Abstract
Odor and fragrances can carry information about an organism and have long been suggested as vital mechanisms that affect or control animal behavior. The use of such chemical signals is widespread in the aquatic environment, and crustaceans such as lobsters, shrimps, crabs, barnacles, and crayfish are known to utilize odor for predator–prey interactions, mating, establishing of dominance or social hierarchies as well as hatching of young and settlement of larvae. Nevertheless, the chemical identity of these behavior-modifying odors remains largely unknown. Here, we briefly review the literature on crustacean chemical signals and describe our approach to identify these using the example of the shore crab (Carcinus maenas) sex pheromones. We describe the principles of a bioassay-driven purification that is combined with a metabolomic approach where differences in the odor profiles of sexually active and inactive crabs are examined. Using such an integrated approach, we identified the female produced signal to be a nucleotide with its production being linked with the female molt (ecdysis). The pheromone enables males to detect the optimal time to mate just after the female molt with the timing of the reproductive event enabling crabs to use a simple, not species-specific, chemical as a sex pheromone. Based on our recent findings, we discuss the implications for future studies on crustacean chemical signaling.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Background and Historical Overview
It has been known for many decades that odors influence animal behavior, including foraging, predator avoidance, alarm response, social dominance, cohort recognition, and courtship. Darwin (1871) initially proposed chemical signals as a key mechanism in mate choice by which sexual selection is promoted. However, it was not until the discovery of the silkworm moth pheromone “bombykol” by Butenandt et al. (1959) that “chemical ecology,” the study of chemical signals that modify an organism’s behavior, was born. To date, over 1,800 pheromones have been identified, but despite their widespread importance, few have been characterized in marine organisms (Wyatt 2003, 2009).
In 1986, JDH was approached by Professor E. Zeeck (Oldenburg University, Germany) upon the challenging idea of identifying a sex pheromone in a marine invertebrate – something that had not been done successfully at that time. Fascinated by the idea, JDH, along with Helga Bartels-Hardege, screened the literature to find a species that we could use. Already a year later, we managed to identify the first sex pheromone in a marine invertebrate and published the work in 1988 (Zeeck et al. 1988). Why did this work so fast? Essentially, there is no simple answer as luck was surely on our side when we decided to pursue the route of not neglecting volatile lipophilic compounds as potential pheromones, but the choice of species to attempt the identification was key in this particular case. A species was sought that had a well-described reproductive behavior to develop a “bioassay-driven purification and identification” strategy, where significant evidence for the role of sex pheromones existed, and where substantial amounts of a chemical cue could be collected.
John Terschak combined an ardor for analytical chemistry with that for the ocean. The School of Fisheries and Ocean Sciences at the University of Alaska Fairbanks supported his graduate work, and seminar attendance was encouraged, even though it was often unrelated to a chemist’s work. During one such talk on standing stocks of commercial crab species, a passing remark was made about a putative female pheromone that had not been isolated or been identified! Down the rabbit-hole he went! The moral: attend seminar, even if it seems unappealing – you never know what white rabbit you may follow or what curious adventure it may initiate. In this case, it led to our combined effort to identify a crustacean sex pheromone.
The key to bioassay-driven purification and identification strategies (see Fig. 19.1) is the recognition of the desired response expressed by the test subject; all too often, animal behavior is subtle or even ambiguous. Dunham, in two comprehensive reviews (Dunham 1978, 1988), attributed the slow progress in crustacean pheromone research to the unreliability of the bioassays employed. For example, the main problem of the frequently used assays based upon an animal’s attraction to an odor source is that it fails to discriminate sex pheromones from other signals such as food cues. Conversely, behavioral assays based on a distinctive sexual behavior, known as a “releaser reaction” (e.g., formation of a mating pair), are a more reliable means of investigating the role of sex pheromones (Dunham 1988; Hardege et al. 2002).
Flowchart of the bioassay-driven purification of chemical signals. Reproduced from Hardege (1999) with kind permission from Springer Science+Business Media
In addition to the ability to differentiate between sexual behaviors (e.g., courtship display) and signals (e.g., food attractants), it is important to employ a receiver that is physiologically and hormonally receptive to a sender’s pheromone (Hayden et al. 2007). Most animals live in complex chemical environments and an individual simultaneously detects multiple chemical signals from a variety of sources (Hazlett 1999, this volume), such as living and dead conspecifics, predators, competitors, and potential mates. From this cacophony, the receiver filters particular messages from the continuous chemical background noise to initiate behavioral responses; as such an animal’s response to chemical stimuli can vary.
Sensitivity to chemical stimuli varies with the internal physiological status of an animal. In crustaceans, this includes molt stage, reproductive status, dominance status, and hunger, all of which are affected by hormonal mechanisms. Physiological differences are often linked to the seasonality of events and controlled via environmental factors such as temperature, light intensity, photoperiod, and circadian cycles, which therefore also influence both the sender and receiver of the chemical signals (Tierney and Atema 1988; Derby 2000; Koehl 2006).
To maximize the effectiveness of signaling, especially for reproductive events, many organisms have developed mechanisms to increase the percentage of individuals in a population that are physiologically in a state to produce and respond to signals as seen, for example, in gregariousness of spiny lobsters (Ratchford and Eggleston 1998) and mass spawning in Nereis (Ram et al. 1999). This mechanism of signal enhancement ensures that more individuals produce sufficient signal strength in a turbulent aquatic environment. Such timing of events may be spectacular, such as the mass spawning events in coral reef invertebrates and the Palolo worm spawning in Western Samoa (Caspers 1984). Timing is achieved as all individuals in a population are exposed to the same environmental cues or undergo the same endocrine rhythms, synchronizing development of the same physiological characteristics.
