Abstract
As a result of a plethora of lab-based studies focusing on primate quadrupedalism, it is well known that compared to most other mammals, primates exhibit distinctive quadrupedal kinematics when moving on artificial “terrestrial” or “arboreal” substrates. However, we have little knowledge of how quadrupedal kinematics are impacted by the complexity of natural habitats, in which pathways may be obstructed, unstable, or vary dramatically in size, orientation, shape, or texture. In this study, we compared data on the quadrupedal kinematics of Saimiri boliviensis in both laboratory and field settings by comparing kinematic responses across laboratory substrates (pole, floor) and natural substrates (branches that varied in size and orientation). Field results indicate that Saimiri boliviensis adjusted to larger branches by increasing limb duty factors, but used a wide variety of gait types (as measured by limb phase) across all branch sizes and orientations, rather than fine tuning limb phase to these aspects of substrate. Lab poles elicited similar average limb phases and duty factors, but reduced gait flexibility compared to branches. Lab studies would benefit from greater complexity of simulated arboreal substrates, and field studies should strive to measure numerous substrate characteristics to most effectively test hypotheses about the adaptive nature of primate locomotion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Primates exhibit a highly diverse and specialized repertoire of locomotor behaviors, indicating that locomotion has played a key role in their evolutionary adaptive strategies. Accordingly, much research has been devoted to understanding the morphological, biomechanical, and ecological factors associated with locomotor variation and evolution across the primate order. The accomplishment of these research goals requires both field and laboratory data. Field studies are critical for providing the ecological context for primate locomotion, documenting the relative frequencies of positional behaviors used by a particular species, the context in which they are used, e.g., travel versus feeding, and the types of substrates on which certain behaviors are preferred. Laboratory studies benefit from the ability to isolate and measure aspects of locomotor biomechanics or morphology and to test specific functional hypotheses about muscle function, bone structure, and locomotor kinematics and kinetics. While field studies have been limited by the inability to use complex equipment to measure locomotor biomechanical variables directly on primate subjects, laboratory studies suffer from the isolation of primate locomotion from the wide variety and complexity of substrates to which primates have become adapted in their natural habitat. These two approaches are complementary; the data provided by one can and should be used to provide key insights into the other. Moreover, recent attempts at overlapping the two methods hold much promise toward providing a more complete analysis of primate locomotion. For example, laboratory studies have worked toward increasing the complexity of substrates on which primates are tested (e.g., Stevens 2006, 2007, chapter 16; Nyakatura et al. 2008) or examining nonstereotypical movements such as turning (e.g., Demes et al. 2006). Conversely, field-based studies of locomotion are experimenting with methods to measure detailed aspects of locomotor morphology and energetics from a distance (Sellers and Crompton 2004; Rothman et al. 2008; Blanchard et al., chapter 10; Pontzer et al., chapter 15).
One type of primate locomotor behavior that has been very well studied is quadrupedalism. Based nearly exclusively on data collected in the lab, the kinematics and kinetics of primate quadrupedalism have been shown to be unusual among mammals, suggesting an adaptive advantage to this form of locomotion early in the evolution of primates. Unlike the quadrupedal walking of most other mammals, primate quadrupedalism is characterized by a preference for diagonal-sequence, diagonal-couplets (DSDC) gait, increased forelimb protraction, reduced vertical ground reaction forces on forelimbs relative to hind limbs, compliant gait, greater limb excursion angles, long stride lengths, and low stride frequencies (Hildebrand 1967; Kimura et al. 1979; Alexander and Maloiy 1984; Reynolds 1985; Demes et al. 1994; Larson et al. 2000, 2001; Li 2000; Cartmill et al. 2007b).
Given the (not unreasonable) assumption that quadrupedal biomechanics measured in the laboratory reflects similar behavior in the wild, evolutionary explanations for the distinctive aspects of primate quadrupedalism have focused on the importance of substrate type. Current consensus states that the unusual aspects of primate quadrupedalism are a biomechanical complex that gave early primates a selective advantage over their mammalian competitors by allowing them exclusive access to resources available in the “fine branch niche” (Cartmill 1972; Larson 1998; Cartmill et al. 2002; Schmitt and Lemelin 2002; Lemelin et al. 2003). Primate locomotor features are viewed as adaptations to movement on branches of narrow diameter and/or nonhorizontal orientation because they are theorized to increase balance and stability, reduce branch oscillations, and enhance the forelimb’s manipulatory abilities (Prost and Sussman 1969; Rollinson and Martin 1981; Demes et al. 1994; Vilensky et al. 1994; Larson 1998; Schmitt 1999; Larson et al. 2000, 2001; Cartmill et al. 2002; Schmitt and Lemelin 2002; Lemelin et al. 2003 Schmitt 2003a, c; Stevens 2003). The convergent expression of these kinematic features, along with the presence of grasping hands and feet in some arboreal marsupials (and their absence in more terrestrial mammals), has provided further support for the importance of small branches in the evolution of primate quadrupedal locomotion (Hildebrand 1976; White 1990; Pridmore 1994; Larson et al. 2000; Schmitt and Lemelin 2002; Lemelin et al. 2003).
Our current view of the adaptive advantage of primate quadrupedalism has benefited greatly from numerous laboratory studies demonstrating that primates exhibit distinctive quadrupedal biomechanics when moving on artificial “terrestrial” versus “arboreal” substrates (Schmitt 1994, 1998, 1999; Schmitt and Hanna 2004; Franz et al. 2005; Wallace and Demes 2008; Young 2009). Researchers have also attempted to analyze the impact of more detailed arboreal environments on primate quadrupedalism by varying the size and/or inclination of simulated branches (usually continuous, smooth, stable poles; Schmitt 2003c; Stevens 2007; Nyakatura et al. 2008; cf. Stevens 2003, 2006). However, we have little knowledge of how quadrupedal kinematics are impacted by the complexity of natural habitats, in which pathways may be obstructed, unstable, or vary dramatically in size, shape, texture, or inclination. To progress toward a better understanding of primate quadrupedalism from an adaptive and evolutionary perspective, it is imperative that we get a broader picture of the variability in substrate use in natural habitats and how aspects of those substrates, e.g., size and inclination, affect quadrupedal kinematics.
