Abstract
Starch-based and composite edible films and coatings can enhance food quality, safety and stability. They can control mass transfer between components within a product, as well as between product and environment. They can improve performance of the product through the addition of antioxidants, antimicrobial agents, and other food additives. Unique advantages of edible films and coatings can lead to the development of new products, such as individual packaging for particular foods, carriers for various food additives, and nutrient supplements. Film materials and their properties have been reviewed extensively in this book and previously (Guilbert 1986; Kester and Fennema 1986; Krochta and De Mulder-Johnson 1997).
Composite films can be formulated to combine the advantages of each component. Biopolymers, such as proteins and polysaccharides, provide the supporting matrix for most composite films, and generally offer good barrier properties to gases, with hydrocolloid components providing a selective barrier to oxygen and carbon dioxide (Guilbert 1986; Kester and Fennema 1986; Drake et al. 1987, 1991; Baldwin 1994; Wong et al. 1992; Baldwin et al. 1997). Lipids provide a good barrier to water vapour (Nisperos-Carriedo 1994; Baldwin et al. 1997), while plasticizers are necessary to enhance flexibility and improve film’s mechanical properties.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Introduction
Starch-based and composite edible films and coatings can enhance food quality, safety and stability. They can control mass transfer between components within a product, as well as between product and environment. They can improve performance of the product through the addition of antioxidants, antimicrobial agents, and other food additives. Unique advantages of edible films and coatings can lead to the development of new products, such as individual packaging for particular foods, carriers for various food additives, and nutrient supplements. Film materials and their properties have been reviewed extensively in this book and previously (Guilbert 1986; Kester and Fennema 1986; Krochta and De Mulder-Johnson 1997).
Composite films can be formulated to combine the advantages of each component. Biopolymers, such as proteins and polysaccharides, provide the supporting matrix for most composite films, and generally offer good barrier properties to gases, with hydrocolloid components providing a selective barrier to oxygen and carbon dioxide (Guilbert 1986; Kester and Fennema 1986; Drake et al. 1987, 1991; Baldwin 1994; Wong et al. 1992; Baldwin et al. 1997). Lipids provide a good barrier to water vapour (Nisperos-Carriedo 1994; Baldwin et al. 1997), while plasticizers are necessary to enhance flexibility and improve film’s mechanical properties.
Composition, microstructure and physical properties of biopolymeric films determine their possible applications. Controlling the film formulation allows tailoring of the mechanical and barrier properties of these materials, improving the efficiency of preservation for packaged foods. The study of film microstructure and interactions between film components provides insight into both fundamental aspects of material science and practical technologies for possible applications. Most methods used in characterization of films in the solid state are based on detection of structural and thermodynamic properties involving the crystalline-amorphous structure. X-ray diffraction is probably the most important technique for observing structural properties of crystalline solid materials, polymers and undoubtedly food materials (Gontard et al. 1993; Krochta and De Mulder-Johnson 1997). Thermodynamic changes can be evaluated by several calorimetric techniques and by thermomechanical analysis (Cherian et al. 1995; Roos 1995; Galietta et al. 1998; García et al. 2000a; Sobral et al. 2001; Mali et al. 2002; Neto et al. 2005). Knowledge of thermodynamic state, molecular mobility and phase transitions are important, since they affect film barrier properties, which define performance of films under conditions of common use and abuse.
The objectives of this chapter are to describe: (a) properties of different starch and composite films and their relation to functionality, with special emphasis on film formulations based on starch, chitosan and methylcellulose; (b) common methods of characterizing composite films and coatings and (c) applications of these biomaterials.
6.2 Formulation of Films and Coatings
Hydrocolloids, proteins, and their derivatives represent the major components of film formulations, and form the primary supporting matrix of films. Functional, organoleptic, nutritional and mechanical properties of an edible film can be modified by addition of other ingredients in minor amounts. A main requirement for film formulation components is that they be “generally recognized as safe” (GRAS) substances, and be used in accordance with good manufacturing practices.
In general, protein films are poor moisture barriers due to the hydrophilic nature of most proteins, but are good barriers to gases such as oxygen and carbon dioxide (Cuq et al. 1998; McHugh and Krochta 1994a). Casein, collagen, zein, wheat gluten, whey proteins, gelatin and soy proteins have been extensively investigated due to their availability and mechanical resistance as components of edible films (McHugh and Krochta 1994a; Were et al. 1999; Oh et al. 2004; Sohail et al. 2006). Edible films and coatings discussed in this chapter are summarized in Table 6.1.
Most commonly used polysaccharides are cellulose derivatives, chitosan, starch, alginate, carrageenan and pectin due to their good film-forming properties. Starches are abundant in nature, commercially available, inexpensive and are totally biodegradable. Thus, they are attractive raw materials for the development of completely degradable products for specific market needs. Among the cellulose derivatives, methylcellulose (MC) has the best film-making properties, high solubility and efficient oxygen and lipid barrier properties (Park et al. 1993; Donhowe and Fennema 1993a, b; Nisperos-Carriedo 1994).
Chitin and its deacetylated product, chitosan, have received a lot of interest in agriculture, biomedicine, biotechnology and the food industry due to their unique properties, biodegradability and bioactivity (Muzzarelli et al. 1988; Kumar 2000; Tharanathan and Kittur 2003). Based on its antifungal, mechanical and oxygen barrier properties (Chen et al. 1996; Caner et al. 1998), chitosan film is a promising active packaging film material (Vermeiren et al. 1999). Production of chitosan from crustacean shells, a byproduct of the seafood industry, is economically feasible (Kumar 2000). However it lacks universal approval for food applications.
Composite biopolymer films can bring about improved mechanical and physical properties if components are structurally compatible. Thus, blending a starch or methylcellulose with chitosan can produce films with interesting properties and novel applications.
Plasticizers are the other components of edible films. These are low molecular weight compounds that are added to soften the rigid structure of films. Plasticizers improve mechanical, barrier and physical properties of biopolymer films (Banker 1966). They must be compatible with film-forming polymers, and reduce intermolecular forces and increase mobility of polymer chains (Donhowe and Fennema 1993c, 1994). Hydrophilic compounds such as polyols (glycerol, sorbitol) and polyethylene glycol are commonly used as plasticizers in hydrophilic film formulations (Gontard et al. 1993). Lipophilic compounds, such as vegetable oils, lecithin and, to a lesser extent, fatty acids, may also act as emulsifiers and plasticizers (Kester and Fennema 1986; Cuppet 1994; Donhowe and Fennema 1994; Hernandez 1994).
Food additives such as antioxidants, antimicrobial agents and nutrients, can be incorporated in film formulations to achieve specific functionalities. This concept of “active films” is a very promising application as it creates new avenues for designing packaging materials.
One of the most popular and oldest techniques to protect specific fruits and vegetables is application of natural wax and lipid coatings. Coatings are intended to protect product against dehydration, attack by fungi, and abrasion during processing and to improve product appearance (Hardenburg 1967; Paull and Chen 1989; Drake and Nelson 1990; Drake et al. 1991; Baldwin 1994; Hagenmaier and Baker 1994; Baldwin et al. 1997). In some products, the oily appearance limits their use (Baker et al. 1994; Baldwin et al. 1997).
Composite starch suspensions can be used as coatings or for preparation of edible films. The most common method of food coating involves spraying or immersion. Coating integrity is a critical factor that depends on, surface tension, adhesion to food substrate and flexibility of the coating. Unplasticized matrices are often brittle and rigid once dried, due to strong interactions between polymer chains that favor crystal development. This brings formation of cracks or and peeling off, especially for irregularly shaped fruits. Addition of a plasticizer can remedy this problem, since it can improve coating flexibility by reducing interactions between polymer chains. However the amount of plasticizer needs to be optimized since its amount can negatively alter the barrier and mechanical properties of a coating. García et al. (1998a, b, 1999, 2000b, 2001) described how they optimized plasticizer in composite coatings. For starch-based formulations, glycerol and sorbitol are common plasticizers used with typical concentrations in coating solutions ranging between 0 and 50 g/L. Maximum plasticizer concentration is limited by surface migration. For example, the maximum amount of glycerol suitable for these starch-based formulations was 20 g/L, while higher concentrations resulted in plasticizer migration to the surface, leading to a sticky coating (García et al. 1998a). For sorbitol, a concentration greater than 20 g/L can led to inconveniently long drying times.
Sunflower oil was included in coating formulations at concentrations between 0 and 8 g/L to enhance water barrier properties. Similar to plasticizers, lipid materials also may migrate to coating surface, depending on coating microstructure. Starch-based coatings containing less than 5 g/L sunflower oil did not exhibit oil migration (García et al. 2001). Wong et al. (1992) reported that similar lipid concentrations did not result in oil migration in chitosan-fatty acid films. However, a marked lipid migration was reported for methylcellulose films (Greener and Fennema 1989; Vojdani and Torres 1989a, b, 1990). Lipid concentration has been optimized for barrier properties by García et al. (2001).
Apart from sunflower oil, a composite coating may also include active ingredients such as antimicrobial agents and antioxidants. Active components remain at the surfaces of food products, where they are needed. The coating matrix limits diffusion of active components into the core of the food and thus, minimizes the amount of additive needed for the product (García et al. 2001).
Another important application of coatings is to reduce oil uptake of products during deep fat frying. Excessive fat in the diet has been linked to coronary heart disease. Thus, coatings applied to food before frying can help in reducing fat absorption. Hydrocolloids, especially cellulose derivatives such as methylcellulose, can be used to minimize oil content of fried foods.
The description of coating formulations used in published works (García et al. 1998a, b, 1999, 2000b, 2001, 2002, 2004a, b) is shown in Table 6.2.
6.3 Preparation and Characterization
Composite films are prepared by using 2 or more hydrocolloids. This requires dissolution of biopolymer molecules. Some polysaccharides, like cellulose derivatives, require solubilization at a higher temperature, while others require dissolution in a pH-regulated medium (e.g., chitosan).
Preparation of starch for the preparation of films requires gelatinization of native starch granules either by cold (e.g., alkaline treatment with NaOH) or thermal treatments. The most common technique to gelatinize starch is through thermal treatment (Mali et al. 2002). However, alkaline dispersion can also be used to alter the size distribution of starch polymers, that brings about a mild degree of starch hydrolysis or depolymerisation (García et al. 2000a; Romero-Bastida et al. 2005).
