Abstract
Biological control of plant-parasitic nematodes can be accomplished either by application of antagonistic organisms, conservation and enhancement of indigenous antagonists, or a combination of both strategies. The application of biological control has been inconsistent in suppressing nematode populations because the efficacy of antagonists is influenced by other soil organisms and the host-plant. Integration of biological control with nematicides, solarization, organic amendments, and crop rotation has also had varied success. Progress in biological control of nematodes has been hampered by the opaque nature of soil, the microscopic size of nematodes and their antagonists, and the complex interactions among soil organisms. Molecular biology offers new tools that will aid in determining which organisms are involved in naturally-suppressive soils, the fate of introduced antagonists, and how populations of indigenous and introduced antagonists change seasonally and with different crop production practices. Moreover, organisms have been engineered to over-express traits that enhance their activity against plant-parasitic nematodes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Current Status of Biological Control
Management of plant-parasitic nematodes in crop production systems currently relies primarily on nematicides, host-plant resistance, and crop rotation. Although many advances have been made in biological control of plant-parasitic nematodes in the last 20 years, it is still scarcely used in nematode management. When we consider the use of biological control for managing nematodes, we typically envision applying some formulated product to the seed, planting furrow, or transplant medium. Historically, there have been few commercial products registered for biological control of plant-parasitic nematodes. If one excludes products containing toxins derived from microorganisms and counts only those products containing viable organisms, then the list is even shorter (Dong and Zhang 2006). Of the eight commercial products containing viable organisms, at least two have been discontinued and three others are formulations of the same fungus (Paecilomyces lilacinus, strain 251). Stirling (1991) provides an in depth analysis of the commercial and organizational barriers to the development of biological control products.
In addition to the lack of commercial biological control organisms, the unreliability and relatively low efficacy of nematode antagonists are major obstacles to the use of biological control for managing plant-parasitic nematodes (Stirling 1991). From a practical standpoint, most growers seek to maximize their profits by selecting nematode management options that provide the greatest increase in yield while keeping input costs low. While it is understood that all management options have a risk of failure, host-plant resistance, rotations with non-host plants, and nematicides typically provide more reliable and effective nematode suppression than biological control. Moreover, nematicides and crop rotation can reduce populations of other plant pests (Timper et al. 2001). Reliability is essential for all nematode management options for which there are input costs because failure to reduce nematode populations can lead to greater monetary losses than if no action was taken to control the nematode. The greater the input cost, the greater the expectation for successful nematode control and yield increase. For any management option, including use of nematode antagonists, low or partial nematode control is less problematic than unreliable control. In the case of partial control, an antagonist could be combined either sequentially (i.e., in different seasons) or simultaneously with other management options to achieve acceptable nematode control (Roberts 1993). Research aimed at understanding the environmental factors affecting reliable and effective biological control of nematodes, as well as research to improve the effectiveness of specific antagonists will be presented later in this chapter.
Though there are major barriers to the utilization of commercially-produced antagonists, evidence suggests that some level of biological control is occurring naturally in many agricultural fields. There are a few well-documented cases of field sites where plant-parasitic nematodes are maintained at very low population densities by one or more indigenous microorganisms (Stirling 1991; Westphal 2005). Suppressive field sites are initially identified because nematode populations are inexplicably low despite conducive soil characteristics and cropping history. However, this phenomenon is not restricted to a few unique field sites. Many agricultural soils may contain organisms which keep nematode populations at a level below that which would occur if those organisms were removed, but because the level of nematode suppression is not dramatic, they may not be readily identified as suppressive. Stirling (1991) states “The possibility that every nematode population is affected to some extent by natural enemies, and that all nematode problems would be much worse in the absence of these antagonists has rarely been seriously considered.” Several studies have identified low to moderate levels of nematode suppression in agricultural soils (Table 11.1). The biological nature of the suppression was determine by comparing nematode multiplication in untreated soil with multiplication in fumigated (Santos et al. 1992; Pyrowolakis et al. 2002) or pasteurized soil (Chen et al. 1996a; McSorley et al. 2006). In one study, a small quantity of test soil was mixed with sterilised soil and then nematode multiplication was compared with that in sterilised soil (Robinson et al. 2008). In many of these field sites, suppression of nematodes was not expected, nor was the suppression clearly attributable to any organism or group of organisms. Evidently, far more soils contain organisms capable of suppressing plant-parasitic nematodes than previously recognized. Are we relying on biological control without being aware of it?
If many agricultural soils contain indigenous organisms capable of reducing populations of plant-parasitic nematodes, then it may be possible to conserve or enhance these organisms by modifying or adopting certain farming practices. Such a strategy is commonly employed in the biological control of insects (Barbosa 1998; Pickett and Bugg 1998) and has also been used in biological control of soil-borne plant pathogens (Mazzola 2007). Therefore, biological control of plant-parasitic nematodes can be accomplished either by introduction of antagonistic organisms to the nematode’s habitat, manipulation of the habitat to conserve and enhance the activity of indigenous antagonists, or a combination of both strategies.
Progress in biological control of nematodes, whether it be via introduction or conservation and enhancement of antagonists, is hampered by the opaque nature of soil, the microscopic size of nematodes and their antagonists, and the complex interactions among soil organisms (Stirling 1991). There have been few tools that would allow nematologists to determine which organisms are involved in naturally-suppressive soils, the fate of introduced antagonists, and how populations of native and introduced antagonists change seasonally and with different crop production practices. In recent years, molecular tools have been developed and are beginning to be used to answer critical questions related to biological control of nematodes. Moreover, organisms can be engineered to over-express certain compounds that enhance their activity against plant-parasitic nematodes.
11.2 Suppressive Soils
11.2.1 Identifying the Organisms Involved
Before tackling a difficult and complex task such as the biological control of nematodes, it is helpful to study systems where antagonistic organisms are regulating populations of plant-parasitic nematodes. The case histories of several classic nematode-suppressive soils are described in detail by Stirling (1991); they include suppression of Heterodera avenae in cereals by Pochonia chlamydosporia and Nematophthora gynophila, Meloidogyne spp. on peach by Dactylella oviparasitica, and M. javanica on grape by Pasteuria penetrans.
Westphal (2005) has recently reviewed techniques for determining whether a soil contains organisms suppressive to nematodes. However, once a soil has been deemed suppressive to nematodes, identifying the causal organisms can be difficult, with the possible exception of P. penetrans. Second-stage juveniles (J2) of Meloidogyne spp. with attached endospores of P. penetrans are readily extracted from soil and there is a good correlation between endospores per J2 and suppression of egg production by the bacterium (Minton and Sayre 1989; Chen et al. 1997; Meyer 2003). Because endospores of P. penetrans are very resistant to environmental extremes, drying and heating of soil can be used to selectively eliminate invertebrate predators and fungal parasites of nematodes, respectively, while autoclaving soil eliminates all organisms including spore-forming bacteria. Weibelzahl-Fulton et al. (1996) used such a technique to demonstrate that P. penetrans was responsible for suppression of Meloidogyne spp. in tobacco.
In most cases, the organisms responsible for nematode suppression are not obvious. Kluepfel et al. (1993) identified two sites in a peach orchard, one suppressive and the other conducive to reproduction of Mesocriconema xenoplax. Compared to steam-heated soil, population densities of the nematode were reduced by 64% and 98% in soil from the conducive and suppressive sites, respectively. Of the 290 pseudomonads isolated from the rhizosphere of peach trees in the suppressive site, seven suppressed populations of M. xenoplax in glasshouse assays. However, no single strain reduced nematode populations to the level found in the suppressive site. The low populations of M. xenoplax in the suppressive site may be due to the concerted action of several antagonistic pseudomonads or to some entirely different organism. This study illustrates the difficulty in assigning causal agents to suppressive soils. Bacteria antagonistic to plant-parasitic nematodes can be isolated from the rhizospheres of many plant species (Kloepper et al. 1992). The presence of antagonistic bacteria, or any other organism for that matter, does not necessarily indicate that they are suppressing nematode populations under field conditions because density of the antagonist and other organisms in rhizosphere can influence the level of biological control (Siddiqui and Ehteshamul-Haque 2001; Siddiqui and Shaukat 2003a, 2005; Weller et al. 2007). The role of the isolated pseudomonads in suppression of M. xenoplax would be strengthened by demonstrating (1) that a subset of these bacteria can suppress the nematode to a similar level as observed in the suppressive site, and (2) that these bacteria are either not present or are present at significantly lower densities in the peach rhizosphere in the conducive site, which was actually moderately suppressive to the nematode.
Recently, a three-phase approach was used to identify the organisms involved in suppression of Heterodera schachtii in a research field (9E) at the University of California, Riverside (Borneman and Becker 2007). The suppressive nature of this field site had been extensively documented (Westphal and Becker 1999, 2000, 2001b). Although several nematode-parasitic fungi including Fusarium oxysporum, Fusarium sp., Dactylella oviparasitica, and P. lilacinus were isolated from cysts in field 9E, it was not clear if one or more of these fungi were responsible for suppressing H. schachtii populations (Westphal and Becker 2001a). Ultimately, a population-based approach was used to identify the organism involved. This approach relied on creating soils with varying levels of suppressiveness and then correlating the abundance of microbial taxa with nematode suppression (Borneman and Becker 2007). In order to reduce the scope of fungal taxa to identify, Yin et al. (2003) focused on the cysts which had been previously shown to harbor the suppressive organism (Westphal and Becker 2001a). In the first phase of the study, oligonucleotide fingerprinting of rRNA genes showed that D. oviparasitica was the dominant fungus in cysts from the two most suppressive soils (Yin et al. 2003). In the second phase of the study, the association between this fungus and nematode suppression was confirmed by developing sequence-selective PCR primers for the three dominant fungal species. Again, D. oviparasitica was the most abundant fungus in the most suppressive soils, but was at low to non-detectable levels in the least suppressive soils. In the final phase of the study, D. oviparasitica isolated from field 9E (strain 50) was introduced into fumigated soil where it suppressed the number of eggs per gram soil of H. schachtii to the same level as the suppressive soil (82%) after 11 weeks in glasshouse pots (Olatinwo et al. 2006c). In fumigated field microplots, D. oviparasitica reduced egg densities of H. schachtii to 91% after 19 weeks compared to microplots without the fungus (Olatinwo et al. 2006b). After an additional 16 weeks, the soil inoculated with D. parasiticia was still as suppressive as the nonfumigated 9E soil (98%). The fungus also reduced populations of H. schachtii by 94–97% in two of four nonfumigated field soils (Olatinwo et al. 2006a). The field soils in which D. oviparasitica did not reduce nematode populations were already highly suppressive to H. schachtii relative to their fumigated counterparts; these soils were collected from fields with a cropping history that included host-plants of the nematode.
11.2.2 Factors Involved in Development of Suppressive Soils
The one characteristic that all of the well-documented nematode-suppressive soils have in common is that they developed in situations where a host-plant for the nematode was present over an extended time such as continuous cultivation of annual crops or in perennial crops (Kluepfel et al. 1993; Weibelzahl-Fulton et al. 1996; Westphal and Becker 1999; Timper et al. 2001; see Stirling 1991 for additional citations). Presumably, the continuous presence of a particular plant-parasitic nematode, initially at high population densities, leads to the build-up of specialized antagonists of that nematode (Kerry and Crump 1998). It is, therefore, not surprising that the organisms typically involved in nematode-suppressive soils are either host-specific Pasteuria spp. or fungal biotypes specialized for parasitizing eggs and sedentary females of cyst and root-knot nematodes (Mauchline et al. 2004; Morton et al. 2003; Siddiqui et al. 2009). Yet suppressive soils do no develop in all perennial systems or in all situations where continuous cropping is practiced (Olatinwo et al. 2006a; Robinson et al. 2008). Is it that these nematode-conducive soils lack key antagonists or is there something in the environment (physical, chemical, or biological) that is limiting the antagonistic organisms?
