Abstract
Although cilostazol, an inhibitor of cyclic nucleotide phosphodiesterase 3 (PDE3), is known to exert a potent antiplatelet function by raising intracellular cAMP concentration, its effect on cerebral microcirculation upon an ischemic insult is not clearly understood. To examine effects of cilostazol on the global ischemic injury in the brain, we first measured the plasma leakage using modified Miles assay after mice had been subjected to 60 min of a bilateral common carotid artery (BCCA) occlusion followed by reperfusion for 4 h. Oral treatment with cilostazol (30 mg/kg) significantly increased plasma leakage. This result led us to examine if the treatment with cilostazol recruits more capillaries leading to an increase in surface area for exchange and oxygen transport to tissues. To do so, we simultaneously measured degrees of tissue hypoxia and vessel perfusion. Pimonidazol was injected intraperitoneally 1 h before sacrifice and capillary patency was assessed by fluorescein isothiocyanate-labeled Lycopersicon esculentum lectin bound to the endothelial surface. Treatment with cilostazol markedly increased the capillary patency which was accompanied by a reduction in the hypoxic area. Although the treatment with cilostazol caused an increase in the flux of plasma proteins across endothelial barrier that may imply an adverse role after a BCCA occlusion, this increase in protein leakage was attributable to the increased surface area for exchange which in turn brought about a reduction in tissue hypoxia. Taken together cilostazol appears to produce a protective effect against the ischemic-reperfusion injury.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Cilostazol exerts a potent antiplatelet function by inhibiting cyclic nucleotide phosphodiesterase 3 (PDE3), resulting in raising intracellular cAMP concentration. Besides inhibiting platelet aggregation, this PDE3 inhibitor is known to possess multiple pharmacological effects including a vasodilatory action on resistance vessels [1] and anti-inflammatory effect on the endothelium [2]. It has been approved by the Japanese health authorities as an antiplatelet agent for the treatment of chronic cerebral infarction since 2001. In recent clinical trials using a double-blind method, a long-term administration of cilostazol has shown effectiveness in preventing the recurrence of lacunar infarction [3, 4]. Since lacunar infarction is a small infarction involving occlusion of small penetrating arterioles, it is necessary to test its effect on the microvasculature. We, therefore, conducted a study to understand the mechanism whereby cilostazol elicits a protective effect on the cerebral microcirculation. To do so, we examined if administration of cilostazol changes plasma leakage and oxygen transport across exchange vessels upon ischemic insult.
2 Materials and Methods
2.1 Animal Preparation
Experiments were performed in adult male C57BL/6 mice weighing 19–23 g (aged 8–10 weeks). The animals were anesthetized with an intraperitoneal injection of α-chloralose (60 mg/kg) and urethane (600 mg/kg) and a femoral vein was cannulated with a polyethylene catheter. Forebrain ischemia was induced by ligation of bilateral common carotid arteries (BCCA) with 5-0 silk sutures for 60 min. Exposure of the BCCA without occlusion was used to produce sham control animals. Animals were killed at variable time points according to the experimental protocols. Cilostazol (30 mg/kg, Otsuka Pharmaceutical) was suspended in 0.5% carboxymethylcellulose solution at a volume of 10 ml/kg. The control groups received the same volume of the corresponding vehicle solution. The drugs were administered orally at 4 h prior to the reperfusion.
2.2 Measurement of Plasma Leakage
Plasma leakage was measured by modified Miles assay as described previously [5]. Briefly, Evans blue (30 mg/kg in 100 µl of PBS) was injected intravenously after removing the ligature on BCCA. At this concentration 98% of the dye should be bound to the plasma protein; thus it can serve as a plasma marker. Four hours after reperfusion, the vasculature was perfused transcardially with 50 mmol/L citrate buffer (pH3.5) for 2 min. The brain was removed, the dye was extracted from tissues with 1 ml of formamide overnight at 55°C, and its amount was determined by spectrophotometry.