In general, preparation of protein-based films requires dissolution of protein in an appropriate solvent, e.g., zein, which is a prolamin, requires dissolution in ethanol (Ghanbarzadeh et al. 2007). Other examples of alcohol-soluble proteins are wheat gluten and fish myofibrillar proteins. Hydration of protein facilitates its solubilization (Sobral et al. 2001).
Cross-linking of proteins with lactic acid, tannic acid, ionized calcium or transglutaminase has been shown to increase resistance of films to water vapour and gas. In addition, preparation of films from aqueous emulsions of proteins and lipids or by lamination of a protein film with lipid can also decrease water vapour permeability (WVP). Lipids are frequently incorporated to reduce water vapour permeability, due to their hydrophobic character. Addition of lipids to a hydrocolloids matrix can be carried out by overcasting, leading to bilayer films, or by emulsion techniques (Baldwin et al. 1997; Shellhammer and Krochta 1997). In the latter case, the suspension can be used to obtain either coatings or films. With solid lipids, such as carnauba wax or saturated fats, the common procedure is to incorporate them via bilayer films, whereas the emulsion technique is preferred for liquid lipids, such as edible oils (Greener and Fennema 1989; Gontard et al. 1994; Hagenmaier and Baker 1994; Shellhammer and Krochta 1997). Emulsion preparation parameters are important factors for obtaining homogenous films and coatings, and will influence performance. For example, in the case of starch-based films, a high stirring velocity produced extensive foaming that could not be eliminated with a vacuum and thus produced low quality coating (García et al. 2001). Another important factor is the lipid to aqueous phase ratio. If the critical ratio is exceeded, migration of lipid may occur.
Plasticizers are often added once biopolymer solubilization has been achieved. Other functional additives (e.g., emulsifiers, antimicrobial or antioxidant agents) are incorporated into formulations after addition of the plasticizer. It must be stressed that each individual additive may require a particular condition for proper and effective incorporation.
As described previously, composite suspensions can be used to obtain either coatings or films. In the case of coatings, surface tension and rheological behavior of the suspension are important factors that will affect suspension spreadability and coating adhesion. Plasticizer and lipid addition, in the case of a starch coating suspension, decrease surface tension (Table 6.3), providing better adhesion to foodstuffs. The surface of vegetables already has low surface tension for natural protection. However, this natural advantage is a drawback for aqueous coating applications (Hershko and Nussinovitch 1998). Modifying the coating formulation to include plasticizers and/or lipids helps decrease surface tension to facilitate adhesion of coating to foodstuffs (Table 6.3). The similarity between liquid and solid film in values of dispersive and polar components influences spreadability of the liquid (Hershko and Nussinovitch 1998). Ghanbarzadeh et al. (2007) reported that sugar addition increases surface tension of zein films, which was attributed to an increase in polar forces and interactions within the film matrix.
Understanding the rheological behavior of film or coating suspensions is critical for process scale-up to ensure that processing requirements and machinability issues can be properly addressed. Rheological properties of composite starch suspensions exhibit pseudoplastic behavior (n < 1), while the Power law rheological model provided a satisfactory behavior fit enough to experimental data (r 2 > 0.96) (García et al. 2006). Plasticizer and lipid addition to corn and amylomaize starch suspensions decreased the flow behavior index and increased the consistency index. Apparent viscosity of corn starch suspensions decreased with both plasticizer and lipid addition (Table 6.3). Measurement of viscosity is important. Spraying requires a low viscosity, while immersion requires a higher viscosity coating solution.
In formulation of composite biopolymer films, it is important to characterize the miscibility of biopolymers and interactions that may occur between them, since these attributes ultimately influence film microstructure. For example, chitosan-starch suspensions show pseudoplastic flow behavior similar to those obtained with chitosan suspensions (Fig. 6.1). The presence of chitosan in the suspension decreased peak viscosity of the suspension. When chitosan was blended with methylcellulose, film solutions also showed pseudoplastic behavior (n < 1). Single component solutions of either MC or CH showed similar values for all rheological parameters; however, composite film solutions had higher apparent viscosities and consistency indexes than those of single component solutions by themselves. These results point to a likely synergistic effect between constituents of the film solution, suggesting a high compatibility and high degree of interaction between the two polymer types. Besides, apparent viscosity of film solutions increased with CH concentration (Table 6.3).
6.3.1 Film Preparation
Films can be prepared by casting, extrusion or lamination, which are similar processes used in the synthetic polymer industry (Stepto 1987). Casting is a common, small-scale production method used to obtain biodegradable films. In this technique, a portion of the film suspension is poured onto acrylic plates, and then dried in a ventilated oven (60°C) to a constant weight (García et al. 1999). This simple technique produces films that can be easily removed from plates, and allows films of variable thickness to be obtained by varying the weight of film suspension applied and the area of the plate onto which films are cast.
Drying conditions (rate and temperature) determine film characteristics (e.g., water content, crystallinity, etc.) that affect film microstructure and properties. Bader and Göritz (1994a, b, c) extensively studied the effect of drying on amylomaize film microstructure, and developed a model relating X-ray diffraction patterns, sorption isotherms and mechanical properties of films. They stressed that drying temperature lead to different X-ray diffraction pattern which are associated with different crystalline developments during storage. Thus, tailoring drying conditions allows controlling of amorphous-crystalline structure, which is strongly related to barrier and mechanical properties.
6.3.2 Film and Coating Characterization
The main attributes involved in characterizing biodegradable films are: optical properties, water-solubility, water sorption/desorption, thickness, microstructure, crystallinity, biopolymer compatibility (composite films), thermal behavior, barrier properties (vapour and gaseous permeabilities) and mechanical behavior.
6.3.2.1 Appearance
The visual properties of a film ultimately determine consumer acceptability of the packaged products. Both subjective and objective techniques are used to characterize optical properties of films, with surface color commonly measured by a colorimeter and opacity determined by spectrophotometry. Polysaccharide films are typically colorless, though those of CH may exhibit a slightly yellow appearance. However, the intensity of the yellowness was negligible in comparison to that of whey protein-based films (Trezza and Krochta 2000a, b). Polysaccharide films are free of many of the problems associated with protein and lipid films, such as Maillard and oxidation reactions (Trezza and Krochta 2000a, b).
Film opacity is a critical property to consider if the film is to be used at a food surface. It can be measured using the method proposed by Gontard et al. (1992), in which the film spectrum is recorded over the visible range. Opacity is estimated as the area under the absorption curve (Au × nm). Transparent films are characterized by low values for area under the absorption curve measurements. Table 6.4 shows that CH films were the most transparent, and CS films the most opaque, increasing opacity with increasing starch concentration. Nevertheless, opacity values for starch-based films were still lower than those reported for wheat gluten films obtained under different solubilization conditions (Gontard et al. 1992). Addition of glycerol reduced starch film opacity, while blending corn starch with CH generated films with reduced opacity, but increased yellowness.
6.3.2.2 Water Solubility
Water solubility is an important factor in determining possible applications for composite biopolymer films. MC films are completely soluble in water, while CH films have lower water-solubility values. Composite MC–CH films had intermediate water-solubility, which decreased with increasing chitosan concentration (Table 6.5). Thus, by adjusting CH concentration in the film formulation, water-solubility of the composite film can be changed to meet requirements of a specific application. A similar trend in water solubility was observed for CH-starch composite films.
6.3.2.3 Water Sorption
Water sorption isotherms are useful for determination of film stability under various conditions, as many film constituents, especially hydrocolloids, are sensitive to relative humidity and temperature. Though sorption isotherms can be measured by several techniques, a simple procedure is described by Spiess and Wolf (1993).
Sorption isotherms for yam starch films (with and without glycerol) and native yam starch were compared at 25°C (Fig. 6.2). All isotherms showed a similar sigmoidal shape (type II isotherm). When samples were conditioned at water activity (aw) values greater than 0.43, the plasticized starch film showed higher equilibrium moisture contents than both the native yam starch powder and the non-plasticized starch film. The hygroscopic characteristics of glycerol-plasticized starch films contributed to an increase in hydrophilicity of the film.
Controlled films showed higher equilibrium moisture content than native yam starch at aw > 0.75. This result could be attributed to the fact that during film production, gelatinization led to a starch molecular reorganization that increased water absorption capacity of the unplasticized film compared to native starch. Unplasticized films showed similar behavior to those reported by other researchers working with high-amylose corn starch (Bader and Göritz 1994 b) and tapioca starch films (Chang et al. 2000).
6.3.2.4 Thickness of Films
Film thickness can be measured with a digital film thickness gauge or by scanning electron microscopy (SEM), the first method being simpler and faster. Correlation between film thickness measured by SEM and a digital film thickness gauge was established for CH-starch films (García et al. 2006). These results are presented in Table 6.6 with a correlation coefficient of 0.9953.
Barrier and mechanical properties depend on film thickness. A wide range of film thickness values has been reported for composite biopolymer films in the literature, indicating its dependence on both film composition and processing parameters.
6.3.2.5 Microstructure
Properties of composite films and coatings depend on several factors such as: the ratio of crystalline to amorphous zones, polymeric chain mobility, and specific interactions between functional groups of polymers and the permeant substance within amorphous zones. Common techniques used to elucidate film structure include SEM, X-ray diffraction, differential scanning calorimetry (DSC), thermomechanical analysis (TMA), dynamic mechanical analysis (DMA) and Fourier transform infrared spectroscopy (FTIR).
6.3.2.6 Scanning Electron Microscopy
SEM may be used to evaluate film homogeneity, layer structure, pores and cracks, surface smoothness and thickness. For example, cross sections of unplasticized corn starch film showed a multi-laminar structure, while those for films containing a plasticizer showed a more compact structure, regardless of the plasticizer used (Fig. 6.3, Table 6.6). SEM images of plasticized films containing lipid exhibited smooth surfaces and a compact structure, indicative of a homogeneous dispersion of lipids within the film matrix.
