Abstract
Early in postnatal development, correlated activity in the hippocampus is characterized by giant depolarizing potentials (GDPs). GDPs are generated by the interplay between glutamate and GABA, which in the immediate postnatal period is depolarizing and excitatory. Here, we review some recent data obtained in our laboratory concerning neuronal signaling at immature MF connections. MF responses were identified on the basis of their strong paired-pulse facilitation, short-term frequency-dependent facilitation and sensitivity to group III mGluR agonist L-AP4. Unlike adulthood, during the first week of postnatal life minimal stimulation of MF evoked responses that were potentiated by flurazepam and abolished by picrotoxin indicating that they were GABAergic. In addition, using a pairing procedure we found that GDPs and associated calcium transients act as coincident detectors for enhancing synaptic efficacy at poorly developed MF-CA3 and MF-interneurons connections. This may be crucial for synaptogenesis and for establishing the adult neuronal circuit.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 γ-Aminobutiric Acid (GABA) Plays a Crucial Role in Developmental Networks
GABA is the main inhibitory transmitter in the adult mammalian CNS. It reduces cell excitability by activating GABAA receptor channels, which are mainly permeable to chloride ions. In addition, GABA inhibits neuronal firing by acting on GABAB receptors coupled to potassium or calcium channels (Cherubini and Conti, 2001). GABA plays a crucial role in many physiological processes including network synchronization and generation of theta and gamma rhythms, thought to be associated with higher cognitive functions (Buzsaki and Draguhn, 2004). Dysfunction of GABAergic signaling leads to several neurological disorders, including epilepsy which is triggered by the unbalance between excitation and inhibition (Roberts, 1986). Interestingly, in the immediate postnatal period, when glutamatergic synapses are still poorly developed (Hosokawa et al., 1994; Tyzio et al., 1999), GABA depolarizes and excites target cells through an outwardly directed flux of chloride (Cherubini et al., 1991; Ben-Ari et al., 1997; Ben-Ari, 2002; Owens and Kriegstein, 2002; Mohajerani and Cherubini, 2005). The intracellular chloride concentration is under control of two main Cl– co-transporters the NKCC1 and KCC2 that enhance and lower [Cl–]i, respectively (Payne et al., 2003). Due to the low expression of the KCC2 extruder at birth, chloride accumulates inside the neuron via NKCC1. The progressive increase in the expression of KCC2 is responsible for the developmental shift of GABA from the depolarizing to the hyperpolarizing direction (Rivera et al., 1999). In the immature hippocampus, the depolarizing action of GABA which occurs well before synapses formation (Demarque et al., 2002) enables the induction of synchronized activity, the so called giant depolarizing potentials or GDPs, which consist in recurrent membrane depolarizations with superimposed fast action potentials, separated by quiescent intervals (Ben-Ari et al., 1989). GDPs which have been proposed to be the in vitro counterpart of “sharp waves” recorded in rat pups during immobility periods, sleep and feeding (Leinekugel et al., 2002) can be considered a primordial form of synchrony between neurons, which precedes more organized forms of activity such as the theta and the gamma rhythms (Buzsaki and Draguhn, 2004). Correlated network activity constitutes a hallmark of developmental networks, well preserved during evolution that has been observed not only in the hippocampus but in almost every brain structure, including the retina (Feller et al., 1997), the neocortex (Owens et al., 1996; Dammerman et al., 2000; Maric et al., 2001), the hypothalamus (Chen et al., 1996), the cerebellum (Yuste and Katz, 1991; Eilers et al., 2001) and the spinal cord (Wang et al., 1994; O’Donovan, 1999).
The depolarizing action of GABA during GDPs results in calcium influx through the activation of voltage-dependent calcium channels and N-methyl-D-aspartate (NMDA) receptors (Leinekugel et al., 1997; Garaschuk et al., 1998). GDPs and associated calcium transients lead to the activation of intracellular signaling pathways thought to contribute to several developmental processes including DNA synthesis, cell migration, morphological maturation and synaptogenesis (Owens and Kriegstein, 2002). More recently, GDPs and associated calcium transients have been shown to act as coincident detectors for enhancing synaptic efficacy at poorly developed synapses (Kasyanov et al., 2004; Mohajerani et al., 2007).
How GDPs are generated is still a matter of debate. In the disinhibited hippocampus, population synchrony has been proposed to depend on an active process consisting in a build up period during which synaptic traffic and cell firing exceeds a certain threshold (Menendez de la Prida et al., 2006). Functionally excitatory synaptic interactions would facilitate neuronal synchronization and the initiation of population bursts (Traub and Wong, 1982; Miles and Wong, 1987; Traub and Miles, 1991). A similar process may be involved in the generation of GDPs early in postnatal life (Menendez de la Prida and Sanchez-Andres, 1999; 2000) when synaptic interactions are facilitated by the excitatory action of GABA (Cherubini et al., 1991; Ben-Ari, 2002).
From the above mentioned examples it emerges that GABA is one of the major players in neuronal development.
