Abstract
Over the last 30 years, adenosine 5’-triphosphate (ATP) has been clearly established as a cotransmitter with noradrenaline and acetylcholine in the peripheral nervous system. More recently, ATP was also identified as a cotransmitter in the central nervous system. In neuronal cultures from postnatal rat spinal cord dorsal horn or embryonic chick and postnatal mouse lateral hypothalamus, ATP was surprisingly found to be synaptically coreleased with the inhibitory neurotransmitter GABA, but not with glutamate. In these preparations, ATP activates excitatory cation-permeable receptors (P2X receptors), whereas GABA stimulates anion-permeable inhibitory GABAA receptors. The corelease of ATP and GABA therefore results in a fast mixed excitatory/inhibitory ATP/GABA cotransmission. Here we review the current knowledge on ATP/GABA cotransmission, the possible interactions of synaptically released ATP and GABA at pre- and postsynaptic sites and discuss the issues related to the role of this mixed cotransmission in the context of physiological and pathological situations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
The possibility that nerve fibres are releasing more than one transmitter has been proposed by Burnstock and colleagues in 1970s (Burnstock 1976), challenging the single transmitter concept known as “Dale’s Principle”, notwithstanding that Dale never defined it as such (Dale 1935). Since then, numerous studies have demonstrated the existence of synaptic cotransmissions in both the peripheral and the central nervous system (Burnstock 2004). Generally, when two transmitters are released by the same neurone, they have either cooperating effects when both are activating ionotropic receptors (e.g. GABA/glycine) or opposite effects with different time-courses inducing a biphasic synaptic current (e.g. glutamate/serotonin) (Johnson 1994). Cotransmissions involving both excitatory and inhibitory fast synaptic components were unknown before the description of a cotransmission involving adenosine 5’-triphosphate (ATP) and γ-aminobutyric acid (GABA) (Jo and Schlichter 1999; Hugel and Schlichter 2000; Jo and Role 2002b), but were subsequently described in the retina for GABA and acetylcholine (ACh) (Duarte et al. 1999) and in the hippocampus for glutamate and GABA (Gutierrez et al. 2003; Gutierrez 2005).
The term “cotransmission” suggests that the two transmitters are released by a single presynaptic neurone and that they are detected by specific receptors located on the postsynaptic neurone. However, both neurotransmitter species are not always stored in the same presynaptic vesicles, and receptors for the two neurotransmitters are not necessarily localized at the same postsynaptic loci. These pre- and postsynaptic heterogeneities offer the possibility of a differential modulation of both components of the cotransmission. Initially, ATP was described as a cotransmitter with noradrenaline (NA) in sympathetic nerves, and acetylcholine (ACh) in parasympathetic nerves (Burnstock 2004, 2006). ATP released by synaptic terminals can activate cation-permeable ionotropic P2X receptors. These receptors are trimers of different subunits (P2X1-P2X7) possessing each two transmembrane segments and displaying no primary sequence homology with other known ionotropic receptors (Khakh and North 2006). The activation of postsynaptic P2X receptors by synaptically released ATP underlies consequently a fast excitatory transmission. Moreover, ATP is rapidly hydrolysed to adenosine 5’-diphosphate (ADP), adenosine 5’-monophosphate (AMP) and adenosine by extracellular ectonucleotidases (Zimmermann 1996; Dunwiddie et al. 1997; Zimmermann 2000). Therefore, in addition to the activation of P2X receptors, the synaptic release of ATP can also induce the subsequent activation of P2Y (by ATP and/or ADP) and adenosine metabotropic receptors.