When corn starch was blended with chitosan, film surfaces appeared smooth (without pores or cracks) and homogeneous (no obvious phase separation), even for formulations that did not include a plasticizer (Fig. 6.4c, Table 6.6). The compact, homogeneous matrix of CS–CH films is an indicator of structural integrity and, consequently, good mechanical properties such as high resistance and elongation at break are expected. Similarly, in the case of MC–CH composite films, a compact structure with no pores or cracks was observed by SEM, even though film formulation did not include a plasticizer (Fig. 6.5). The composite film showed multilayered structure (Fig. 6.5c), though both CH and MC are compatible polymers that could limit chain mobility.
When dealing with coatings, characterization of microstructure provides important insight into performance of the different formulations. Light microscopy is used for evaluation of coating adhesion, thickness and uniformity, while estimation of coating integrity is conducted by SEM. Improved visualization of starch-based coatings using light microscropy may be achieved via staining with iodine solution. Coating thicknesses varied between 40 and 50 μm for all tested formulations (García et al. 1998a, b).
6.3.2.7 Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR) is a useful technique to supplement microstructural characterization of composite films, since it may be used to evaluate interactions between film components. Figure 6.6a shows the IR spectra for films of MC, CH and a 50:50 CH–MC blend. The positions of peaks of the single component spectrum is similar to those described by different authors (Chen et al. 2003; Sionkowska et al. 2004; Wu et al. 2005; Zaccaron et al. 2005). Spectra of compo-site films show characteristic peaks of both polymers, and also small shifts in the positions of the 1,500–1,700 cm−1 bands, which are related to amino and carbonyl groups. Figure 6.6b shows the effect of chitosan concentration in CH–MC blends with an increasing CH concentration, while spectra of composite films approach that of the CH film. Further, composite films showed a broad band between 2,985 and 3,600 cm−1 corresponding to the N–H and –OH superposition of chitosan (Sionkowska et al. 2004), while MC film showed only the characteristic –OH stretching at 3,457 cm−1 (Zaccaron et al. 2005).
According to Wanchoo and Sharma (2003), hydrogen bonding or other interactions between chemical groups of dissimilar polymers should theoretically cause a shift in the peak position of participating groups. In composite MC–CH films, this behavior is observed for –OH stretching, since this peak shifted from 3,463 cm−1 in MC films toward 3,450 cm−1 in CH-MC composite mixtures with increasing CH proportion. This observation could indicate that the –OH group movement is compromised when another polymer is present. A shift was also observed in CH amino group (–NH2) band at 1,560 cm−1 as the amount of MC decreased in composite films. However, other characteristic peaks, including those at 1,411 cm−1 (OH vibrations) and 2,904 cm−1 (C–H stretching) assigned by Pawlak and Mucha (2003) and Zaccaron et al. (2005) did not show a significant shift. These results suggest a mild interaction between CH and MC that might be attributed to their similar chemical and linear structures.
6.3.2.8 X-ray Diffraction
X-ray diffraction patterns of edible films, either of single component or composite materials, show an amorphous-crystalline structure characterized by sharp peaks associated with the crystalline diffraction and an amorphous zone. The higher the amorphous zone, the lower the crystallinity. Amorphous regions within the sample can be estimated by the area between the smooth curve drawn following the scattering hump and the baseline joining the background within the low- and high-angle points. The crystalline fraction can be estimated by the relative area of the upper regions above the smooth curve (Snyder and Bish 1989; Köksel et al. 1983). In general, crystallinity of composite films is dependent on the following processing conditions: (1) biopolymer source and plasticizer, (2) completeness of biopolymer dissolution in water, (3) conditions of film drying (rate and temperature), and (4) final moisture content of the samples (Van Soest et al. 1996; Van Soest and Vliegenthart 1997). The shape and width of the diffraction profile are determined by both the mean crystal size (and distribution of crystal size within the specimen) and the particular imperfections of the crystalline lattice (Klug and Alexander 1974).
X-ray diffraction can be used to track recrystallization of film polymers during storage. In the case of starch-based films, peak width decreased slightly and peak intensities increased, indicating a growth in crystallite size corresponding to a slow recrystallization process. X-ray diffraction patterns of composite films generally represent a mixture of component features in which the characteristic peaks of individual components can be identified. For example, X-ray diffractograms of composite MC–CH films are similar to those obtained for MC alone, although peak intensity at 2θ = 8° (which is distinctive of MC films) decreased with an increasing CH concentration, suggesting that CH interferes with MC ordering (Fig. 6.7). Similar results were reported for CH-gelatin films by Chen et al. (2003), who attributed the decreased crystallinity of the composites to reduced hydrogen bonding in CH molecules, leading to an amorphous structure for the polyelectrolyte complex.
Film preparation techniques also may lead to development of different matrix structures. For films based on thermal gelatinization of starch, X-ray diffraction patterns of yam starch films exhibited a B-type crystalline packing arrangement, characteristic of starch from tubers (Roos 1995). This pattern remained virtually unchanged over the course of storage. Starch and glycerol contents did not markedly influence the X-ray pattern of these films (Mali et al. 2002). In contrast, when films were obtained from starch solubilized by alkaline treatment, X-ray diffraction patterns showed that starch films exhibited a crystalline structure of higher stability, namely an A-type starch pattern (Fig. 6.8). This shift in the crystalline pattern was also observed for amylomaize films by Bader and Göritz (1994a, c) and was attributed to film drying conditions. For drying temperatures up to 60°C, diffractograms of films exhibited a B-type crystalline pattern, while those dried at 100°C exhibited diffractograms corresponding to A-type crystals. As film drying temperature increased over the range of 60–100°C, film diffractograms exhibited a continuous transition from B- to A-type crystals. The crystallinity of starch films was primarily related to the amylose content of the starch source, and depended on both drying and storage conditions. High water content favors chain interactions and, thus, crystallization within starch films. In hydrocolloid films, as described for starch, water has an additional role, since it is incorporated in a structural active state through inter- and intramolecular hydrogen bondings (Even and Carr 1978).
Starch films without plasticizer showed higher crystallinity (higher peaks) than those containing plasticizer, which resulted in a larger amorphous zone and lower peaks (Fig. 6.9). Films containing plasticizers also retained a stable diffraction pattern during initial stages of storage, while unplasticized starch films required more time to reach a stable diffraction pattern (García et al. 2000a). Addition of sunflower oil did not strongly modify X-ray pattern of films during storage (Fig. 6.9b). Nevertheless, the presence of oil facilitated polymeric chain mobility, allowing rapid development of the most stable structure, and also reduced crystal growth by interfering with the polymeric chain arrangement.
6.3.2.9 Differential Scanning Calorimetry of Films
Evolution of the film matrix crystalline structure during storage can also be evaluated by DSC. As described in the results of X-ray diffraction, film preparation and storage conditions impact the thermal properties of composite films. Films obtained by starch thermal treatment, such as those formulated with yam starch, did not show any peaks in DSC thermograms at the beginning of the storage time, indicating that starch gelatinization during film production was complete. With storage (90 days at 20°C and 64% relative humidity), these films did not show any peaks in DSC thermograms (between 40° and 120°C), indicating stability of the matrix (Mali et al. 2002).
However, starch films obtained by alkaline treatment showed an endothermic transition with a peak temperature around 50°C during storage (90 days). This peak became narrower and its melting temperature and the corresponding enthalpy (∆H) increased with increasing storage time (Fig. 6.10). This, DSC transition could be associated with several processes, such as the crystal growth of short chains (products of hydrolysis) and recrystallization of amylose or other long lateral amylopectin chains.
The presence of plasticizers in films limited crystal growth and recrystallization similar to that previously described for X-ray analysis, leading to lower enthalpy values (∆H) and peak melting temperatures (Fig. 6.10). Sorbitol is a good plasticizer for starch, since its molecular structure is similar to glucose units of starch chains, increasing the chance to interact with polymeric chains (García et al. 2000a). Additives may interfere with polymeric chain association due to steric hindrances, and in these cases film crystallinity will likely decrease.
6.3.2.10 Glass Transition Temperature
The glass transition temperature (T g) is strongly dependent on both the film composition and moisture content, and can denote stability of a film. As water content of an amorphous material increases, T g decreases. Thus, for a given storage temperature, an amorphous matrix at low water content (in the glassy state) remains stable to molecular change, while the same matrix at higher moisture content, corresponding to the rubbery state, can undergo molecular reorganization. In the rubbery state, polymer chains possess sufficient mobility for crystal growth and perfection. Thus, knowledge of T g is important, since it impacts mechanical and barrier properties of a film under specific conditions of application and storage.
Both DSC and TMA techniques are commonly used to estimate T g, though T g values obtained by DSC are slightly higher than those determined by TMA. Chang and Randall (1992) stressed that the sensitivity of TMA is superior to that of DSC for defining glass transition temperatures. Dynamic mechanical thermal analysis (DMTA) may also be used for determination of T g. Table 6.7 provides some literature-reported T g values for various film formulations. Plasticization decreases intermolecular forces between polymer chains and, consequently, reduces T g (Guilbert and Gontard 1995). For composite starch matrices, T g values for films without plasticizer were higher than those of films containing glycerol (Table 6.7). In this regard, a similar trend was observed for films formulated with other polysaccharides (Debeaufort and Voilley 1997) and proteins of varied origin (Cherian et al. 1995; Galietta et al. 1998; Sobral et al. 2001).
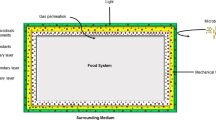
6.3.2.11 Barrier Properties
Barrier properties ultimately determine the ability of films and coatings to improve storage or shelf life of various food products. Common measurements include water vapour and gas permeabilities, which are strongly related to film structure, since permeants generally, move through a film or coating via its amorphous zones. Film components, especially plasticizers, affect both barrier and mechanical properties, because they modify film structure, chain mobility and diffusion coefficients of permeants.
6.3.2.11.1 Water Vapour Permeability
Determination of water vapour permeability (WVP) is strongly dependent on measurement conditions, such as temperature and the gradient of water vapour pressure. Coated biomaterials, such as fruits and vegetables, are characterized by irregular shapes and high water contents that make WVP measurements difficult. To overcome this challenge, García et al. (1998a, b) used a biological model (coated sliced carrots) that allowed surface area calculations to be accounted for WVP determinations of starch-based coatings (García et al. 1998a, b). WVP testing of films may be conducted according to method E96 (ASTM 1996a) that includes some modifications introduced by Gennadios et al. (1994). Since barrier thickness strongly affects water vapour transport through the film matrix, it is necessary to consider film thickness in WVP calculations. In addition, WVP determinations can be performed using the PERMATRAN, a specific instrument developed by MOCON for analysis of synthetic film materials. It is interesting to note that WVP results for coatings (applied on fruits) and films have been shown to be similar, even though the measuring techniques and driving forces for both systems are different. These findings validate the use of films to characterize barrier properties of coatings (García et al. 1998b, 2000b, 2004c ).