In this chapter we will review some recent data obtained in our laboratory demonstrating that, during the first week of postnatal life, the axons of dentate gyrus granule cells, the mossy fibers, which in adult are glutamatergic, release into CA3 pyramidal cells and GABAergic interneurons mainly GABA. In addition, we will provide evidence that GABA-mediated GDPs act as coincidence detectors for enhancing synaptic efficacy at mossy fiber-CA3 synapses.
6.2 Mossy Fiber Synapses
The axons of granule cells have been originally called “mossy” by Ramon y Cajal because of their particular appearance at the light microscopic level that reminds, as the mossy fibers in the cerebellum, the shape of the moss on trees (Ramon y Cajal, 1911). Thus, unlike other principal cells, mossy fibers (MF) give rise to large en passant swellings and terminal expansions on CA3 principal neurons or mossy cells seen as giant boutons at the electron microscopic level. These presynaptic swellings adapt very well to specialized postsynaptic elements present on proximal dendrites of CA3 principal cells, called thorny excrescences. The MF synaptic complex contains multiple active zones (up to 50) associated with postsynaptic densities. In addition MF make synaptic contacts with GABAergic interneurons present in the hilus and in the CA3 area and these represent the majority of all MF connections (Frotscher et al., 2006). In a seminal paper, Acsády et al. (1998) demonstrated that, MF connections with interneurons have either the shape of small boutons or filopodial extensions. Differences in morphology between MF terminals at principal cells and interneurons may account for the distinct functional properties of these synapses which appear to be regulated in a target specific way (Nicoll and Schmitz, 2005). Interestingly, at principal cell synapses, giant boutons develop gradually during the first 21 days (Amaral and Dent, 1981). A light and electron microscopic study has shown that, during the first postnatal days, at the time when our study was performed, immature axons terminate in very small, spherical expansions, which establish both symmetric and asymmetric contacts with pyramidal cell dendrites (Fig. 6.1).
Schematic representation of a mossy fiber (blue) making synaptic contacts with a GABAergic interneuron (red) and a pyramidal cell (green) during the first week of postnatal life (P0–P6) and in adulthood. (Modified from Amaral and Dent, 1981). (See Color Plate 4)
These contacts are made several days before the development of thorny excrescences (Stirling and Bliss, 1978; Amaral and Dent, 1981). Expansions markedly increase in size by day 9 while maintaining a relatively spherical shape. During this period, pyramidal cell dendrites show a marked lateral growth and fingers which began indenting into MF expansions. This period is also characterized by an increased number and densities of synaptic vesicles.
In adults, the MF input to CA3 is glutamatergic and comprises the second synapse of the classical trisynaptic hippocampal circuit. Glutamate acts mainly on postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors (Henze et al., 2000). It is stored in synaptic vesicles with zinc, which is co-released with glutamate upon nerve stimulation and is known to down regulate both N-methyl-D-aspartate and GABAA receptors (Westbrook and Mayer, 1987). Besides glutamate, MFs release other substances including dynorphin, known to modulate glutamate release via presynaptic receptors (Weisskopf et al., 1993). The latter is stored on large dense-core vesicles, which also contain other peptides such as enkephalins, cholecystokinin and neuropeptide Y (Henze et al., 2000). Moreover, MF are endowed with a variety of different receptors (autoreceptors) whose tonic activation has been shown to depress or enhance transmitter release, respectively. Thus, activation of A1 adenosine receptors (Moore et al., 2003), GABAB receptors (Hirata et al., 1992) and type II/III metabotropic glutamate receptors (mGluR,) reduces transmitter release (Kamiya et al., 1996; Shigemoto et al., 1997) while activation of kainate receptors facilitates transmitter release (Schmitz et al., 2001).
In pathological conditions, MFs can release GABA in addition to glutamate. Thus, in the hippocampus of epileptic animals (Gutierrez and Heinemann, 2001; Romo-Parra et al., 2003) monosynaptic GABAergic inhibitory postsynaptic potentials (IPSPs) occur in principal cells in response to dentate gyrus stimulation. Seizures are associated with a transient upregulation of the GABAergic markers GAD65 and GAD67 (Schwarzer and Sperk, 1995; Sloviter et al., 1996) as well as the mRNA for the vesicular GABA transporter, VGAT (Lamas et al., 2001). Interestingly, both GAD67 and its product GABA appear to be constitutively expressed in MF. Further evidence suggests that MFs can release glutamate and GABA also in physiological conditions. Hence, Walker et al. (2001) and Gutierrez et al. (2003) have demonstrated the presence of both monosynaptic GABAergic and glutamatergic responses following activation of granule cells in the dentate gyrus in juvenile guinea pigs and rats. While these pieces of work will be the object of other chapters in this book, here we will focus on the first week of postnatal life when the main neurotransmitter released by the MF has been found to be GABA. It should be stressed that, due to the complexity of dentate gyrus-CA3 circuitry (Henze et al., 2000), studying pure MF synaptic responses with electrophysiological approaches is quite difficult. This task is, at least partially facilitated in neonatal animals where the small size of neurons and the relatively low extension of dendritic branches allow good space clamp conditions.