Unfortunately, the complexity of factors that control an individual’s sensitivity toward chemical signals provides significant challenges in the development of biological assays producing reliable data that can be compared and reproduced. Ideally, most factors influencing an individual’s responses should be understood by the time an attempt is made to identify a pheromone (Fig. 19.2); this is hardly ever possible, but in general, the simpler the mechanisms that control an organism’s behavior, the more reliable an assay that can be developed.
Flow chart of environmental control of spawning in Nereis. “Timing” is used to describe factors influencing the maturation of a population; “Zeitgeber” is the terminus used to describe the factors controlling the date and location of the reproductive event; pheromone boxes show involvement of the chemical signals. Reproduced from Hardege et al. (1998) with kind permission from Ecoscience, Kanada
2 Nereidid Polychaetes as a Model for Marine Chemical Ecology
We began identifying marine invertebrate sex pheromones using polychaete worms (Platynereis dumerillii and Nereis succinea). These worms are semelparous, dying after a single reproductive event for which they undergo a metamorphosis into a reproductive stage, the heteronereis. This highly specialized heteronereis leaves its living tube and swims toward the water surface to locate a partner. Upon meeting the opposite sex, gametes are released into the free water column in a “nuptial dance” (Hardege et al. 1990). As a result of having only one opportunity to reproduce, these worms utilize a mass spawning event where a significant proportion of a population participates, thus maximizing the likelihood to meet a partner. The coordination of individual maturation and mass courtship relies on the simultaneous timing of complex environmental and endocrine factors. Because these worms are easy to culture, reproduce in the laboratory under controlled conditions, and their behavior is unambiguous (performance of a nuptial dance with an observable release of gametes), they are ideal organisms to identify sex pheromones through the use of straightforward bioassays (Zeeck et al. 1988; Hardege 1999).
Using a bioassay-driven purification strategy (Fig. 19.1), a number of sex pheromones were identified including such diverse molecules as ketones, uric acid, peptides, and l-ovothiol-A (Zeeck et al. 1988; Röhl et al. 1999; Ram et al. 1999). This has led to recent studies investigating the biological functions of pheromones, such as mate tracking and mate choice (Ram et al. 2008).
3 Sex Pheromones in Crustaceans
Crustacean pheromones have been the topic of a large number of studies since Ryan (1966) first demonstrated the existence of a female sex pheromone in Portunus sanguinolentus. The list of crustacean species for which there is evidence of a sex pheromone is extensive (see Hardege et al. 2002), but to date the chemical identity of these compounds remains largely unknown. Early studies suggested a pheromone role for the molting hormone 20-hydroxyecdysone (20HE) (Kittredge et al. 1971), as well as the peptide arthropodin (Dunham 1988). Both were dismissed based on experiments using a range of species, including Carcinus maenas (Eales 1974; Seifert 1982; Gleeson et al. 1984). The recent tentative identification of a novel ceramide in the hair crab Erimacrus isenbeckii (Asai et al. 2000) also proved inconclusive since behavioral assays did not support this theory and ceramides were not detectable at biologically relevant concentrations in urine (Asai et al. 2000). Biogenic amines, such as serotonin and dopamine, have been found to influence behavior in crustaceans (Beltz 1999). Wood and Derby (1996) showed that when injected directly into the hemolymph, dopamine induced the mating posture in the male blue crab (Callinectes sapidus), characteristic of its courtship display. Huber et al. (1997), through injection of radiolabelled serotonin, determined that it is not excreted in the urine, but its three major metabolic compounds are; as such, the role of biogenic amines as sex pheromones remains unclear. Some crustaceans, especially those that occur in high-density populations such as shrimps, may not require signals sent over a distance. Instead, they may use contact pheromones coating on the body surface for mate recognition (Zhang and Lin 2006), the nature of which remains unknown (see Bauer, Chap. 14).
In many crustaceans, females carry their young until the larvae hatch in unison with the female contracting its abdomen rapidly, a behavior known as the pumping response. Forward et al. (1987) identified tri-peptides from the eggs of the mud crabs (Rhithropanopeus harrisii) that induced this stereotypical hatching behavior and found the peptide to work at extremely low concentrations (Pettis et al. 1993).
Once the larvae of a marine crustacean are reaching the final stages of their planktonic life, they must find a suitable place to settle. Attractions toward and assessment of potential settlement sites possibly involve chemical settlement cues. Barnacles often form dense populations on manmade structures such as dock pilings as well as the hulls of ships causing increased drag and, consequently, significant operating costs in terms of an increased use of energy. Barnacle settlement was studied intensively, and the cuticular glycoprotein arthropodin could be identified as a gregarious settlement cue (Clare and Matsumura 2000).
The behavioral use of chemoreception in crustaceans is best investigated with respect to feeding stimulants with a large number of these cues identified in various species including shore crabs (Hayden et al. 2007; Weissburg, Chap. 4).
The use of novel compounds as chemical signals is actually unlikely, as this would require a de novo synthesis for the purpose of producing a chemical cue. With the multitude of behaviors mediated via chemical cues and the large number of species using these, especially insects, this would presumably require millions of unique compounds each with their own synthesis and receptor system (Wyatt 2003). Most chemical signals are related to the physiological state of the sender, such as freshly molted in many decapod crabs (Hardege et al. 2002). Signal specificity in such situations is often achieved through the use of multicomponent pheromone bouquets (Wyatt 2003) that may also include multimodal communication system such as visual or acoustic signals. Equally important could be that a complex behavior that involves multiple steps such as crustacean mating (i.e., attraction from distance, formation of a mating pair or mating stance, attempted copulation) is coordinated through a series of signals that provide a species-specific signaling code. This would prevent mistakes and allow for the use of a combination of simple, nonspecific compounds for every individual step of the process. This “chemical combination lock” type hypothesis is discussed recently in a review by Hay (2009; see also Hay, Chap. 3) and could, for example, involve contact pheromones as shown in shrimps (Caskey et al. 2009). Alternatively, as we have shown in nereidid polychaetes (Ram et al. 2008) external (mainly environmental) cues may bring about species specificity by separating breeding species in space and time, with “timing” negating the need for highly specific sex pheromones.