Coatings without plasticizer often yield significantly higher WVP values than those with plasticizer (Table 6.8). This difference is attributed to the presence of pores and cracks in unplasticized coatings, as observed by light microscopy and SEM (García et al. 1999). In the absence of cracks or pores, a plasticized film generally would be expected to exhibit higher WVP than an unplasticized film (Banker 1966). These results could be related to structural modifications to the starch network produced by the plasticizer and to the hydrophilic character of glycerol, which favors the absorption and desorption of water molecules to promote permeability. Gontard and coworkers (1993) working on wheat gluten films found similar results on the effect of glycerol. Sorbitol generally resulted in lower WVP values compared to glycerol in plasticized starch-based coatings (Table 6.8e). McHugh and Krochta (1994a, b, c) reported similar results for alginate and pectin films using the same plasticizers at the same concentrations.
Amylomaize films and coatings, which possessed higher amylose content, denser matrix structure and higher degree of crystallinity than those of standard corn starch, also exhibited relatively lower WVP values (Figs. 6.8, 6.10). A similar trend has been observed by others (Noel et al. 1992; Miles et al. 1985a, b). How-ever, the effect of amylose content on the WVP of the starch film matrix was minimized by incorporation of sunflower oil to the coating formulation (Table 6.8). Addition of sunflower oil significantly decreased WVP of starch-based films and coatings. Baldwin and coworkers (1997) reviewed lipid effects on other non-starch composite films. As water vapour transfer generally occurs through the hydrophilic portion of a film, WVP is dependent on the hydrophilic-hydrophobic ratio of film components (Hernandez 1994). In general, WVP increases as polarity and degree of unsaturation/branching of the incorporated lipid increases, though water absorption properties of polar components of the film must also be considered (Gontard et al. 1994).
Yam starch film WVP is lower than those of many edible, biodegradable films such as wheat gluten plasticized with glycerol and amylose and hydroxypropyl methylcellulose containing plasticizer and oil, (Gennadios et al. 1994). In general, starch films exhibit a lower order of magnitude of WVP compared both to protein films and other polysaccharide-based films reported in literature (García et al. 1999, 2001, 2004c; McHugh and Krochta 1994a, b, c; McHugh et al. 1994; Parra et al. 2004; Parris et al. 1995). In comparison to synthetic polymers, starch films have WVP values similar to those of cellophane, but higher than low density polyethylene, the most common synthetic film.
García et al. (2004b) studied the effect of composition on CH–MC composite biopolymer films. Permeabilities of these composite biopolymer films did not differ significantly from those of single component films (Table 6.8). This result, together with X-ray analysis, provided evidence that matrices of composite films were very similar to those based on the single polymers. Table 6.8 also shows WVP values of composite films formulated with chitosan and corn starch. Addition of glycerol decreased WVP of CS films as described previously. McHugh and Krochta (1994c) found similar results for alginate and pectin films. Blending CS with CH decreased WVP of composite films. Plasticized composite CS–CH films have WVP values that were similar to those of CH films, thus blending CH with CS is a good alternative to maintain barrier properties and reduce costs (García et al. 2006).
A summary of WVP values for several hydrophilic edible films and synthetic polymer films is presented in Table 6.9. In comparison to other commercial biopolymers, chitosan films exhibit relatively low WVP. Composite films of CH, MC and CS attain WVP values close to those of cellophane (Table 6.8), as might be expected, due to similar chemical structures of these polymers (Shellhammer and Krochta 1997). However, WVP values of composite films are not comparable with those of synthetic films, such as low density polyethylene (LDPE), (Smith 1986), the primary polymer used in the food packaging industry (Table 6.9).
6.3.2.11.2 Gas Permeability
Measurement of film or coating permeability to CO2 and O2 is essential to understanding quality and physiological aspects of coated fruit products during storage. In the case of coatings, gas permeability is commonly measured using isolated films rather than coated products. This simplification is based on the finding that WVP results for coatings and films are similar (García et al. 2000b).
Different methods are used to determine gas permeability of films. Oxygen permeability can be measured using commercial instruments, such as the OXTRAN developed by Mocon Company. In addition, CO2 and O2 permeabilities of films can be assessed by using a specially designed cell (García et al. 2000a, b, 2001). This quasi-static method is based on measurement of gas diffusing through a film, which is quantified by a gas chromatograph. As was previously described for WVP determinations, calculated values for gaseous permeability must account for film thickness. Film and coating integrity is a must for attainment of good gas barrier properties. Starch films without plasticizers exhibit much higher CO2 and O2 permeabilities and also higher standard deviation coefficients than the same formulations containing plasticizers (Table 6.10). This result was attributed to the presence of pores within films without plasticizers, resulting in a lack of film integrity, as determined by SEM (García et al. 1999, 2000a).
Starch films with sorbitol exhibit lower oxygen permeability values than those formulated with glycerol (Table 6.10). Sorbitol combined with an amylomaize film matrix provided the lowest gas permeability values for all materials tested (Table 6.10). McHugh and Krochta (1994a, b) found similar results working on milk and whey protein films.
Table 6.10 shows that O2 permeabilities for starch-based films were much lower than those of CO2, indicating variable permeability of these films to select gases. This effect can be attributed to a higher solubility of CO2 than O2 in the film matrix (Young 1984; McHugh and Krochta 1994a). Similar results were obtained by Arvanitoyannis et al. (1994) while working with potato and rice starch films. Further, the addition of lipid, which is necessary to reduce water vapour permeability, maintained the selective gas permeability properties of the starch-based films (Table 6.10).
Synthetic film materials, such as LDPE, show lower gas permeabilities (2.16 × 10−11 and 9.45×10−11 cm3 m−1 s−1 Pa−1 for O2 and CO2, respectively) than starch-based films. However, LDPE has a relatively low CO2 to O2 permeability ratio (≈4), compared to an average ratio of 13 for starch-based films (Cuq et al. 1998; García et al. 1998a, b). Cuq et al. (1995) compared CO2 to O2 permeability ratios of several synthetic and edible films, and reported that edible films show higher selectivities than synthetic films, with ratios for edible films ranging from 8 to 30. Development of composite edible films and coatings with selective gas permeabilities could be very promising for controlling respiratory exchange and improving conservation of fresh or minimally-processed vegetables (Cuq et al. 1998; García et al. 1998a, b).
Table 6.11 shows that the CO2 permeability of starch-based films decreased with increasing storage time (García et al. 2000a). Gas and vapour permeabilities of films and coatings depend on several factors such as: (1) ratio of crystalline to amorphous zones, (2) polymeric chain mobility, and (3) specific interactions between the functional groups of the polymers and gases within the amorphous zones. Gaseous permeability measurements of starch stored films correlate with the increase in the crystallinity reported by DSC and X-ray diffraction (Fig. 6.8–6.10). According to Donhowe and Fennema (1993b), film permeability increases with a decreasing crystalline to amorphous zone ratio, since permeation occurs through amorphous zones of the film.
6.3.2.12 Mechanical Properties
Mechanical properties of films and coatings are dependent on additive-matrix interactions, and are also strongly affected by physical, chemical and temperature conditions, which influence film stability and flexibility. Mechanical performance of a film is usually characterized by deformation at break (extension at the moment of rupture, mm), percent elongation at break (deformation divided by initial probe length and multiplying by 100%), tensile strength (force at rupture divided by film cross section, MPa) and elastic modulus (slope of force-deformation curve, N/mm) according to the ASTM D882-91 method (1996b). These can be determined using a texturometer or an Instron machine. These parameters are related to the stretching ability of the film. It is well known that the environmental conditions during production, storage and usage of these materials affect their mechanical properties. Ageing phenomena occurring within the useful lifetime of films also cause great losses to mechanical properties, particularly with regards to film elongation.
Plasticizers interfere with polymeric chain association facilitating their slipping and, thus, decreasing rigidity of the network, producing a less ordered film structure. In the case of amylomaize films, high values of elastic modulus were obtained (Fig. 6.11). These results were attributed to their high amylose content. According to García and coworkers (2001), the plasticizer-matrix interaction showed similar trends for both corn starch and amylomaize films, though plasticized corn starch films were more flexible than those of amylomaize. The combination of lipid and plasticizer addition increased film flexibility, and reduced the effect of starch type on film mechanical properties.
The stress–strain curves for composite films formulated with chitosan (CH) and corn starch (CS) without plasticizer showed a pattern typical of brittle materials (Sarantopoulos et al. 2002; Mali et al. 2005a, b). This characteristic is more evident in CH films than in CS films (Fig. 6.12). Blends of chitosan and corn starch did not show significant differences in film deformation from single component films, although film stress of composite films decreased. These results would indicate that interactions between polymeric chains are weaker, reducing rigidity of the matrix, although both hydrocolloids are compatible (García et al. 2006).
Plasticized films exhibit the stress-strain behavior of ductile polymers compared with unplasticized films (Fig. 6.12). Corn starch films plasticized with glycerol (CS + G) exhibited the most flexible behavior (García et al. 2006). Similar results were obtained by Laohakunjit and Noomhorm (2004) and Mali and coworkers (2005b) for cassava starch films plasticized with glycerol or sorbitol.
For composite films (CS + CH + G), a lower plasticizing effect of glycerol was observed when compared with starch matrices (CS + G). Composite MC–CH films also exhibited different behavior patterns under tensile tests. CH films showed high resistance at break, while MC was more flexible, exhibiting composite films with intermediate percent elongation values (Fig. 6.13) (Pinotti et al. 2007). Similar mechanical properties have been reported in the literature for MC films (Donhowe and Fennema 1993a, b, c; Park et al. 1993, 1994; Debeaufort and Voilley 1997). For CH films, most literature reported values for tensile tests were higher than those obtained by Pinotti et al. (2007). These differences may be due to variations in CH composition and/or suppliers and film preparation (Chen and Lin 1994; Butler et al. 1996; Caner et al. 1998). Caner et al. (1998) reported a wide range of elongation values (14–70%) for CH films, depending on the acid used for chitosan solubilization, plasticizer type, and storage time.