6.3 Criteria for Identifying Single Mossy Fiber Responses
The best way for studying synaptic transmission at given synapses is to record simultaneously from interconnected pre and postsynaptic cells. However, in the case of MF synapses this approach is very difficult due to the very low probability of finding interconnected granule cells and CA3 pyramidal neurons. An alternative approach consists in recording single fiber responses (Jonas et al., 1993; Allen and Stevens, 1994). With this technique, a small stimulating electrode is placed in the granule cell layer and the stimulation intensity is decreased until only a single axon is activated. This is achieved when the mean amplitude of the postsynaptic currents and failure probability remain constant over a range of stimulus intensities near threshold for detecting a response. Small movements of the stimulating electrode 20–30 μm away from the initial location, lead to the loss of the evoked response. The example of Fig. 6.2 shows average traces of synaptic currents evoked in a CA3 principal cell in response to stimulation of granule cells in the dentate gyrus with different intensities. An abrupt increase in the mean peak amplitude of synaptic currents can be detected by increasing the strength of stimulation. This all-or-none behavior suggests that only a single granule cell is stimulated. When the stimulation intensity is turned down the probability of failures in synaptic transmission is near 1.
(a) Unitary synaptic currents evoked in a P3 CA3 principal cell by minimal stimulation of granule cells in the dentate gyrus. Each trace is the average of 15–20 responses. Holding potential –70 mV. The peak amplitude of synaptic currents represented in A is plotted against different stimulus intensities in (b). Note the all-or-none appearance of synaptic currents with increasing stimulus intensities. Bars are SEM. Dashed lines connect the mean values of individual points within the same group. (c), (d) Latency and rise time distributions of individual currents evoked in another CA3 pyramidal cell in the presence of a solution containing a Ca2+/Mg2+ ratio of 2:1.3 (C) or 1:3 (D). Note the unimodal distributions of latencies and rise times of individual responses that did not change when the extracellular Ca2+/Mg2+ ratio was changed. Modified from Safiulina et al. (2006)
In addition, the latency and the shape of individual synaptic responses should remain constant for repeated stimuli. Between P0 and P6, MF-evoked synaptic currents occurred with a latency of 3.8 ± 0.3 ms in principal cells and 3.4 ± 0.4 ms in interneurons. The latencies distribution was unimodal and narrow with an average standard deviation of 0.31 ± 0.03 ms (n = 10; Fig. 6.2). Moreover, the latency as well as the rise time of synaptic responses remained constant when the extracellular Ca2+/Mg2+ concentration ratio was reduced from 2:1.3 to 1:3 (Fig. 6.2) further supporting the monosynaptic nature of synaptic currents. The 10–90% rise time was 3.5 ± 0.8 ms in principal cells (n=12) and 3.1 ± 0.4 ms in interneurons (n= 8; see also Walker et al., 2001).
MF inputs to principal cells or interneurons were identified on the basis of their sensitivity to group III mGluR agonist 2-amino-4-phosphonobutyric acid (L-AP4; Fig. 6.3 ). In this regard, neonatal rats behave differently from adult animals which are sensitive to: (2S,2'R,3'R)-2-(2',3'-Dicarboxycyclopropyl)glycine (DCG-IV) but insensitive to L-AP4 (Lanthorn et al., 1984). It should be stressed, however, that both group II and III mGluRs have been found on rat MF terminals: while group III mGluRs are located predominantly in presynaptic active zones, group II are in preterminal rather than terminal portions of axons (Shigemoto et al., 1997). The inability of DCG-IV in modulating MF responses in immature neurons can be attributed to the different expression and/or location of group II/III mGluRs early in postnatal development. One intriguing aspect to be considered is how mGluRs are activated early in postnatal days, when MFs seem to release only GABA. One possibility is that they are constitutively activated by ambient glutamate present in the extracellular medium. However, more work should be done in order to elucidate this point.
Minimal stimulation of granule cells in the dentate gyrus evokes GABAA-mediated monosynaptic responses in CA3 pyramidal cells. (a) Amplitude of synaptic responses (dots) evoked by stimulation of granule cells in the dentate gyrus are plotted against time in control, during bath application of flurazepam 3 μM (FLZM), L-AP4 (10 μM) and picrotoxin 100 μM (PTX). The inset above show examples of average traces, taken in different experimental conditions (each is the average of at least 20 traces including failures). Note that the currents were enhanced by FLZM, reduced in amplitude by L-AP4 and blocked by PTX. (b) Normalized responses obtained in the absence (1) or in the presence (2) of FLZM. Note the slow down of the deactivation kinetics of synaptic currents with FLZM. (c) Each column represents the mean amplitude (± SEM) of MF-evoked synaptic currents recorded from 5 CA3 principal cells in control, in the presence of FLZM, L-AP4 and PTX. (d) Decay kinetics (τ mean) of synaptic currents recorded in control and in the presence of FLZM (n = 5) × p<0.05
Interestingly, monosynaptic inhibitory responses obtained in principal cells by stimulating GABAergic interneurons were found to be insensitive to mGluR agonists (Walker et al., 2001; Doherty et al., 2004; Kasyanov et al., 2004; Gutierrez, 2005; Fig. 6.5). L-AP4-sensitive interneurons have been described in guinea pig hippocampus (Semyanov and Kullmann, 2000). Here, L-AP4, at the concentration of 50 μM (5 times higher than that used in the present experiments) was able to reduce the amplitude of IPSCs of ˜50%. This raises the possibility that different mGluR subtypes are expressed in different animal species.
