Abstract
Due to its high ocular transduction, low immune clearance and capability to bypass the brain blood barrier, adeno-associated virus-9 (AAV9) has been regarded as a promising vector for retinal disease gene therapy. We recently demonstrated that AAV9 efficiently transduces the retinal outer plexiform layer (OPL). The OPL consists of synapses formed between axons of the rod and cone photoreceptors (cell bodies in the outer nuclear layer, ONL) and dendrites of bipolar and horizontal cells (cell bodies in the inner nuclear layer, INL). It is not clear whether AAV9 transduces the OPL through the photoreceptors in the ONL or through bipolar and horizontal cells in the INL. To map the subcelluar pathway(s) involved in AAV9-mediated OPL transduction, we delivered subretinally AAV9.CMV.eGFP, an AAV vector carrying the enhanced green fluorescent protein gene (eGFP, 1 × 1010 viral genome particles in microliter), to young (21-day-old) and adult (2- to 3-month-old) C57BL/6 mice. Four weeks after subretinal injection, eGFP expression was examined on retinal cryosections. PSD95 (postsynaptic density protein, a marker for photoreceptor terminals), CtBP2 (C-terminal binding protein 2, a marker for the photoreceptor synaptic ribbon), PKCalpha (protein kinase Cα, a marker for rod bipolar cells), and calbindin (a marker for horizontal cells) were localized by immunofluorescence staining. In AAV9 infected retina, eGFP expression was seen in the retinal pigment epithelia, photoreceptor inner segments, ONL, OPL, Müller cells in the INL, inner plexiform layer and ganglion cell layer. Interestingly, eGFP expression co-localized with PSD95 and CtBP2, but not with PKCalpha and calbindin. Our results suggest that AAV9 transduces the photoreceptor side of the synapses in the OPL rather than the dendrites of bipolar and horizontal cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In the past decade, the world has witnessed tremendous progress in the treatment of inherited retinal degenerations. While no long ago such conditions were regarded untreatable and incurable by any means, gene therapy has preserved retinal morphology and restored retinal functions in several animal models of retinal degenerations (Ali et al. 2000; Acland et al. 2001; Allocca et al. 2006; Alexander et al. 2007). Promising results have also been reported recently in Leber’s Congenital Amaurosis (LCA) patients (Bainbridge et al. 2008; Cideciyan et al. 2008; Hauswirth et al. 2008; Maguire et al. 2008).
To develop more efficient and safe vectors for retinal gene therapy, many groups have begun to evaluate new gene vectors. Among the newly described AAV serotypes, AAV serotype-9 (AAV9) stands out as a particularly attractive vehicle because of its superior performance (Gao et al. 2004, 2005). It was recently reported that AAV9 transduction efficiency can be 200-fold higher than that of other AAV serotypes (Inagaki et al. 2006; Limberis and Wilson 2006; Pacak et al. 2006; Bostick et al. 2007; Vandendriessche et al. 2007). In addition, AAV9 is capable to bypass the brain blood barrier (Foust et al. 2009), an important feature that may be utilized to treat a wide range of neural degenerations in the central never system. Therefore, it is imperative to understand details of this novel AAV vector.
In the eye, two previous studies suggest that subretinal administration of AAV9 lead to robust transduction in the retinal pigment epithelium (RPE), the photoreceptors (including the outer and inner segments, the cell bodies in the outer nuclear layer, ONL), the Müller cells in the inner nuclear layer (INL), and the retinal ganglion cell (RGC) layer (Allocca et al. 2007; Lebherz et al. 2008). Using three different genes and two different promoters, we found that AAV9 also transduces the two retinal synaptic layers (the outer plexiform layer OPL, and the inner plexi-form layer IPL), a special characteristic that was rarely documented with all AAV serotypes (Lei et al. 2009). Here, we further showed that AAV9-mediate expression co-localized with two photoreceptor terminal markers PSD95 and CtBP2, but not with rod ON bipolar cell and horizontal cell markers. Our results suggest that AAV9 mediated OPL transduction is through the photoreceptors but not the neurons in the INL.