Very little is known about the organisms involved in or the conditions contributing to moderately suppressive soils. Moderately suppressive soils sometimes have no history of the nematodes they suppress and are not necessarily associated with long-term presence of a host plant (Santos et al. 1992; Chen et al. 1996a; Pyrowolakis et al. 2002). Cook and Baker (1983) differentiate between specific and general soil suppressiveness for plant pathogens. General suppression is caused by the total biological activity of a soil and is a characteristic of most soils, whereas specific suppression is due to an individual or select group of organisms antagonistic to a specific pathogen. With regard to nematodes, there is little evidence for or against a suppressive soil community. Because plant-parasitic nematodes do not compete for organic matter with other microorganisms, they may be less affected by saprophytic organisms than many facultative plant pathogens. Moreover, although suppressive soils are not rare, they are not found in the majority of tested field sites (Table 11.1). Other than the magnitude of nematode suppression, there may be little difference between highly suppressive and moderately suppressive soils; in both cases, suppression may be caused by an individual or a select group of antagonists. The population-based approach used to identify D. oviparasitica as the organism responsible for suppression of H. schachtii in field 9E could be used to identify the organisms involved in moderately suppressive soils. Following a survey of six agricultural fields, Bent et al. (2008) identified one soil that suppressed M. incognita populations by 80–89% compared to fumigated soil. Using several different methods for creating a range of nematode-suppressive environments, reductions in M. incognita populations had the strongest negative correlation with P. chlamydosporia based on oligonucleotide fingerprinting of rRNA genes. Sequence-selective PCR primers confirmed the association between P. chlamydosporia rRNA and suppression of M. incognita densities. Further studies are needed to show that this fungus is capable of reducing M. incognita populations to the same level as the suppressive soil.
11.3 Application of Antagonists
There are a large number of studies conducted in glasshouse pots demonstrating high levels of nematode suppression with antagonistic organisms. Most of these studies utilized heat-treated or fumigated soil to eliminate resident plant-parasitic nematodes and plant pathogens. While studies using heated-treated or fumigated soil are regarded as a necessary first step toward identifying potential biological control organisms, they can provide unrealistic expectations for nematode suppression. Many fungi and bacteria grow and survive better in soil that has been partially or completely sterilised because of reduced competition, predation, and antibiotic production, and because of increased organic substrates from dead organisms. Furthermore, most planting pots restrict the biological control arena and provide a greater opportunity for the antagonist and nematode to interact than under field conditions. Therefore, in this section, only studies conducted in natural soil will be presented, with emphasis on microplot and field applications published after 1990. Stirling (1991) reviewed earlier attempts to release antagonistic organisms for biological control of nematodes.
11.3.1 Bacteria
Pasteuria penetrans has been the most commonly applied bacterium for the biological control of plant-parasitic nematodes (Chen and Dickson 1998). Application of endospores or dried plant material containing spore-filled females have been used to infest field and microplot soil because of the difficulty of in vitro culture of this fastidious organism. However, recent advances in fermentation culture of Pasteuria spp. may lead to large-scale applications of endospores (Smith et al. 2004). In microplots infested with M. arenaria, application of 100,000 and 10,000 endospores/g soil reduced root galling of peanut by 81% and 61%, respectively, 2 years after initial application (Chen et al. 1996b). After 3 years, root galling was reduced even in plots initially infested with only 1,000 and 3,000 endospores/g soil (Chen and Dickson 1998). Kariuki and Dickson (2007) used dried roots from an infested field site to transfer P. penetrans to another field site. Three years after infestation of the new field site, root galling on peanut was reduced to the same level as in plots fumigated with 1,3-dichloropropene. In a large multi-national project, eight microplot and field studies were conducted to test the hypothesis that intensive cropping of Meloidogyne-susceptible crops would lead to an increase in abundance of P. penetrans endospores and suppression of the nematode population (Trudgill et al. 2000). However, nematode suppression was only documented in three trials where an exotic isolate of P. penetrans had been introduced to supplement an indigenous isolate present at low background levels. Because the indigenous P. penetrans in the trials failed to increase following repeated cropping of a host, the authors speculated that the nematode populations had undergone selection for reduced attachment of endospores (Tzortzakakis et al. 1996) leading to low equilibrium levels of parasitism. In two other trials, application of an exotic isolate did not suppress Meloidogyne populations suggesting that the environment may not have been conducive for the bacterium.
In addition to P. penetrans, a diverse group of bacteria have been applied for control of plant-parasitic nematodes. Some of these bacteria are referred to as rhizobacteria because of their close association with plant roots. In a glasshouse experiment, two strains of Burkoldera cepacia suppressed the numbers of M. incognita eggs on bell pepper by 60–69% (Meyer et al. 2001). However, in two separate field experiments, a commercial preparation of B. cepacia failed to reduce populations of H. glycines on soybean (Noel 1990). Although B. cepacia is considered a rhizosphere colonizer, a foliar application of a commercial formulation reduced the number of Aphelenchoides fragariae on hosta foliage under glasshouse conditions (Jagdale and Grewal 2002). In a microplot study, the rhizobacteria Pseudomonas fluorescens strain CHA0 and P. aeruginosa strain IE-6S+, and the root-nodulating bacterium Bradyrhizobium japonicum suppressed the number of galls on tomato caused by M. javanica by 28–43% (Siddiqui and Shaukat 2002). Similarly, in a field trial, seed treatments with two isolates of P. aeruginosa reduced Heterodera cajani in sesame by up to 58% and increased yield (Kumar et al. 2009). Populations of Pratylenchus penetrans in glasshouse pots were suppressed by P. chloroaphis strain Sm3 on strawberry in six different field soils; however, suppression only averaged 28% compared to soils without the bacterium (Hackenberg et al. 2000). Chen et al. (2000) demonstrated that both Streptomyces costaricanus and a nematode-antagonistic strain of Bacillus thuringiensis were able to reduce galling and egg production of M. hapla and increase lettuce head weight in microplots. In a field study, tomato and pepper were grown in a potting mix containing strains of rhizobacteria formulated with chitin before transplanting in a field infested with M. incognita. None of five bacterial formulations were able to suppress the nematode on tomato; however, one formulation containing Bacillus subtilis strain GBO3 and B. cereus strain C4 suppressed root galling on pepper (Kokalis-Burelle et al. 2002). Similarly, a commercial formulation containing B. subtilis strain GBO3, B. amyloliquefaciens strain GB99, and chitin reduced galling by Meloidogyne sp. on tomato in field plots (Kokalis-Burelle and Dickson 2003). In both studies, only slight reductions in galling were observed on the pepper and tomato. In a commercial glasshouse naturally infested with M. incognita, Giannakou et al. (2004) showed in three separate experiments that a commercial formulation of B. firmus suppressed galling and numbers of juveniles in soil by 52–64%. A broadcast application of the formulation was more effective than a banding application. In another study, a wettable powder formulation of B. firmus reduced galling by 54–65% in a tomato nursery when used at the recommended rates (Terefe et al. 2009). The formulations of B. firmus used in these studies contained 97% plant and animal extracts; therefore, it is unclear whether nematode suppression was due to the bacterium, stimulation of other antagonistic organisms, or toxic products from the degradation of organic matter.
11.3.2 Fungi
Most field and microplot studies testing fungi for biological control of nematodes after 1990 have been conducted with parasites of sedentary stages such as the eggs, developing juveniles and females of cyst and root-knot nematodes. Paecilomyces lilacinus, P. chlamydosporia, and Trichoderma spp. are all common soil inhabitants and some strains are aggressive parasites of sedentary stages of nematodes (Siddiqui and Mahmood 1996; Sharon et al. 2001, 2007). Trichoderma spp. may also produce toxic metabolites (Khan and Saxena 1997; Sharon et al. 2001).
Paecilomyces lilacinus strain 251 is registered for biological control of nematodes in several countries (Atkins et al. 2005). An overview of biological control attempts from 1991 to 1995 using strains of this fungus has been published (Siddiqui and Mahmood 1996). Lara Martez et al. (1996) showed that P. lilacinus reduced numbers of M. incognita J2 in field-grown tomato by 70% and 41% when applied at transplant and 2 weeks after transplanting, respectively. However, the fungus was not able to suppress populations of R. reniformis or Helicotylenchus dihystera. In another field study, P. lilacinus suppressed galling of tomato by M. incognita by 39% when applied at transplant (Goswami et al. 2008). On golf-coarse greens, a commercial formulation of P. lilacinus failed to reduce densities of M. marylandi in two experiments (Starr et al. 2007). Similarly, in greenhouse soil heavily infested with M. incognita, strain 251 did not reduce galling in tomato (Kaşkavalci et al. 2009). However, in a commercial plastic house, the fungus was as effective as oxamyl in reducing J2 densities at mid season and harvest of cucumber compared to the untreated control (Anastasiadis et al. 2008).
Pochonia chlamydosporia is associated with nematode-suppressive soils and has been effective in the biological control of root-knot and cyst nematodes in glasshouse pots (Kerry 1995, 2001). Siddiqui and Mahmood (1996) have reviewed studies utilizing this fungus for control of nematodes from 1991 to 1996. When applied at planting as a kaolin formulation, P. chlamydosporia was unable to suppress root galling from Meloidogyne spp. or numbers of J2 in four field experiments with tomato (Stirling and Smith 1998). Sorribas et al. (2003) applied two isolates, one native and the other exotic, of P. chlamydosporia for control of M. javanica in plastic houses infested with the nematode. A single application of the fungus 10 weeks after planting tomato had no effect on root galling or egg production by the nematode. When the fungus was applied weekly for 6 weeks, both isolates were equally effective in reducing galling on tomato, but the native isolate parasitized more eggs than the exotic isolate (30% vs 5%) and reduce densities of healthy eggs by 50%, whereas the exotic strain had no effect on egg densities. Nevertheless, root-gall ratings were quite high despite significant suppression by the fungus. Colonization of the rhizosphere of tomato by the native isolate was 15X greater than the exotic isolate suggesting that the former was better adapted to the local habitat. In a field site infested with Globodera pallida, P. chlamydosporia strain B1357 reduced final nematode numbers by 48–51% but did not increase potato yield relative to the untreated control (Tobin et al. 2008b). Wei et al. (2009) used a screening strategy based on protease and chitinase production to identify fungi with the greatest potential for nematode suppression. Three of the isolates selected using this strategy, two P. lilacinus and one P. chlamydosporia, suppressed root galling from Meloidogyne sp. in field-grown tomato by 48–61% and increased yields by a similar percentage.