In this study, we compare data on the quadrupedal kinematics of Bolivian squirrel monkeys (Saimiri boliviensis) in both laboratory and field settings, i.e., Cocha Cashu Biological Station, Manu National Park, Peru. In many ways, Saimiri boliviensis is an excellent species with which to explore the adaptive significance of primate gait kinematics. First, squirrel monkeys at Manu are exceedingly active, frequently traveling 2–5 km per day in order to evade predators and gain access to distributed foraging resources (Terborgh 1983; Mitchell 1990). In fact, squirrel monkeys are the most itinerant primates at Manu, with home ranges more than twice as large as similarly-sized primates at the site, e.g., Cebus and Saguinus (Terborgh 1983; Mitchell 1990). Second, squirrel monkeys frequently travel and forage on a variety of substrates that vary widely in diameter and orientation (Terborgh 1983; Boinski 1989; Fontaine 1990; Mitchell 1990; Arms et al. 2002). Finally, previous laboratory studies have provided conflicting data on the predominant pattern of interlimb coordination in squirrel monkeys. Prost and Sussman (1969) and Vilensky and colleagues (Vilensky and Patrick 1985; Vilensky et al. 1994) found that squirrel monkeys primarily used lateral sequence gaits when walking on declined and level substrates, but diagonal sequence gaits on inclined surfaces. In contrast, more recent observations indicate that diagonal sequence gaits predominate on all substrates (Arms et al. 2002; Schmidt 2005; see also Youlatos, chapter 14, on howlers).
Our objectives are to:
-
1)
Provide additional field-based data documenting the range of variation of substrate size and orientation utilized by squirrel monkeys during quadrupedal walking and running in a natural habitat;
-
2)
Provide data on footfall patterns and interlimb timing utilized by Saimiri for comparison to previous laboratory studies;
-
3)
Assess whether artificial arboreal substrates capture similar quadrupedal behavior when compared to locomotion in natural habitats;
-
4)
Evaluate the degree to which quadrupedal kinematics are “fine-tuned” to substrate characteristics; i.e., is kinematic variation across substrates greater than that within substrates?
Materials and Methods
Laboratory Data
J. Young collected laboratory data at the Center for Neotropical Primate Research and Resources (CNPRR, Mobile, AL). All procedures were approved by the CNPRR Institutional Animal Care and Use Committee (IACUC). The sample consisted of five female squirrel monkeys, ranging in age from 104 to 302 days and body mass from 218 to 535 g. Monkeys were filmed with a high-speed digital video camera (MotionMeter 1000, Redlake MASD, San Diego, CA) at 250 Hz as they traversed a 2.75 m × 0.3 m × 0.53 m runway. The floor of the runway was constructed from vinyl-coated plywood (Omega Signboard, Laminators Incorporated, Hatfield, PA). The top and front walls of the runway were formed from a single piece of angled Plexiglas, allowing the subject to be easily lighted and filmed. Depending on experimental condition, e.g. floor versus pole, monkeys traversed either the flat runway floor or a 3.2 cm diameter PVC pipe elevated 10.7 cm above the surface of the runway. Both substrates were coated with a mixture of polyurethane and nonskid paint additive (Behr Process Corporation, Santa Ana, CA) in order to increase traction.
Before the beginning of each squirrel monkey experiment, individuals were weighed and the skin over the approximate centers of rotation of the shoulder and the hip were shaved and marked with retro-reflective tape, a procedure that did not require the use of anesthesia. Video files were imported into the MATLAB DLT Dataviewer 2 digitizing platform (Hedrick 2007) for coding of kinematic variables. More details about the experimental apparatus and procedure can be found in Young (2009).
Field Data
We collected field data from videotapes taken by A. Souther, of Saimiri boliviensis moving in its natural forest habitat at Cocha Cashu Biological Station, Manu National Park, Peru. Manu National Park sits on the bank of a large river (the Rio Manu) and consists of undisturbed primary forest encompassing several different vegetation types, from riparian successional vegetation, to dense lacustrine swamps, to high ground tropical forests. Because Saimiri is not a habitat specialist (Boinski et al. 2002), but rather ranges widely over several microhabitats during the course of a day, the variety of vegetation types ensures that individuals encounter a diversity of substrate sizes and inclinations during daily travel. We collected video data over a period of 2 months in September–October 1998. Individual subjects were not identified, so the number of individuals or their ages is unknown, although the sample does appear to include some juveniles with fully independent locomotion. Because the period of study corresponds to the beginning of the wet season at Manu, when most births take place (Terborgh 1983; Mitchell 1990), any juveniles filmed would have been no younger than ca. 10–12 months old. We filmed monkeys with a hand-held camcorder (Canon ES5000) at 30 Hz. Video fields were subsequently split, resulting in an effective frame rate of 60 Hz. We selected usable video clips, i.e., those in which the camera was close enough for good visibility of limbs and trunk, and then imported them into Peak Motus (v. 9.2, Vicon Motion Systems, Oxford, UK) for coding of kinematic variables.
Kinematic Variables
The kinematic variables used in this study represent a subset of those that have been shown to vary with substrate type in previous laboratory studies and that were also easily measured from the field videos.
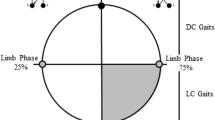
Limb phase: Limb phase describes both footfall sequence and interlimb timing, i.e., couplets (Hildebrand 1966, 1976). Divisions between named gaits, e.g., DSDC, LSDC, are a slight modification of the divisions of Hildebrand (1966, 1976) and follow those of Cartmill et al. (2002), in which values between 50 and 75 are designated as diagonal sequence, diagonal couplets (DSDC) gaits; values between 25 and 50 are lateral sequence, diagonal couplets (LSDC) gaits; and values between 0 and 25 are lateral sequence, lateral couplets (LSLC) gaits. Although limb phase is usually calculated based on the time lag between ipsilateral limb touchdown events (Hildebrand 1967), theory and data indicate that when forelimb and hind limb duty factors are unequal, calculating limb phase from mid-support events provides a more accurate description of interlimb coordination (Hildebrand 1976; Griffin et al. 2004). Therefore, because forelimb and hind limb duty factors were highly divergent across substrate categories (paired t-tests: all p < 0.001), we calculated limb phase as the proportion of stride duration separating hind limb and ipsilateral forelimb mid-support events (where mid-support is defined as the midpoint between touchdown and lift-off).