2 AAV9-Mediated Gene Transfer in the Retina
Two recent studies evaluated AAV9 transduction in the retina following subretinal administration (Allocca et al. 2007; Lebherz et al. 2008). Both studies demonstrated efficient transduction of the RPE and Müller cells (Allocca et al. 2007; Lebherz et al. 2008). However, one group found photoreceptor transduction (Allocca et al. 2007), whereas the other group did not detect photoreceptor transduction (Lebherz et al. 2008). The reasons for these differences are unknown but may relate to experimental designs such as animal age, the promoter, the reporter gene and the time frame of observation.
To further characterize AAV9 mediated transduction in the retina, we performed a comprehensive study in mice. First, we injected subretinally an RSV promoter driving alkaline phosphatase (AP) reporter gene vector (AAV9.RSV.AP, 1 μl, 1 × 109 viral genome particles). We found widespread (peripheral-central-peripheral) and throughout (from RPE to RGC) transduction in mice ranging from 3- to 12-week-old. AP expression was observed in the RPE, ONL, INL, OPL, IPL, RGC layer and Müller cells but not the outer and inner segments of the photoreceptor. Remarkably, two retinal synaptic layers (OPL and IPL) were highly transduced (Lei et al. 2009).
To exclude the potential bias from the transgene and/or the promoter, we performed another study with the AAV9.CMV.eGFP vector (1 μl, 1 × 1010 viral genome particles). Four weeks after subretinal injection, we observed intense eGFP expression in the RPE, ONL and to less extent in the OPL, Müller cells in the INL, IPL, and RGC layer in all experimental mice (3- to 12-week-old) (Fig. 77.1). Similar to those observed in AAV9.RSV.AP injected eyes (Lei et al. 2009), eGFP expression was widespread and throughout the retina. Our results are consistent with those of Allocca et al and confirm that AAV9 indeed transduces the photoreceptors (Allocca et al. 2007). Similar to our findings with the AV.RSV.AP vector, we observed eGFP expression in the OPL and IPL, the two retinal synaptic layers. Table 77.1 summarizes the retinal tropism of AAV9 mediated gene transduction after subretinal injection.
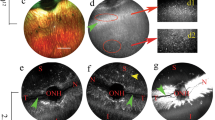
Mouse retinal eGFP expression 4 weeks after subretinal delivery of 1 μl AAV9.CMV.eGFP vector (1 × 1010 viral genome particles). Panels A and B were from the same field except that panel B was taken with a shorter exposure time. Panel C shows schematic outline of the OPL synapse structure. Panel D shows an enlarge view of the boxed area in panel B. The highest eGFP expression was seen in the ONL and RPE layers. Substantial eGFP expression was also found in the OPL, INL, IPL, and RGC layers and the Müller cells. In panels B and D, eGFP expression is mainly localized to the distal portion of the OPL. (RGC, retinal ganglion cell; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer)
3 The Sub-Cellular Location of AAV9 Transduction in the OPL
The finding that AAV9 mediated efficient expression in the OPL is intriguing. Pathology in the OPL is associated with a wide range of retinal diseases (Miyake et al. 1986; Alexander et al. 1992; Fitzgerald et al. 1994; Dryja et al. 2005; Chang et al. 2006). Therefore, AAV9 may be a candidate gene therapy vector for these disorders. Unfortunately, few studies have thoroughly evaluated AAV transduction in the OPL.
To investigate the pattern of AAV9-mediated OPL transduction, we stained AAV9.CMV.eGFP infected eyes with CtBP2 (C-terminal binding protein 2, a marker for the photoreceptor synaptic ribbon), PSD95 (postsynaptic density protein, a marker for the photoreceptor terminals), PKCα (protein kinase C alpha, a marker for the rod bipolar cells), and calbindin (a marker for the horizontal cells). Nuclei were revealed with 4ʹ, 6 diamidino-2-phenylindole dihydrocholoride (DAPI). With shorter exposure time eGFP expression was only seen in the RPE and photoreceptors. Interestingly eGFP co-localized with CtBP2 (Lei et al. 2009) and PSD95 but not with PKCα and calbindin (Fig. 77.2). These results strongly suggest that the observed eGFP expression in the OPL is from the photoreceptor terminals, while the rod bipolar cell and horizontal cell dendrites play minimal role.