For CH–MC composite films, tensile strength and elastic modulus increased with an increasing CH concentration (Fig. 6.13b); accordingly, relative deformation decreased (Fig. 6.13a). The blending of CH with MC enhanced film deformation and increased film flexibility, avoiding the need for plasticizer addition. With regard to puncture tests, the elastic modulus showed the same trend as in tensile tests (Fig. 6.13b). However, all composite films have a similar values of deformation at break, regardless of CH:MC ratio (Fig. 6.13a). Thus, CH may serve as a partial replacement of MC for generation of composite films with good puncture resistance and economic benefit, since MC is itself an expensive commodity.
Table 6.12 compares mechanical properties determined for both biodegradable and synthetic films under identical test conditions. Tensile strength values for composite films were in the range of those for LDPE and HDPE, but were lower than that obtained for cellophane. However, synthetic polymers like LDPE and HDPE exhibited much greater values for elongation at break. Similar results were reported for synthetic films by Cunningham et al. (2000).
6.4 Film and Coating Applications
Various coating applications reported in literature have utilized vegetable, meat, seafood and bread dough products among others (Olorunda and Aworth 1984; Drake et al. 1987, 1991; Dhalla and Hanson 1988; Avena-Bustillos et al. 1993, 1994; Baldwin et al. 1997; García et al. 1998a, b). Since most coating applications are reported for fruits and vegetables, several specific examples will be highlighted here. The diverse array of fruits and vegetables offers many challenges to coating applications that include adhesion of coating to slippery (e.g., tomatoes, mushrooms), irregular or rough (e.g., strawberries) surfaces. These will require addition of ingredients to formulations that will reduce coating solution surface tension to ensure coating uniformity and absence of void holes. In the case of starch-based coatings, those containing plasticizer have been shown to be homogeneous, and covered the whole surface of strawberries, including the achenes (García et al. 1998a). On the other hand, coatings without plasticizer were brittle and possessed undesirable cracks, as revealed by iodine staining of the film surface (García et al. 1998a).
Strategies to extend post-harvest quality of fruits and vegetables need to address several key challenges, like extending maturation and senescence periods, minimizing dehydration and reducing the onset and rate of microbial growth. Edible films and coatings can offer simultaneous solutions to vegetables, such as Brussels sprouts, tomatoes, cucumbers, and red peppers (El Gaouth et al. 1991a, b; Viña et al. 2007), and for fruits like bananas (Banks 1984), apples (Drake et al. 1987), and mangoes (Dhalla and Hanson 1988). Mali and Grossmann (2003) proposed the use of yam starch films as packaging for strawberries contained within plastic trays, and compared performance of these films with that of PVC. Although the PVC film provided better weight and firmness retention for strawberries, yam starch films could be considered a viable packaging alternative.
6.4.1 Application of Coatings to Highly Perishable Fruits
A good example is application of composite active coatings to extend storage life of strawberries, which are highly perishable fruits with only a short period of annual production and high susceptibility to fungal infection (El Gaouth et al. 1991a; García et al. 1998a, b, 2001). Cold gelatinization of starch was the chosen alternative to comply with both requirements: (1) the dipping procedure selected for coating application, and (2) the heat sensitive characteristics of strawberries. Formulations of the tested starch-based coatings are shown in Table 6.2.
6.4.2 Microbiological Analysis
Surface microbial growth is the main cause of spoilage for many food products. The microorganisms that grew on strawberries were mainly yeasts, molds and sugar-fermenting bacteria. Figure 6.14 demonstrates need of a composite coating to decrease microbial counts, as the unplasticized coating did not differ significantly from the control in this regard. Coatings containing potassium sorbate, a well-known, effective antifungal agent, significantly decreased (P < 0.05) yeast and mold counts on coated strawberries. Since the undissociated form of sorbic acid is the active antimicrobial agent, citric acid was added to the coating formulation to increase potassium sorbate effectivity. Fig. 6.14 shows that 0.2 g/L potassium sorbate used in conjunction with citric acid was the most effective formulation for decreasing microbial counts. Use of active coatings lowers the amount of preservative required to achieve the same antimicrobial efficacy as traditional techniques (Guilbert 1986; Guilbert et al. 1997; Vojdani and Torres 1989a, b).
Effect of coating formulation on growth of yeasts and molds (Log CFU/g fruit) in strawberries stored at 0°C and 84%RH (Control: uncoated fruits, (open diamond); composition of coating formulation: (open circle) amylomaize without plasticizer; (open square) amylomaize and 20 g/L sorbitol; (open up triangle) amylomaize, 20 g/L sorbitol and 0.2 g/L potassium sorbate and (open down triangle) amylomaize, 20 g/L sorbitol, 0.2 g/L potassium sorbate and citric acid; bars indicate standard error)
Storage life, defined as the time necessary to reach 106 CFU/g fruit, was 14 days for uncoated fruit stored at 0°C. Coatings containing sorbitol extended storage life to 21 days. At the maximum storage time evaluated (28 days), coating formulations containing both sorbitol and potassium sorbate still exhibited microbial counts below the storage life limit of 106 CFU/g of fruit. While addition of potassium sorbate improved antimicrobial characteristics of the starch coatings, addition of both citric acid and potassium sorbate further enhanced antimicrobial effectiveness, yielding a composite coating with a storage life of more than 28 days (Fig. 6.14).
6.4.3 Quality Attributes of Strawberries
All plasticized coatings effectively reduced strawberry weight loss during storage (Fig. 6.15). Significant differences (P < 0.05) were observed among the weight loss values for uncoated strawberries and those coated with common corn or high-amylose corn starch, with lowest weight loss values obtained with amylomaize. Weight losses of fruits coated with starch formulations without plasticizer were similar to those of controlled fruit, due to presence of pores and cracks. Coatings with sorbitol led to significantly lower fruit weight losses than glycerol, regardless of starch type. However, weight losses were unacceptable after 3 weeks of storage for coated fruits without lipid. Addition of sunflower oil was necessary to reduce weight loss and increase storage life, provided that microbial counts could be maintained below the established limit (106 CFU/g fruit) even at 28 days of storage. The maximum weight loss reduction (63.2%) after 28 days of storage was obtained for coating formulations that included sunflower oil (Fig. 6.15). Avena-Bustillos et al. (1997), working on apples and celery treated with caseinate and acetylated monoglyceride coating, found a 75% increase in resistance to water vapour transfer and consequently, a decrease in weight loss of coated vegetables compared to uncoated controls. Ultimately, changes in texture and appearance are the most important factors that determine post-harvest storage life of fruits and vegetables. The rate and extent of firmness loss during ripening of soft fruits like strawberries is also another factor of primary importance. According to Manning (1993), fruit softening is attributed to degradation of components of the cell wall, primarily pectic substances, due to activity of specific enzymes such as polygalacturonase. For both control and coated fruits, breaking force decreased as a function of storage time. Coating formulations that minimized weight loss also maintained better firmness, since the firmness attribute is highly influenced by water content.
Effect of coating formulation on weight loss of control and coated strawberries during storage at 0°C and 84%RH. (Control: uncoated fruits, (open circle); composition of coating formulation: (open square) amylomaize without plasticizer; (open up triangle) amylomaize with 20 g/L sorbitol and (open diamond) amylomaize with 20 g/L sorbitol and 2 g/L sunflower oil)
The effect of coatings on surface color modifications of strawberries was analyzed, because this quality attribute may also determine consumer acceptability of fruit. Glycerol or sorbitol significantly (P < 0.05) delayed development of surface color, which resulted in a slight change in color lightness with time. Coating formulations containing glycerol gave better surface color results compared to those with sorbitol. Lipid addition did not significantly modify (P < 0.05) surface color results, regardless of the plasticizer used in formulations.
Active starch-based coatings containing antimicrobial agents, plasticizer and lipid improved barrier properties to water vapour (Table 6.8), reduced microbial growth and showed selective gas permeability (Table 6.10), and thus, extended storage life of strawberries. The best coating formulation in our case contained high-amylose starch, sorbitol, sunflower oil and potassium sorbate plus citric acid as antimicrobial agents.
6.4.4 Physiological Modifications
Physiological parameters, including titratable acidity, pH, anthocyanin level and sugar content, are good indicators of fruit maturation and senescence. Coatings may be used to alter the natural physiological behavior, modifying organoleptic characteristics of fruits such as color, taste or flavor.
In coated strawberries, these physiological parameters were slowed down, but did reach commercially acceptable values (García et al. 1998b). These results indicate that starch-based coatings retard metabolic reactions, and thus, senescence of coated fruits is delayed. The oxygen and carbon dioxide barriers lead to a reduction in respiration rate by limiting exposure to ambient oxygen, increasing internal carbon dioxide, delaying ripening and senescence, and extending the storage life of treated fruits (Baldwin 1994; Avena-Bustillos et al. 1993, 1994; Drake et al. 1991; García et al. 1998a, b).
Another important parameter in fruits is the degree of maturity reached at the time of application of the coating. For example, when composite starch-based coatings were applied on strawberries at 25, 50, 75 and 100% color maturity ripening stages, 75% was selected as the optimum degree of maturity for prolonging storage life. In this case, development of appropriate anthocyanin content, reducing and non-reducing sugar levels and titratable acidity of strawberries were slowed down during storage, reaching commercially acceptable values at the end of the storage period. The delay in development of physiological parameters observed in coated fruits, regardless of the degree of maturity, was attributed to the differential gaseous permeability of films and influence on respiratory activity.