2.3 Evaluation of Capillary Patency
To evaluate capillary patency, we labeled plasma with dichlorotriazinylamino fluorescein (DTAF; Sigma-Aldrich) as described previously [6]. DTAF conjugated with bovine serum albumin was injected into a saphenous vein. Thirty seconds after completion of the injection, each mouse was decapitated and the brain was fixed in 80% ethanol for 24 h. Coronal brain slices, 50 µm in thickness, were prepared with a vibratome and examined under a fluorescence microscope. Photographs were taken at the level of bregma and at 3 mm caudal from bregma. Each photograph was analyzed to calculate an index for capillary patency that is defined as % of the perfused area divided by the total cross-sectional area.
2.4 Evaluation of Hypoxia and Vessel Perfusion
To assess hypoxic region, Hypoxyprobe-1 (60 mg/kg, Chemicon) was injected intraperitoneally 1 h before sacrifice. To mark vessel perfusion, mice were injected via a femoral vein with FITC labeled Lycopersicon esculentum lectin (4 mg/kg, Vector Laboratories) and lectin was circulated for 1 min. Brain tissues were processed for histochemical analysis [7]. Tissues were stained for anti-Hypoxyprobe-1 and for lectin bound to the endothelial cell surface. Furthermore, endothelial cells were visualized by anti-CD31 antibody.
2.5 Image Analysis
The collected images were processed by the digital imaging software (MetaMorph 6.1, Universal Imaging Corporation). Subsequently, the pixel-based data were converted into gray levels. To determine cross-sectional area of the perfused vessels, a certain grayscale value was applied as a cutoff point and grayscale values above the cutoff were treated as the area being perfused.
3 Results
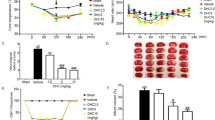
First, we examined whether cilostazol modulates permeability properties of blood brain barrier (BBB) upon ischemic insult. To do so, we compared the flux of plasma protein across BBB using modified Miles assay. Ischemic insult induced by BCCA occlusion elevated leakage of plasma from the vasculature (Fig. 1a, black bar). We initially predicted that treatment of this PDE3 inhibitor could reduce plasma leakage by tightening the BBB through raising intracellular cAMP concentration in the endothelium. On the contrary, the plasma leakage in the cilostazol-treated group (Fig. 1a, gray bar) was greater than that in the non-treated group. This finding led us to examine the possibility that cilostazol recruits more capillaries being perfused leading to an increase in the surface area for exchange of hydrophilic solutes during ischemic reperfusion. No difference in capillary patency was found in the sections at bregma. By contrast, in the cortical area at 3-mm caudal from bregma, cilostazol caused a significant increase in capillary patency (Fig. 1b).
Effects of the cilostazol treatment on plasma leakage and on capillary patency upon ischemic insult. a. Spectrophotometric measurement of amount of extravasated Evans blue in the brain 4 h after ischemic reperfusion (IR). Baseline leakage (white bar) was elevated after IR (black bar). Treatment with cilostazol (gray bar) caused a further elevation. b. Quantification of capillary patency in the three different sections of the brain. *Significantly greater leakage in treated group than in corresponding untreated group, P < 0.05
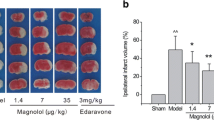
To investigate whether this increase in capillary patency brings about optimizing tissue oxygenation, hypoxic areas were compared. As shown in Fig. 2a, a non-perfused region appeared to show extensive hypoxia as indicated by a mirror image of lectin-negative and hypoxyprobe stainings. Histologic assessment showed significantly less extensive hypoxia in cilostazol-treated group than that in control (Fig. 2b, c), indicating more functional capillaries were being perfused when treated with cilostazol during ischemic insult. There was a correlation between the degree of capillary patency and the extent of tissue being hypoxic.