Subcellular localization of AAV9 transduction in the OPL. Immunofluorescence staining of PKCα, calbindin and PSD95 in AAV9.CMV.eGFP infected retina (1 × 1010 viral genome particles). PKCα and calbindin are the markers for the rod bipolar cells and horizontal cells (and their dendrites in the proximal portion of the OPL) respectively. PSD95 is a marker for the photoreceptor terminals (distal portion of the OPL). In the overlay images eGFP only co-localize with PSD95
4 AAV9-Mediated Retinal Gene Transfer in mdx 3cv Mice
Mdx 3cv mice are models for Duchene muscular dystrophy (DMD), a lethal childhood genetic disease caused by mutations in the dystrophin gene (Pillers et al. 1995; Pillers et al. 1999). Besides muscle disease, DMD patients also suffer from pathology in other tissues including the retina. A 260 kD dystrophin isoform (Dp260) is normally expressed in the photoreceptor terminals in the OPL. Dp260 expression is lost in the eyes of DMD patients and mdx 3cv mice (Pillers et al. 1993; Schmitz and Drenckhahn 1997; Jastrow et al. 2006). Absence of Dp260 has been associated with the abnormal electroretinogram seen in DMD patients, such as reduced b-wave amplitude and prolonged implicit time.
To determine whether AAV9 can be used to deliver a therapeutic gene to the OPL, we performed subretinal injection of AAV9.CMV.△R4/△C vector in mdx 3cv mice. The 3.8 kb △R4/△C microgene encodes a truncated dystrophin. This microgene has been extensively studied as a candidate gene for DMD gene therapy (Harper et al. 2002). At 5 weeks after subretinal injection, we examined dystrophin expression by immunofluorescence staining. Two epitope-specific antibodies were used in the study. The Dys-2 antibody recognizes endogenous Dp260 in the wild type retina, the Dys-3 antibody only reacts with micro-dystrophin. Consistent with our findings with the reporter AAV vectors, we observed robust microgene expression in the OPL of the injected mdx 3cv mice (Lei et al. 2009). These results raise the hope of using AAV9 to treat retinal diseases that are associated with defects in the photoreceptor terminals.
5 Subretinal Injection of AAV9 Vector Did Not Cause Acute Retinal Damage
We also examined whether subretinal injection of AAV9 vector causes acute damages to the retina. At 5 weeks after delivery of AAV9.RSV.AP or a saline control, we examined retinal histology and recorded dark- and light-adapted electroretinogram in the mouse eyes. Compared with untreated eyes, neither saline nor AAV9 RSV.AP resulted in appreciable morphology alterations. We obtained similar measurements in the thresholds and amplitudes of the dark-adapted ERG a-wave, b-wave and light-adapted b-wave in AAV injected and saline injected eyes (Lei et al. 2009). Our results suggest that rod and cone photoreceptor and bipolar cell functions are not affected by subretinal delivery of AAV9 vectors.
6 Conclusions
Using three different genes and two different promoters, we confirmed that subretinal delivery of AAV9 mediates robust photoreceptor gene transduction. AAV9 vectors also efficiently ferry transgene products to the photoreceptor terminals in the OPL. Our data indicated that AAV9 may be a promising vector for retinal disease gene therapy, especially for disorders that primarily affect the RPE, photoreceptors and the OPL.