Duty factor: Duty factor is the proportion of stride duration that a limb is in contact with the substrate. Because duty factors in hind and forelimbs often differ, we report the mean duty factor across all four limbs as an index of the overall response to variation in substrate type, size, and orientation.
Relative speed: Owing to the lack of absolute scale in our field videos, we measured relative speed as trunk lengths per second for both laboratory and field data. For field data, we measured trunk length as the distance between shoulder and hip joints. We calculated relative speed by scaling trunk length to the distance traversed by the individual on the substrate during a full stride, i.e., relative trunk length, and dividing by stride duration.
In the laboratory, absolute speed (in meters per second) was calculated from the displacement of either the hip or the shoulder, depending on marker visibility. After transforming raw pixel coordinates into meters using a standard calibration object, we used linear least-squares regressions of corrected displacement data on time to calculate overall speed across each stride. We then calculated trunk length as the mean distance between the hip and shoulder across six stride events, e.g., forelimb and hind limb touchdown, mid-support and lift-off. We calculated relative speed as the quotient of absolute speed divided by trunk length. The range of relative speeds sampled was similar across both conditions (field: 0.71–5.0; laboratory 2.4–5.0), allowing comparisons of other variables with respect to speed.
Symmetry: In a perfectly symmetrical walk or run, a fore- or hind limb contacts the ground at exactly 50% of the interval of time between footfalls of the contralateral fore or hind limb (Hildebrand 1966). For this study, we excluded asymmetrical strides that were obviously gallops, bounds, or half-bounds, i.e., with whole-body aerial phases. Because perfect symmetry is rare even in gaits normally classified as “symmetrical,” we included walking or running gaits that deviated from perfect symmetry. The average fore-hind symmetry values in our dataset ranged predominantly from 40% to 60%, i.e., 97% of all strides in the data set (Fig. 17.1), matching previous boundaries used to define symmetry (Schmitt et al. 2006). Nevertheless, as long as there was no whole-body aerial phase, we did not exclude strides with symmetry values below or above this range, as we feel this captures the more naturalistic locomotor behavior of the animals.
Substrate Variables
In the field, we quantified substrates with respect to orientation and relative size. We determined substrate orientation by digitizing two endpoints of the substrate traversed by the individual for each stride included in the sample, and calculating its angular orientation relative to the horizontal plane. For categorical comparisons, “horizontal” included all substrates with orientations between –10 and +10 degrees. We categorized substrates with all other negative angles as declines and substrates with all other positive angles were categorized as inclines. Substrate orientations sampled ranged from –69° to +89°. Actual substrate sizes were unknown because the video images lacked an absolute scale. Therefore, we digitized substrate diameters, and calculated relative substrate size as substrate diameter divided by the animal’s trunk length. Schultz (1963) reported that among squirrel monkeys, foot length is typically 41% of trunk length. Assuming the feet would need to grasp across at least half the circumference of a branch for it to be considered easily graspable (Cartmill 1974), we estimated that Saimiri would have more difficulty grasping branches with a circumference greater than 80% of trunk length. Since circumference is equal to π*diameter, we categorized relative substrate sizes (expressed as a proportion of trunk length) as “small” when below 0.25 and “large” when above 0.25. Overall, relative substrate sizes from the field data ranged from 0.04 to 0.73. In the lab, substrates consisted of an elevated 3.2 cm pole and the flat floor of the test runway, both of which were horizontal. So that “arboreal” laboratory and field data could be compared directly, the relative diameter of the pole was also expressed as a proportion of trunk length. Relative substrate size of the pole across all laboratory subjects ranged from 0.20 to 0.25 and was therefore comparable to a “small” branch.
Statistical Analyses
We used χ2 tests of goodness of fit to examine proportional differences in categorical gait type, i.e., LSLC, LSDC, DSDC, attributable to substrate type, orientation, and relative size. Limb phase was non-normally distributed across most of our categorical subgroups. Categorical differences in limb phases were therefore examined using either nonparametric Kruskal-Wallis tests, supplemented by post hoc Wilcoxon rank-sum tests with a Sequential Bonferroni correction (Rice 1988), or rank-based analyses of covariance (ANCOVA: Conover and Iman 1981), specifying duty factor as the covariate. Mean duty factor, in contrast, was normally distributed across most subgroups. Because duty factor correlates strongly with relative speed across conditions (see later), we examined categorical differences in duty factor using ANCOVAs, specifying relative speed as the covariate. Post hoc analyses following significant ANCOVAs were examined using Tukey’s T-method (Sokal and Rohlf 1995). Finally, we examined associations between continuous kinematic and substrate parameters using either Spearman rank-order (ρ) or Pearson product-moment correlations (r), depending on data normality.
Results
Comparison of laboratory- and field-based data on quadrupedal locomotion in Saimiri boliviensis
Limb Phase
In both laboratory and field, Saimiri boliviensis most frequently used DSDC gaits (χ2 [4] = 35.7, p < 0.001). Nevertheless, it is notable that squirrel monkeys also used LSDC or LSLC gaits in all three conditions, i.e., pole, floor, branches, albeit in lower frequencies (Fig. 17.2a, b). Limb phase correlates significantly negatively with duty factor on both branches and pole (branches: ρ = –0.53, p < 0.001; pole: ρ = –0.50, p < 0.001) but not on the floor (ρ = 0.12, p = 0.24). The lack of correlation between limb phase and duty factor during locomotion on the floor is due to the squirrel monkeys’ flexible use of both DS and LS gaits at all duty factors. After controlling for the influence of duty factor when appropriate, we found Saimiri boliviensis to have used significantly higher average limb phases on the pole than on the floor (US[101,71] = 4627, p < 0.01), but statistically similar average limb phases on the pole and on branches (F [1,117] = 1.0, p = 0.32; Fig. 17.2c, Table 17.1). Limb phase did not differ between branches and the floor, perhaps due to increased variability in these conditions. On the floor, where balance issues are nonexistent, and hands and feet are not employed in grasping, any functional constraints on limb phase are likely alleviated, freeing the monkeys to utilize a wider variety of gaits (Vilensky and Larson 1989; Schmidt 2005). On the other hand, the variability of limb phase on branches cannot be interpreted without a more in-depth analysis of substrate variation encompassed by this category (see natural substrate section later).