It seems evident that to achieve pheromone identification, a clear biological assay relying upon unambiguous behavior coupled with a chemical identification strategy, testing each successive purification step, is critical. Additionally, the use of biologically relevant samples such as “conditioned seawater” that contains compounds released into the environment at biologically relevant concentrations should be used.
4 Carcinus maenas, an Ideal Organism to Attempt Pheromone Identification
As described above, mating in the shore crab (C. maenas, synonym: European green crab) is restricted to the time of female molting (ecdysis) when males mate with soft-bodied females. The male guards the female several days before mating, defending her against predators and other competitor males by holding her under his abdomen in a cradling position (Fig. 19.3). It was hypothesized that guarding behavior is induced by sex pheromones emitted by the female (Ryan 1966; Ekerholm and Hallberg 2005). Copulation usually occurs a few hours after the molt (Hartnoll 1969), and it is therefore expected that elaborate methods to signal a potential mate have evolved to take advantage of the limited opportunity (Bamber and Naylor 1996).
Pair formation is a clear, unmistakable behavioral response, which can form the basis of a behavioral assay for pheromones. The coincidence of molt and reproduction further reduces the likelihood of other signals, such as feeding, to interfere with this behavior. The short window of mating opportunity also has two other important advantages. First, collecting pre-copula pairs provides specimens that are known to be both releasing as well as receptive to the signals; as such, the availability of a source and sender (female) for pheromones and a receiver (male) that is receptive to the cues is guaranteed (Hardege et al. 2002). The second major advantage of pair formation at the time of molt is the capacity to investigate potential chemical compounds, which are a direct result of the biochemistry associated with the current physiological state of the sender. For crustaceans such as C. maenas, the signal molecule could potentially be a metabolic by-product of the female molting process. This would focus the search to those compounds that change in the female’s body during the molt period, thus enabling a “metabolomic” approach (see Kamio and Derby, Chap. 20) as well as a bioassay-driven purification of the cue(s) concurrently.
5 Sex Pheromones in Carcinus maenas – The Current State of Knowledge
Our efforts to identify the female sex pheromone in the shore crab were not the first attempts to do so. As such, we initially focused on evaluating previously hypothesized cues (candidate approach). The molting hormone 20-hydroxyecdysone (also known as 20HE and crustecdysone) is linked to ovarian development and was proposed to function as a sex pheromone signal in several decapod species (Kittredge et al. 1971).
Although early studies indicated that 20HE functioned as a pheromone for species such as C. sapidus, later evidence showed that this is not the case; we also tested the compound and could not elicit pair formation (Hardege et al. 2002). Male crabs do detect 20HE, as is often reported; however, it is not the compound responsible for mate attraction. Instead, 20HE can be linked to a different ecological consequence of its molt cycle-related release into the environment; it functions as a sex-specific feeding deterrent emitted by females preventing cannibalism by males (Fig. 19.4; Tomaschko 1994; Hayden et al. 2007). As such, 20HE plays a role in the pheromone bouquet controlling the reproductive behavior of the shore crab, albeit not as an initiator of pair formation.
The role of 20HE as feeding deterrent in males. The crabs were exposed to increasing concentrations of the food attractant glycine (from 10−6 to 10−2 M) against a constant concentration of the potential feeding deterrent 20HE (10−4 M). Ten specimens per sex were tested for each data point, and the experiments were repeated four times (n = 40 per data point). Data presented show the mean and standard error of mean. 20HE increasingly inhibits feedings response at lower concentrations of glycine (Chi-Square, two-tailed, asterisk P < 0.01; double asterisk P < 0.001). Reproduced from Hayden et al. (2007) with kind permission from Elsevier
To identify the female-produced sex pheromone, we used a bioassay-driven purification similar to that for Nereis pheromones. We combined this with a literature-driven metabolomic approach focused primarily on the biochemistry associated with ecdysis. This was based on the hypothesis that compounds linked to the physiological changes that appear during the molt process would be prime candidates to signal the event to other individuals. As outlined earlier, the bioassay to unambiguously test for the sex pheromone activity of any purified compounds presented the first major hurdle. We chose the reproductive-specific cradling behavior (Fig. 19.3) to develop such an assay (Hardege et al. 2002). The premise was to replace the female with an object that bears as little resemblance to her as possible (to eliminate any possible visual cues) and “mask” this object with the “scent” of a female that is about to reproduce (i.e., has just molted) (see Fig. 19.5). Using only pairs collected in the field should then almost guarantee bioactive samples from the females and positive behavioral responses in the males.
Unlike the nereidid polychaetes that devote all their resources to reproduction, shore crabs maintain other behavioral and physiological functions. They are continuously exposed and respond to a variety of chemical stimuli, such as other individuals, feeding stimulants, and predator odor. Consequently, hunger, social interaction, and seasonality have significant effects upon pheromone communication in crustaceans (Hayden et al. 2007), introducing complex challenges for biological assays. Male responses to female cues are rarely completely efficient, mainly because the physiological states of receiving males vary greatly. For example, social hierarchies as described in lobster and crayfish (e.g., see Breithaupt, Chap. 13) result in dominant and subordinate males that potentially respond differently to females (Johansson and Jones 2007). A male’s age and size significantly affects its success in aggressive encounters and, potentially, in mate choice (Huntingford et al. 1995; Sneddon et al. 1997; Smallegange and van der Meer 2007). Exposure to pheromones, in turn, also affects male–male aggressive encounters (Sneddon et al. 2003; Fletcher and Hardege 2009).