L-AP4-insensitive synaptic currents elicited in principal cells by stimulation of the granule cells in the dentate gyrus. (a) Left: diagram showing a GABAergic interneurons impinging into a CA3 pyramidal cell. Right: average of 20 individual responses (including failures) to paired stimuli. (b) Average of 20 individual traces evoked in control conditions, in the presence of L-AP4 and L-AP4 plus PTX. Note lack of effect of L-AP4. (c) Average of 20 individual traces from another pyramidal cell obtained in control or in the presence of FLZM. On the right the two normalized traces are superimposed. (d) Summary data showing the amplitude of synaptic currents obtained from 4 pyramidal cells in different experimental conditions normalized to the respective controls (dashed line). Modified from Safiulina et al. (2006)
MF inputs were also identified on the basis of their strong paired pulse facilitation and short-term frequency-dependent facilitation (Salin et al., 1996). Strong paired pulse facilitation was observed particularly at MF-CA3 synapses, which were often “silent” in response to the first stimulus. At MF-interneuron synapses both paired pulse facilitation and depression were observed while at interneuron-CA3 or interneuron-interneuron synapses the most common feature was paired pulse depression (Fig. 6.5).
The degree of frequency-dependent facilitation is another peculiar aspect of MF responses which probably depends on the enhanced probability of neurotransmitter release following the large rise of intraterminal calcium concentration and activation of calcium/calmodulin-dependent kinase II (Salin et al., 1996). Alternatively, synaptic facilitation may occur as the consequence of the progressive and local saturation of calcium by the endogenous fast calcium buffer calbindin, which is highly expressed in MF terminals. This would produce a gradual increase in calcium concentration at releasing sites (Blatow et al., 2003). In our case, frequency facilitation occurred already when the stimulation frequency was shifted from 0.05 to 0.3 Hz. However, the increment in size of synaptic responses was larger at MF-CA3 principal cell connections than at MF-interneuron synapses (see also Toth et al., 2000).
6.4 GABA Is the Main Neurotransmitter Released by MF Early in Postnatal Life
As shown in the illustrative example of Fig. 6.3, minimal stimulation of granule cells in the dentate gyrus was able to evoke in CA3 principal cells monosynaptic currents that were completely blocked by picrotoxin or bicuculline, suggesting that they were mediated by GABAA receptors. As classical MF responses, synaptic currents exhibited strong paired pulse facilitation. Moreover, they were highly sensitive to L-AP4 and underwent short-term frequency-dependent facilitation. As expected for GABAA-mediated responses MF-evoked synaptic currents were potentiated by NO-711, a blocker of the GABA transporter GAT-1 and by flurazepam, an allosteric modulator of GABAA receptors.
Similar results were found for MF making synaptic contacts with GABAergic interneurons. In this case however, in agreement with previous findings (Toth et al., 2000), changing the stimulation frequency from 0.05 to 0.33 Hz, induced only a moderate facilitation of synaptic responses. This can be attributed to the fact that, in contrast with principal cells, MF contacting interneurons comprise only a single release site (Acsady et al., 1998). In a few cases, a depression of synaptic currents was also observed.
Pressure application of glutamate to granule cells dendrites in stratum moleculare (in the presence of the AMPA/kainate receptor antagonist 6,7-Dinitroquinoxaline-2,3-dione (DNQX) to prevent the recruitment of GABAergic interneurons) induced in target cells barrages of L-AP4 sensitive currents that were completely abolished by picrotoxin. It is therefore likely that activation of NMDA receptors localized on granule cells dendrites in stratum moleculare causes a membrane depolarization and the release of GABA from MF terminals. This was supported by the observation that, blocking NMDA receptors with D-(-)-2-Amino-5-phosphonopentanoic acid (D-APV) prevented the effects of chemical stimulation of granule cell dendrites on CA3 principal cells and GABAergic interneurons. Moving the pressure pipette few μm away toward the hilus to activate hilar interneurons caused barrage of synaptic currents that were insensitive to L-AP4 but were blocked by picrotoxin, implying that they were mediated by the release of GABA from GABAergic interneurons.
Additional fibers releasing both glutamate and GABA into CA3 principal cells and interneurons could be recruited by increasing the strength of stimulation (Safiulina et al., 2006). In comparison with MF-induced GABAergic currents, glutamatergic responses occurred with a shorter latency. However, these responses involved MF synapses, since they were reversibly depressed by L-AP4 and were abolished when DNQX (10 μM) was added to picrotoxin.