In this review we will deal with general questions relative to excitatory or inhibitory cotransmissions, such as those related to the storage and the co-release of the cotransmitters. In the context of the issue of codetection, we will focus on the ATP/GABA cotransmission. This point is important because unlike other neurotransmitters, ATP can be metabolized in the synaptic cleft to other purines such as adenosine which also act as neuromodulators. In this respect we will address the issues of codetection by ionotropic and metabotropic receptors located post- and/or presynaptically. We will also discuss how the localization of the receptors within the synapse and the metabolism of ATP might influence the detection of miniature ATPergic postsynaptic currents. Finally, we will envisage the potential role of the ATP/GABA cotransmission in physiological and/or pathological situations. The major points that will be addressed are summarized in Fig. 11.1.
The ATP/GABA cotransmission. ATP and GABA are coreleased from the presynaptic terminal and act at postsynaptic ionotropic excitatory ATP receptors (P2X receptors) and inhibitory anionic GABAA receptors. (a) In the dorsal horn of the spinal cord, the synaptic corelease of ATP and GABA is modulated by presynaptic autoreceptors. ATP can facilitate GABA release by acting at presynaptic P2X receptors. Inhibition of corelease involves metabotropic GABAB receptors and A1 adenosine receptors which act by a partially convergent presynaptic mechanism. Adenosine is generated by the extracellular hydrolysis of ATP by ectonucleotidases. (b) Possible anatomical substrates and scenarios of ATP/GABA cotransmission. ATP and GABA might be costored in the same vesicle or stored in different vesicles. Postsynaptic P2X and GABAA receptors might be colocalized in front of the same release sites or segregated in different synapses. (c) The mixed excitatory/inhibitory ATP/GABA cotransmission can finely tune the balance of excitation and inhibition converging on the same neuron by shifting the net equilibrium toward inhibition or excitation, depending on the relative weight of the ATPergic and GABAergic components of the cotransmission. (See Color Plate 14)
11.2 ATP/GABA Synaptic Cotransmission
Neurones from postnatal rat spinal cord dorsal horn, and from both embryonic chick and postnatal mouse lateral hypothalamus maintained in dissociated cell culture form functional excitatory and inhibitory synaptic connections. The electrophysiological properties and the characteristics of synaptic transmissions in these cultures are similar to those observed in neurones from native networks (Jo et al. 1998b; Jo et al. 1998a). A subpopulation of cultured neurones is excitatory in nature, involving glutamate as a neurotransmitter. Excitatory glutamatergic transmission is always entirely blocked by AMPA/kainate and NMDA receptor antagonists (Jo et al. 1998b). Another subpopulation of neurones is constituted of inhibitory interneurones, and uses GABA and/or glycine as transmitters (Hugel and Schlichter 2000). Electrical stimulation of GABAergic neurones induces fast evoked postsynaptic currents (ePSCs) that are not always completely blocked by GABAA receptor antagonists (Fig. 11.2a ). The residual ePSC component is however at least partially blocked by P2X receptors antagonists (Jo and Schlichter 1999; Hugel and Schlichter 2000). The GABAergic and the purinergic (ATPergic) components can be easily separated by setting the equilibrium potential for chloride ions (ECl) at –90 mV and that for cations (Ecations) at 0 mV (Fig. 11.2b–d). Under these conditions, the P2X antagonist-sensitive ePSC component can be recorded in isolation at a holding potential of –90 mV, revealing the cationic nature of the conductance. A detailed study on the properties of ePSCs showed that both the GABAergic and the P2X receptor-mediated components had the same latency and the same stimulation threshold, demonstrating that a single presynaptic neurone was releasing GABA and ATP. Altogether, these data demonstrate that ATP is coreleased with GABA but never with glutamate in the dorsal horn of the spinal cord and the lateral hypothalamus. This type of corelease is observed only in a subpopulation of GABAergic neurones (Jo and Schlichter 1999; Hugel and Schlichter 2000).