Gait variation in Saimiri boliviensis across two laboratory substrates (pole, floor) and all natural substrates (branch). (a) Limb phase values for all strides plotted against mean duty factor. (b) Frequencies of each gait type. LS = lateral sequence, DS = diagonal sequence, DC = diagonal couplets, LC = lateral couplets. See text for definitions of limb phase values included in each gait category. (c) Box-and-whisker plots of limb phase, in which dark lines represent the median, gray boxes represent the interquartile range, dotted lines are 1.5 × interquartile range, and the circles are outliers
Speed and Duty Factor
Consistent with the results of numerous laboratory studies, duty factor and relative speed had an inverse relationship across lab-based and field-based data (branch: r = –0.62, p < 0.001; pole: r = –0.68, p < 0.001; floor: r = –0.53, p < 0.001; Fig. 17.3). However, at a given relative speed, Saimiri boliviensis used higher duty factors on natural substrates than either the pole or floor (F [2,219] = 5.6, p < 0.01). In other words, limb contact times were longer relative to stride duration on branches than on smooth poles or on a flat surface, even after controlling for the effects of speed (Fig. 17.3, Table 17.1).
Effects of Natural Substrate Variation on Quadrupedal Locomotion in Saimiri boliviensis
Substrate Orientation
Limb phase: Substrate orientation had no clear effect on limb phase. Squirrel monkeys used DSDC, LSDC, and LSLC gaits on inclining, declining, and horizontal branches but DSDC gaits were highly preferred on each type (Fig. 17.4a, b). There was no correlation between substrate angle and limb phase (ρ = 0.006, p = 0.96), and average limb phases did not differ across categories of substrate orientation (H [2 ] = 0.143, p = 0.931; Fig. 17.4c, Table 17.1).
Gait variation in Saimiri boliviensis across inclined, declined, and horizontal natural substrates. (a) Limb phase values for all strides plotted against mean duty factor. (b) Frequencies of each gait type. LS = lateral sequence, DS = diagonal sequence, DC = diagonal couplets, LC = lateral couplets. See text for definitions of limb phase values included in each gait category. (c) Box-and-whisker plots of limb phase, in which dark lines represent the median, gray boxes represent the interquartile range, dotted lines are 1.5 × interquartile range, and the circles are outliers
Speed and duty factor: Relative speed did not correlate with substrate orientation (r = –0.15, p = 0.29), indicating that squirrel monkeys used similar ranges of relative speed across declining, inclining, and horizontal branches. At a given relative speed, duty factors tended to be higher on inclines than on horizontal or declined branches (Fig. 17.5, Table 17.1), but this difference was not significant (F [2,44] = 1.2, p = 0.31).
Relative Substrate Size
Limb phase: Relative substrate size had no clear effect on limb phase. Squirrel monkeys used DSDC, LSDC, and LSLC gaits on both small and large substrates (Fig. 17.6a, b). Similar to the results for substrate orientation, squirrel monkeys used predominantly DSDC gaits on both large and small substrates. There was no correlation between limb phase and relative substrate size (ρ = 0.15, p = 0.36), and average limb phases did not differ significantly between small and large branches (U S [34,13] = 124, p = 0.36; Fig. 17.6c, Table 17.1).
Gait variation in Saimiri boliviensis across small and large natural substrates. (a) Limb phase values for all strides plotted against mean duty factor. (b) Frequencies of each gait type. LS = lateral sequence, DS = diagonal sequence, DC = diagonal couplets, LC = lateral couplets. See text for definitions of limb phase values included in each gait category. (c) Box and whiskers plots of limb phase, in which dark lines represent the median, gray boxes represent the interquartile range, dotted lines are 1.5 × interquartile range, and the circles are outliers
Speed, duty factor: Squirrel monkeys did not modulate their speed in a consistent manner with respect to substrate size; relative speed and substrate size were uncorrelated (r = 0.02, p = 0.90). However, duty factor did vary with relative substrate size, even after controlling for the effects of speed (F [2,114] = 9.3, p < 0.001). At a given relative speed, monkeys used significantly higher mean duty factors on large branches relative to small substrates, whether they are small branches or similarly sized poles (large branches – small branches: t [55] = 2.8, p < 0.05; large branches – pole: t [82] = 4.3, p < 0.001; Fig. 17.7, Table 17.1). Moreover, among all branches classified as “large,” mean duty factor correlated significantly positively correlated with substrate size (r = 0.73, p < 0.01; Fig. 17.8). Although sample sizes were reduced when we examined limbs separately, both hind and forelimb duty factors increased with substrate size, and the correlation was stronger in the forelimb (hind: r = 0.55, p = 0.051; fore: r = 0.71, p = 0.049).
Summary of Results
-
1.
Overall, limb phases did not differ with respect to substrate; DSDC gait was highly preferred by Saimiri on all substrates, in the laboratory as well as in its natural habitat.
-
2.
LSDC and LSLC gaits were used occasionally by Saimiri on natural substrates of all three orientations (horizontal, incline, decline) and both sizes (small, large), and on a flat laboratory surface. LSLC, but not LSDC gaits, were used on the laboratory pole.
-
3.
Variation in branch orientation or relative size did not affect relative speed.
-
4.
Relative substrate size, but not substrate orientation, affected mean duty factor. At a given speed, Saimiri used significantly higher mean duty factors, i.e., relative contact times, on large branches than on small branches or poles of similar diameter to small branches, and mean duty factors increased as large branches increased in relative size.