With so many environmental, seasonal, and physiological factors affecting both sender and receiver, there exists an overarching need to standardize as many external parameters as possible when collecting pheromone samples and testing for pheromone activity. As such, we restricted bioassays to the main reproductive season (June to September) using mainly large, dominant males, as they were more likely to respond to female signals (Sneddon et al. 2003). Both negative (filtered sea water) and positive (female conditioned water) controls were used when testing fractions. Bioassays were also undertaken “blind,” with the observer of a test not informed as to the exact nature of the sample being tested. This was done to reduce observer bias. We used a variety of different behavioral and physiological assays to record pheromone activity, including electrophysiology, signal visualization, heart rate, and ventilation rate, to verify the different aspects of pheromone responses. Since pair formation and cradling behavior (see Fig. 19.3) represent a late step in the mating process prior to which the mating partners must first meet, we also included a classical olfactometer bioassay (see Fig. 10.2, Thiel, Chap. 10) to test distance functions (i.e., attraction) of the sex pheromone.
6 The Identification of Crustacean Sex Pheromones
There exist a number of plausible approaches to the chemical characterization of signal molecules. The definition of these is not entirely strict, and often an approach is chosen that includes elements from all these, but in principle, one can distinguish between:
-
(a)
Candidate approach: This method has been used in a number of pheromone studies in aquatic organisms, including fish (Stacey and Sorensen 2002), as well as in examining levels of biogenic amines in urine of crayfish during antagonistic behaviors. For this, synthetically available compounds that potentially function as pheromones are tested by exposing the target species to various cue concentrations. For example, sex hormone levels of fish increase during maturation in both sexes, and it was plausible that these steroids could signal status of maturity to the opposite sex and function as sex pheromones, and they should be released into the environment. Starting with goldfish (Carassius auratus), a number of steroid sex pheromones have now been characterized in a variety of fish species (Stacey and Sorensen 2006).
-
(b)
Metabolomic approach: This approach is based on significant advances over the past 20 years in the chemical analysis of natural products and the ability to use complex computational methods to compare odor fingerprints (Kamio and Derby, Chap. 20). It involves chemically screening a bioactive sample for compounds that are different (present, missing, or significantly changed in concentration) to a sample of similar origin that is not bioactive. Using crustaceans as an example, this could mean comparing urine of female crabs before the molt, at the time of the molt, and after the molt and examine which chemicals change. Once identified, these compounds would then be the ideal candidates for inclusion in bioassays. Despite its simplicity as an idea, examples of successful implementation are limited, as this approach is still in its infancy (Kamio and Derby, Chap. 20).
-
(c)
Bioassay-driven purification: This approach was used for the vast majority of pheromone identifications in terrestrial insects where the transmission medium (air) limits the variety of molecules that can be potential pheromones and where the purification processes, extraction and analysis via gas chromatography (GC), and GC-MS, are long established (see Wyatt 2003). Aquatic signals are less well understood; they are often water-soluble (polar) molecules and are difficult to isolate from the transmission medium (water). This is especially true for seawater where its ionic strength and buffer capacity interfere with standard chromatographic methods. The philosophy of a bioassay-driven purification is that the ultimate detector for a chemical signal is not an electronic sensor, but the animal itself. For purifications testing against the potential to invoke a response in the whole organism, it is the animal’s receptors that are validating a chemical’s bioactivity.
Although this seems logical, such an approach also bears a number of problems such as:
-
A proven bioassay is a prerequisite and with many organisms reproducing seasonally this could reduce the timeframe for analytical work.
-
Extraction of organic molecules often involves solvents that could interfere with an animal’s response when used in a bioassay.
-
Isolating separate compounds can reduce the bioactivity in a sample as a significant percentage of signals are made of a bouquet of compounds having synergistic effects; removing just one can reduce or even eliminate a response, making assays difficult to interpret.
Regardless of which strategy is used to identify pheromones, all share common problems that have created significant disagreements related to published pheromone structures. In chromatography for example, the largest peak is often not the most important in terms of pheromone activity, but likely to be an unrelated compound of an organism’s physiology; in bioassays the most active cue in the tests may not be the one found in the environment. For example, an active compound might be isolated from body fluids, but that compound may not be the one actually released into the environment.
To avoid the many pitfalls, we sought to acquire basic chemical knowledge on the stability, solubility, and size of the compounds before attempting pheromone identification. We designed a biological assay that tests for an unambiguous, fast releaser-type reaction, namely, the induction of the mate-guarding behavior. It is important to highlight that any bioassay needs to be fit for its purpose; when long-term changes in hormonal status are the biological effect (as in the induction of maturation via sex hormones in fish), such effects cannot be tested with an immediate releaser-type assay such as an animal’s behavior. To avoid this mistake, long-term assays with controlled exposure to cues over time are required, thus making these studies more complex.