Various species of Trichoderma are antagonistic to plant-parasitic nematodes (Sharon et al. 2007). In microplots, T. harzianum did not reduce galling of M. incognita on eggplant, but 30% of the females in the roots were infected by the fungus (Rao et al. 1998). Parasitism of females was increased to 51% when the fungus was formulated with castor cake extract. In a field site infested with M. incognita, T. harzianum reduced galling on tomato roots by 47% compared to untreated plots (Goswami et al. 2008). Application of T. pseudokoningii did not reduce galling on soybean from M. incognita or increase grain yield in a field study (Oyekanmi et al. 2007). However, in a pot experiment, the same fungus reduced the number of egg masses even though galling was not reduced. Two endophytic strains of T. atroviride suppressed populations of Radopholus similis in banana (Pocasangre et al. 2007). Maehara (2008) demonstrated that Trichoderma sp. 3, when inoculated into pine logs, decreased the number of Bursaphelenchus xylophilus carried by Monochamus beetles. Trichoderma spp. appear to have an indirect effect on B. xylophilus in pine logs by competing with the blue-stain fungus which is an ideal food source for the nematode, but may also have a direct antagonistic effect on the nematode.
The mobile vermiform stages of nematodes have also been targets for biological control. Conidia of Hirsutella rhossiliensis adhere to the cuticle of passing nematodes and penetrate the cuticle via a germ tube. Tedford et al. (1993) introduced H. rhossiliensis into microplots in the form of infected nematodes. Although the fungus became established in the microplots, it failed to reduce the number of H. schachtii in sugarbeet or M. javanica in tomato. The authors speculated that exposure of J2 to the adhesive conidia was limited because of the short distance the juveniles exiting from egg masses needed to travel to re-infect the root. In another microplot study, H. rhossiliensis, formulated as hyphae in alginate pellets, was tested for its ability to reduce infection of cabbage seedlings by H. schachtii (Jaffee et al. 1996). In this test, the infective juveniles had to move through soil because they were not hatching from egg masses on the root. However, the fungus failed to reduce root invasion. In observation chambers containing field soil, the pelletized hyphae sometimes appeared to have been eaten and the fungal colonies growing from the pellets were smaller than in chambers containing heat-treated soil, suggesting biotic inhibition of the fungus. In a third microplot study, pelletized hyphae of H. rhossiliensis was compared to pelletized hyphae of two trapping fungi, Monacrosporium gephyropagum and M. ellipsosporum (Jaffee and Muldoon 1997). These two fungi trap nematodes by adhesive hyphae and adhesive knobs, respectively. Of the three fungi, only M. gephyropagum suppressed invasion of tomato seedlings by M. javanica and improved seedling emergence and root growth. Alginate pellets containing the fungi did not persist over the 20 day observation period. The effectiveness of M. gephyropagum in this study may be due to its rapid growth and capture of nematodes before the pellets were consumed by grazing microfauna.
11.3.3 Nematodes
The potential of predatory nematodes for biological control of plant-parasitic nematodes has been reviewed by Khan and Kim (2007). Predatory nematodes have not received much attention for biological control of plant-parasitic nematodes because of the difficulty in mass culturing due to low fecundity, long life cycle, complex culture conditions, and cannibalism. Diplogasterid nematodes have advantages over other predatory nematodes in that they have high reproductive rates, short life cycles, and can be cultured on bacteria (Bilgrami et al. 2008). Recently, the diplogasterid nematode Mononchoides gaugleri was evaluated in field microplots for suppression of plant-parasitic nematodes in turf grass. The predator reduced total populations of plant-parasitic nematodes 30 days after application, but individual genera were differentially affected. Populations of Ditylenchus sp., Aphelenchoides sp., Tylenchorhynchus sp., and Tylenchus sp. were reduced by 45%, 40%, 35%, and 20%, respectively; whereas Hoplolaimus sp. and Helicotylenchus sp. were not affected by the predator.
Entomopathogenic nematodes in the genera Steinernema and Heterorhabditis can suppress populations of plant-parasitic nematodes. Suppression may involve one or more of the following mechanisms: interference competition at the root surface (Bird and Bird 1986), stimulation of nematode antagonists (Ishibashi and Kondo 1986), allelochemicals from the symbiotic bacteria associated with these nematodes, or induction of systemic resistance in the plant (Jagdale et al. 2002, 2009). In turf grass plots, a mixture of S. carpocapsae and H. bacteriophora reduced Tylenchorhynchus spp. by 50–59% under irrigated but not non-irrigated conditions 5 weeks after application (Smitley et al. 1992). Application of S. riobrave to turf grass at two golf courses suppressed populations of Meloidogyne sp., Belonolaimus longicaudatus, and Criconemella sp. by 84–100% at 4 and 8 weeks after treatment (Grewal et al. 1997). In two different studies, S. riobrave and S. carpocapsae reduced several genera of plant-parasitic nematodes on boxwood for a least 30 days following treatment (Jagdale et al. 2002; Perez and Lewis 2006). However, S. riobrave failed to reduce the populations of M. xenoplax on peach grown in glasshouse pots and pecan in microplots (Nyczepir et al. 2004).
11.3.4 Biotic and Abiotic Factors Modifying Efficacy
The environment to which a biological control organism is introduced can play a large role in the success or failure of that organism to reduce populations of plant-parasitic nematodes. Antagonism from other organisms is often cited as the cause of poor nematode control in field soils compared to partially or completely sterilised soil. The organisms involved in antagonism are mostly unknown, but are assumed to be competitors for organic matter, a supplemental food source for many biological control organisms (Mankau 1962; Cook and Baker 1983). However, competition is not the only hostile encounter biological control organisms face when applied to soil. In microplot experiments, collembolans and enchytraeid worms were observed in the vicinity of partially consumed pellets containing nematophagous fungi (Jaffee and Muldoon 1997; Jaffee et al. 1996). Using soil cages of different mesh sizes, Jaffee et al. (1997) and Jaffee (1999) demonstrated that exclusion of enchytraeids and microarthropods increased the persistence of nematophagous fungi growing from alginate pellets. However, smaller organisms (e.g., fungi, bacteria, nematodes, protozoa, etc.), which were not excluded, still reduced persistence of the fungi compared to heat-treated soil.
Microorganisms also release compounds which can inhibit biological control. Diffusible compounds from two soil communities reduced growth of P. chlamydosporium and P. lilaciunus (Monfort et al. 2006). Bacillus sp. strain H6, isolated from a fungistatic soil, produces iturin A-like compounds which caused swelling in the conidia and germ tubes of nematophagous fungi (Li et al. 2007). The egg masses of Meloidogyne spp. may also harbor microflora inhibitory to biological control. Kok et al. (2001) isolated 122 bacteria and 19 fungi from egg masses and found that 23% and 74%, respectively, were antagonistic to P. chlamydosporia. The production of DAPG (2,4-diacetylphloroglucinol) by P. fluorescens is involved in suppression of cyst and root-knot nematodes (Cronin et al. 1997; Siddiqui and Shaukat 2003c). Metabolites from several common soil fungi have been shown to inhibit expression of DAPG (Notz et al. 2002; Siddiqui and Shaukat 2003a, 2005; Siddiqui et al. 2004). Moreover, the presence of some of these fungi (Fusarium solani, Rhizoctonia solani, and Aspergillus quadrilineatus) in soil reduced the ability of the bacterium to suppress populations of Meloidogyne spp. on tomato (Siddiqui and Shaukat 2003a, 2005; Siddiqui et al. 2004).
Isolates of nematophagous fungi differ in their sensitivity to biological inhibition. Saprotrophic growth of five P. chlamydosporia isolates was compared in two soils (Monfort et al. 2006). In both soils, isolate 5 was the least affected by soil microorganisms, with a reduction in growth of 57–72% compared to sterilised soil. Growth of isolate 4624 was suppressed less in the Lancelin than in the Biar soil; growth of all other isolates was suppressed by 83–98% compared to sterilised soil. In another study, microbial inhibition of P. chlamydosporia isolates was very low, ranging from 0% to 37% when tested in two different soils (Siddiqui et al. 2009). There was also a negative correlation between saprotrophic growth and parasitism of eggs suggesting that there may be a trade-off between these two traits. However, in another study, there was no correlation between saprotrophic and parasitic abilities (data presented in Siddiqui et al. 2009).
Soil microorganisms can sometimes enhance biological control of plant-parasitic nematodes. In attachment assays, the presence of some bacterial isolates originating from both soil and gall tissue increased attachment of P. penetrans endospores to J2 of Meloidogyne spp. (Duponnois and Ba 1998; Duponnois et al. 1999). In a glasshouse experiment, one of the bacterial isolates, Enterobacter cloacae, when combined with P. penetrans for control of M. incognita on tomato, reduced the number of egg masses on the roots by 36% and increased the number of endospores produced in roots compared to treatments with only P. penetrans (Duponnois et al. 1999). Although the mechanism is unclear, enzymes produced by E. cloacae and other bacteria may modify either the nematode cuticle or the endospore sporangial wall or exosporium to increase attachment. While a number of fungi can inhibit expression of DAPG by P. fluorescens strain CHA0, Pythium ultimum, Aspergillus niger, and T. harzianum can enhance expression of the antibiotic (Notz et al. 2001; Siddiqui and Shaukat 2004; Siddiqui et al. 2004). However, neither A. niger or T. harzianum were able to significantly increase suppression of M. javanica on tomato compared to the bacterium alone (Siddiqui and Shaukat 2004; Siddiqui et al. 2004).
Biological control of Meloidogyne spp. by parasites of sedentary stages has been suggested to be more effective on plants that are poor hosts for the nematode because small galls leave egg masses exposed on the root surface and fewer eggs are produced than on good hosts (Stirling et al. 1979; De Leij and Kerry 1991). Bourne et al. (1996) demonstrated this principle with P. chlamydosporia, which provided greater suppression of M. incognita on the poorer host potato than on the better host tomato despite greater fungal colonization of the tomato roots. Although colonization of the rhizosphere by P. chlamydosporia differs among host-plants, there was no relationship between abundance of the fungus on roots and the rate of parasitism (Bourne and Kerry 1999). Colonization of roots by P. fluorescens strain CHA0 differed among host-plants and among cultivars of soybean, but degree of colonization was not related to suppression of root galling by M. incognita (Siddiqui and Shaukat 2003b). Strain CHA0 suppressed galling on all crops except chili, which was a relatively poor host compared to the other crops.
Abiotic factors that can influence the level of biological control include temperature, soil type, moisture, and rainfall/irrigation. In glasshouse pots using field soil, a commercial formulation of Paecilomyces lilacinus strain 251 suppressed galling and egg masses of M. hapla on tomato by 66–90% when daytime temperatures were 23–25°C, but was much less effective when the daytime temperature was 21°C (Kiewnick and Sikora 2006). Establishment in the rhizosphere of P. chlamydosporia and nematode suppression by the fungus was greater in peaty sand than in loamy sand or sand (De Leij et al. 1993); however, in another study, there was no difference in colonization of a compost, sandy loam, and loamy sand soil by the fungus (Siddiqui et al. 2009). Soil type can also influence retention of Pasteuria penetrans endospores in the root zone and acquisition by Meloidogyne J2. The bacterium occurs more frequently in sandy soils than in finer-textured soils (Spaull 1984); the mobility of J2 in sandy soils likely allows for greater acquisition of endospores. In a pot experiment, the percentage of M. incognita females infected with P. penetrans was greater in a sandy soil than in a sandy clay soil (Carneiro et al. 2007). However, leaching of endospores is also greater in sandy soils than in finer-textured soils. Under a drip system, 76% of endospores leached 10 cm after 24 h in sand, and with increasing clay content, there was a decrease in the percentage of endospores leached (Dabire and Mateille 2004). Soils with clay content between 10% and 30% were considered optimal for biological control with P. penetrans.
