DM, Holler JF, Crouch SR (2000) Analytical chemistry: an introduction, 7th edn. Brooks/Cole, Pacific Grove, CA
Wehr HM, Frank JF (eds) (2004) Standard method for examination of dairy products, 17th edn. American Public Health Association, Washington, DC
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Sadler, G.D., Murphy, P.A. (2010). pH and Titratable Acidity. In: Food Analysis. Food Analysis. Springer, Boston, MA. https://doi.org/10.1007/978-1-4419-1478-1_13
Download citation
DOI: https://doi.org/10.1007/978-1-4419-1478-1_13
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4419-1477-4
Online ISBN: 978-1-4419-1478-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)