6.5 Coating Application on Fried Foods
As mentioned previously, another novel application of coatings is to reduce oil uptake of products during deep-fat frying. Cellulose derivatives, such as methyl cellulose (Table 6.2), can be used to decrease oil content of food products during frying. Hydrocolloids with thermal gelling or thickening properties, like proteins and carbohydrates, have been investigated for this purpose. Williams and Mittal (1999a, b) found that methylcellulose (MC) films provided the best oil barrier properties, and reduced fat uptake more than hydroxypropylcellulose and gellan gum films in pastry mix applications. Mallikarjunan et al. (1997) stressed that products coated with cellulose derivatives form a protective layer at the surface of food products during initial stages of frying, due to thermal gelation of the hydrocolloid at temperatures above 60°C. This protective layer limits transfer of moisture and fat between the sample and the frying medium. MC formulations that included sorbitol as plasticizer were applied to dough discs and potato strips to reduce oil uptake during deep fat frying (García et al. 2002, 2004a). Coating application did not modify textural characteristics or sensory properties of the fried samples. MC coating formulations were the most effective, reducing oil uptake between 35 and 40% depending on the product. To obtain a coating with adequate adherence and integrity, it is necessary to incorporate a plasticizer, sorbitol being the most effective in the case of MC coatings. SEM micrographs showed good adhesion between the coating and the dough, and no significant differences between coated and uncoated samples were detected by the sensory panel.
6.6 Future Trends
The use of coatings and packaging films by the food industry has become a topic of great interest, because of their potential for increasing shelf life of many food products (Ahvenainen 2003; Coles et al. 2003; Giles and Bain 2001; Hernandez et al. 2000). By selecting the right materials and packaging technologies, it is possible to maintain product quality and freshness of food products (Brown 1992; Stewart et al. 2002). Nowadays, a large part of materials used in packaging industries is produced from fossil fuels, which are practically not degradable. Preservation of natural resources and recycling has led to a renewed interest in biomaterials and renewable raw materials. A concerted effort to extend shelf life and to enhance food quality, while reducing packaging waste, has encouraged exploration of new bio-based packaging materials, such as edible and biodegradable films from renewable resources (Albertsson and Karlsson 1995; Trznadel 1995; Tharanathan and Kittur 2003). However, like conventional packaging, bio-based packaging has to supply a number of important functions with regards to food applications, including containment and protection, maintenance of sensory quality and safety, and labeling (Robertson 1993).
Polar biopolymers, such as polysaccharides and proteins, are being studied as prospective replacements for synthetic polymers in the film and plastic industries. Their potential benefits are both environmental and cost-related. Traditional techniques for processing thermoplastic synthetic polymers have been adapted to hydrophilic polymers like starch and gelatin.
To date, use of biodegradable films for food packaging has been strongly limited because of the poor barrier properties and weak mechanical properties shown by natural polymers. For this reason, natural polymers are frequently blended with other synthetic polymers or, less frequently, chemically modified with the aim of extending their applications (Guilbert et al. 1997; Petersen et al. 1999). Recent advances in genetic engineering and composite science offer significant opportunities for improving materials from renewable resources with enhanced support of global sustainability, although use of materials obtained by genetic modification is still controversial.
For scientists, the real challenge lies in finding applications that would consume sufficiently large quantities of these materials to reduce their cost, allowing biodegradable polymers to compete economically in the market. Starch and its derivates are promising raw materials, because they are renewable, widely available and relatively low cost (Gonera and Cornillon 2002; Smits et al. 1998). As a packaging material, starch alone does not form films with appropriate mechanical properties, unless it is first plasticized or chemically modified. When starch is treated in an extruder by application of both thermal and mechanical energy, it can be converted to a thermoplastic material. In production of thermoplastic starches, plasticizers are expected to efficiently reduce intra-molecular hydrogen bonds and to provide stability to product properties. There are many opportunities for using starch as packaging materials (Kim and Pometto 1994). As described previously, starch can be employed as packaging for fruits, vegetables, snacks, or other dry products. For example, Novamont, an Italian company, commercializes EverCornTM, a starch-based material available as films or bags. Similarly, Bioenvelope, a Japanese company commercializes Bio-PTM, another starch-based film. In these applications, however, efficient mechanical, oxygen and moisture protection is needed, as thermoplastic starch alone cannot meet all these requirements. Because of the hydrophilicity of the starch, performance changes during and after processing, due to water content changes. To overcome this drawback, many different routes have been reported. Some biodegradable materials like Mater-BiTM, produced by Novamont, are available in the market, which is based on 60% starch and 40% alcohol. This is mainly commercialized in Europe and America.
Today’s excellent performance of traditional plastics is an outcome of continued R&D efforts of the last several years. However, existing biodegradable polymers have come to public view only recently. Prices of biodegradable biopolymers can be realized through continued research and development efforts focused on optimizing composition and functionality to improve performance of edible films and coatings.
References
Ahvenainen R (2003) Novel food packaging techniques. Boca Raton, FL: CRC Press LLC
Albertsson AC, Karlsson S (1995) Degradable polymers for the future. Acta Polymers, 46, 114–123
Arvanitoyannis I, Kalichevsky M, Blanshard JMV, Psomiadou E (1994) Study of diffusion and permeation of gases in undrawn and uniaxially drawn films made from potato and rice starch conditioned at different relative humidities. Carbohydr. Polym. 24, 1–15
ASTM (1996a) Standard test methods for water vapour transmission of material, E96 – 95. Annual book of ASTM. Philadelphia, PA: American Society for Testing and Materials
ASTM (1996b) Standard test methods for tensile properties of thin plastic sheeting, D882-91. Annual Book of ASTM. Philadelphia, PA: American Society for Testing and Materials
Avena-Bustillos RJ, Cisneros-Zeballos LA, Krochta JM, Saltveit ME (1993) Optimization of edible coatings on minimally processed carrots using response surface methodology. Am. Soc. Agric. Eng. , 36 3, 801–805
Avena-Bustillos RJ, Krochta JM, Saltveit ME, Rojas-Villegas RJ, Sauceda-Pérez JA (1994) Optimization of edible coating formulations on Zucchini to reduce water loss. J. Food Eng. 21, 197–214
Avena-Bustillos RJ, Krochta JM, Saltveit ME (1997) Water vapour resistance of red delicious apples and celery sticks coated with edible caseinate-acetylated mono-glyceride films. J. Food Sci. 62, 2, 351–354
Avena-Bustillos RJ, Olsen CW, Olson DA, Chiou B, Yee E, Bechtel PJ, McHugh TH (2006) Water vapour permeability of mammalian and fish gelatin films. J. Food Sci. 71, 4, 202–207
Bader HG, Göritz D (1994a) Investigations on high amylose corn starch films. Part 1: wide angle X-ray scattering (WAXS). Starch/Stärke 46, 6, 229–232
Bader HG, Göritz D (1994b) Investigations on high amylose corn starch films. Part 2: water vapour sorption. Starch/Stärke 46, 7, 249–252
Bader HG, Göritz D (1994c) Investigations on high amylose corn starch films. Part 3: stress strain behaviour. Starch/Stärke 46, 11, 435–439
Baker RA, Baldwin EA, Nisperos-Carriedo MO (1994) Edible coatings for processed foods. In: Krochta JM, Baldwin EA, Nisperos-Carriedo M (Eds.) Edible Coatings and Films to Improve Food Quality. Technomic Pub. Co., Lancaster, PA, pp. 89–104
Baldwin EA (1994) Edible coatings for fruits and vegetables, past, present and future. In: Krochta JM, Baldwin EA, Nisperos-Carriedo MO (Eds.) Edible Coatings and Films to Improve Food Quality. Technomic Pub. Co., Lancaster, PA, pp. 25–64
Baldwin EA, Nisperos-Carriedo MO, Hagenmaier RD, Baker RA (1997) Use of lipids in coatings for food products. Food Technol. 51, 6, 56–64
Banker GS (1966) Film coating, theory and practice. J. Pharm. Sci. 55, 81
Banks NH (1984) Some effects of TAL Pro-Long coatings on ripening bananas. J. Exp. Bot. 35, 127
Brown WE (1992) Plastics in Food Packaging. Properties, Design, and Fabrication. New York, NY: Marcel Dekker
Butler B, Vergano R, Testin R, Bunn J, Wiles J (1996) Mechanical and barrier properties of edible chitosan films as affected by composition and storage. J. Food Sci. 61, 5, 953–961
Caner C, Vergano P, Wiles J (1998) Chitosan film mechanical and permeation properties as affected by acid, plasticizer, and storage. J. Food Sci. 63, 6, 1049–1053
Chang BS, Randall CS (1992) Use of subambient thermal analysis of optimize protein lyophilization. Cryobiology 29, 632–656
Chang YP, Cheah PB, Seow CC (2000) Plasticizing – antiplasticizing effects of water on physical properties of tapioca starch films in the glassy state. J. Food Sci. 65, 3, 445–451
Chen RH, Lin JH (1994) Relationships between the chain flexibilities of chitosan molecules and the physical properties of their casted films. Carbohydr. Polym. 24, 41–46
Chen M, Yeh H, Chiang B (1996) Antimicrobial and physicochemical properties of methylcellulose and chitosan films containing a preservative. J. Food Process Preserv. 20, 379–390