Purification and concentration of chemical signals from aquatic sources carry a number of potential problems. A basic decision is to either start with a body fluid (urine, bile, macerates) that is likely to contain the chemical signal, or use “conditioned water” samples. The use of conditioned water has the advantage that, theoretically, only those compounds that are released into the environment can act as a chemical signal. Unfortunately for marine organisms, conditioned water samples contain substantial amounts of ionic compounds, mainly sodium chloride, which interferes with most chemical extraction techniques. Since the concentrations of released chemical signals are quite low, they are often below detection limits for analytical tools such as nuclear magnetic resonance (NMR) spectroscopy and even Mass spectrometry (MS). This requires the concentration of samples and the removal of salts, preferably at the same time. As seen for the Carcinus pheromone, the use of desalting media and gel chromatography unfortunately also removes the bioactive compounds (Hardege et al. 2002). Solid phase extraction is a promising technology, but cue stability (Röhl et al. 1999) and the limited solubility of potential pheromone compounds in organic solvents may continue to make sample preparation difficult for some time. Furthermore, many compounds show little or no UV or fluorescence absorbance commonly used in high performance liquid chromatography (HPLC) peak detection, and others, such as carbohydrates, are difficult to ionize in MS.
The extraction of pheromones from body fluids brings different challenges, as these are literally a chemical cocktail containing a massive diversity of compounds. A majority of these compounds are unrelated to the pheromone or may never be released into the environment. Additionally, some compounds may interfere with each other or serve as receptor antagonists or agonists and change receptor responses. Moreover, cues are often only produced during specific communication events such as the induced alarm pheromone production in aphids (Pickett and Griffiths 1980) and sex pheromones in polychaetes (Röhl et al. 1999), thus all that can be found in body fluids are the biological precursors of the actual cues.
For the purification of compounds, methods including molecular filtration, solid phase extraction (SPE, SPME), solvent extraction, and a variety of basic chromatographic techniques (thin layer, low pressure, ion exchange, size exclusion, etc.), HPLC, and GC (with derivatization of nonvolatile compounds) can be used. Additionally, instrumentation to identify compounds is available, such as the different spectrometric applications, including infrared (IR), mass (MS), ultra-violet and visible (UV-Vis), and NMR spectroscopy. In recent years, the so-called “hyphenated techniques” (combined chromatographic and spectral methods such as GC-MS and LC-MS) have produced powerful new analytical tools providing the separation, detection, identification, and quantification of analytes. Specifically for chemical signals, these applications can even be combined with biological assays employing electrophysiological detectors that make direct use of an organism’s olfactory system, as is done with electro-antennograms (EAG), electroantennographic detectors (EAD), and electro-olfactograms (EOG).
For anyone attempting pheromone identification, this diverse range of techniques and potential pitfalls represents a difficult and complex challenge to select the appropriate purification, fractionation, and identification strategies relevant to the compounds under investigation.
7 The Identification of Sex Pheromones in the Shore Crab, Carcinus maenas
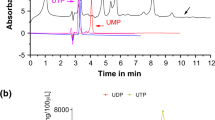
For the purification of the female sex pheromone in C. maenas, we began with “conditioned seawater” as well as whole urine samples (Hardege et al. 2002). Aliquots of these samples were then ultrafiltered to eliminate large molecules, such as proteins, that would most likely not serve as a pheromone compound. The filtered samples were then separated via HPLC using a Phenomenex Synergi Fusion RP column (4.6 × 250 mm). This provided separation from the unretained inorganic salt peak when using an isocratic mobile phase of 0.2 M KH2PO4 (pH 5.5) with a flow rate of 1 mL/min at 28°C (Hardege, unpulished data). We used an Agilent 1100 HPLC system with quaternary pump, degasser, autosampler (100 μL loop), temperature controlled column compartment, and DAD (diode-array detector) scanning between 200 and 300 nm. Once we had purified and identified the cue using supplemental data from LC-MS and NMR, we verified its identity using a synthetic analog by coinjecting it onto a number of different HPLC columns (Hardege, unpublished data). This coinjection of synthetic compounds enabled a positive identification of the compound as the nucleotide, uridine diphosphate (UDP) (Fig. 19.6).
HPLC analysis of the female-produced sex pheromone isolated from female conditioned seawater (Hardege, unpublished data). The chromatogram shows the HPLC analysis of female C. maenas urine at 2-day post-moult (black line), and synthetic pheromone, UDP (red line). HPLC conditions used were: Phenomenex RP Fusion column (4.6 × 250 mm); mobile phase: 0.2 M KH2PO4 buffer, pH 5.5; 1 mL min−1. Synthetic pheromone UDP (for structure see insert) was at a concentration of 10−5 M. The unresolved shoulder peak in the female sample at 3.3 min represents both tautomeric forms of UDP
Synthetic UDP and nucleotide analogs were tested using both olfactometry and guarding stance assays (Hardege et al. 2002) with responses being elicited at biologically appropriate concentrations of 10−5 M (Hardege unpublished data). UDP was found to be not only an attractant but also induces the mating behavior, thus representing the first chemically characterized crustacean sex pheromone (Hardege, unpublished data). When examining the seasonality of feeding and sex pheromone responses in shore crabs, we had found earlier that only during the summer mating season do significant percentages of males respond to female cues (Hayden et al. 2009). Similarly, synthetic nucleotides elicit a full response in males only within the summer reproductive season (Hardege, unpulished data). Through the use of electrophysiological assays, we found that males are still able to detect UDP in autumn and spring, but no behavioral responses were elicited upon exposure to UDP outside the normal summer mating period.
Willig (1974) measured the molting hormone (20HE) levels in the hemolymph and urine of female shore crabs over the course of the molt cycle. Unlike the pheromone release pattern that increases slightly after molt (Bamber and Naylor 1996), 20HE levels drop dramatically in the days just prior to ecdysis. As male shore crabs detect both compounds, the receiver (male) could gain information on the molt stage of the female and use it for mate choice. The concept of the receiver of a chemical message being able to utilize the information to time a response is known in male fish (Sorensen and Stacey 1999) and is considered a form of “chemical spying.” Chemical spying and male mate choice are quite plausible in Carcinus since it has been shown that males differ in quality (mainly size) and position in social hierarchies (Sneddon et al. 1997, 2003).