Discussion
Previous laboratory studies on Saimiri assessed quadrupedal kinematics while subjects walked on flat horizontal or flat inclined “boards” (Prost and Sussman 1969); flat horizontal, inclined, or declined treadmills (Vilensky and Patrick 1985; Vilensky et al. 1994); horizontal wooden poles (Schmidt 2005); or a variety of substrates (Arms et al. 2002). Previous field studies of locomotion in Saimiri have provided ecological data on the relative frequency of quadrupedal locomotion on various substrates (Fleagle and Mittermeier 1980; Boinski 1989; Fontaine 1990; Mitchell 1990; Johnson and Shapiro 1998; Youlatos 1999). Our study expands on previous work by providing additional lab-based data, in conjunction with the first quantitative analysis of quadrupedal kinematics in Saimiri in a natural habitat. This allows us to assess 1) the consistency of results across several laboratory-based studies, 2) the extent to which squirrel monkeys adjust their quadrupedal gait characteristics in response to substrate variation in size and angular orientation, and 3) the extent to which lab-based data represent natural locomotor behavior. Our data also provides insight on the benefits and limitations of both laboratory- and field-based kinematic analysis.
Our laboratory analysis tested horizontal substrates only. Prost and Sussman (1969) reported that Saimiri used LS gaits 63% of the time on level ground, and Vilensky et al. (1994) found exclusively LS gaits on a horizontal treadmill. Schmidt (2005) found that Saimiri used DS gaits exclusively on a 3 cm horizontal pole, and Arms et al. (2002) found that Saimiri used DS gaits nearly exclusively (95% of all strides) on a variety of laboratory-constructed substrates. Our laboratory data do not correspond to any of these previous studies. On the floor, rather than preferring LS gaits, our squirrel monkeys preferred DSDC gaits (57% of the time), even though they used LSLC fairly often (38%) and LSDC occasionally (5%). On our pole, rather than using DSDC gaits exclusively, squirrel monkeys used DSDC 87% of the time, and LSLC gaits the rest of the time. The variability of gait choice on horizontal flat surfaces both within our study and across other laboratory studies is consistent with Schmidt’s (2005) observation that if DSDC gait evolved for an arboreal adaptive advantage, primates’ limb phases should be less constrained in situations where grasping is not employed. Schmidt’s (2005) explanation is also consistent with her laboratory animals’ exclusive use of DS gaits on a pole, but begs the question as to why our squirrel monkeys used LSLC gaits in conjunction with DSDC gaits on a pole. It is possible that the use of LSLC gaits in our squirrel monkeys was attributable to the fact that they were infants and juveniles, as other primates have been shown to use this type of gait as a transitory ontogenetic phase (Hurov 1982; Nakano 1996; Shapiro and Raichlen 2005, 2006). We cannot exclude this explanation without further analysis, but at a minimum, our data do not reveal a strict correlation of age with limb phase. It is also possible that the difference in pole data between our study and that of Schmidt (2005) simply expresses the flexibility of gait choice in primates (Vilensky and Larson 1989; Vilensky and Moore 1992).
Our field analysis allowed us to test the effects of relative substrate size and orientation on quadrupedal kinematics. Current hypotheses emphasize that DS gait and other unusual aspects of primate quadrupedalism, e.g., accentuated forelimb protraction at touchdown, higher peak vertical forces on hind limbs than forelimbs, increased limb yield, long stride lengths, low stride frequencies, long limb contact times, most likely evolved because they provide a particular advantage for navigating “small” branches (Larson 1998; Schmitt and Lemelin 2002; Cartmill et al. 2002, 2007a,b). This view is supported by laboratory studies demonstrating that when primates switch from the floor to an artificial arboreal substrate such as a pole, or from larger to smaller poles, at least some aspects of their kinematics become more “primate-like” (Schmitt 1999, 2003b; Schmitt and Hanna 2004; Stevens 2007; Wallace and Demes 2008). Specific to the variables addressed in our study, laboratory studies have shown that DS gait increases in frequency on poles compared to floors (Wallace and Demes 2008; this study), and on inclines compared to declining or horizontal substrates (Prost and Sussman 1969; Vilensky et al. 1994; Stevens 2003; Nyakatura 2008). Duty factors (or limb contact times) have been shown to increase on poles compared to floors (Schmitt 1999), on relatively smaller compared to larger poles, and on declines (Stevens 2003). Therefore, previous laboratory studies combined with evolutionary hypotheses would lead to the prediction that limb phase should increase on relatively small and/or inclined substrates, and duty factor, i.e., relative limb contact time, should increase on relatively small and declined substrates.
To the contrary, at Manu, squirrel monkeys did not adjust limb phase in any consistent manner in response to changing substrate size or orientation. The influence of substrate size on limb phase has not been widely studied. However, contrary to the consensus view of primate quadrupedalism, Dunbar and Badam (2000) observed that juvenile macaques in a natural setting preferred DS on the large end of branches and LS on the smaller, distal stems. Our field results are more consistent with the only primate laboratory study directly assessing limb phase and relative substrate size (Stevens 2007) in which six strepsirrhines did not alter limb phase patterns on small versus large poles. In all other respects, however, our field study’s results are not consistent with most primate laboratory studies or ecologically based evolutionary hypotheses. In Saimiri boliviensis, DS does not appear to be particularly (nor exclusively) functionally associated with smaller or inclined substrates; it is used just as frequently on horizontals, declines and relatively large substrates. In addition, Saimiri boliviensis occasionally uses LS gaits on substrates of both sizes and all orientations. This could represent random flexibility and lack of “fine-tuning,” but it is also possible that instances of LS could be associated with substrate variables not measured here, such as branch surface continuity or branch compliance. In fact, Stevens (2006) showed that Loris tardigradus changed its limb phase when laboratory substrates were manipulated to challenge stability, i.e., rotated or displaced in different planes. Measuring substrate compliance and displacement is rare in field studies (e.g., Demes et al. 1996), but continued work in this area would enhance our understanding of variation in primate kinematics.
The fact that Saimiri boliviensis employed the longest limb relative contact times (duty factors) on the largest substrates is not consistent with adaptive hypotheses indicating that primates employ this kinematic adjustment to enhance stability and decrease branch oscillations on small, terminal branches. By comparison, Stevens (2003) found that strepsirrhines used higher duty factors on smaller substrates, but the substrate size effect was subtle and variable across species. Although it seems counterintuitive that large branches should present more of a functional challenge to squirrel monkeys than smaller ones, we hypothesize that larger duty factors may be a response to the increasing difficulty of grasping as branch circumference increases relative to hand or foot size. Certainly, we need more data from both laboratory and field to further test this hypothesis. Further study could also help reconcile the fact that we found no significant change in duty factors with substrate orientation, contra Stevens’ (2003) observations of increased duty factors on declines in strepsirrhines and observations of Nyakatura et al. (2008) of increased hind/fore duty factor ratios on inclines in cotton-top tamarins.