In additional experiments a low chloride intracellular solution (ECl –90 mV) was used to simultaneously record AMPA- and GABAA-mediated synaptic currents at room temperature (22–24°C) to avoid the activation of polysynaptic pathways and GDPs. Thus, at –50 mV, AMPA-mediated synaptic responses were detected as inward currents while GABAA-mediated responses as outward currents (Fig. 6.4 ). The Figure shows also that, in comparison with AMPA-mediated synaptic currents, those mediated by GABA occurred more frequently. While at –80 mV, close to the chloride reversal potential, pure AMPA-mediated responses could be detected, at –30 mV pure GABAA-mediated synaptic responses. Both components were sensitive to L-AP4 and were blocked by the respective receptor antagonists.
Glutamatergic and GABAergic currents evoked in principal cells by stimulation of the granule cells in the dentate gyrus at room temperature (to avoid the activation of GDPs; see Ben-Ari et al. 1989). (a) Average responses evoked at three different holding potentials in a CA3 pyramidal cell recorded with a low chloride intrapipette solution (ECl –90 mV). Note the biphasic currents at –50 mV and the isolated GABAergic and glutamatergic components at –30 and –80 mV, respectively. The two components were reduced in amplitude by L-AP4 and were selectively blocked by the AMPA and GABAA receptor blockers, SYM-2206 (20 μM) and PTX (100 μM), respectively (b) Individual traces from one single cell recorded at –50 mV showing inward, outward, biphasic responses and response failures (c) Summary data (n=5) showing the mean amplitude of GABAergic (black columns) and glutamatergic (white columns) currents in control, in the presence of L-AP4, L-AP4 plus SYM-2206 and L-AP4 plus SYM-2206 and picrotoxin. (d) Each column represents the relative frequency of each type of response for 3 cells. Modified from Safiulina et al. (2006)
Synaptic currents fluctuated between outward, biphasic and inward and were intermingled with response failures. This suggests that GABA and glutamate can be released independently from the same fiber.
The possibility that the same fiber can release different neurotransmitters has been well documented in several brain structures including the retina (O’ Malley and Masland, 1989) and the spinal cord (Jonas et al., 1998). In particular, GABA has been reported to be released from excitatory inputs in CA3 pyramidal cells (Walker et al., 2001; Gutierrez et al., 2003) whereas glutamate from inhibitory terminals in the lateral superior olive in the developing auditory system (Gillepsie et al., 2005).
In contrast with MF responses, synaptic currents mediated by GABA released from GABAergic interneurons were insensitive to L-AP4 and DNQX but were blocked by bicuculline or picrotoxin (Fig. 6.5 ). These responses were probably generated by interneurons projecting to principal cells or interneurons sending collaterals to the granule cells into the dentate gyrus. They occurred with high probability and exhibited a strong paired-pulse depression in response to two closely spaced stimuli. Moreover, in comparison with MF responses they were potentiated by flurazepam in a more pronounced way (Fig. 6.5).
Altogether these experiments are in line with previous reports showing the sequential expression of GABAergic and glutamatergic synapses early in postnatal development (Hosokawa et al., 1994; Tyzio et al., 1999) and clearly demonstrate that GABA is the main neurotransmitter released from MFs during postnatal development.
Further evidence in favor of GABA as a transmitter at MF synapses is the observation that the vesicular GABA transporter VGAT was found in MF terminals (Safiulina et al., 2006; see also Chaudhry et al., 1998). However, in order to generate synaptic responses, GABA should not only be present in synaptic vesicles and released in an activity-dependent manner but should bind to postsynaptic GABAA receptors. Although evidence for the presence of GABAA receptors facing immature MF terminals early in development is still lacking, a previous study on adult rats has demonstrated the presence of AMPA and GABAA receptors co-localized in front of the respective active zones (Bergersen et al., 2003). GABA may also spill out to activate neighboring extrasynaptic GABAA receptors localized away from the release sites as suggested for juvenile guinea pigs and rats (Walker et al., 2001). However, the relatively fast rise time of GABAergic responses found in the present experiments, similar to that of glutamatergic synaptic currents makes this hypothesis unlikely.
Overall, these data support the hypothesis that early in development, MF contain two different sets of low and high threshold fibers releasing GABA and GABA plus glutamate respectively. While the first would disappear with maturation the second would persist longer or would reappear in pathological conditions such as in epilepsy (Walker et al., 2001; Gutierrez et al., 2003). Comparable to our results it has been recently shown that minimal stimulation of the granule cell layer in hippocampal slices obtained from P4–P6 mice evoked GABAergic currents that were insensitive to 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), D-APV but were blocked by picrotoxin (Uchigashima et al., 2007). According to these authors, these currents were due to GABA released from low threshold GABAergic interneurons. It should be stressed however, that unlike putative MF responses described here, which occurred with very low probability (responses were often silent to first pulse) and exhibited strong short-term frequency-dependent facilitation (see Fig. 6.6 ), those reported by Uchigashima et al. (2007) displayed only minimal facilitation.