11.3.5 Integration of Biological Control with Other Management Tactics
Integrated pest management utilizes multiple management tactics within a growing season or in different seasons to reduce pest populations (Roberts 1993). Because nematode control in integrated management systems does not rely solely on one management tactic, partially effective tactics such as biological control can be combined to lower nematode populations below the damage threshold. However, attempts to integrate biological control with nematicides, host-plant resistance, crop rotation, solarization of soil, other antagonists, and soil amendments have generated mixed results.
Nematophagous fungi and P. penetrans are generally compatible with non-fumigant nematicides and some fumigants such as 1,3-dichloropropene. Nematicides do not usually have an adverse effect on these organisms (Mankau and Prasad 1972; Jacobs et al. 2003) and may even enhance parasitism. Brown and Nordmeyer (1985) suggested that aldicarb and carbofuran increased movement of M. javanica J2 and acquisition of endospores leading to a synergistic reduction in galling when the nematicides were combined with P. penetrans. However, the frequency of endospores attached to J2 in a field study was not influenced by the application of aldicarb (Timper et al. 2001). Applications of oxamyl and P. penetrans to tomato had an additive effect on reducing egg production by Meloidogyne spp., but acted synergistically in the subsequent cucumber crop (Tzortzakakis and Gowen 1994). Fungal parasites of sedentary stages cannot protect plant seedlings from nematode invasion and early-season damage, but will often proliferate in the rhizosphere during the growing season (Stirling and Smith 1998; Sorribas et al. 2003; Tobin et al. 2008b). Nematicides could be used in conjunction with these fungi to reduce initial nematode populations while the antagonist reduces egg production and viability leading to lower nematode populations for the succeeding crop. In three separate studies evaluating the combined application of oxamyl and P. chlamydosporia (Tzortzakakis 2000; Tzortzakakis and Petsas 2003; Verdejo-Lucas et al. 2003), only one study demonstrated that the fungus provided additional suppression of M. javanica galling and egg production over the nematicide alone (Verdejo-Lucas et al. 2003). In a field study, both fosthiazate and P. chlamydosporia suppressed final population densities of potato cyst nematodes, but there was no additive effect of the two control tactics (Tobin et al. 2008b).
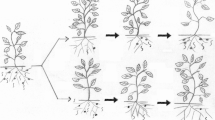
Very little research has been done to evaluate the effectiveness of combining biological control with host-plant resistance and crop rotation. A nematode antagonist could be applied to a moderately resistant cultivar or to a susceptible cultivar following rotation with a resistant cultivar or non-host crop. Samac and Kinkel (2001) tested a strain of Streptomyces sp. for biological control of Pratylenchus penetrans on resistant and susceptible alfalfa. Nematode suppression by the resistant cultivar and Streptomyces sp. was additive and together they provided >90% control. In plastic houses where susceptible tomato followed resistant tomato in a single growing season, P. chlamydosporia failed to suppress M. javanica on the susceptible tomato (Tzortzakakis and Petsas 2003). The fungus, however, was effective in reducing M. incognita on tomato when it was used in a rotation system involving two poor hosts of the nematode, bean and Chinese cabbage (Atkins et al. 2003c). In that study, P. chlamydosporia, var. catenulate was applied before the bean crop, where it suppressed final densities of J2 in the soil, persisted through the cabbage crop, and prevented population increase on the tomato crop (Fig. 11.1). Egg parasitism on tomato was >70%.
Numbers of second-stage juveniles of Meloidogyne incognita in untreated soil and treated with Pochonia chlamydosporia var. catenulate applied before the bean crop in the rotation (Atkins et al. 2003c)
Soil solarization can not only reduce pest populations, but also alters the microbial community, both qualitatively and quantitatively, which may lead to less competition and antagonism of an introduced biological control organism (Katan and DeVay 1991). Kluepfel et al. (2002) used such a strategy to enhance survival and efficacy of Pseudomonas synxantha strain BG33R, a bacterial antagonist of M. xenoplax. Populations of the nematode were lower in plots that received both solarization and BG33R than in solarization alone. Pasteuria penetrans and solarization were also additive in suppression of root-galling and egg production by Meloidogyne spp. on cucumber. However, Bacillus firmus did not provide any additional control of M. incognita on cucumber when combined with solarization even though the bacterium suppressed root galling without solarization (Giannakou et al. 2007). Paecilomyces lilacinus reduced final populations of Meloidogyne spp. on cucumber, but was not effective in suppressing nematode populations when combined with solarization (Anastasiadis et al. 2008).
Combining different antagonists of nematodes may improve the level and consistency of biological control. Selected combinations may vary in their mode of action, the stage of nematode affected, activity under different soil conditions, and ability to control different pests. A review of studies combining biological control organisms prior to 2002 has been published (Meyer and Roberts 2002). In a field study, application of three bacteria, P. fluorescens, P. aeruginosa, and Bradyrhizobium japonicum, with different modes of action, suppressed galling of tomato by M. javanica both individually and in combination; however, the combinations did not provide greater suppression than the most effective bacterium in the group (Siddiqui and Shaukat 2002). Khan et al. (2006) evaluated P. lilacinus and Monacrosporium lysipagum, fungal parasites of sedentary and migratory stages, respectively, for control of three different nematodes. Combination of the two fungi did not increase the level of nematode suppression of M. javanica on tomato or H. avenae on barley compared to individual applications; however, it appeared that the two fungi had an additive effect on suppression of R. reniformis on banana. Combined applications of B. japonicum, Trichoderma pseudokoningii, and Glomus mossae to soybean did not improve suppression of M. incognita over single species applications (Oyekanmi et al. 2007). Of the four fungi tested by Goswami et al. (2008), only the combination of T. harzianum and Acromonium strictum had an additive effect on suppression of root galling by M. incognita.
Organic amendments have been used to suppress plant-parasitic nematodes (Akhtar and Malik 2000). Though the mechanism of suppression is not always clear, it can involve release of toxic compounds and stimulation of antagonistic organisms. A number of studies have examined dual applications of organic amendments and biological control organisms for integrated management of plant-parasitic nematodes. Amendments specifically used to enhance the survival and proliferation of biological control organisms will be covered in the next section. Application of neem cake and T. harzianum had an additive effect on suppression of Tylenchulus semipenetrans on citrus in pots (Parvatha Reddy et al. 1996). In a field study, however, the combined application of neem and the fungus was not different from application of neem alone in suppression of M. incognita galling on eggplant (Rao et al. 1998). Suppression of M. hapla in soil amended with chitin was not increased by the application of single or multiple species of antagonistic fungi and bacteria (Chen et al. 1999). Likewise, the efficacy of B. thuringiensis, Paecilomyces marquandii, and Streptomyces costaricanus was not increased by any of the organic amendments, including chitin, though each organism alone reduced galling and reproduction of M. hapla on lettuce (Chen et al. 2000).
11.4 Conservation and Enhancement of Indigenous and Introduced Antagonists
Where integrated management seeks to supplement biological control with other nematode control tactics, the goal of conservation and enhancement is to avoid practices that are harmful to antagonists (conservation) and promote practices that increase the survival, abundance, and activity of antagonists (enhancement). A large proportion of the research effort in biological control of nematodes has been directed toward application of antagonists for nematode control during a single cropping cycle and little effort has been given to determining which agricultural practices have positive or negative effects on indigenous and introduced antagonists.
11.4.1 Pesticides
In biological control of insect pests, conservation has emphasized reduced application of broad spectrum insecticides which negatively impact parasitoids and predators (Ruberson et al. 1998). However, there is little information, particularly from field studies, on the impact of pesticides on antagonists of nematodes (Stirling 1991). Fungicide applications could potentially reduce the activity of nematophagous fungi. Recently, Tobin et al. (2008a) demonstrated that the fungicide azoxystrobin had a negative impact on densities of P. chlamydosporia in the soil and rhizosphere, but the fungus showed some recovery 49 days after application. The impact of the fungicide on nematode suppression by P. chlamydosporia was not tested. Application of captafol resulted in greater nematode reproduction compared to untreated soil, presumably because the fungicide reduced fungal antagonists of the nematode (Muller 1985). Egg parasitism was not affected by captafol; however, in the untreated soil, a significant proportion of juveniles were parasitized by H. rhossiliensis. When egg and juvenile parasitism (primarily by H. rhossiliensis) was evaluated 8 months after fumigation with 1,3-dichloropropene (1,3-D), there was no difference in parasitism of either stage between fumigated and unfumigated treatments.
Pasteuria penetrans is tolerant of many pesticides including 1,3-D (Chen and Dickson 1998; Mankau and Prasad 1972; Stirling 1984). The bacterium, however, is sensitive to chloropicrin. Kariuki and Dickson (2007) found that the percentage of females infected by P. pentrans in plots treated with chloropicrin was less than half the percentage in untreated plots. Moreover, root galling from M. arenaria on peanut was greater in the chloropicrin than in the untreated plots (1.1 vs 4.2 on a 0–10 scale).
Nematicides can reduce abundance of omnivorous and predatory nematodes. Population densities of these nematodes were severely suppressed following application of 1,3-D (Fig. 11.2). Populations of omnivorous nematodes partially recovered by mid season, but populations of predatory nematodes remained low and even showed residual effects from application of the fumigant in the previous spring (Timper, Jagdale, Davis, unpublished). It is not known whether the omnivores and predators were regulating populations of plant-parasitic nematodes and if they were, whether suppression was disrupted by 1,3-D.
Population densities of omnivorous and predatory nematodes in cotton plots without nematicide and treated with 1,3-dichloropropene (1,3-D). The fumigant was applied 2 weeks before planting in the spring. Nematodes were sampled immediately before fumigation, after planting, and midway through the season. The fumigant had also been applied the previous spring. Differences between the control and 1,3-D are indicated by ** (P < 0.01) and *** (P < 0.001) (Timper, Jagdale, Davis, unpublished)
11.4.2 Organic Amendments
The application of organic amendments is the most commonly used tactic for enhancing the abundance and activity of antagonists of nematodes. This topic has been reviewed by Akhtar and Malik (2000). The organic matter can be used as a substrate for growth of antagonists or it can stimulate populations of microbivorous nematodes which can serve as hosts or prey for antagonists (van den Boogert et al. 1994; Jaffee 2006). Nevertheless, application of a manure/sawdust mixture did not enhance the activity of Arthrobotrys dactyloides or P. chlamydosporia (Stirling and Smith 1998). Likewise, various plant and manure amendments did not increase parasitism of M. xenoplax by H. rhossiliensis (Jaffee et al. 1994). Incorporation of Crotolaria juncea into soil increased the abundance of nematode-trapping fungi, particularly in soil with high organic matter (Wang et al. 2002, 2003, 2004). Suppression of R. reniformis by C. juncea amendments in six soils was correlated with nematode-trapping fungi and egg parasitism by fungi (Wang et al. 2003). In another study, incorporation of mustard, oil radish, and rape increased parasitism of H. schachtii eggs in one field site, but decreased it in another field site (Pyrowolakis et al. 1999). The enhancing effect of the three crucifers in the one field site may be due to the greater fungal diversity in that site compared to the other site. In vineyard soil, addition of dried grape or alfalfa leaves to soil increased microbivorous nematodes, but did not have a consistent effect on trapping activity of nematophagous fungi (Jaffee 2002, 2004). Although abundance of Arthrobotrys oligospora increased with addition of leaves, trapping activity did not increase. The response of Dactylellina candidum (=D. haptotyla) to the organic matter was more erratic. Abundance of the fungus was correlated with trapping activity; however, the leaf material did not always stimulate abundance or activity. Amending soil with sugarcane trash reduced population densities of Pratylenchus zeae and Tylenchorhynchus annulatus and increased densities of omnivorous and predatory nematodes three to eightfold (Stirling et al. 2005). An unidentified trapping fungus was also found only in soil amended with sugarcane trash suggesting a possible involvement in suppression of the plant parasites.