Since female pheromone production levels are directly linked to the molt stage, we also investigated whether the female pheromone UDP could be a metabolic by-product of the female molting process – specifically chitin biosynthesis. Figure 19.7 shows that the final step of chitin biosynthesis from UDP-N-acetylglucosamine releases UDP. As such, increasing levels of UDP in female urine should exist postmolt and signal to males that a female is approaching the end of the period during which they can mate. As shown by Bamber and Naylor (1996), this is the period when females are most attractive to males.
Biosynthesis of Chitin – a potential source of the nucleotide pheromone UDP. Modified and corrected from Mansecal (1999)
As social hierarchies are well known in crustaceans, we also tested the responses of males toward synthetic pheromone after they were exposed to male–male encounters. We found that winners and loser of fights respond differently to female sex pheromone (Fletcher and Hardege 2009). While winners of fights took slightly longer after a fight before responding to female cues than if not subjected to a bout, losers often did not respond to female pheromone at all and, even if responding, took significantly longer before approaching (Fig. 19.8). Since female crabs also show multiple paternity of their egg clutches (Thonet, unpublished data), investment in mate guarding may not be the same for males of different social status. Van der Meeren (1994) showed that dominant males gather in mating territories (hotspots) and smaller males roam in the vicinity attempting to intercept females that move toward these mating grounds. After forming a pair, the couple usually moves away from hotspots, and males are known to bury their female partners in the sediment, presumably as a mechanism to prevent further transmission of pheromone that, in turn, would attract male competitors; this movement is particularly important for small males in possession of a female, because they could be easily displaced by large males (Sneddon et al. 2003). It remains to be studied whether the first male to mate fathers the majority of the offspring. Additionally, it is unknown if large, dominant males roam and mate first, last, or just before the female hardens. The most effective mating strategy may vary based on a number of factors yet unknown (Fletcher and Hardege 2009). Responses of receivers of signals vary, and the complexities in sender–receiver relationships will be both challenging and fascinating to study using identified synthetic cues (Johansson and Jones 2007).
Responses of subordinate and dominant males to synthetic nucleotide pheromone UDP at 10−5 M. Data show time interval between pheromone presentation and response for prefight (filled square) and postfight males (open square). Reproduced from Fletcher and Hardege (2009) with kind permission from Elsevier
UDP is a relatively widespread molecule as it occurs in almost all organisms. If produced as a secondary metabolite during the molt process, it should have relatively little species specificity. Table 19.1 shows that a number of species, but not all, responded to UDP (Bublitz et al. 2008). As such, we propose that the Carcinus pheromone is reaction-specific, alerting a male to the presence of a molting female. Species-specificity is most likely based on environmental timing ensuring premating species isolation. Since its production is linked to the message (molt), is energetically costly (UDP is an energy carrier) and might even attract potential predators, it should qualify as an honest signal (Zahavi and Zahavi 1997) indicating the physiological state and quality of the sender (Hardege, unpublished data).
8 Future Implications and Follow-Up Research Potential
Having identified UDP as the first crustacean sex pheromone, we can now use this knowledge to design experiments that further our understanding of the behavior and evolution of chemical communication in crustaceans. While the identification of nucleotide pheromones represents a major breakthrough in marine chemical ecology, it is evident that more structural information on crustacean pheromones is needed before we can fully address interesting questions such as the evolution of chemical signals. The use of glutathione derivatives as sex pheromones in nereidid polychaetes (Ram et al. 1999) and of nucleotides in a crustacean (Hardege, unpublished data), both of which are known feeding stimulants (Hayden et al. 2007), shows that one potential route for chemical signals to evolve is from feeding cues (Macías-Garcia and Ramirez 2005).
An emerging field with respect to marine organisms is the potential influence of environmental and ecological factors such as pollution on chemical signaling. Interference with chemical signaling systems, or “pheromone disruption,” may occur within any of the stages of signaling, either during biosynthesis, release, transmission through the environment, reception, or signal transduction. Endocrine-disrupting chemicals are already known to leach into the environment through waste treatment facilities and, other means (both point- and non-point-sources) and interfere with the hormone systems of a variety of animals (Rodríguez et al. 2007). These well-studied pollutants could potentially also have dramatic consequences for chemical communication in crustaceans and other marine species (Beckmann et al. 1995).
Knowledge of the chemical structures of pheromones and their exact biological functions potentially allows their use in population management, especially in aquaculture and pest control (Barki et al., Chap. 25). Well established for insects in terrestrial systems (Wyatt 2003; Baker, Chap. 27), integrated pest management approaches in aquatic systems have not yet been widely pursued, with the current exception of the control of sea lampreys in the Great Lakes (Li et al. 2002; Wagner et al. 2006; Chung-Davidson et al., Chap. 24). This first, promising step for aquatic systems could potentially be followed by a similar endeavor for crustaceans, especially Carcinus, which is a major globally invasive marine species threatening endemic species, including those in the pristine environments of Alaska and Australia (Grosholz and Ruiz 1995). Integrated pest management utilizing chemical cues such as attractants and deterrents could be one mechanism to slow their spread.
For decades, pheromone research in aquatic organisms, especially in marine systems, has lagged behind the immense progress made in terrestrial insects and vertebrates. It is encouraging to see that significant progress is now being made in systems such as fish pheromones, brown algae, polychaetes, nudibranchs (Li et al. 2002; Hardege 1999; Painter et al. 1998) and, most recently, in crustaceans. With increasing knowledge of purification and extraction strategies, it can be expected that more breakthroughs will follow, enabling new evolutionary, physiological, neurobiological, and molecular studies that will only add depth to this exciting and fast-growing field of research.