Synaptic corelease of ATP and GABA. (a) In cultured neonatal neurones from the superficial layers of the dorsal horn of the spinal cord, electrical stimulation of a single presynaptic neurone in the presence of bicuculline (10 µM), strychnine (1 µM), CNQX (10 µM) and D-APV (50 µM) induced an inward postsynaptic current in half of the postsynaptic neurones recorded (trace i). This inward postsynaptic current was blocked by suramin (30 µM). (b) Setting ECl to –90 mV and Ecations to 0 mV allowed the isolation of the cationic ATPergic component at a holding potential (HP) of –90 mV and the GABAergic component at a HP of 0 mV. (c) The GABAergic component recorded at HP = 0 mV was completely blocked by SR95531 (gabazine; 5 µM), a competitive antagonist of GABAA receptors. (d) The ATPergic component recorded at HP = –90 mV was blocked by suramin (30 µM). Adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience, Jo and Schlichter, 1999, copyright, 1999
11.2.1 General Considerations on Costorage and Corelease
ATP is present in the cytoplasm of all cell types at concentrations in the millimolar range, and is accumulated in synaptic vesicles (Sperlagh and Vizi 1996). However, the mechanisms involved in vesicular uptake of ATP are still unclear. Recent results obtained on brain synaptosomes suggest that ATP import into synaptic vesicles might involve an ADP/ATP translocase related to the mitochondrial nucleotide translocase (Gualix et al. 1999). Since ATP is present in all synaptic vesicles, it follows that ATP must be costored with classical neurotransmitters and probably with neuropeptides as well. Therefore, ATP and its cotransmitters are likely to be coreleased during fusion of single vesicles with the presynaptic membrane. However, costorage in the same vesicle does not necessarily imply that both cotransmitters are released simultaneously and at the same amounts. Indeed, a recent study on pancreatic β cells indicates that, although different cotransmitters (ATP, serotonin and GABA) are coreleased, a differential release can occur, depending on the properties of the fusion pore (Braun et al. 2007). For example, it appears that GABA and serotonin can easily exit the vesicle during partial opening of the fusion pore (e.g. “kiss and run” exocytosis) whereas ATP does not easily leave the vesicle under these conditions. A similar situation might also apply to synaptic vesicle exocytosis at central synapses (Gandhi and Stevens 2003).
11.2.2 Are ATP and GABA Coreleased From Common or Distinct Synaptic Vesicles?
Although the existence of a pure purinergic transmission has been suggested (Sperlagh and Vizi 1996), it was observed only in a few instances in the CNS (Robertson and Edwards 1998; Pankratov et al. 2007). Instead, in the peripheral nervous system as well as in the central nervous system, ATP appears to be primarily a cotransmitter rather than a principal transmitter (Burnstock 2004). Indeed, in all experiments in which stimulation of a single presynaptic neurone could be performed and electrically-evoked postsynaptic P2X receptor-mediated EPSCs were recorded, the wash out of ionotropic GABA or glutamate receptor antagonists systematically revealed the presence of synaptic GABA or glutamate corelease (Fig. 11.2a) (Jo and Schlichter 1999; Hugel and Schlichter 2000; Mori et al. 2001). Nevertheless, this does not exclude that the release of ATP and of its cotransmitter involves separate vesicular pools and/or separate presynaptic terminals (Fig. 11.1b). The definitive answer to this question relies on the analysis of miniature postsynaptic currents. Separate vesicular storage should generate separate miniature currents, whereas corelease from the same vesicle should generate mixed miniature currents with a component due to ATP and another due to the cotransmitter. Unfortuantely, this issue is extremely difficult to address since it is linked to the problem of detection of ATP by P2X receptors at the postsynaptic level (see below section 11.2.3).