11.4.3 Crop Rotation
Population densities of biological control organisms can be influenced by the species of crop planted. The most straight-forward example of this is the continuous cultivation of a crop leading to an increase in specific antagonists of a plant-parasitic nematode on that crop. Rotating non-host crops for Meloidogyne spp. resulted in lower densities of P. penetrans relative to continuous cropping of a host crop (Madulu et al. 1994; Timper et al. 2001). Other plant-antagonist interactions are more unexpected. In a nematode suppressive soil, later shown to be caused by D. oviparasitica, suppression of H. schachtii was decreased following both wheat and fallow, but not following nematode resistant sugar beet or radish indicating these plants could support the fungus in the absence of nematodes (Westphal and Becker 2001b). Rumbos and Kiewnick (2006) determined the effect of different plant species on the persistence of P. lilacinus and found that only bean significantly reduced persistence compared to fallow soil. Perhaps the bean rhizosphere contained organisms antagonistic to the fungus.
11.4.4 Tillage
Tillage changes the physical, chemical, and biological components of soil (Kladivko 2001). However, few studies have examined the effect of tillage on antagonists of nematodes. Bernard et al. (1996) sampled six different tillage treatments for fungi associated with Heterodera glycines and for rates of egg and female parasitism. In the monthly samples, no tillage treatment consistently supported more egg parasitism. When the monthly samples were combined, disc-tilled plots had greater egg parasitism than no-till plots. Paecilomyces lilacinus, the most prevalent fungus, parasitized more eggs in disc than in no till plots, whereas P. chlamydosporia parasitized more eggs in plots that where moldboard plowed. Tillage may have a negative impact on P. penetrans, particularly early in the season when root growth is shallow. At planting, tillage reduced the density of endospores in the upper 10 cm of soil, but tended to increase endospore densities below 10 cm (Talavera et al. 2002). At harvest, the effects of tillage on endospore densities in the upper 10 cm disappeared.
11.4.5 Organic Production Systems
A few studies have evaluated the impact of substantial changes in production practices, such as organic farming, on the level of biological control or on densities of antagonists. Organic farming replaces synthetic fertilizers and pesticides with organic fertilizers (plant material and animal manure), crop rotation, and resistant cultivars. Persmark (1997) sampled 11 pairs of organically and conventionally managed farms and found no difference between the two management systems in either the densities of nematode-trapping fungi, numbers of nematodes in the rhizosphere of pea, or organic matter. In a field plot experiment, organically managed plots had more species of nematophagous fungi and two species, Arthrobotrys dactyloides and Nematoctonus leiosporus, were more abundant than in conventionally managed plots (Jaffee et al. 1998). However, soils from organic and conventionally managed plots did not differ in level of suppression of M. javanica. In another similar study, the number of species of nematophagous fungi was not different in organically and conventionally managed plots (Timm et al. 2001). The only two fungi, N. leiosporus and Meristacrum sp. that were found more frequently in the organic plots were present at very low densities.
11.5 Using Molecular Techniques to Improve Biological Control
11.5.1 Detection and Quantification of Antagonists and Their Biological Control Activity
From the preceding sections, it is apparent that the abundance and biological control activity of antagonists can be influenced by other soil organisms, plant species, and agricultural practices such as pesticide application, organic amendments, tillage, and crop rotation. Rapid, sensitive and reliable methods for quantifying population densities of antagonists are needed to advance our knowledge of the environmental factors affecting biological control of nematodes. Ultimately, such knowledge will improve the efficacy and consistency of nematode suppression. Non-molecular techniques for detecting and quantifying antagonists of nematodes include extraction of spores, selective media, most probable number procedures, bioassays, and enzyme-linked immunosorbent assays (Stirling 1991; Fould et al. 2001; Schmidt et al. 2003). All of these techniques have one or more limitations; they can be time and labor intensive, or lack suitable specificity or sensitivity. Competitive and real-time PCR techniques have the potential for rapid, sensitive, culture independent and highly specific quantification of antagonists (Okubara et al. 2005). Sufficiently pure DNA can be extracted from soil and plant tissue using relatively simple and rapid methods utilizing commercial extraction kits. Recently, sequence-specific primers have been developed for either the ITS region or for specific genes from P. chlamydosporia, P. lilacinus, Plectosphaerella cucumerina, D. oviparasitica, H. rhossiliensis, nematode-trapping fungi (Orbiliales), and Pasteuria penetrans (Hirsch et al. 2001; Atkins et al. 2003c, 2005; Yin et al. 2003; Schmidt et al. 2004; Zhang et al. 2006; Smith and Jaffee 2009). These primers showed a high degree of specificity for the organisms for which they were developed. When quantitative PCR techniques were compared with direct plating onto selective media for quantification of P. chlamydosporia, P. lilacinus, P. cucumerina, and nematode-trapping fungi (Orbiliales) the PCR techniques were found to be more sensitive (Mauchline et al. 2002; Atkins et al. 2003a, 2005; Smith and Jaffee 2009). However, all four of these studies emphasized the importance of using quantitative PCR techniques in conjunction with plating onto selective media or bioassays.
Perhaps the greatest advantage of DNA-based detection methods is the potential for differentiating biotypes and strains of a biological control organism. With P. penetrans, Schmidt et al. (2004) found greater sequence heterogeneity in the sporulation gene sigE than in 16S rDNA and suggested that species- and biotype-specific probes could be developed from this gene. Specific primer sets have been developed to distinguish between two morphologically similar varieties of P. chlamydosporia, var. catenulate and var. chlamydosporia (Atkins et al. 2003b; Hirsch et al. 2000). Siddiqui et al. (2009) developed PCR primers based on the vcp1 gene to differentiate biotypes of P. chlamydosporia from cyst and root-knot nematodes. These primers were able to identify the original nematode host from which the fungus was isolated. PCR fingerprinting with arbitrary primers has also been used to determine variation within populations of P. chlamydosporia var. chlamydo-sporia (Manzanilla-López et al. 2009a). Unexpectedly, little genetic variation was detected in populations of the fungus at two different locations where it was parasitizing eggs of M. incognita. Biotype-specific probes and PCR fingerprinting could be used to determine which biotypes prevail against different host nematodes and under different environmental conditions (Atkins et al. 2009; Manzanilla-López et al. 2009b). Recently, SCAR-PCR primers were developed to detect specific strains of P. lilacinus and P. chlamydosporia (Zhu et al. 2006). Further research is needed to determine whether these markers can discriminate these strains from background populations of the same species in the field.
Quantitative PCR (qPCR) techniques are not without limitations. DNA from moribund or recently dead propagules can be amplified leading to an overestimation of viable propagules. Extraction of RNA from soil could be combined with DNA extraction to provide a more accurate assessment of viable cells, but further research in this area is necessary (Atkins et al. 2003a). Interpreting the results of qPCR for filamentous fungi is also complicated by the presence of multiple stages such as conidia, hyphae, and chlamydospores. When plating or direct counting techniques are used, chlamydospores and mycelial fragments are counted as single propagules; however, with qPCR each of the cells making up the structure contribute DNA. In other words, qPCR quantifies fungal biomass whereas dilution plating quantifies propagules (or cfu). Germination of chlamydospores and subsequent sporulation may not increase the amount of DNA detected, but would increase the number of propagules (Mauchline et al. 2002). Such shifts in fungal life stages could only be deduced with a combination of plating and qPCR. The level of biological control activity cannot be determined with either qPCR or plating (except of infected cadavers). Based on qPCR and plating on selective medium, populations of P. lilacinus were found to be greater in the Spalding location than in the Ely location; however, parasitism of G. pallida eggs in a bioassay was similar in both locations (Atkins et al. 2005). In contrast, there was a strong correlation between results of qPCR and assay nematodes parasitized by H. rhossiliensis (Zhang et al. 2006). In this comparison, the soil for both the parasitism assay and the qPCR was inoculated with the fungus in the laboratory and left undisturbed until the assay nematodes were extracted. More realistically, soil would be collected from a field site, a process which inactivates the conidia of H. rhossiliensis (McInnis and Jaffee 1989). Following such a disturbance, fungal reserves must be used to produce fewer new conidia which would then be quantified in the parasitism assay. However, qPCR would be able to detect both the hyphae and detached conidia from freshly collected soil.
Abundance of an organism is not always an indication of biological control activity. This is predominantly an issue with organisms that are competitive soil saprophytes because they may not depend on nematodes for nutrition. For some of these organisms, such as parasites of sedentary stages and certain trapping fungi, biological control activity can be monitored by recovering infected stages of nematodes (Atkins et al. 2009). However, quantifying biological control activity of bacteria that produce toxins and some trapping fungi is difficult (Jaffee 2004), particularly in field studies. Reporter genes could be used to monitor gene expression involved in antibiotic production, trap formation, and parasitism. The reporter gene lacZ, encoding for B-galactosidase, has been used to study expression of the antibiotic DAPG by Pseudomonas fluorescens in the rhizosphere of plants (Notz et al. 2001, 2002). Using a strain of the bacterium carrying a translational phlA ‘-’ lacZ fusion, expression of DAPG was found to be greater in monocots than dicots, influenced by plant cultivar and age, stimulated in the presence of Pythium ultimum, and depressed in the presence of fusaric acid-producing strains of Fusarium oxysporum. Recently, the gene encoding for green fluorescent protein was used along with flow cytometry to visualize and quantify expression of DAPG in situ on plant roots (de Werra et al. 2008). With improved knowledge of the genes involved in trap formation and nematode infection, reporter genes may be used to monitor biological control activity of nematophagous fungi. For example, a reporter gene could be used with the PII gene in A. oligospora, which encodes for an extracellular serine protease and is involved in nematode trapping (Ahman et al. 2002), to determine the conditions under which the fungus becomes parasitic. Ahren et al.(2005) used microarray analysis to determine which genes were up-regulated in the adhesive knobs of Monacrosporium haptotylum (syn. Dactylellina candidum). A reporter gene could be fused with one of the genes specifically expressed in the adhesive knobs to quantify trap formation under different production practices (e.g., organic vs conventional production).
11.5.2 Trait Enhancement
Improvements in biological control have been achieved by genetically engineering organisms for overexpression of traits involved in pathogenicity or nematicidal activity. Transgenic lines of Trichoderma atroviride carrying multiple copies of the prb1 gene, which encodes for a 31-kDa proteinase (Prb 1), were tested for suppression of M. javanica on tomato (Sharon et al. 2001). Of the four transformed strains, only P-2 was more effective than the wild-type strain in reducing root galling. The P-2 strain was similar to the wild-type strain in nematicidal activity, but showed improved ability to penetrate egg masses and colonize eggs in vitro. Arthrobotrys oligospora produces an extracellular serine protease designated PII which is thought to be involved in penetration of the nematode cuticle or tissue digestion within the host (Ahman et al. 2002). Transformed strains of the fungus containing additional copies of the pII gene produced more traps and had an increase rate of capture compared to the wild-type strain. Siddiqui and Shaukat (2003c) demonstrated that a DAPG over-producing strain of P. fluorescens was more effective in reducing root galling from M. javanica in tomato than the wild-type strain CHA0. In addition to improving the effectiveness of biological control, these enhanced strains of antagonists may be able to suppress nematode populations when applied at much lower rates, and cost, than wild-type strains.