Because we studied the same species in the laboratory and field, we can evaluate the extent to which laboratory data are representative of more natural behavior. As discussed earlier, although the distribution of gait types used on the floor and on branches (combined) are more similar than the distribution of gait types used on the pole, variability of gait selection on the floor has little to do with arboreality and more to do with freedom from constraints associated with balancing the body. With respect to gait variability on branches, our study did not find a significant influence of relative substrate size or orientation. However, it is possible that gait choice on branches is correlated with substrate variables we have not measured here, such as discontinuity or branch flexibility. After correcting for differences in duty factor, limb phases on the pole were similar on average to those observed on branches. However, the fact that gait choice was less variable on the pole than on branches, suggests that a single, stable, horizontal pole does not adequately capture the full extent of gait flexibility in Saimiri boliviensis. Nevertheless, some aspects of the arboreal environment are captured well by the use of horizontal poles in laboratory studies, as indicated by squirrel monkeys’ use of similar mean duty factors (at a given relative speed) on the pole compared to branches of the same size range.
The benefits of laboratory studies are that animals are more easily filmed and kinematic variables are more easily and accurately measured than in the field. In addition, unique biomechanical hypotheses can be tested by coaxing subjects to move on substrates they might naturally avoid, or by artificially changing their biomechanical properties (e.g., Young et al. 2007). Primate laboratory studies are limited however, by the difficulty of housing or collecting data on more than a few individuals at a time, and by the difficulty of mimicking the complexity of an arboreal environment. Field studies allow one to collect data on many more individuals simultaneously and to test how “fine-tuned” primate locomotion is to the wide variety of substrate challenges found in the natural environment. In addition, field studies can be used to assess how kinematic characteristics might actually affect performance and therefore, evolutionary fitness (Arnold 1983). The disadvantages of locomotor field studies are that animals are difficult to film because they are either far away or obscured by foliage, and some variables cannot be measured without a fair amount of difficulty, e.g., substrate reaction forces.
The best solution is to use laboratory studies to test specific hypotheses in a controlled setting, while using field studies to evaluate the “messiness” of real locomotion and as a guide for selecting appropriate substrates for the lab. As a start, our comparative analysis has revealed that in order to capture the full range of quadrupedal kinematics employed by primates, laboratory studies would benefit from using a wider range of simulated arboreal substrates. Our field results suggest that varying substrate size may be even more critical than substrate orientation, while results from several laboratory studies imply that variation in substrate orientation is also very functionally informative. Of course, substrate variation is much more complex than simply size or orientation. Primates face other arboreal challenges such as discontinuous pathways and unstable branches. We were not able to assess these here, but such factors might have accounted for a portion of the kinematic variation we discovered. Although the true complexity of natural substrates and/or irregular locomotor movements are difficult to measure in the field, it is promising to see efforts to address some of these factors in a controlled laboratory setting (e.g., Stevens 2003, 2006; Demes et al. 2006; Higurashi et al. 2008).
Summary and Conclusions
To summarize, our field study of quadrupedal kinematics revealed that Saimiri boliviensis did not “fine-tune” its limb phases in a consistent manner with respect to relative substrate size or substrate orientation, but this species did significantly increase its duty factors in response to increased branch size. Our laboratory study revealed (not surprisingly) that quadrupedal kinematics on the floor is not a good representation of an arboreal primate’s behavior in a natural habitat. Our laboratory monkeys used comparable duty factors and average limb phases when moving on the horizontal pole and similarly sized arboreal branches, but showed less variability in limb phase on the pole. Therefore, we conclude that laboratory studies can certainly be improved by incorporating more varied substrates, particularly with respect to size.
The flexibility exhibited by Saimiri boliviensis in limb phase across different natural substrates contradicts some previous laboratory studies on Saimiri and other primates that have found a clear effect of substrate orientation on limb phase, i.e., higher limb phases on inclines and lower limb phases on declines. It is also somewhat inconsistent with the view that DS gait in primates is functionally preferable to other gaits on relatively small branches, since both DS and LS gaits were used on small and large branches. Similarly, our results for duty factor are the opposite of what one would expect if primates were most challenged by stability on relatively small branches. There are several implications of the fact that our field results stand in distinction to laboratory studies. It is possible that our field study has revealed the need to examine aspects of substrate variation (in the laboratory or field) beyond size and orientation in order to determine what is driving kinematic variability in this species. Alternatively, Saimiri may happen to be a particularly flexible primate that does not require fine-tuning of its kinematic features to navigate complex and changing substrates. Either way, future studies of primate quadrupedalism, whether in the laboratory or the field, would benefit from a consideration of the unique biomechanical challenges presented by a complex natural environment as well as the distinctive approaches individual species may exhibit to those challenges.
Acknowledgments
We thank the staff at the Center for Neotropical Primate Research and Resources (CNPRR, Mobile, AL) for assistance with data collection and animal care. We also thank staff and researchers at Manu National Park, Peru for permission to conduct this research at Cocha Cashu Biological Station, and for assistance with videotaping Saimiri boliviensis. Special thanks to Teresa Burgess, Abbas Haidry, and Parham Daghighi for assistance with video analysis. Laboratory research was supported by the L.S.B. Leakey Foundation (Grant 38648).
Abbreviations
- DS:
-
diagonal sequence
- DSDC:
-
diagonal-sequence, diagonal-couplets
- LS:
-
lateral sequence
- LSDC:
-
lateral-sequence, diagonal-couplets
- LSLC:
-
lateral-sequence, lateral-couplets
- p :
-
probability level
- r :
-
Pearson product-moment correlation
- rho (ρ) :
-
Spearman rank-order correlation
References
Alexander RM, Maloiy GMO (1984) Stride lengths and stride frequencies of primates. J Zool Lond 202:577–582.