References
Acland GM, Aguirre GD, Ray J et al (2001) Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 28:92–95
Alexander JJ, Umino Y, Everhart D et al (2007) Restoration of cone vision in a mouse model of achromatopsia. Nat Med 13:685–687
Alexander KR, Fishman GA, Peachey NS et al (1992) ‘On’ response defect in paraneoplastic night blindness with cutaneous malignant melanoma. Invest Ophthalmol Vis Sci 33:477–483
Ali RR, Sarra GM, Stephens C et al (2000) Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet 25:306–310
Allocca M, Mussolino C, Garcia-Hoyos M et al (2007) Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol 81:11372–11380
Allocca M, Tessitore A, Cotugno G et al (2006) AAV-mediated gene transfer for retinal diseases. Expert Opin Biol Ther 6:1279–1294
Bainbridge JW, Smith AJ, Barker SS et al (2008) Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 358:2231–2239
Bostick B, Ghosh A, Yue Y et al (2007) Systemic AAV9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther 14:1605–1609
Chang B, Heckenlively JR, Bayley PR et al (2006) The nob2 mouse, a null mutation in Cacna1f: anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Vis Neurosci 23:11–24
Cideciyan AV, Aleman TS, Boye SL et al (2008) Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A 105:15112–15117
Dryja TP, McGee TL, Berson EL et al (2005) Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci U S A 102:4884–4889
Duan D, Yue Y, Yan Z et al (2000) Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest 105:1573–1587
Fitzgerald KM, Cibis GW, Giambrone SA et al (1994) Retinal signal transmission in Duchenne muscular dystrophy: evidence for dysfunction in the photoreceptor/depolarizing bipolar cell pathway. J Clin Invest 93:2425–2430
Foust KD, Nurre E, Montgomery CL et al (2009) Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27:59–65
Gao G, Vandenberghe LH, Alvira MR et al (2004) Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol 78:6381–6388
Gao G, Vandenberghe LH, Wilson JM (2005) New recombinant serotypes of AAV vectors. Curr Gene Ther 5:285–297
Harper SQ, Hauser MA, DelloRusso C et al (2002) Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med 8:253–261
18. Hauswirth WW, Aleman TS, Kaushal S, et al. (2009) Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. 19(10):979–990
Inagaki K, Fuess S, Storm TA et al (2006) Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 14:45–53
Jastrow H, Koulen P, Altrock WD et al (2006) Identification of a beta-dystroglycan immunoreactive subcompartment in photoreceptor terminals. Invest Ophthalmol Vis Sci 47: 17–24
Lebherz C, Maguire A, Tang W et al (2008) Novel AAV serotypes for improved ocular gene transfer. J Gene Med 10:375–382
Lei B, Zhang K, Yue Y et al (2009) Adeno-associated virus serotype-9 efficiently transduces the retinal outer plexiform layer. Mol Vis 15:1374–1382.
Limberis MP, Wilson JM (2006) Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci U S A 103:12993–12998
Maguire AM, Simonelli F, Pierce EA et al (2008) Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 358:2240–2248
Miyake Y, Yagasaki K, Horiguchi M et al (1986) Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol 104:1013–1020
Pacak CA, Mah CS, Thattaliyath BD et al (2006) Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res 99:e3–e9
Pillers DA, Bulman DE, Weleber RG et al (1993) Dystrophin expression in the human retina is required for normal function as defined by electroretinography. Nat Genet 4:82–86
Pillers DA, Weleber RG, Green DG et al (1999) Effects of dystrophin isoforms on signal transduction through neural retina: genotype-phenotype analysis of duchenne muscular dystrophy mouse mutants. Mol Genet Metab 66:100–110
Pillers DM, Weleber RG, Woodward WR et al (1995) mdxCv3 mouse is a model for electroretino-graphy of Duchenne/Becker muscular dystrophy. Invest Ophthalmol Vis Sci 36:462–466
Schmitz F, Drenckhahn D (1997) Localization of dystrophin and beta-dystroglycan in bovine retinal photoreceptor processes extending into the postsynaptic dendritic complex. Histochem Cell Biol 108:249–255
Vandendriessche T, Thorrez L, Acosta-Sanchez A et al (2007) Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost 5:16–24
Zhang SH, Wu JH, Wu XB et al (2008) Distinctive gene transduction efficiencies of commonly used viral vectors in the retina. Curr Eye Res 33:81–90
Acknowledgments
This work was supported in part by Research Board of the University of Missouri; National Institutes of Health grant NIH AR49419, and a grant from the Muscular Dystrophy Association. We thank Mrs. Chun Long for technical assistant, Drs. Guangping Gao and James Wilson for providing the AAV9 packaging plasmid pRep2/Cap9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Lei, B., Zhang, K., Yue, Y., Ghosh, A., Duan, D. (2010). Adeno-Associated Virus Serotype-9 Mediated Retinal Outer Plexiform Layer Transduction is Mainly Through the Photoreceptors. In: Anderson, R., Hollyfield, J., LaVail, M. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 664. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-1399-9_77
Download citation
DOI: https://doi.org/10.1007/978-1-4419-1399-9_77
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-1398-2
Online ISBN: 978-1-4419-1399-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)