Chen M, Deng J, Yang F, Gong Y, Zhao N, Zhang X (2003) Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials 24, 2871–2880
Cherian G, Gennadios A, Weller C, Chinachoti P (1995) Thermomechanical behavior of gluten films; effect of sucrose, glycerin and sorbitol. Cereal Chem. 72, 1, 1–6
Coles R, McDowell D, Kirwan MJ (2003) Food packaging technology. Oxford, UK: Blackwell Publishing Ltd
Cunningham P, Ogale A, Dawson P, Acton J (2000) Tensile properties of soy protein isolate films produced by a thermal compaction technique. J. Food Sci. 65, 4, 668–671
Cuppet SL (1994) Edible coatings as carriers of food additives, fungicides and naturals antagonists. In: Krochta JM, Baldwin EA, Nisperos-Carriedo M (Eds.) Edible Coatings and Films to Improve Food Quality. Technomic Pub. Co., Lancaster, PA, pp. 121–137
Cuq B, Gontard N, Guilbert S (1995) Edible films and coatings as active layers. In: Rooney ML (Ed.) Active Food Packaging. Chapman & Hall, UK, pp. 111–142
Cuq B, Gontard N, Guilbert S (1998) Proteins as agricultural polymers for packaging production. Cereal Chem. 75, 1, 1–9
Dhalla R, Hanson SW (1988) Effect of permeability coatings on the storage life of fruits. II. Pro-long treatments of mangoes (Mangifera indica L. cv. Julie). Int. J. Food Sci. Technol. 23, 107–112
Debeaufort F, Voilley A (1997) Methylcellulose-based edible films and coatings: 2. Mechanical and thermal properties as a function of plasticizer content. J. Agric. Food Chem. 45, 685–689
Donhowe IG, Fennema OR (1993a) Water vapour and oxygen permeability of wax films. J. Am. Oil Chem. Soc. 70 (9), 867–873
Donhowe IG, Fennema O (1993b) The effects of solution composition and drying temperature on crystallinity, permeability and mechanical properties of methylcellulose films. J. Food Process Preserv. 17, 231–246
Donhowe IG, Fennema O (1993c) The effects of plasticizers on crystallinity, permeability and mechanical properties of methylcellulose films. J. Food Process Preserv. 17, 247–258
Donhowe IG, Fennema OR (1994) Edible films and coatings: characteristics, formation, definitions and testing methods. In: Krochta JM, Baldwin EA, Nisperos-Carriedo M (Eds.) Edible Coatings and Films to Improve Food Quality, Technomic Pub. Co., Lancaster, PA, pp. 1–21
Drake SR, Fellman JK, Nelson JW (1987) Post-harvest use of sucrose polyesters for extending the shelf-life of stored Golden Delicious apples. J. Food Sci. 52, 5, 1283–1285
Drake SR, Nelson JW (1990) Storage quality of waxed and nonwaxed “Delicious” and “Golden Delicious” apples. J. Food Qual. 13, 331–334
Drake SR, Cavalieri R, Kupferman EM (1991) Quality attributes of “D’Anjou pears after different wax drying and refrigerated storage. J. Food Qual. 14, 455–465
El Gaouth A, Arul J, Ponnampalam R, Boulet M (1991a) Chitosan coating effect on storability and quality of fresh strawberry. J. Food Sci. 56, 6, 1618–1620
El Gaouth A, Arul J, Ponnampalam R, Boulet M (1991b) Use of chitosan coating to reduce water loss and maintain quality of cucumber and bell pepper fruits. J. Food Process Preserv. 15, 339–368
Even WR, Carr SH (1978) Micromechanical fracture analysis of amylose. Polymer 19, 583–588
Galietta G, Giola DD, Guilbert S, Cuq B (1998) Mechanical and thermomechanical properties of films based on whey protein as affect by plasticizer and crosslinking agents. J. Dairy Sci. 81, 3123–3130
García MA, Martino MN, Zaritzky NE (1998a) Starch-based coatings: effect on refrigerated strawberry (Fragaria× Ananassa) quality. J. Sci. Food Agric. 76, 411–420
García MA, Martino MN, Zaritzky NE (1998b) Plasticizer effect on starch-based coatings applied to strawberries (Fragaria × Ananassa). J. Agric. Food Chem. 46, 3758–3767
García MA, Martino MN, Zaritzky NE (1999) Edible starch films and coatings characterization: sem, water vapour and gas permeabilities. J. Scanning Microsc. 21, 5, 348–353
García MA, Martino MN, Zaritzky NE (2000a) Microstructural characterization of plasticized starch-based films. Starch/ Stärke 52, 4, 118–124
García MA, Martino MN, Zaritzky NE (2000b) Lipid addition to improve barrier properties of edible starch-based films and coatings. J. Food Sci. 65, 6, 941–947
García MA , Martino MN, Zaritzky NE (2001) Composite starch-based coatings applied to strawberries (Fragaria × ananassa). Nahrung/Food 45, 4, 267–272
García MA, Ferrero C, Bértola N, Martino MN, Zaritzky NE (2002) Edible coatings from cellulose derivatives to reduce oil uptake in fried products. Innov. Food Sci. Emerg. Technol. 3–4, 391–397
García MA, Ferrero C, Campana A, Bértola N, Martino M, Zaritzky N (2004a). Methylcellulose coatings reduce oil uptake in fried products. Food Sci. Technol. Int. 10, 5, 339–346
García MA, Martino MN, Zaritzky NE (2004b) Research advances in edible coatings and films from starch. In: Mohan RM (Ed.) Research Advances In Food Science. Global Research Network. Research Advances in Food Science 4, 107–128
García MA, Pinotti A, Martino MN, Zaritzky NE (2004c) Characterization of composite hydrocolloid films. Carbohydr. Polym. 56, 3, 339–345
García MA, Pinotti A, Zaritzky NE (2006) Physicochemical, water vapour barrier and mechanical properties of corn starch and chitosan composite films. Starch/Starke 58, 9, 453–463
Gennadios A, Weller CL, Gooding CH (1994) Measurement errors in water vapour permeability of highly permeable, hydrophilic edible films. J. Food Eng. 21, 395–409
Ghanbarzadeh B, Musavi M, Oromiehie AR, Rezayi K, Razmi E, Milani J (2007) Effect of plasticizing sugars on water vapour permeability, surface energy and microstructure properties of zein films. LWT 40, 1191–1197
Giles GA, Bain DA (2001) Technology of Plastics Packaging for the Consumer Market. Boca Raton, FL: CRC Press LLC
Gonera A, Cornillon P (2002) Gelatinization of starch/gum/sugar system studied by using DSC, NMR and CSLM. Starch/Sta¨rke, 54, 508–516
Gontard N, Guilbert S, Cuq B (1992) Edible wheat gluten films: influence of the main process variables on film properties using response surface methodology. J. Food Sci. 57, 1, 190–199
Gontard N, Guilbert S, Cuq JL (1993) Water and glycerol as plasticizers affect mechanical and water vapour barrier properties of an edible wheat gluten film. J. Food Sci. 58, 1, 206–211
Gontard N, Duchez C, Cuq JL, Guilbert S (1994) Edible composite films of wheat gluten and lipids: water vapour permeability and other physical properties. Int. J. Food Sci. Technol. 19: 39–50
Greener IK, Fennema OR (1989) Evaluation of edible, bilayer films for use as moisture barriers for foods. J. Food Sci. 54, 1400–1406
Guilbert S (1986) Technology and application of edible protective films. In: Mathlouthi M (Ed.) Food Packaging and Preservation. Theory and Practice. Elsevier Applied Science Publishing Co. London, UK, pp. 371–394
Guilbert S, Gontard N (1995) Technology and applications of edible protective films. In: VII Biotechnology and Food Research – “New Shelf-Life Technologies and Safety Assessments”. Helsink, Finland, pp. 49–60
Guilbert S, Cuq B, Gontard N (1997) Recent innovations in edible and/or biodegradable packaging materials. Food Addit. Contam. 14, 6, 741–751
Hagenmaier RD, Baker RA (1994) Wax microemulsions and emulsions as citrus coatings. J. Agric. Food Chem. 42, 899–902.
Hardenburg RE (1967) Wax and related coatings for horticultural products – a bibliography. Agric. Res. Serv. Bull. 965. Cornell University, Ithaca, NY
Hernandez E (1994) Edible coatings for lipids and resins. In: Krochta JM, Baldwin EA, Nisperos-Carriedo M (Eds.) Edible Coatings and Films to Improve Food Quality. Technomic Pub. Co., Lancaster, PA, pp. 279–304
Hernandez R, Selke SEM, Cultler J (2000) Plastics Packaging: Properties, Processing, Applications, Regulations. Munich, Germany: Hanser Gardner Publications
Hershko V, Nussinovitch A (1998) Relationship between hydrocolloid coating and mushroom structure. J. Agric. Food Chem. 46, 8, 2988–2997
Iijimaa M, Nakamura K, Hatakeyama T, Hatakeyamab H (2000) Phase transition of pectin with sorbed water. Carbohydr. Polym. 41, 101–106
Kester JJ, Fennema OR (1986) Edible films and coatings: a review. Food Technol. 12, 47–59
Kim M, Pometto III OR (1994) Food packaging potential of some novel degradable starchepolyethylene plastics. J. Food Prot., 57, 1007–1012
Klug HP, Alexander LE (1974) Crystallite size and lattice strains from line broadening. In: X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials. New York, USA: Wiley-Intersciences, pp. 618–708
Köksel H, ahbaz F Özboy Ö (1983) Influence of wheat-drying temperatures on the birefringence and X-ray diffraction patterns of wet-harvested wheat starch. Cereal Chem. 70, 4, 481–483
Krochta JM, De Mulder-Johnson C (1997) Edible and biodegradable polymer films: challenges and opportunities. Food Technol. 51, 2, 61–77
Kumar MNVR (2000) A review of chitin and chitosan applications. React Funct. Polym. 46, 1–27
Laohakunjit N, Noomhorm A (2004) Effect of plasticizers on mechanical properties of rice starch film. Starch/Stärke 5, 8, 348–356
Mali S, Grossmann MVE (2003) Effects of yam starch films on storability and quality of fresh strawberries (Fragaria ananassa). J. Agric. Food Chem. 51, 24, 7005–7011
Mali S, Grossmann MV, García MA, Martino MN, Zaritzky NE (2002) Microstructural characterization of yam starch films. Carbohydr. Polym. 50, 4, 379–386
Mali S, Grossmann MV, García MA, Martino M, Zaritzky N (2005a) Mechanical and thermal properties of yam starch films. Food Hydrocol. 19, 157–164
Mali S, Sakanaka LS, Yamashita F, Grossmann MVE (2005b) Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr. Polym. 60, 283–289
Mallikarjunan P, Chinnan MS, Balasubramaniam VM, Phillips RD (1997) Edible coatings for deep-fat frying of starchy products. LWT 30, 709–714
Manning K (1993) Soft fruits. In: Seymour GB, Taylor JE, Tucker GA (Eds.) Biochemistry of Fruit Ripenning. Chapman & Hall, London, pp. 347–373
McHugh TH, Krochta JM (1994a) Milk-protein-based edible films and coatings. Food Technol. 48, 1, 97–103
McHugh TH, Krochta JM (1994b) Sorbitol- vs glycerol- plasticized whey protein edible films: integrated oxygen permeability and tensile property evaluation. J. Agric. Food Chem. 42, 4, 841–845.