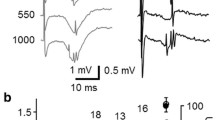
Pairing-induced the appearance of synaptic responses in “presynaptically silent neurones” (a) GDPs recorded from a CA3 pyramidal cell in current clamp mode from the hippocampus of a P2 old rat. On the right, a single GDP is shown on an expanded time scale. Note the absence of spikes riding on the top of GDP due to block of the sodium channel with QX 314. The rising phase of GDPs (between the dotted lines) was used to trigger synaptic stimulation (Stim). (b) Amplitudes of synaptic responses (dots) evoked by minimal stimulation of MF before and after pairing (arrow at time 0) are plotted against time. The traces above the graph represent individual responses evoked before and after pairing in different experimental conditions as indicated. This synapse was considered “presynaptically” silent because did not exhibit any response to the first stimulus over 48 trials (at 0.1 Hz) but occasional (two) responses to the second one. For clarity after “pairing” only synaptic responses evoked by the first stimulus are shown. C and D. Mean excitatory postsynaptic current (EPSC) amplitude (c) and mean percentage of successes (d) before and after pairing for the cell shown in B. Modified from Kasyanov et al. (2004)
Paired recordings from granule cells and postsynaptic neurons would probably allow better understanding how MF establish their contact at early developmental stages. However, we cannot exclude the possibility that, during the first week of postnatal life, synaptic currents evoked by minimal stimulation of granule cell in the dentate gyrus are generated by GAD or GAD mRNA positive interneurons, which early in development migrate to the upper and middle portions of the granule layer cells (Dupuy and Houser, 1997) while maintaining all the functional properties of later appearing glutamatergic MF responses.
6.5 GDPs as Coincidence Detectors for Enhancing Synaptic Efficacy at Low Probability MF-CA3 Synapses
To assess whether synchronized network activity such as GDPs are able to modify MF connections in an associative type of manner, we have developed a “pairing” procedure consisting of correlating GDPs-associated calcium rise in the postsynaptic cell with stimulation of MF (Kasyanov et al., 2004). To this purpose, after a control period of 5–10 min, the patch was switched from voltage-clamp to current-clamp mode and MF responses were paired for 5 min with GDPs. “Pairing” consisted in triggering MF stimulation with the rising phases of GDPs (Fig. 6.6). After this period the patch was switched back to voltage clamp mode and synaptic currents were recorded as in control. As illustrated in Fig. 6, in the case of presynaptically silent synapses (Gasparini et al., 2000), the pairing protocol caused the appearance of responses to the first stimulus and increased the number of successes to the second one.
In the case of non-silent low probability synapses, the pairing procedure produced a strong and persistent potentiation of MF responses, which was associated with a significant increase in the number of successes and in double pulse experiments, with a significant reduction in the paired-pulse ratio and a significant increase in the inverse squared value of the coefficient of variation. This suggests that an increased probability of transmitter release accounts for the persistent increase in synaptic efficacy.
In the absence of pairing no significant changes in synaptic efficacy could be detected. Moreover, when the interval between GDPs and MF stimulation was progressively increased, the potentiation declined and reached the control level when presynaptic signals were activated 2–3 s after GDPs (Kasyanov et al., 2004). In addition we found that pairing-induced long-lasting increase in synaptic efficacy was prevented when cells were loaded with the calcium chelator BAPTA or when nifedipine was added to the extracellular medium. In contrast, the NMDA receptor antagonist D-APV failed to prevent pairing-induced potentiation, indicating that early in postnatal life calcium rise through voltage-dependent calcium channel activated by the depolarizing action of GABA during GDPs is the common trigger for activity-dependent changes in synaptic efficacy.
In conclusions, during development, coincident detection provided by GDPs can be important for enhancing synaptic transmission at emerging MF-CA3 connections in a Hebbian type of way. How this may contribute to the wiring and proper assembly of adult networks remains to be determined.
Abbreviations
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CNS:
-
Central Nervous System
- CNQX:
-
6-cyano-7-nitroquinoxaline-2,3-dione
- D-APV:
-
D-(-)-2-Amino-5-phosphonopentanoic acid
- DCG-IV:
-
(2S,2'R,3'R)-2-(2',3'-Dicarboxycyclopropyl)glycine
- DNQX:
-
6,7-Dinitroquinoxaline-2,3-dione
- GAT-1:
-
a high-affinity GABA plasma membrane transporter
- GABA:
-
γ-Amino-butyric acid
- GAD:
-
glutamic acid decarboxylase
- GDPs:
-
Giant Depolarizing Potentials
- IPSC:
-
inhibitory postsynaptic current
- KCC2:
-
neuronal Potassium-Chloride cotransporter
- L-AP4:
-
2-amino-4-phosphonobutyric acid
- MF:
-
Mossy fibers
- mGluR:
-
metabotropic glutamate receptors
- NKCC1:
-
Sodium, Potassium Chloride cotransporter
- NMDA:
-
N-methyl-D-aspartate
- P:
-
postnatal day
- VGAT:
-
Vesicular GABA Transporter
References
Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G (1998) GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci 18:3386–3403
