Abstract
Recent evidence reports that high doses of O2 administered via hyperbaric oxygen therapy (HBOT) improve the return of spontaneous circulation (ROSC), and the outcome of damage to the heart following a 25 min normothermic cardiac arrest. However, excessive O2 during HBOT can be toxic. Near infrared absorbance spectroscopy (NIRS) measures and determines when cytochrome oxidase (aa3), the O2 end user, changes from reduced to oxidized, signifying adequate dosage. Present NIRS monitoring methods do not account for change in scattering expected in severe anoxia. Given this limitation, we simultaneously measured changes in intensity and scattering that occurred over time after 830 nm light traveled 4.25 cm through brain tissue during both normoxia and anoxia. Results indicated increased intensity and scattering during anoxia with correlation between the two, demonstrating that scattering does not remain constant and is associated with intensity. With this additional insight in concurrent scattering and intensity change during anoxia, we believe improvements can be made to our aa3 measuring technique resulting in a method to ascertain adequate O2 dosage during HBOT.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
A recent publication reported that use of HBOT during resuscitation results in ROSC after 25 min of normothermic arrest [1]. These results are possible because HBOT raises atmospheric pressure, increasing O2 solubility in plasma and providing a higher O2 content at the mitochondrial site of O2 utilization. Because such high O2 dosages are necessary after cardiac arrest and excessive oxygen is toxic, beneficial, but not excessive dosages are necessary for clinical therapy. Currently, no method exists to measure nontoxic, beneficial O2 dosage.
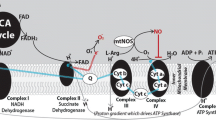
Jobsis first discovered a NIRS technique to measure adequate O2 dosage [2]. He used relative transparency of tissue and Beer’s equation to interrogate brain tissue metabolism non-invasively. NIRS monitors three chromophores: oxygenated hemoglobin (HbO2), deoxygenated hemoglobin (Hb), and aa3. Neither Hb nor HbO2 are helpful to determine nontoxic dosages of O2. Monitoring the redox of aa3 appears to be a useful indicator of brain tissue O2 dosage. Lack of O2 causes aa3 reduction (i.e. buildup of electrons); however, when adequate O2 has removed the excessive electron population, aa3 becomes oxidized. A reliable aa3 signal marking a change from reduced to oxidized would determine adequate O2 dosage during HBOT resuscitation.
In 1988, Jobsis advised that the NIRS technology was ready for measuring equipment to be produced [3]. This publication reported that the halfwidth of the aa3 spectrum measured in mitochondrial and bloodless in situ rat heads (i.e. scattering media) was 75% of the measured halfwidth in purified aa3 (i.e. non scattering media). Although halfwidth reduction was observed Jobsis did not elaborate on this phenomenon. He and others produced algorithms for aa3 signal extraction based on Beer’s equation, but assumed scattering to be constant. Our laboratory became interested in using NIRS for HBOT, but found in vivo aa3 measurements problematic and controversial. Problems included the following: a weak aa3 signal, the use of Beer’s equation in a scattering media, and Hb signal contamination [4]. In 2002, we proposed a method for aa3 signal extraction using a Fortier filtering technique (FFT), which did not require Beer’s equation [5]. We observed that the spectral peak half width was reduced as anoxia progressed. If the aa3 spectrum was also changing, it would cause errors in the FFT.
Kawauchi reported simultaneous measurements in scattering and intensity during anoxia, which was correlated to reduction of aa3 in vivo [6] and later attributed the change in scatter to mitochondrial shrinkage and dendrite swelling [7]. These publications led our team to hypothesize that FFT may have been confounded by a changing aa3 spectrum due to scatter. To examine these effects, we used a scatter model in swine, which was compared to a phantom experiment. Concurrent observation of change in intensity and scatter at 830 nm could lead to the elucidation of how it might affect our FFT extraction and allow adequate O2 dosage measurements.
2 Materials and Methods
Six pigs (30 ± 2 kg) were anesthetized continuously throughout the experiment with Telazol/Xylazine in accordance with our Institutional Animal Care and Use Committee approved protocol. The animals were intubated and provided with 100% O2. The scalp was removed and a template attached to the skull with 3 bone screws to maintain a precise distance between optodes for each animal. The template’s 12 mm diameter input optode was positioned on the midline of the skull 20 mm rostral to the bregma, providing a guide to precisely locate a 5 mm hole, which was drilled through the skull, 40 mm rostral to bregma and 9 mm left of midline. The dura was punctured and a collection optode placed 20 mm below dural tissue.