Effects of the cilostazol treatment on oxygenation of the tissue upon ischemic insult. a. Hypoxia was found in the region of non-perfused area. This coronal section was stained for CD31 (left), L. esculentum lectin (middle) and hypoxyprobe (right) to mark vasculature, perfused vessels, and tissue hypoxia, respectively. Scale bar = 100 µm. b. Comparison of hypoxic area with and without the treatment of cilostazol. These sections were cut at 3-mm caudal from bregma. Scale bar = 2 mm. c. Quantification of hypoxic area. The treatment of cilostazol upon IR reduced hypoxic area. *, P < 0.05
4 Discussion
In this study, we investigated the mechanisms of actions of cilostazol in an acute model of cerebral infarction. Main findings are: (i) that treatment with cilostazol increases protein flux across microvessels in the brain upon ischemia; and (ii) that it enhances oxygen transport to the brain parenchyma which is linked with an increase in the functional capillary density during ischemia.
From the point of view of transcapillary solute exchange, increased flux of plasma protein seen in the cilostazol treated animal can be accounted for by multiple factors. They are: (i) the surface area of exchange vessels; (ii) the endothelial permeability; (iii) the difference in the solute concentration across the exchange surface; (iv) the convective transport; and (v) the rate of delivery of the solute dissolved in the blood [8]. Although we are unable to demonstrate a causal relation between the increased plasma leakage and each factor listed above, the data showing an increase in capillary patency enables us to conclude that cilostazol causes protein flux to increase by recruiting more surface area for the exchange upon ischemic injury in the brain.
Previous studies indicate that cilostazol can elicit protective effects on the cerebral injury in ischemic stroke [3, 4]. Based on our result that cilostazol causes a reduction in hypoxic area after ischemia, it can be speculated that the treatment of this kind increases the oxygen delivery to the tissue. Considering the fact that reactive oxygen species are generated from oxygen, outcomes after increasing oxygen delivery might be two-fold and caution must be taken when we interpret current results.
In summary, cilostazol has a broad spectrum of pharmacological effects that may work together to improve tissue perfusion upon ischemic insult. Further studies are required to elucidate the mechanisms of this phenomenon.
References
Yashiro Y, Ohhashi T. Effects of cilostazol, a selective cyclic AMP phosphodiesterase inhibitor on isolated rabbit spinal arterioles. Jpn J Physiol 2002;52:471–477.
Torii H, Kubota H, Ishihara H, Suzuki M. Cilostazol inhibits the redistribution of the actin cytoskeleton and junctional proteins on the blood-brain barrier under hypoxia/reoxygenation. Pharmacol Res 2007;55:104–110.
Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E et al. Cilostazol Stroke Prevention Study: A placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis 2000;9:147–157.
Huang Y, Cheng Y, Wu J, Li Y, Xu E, Hong Z et al. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. Lancet Neurol 2008;7:494–499.
Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999;286:2511–2514.
Kitagawa K, Matsumoto M, Mabuchi T, Yagita Y, Ohtsuki T, Hori M et al. Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab 1998;18:1336–1345.
Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol 2004;165:35–52.
Michel CC, Curry FE. Microvascular permeability. Physiol Rev 1999;79:703–761.
Acknowledgments
Supported by Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research 19500329, Health Labour Sciences Research Grant, Research on Advanced Medical Technology from the Ministry of Health Labour and Welfare (to M. K.), and Grant-in-Aid for Creative Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (to M.S.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this paper
Cite this paper
Morikawa, T., Hattori, K., Kajimura, M., Suematsu, M. (2010). The Effects of Cilostazol on Tissue Oxygenation upon an Ischemic-reperfusion Injury in the Mouse Cerebrum. In: Takahashi, E., Bruley, D. (eds) Oxygen Transport to Tissue XXXI. Advances in Experimental Medicine and Biology, vol 662. Springer, Boston, MA. https://doi.org/10.1007/978-1-4419-1241-1_12
Download citation
DOI: https://doi.org/10.1007/978-1-4419-1241-1_12
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4419-1239-8
Online ISBN: 978-1-4419-1241-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)