It has been emphasized that a differential modulation of the release of the cotransmitters indicates that the transmitters are stored in different vesicles. In the retina, the involvement of separate vesicle pools releasing ACh and GABA is strengthened by the fact that the Ca2+-dependent release of the two cotransmitters depend on distinct voltage-dependent Ca2+ channel types (Duarte et al. 1999). In this preparation, the release of both cotransmitters is also differentially affected by presynaptic adenosine receptors (Duarte et al. 1999). However, as mentioned above, differential release of cotransmitters packaged in the same vesicle might occur, depending on the type of exocytosis, and more precisely of the size of the fusion pore (Gandhi and Stevens 2003; Braun et al. 2007). Therefore, differential modulation of release does not necessarily imply that the transmitters are contained in distinct vesicles. Moreover, in cultured embryonic chick lateral hypothalamic neurones, nicotinic receptor stimulation selectively facilitates synaptic GABA release whereas stimulation of muscarinic receptors increases ATP release (Fig. 11.3 ) (Jo and Role 2002a). This differential modulation occurs despite the detection of mixed ATP/GABA miniature PSCs in this preparation (Fig. 11.4 ), which indicates the copackaging and corelease of ATP and GABA from the same vesicles at least at a subset of synapses. Alternatively, the differential modulatory effect of nicotinic and muscarinic agonists might concern a subset of synapses releasing only GABA and ATP, or synapses coreleasing both transmitters but at which only a single detection element (i.e. GABAA or P2X receptors) is present at the postsynaptic membrane facing the release site.
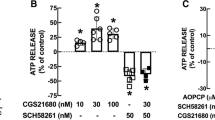
Activity-dependent (phasic) activation of presynaptic A1 and GABAB autoreceptors in neurones coreleasing GABA and ATP but not in neurones releasing only GABA. Effects of coapplication of the A1 antagonist (8-Cyclopentyl-1,3-dipropylxanthine, DPCPX, 1 µM) and the GABAB antagonist (CGP 54626, 1 µM) on the amplitude of eIPSCs evoked by a train of eight pulses (2.5 Hz, arrow heads) applied to the cell body of a presynaptic neurone coreleasing GABA and ATP (a) or of a neurone releasing only GABA (b). c-d. histograms of the percentage of GABAergic eIPSC facilitation by application of DPCPX and CGP 54626 for each of the eight stimulation pulses in neurones coreleasing ATP and GABA (c) and in neurones releasing GABA only (d). The amplitudes of the effect on the sixth and seventh eIPSCs in (c)were found to be significantly different from that on the other eIPSCs (one-way ANOVA with repeated measures, P < 0.05 and post hoc comparison with Duncan test, P < 0.05). There was no statistically significant difference in the case of neurones releasing only GABA (d). The HP was 0 mV. Dotted lines indicate the amplitude of the eIPSCs before applications of DPCPX and CGP 54626. Modified with permission from Hugel and Schlichter, 2003, The Journal of Physiology, Copyright Blackwell Publishing
Differential cholinergic modulation of ATP/GABA cotransmission in lateral hypothalamus. a-b Activation of nicotinic ACh receptors facilitates GABAergic but not ATPergic transmission. a. Evoked GABAergic eIPSCs isolated at HP = 0 mV before, during and after application of nicotine (0.5 µM). Nicotine increased the amplitude of eIPSCs and changed the value of the ratio between the amplitude of the two consecutive eIPSCs, indicating a presynaptic action b. Evoked ATPergic eEPSCs isolated at HP = –70 mV were not modified by application of nicotine (0.5 µM). c-d. Activation of muscarinic ACh receptors depressed GABAergic transmission and facilitated purinergic transmission. c. Evoked GABAergic eIPSCs isolated at HP = 0 mV were depressed by application of mucarine (10 µM). b. Evoked ATPergic eEPSCs isolated at HP = –70 mV were potentiated by application of mucarine (10 µM). In both cases, a change in the ratio of amplitudes of eIPSCs or eEPSCs was observed indicating a presynaptic action of muscarine. Modified with permission from Jo and Role, 2002a, The Journal of Neurophysiology, American Physiological Society
11.2.3 Codetection of ATP and GABA Corelease by Ionotropic Receptors: The Puzzling Issue of Miniature P2X Receptor-Mediated Postsynaptic Currents
Electrical stimulation of individual presynaptic neurones has revealed the existence of a functional ATPergic synaptic transmission in many structures of the peripheral and central nervous system (Burnstock 2004). Surprisingly, spontaneous ATPergic EPSC and particularly ATPergic miniature synaptic currents (mEPSCs) have only rarely been described in the central nervous system. In certain cases, increasing neuronal excitability and the release probability of neurotransmitters with a bath solution containing high concentrations of K+ and Ca2+ allowed the observation of ATPergic EPSCs in the presence of tetrodotoxin (Edwards et al. 1992). Observation of ATPergic mEPSCs may also depend on the preparation and/or on the species, because ATPergic mEPSCs have been recorded in hypothalamic cultured chick embryonic neurones but were undetectable in hypothalamic neurones cultured from postnatal mice (Jo and Role 2002b). This difficulty to record ATPergic mEPSCs might be explained by peculiarities of ATP fate in the synaptic cleft and/or of the organization of the postsynapse.