After assessing for a normal and stable condition, 100 control spectra were taken while the animal was breathing 100% O2. Breathing gas was changed to 100% N2 immediately after control spectra were completed. Recording continued with 200 experimental spectra while the animal’s condition progressed from normoxia to anoxia, and finally death. Spectra identification numbers were noted when mean arterial pressure (η) fell below 50 mmHg (see Figs. 1a and 2) and when cardiac arrest occurred (i.e. beginning of phase III). After death, a 65 mm diameter portion of the skull was trephinized (centered at the template) for use in the phantom experiments.
(a) Change in NFS (1/change area under a normalized S3P) (n = 6) in vivo is plotted against time while a steady state (1%) spectral pulse (830 nm filtered light × 20 nm halfwidth) has traversed 4.25 ± .09 cm through brain tissue. The time course on the x-axis (in 5 min increments) is divided into three phases: Phase I – Normoxia (control period while breathing 100% O2). Note: O2 was substituted for air to compensate for probe insertion brain trauma; Phase II – Anoxia (experimental period while breathing 100% N2 (condition moved from normoxia to anoxia and finally cardiac arrest)); and Phase III – Post Cardiac Arrest (breathing 100% N2). The data were normalized by determining the average scattering values during the 50 min control period and dividing all measured scattering values by this calculated average. Repeated measures ANOVA indicated that normoxia measurements were significantly different from anoxia (p < 0.001). Label (η) is the time point in phase II when MAP fell below 50 mmHg. (b) Phantom data for NFS (n = 6) demonstrate that increased NFS (1/change area under a normalized S3P) occurs when the concentration of scattering media is increased from water to 100% whole milk
Change in intensity (n = 6) is plotted against time while a steady state (1%) spectral pulse (830 nm filtered light × 20 nm halfwidth) has traversed 4.25 ± .09 cm through brain tissue in vivo. See Fig. 1a for time scale details and normalization process. Repeated measure ANOVA indicated that normoxia measurements were significantly different from anoxia (p < 0.001). Label (η) is the time point in phase II when MAP fell below 50 mmHg
A phantom experiment measured identical conditions in various concentrations of whole milk and water. Light was directed to traverse the same distance through the scattering media as in vivo. A series of 20 spectra were taken at each concentration and repeated six times to insure repeatability. The effect of absorbance was measured by adding India ink to 100% whole milk, (i.e. 4 increments of 1–4 µL) (n = 6) while the mixture was magnetically stirred.
A stabilized 250 W Quartz Tungsten Halogen lamp with an additional intensity stabilizer provided stable light within 1%. The light was then directed through an 830 nm filter (20 nm halfwidth), resulting in a steady state spectral pulse (SSSP or S3P) centered at 830 nm with a measured power of 50 mW. The S3P was directed into the skull, dura and brain and collected with a custom fiber optics probe and processed with a liquid N2 CCD spectrophotometer (integration time 29 s with 1 s interval) and stored on a personal computer.
Spectra were extracted from the data at the beginning of each 5 min interval and processed using Grams32 AI software. For intensity measurements the peak of the spectra was considered the intensity value. Normalized forward scattering (NFS) measurements were determined. Each spectrum was normalized to evaluate area change by multiplying by 60,000/intensity measurement (see above) to raise the peak to a fixed value of 60,000 data units while maintaining identical geometrical proportions. Integration provided area under the spectra which represented any broadening or narrowing effects by scattering on the original S3P entering the brain. The intensity and measured area (i.e. NFS) of all control measurements were averaged, representing the 100% normalized value. All other measurements were divided by this value to give their relative normalized values.