Arms A, Voges D, Fischer MS, Preuschoft H (2002) Arboreal locomotion in small new-world primates. Z Morphol Anthropol 83:243–263.
Arnold SJ (1983) Morphology, performance and fitness. Am Zool 23:347–361.
Boinski S (1989) The positional behavior and substrate use of squirrel monkeys: ecological implications. J Hum Evol 18:659–677.
Boinski S, Sughrue K, Selvaggi L, Quatrone R, Henry M, Cropp S (2002) An expanded test of the ecological model of primate social evolution: competitive regimes and female bonding in three species of squirrel monkeys (Saimiri oerstedii, S. boliviensis, and S. sciureus). Behaviour 139:227–261.
Cartmill M (1972) Arboreal adaptations and the origin of the order Primates. In: Tuttle R (ed), The Functional and Evolutionary Biology of Primates. Chicago, Aldine 97–122.
Cartmill M (1974) Pads and claws in arboreal locomotion. In: Jenkins FA (ed), Primate Locomotion. New York, Academic Press 45–83.
Cartmill M, Lemelin P, Schmitt D (2002) Support polygons and symmetrical gaits in mammals. Zool J Linn Soc 136:401–420.
Cartmill M, Lemelin P, Schmitt D (2007a) Understanding the adaptive value of diagonal-sequence gaits in primates: a comment on Shapiro and Raichlen, 2005. Am J Phys Anthropol 133:822–825.
Cartmill M, Lemelin P, Schmitt D (2007b) Primate gaits and primate origins. In: Ravosa MJ, Dagosto M (eds), Primate Origins: Adaptations and Evolution. New York, Springer 403–435.
Conover WJ, Iman RI (1981) Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat 35:124–133.
Demes B, Larson SG, Stern JT Jr, Jungers WL, Biknivicius AR, Schmitt D (1994) The kinetics of primate quadrupedalism: “hindlimb drive” reconsidered. J Hum Evol 26:353–374.
Demes B, Jungers WL, Fleagle JG, Wunderlich RE, Richmond BG, Lemelin P (1996) Body size and leaping kinematics in Malagasy vertical clingers and leapers. J Hum Evol 31:367–388.
Demes B, Carlson KJ, Franz TM (2006) Cutting corners: the dynamics of turning behaviors in two primate species. J Exp Biol 209:927–937.
Dunbar DC, Badam GL (2000) Locomotion and posture during terminal branch feeding. Int J Primatol 21:649–669.
Fleagle JG, Mittermeier RA (1980) Locomotor behavior, body size, and comparative ecology of seven Surinam monkeys. Am J Phys Anthropol 52:301–314.
Fontaine R (1990) Positional behavior in Saimiri boliviensis and Ateles geoffroyi. Am J Phys Anthropol 82:485–508.
Franz TM, Demes B, Carlson KJ (2005) Gait mechanics of lemurid primates on terrestrial and arboreal substrates. J Hum Evol 48:199–217.
Griffin TM, Main RP, Farley CT (2004) Biomechanics of quadrupedal walking: how do four-legged animals achieve inverted pendulum-like movements? J Exp Biol 207:3545–3558.
Hedrick T (2007) “DLT Data Viewer 2”, Digitizing and DLT in MATLAB. http://www.unc.edu/∼thedrick/software1.html
Higurashi Y, Hirasaki E, Kumakura H (2008) Gaits of Japanese macaques (Macaca fuscata) on a horizontal ladder and arboreal stability. Am J Phys Anthropol 138(4): 448–457.
Hildebrand M (1966) Analysis of the symmetrical gaits of tetrapods. Folia Biotheoretica 1–22.
Hildebrand M (1967) Symmetrical gaits of primates. Am J Phys Anthropol 26:119–130.
Hildebrand M (1976) Analysis of tetrapod gaits: general considerations and symmetrical gaits. In: Herman RM, Grillner S, Stein PSG et al (eds), Neural Control of Locomotion. New York, Plenum Press 203–236.
Hurov JR (1982) Diagonal walking in captive infant vervet monkeys. Am J Primatol 2:211–213.
Johnson SE, Shapiro LJ (1998) Positional behavior and vertebral morphology in atelines and cebines. Am J Phys Anthropol 105:333–354.
Kimura T, Okada M, Ishida H (1979) Kinesiological characteristics of primate walking: its significance in human walking. In: Morbeck ME, Preuschoft H and Gomberg N (eds), Environment, Behavior and Morphology: Dynamic Interactions in Primates. New York, G Fischer 297–311.
Larson SG (1998) Unique aspects of quadrupedal locomotion in nonhuman primates. In: Strasser E, Fleagle J, Rosenberger A, McHenry H (eds), Primate Locomotion. New York, Plenum Press 157–173.
Larson SG, Schmitt D, Lemelin P, Hamrick M (2000) Uniqueness of primate forelimb posture during quadrupedal locomotion. Am J Phys Anthropol 112:87–101.
Larson SG, Schmitt D, Lemelin P, Hamrick M (2001) Limb excursion during quadrupedal walking: how do primates compare to other mammals? J Zool Lond 255:353–365.
Lemelin P, Schmitt D, Cartmill M (2003) Footfall patterns and interlimb coordination in opossums (Family Didelphidae): evidence for the evolution of diagonal sequence gaits in primates. J Zool Lond 260:423–429.
Li Y (2000) Arboreal primates and the origin of diagonal gait. Acta Anthropological Sinica (Suppl) 19:83–89.
Mitchell CL (1990) The ecological basis for female social dominance: a behavioral study of the squirrel monkey (Saimiri sciureus) in the wild. PhD Dissertation, Princeton University, NJ.
Nakano Y (1996) Footfall patterns in the early development of the quadrupedal walking of Japanese macaques. Folia Primatol 66:113–125.
Nyakatura J, Fischer MS, Schmidt M (2008) Gait parameter adjustments of cotton-top tamarins (Saguinus oedipus, Callitrichidae) to locomotion on inclined arboreal substrates. Am J Phys Anthropol 135:13–26.
Pridmore PA (1994) Locomotion in Dromiciops australis (Marsupialia: Microbiotheriidae). Austral J Zool 42:679–699.
Prost JH, Sussman RW (1969) Monkey locomotion on inclined surfaces. Am J Phys Anthropol 31:53–58.