11.2.3.1 Ectonucleotidases and ATP Transients in the Synaptic Cleft
Whereas the dwell time of most transmitters within the synaptic cleft is controlled by specific transporters expressed by neurones and/or glial cells, the concentration profile of ATP within the cleft depends on the activity of specific hydrolytic enzymes, as it is the case for ACh. The enzymes metabolising ATP in the extracellular space are grouped under the generic term of ectonucleotidases (Zimmermann 1996, 2000). Interestingly, while degrading ATP in the synaptic cleft, ectonucleotidases are producing agonists for purinergic metabotropic receptors, i.e., ADP, AMP and adenosine. Most ectonucleotidases are anchored in the plasma membrane, but unidentified soluble ectonucleotidases were shown to be coreleased with ATP in the peripheral nervous system (Todorov et al. 1997; Westfall et al. 2002). In acute hippocampal slices, 50% and 96% of ATP is converted to adenosine within 200 ms and 1 s, respectively (Dunwiddie et al. 1997). Therefore, a synchronous release of several vesicles might be required to transiently saturate the ectonucleotidases in order to reach within the synaptic cleft an ATP concentration sufficient to activate postsynaptic P2X receptors. This might also explain why ATPergic mEPSCs are difficult to detect.
Results from studies on the peripheral autonomic nervous system in which ATP is coreleased with ACh or NA, or from neuroendocrine chromaffin cells, have shown that the intravesicular concentration of ATP is generally lower than that of its cotransmitter. Estimated ratios range from 2:1 to 50:1 which represent intravesicular concentrations of ATP between 1 and 200 mM (Sperlagh and Vizi 1996). This suggests that even in the case of complete exocytosis of a vesicle, the concentration transient of ATP in the synaptic cleft will be significantly smaller than that of the cotransmitter. This might explain in part why miniature postsynaptic currents due to the activation of P2X receptors are rarely observed. In addition, the concentration of ATP in the synaptic cleft is likely to be further decreased by the action of extracellular ectonucleotidases which rapidly metabolize ATP (Zimmermann 1996; Dunwiddie et al. 1997; Ghildyal et al. 2006). It can be argued that the enzymatic action of the ectonucleotidases is slow compared to the fast diffusion of ATP in the synaptic cleft. Nevertheless, the binding of ATP to ectonucleotidases (which is a fast process) might act as a buffer and contribute to reduce the concentration of free available ATP in the synaptic cleft. In line with these speculations, it has been recently shown that the ectonucleotidase inhibitor ARL 67156 strongly potentiates purinergic synaptic potentials in the guinea-pig vas deferens (Ghildyal et al. 2006). In this context, it should also be emphasized that since ATP and its nonpeptidic cotransmitter are transported by separate vesicular transporters, a differential regulation of the expression or of the activity of these transporters would result in a change in the ratio of ATP to cotransmitter content of the synaptic vesicles.