References
Asai N, Fusetani N, Matsunaga S, Sasaki J (2000) Sex pheromones of the hair crab Erimacrus isenbeckii. Part 1: isolation and structures of novel ceramides. Tetrahedron 56:9895–9899
Bamber SD, Naylor E (1996) Chemical communication and behavioral interaction between sexually mature male and female shore crabs (Carcinus maenas). J Mar Biol Assoc UK 76:691–699
Beckmann M, Hardege JD, Zeeck E (1995) Effects of the volatile fraction of crude oil on the reproductive behaviour of nereids (Annelida, Polychaeta). Mar Environ Res 40:267–276
Beltz BS (1999) The distribution and functional anatomy of amine containing neurons in decapod crustaceans. Microsc Res Tech 44:105–120
Bublitz R, Sainte-Marie B, Newcomb-Hodgetts C, Fletcher N, Smith M, Hardege JD (2008) Interspecific activity of sex pheromone of the European shore crab (Carcinus maenas). Behaviour 145:1465–1478
Butenandt A, Beckmann R, Stamm D, Hecker E (1959) Über den Sexuallockstoff des Seidenspinners Bombyx mori. Reindarstellung und Konstitution. Z Naturforsch 14:283–284
Caskey JD, Hasenstein KH, Bauer RT (2009) Studies on contact sex pheromones of the caridean shrimp Palaemonetes pugio: I. Cuticular hydrocarbons associated with mate recognition. Inv Reprod Dev 53:93–103
Caspers H (1984) Spawning periodicity and habitat of the Palolo worm Eunice viridis (Polychaeta: Eunicidae) in the Samoan Islands. Mar Biol 79:229–236
Clare AS, Matsumura K (2000) Nature and perception of barnacle settlement pheromones. Biofouling 15:57–71
Darwin C (1871) The descent of man, and sexual selection in relation to sex. John Murray, London
Derby CD (2000) Learning from spiny lobsters about chemosensory coding of mixtures. Physiol Behav 69:203–209
Dunham PJ (1978) Sex pheromones in Crustacea. Biol Rev 53:555–583
Dunham PJ (1988) Pheromones and behaviour in Crustacea. In: Laufer H, Downer R (eds) Endocrinology of selected invertebrate types. Alan R. Liss, New York, pp 375–392
Eales AJ (1974) Sex pheromone in the shore crab Carcinus maenas, and the site of its release from females. Mar Behav Physiol 2:345–355
Ekerholm M, Hallberg E (2005) Primer and short-range releaser pheromone properties of premolt female urine from the shore crab Carcinus maenas. J Chem Ecol 31:1845–1864
Fletcher N, Hardege JD (2009) The cost of conflict: agonistic encounters influence responses to chemical signals in the shore crab, Carcinus maenas. Anim Behav 77:357–361
Forward RB Jr, Ritttschof D, De Vries MC (1987) Peptide pheromones synchronize crustacean egg hatching and larval release. Chem Senses 12:491–498
Gleeson RA, Adams MA, Smith AB (1984) Characterisation of a sex pheromone in the blue crab Callinectes sapidus. J Chem Ecol 10:913–921
Grosholz ED, Ruiz GM (1995) Spread and potential impact of the recently introduced European green crab, Carcinus maenas, in central California. Mar Biol 122:239–247
Hardege JD (1999) Nereid polychaetes as model organism for marine chemical ecology: a review. Hydrobiologia 402:145–161
Hardege JD, Bartels-Hardege HD, Zeeck E, Grimm FT (1990) Induction of swarming in Nereis succinea. Mar Biol 104:291–295
Hardege JD, Mueller CT, Beckmann M, Bartels-Hardege HD, Bentley MG (1998) Timing of reproduction in marine polychaetes: the role of sex pheromones. Ecoscience 5:395–404
Hardege JD, Jennings A, Hayden D, Müller CT, Pascoe D, Bentley MG, Clare AS (2002) Novel behavioural assay and partial purification of a female-derived sex pheromone in Carcinus maenas. Mar Ecol Prog Ser 244:179–189
Hartnoll RG (1969) Mating in the Brachyura. Crustaceana 16:161–181
Hay ME (2009) Marine chemical ecology: chemical signals and cues structure marine populations, communities, and ecosystems. Annu Rev Mar Sci 1:193–212
Hayden D, Jennings A, Mueller C, Pascoe D, Bublitz R, Webb H, Breithaupt T, Watkins L, Hardege JD (2007) Sex specific mediation of foraging in the shore crab, Carcinus maenas. Horm Behav 52:162–168
Hazlett BA (1999) Responses to multiple chemical cues by the crayfish Orconectes virilis. Behaviour 136:161–177
Huber R, Smith K, Delago A, Isaksson K, Kravitz EA (1997) Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc Natl Acad Sci U S A 94:5939–5942
Huntingford FA, Taylor AC, Smith IP, Thorpe KE (1995) Behavioural and physiological studies of aggression in swimming crabs. J Exp Mar Biol Ecol 193:21–39
Johansson BG, Jones TM (2007) The role of chemical communication in mate choice. Biol Rev 82:265–289
Kittredge JS, Terry M, Takahashi FT (1971) Sex pheromone activity of the moulting hormone crustecdysone on male crabs (Pachygrapsus crassipes, Cancer antennarius and Cancer anthonyi). Fish Bull 69:337–343
Koehl MAR (2006) The fluid mechanics of arthropod sniffing in turbulent odor plumes. Chem Senses 31:93–105
Li W, Scott AP, Siefkes MJ, Yan H, Liu Q, Yun SS, Gage DA (2002) Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science 296:138–141