References
Ahman J, Johansson T, Olsson M et al (2002) Improving the pathogenicity of a nematode-trapping fungus by genetic engineering of a subtilisin with nematotoxic activity. Appl Environ Microbiol 68:3408–3415
Ahren D, Tholander M, Fekete C et al (2005) Comparison of gene expression in trap cells and vegetative hyphae of the nematophagous fungus Monacrosporium haptotylum. Microbiology 151:789–803
Akhtar M, Malik A (2000) Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: a review. Bioresour Technol 74:35–47
Anastasiadis IA, Giannakou IO, Prophetou-Athanasiadou DA et al (2008) The combined effect of the application of a biocontrol agent Paecilomyces lilacinus, with various practices for the control of root-knot nematodes. Crop Prot 27:352–361
Atkins SD, Clark IM, Sosnowska D et al (2003a) Detection and quantification of Plectos-phaerella cucumerina, a potential biological control agent of potato cyst nematodes, by using conventional PCR, real-time PCR, selective media, and baiting. Appl Environ Microbiol 69:4788–4793
Atkins SD, Hidalgo-Diaz L, Clark IM et al (2003b) Approaches for monitoring the release of Pochonia chlamydosporia var. catenulata, a biocontrol agent of root-knot nematodes. Mycol Res 107:206–212
Atkins SD, Hidalgo-Diaz L, Kalisz H et al (2003c) Development of a new management strategy for the control of root-knot nematodes (Meloidogyne spp) in organic vegetable production. Pest Manag Sci 59:183–189
Atkins SD, Clark IM, Pande S et al (2005) The use of real-time PCR and species-specific primers for the identification and monitoring of Paecilomyces lilacinus. FEMS Microbiol Ecol 51:257–264
Atkins SD, Peteira B, Clark IM (2009) Use of real-time quantitative PCR to investigate root and gall colonisation by co-inoculated isolates of the nematophagous fungus Pochonia chlamydosporia. Ann Appl Biol 155:143–152
Barbosa P (1998) Conservation biological control. Academic, San Diego
Bent E, Loffredo A, McKenry MV et al (2008) Detection and investigation of soil biological activity against Meloidogyne incognita. J Nematol 40:109–118
Bernard EC, Self LH, Tyler DD (1996) Fungal parasitism of soybean cyst nematode, Heterodera glycines (Nemata: Heteroderidae), in differing cropping-tillage regimes. Appl Soil Ecol 5:57–70
Bilgrami AL, Brey C, Gaugler R (2008) First field release of a predatory nematode, Mononchoides gaugleri (Nematoda: Diplogastrida), to control plant-parasitic nematodes. Nematology 10:143–146
Bird AF, Bird J (1986) Observations on the use of insect parasitic nematodes as a means of biological control of root-knot nematodes. Int J Parasitol 16:511–516
Borneman J, Becker JO (2007) Identifying microorganisms involved in specific pathogen suppression in soil. Annu Rev Phytopathol 45:153–172
Bourne JM, Kerry BR (1999) Effect of the host plant on the efficacy of Verticillium chlamydosporium as a biological control agent of root-knot nematodes at different nematode densities and fungal application rates. Soil Biol Biochem 31:75–84
Bourne JM, Kerry BR, De Leij F (1996) The importance of the host plant on the interaction between root-knot nematodes (Meloidogyne spp.) and the nematophagous fungus, Verticillium chlamydosporium Goddard. Biocontrol Sci Technol 6:539–548
Brown SM, Nordmeyer D (1985) Synergistic reduction in root galling by Meloidogyne javanica with Pasteuria penetrans and nematicides. Rev Nematol 8:285–286
Carneiro R, de Mesquita LFG, Cirotto PAS et al (2007) The effect of sandy soil, bacterium dose and time on the efficacy of Pasteuria penetrans to control Meloidogyne incognita race 1 on coffee. Nematology 9:845–851
Chen ZX, Dickson DM (1998) Review of Pasteuria penetrans: biology, ecology, and biological control potential. J Nematol 30:313–340
Chen SY, Dickson DW, Mitchell DJ (1996a) Population development of Heterodera glycines in response to mycoflora in soil from Florida. Biol Control 6:226–231
Chen ZX, Dickson DW, McSorley R et al (1996b) Suppression of Meloidogyne arenaria race 1 by soil application of endospores of Pasteuria penetrans. J Nematol 28:159–168
Chen ZX, Dickson DW, Mitchell DJ et al (1997) Suppression mechanisms of Meloidogyne arenaria race 1 by Pasteuria penetrans. J Nematol 29:1–8
Chen J, Abawi GS, Zuckerman BM (1999) Suppression of Meloidogyne hapla and its damage to lettuce grown in a mineral soil amended with chitin and biocontrol organisms. J Nematol 31:719–725
Chen J, Abawi GS, Zuckerman BM (2000) Efficacy of Bacillus thuringiensis, Paecilomyces marquandii, and Streptomyces costaricanus with and without organic amendments against Meloidogyne hapla infecting lettuce. J Nematol 32:70–77
Cook RJ, Baker KF (1983) The nature and practice of biological control of plant pathogens. The American Phytopathological Society, St. Paul
Cronin D, MoenneLoccoz Y, Fenton A et al (1997) Role of 2, 4-diacetylphloroglucinol in the interactions of the biocontrol pseudomonad strain F113 with the potato cyst nematode Globodera rostochiensis. Appl Environ Microbiol 63:1357–1361
Dabire KR, Mateille T (2004) Soil texture and irrigation influence the transport and the development of Pasteuria penetrans, a bacterial parasite of root-knot nematodes. Soil Biol Biochem 36:539–543
De Leij FAAM, Kerry BR (1991) The nematophagous fungus Verticillium chlamydosporium as a potential biological control agent for Meloidogyne arenaria. Rev Nematol 14:157–164
De Leij FAAM, Kerry BR, Dennehy JA (1993) Verticillium chlamydosporium as a biological control agent for Meloidogyne incognita and Meloidogyne hapla in pot and micro-plot tests. Nematologica 39:115–126
de Werra P, Baehler E, Huser A et al (2008) Detection of plant-modulated alterations in antifungal gene expression in Pseudomonas fluorescens CHA0 on roots by flow cytometry. Appl Environ Microbiol 74:1339–1349
Dong LQ, Zhang KQ (2006) Microbial control of plant-parasitic nematodes: a five-party interaction. Plant Soil 288:31–45
Duponnois R, Ba AM (1998) Influence of the microbial community of a Sahel soil on the interactions between Meloidogyne javanica and Pasteuria penetrans. Nematologica 44:331–343
Duponnois R, Amadou MB, Mateille T (1999) Beneficial effects of Enterobacter cloacae and Pseudomonas mendocina for biological control of Meloidogyne incognita with the endospore-forming bacterium Pasteuria penetrans. Nematology 1:95–101
Fould S, Dieng AL, Davies KG et al (2001) Immunological quantification of the nematode parasitic bacterium Pasteuria penetrans in soil. FEMS Microbiol Ecol 37:187–195
Gaspard JT, Jaffee BA, Ferris H (1990) Meloidogyne incognita survival in soil infested with Paecilomyces lilacinus and Verticillium chlamydosporium. J Nematol 22:176–181
Giannakou IO, Dimitrios G, Karpouzas DG et al (2004) A novel non-chemical nematicide for the control of root-knot nematodes. Appl Soil Ecol 26:69–79
Giannakou IO, Anastasiadis IA, Gowen SR et al (2007) Effects of a non-chemical nematicide combined with soil solarization for the control of root-knot nematodes. Crop Prot 26:1644–1654
Goswami J, Pandey RK, Tewari JP et al (2008) Management of root knot nematode on tomato through application of fungal antagonists, Acremonium strictum and Trichoderma harzianum. J Environ Sci Heal B 43:237–240
Grewal PS, Martin WR, Miller RW et al (1997) Suppression of plant-parasitic nematode populations in turfgrass by application of entomopathogenic nematodes. Biocontrol Sci Technol 7:393–399
Hackenberg C, Muehlchen A, Forge T et al (2000) Pseudomonas chlororaphis strain Sm3, bacterial antagonist of Pratylenchus penetrans. J Nematol 32:183–189
Hirsch PR, Mauchline TH, Mendum TA et al (2000) Detection of the nematophagous fungus Verticillium chlamydosporium in nematode-infested plant roots using PCR. Mycol Res 104:435–439
Hirsch PR, Atkins SD, Mauchline TH et al (2001) Methods for studying the nematophagous fungus Verticillium chlamydosporium in the root environment. Plant Soil 232:21–30
Ishibashi N, Kondo E (1986) Steinernema feltiae (DD-136) and S. glaseri: persistence in soil and bark compost and their influence on native nematodes. J Nematol 18:310–316
Jacobs H, Gray SN, Crump DH (2003) Interactions between nematophagous fungi and consequences for their potential as biological agents for the control of potato cyst nematodes. Mycol Res 107:47–56
Jaffee BA (1999) Enchytraeids and nematophagous fungi in tomato fields and vineyards. Phytopathology 89:398–406
Jaffee BA (2002) Soil cages for studying how organic amendments affect nematode-trapping fungi. Appl Soil Ecol 21:1–9
Jaffee BA (2004) Do organic amendments enhance the nematode-trapping fungi Dactylellina haptotyla and Arthrobotrys oligospora? J Nematol 36:267–275
Jaffee BA (2006) Interactions among a soil organic amendment, nematodes, and the nematode-trapping fungus Dactylellina candidum. Phytopathology 96:1388–1396
Jaffee BA, Muldoon AE (1997) Suppression of the root-knot nematode Meloidogyne javanica by alginate pellets containing the nematophagous fungi Hirsutella rhossiliensis, Monacrosporium cionopagum and M. ellipsosporum. Biocontrol Sci Technol 7:203–217
Jaffee BA, Ferris H, Stapleton JJ et al (1994) Parasitism of nematodes by the fungus Hirsutella rhossiliensis as affected by certain organic amendments. J Nematol 26:152–161
Jaffee BA, Muldoon AE, Westerdahl BB (1996) Failure of a mycelial formulation of the nematophagous fungus Hirsutella rhossiliensis to suppress the nematode Heterodera schachtii. Biol Control 6:340–346
Jaffee BA, Muldoon AE, Didden WAM (1997) Enchytraeids and nematophagous fungi in soil microcosms. Biol Fertil Soils 25:382–388
Jaffee BA, Ferris F, Scow KM (1998) Nematode-trapping fungi in organic and conventional cropping systems. Phytopathology 88:344–350
Jagdale GB, Grewal PS (2002) Identification of alternatives for the management of foliar nematodes in floriculture. Pest Manag Sci 58:451–458
Jagdale GB, Somasekhar N, Grewal PS et al (2002) Suppression of plant-parasitic nematodes by application of live and dead infective juveniles of an entomopathogenic nematode, Steinernema carpocapsae, on boxwood (Buxus spp.). Biol Control 24:42–49
Jagdale GB, Kamoun S, Grewal PS (2009) Entomopathogenic nematodes induce components of systemic resistance in plants: biochemical and molecular evidence. Biol Control 51:102–109
Kariuki GM, Dickson DW (2007) Transfer and development of Pasteuria penetrans. J Nematol 39:55–61
Kaşkavalci G, Tüzel Y, Dura O et al (2009) Effects of alternative control methods against Meloidogyne incognita in organic tomato production. Ekoloji 18:23–31