Allen C, Stevens CF (1994) An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci U S A 91:10380–10383
Amaral DG, Dent JA (1981) Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol 195:51–86
Ben-Ari Y (2002) Excitatory actions of GABA during development: the nature of the nurture. Nature Rev Neurosci 3:728–739
Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL (1989) Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol (Lond) 416:303–325
Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL (1997) GABAA, NMDA and AMPA receptors: a developmentally regulated “menage a trois”. Trends Neurosci 20:523–529
Bergersen L, Ruiz A, Bjaalie JG, Kullmann DM, Gundersen V (2003) GABA and GABAA receptors at hippocampal mossy fibre synapses. Eur J Neurosci 18:931–941
Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A (2003) Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron 38:79–88
Buzsaki G, Draguhn A. (2004) Neuronal oscillations in cortical networks. Science 304:1926–1929
Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J (1998) The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci 8:9733–9750
Chen G, Trombley PQ, van den Pol AN (1996) Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol 494:451–464
Cherubini E, Gaiarsa JL, Ben-Ari Y (1991) GABA: an excitatory transmitter in early postnatal life. Trends Neurosci 14:515–519
Cherubini E and Conti F (2001) Generating diversity at GABAergic synapses. Trends Neurosci 24:155–162
Dammerman RS, Flint AC, Noctor S, Kriegstein AR (2000) An excitatory GABAergic plexus in developing neocortical layer 1. J Neurophysiol 84:428–434
Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y and Aniksztejn L (2002) Paracrine intercellular communication by a Ca2+ – and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron 36:1051–1061
Doherty JJ, Alagarsamy S, Bough KJ, Conn PJ, Dingledine R, Mott DD (2004) Metabotropic glutamate receptors modulate feedback inhibition in a developmentally regulated manner in rat dentate gyrus. J Physiol 561:395–401
Dupuy ST, Houser CR (1997) Developmental changes in GABA neurons of the rat dentate gyrus: an in situ hybridization and birthdating study. J Comp Neurol 389:402–418
Eilers J, Plant TD, Marandi N, Konnerth A (2001) GABA-mediated Ca2+ signalling in developing rat cerebellar Purkinje neurones. J Physiol 536:429–437
Feller MB, Butts DA, Aaron HL, Rokhsar DS, Shatz CJ (1997) Dynamic processes shape spatiotemporal properties of retinal waves. Neuron 19:293–306
Frotscher M, Jonas P, Sloviter RS (2006) Synapses formed by normal and abnormal hippocampal mossy fibers. Cell Tissue Res 326:361–367
Garaschuk O, Hanse E, Konnerth A (1998) Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol (London) 507:219–236
Gasparini S, Saviane C, Voronin LL, Cherubini E (2000) Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release? Proc Natl Acad Sci U S A 97:9741–9746
Gillespie DC, Kim G, Kandler K (2005) Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci 8:332–338
Gutierrez R (2005) The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci 28:297–303
Gutierrez R, Heinemann U (2001) Kindling induces transient fast inhibition in the dentate gyrus--CA3 projection. Eur J Neurosci 13:1371–1379
Gutierrez R, Romo-Parra H, Maqueda J, Vivar C, Ramirez M, Morales MA, Lamas M (2003) Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J Neurosci 23:5594–5598
Henze DA, Urban NN, Barrionuevo G (2000) The multifarious hippocampal mossy fiber pathway: a review. Neuroscience 98:407–427
Hirata K, Sawada S, Yamamoto C (1992) Quantal analysis of suppressing action of baclofen on mossy fiber synapses in guinea pig hippocampus. Brain Res 578:33–40
Hosokawa Y, Sciancalepore M, Stratta F, Martina M and Cherubini E (1994) Developmental changes in spontaneous GABAA-mediated synaptic events in rat hippocampal CA3 neurones. Eur J Neurosci 6:805–813
Jonas P, Major G, Sakmann B (1993) Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol 472:615–663
Jonas P, Bischofberger J, Sandkuhler J (1998) Corelease of two fast neurotransmitters at a central synapse. Science 281:419–424
Kamiya H, Shinozaki H, Yamamoto C (1996) Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol 493:447–455
Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E (2004) GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc Natl Acad Sci USA 101:3967–3972
Lamas M, Gomez-Lira G, Gutierrez R (2001) Vesicular GABA transporter mRNA expression in the dentate gyrus and in mossy fiber synaptosomes. Brain Res Mol Brain Res 93:209–214
Lanthorn TH, Ganong AH, Cotman CW (1984) 2-Amino-4-phosphonobutyrate selectively blocks mossy fiber-CA3 responses in guinea pig but not rat hippocampus. Brain Res 290:174–178
Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R (1997) Ca2+ oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron 18:243–255
Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y and Buzsaki G (2002) Correlated bursts of activity in the neonatal hippocampus in vivo. Science 296:2049–2052
Maric D, Liu QY, Maric I, Chaudry S, Chang YH, Smith SV, Sieghart W, Fritschy JM, Barker JL (2001) GABA expression dominates neuronal lineage progression in the embryonic rat neocortex and facilitates neurite outgrowth via GABA(A) autoreceptor/Cl- channels. J Neurosci 21:2343–2360
Menendez de la Prida L, Sanchez-Andres JV (1999) Nonlinear frequency-dependent synchronization in the developing hippocampus. J Neurophysiol 82:202–208