McHugh TH, Krochta JM (1994c) Permeability properties of edible films. In: Krochta JM, Baldwin EA, Nisperos-Carriedo M (Eds.) Edible Coatings and Films to Improve Food Quality. Technomic Pub. Co., Lancaster, PA, pp 139–187
McHugh TH, Aujard JF, Krochta JM (1994) Plasticized whey protein edible films: water vapour permeability properties. J. Food Sci. 59, 2, 416–419
Miles MJ, Morris VJ, Ring SG (1985a) Gelation of amylose. Carbohydr. Res. 135, 257–269
Miles MJ, Morris VJ, Orford PD, Ring SG (1985b) The roles of amylose and amylopectin in the gelation and retrogradation of starch. Carbohydr. Res. 135, 271–281
Muzzarelli R, Baldassarre V, Conti F, Ferrara P, Biagini G, Gazzanelli G, Vasi V (1988) Biological activity of chitosan: ultrastructural study. Biomaterials 9, 3, 247–252
Neto CGT, Giacomettib JA, Jobb AE, Ferreirab FC, Fonsecaa JLC, Pereiraa MR (2005) Thermal analysis of chitosan based networks. Carbohydr. Polym. 62, 97–103
Nisperos-Carriedo MO (1994) Edible coatings and films based on polysaccharides. In: Krochta JM, Baldwin EA, Nisperos-Carriedo MO (Eds.) Edible Coatings and Films to Improve Food Quality. Technomic Pub. Co., Lancaster, PA, pp. 305–330
Noel TR, Ring SG, Whittman MA (1992) The structure and gelatinization of starch [Review]. Food Sci. Technol. Today, 6, 159
Oh JH, Wang B, Field PD, Aglan HA (2004) Characteristics of edible films made from dairy proteins and zein hydrolysate cross-linked with transglutaminase. Int. J. Food Sci. Technol. 39, 1–8
Olorunda AO, Aworh OC (1984) Effects of Pro-long, a surface coating agent, on the shelf-life and quality attributes of plantain. J. Sci. Food Agric. 35, 573–578
Park H, Weller C, Vergano P, Testin R (1993) Permeability and mechanical properties of cellulose-based edible films. J. Food Sci. 58, 6, 1361–1364, 1370
Park HJ, Bunn JM, Weller CL, Vergano PJ, Testin RF (1994) Water vapour permeability and mechanical properties of grain protein-based films as affected by mixtures of polyethylene glycol and glycerin plasticizers. Trans ASAE 37, 4, 1281–1285
Parra DF, Tadini CC, Ponce P, Lugão AB (2004) Mechanical properties and water vapour transmission in some blends of cassava starch edible films. Carbohydr. Polym. 58, 475–481
Parris N, Coffin DR, Joubran RF, Pessen H (1995) Composition factors affecting the water vapour permeability and tensile properties of hydrophilic films. J. Agric. Food Chem. 43, 1432–1435
Paull RE, Chen NJ (1989) Waxing and plastic wraps influence water loss from papaya fruit during storage and ripening. J. Am. Soc. Hortic. Sci. 114, 937–942
Pawlak A, Mucha M (2003) Thermogravimetric and FTIR studies of chitosan blends. Thermochimica Acta, 396, 153–166
Petersen K, Nielsen PV, Bertelsen G, Lawther M, Olsen MB, Nilssonk NH (1999) Potential of biobased materials for food packaging. Trends Food Sci. Technol. 10, 52–68
Pinotti A, García MA, Martino MN, Zaritzky NE (2007) Study on microstructure and physical properties of composite films based on chitosan and methylcellulose. Food Hydrocol. 21, 1, 66–72
Pommet M, Redl A, Guilbert S, Morel MH (2005) Intrinsic influence of various plasticizers on functional properties and reactivity of wheat gluten thermoplastic materials. J. Cereal Sci. 42, 81–91
Robertson GL (1993) Food Packaging. Principles and Practice. New York, NY: Marcel Dekker
Romero-Bastida CA, Bello-Pérez LA, García MA, Martino MN, Solorza-Feria J, Zaritzky NE (2005) Mechanical and microstructural characterization of films prepared by thermal or cold gelatinization of non-conventional starches. Carbohydr. Polym. 60, 2, 235–244
Roos YH (1995) Phase Transitions in Foods. Taylor, S.L. Academic Press San Diego, CA, USA
Sarantopoulos C, de Oliveira M, Padula Coltro L, Vercelino Alves RM, Correa García E (2002) Embalagens Plásticas Flexíveis. Centro de Tecnología de Embalagem. Campinas, Brasil
Shellhammer TH, Krochta JM (1997) Whey protein emulsion film performance as affected by lipid type amount. J. Food Sci. 62, 2, 390–394
Sionkowska A, Wisniewski J, Skopinska J, Kennedy CJ, Wess TJ (2004) Molecular interactions in collagen and chitosan blends. Biomaterials, 25, 795–801
Smith SA (1986) Polyethylene, low density. In: Bakker M. (Ed.) The Wiley Encyclopedia of Packaging Technology. John Wiley & Sons, New York, pp. 514–523
Smith JP, Hoshino J, Abe Y (1995) Interactive packaging involving sachet technology. In: Rooney ML (Ed.) Active Food Packaging. Blackie Academic and Professional, Glasgow, pp. 143–173
Smits ALM, Ruhnau FC, Vliegenthart JFG (1998) Ageing of starch based systems as observed by FT-IR and solid state NMR spectroscopy. Starch/Starke, 50, 11–12, 478–483
Snyder RL, Bish DL (1989) Quantitative analysis. In: Bish DL, Post JE (Eds.) Modern Powder Diffraction. Mineralogical Society of America, Washington, DC, pp. 101–144
Sobral PJA, Menegalli FC, Hubinger MD, Roques MA (2001) Mechanical, water vapour barrier and thermal properties of gelatin based edible films. Food Hydrocol. 15, 423–432
Sohail SS, Wang B, Biswas MAS, Oh JH (2006) Physical, morphological, and barrier properties of edible casein films with wax applications. J. Food Sci. 71, 4, 255–259
Spiess WEL, Wolf WR (1993) The results of the COST 90 project on water activity. In: Jowitt R, Escher F, Hallstrom FB, Meffert MF, Spiess WEL, Vos G (Eds.) Physical Properties of Foods. Applied Science Publishers, London, pp. 65–91
Stepto RFT, Tomka I (1987) Injection moulding of natural hydrophilic polymers in the presence of water. Chimia 41, 3, 76–81
Stewart CM, Tompkin RB, Cole MB (2002) Food safety: new concepts for the new millennium. Innov. Food Sci. Emerg. Technol. 3, 105–112
Tharanathan NR, Kittur SF (2003) Chitin-the undisputed biomolecule of great potential. Crit. Rev. Food Sci. 43, 61–83
Trezza TA Krochta JM (2000a) The gloss of edible coatings as affected by surfactants, lipids, relative humidity and time. J. Food Sci. 65, 4, 658–662
Trezza TA, Krochta JM (200b) Color stability of edible coatings during prolonged storage. J. Food Sci. 65, 7, 1166–1169
Trznadel M (1995) Biodegradable polymer materials. Int. Polym. Sci. Technol. 22, 12, 58–65
Van Soest JJG, Vliegenthart JFG (1997) Crystallinity in starch plastics: consequences for material properties. Trends Biotechnol. 15, 208–213
Van Soest JJG, Hulleman SHD, de Wit D, Vliegenthart JFG (1996) Crystallinity in starch bioplastics. Ind. Crops Prod. 5, 11–22
Vermeiren L, Devlieghere F, van Beest M, de Kruijf N, Debevere J (1999) Developments in the active packaging of foods. Trends Food Sci. Technol. 10, 77–86
Viña SZ, Mugridge A, García MA, Ferreyra RM, Martino MN, Chaves AR, Zaritzky NE (2007) Quality of refrigerated Brussels sprouts. Effect of plastic packaging film and edible starch coatings. Food Chem. 103, 701–709
Vojdani F, Torres JA (1989a) Potassium sorbate permeability of methylcellulose and hydroxypropylmethylcellulose multi-layer films. J. Food Process. Preserv. 13, 417–430
Vojdani F, Torres JA (1989b) Potassium sorbate permeability of polysaccharide films: chitosan, methylcellulose and hydroxypro-pylmethylcellulose. J. Food Process. Eng. 12, 33–48
Vojdani F, Torres JA (1990) Potassium sorbate permeability of methylcellulose and hydroxypropylmethylcellulose coatings: effect of fatty acids. J. Food Sci. 55, 3, 841–846
Wanchoo RK, Sharma PK (2003) Viscometric study on the compatibility of some water-soluble polymer-polymer mixtures. Eur. Polym. J. 39, 1481–1490
Were L, Hettiarachachy NS, Coleman M (1999) Properties of cysteine-added soy protein-wheat gluten films. J. Food Sci. 64, 3, 514–518
Williams R, Mittal GS (1999a) Water and fat transfer properties of polysaccharide films on fried pastry mix. LWT, 32, 440–445
Williams R, Mittal GS (1999b) Low-fat fried foods with edible coatings: modeling and simulation. J. Food Sci. 64(2), 317–322
Wong DWS, Gastineau FA, Gregorski KS, Tillin SJ, Pavlath AE (1992) Chitosan-lipids films: microstructure and surface energy. J. Agric. Food Chem. 40, 540–544
Wu T, Zivanovic S, Draughon FA Conway WS, Sams CE (2005) Physicochemical properties and bioactivity of fungal chitin and chitosan. J. Agric. Food Chem. 53, 3888–3894
Young AH (1984) Fractionation of Starch. In: Whistler RL, Be Miller JN, Paschall EF (Eds.) Starch, Chemistry and Technology, 2nd edn. Academic Press, Orlando, FL, USA, pp. 249–283
Zaccaron C, Oliveira R, Guiotoku M, Pires A, Soldi V (2005). Blends of hydroxypropyl methylcellulose and poly(1-vinylpyrrolidone-co-vinyl acetate): miscibility and thermal stability. Polym. Degrad. Stab. 90, 1, 21–27
Acknowledgments
The financial support provided by CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), Agencia Nacional de Promoción Científica y Tecnológica and Universidad Nacional de La Plata is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
García, M.A., Pinotti, A., Martino, M.N., Zaritzky, N.E. (2009). Characterization of Starch and Composite Edible Films and Coatings. In: Huber, K., Embuscado, M. (eds) Edible Films and Coatings for Food Applications. Springer, New York, NY. https://doi.org/10.1007/978-0-387-92824-1_6
Download citation
DOI: https://doi.org/10.1007/978-0-387-92824-1_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-92823-4
Online ISBN: 978-0-387-92824-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)