Reynolds TR (1985) Mechanics of increased support of weight by the hindlimbs in primates. Am J Phys Anthropol 67:335–349.
Rice WR (1988) Analyzing tables of statitical tests. Evolution 43:223–225.
Rollinson J, Martin RD (1981) Comparative aspects of primate locomotion, with special reference to arboreal cercopithecines. Symposia of the Zoological Society of London 48:377–427.
Rothman J, Chapman C, Twinomugisha D, Wasserman MD, Lambert JE, Goldberg TL (2008) Measuring physical traits of primates remotely: the use of parallel lasers. Am J Primatol 70:1191–1195.
Schmidt M (2005) Quadrupedal locomotion in squirrel monkeys (Cebidae: Saimiri sciureus): a cineradiographic study of limb kinematics and related substrate reaction forces. Am J Phys Anthropol 128:359–370.
Schmitt D (1994) Forelimb mechanics as a function of substrate type during quadrupedalism in two anthropoid primates. J Hum Evol 26:441–457.
Schmitt D (1998) Forelimb mechanics during arboreal and terrestrial quadrupedalism in Old World monkeys. In: Strasser E, Fleagle J, Rosenberger A, McHenry H (eds), Primate Locomotion: Recent Advances. New York, Plenum Press 175–200.
Schmitt D (1999) Compliant walking in primates. J Zool Lond 248:149–160.
Schmitt D (2003a) Evolutionary implications of the unusual walking mechanics of the common marmoset (C. jacchus). Am J Phys Anthropol 122:28–37.
Schmitt D (2003b) Mediolateral reaction forces and forelimb anatomy in quadrupedal primates: implications for interpreting locomotor behavior in fossil primates. J Hum Evol 44:47–58.
Schmitt D (2003c) Substrate size and primate forelimb mechanics: implications for understanding the evolution of primate locomotion. Int J Primatol 24:1023–1036.
Schmitt D, Lemelin P (2002) Origins of primate locomotion: gait mechanics of the woolly opossum. Am J Phys Anthropol 118:231–238.
Schmitt D, Hanna JB (2004) Substrate alters forelimb to hindlimb peak force ratios in primates. J Hum Evol 46:239–254.
Schmitt D, Cartmill M, Griffin TM, Hannah JB, Lemelin P (2006) Adaptive value of ambling gaits in primates and other mammals. J Exp Biol 209:2042–2049.
Schultz AH (1963) Relations between the lengths of the main parts of the foot skeleton in primates. Folia Primatol 1:150–171.
Sellers W, Crompton R (2004) Automatic monitoring of primate locomotor behaviour using accelerometers. Folia Primatol 75:279–293.
Shapiro LJ, Raichlen DA (2005) Lateral sequence walking in infant Papio cynocephalus: implications for the evolution of diagonal sequence walking in primates. Am J Phys Anthropol 126:205–213.
Shapiro LJ, Raichlen DA (2006) Limb proportions and the ontogeny of quadrupedal walking in infant baboons (Papio cynocephalus). J Zool 269:191–203.
Sokal RR, Rohlf FJ (1995) Biometry. New York, WH Freeman.
Stevens NJ (2003) The influence of substrate size, orientation and compliance upon prosimian arboreal quadrupedalism. PhD Dissertation, Stony Brook University, New York.
Stevens NJ (2006) Stability, limb coordination and substrate type: the ecorelevance of gait sequence pattern in primates. J Exp Zool 305A:953–963.
Stevens NJ (2007) The effect of branch diameter on primate gait sequence pattern. Am J Primatol 70:1–7.
Terborgh J (1983) Five New World Primates: a Study in Comparative Ecology. Princeton, NJ, Princeton University Press.
Vilensky JA, Patrick MC (1985) Gait characteristics of two squirrel monkeys, with emphasis on relationships with speed and neural control. Am J Phys Anthropol 68:429–444.
Vilensky JA, Larson SG (1989) Primate locomotion: utilization and control of symmetrical gaits. Annu Rev Anthrop 18:17–35.
Vilensky JA, Moore AM (1992) Utilization of lateral- and diagonal-sequence gaits at identical speeds by individual vervet monkeys. In: Matano S, Tuttle RH, Ishida H, Goodman M (eds), Topics in Primatology, Vol. 3: Evolutionary Biology, Reproductive Endocrinology and Virology. Tokyo, University of Tokyo Press 129–137.
Vilensky JA, Moore AM, Libii JN (1994) Squirrel monkey locomotion on an inclined treadmill: implications for the evolution of gaits. J Hum Evol 26:375–386.
Wallace IJ, Demes B (2008) Symmetrical gaits of Cebus apella: implications for the functional significance of diagonal sequence gait in primates. J Hum Evol 54:783–794.
White TD (1990) Gait selection in the brush-tail possum (Trichosurus vulpecula), the northern quoll (Dasyurus hallucatus), and the virginia opossum (Didelphis virginiana). J Mammal 71:79–84.
Youlatos D (1999) Comparative locomotion of six sympatric primates in Ecuador. Ann Sci Nat Zool Biol Anim 20:161–168.
Young JW, Patel BA, Stevens NJ (2007) Body mass distribution and gait mechanics in fat-tailed dwarf lemurs (Cheirogaleus medius) and patas monkeys (Erythrocebus patas). J Hum Evol 53:26–40.
Young JW (2008) Ontogeny of locomotion in Saimiri boliviensis and Callithrix jacchus: implications for primate locomotor ecology and evolution. PhD dissertation, Stony Brook University, New York.
Young JW (2009) Substrate determines asymmetrical gait dynamics in marmosets (Callithrix jacchus) and squirrel monkeys (Saimiri boliviensis). Am J Phys Anthropol 138: 403–420.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Shapiro, L.J., Young, J.W., Souther, A. (2011). Quadrupedal Locomotion of Saimiri boliviensis: A Comparison of Field and Laboratory-based Kinematic Data. In: D'Août, K., Vereecke, E. (eds) Primate Locomotion. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-1420-0_17
Download citation
DOI: https://doi.org/10.1007/978-1-4419-1420-0_17
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-1419-4
Online ISBN: 978-1-4419-1420-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)