Katan J, DeVay JE (1991) Soil solarization. CRC Press, Boca Raton
Kerry BR (1995) Ecological considerations for the use of the nematophagous fungus Verticillium chlamydosporium, to control plant parasitic nematodes. Can J Bot 73:65–70
Kerry BR (2001) Exploitation of the nematophagous fungus Verticillium chlamydosporium Goddard for the biological control of root-knot nematodes (Meloidogyne spp.). In: Butt TM, Jackson C, Magan N (eds) Fungi as biocontrol agents. CAB International, Wallingford
Kerry BR, Crump DH (1998) The dynamics of the decline of the cereal cyst nematode, Heterodera avenae, in four soils under intensive cereal production. Fund Appl Nematol 21:617–625
Khan Z, Kim YH (2007) A review on the role of predatory soil nematodes in the biological control of plant parasitic nematodes. Appl Soil Ecol 35:370–379
Khan TA, Saxena SK (1997) Effect of root-dip treatment with culture filtrates of on root penetration, development and reproduction of Meloidogyne javanica on tomato. Int J Nematol 7:85–88
Khan A, Williams KL, Nevalainen HKM (2006) Control of plant-parasitic nematodes by Paecilomyces lilacinus and Monacrosporium lysipagum in pot trials. Biocontrol 51:643–658
Kiewnick S, Sikora RA (2006) Evaluation of Paecilomyces lilacinus strain 251 for the biological control of the northern root-knot nematode Meloidogyne hapla Chitwood. Nematology 8:69–78
Kladivko EJ (2001) Tillage systems and soil ecology. Soil Tillage Res 61:61–76
Kloepper JW, Rodriguez KR, McInroy JA et al (1992) Rhizosphere bacteria antagonistic to soybean cyst Heterodera glycines and root-knot Meloidogyne incognita nematodes identification by fatty acid analysis and frequency of biological control activity. Plant Soil 139:75–84
Kluepfel DA, McInnis TM, Zehr EI (1993) Involvement of root-colonizing bacteria in peach orchard soils suppressive of the nematode Criconemella xenoplax. Phytopathology 83:1240–1245
Kluepfel DA, Nyczepir AP, Lawrence JE et al (2002) Biological control of the phytoparasitic nematode Mesocriconema xenoplax on peach trees. J Nematol 34:120–123
Kok CJ, Papert A, Hok-A-Hin CH (2001) Microflora of Meloidogyne egg masses: species composition, population density and effect on the biocontrol agent Verticillium chlamydosporium (Goddard). Nematology 3:729–734
Kokalis-Burelle N, Dickson DW (2003) Effects of soil fumigants and BioYieldTM on root-knot nematode incidence and yield of tomato. In: Proceedings of international research conference on methyl bromide: alternatives and emissions reductions, San Diego, pp 50.51–50.53
Kokalis-Burelle N, Vavrina CS, Rosskopf EN et al (2002) Field evaluation of plant growth-promoting Rhizobacteria amended transplant mixes and soil solarization for tomato and pepper production in Florida. Plant Soil 238:257–266
Kumar T, Wahla V, Pandey P et al (2009) Rhizosphere competent Pseudomonas aeruginosa in the management of Heterodera cajani on sesame. World J Microbiol Biotechnol 25:277–285
Lara Martez J, Acosta N, Betancourt C et al (1996) Biological control of Meloidogyne incognita in tomato in Puerto Rico. Nematropica 26:143–152
Li L, Mo MH, Qu Q et al (2007) Compounds inhibitory to nematophagous fungi produced by Bacillus sp strain H6 isolated from fungistatic soil. Eur J Plant Pathol 117:329–340
Madulu JD, Trudgill DL, Phillips MS (1994) Rotational management of Meloidogyne javanica and effects on Pasteuria penetrans and tomato and tobacco yields. Nematologica 40:438–455
Maehara N (2008) Reduction of Bursaphelenchus xylophilus (Nematoda: Parasitaphelen-chidae) population by inoculating Trichoderma spp. into pine wilt-killed trees. Biol Control 44:61–66
Mankau R (1962) Soil fungistasis and nematophagous fungi. Phytopathology 52:611–615
Mankau R, Prasad N (1972) Possibilities and problems in the use of a sporozoan endoparasite for biological control of plant-parasitic nematodes. Nematropica 2:7–8
Manzanilla-López RH, Atkins SD, Clark IM et al (2009a) Measuring abundance, diversity and parasitic ability in two populations of the nematophagous fungus Pochonia chlamydosporia var. chlamydosporia. Biocontrol Sci Technol 19:391–406
Manzanilla-López RH, Clark IM, Atkins SD et al (2009b) Rapid and reliable DNA extraction and PCR fingerprinting methods to discriminate multiple biotypes of the nematophagous fungus Pochonia chlamydosporia isolated from plant rhizospheres. Lett Appl Microbiol 48:71–76
Mauchline TH, Kerry BR, Hirsch PR (2002) Quantification in soil and the rhizosphere of the nematophagous fungus Verticillium chlamydospotium by competitive PCR and comparison with selective plating. Appl Environ Microbiol 68:1846–1853
Mauchline TH, Kerry BR, Hirsch PR (2004) The biocontrol fungus Pochonia chlamydosporia shows nematode host preference at the infraspecific level. Mycol Res 108:161–169
Mazzola M (2007) Manipulation of rhizosphere bacterial communities to induce suppressive soils. J Nematol 39:213–220
McInnis TM, Jaffee BA (1989) An assay for Hirsutella rhossiliensis spores and the importance of phialides for nematode inoculation. J Nematol 21:229–234
McSorley R, Wang KH, Kokalis-Burelle N et al (2006) Effects of soil type and steam on nematode biological control potential of the rhizosphere community. Nematropica 36:197–214
Meyer SLF (2003) United States Department of Agriculture – Agricultural Research Service research programs on microbes for management of plant-parasitic nematodes. Pest Manag Sci 59:665–670
Meyer SLF, Roberts DP (2002) Combinations of biocontrol agents for management of plant-parasitic nematodes and soilborne plant-pathogenic fungi. J Nematol 34:1–8
Meyer SLF, Roberts DP, Chitwood DJ et al (2001) Application of Burkholderia cepacia and Trichoderma virens, alone and in combinations, against Meloidogyne incognita on bell pepper. Nematropica 31:75–86
Minton NA, Sayre RM (1989) Suppressive influence of Pasteuria penetrans in Georgia USA soils on reproduction of Meloidogyne arenaria. J Nematol 21:574–575
Monfort E, Lopez-Llorca LV, Jansson HB et al (2006) In vitro soil receptivity assays to egg-parasitic nematophagous fungi. Mycol Prog 5:18–23
Morton CO, Hirsch PR, Peberdy JP et al (2003) Cloning of and genetic variation in protease VCP1 from the nematophagous fungus Pochonia chlamydosporia. Mycol Res 107:38–46
Muller J (1985) The influence of two pesticides on fungal parasites of Heterodera schachtii. Colloques INRA 31:225–231
Noel GR (1990) Inability of a seed treatment with Pseudomonas cepacia to control Heterodera glycines on soybean. J Nematol 22:792–794
Notz R, Maurhofer M, Schnider-Keel U et al (2001) Biotic factors affecting expression of the 2, 4-diacetylphloroglucinol biosynthesis gene phlA in Pseudomonas fluorescens biocontrol strain CHA0 in the rhizosphere. Phytopathology 91:873–881
Notz R, Maurhofer M, Dubach H et al (2002) Fusaric acid-producing strains of Fusarium oxysporum alter 2, 4-diacetylphloroglucinol biosynthetic gene expression in Pseudomonas fluorescens CHA0 in vitro and in the rhizosphere of wheat. Appl Environ Microbiol 68:2229–2235
Nyczepir AP, Shapiro-Ilan DI, Lewis EE et al (2004) Effect of entomopathogenic nematodes on Mesocriconema xenoplax populations in peach and pecan. J Nematol 36:181–185
Okubara PA, Schroeder KL, Paulitz TC (2005) Real-time polymerase chain reaction: applications to studies on soilborne pathogens. Can J Plant Pathol 27:300–313
Olatinwo R, Becker JO, Borneman J (2006a) Suppression of Heterodera schachtii populations by Dactylella oviparasitica in four soils. J Nematol 38:345–348
Olatinwo R, Borneman J, Becker JO (2006b) Induction of beet-cyst nematode suppressiveness by the fungi Dactylella oviparasitica and Fusarium oxysporum in field microplots. Phytopa-thology 96:855–859
Olatinwo R, Yin B, Becker JO et al (2006c) Suppression of the plant-parasitic nematode Heterodera schachtii by the fungus Dactylella oviparasitica. Phytopathology 96:111–114
Oyekanmi EO, Coyne DL, Fagade OE et al (2007) Improving root-knot nematode management on two soybean genotypes through the application of Bradyrhizobium japonicum, Trichoderma pseudokoningii and Glomus mosseae in full factorial combinations. Crop Prot 26:1006–1012
Parvatha Reddy P, Rao MS, Nagesh M (1996) Management of the citrus nematode, Tylenchulus semipenetrans, by integration of Trichoderma harzianum with oil cakes. Nematol Medit 24:265–267
Perez EE, Lewis EE (2006) Use of entomopathogenic nematodes and thyme oil to suppress plant-parasitic nematodes on English boxwood. Plant Dis 90:471–475
Persmark L (1997) Ecology of nematophagous fungi in agricultural soils. Ph.D. thesis, Lund University, Lund
Pickett CH, Bugg RL (1998) Enhancing biological control. University of California Press, Berkeley
Pocasangre LE, zum Felde A, Canizares C et al (2007) Field evaluation of the antagonistic activity of endophytic fungi towards the burrowing nematode, Rhadopholus similis, in plantain in Latin America. In: ISHS/ProMusa symposium: Recent advances in banana crop protection for sustainable production and improved livelihoods, White River
Pyrowolakis A, Schuster RP, Sikora RA (1999) Effect of cropping pattern and green manure on the antagonistic potential and the diversity of egg pathogenic fungi in fields with Heterodera schachtii infection. Nematology 1:165–171
Pyrowolakis A, Westphal A, Sikora RA et al (2002) Identification of root-knot nematode suppressive soils. Appl Soil Ecol 19:51–56
Rao MS, Parvatha Reddy P, Nagesh M (1998) Evaluation of plant based formulations of Trichoderma harzianum for the management of Meloidogyne incognita on egg plant. Nematol Medit 26:59–62
Roberts PA (1993) The future of nematology: integration of new and improved management strategies. J Nematol 25:383–394
Robinson AF, Westphal A, Overstreet C et al (2008) Detection of suppressiveness against Rotylenchulus reniformis in soil from cotton (Gossypium hirsutum) fields in Texas and Louisiana. J Nematol 40:35–38
Ruberson JR, Nemoto H, Hirose Y (1998) Pesticides and conservation of natural enemies in pest management. In: Barbosa P (ed) Conservation biological control. Academic, San Diego
Rumbos CI, Kiewnick S (2006) Effect of plant species on persistence of Paecilomyces lilacinus strain 251 in soil and on root colonization by the fungus. Plant Soil 283:25–31
Samac DA, Kinkel LL (2001) Suppression of the root-lesion nematode (Pratylenchus penetrans) in alfalfa (Medicago sativa) by Streptomyces spp. Plant Soil 235:35–44