Macías-Garcia C, Ramirez E (2005) Evidence that sensory traps can evolve into honest signals. Nature 434:501–505
Mansecal R (1999) Chitin. In: Polymer data handbook. Mark JE (ed) Oxford University Press, New York, pp 67–69
Painter S, Clough B, Garden RW, Sweedler JV, Nagle GT (1998) Characterization of Aplysia attraction, the first water-borne peptide pheromone in invertebrates. Biol Bull 194:120–131
Pettis RJ, Erickson BW, Forward RB, Rittschof D (1993) Superpotent synthetic tripeptide mimics of the mud-crab pumping pheromone. Int J Pept Protein Res 42:312–319
Pickett JA, Griffiths DC (1980) Composition of aphid alarm pheromones. J Chem Ecol 6:349–360
Ram JL, Mueller CT, Beckmann M, Hardege JD (1999) The spawning pheromone cysteine-glutathione disulfide (“Nereithione”) arouses a multicomponent nuptial behaviour and electrophysiological activity in Nereis succinea males. FASEB J 13:945–952
Ram JL, Fei X, Danaher SM, Lu S, Breithaupt T, Hardege JD (2008) Finding females: pheromone-guided reproductive tracking behavior by male Nereis succinea in the marine environment. J Exp Biol 211:757–765
Ratchford SG, Eggleston DB (1998) Size- and scale-dependent chemical attraction contribute to an ontogenetic shift in sociality. Anim Behav 56:1027–1034
Rodríguez EM, Medesani DA, Fingerman M (2007) Endocrine disruption in crustaceans due to pollutants: a review. Comp Biochem Physiol A 146:661–671
Röhl I, Schneider B, Schmidt B, Zeeck E (1999) L-Ovothiol A: the egg release pheromone of the marine polychaete Platynereis dumerilii: Annelida: Polychaeta. Z Naturforsch 54:1145–1147
Ryan EP (1966) Pheromone: evidence in decapod Crustacea. Science 151:340–341
Seifert P (1982) Studies on the sex pheromone of the shore crab, Carcinus maenas, with special regard to ecdysone excretion. Ophelia 21:147–158
Smallegange IM, Van der Meer J (2007) Interference from a game theoretical perspective: shore crabs suffer most from equal competitors. Behav Ecol 18:215–221
Sneddon LU, Huntingford FA, Taylor AC (1997) Weapon size versus body size as a predictor of winning in fight between shore crabs, Carcinus maenas. Behav Ecol Sociobiol 41:237–242
Sneddon LU, Huntingford FA, Taylor AC, Clare AS (2003) Female sex pheromone-mediated effects on behaviour and consequences of male competition in the shore crab (Carcinus maenas). J Chem Ecol 29:55–70
Sorensen PW, Stacey NE (1999) Evolution and specialization in fish hormonal pheromones. In: Johnston RE, Müller-Schwarze D, Sorensen PW (eds) Advances in chemical signals in vertebrates. Kluwer Publishers, Amsterdam, pp 15–47
Stacey N, Sorensen P (2002) Hormonal pheromones in fish. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT (eds) Non-mammalian hormone-behavior system. Harcourt Publishers, London, pp 375–434
Stacey NE, Sorensen P (2006) Reproductive pheromones. In: Sloman KA, Balshine S, Wilson RI (eds) Behaviour and physiology of fish. Elsevier, Amsterdam, pp 359–412
Tierney AJ, Atema J (1988) Amino-acid chemoreception – effects of pH on receptors and stimuli. J Chem Ecol 14:135–141
Tomaschko KH (1994) Ecdysteroids from Pycnogonum litorale (Arthropoda, Pantopoda) act as a chemical defense against Carcinus maenas (Crustacea, Decapoda). J Chem Ecol 20:1445–1455
Van der Meeren GI (1994) Sex- and size-dependent mating tactics in a natural population of shore crabs Carcinus maenas. J Anim Ecol 63:307–314
Wagner CM, Jones ML, Twohey MB, Sorensen PW (2006) A field test verifies that pheromones can be useful for sea lamprey (Petromyzon marinus) control in the Great Lakes. Can J Fish Aquat Sci 63:475–479
Willig A (1974) Die Rolle der Ecdyosne im Häutungszyklus der Crustaceen. Fortschr Zool 22:55–74
Wood DE, Derby CD (1996) Distribution of dopamine-like immunoreactivity suggests a role for dopamine in the courtship display behavior of the blue crab, Callinectes sapidus. Cell Tissue Res 285:321–330
Wyatt TD (2003) Pheromones and animal behavior. Cambridge University Press, Cambridge
Wyatt TD (2009) Fifty years of pheromones. Nature 457:262–263
Zahavi A, Zahavi A (1997) The handicap principle: a missing piece in Darwin’s puzzle. Oxford University Press, Oxford
Zeeck E, Hardege JD, Bartels-Hardege HD, Wesselmann G (1988) Sex pheromone in a marine polychaete: determination of the chemical structure. J Exp Zool 246:285–292
Zhang D, Lin J (2006) Mate recognition in a simultaneous hermaphroditic shrimp, Lysmata wurdemanni (Caridea: Hippolytidae). Anim Behav 71:1191–1196
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Hardege, J.D., Terschak, J.A. (2010). Identification of Crustacean Sex Pheromones. In: Breithaupt, T., Thiel, M. (eds) Chemical Communication in Crustaceans. Springer, New York, NY. https://doi.org/10.1007/978-0-387-77101-4_19
Download citation
DOI: https://doi.org/10.1007/978-0-387-77101-4_19
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-77100-7
Online ISBN: 978-0-387-77101-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)