3 Results and Discussion
Our swine model data accompanied with phantom NFS and intensity data show at 830 nm, scattering does not remain constant and correlates with normalized intensity during the normoxia to anoxia transition. The concurrent changes in NFS and normalized intensity as S3P traveled 4.25 ± 0.09 cm through the brain (from dura to collection optode) are shown in Figs. 1a and 2. Figure 1b shows the results of phantom NFS measurements and the effect of increasing concentration of scatter media. Notice a 0.1 change in NFS when transitioning from clear water to milk, but a 0.25 NFS change in vivo when shifting from normoxia to anoxia (Fig. 1a), implying a large change in scatter. Phantom absorbance studies indicated no effect on NFS as intensity dropped from 100 to 10% by stepwise increasing absorption media. Our method to measure NFS is novel but uses a narrowing effect on peak halfwidth reported by Jobsis [4], which we believe is caused by increasing scatter. To ensure that results were not caused by artifact due to our novel method of measuring NFS, we preformed a correlation analysis comparing normoxia and anoxia (Fig. 3)
In Fig. 1a, forward scattering over Phase I is constant but oscillates about the mean value. In Fig. 2, intensity over Phase I decreases approximately 0.125. This decrease is likely due to tissue recovery (i.e. aa3 oxidation) in the neighborhood of the collection optode trauma. In Fig. 1a at the beginning of phase II, scatter reverses from increasing to decreasing, but at 55 min again reverses to a rapid increasing rate, and at 65 min continues to rise at a slower rate. In Fig. 2, during Phase II, intensity rapidly decreases; we believe this is due to increased blood volume during initial hypoxia, but at 55 min reverses to a rapidly increasing value due to the reduction of aa3. In both scatter and intensity the bidirectional change is centered at the time point when MAP (η) falls below 50 mmHg. In Figs. 1b and 2 during Phase III, scatter continues to increase, but less rapidly, and intensity continues to increase until 120 min, then remains constant.
Kawauchi [7] reported tridirectional changes in scattering in rats during anoxia. Many investigators have reported bi and tridirectional changes in intensity using tissue slices during hypoxic episodes. We agree with their assessment that change in scattering is probably due to mitochondrial shrinkage and cellular swelling [8].
There is no question that HBOT resuscitation requires a method to ascertain adequate but not excessive dosage, and monitoring aa3 by NIRS has potential. With the evidence that change in scatter is affecting the aa3 spectrum, we believe we can improve our FFT signal extraction method, allowing a precise determination of aa3 conversion from reduced to oxidized signaling adequate O2 dosage.
References
Van Meter K, Sheps S, Kriedt F et al. (2008) Hyperbaric oxygen improves rate of return of spontaneous circulation after prolonged normothermic porcine cardiopulmonary arrest. Resus 78(2):200–214.
Jobsis F (1977) Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198:1264–1267.
Jobsis F, Paintadosi C, Sylvia A et al. (1988) Near infrared monitoring of cerebral oxygen sufficiency. Neurol Res 10(1):7–17 .
Springett R, Newman J, Cope M et al. (2000) Oxygen dependency and precision of cytochrome oxidase signal from full spectral NIRS of the piglet brain. Am J Physiol Heart Circ Physiol 279:H2202–H2209.
Kriedt F, Walker C, Swanson H et al. (2002) Application of Fortier filtering techniques for determining redox change in cerebral cortical cytochrome oxidase. EMBS/BMES Conference. Proceedings of the Second Joint 3(23–26):2257–2258.
Kawauchi S, Sato S, Ooigawa H et al. (2007) Correlation between light scattering and reduction level of cytochrome oxidase in perfused brains of rats. IFMBE Proceedings 14(2):1281-1283.
Kawauchi S, Sato S, Ooigawa H et al. (2008) Simultaneous measurement of light absorption due to reduction of cytochrome oxidase and light scattering in rat brains during loss of tissue viability. Appl Opt 47(22):4164–4176.
Aitken P, Fayuk D, Somjen G et al. (1999) Use of intrinsic optical signals to monitor physiological changes in brain tissue slices. Methods 18:91–103.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this paper
Cite this paper
Kriedt, F., Kriedt, C., Patterson, C., Van Meter, K. (2010). Determination of Oxygen Dosage Effects on Cytochrome Oxidase after Anoxia in Brain. In: Takahashi, E., Bruley, D. (eds) Oxygen Transport to Tissue XXXI. Advances in Experimental Medicine and Biology, vol 662. Springer, Boston, MA. https://doi.org/10.1007/978-1-4419-1241-1_27
Download citation
DOI: https://doi.org/10.1007/978-1-4419-1241-1_27
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4419-1239-8
Online ISBN: 978-1-4419-1241-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)