Santos MA, Ferraz S, Muchovej JJ (1992) Evaluation of 20 species of fungi from Brazil for biocontrol of Meloidogyne incognita race 3. Nematropica 22:183–192
Schmidt LM, Preston JF, Dickson DW et al (2003) Environmental quantification of Pasteuria penetrans endospores using in situ antigen extraction and immunodetection with a monoclonal antibody. FEMS Microbiol Ecol 44:17–26
Schmidt LM, Preston JF, Nong G et al (2004) Detection of Pasteuria penetrans infection in Meloidogyne arenaria race 1 in planta by polymerase chain reaction. FEMS Microbiol Ecol 48:457–464
Sharon E, Bar-Eyal M, Chet I et al (2001) Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Phytopathology 91:687–693
Sharon E, Chet I, Viterbo A et al (2007) Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. Eur J Plant Pathol 118:247–258
Siddiqui IA, Ehteshamul-Haque S (2001) Suppression of the root rot-root knot disease complex by Pseudomonas aeruginosa in tomato: the influence of inoculum density, nematode populations, moisture and other plant-associated bacteria. Plant Soil 237:81–89
Siddiqui ZA, Mahmood I (1996) Biological control of plant parasitic nematodes by fungi: a review. Bioresour Technol 58:229–239
Siddiqui MA, Shaukat SS (2002) Mixtures of plant disease suppressive bacteria enhance biological control of multiple tomato pathogens. Biol Fertil Soils 36:260–268
Siddiqui IA, Shaukat SS (2003a) Non-pathogenic Fusarium solani represses the biosynthesis of nematicidal compounds in vitro and reduces the biocontrol of Meloidogyne javanica by Pseudomonas fluorescens in tomato. Lett Appl Microbiol 37:109–114
Siddiqui IA, Shaukat SS (2003b) Plant species, host age and host genotype effects on Meloidogyne incognita biocontrol by Pseudomonas fluorescens strain CHA0 and its genetically-modified derivatives. J Phytopathol 151:231–238
Siddiqui IA, Shaukat SS (2003c) Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: importance of bacterial secondary metabolite, 2, 4-diacetylpholoroglucinol. Soil Biol Biochem 35:1615–1623
Siddiqui IA, Shaukat SS (2004) Trichoderma harzianum enhances the production of nematicidal compounds in vitro and improves biocontrol of Meloidogyne javanica by Pseudomonas fluorescens in tomato. Lett Appl Microbiol 38:169–175
Siddiqui IA, Shaukat SS (2005) Phenylacetic acid-producing Rhizoctonia solani represses the biosynthesis of nematicidal compounds in vitro and influences biocontrol of Meloidogyne incognita in tomato by Pseudomonas fluorescens strain CHA0 and its GM derivatives. J Appl Microbiol 98:43–55
Siddiqui IA, Shaukat SS, Khan A (2004) Differential impact of some Aspergillus species on Meloidogyne javanica biocontrol by Pseudomonas fluorescens strain CHA0. Lett Appl Microbiol 39:74–83
Siddiqui IA, Atkins SD, Kerry BR (2009) Relationship between saprotrophic growth in soil of different biotypes of Pochonia chlamydosporia and the infection of nematode eggs. Ann Appl Biol 155:131–141
Smith ME, Jaffee BA (2009) PCR primers with enhanced specificity for nematode-trapping fungi (Orbiliales). Microb Ecol 58:117–128
Smith KS, Hewlett TE, Griswold S (2004) Pasteuria for nematode control: development of a commercial production process. In: Proceedings of the 2004 annual international research conference on methyl bromide alternatives and emissions reductions Orlando, #44. http://www.mbao.org/2004/Proceedings04/mbrpro04.html
Smitley DR, Warner FW, Bird GW (1992) Influence of irrigation and Heterorhabditis bacteriophora on plant-parasitic nematodes in turf. J Nematol 24:637–641
Sorribas FJ, Ornat C, Galeano M et al (2003) Evaluation of a native and introduced isolate of Pochonia chlamydosporia against Meloidogyne javanica. Biocontrol Sci Technol 13:707–714
Spaull VW (1984) Observations of Bacillus penetrans infecting Meloidogyne in sugarcane fields in South Africa. Rev Nematol 7:277–282
Starr JL, Ong KL, Huddleston M et al (2007) Control of Meloidogyne marylandi on bermudagrass. Nematropica 37:43–49
Stirling GR (1984) Biological control of Meloidogyne javanica with Bacillus penetrans. Phytopathology 74:55–60
Stirling GR (1991) Biological control of plant-parasitic nematodes: progress problems and prospects. C.A.B International, Wallingford
Stirling GR, Smith LJ (1998) Field tests of formulated products containing either Verticillium chlamydosporium or Arthrobotrys dactyloides for biological control of root-knot nematodes. Biol Control 11:231–239
Stirling GR, McKenry MV, Mankau R (1979) Biological control of root-knot nematodes (Meloidogyne spp.) on peach. Phytopathology 69:806–809
Stirling GR, Wilson EJ, Stirling AM et al (2005) Amendments of sugarcane trash induce suppressiveness to plant-parasitic nematodes in a sugarcane soil. Aust Plant Pathol 34:203–211
Talavera M, Mizukubo T, Ito K et al (2002) Effect of spore inoculum and agricultural practices on the vertical distribution of the biocontrol plant-growth-promoting bacterium Pasteuria penetrans and growth of Meloidogyne incognita-infected tomato. Biol Fertil Soils 35:435–440
Tedford EC, Jaffee BA, Muldoon AE et al (1993) Parasitism of Heterodera schachtii and Meloidogyne javanica by Hirsutella rhossiliensis in microplots over two growing seasons. J Nematol 25:427–433
Terefe M, Tefera T, Sakhuja PK (2009) Effect of a formulation of Bacillus firmus on root-knot nematode Meloidogyne incognita infestation and the growth of tomato plants in the greenhouse and nursery. J Invertebr Pathol 100:94–99
Timm L, Pearson D, Jaffee B (2001) Nematode-trapping fungi in conventionally and organically managed corn-tomato rotations. Mycologia 93:25–29
Timper P, Minton NA, Johnson AW et al (2001) Influence of cropping systems on stem rot (Sclerotium rolfsii), Meloidogyne arenaria, and the nematode antagonist Pasteuria penetrans in peanut. Plant Dis 85:767–772
Tobin JD, Haydock PPJ, Hare MC et al (2008a) The compatibility of the fungicide azoxystrobin with Pochonia chlamydosporia, a biological control agent for potato cyst nematodes (Globodera spp.). Ann Appl Biol 152:301–305
Tobin JD, Haydock PPJ, Hare MC et al (2008b) Effect of the fungus Pochonia chlamydosporia and fosthiazate on the multiplication rate of potato cyst nematodes (Globodera pallida and G. rostochiensis) in potato crops grown under UK field conditions. Biol Control 46:194–201
Trudgill DL, Bala G, Blok VC et al (2000) The importance of tropical root-knot nematodes (Meloidogyne spp.) and factors affecting the utility of Pasteuria penetrans as a biocontrol agent. Nematology 2:823–845
Tzortzakakis EA (2000) The effect of Verticillium chlamydosporium and oxamyl on the control of Meloidogyne javanica on tomatoes grown in a plastic house in Crete, Greece. Nematol Medit 28:249–254
Tzortzakakis EA, Gowen SR (1994) Evaluation of Pasteuria penetrans alone and in combination with oxamyl, plant resistance and solarization for control of Meloidogyne spp. on vegetables grown in greenhouses in Crete. Crop Prot 13:455–462
Tzortzakakis EA, Petsas SE (2003) Investigation of alternatives to methyl bromide for management of Meloidogyne javanica on greenhouse grown tomato. Pest Manag Sci 59:1311–1320
Tzortzakakis EA, Gowen SR, Goumas DE (1996) Decreased ability of Pasteuria penetrans spores to attach to successive generations of Meloidogyne javanica. Fund Appl Nematol 19:201–204
van den Boogert PHJF, Velvis H, Ettema CH et al (1994) The role of organic matter in the population dynamics of the endoparasitic nematophagous fungus Drechmeria coniospora in microcosms. Nematologica 40:249–257
Verdejo-Lucas S, Sorribas FJ, Ornat C et al (2003) Evaluating Pochonia chlamydosporia in a double-cropping system of lettuce and tomato in plastic houses infested with Meloidogyne javanica. Plant Pathol 52:521–528
Wang KH, Sipes BS, Schmitt DP (2002) Management of Rotylenchulus reniformis in pineapple, Ananas comosus, by intercycle cover crops. J Nematol 34:106–114
Wang KH, Sipes BS, Schmitt DP (2003) Enhancement of Rotylenchulus reniformis suppressiveness by Crotalaria juncea amendment in pineapple soils. Agric Ecosyst Environ 94:197–203
Wang KH, McSorley R, Gallaher RN (2004) Effect of Crotalaria juncea amendment on squash infected with Meloidogyne incognita. J Nematol 36:290–296
Wei BQ, Xue QY, Wei LH et al (2009) A novel screening strategy to identify biocontrol fungi using protease production or chitinase activity against Meloidogyne root-knot nematodes. Biocontrol Sci Technol 19:859–870
Weibelzahl-Fulton E, Dickson DW, Whitty EB (1996) Suppression of Meloidogyne incognita and M. javanica by Pasteuria penetrans in field soil. J Nematol 28:43–49
Weller DM, Landa BB, Mavrodi OV et al (2007) Role of 2, 4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol 9:4–20
Westphal A (2005) Detection and description of soils with specific nematode suppressiveness. J Nematol 37:121–130
Westphal A, Becker JO (1999) Biological suppression and natural population decline of Heterodera schachtii in a California field. Phytopathology 89:434–440
Westphal A, Becker JO (2000) Transfer of biological suppressiveness against Heterodera schachtii. Phytopathology 90:401–406
Westphal A, Becker JO (2001a) Components of soil suppressiveness against Heterodera schachtii. Soil Biol Biochem 33:9–16
Westphal A, Becker JO (2001b) Soil suppressiveness to Heterodera schachtii under different cropping sequences. Nematology 3:551–558
Yin B, Valinsky L, Gao XB et al (2003) Identification of fungal rDNA associated with soil suppressiveness against Heterodera schachtii using oligonucleotide fingerprinting. Phytopa-thology 93:1006–1013
Zhang L, Liu XZ, Zhu SF et al (2006) Detection of the nematophagous fungus Hirsutella rhossiliensis in soil by real-time PCR and parasitism bioassay. Biol Control 36:316–323
Zhu ML, Mo MH, Xia ZY et al (2006) Detection of two fungal biocontrol agents against root-knot nematodes by RAPD markers. Mycopathologia 161:307–316
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Timper, P. (2011). Utilization of Biological Control for Managing Plant-Parasitic Nematodes. In: Davies, K., Spiegel, Y. (eds) Biological Control of Plant-Parasitic Nematodes:. Progress in Biological Control, vol 11. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-9648-8_11
Download citation
DOI: https://doi.org/10.1007/978-1-4020-9648-8_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-1-4020-9647-1
Online ISBN: 978-1-4020-9648-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)