Menendez de la Prida L, Sanchez-Andres JV (2000) Heterogeneous populations of cells mediate spontaneous synchronous bursting in the developing hippocampus through a frequency-dependent mechanism. Neuroscience 97:227–241
Menendez de la Prida LM, Huberfeld G, Cohen I, Miles R (2006) Threshold behavior in the initiation of hippocampal population bursts. Neuron 49:131–142
Miles R, Wong RKS (1987) Latent synaptic pathways revealed after tetanic stimulation in the hippocampus. Nature 329:724–726
Mohajerani MH and Cherubini E (2005) Spontaneous recurrent network activity in organotypic rat hippocampal slices. Eur J Neurosci 22:107–118
Mohajerani MH, Sivakumaran S, Zacchi P, Aguilera P, Cherubini E (2007) Correlated network activity enhances synaptic efficacy via BDNF and the ERK pathway at immature CA3–CA1 connections in the hippocampus. Proc Natl Acad Sci U S A 104:13176–13181
Moore KA, Nicoll RA, Schmitz D (2003) Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A 100:14397–14402
Nicoll RA, Schmitz D (2005) Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci 6:863–876
O’Donovan MJ (1999) The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol 9:94–104
O’Malley DM, Masland RH (1989) Co-release of acetylcholine and gamma-aminobutyric acid by a retinal neuron. Proc Natl Acad Sci U S A 86:3414–3418
Owens DF, Boyce LH, Davis MBE, Kriegstein AR (1996) Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci 16:6414–6423
Owens DF, Kriegstein AR (2002) Is there more to GABA than synaptic inhibition? Nature Rev Neurosci 3:715–727
Payne JA, Rivera C, Voipio J, Kaila K (2003) Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci 26:199–206
Ramon y Cajal SR (1911) Histologie du Système Nerveux de l’Homme et des Vertébrés,vol. II. Maloine, Paris
Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, et al (1999) The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–255
Roberts E (1986) Failure of GABAergic inhibition: a key to local and global seizures. Adv Neurol 44:319–341
Romo-Parra H, Vivar C, Maqueda J, Morales MA, Gutierrez R (2003) Activity-dependent induction of multitransmitter signaling onto pyramidal cells and interneurons of hippocampal area CA3. J Neurophysiol 89:3155–3167
Salin PA, Scanziani M, Malenka RC, Nicoll RA (1996) Distinct short-term plasticity at two excitatory synapses in the hippocampus Proc Natl Acad Sci U S A 93:13304–13309
Safiulina VF, Fattorini G, Conti F, Cherubini E (2006) GABAergic signaling at mossy fiber synapses in neonatal rat hippocampus. J Neurosci 26:597–608
Schmitz D, Mellor J, Nicoll RA (2001) Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001 Mar 9; 291(5510):1972–1976
Schwarzer C, Sperk G (1995) Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat. Neuroscience 69:705–709
Semyanov A, Kullmann DM (2000) Modulation of GABAergic signaling among interneurons by metabotropic glutamate receptors. Neuron 25:663–672
Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N (1997) Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci 17:7503–7522
Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL (1996) Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol 373:593–618
Stirling RV, Bliss TV (1978) Hippocampal mossy fiber development at the ultrastructural level. Prog Brain Res 48:191–198
Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ (2000) Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci 20:8279–8289
Traub RD, Wong RK (1982) Cellular mechanism of neuronal synchronization in epilepsy. Science 216:745–747
Traub RD, Miles R (1991) Multiple modes of neuronal population activity emerge after modifying specific synapses in a model of the CA3 region of the hippocampus. Ann N Y Acad Sci 627:277–290
Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H and Aniksztejn L (1999) The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci 19:10372–10382
Uchigashima M, Fukaya M, Watanabe M, Kamiya H (2007) Evidence against GABA release from glutamatergic mossy fiber terminals in the developing hippocampus. J Neurosci 27:8088–8100
Walker MC, Ruiz A, Kullmann DM (2001) Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron 29:703–715
Wang J, Reichling DB, Kyrozis A, MacDermott AB (1994) Developmental loss of GABA- and glycine-induced depolarization and Ca2+ transients in embryonic rat dorsal horn neurons in culture. Eur J Neurosci 6:1275–1280
Weisskopf MG, Zalutsky RA, Nicoll RA (1993) The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature 362:423–427
Westbrook GL, Mayer ML (1987) Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature 328:640–643
Yuste R, Katz LC (1991) Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron 6:333–344
Acknowledgments
The authors are grateful to Drs. A. Kasyanov, G. Fattorini and F. Conti for participating in some experiments. The original research work was supported by grants from Ministero Istruzione, Universita’, Ricerca (MIUR, Italy) and the European Union.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Safiulina, V.F., Mohajerani, M.H., Sivakumaran, S., Cherubini, E. (2009). GABA is the Main Neurotransmitter Released from Mossy Fiber Terminals in the Developing Rat Hippocampus. In: Gutierrez, R. (eds) Co-Existence and Co-Release of Classical Neurotransmitters. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-09622-3_6
Download citation
DOI: https://doi.org/10.1007/978-0-387-09622-3_6
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-0-387-09621-6
Online ISBN: 978-0-387-09622-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)