11.2.3.2 Distribution of Postsynaptic P2X and GABAA Receptors
The difficulty to record ATPergic mEPSCs might also be linked to the organization of the postsynapse (Fig. 11.1b). Both morphological and electrophysiological studies indicate an uneven distribution of P2X receptors within the neuronal membrane. P2X receptors are rarely found in excised-patches from ATP-responsive neurones of medial habenula slices (Edwards et al. 1992), but when they are found in outside-out patches from dentate gyrus granule cells, they are grouped, suggesting that P2X receptors are organized in clusters (Wong et al. 2000). In the hippocampus and the cerebellum, P2X2, P2X4 and P2X6 subunits clusters have been localized to the periphery of glutamatergic postsynaptic densities (Rubio and Soto 2001). Such perisynaptic localizations increase the time of diffusion of ATP to its receptors and therefore the probability of ATP hydrolysis by ectonucleotidases.
11.2.3.3 Functional interactions between GABAA and P2X receptors
Co-activation of P2X2 and GABAA receptors co-expressed in Xenopus oocytes leads to a functional cross-inhibition (Boue-Grabot et al. 2004) (see section below). Similar observations have been made in sympathetic (Karanjia et al. 2006) and sensory (Sokolova et al. 2001) neurones which naturally express P2X and GABAA receptors. This phenomenon involves a direct protein-to-protein interaction of the two receptor types (Boue-Grabot et al. 2004). Interestingly, when GABAA receptors include a γ subunit, which is the case of all synaptic receptors in the dorsal horn and the lateral hypothalamus (Keller et al. 2001; Jo and Role 2002b; Keller et al. 2004), the inhibition becomes unidirectional (Boue-Grabot et al. 2004). Indeed, under these conditions, GABAA receptors still inhibit the activity of P2X receptors but the reverse is no longer observed. Such an inhibition might occur during synaptic corelease of ATP and GABAA when spatially close P2X and GABAA receptors are concomitantly activated.
In neuronal cultures from the chick hypothalamus, a single postsynaptic neurone receiving synaptic inputs from a presynaptic neurone coreleasing ATP and GABA, displays “pure” GABAergic mIPSCs and mixed ATP/GABA mPSCs but “pure” P2X-receptor-mediated ATPergic mEPSCs are never recorded (Jo and Role 2002b). However, after pharmacological blockade of GABAA receptors with β-hydrastine, P2X-receptor-mediated PSCs are detected. This situation could illustrate the functional inhibition of P2X receptors by the activation of GABAA receptors during synaptic corelease of ATP and GABA (Boue-Grabot et al. 2004). Preventing the activation of GABAA receptors would therefore allow the synaptic activation of P2X receptors. Although this seems to be the case in chick hypothalamic neurones (Jo and Role 2002b), it does not apply to other preparations were ATP and GABA corelease has been described. In cultures of the dorsal horn of the spinal cord, blockade of GABAA receptors by bicuculline did not reveal miniature P2X receptor-mediated EPSCs, but β-hydrastine was not tested in the same situation (Jo and Schlichter 1999). Moreover, since this phenomenon is due to a direct protein-protein interaction, it implies that GABAA and P2X receptors must be in close spatial proximity.
11.2.4 Codetection of ATP and GABA Corelease by Presynaptic Ionotropic and Metabotropic Receptors
Up to this stage, we have only considered the detection/codetection of synaptically released transmitters/cotransmitters by ionotropic receptors expressed by the postsynaptic neurone. Alternatively, the cotransmitters could be detected by presynaptic ionotropic receptors or/and metabotropic G-protein coupled receptors, i.e GABAB receptors for GABA and P2Y receptors for ATP/ADP. Moreover, adenosine resulting from rapid degradation of ATP by ectonucleotidases can bind to adenosine receptors (Fig. 11.1a) (Zimmermann 1996; Dunwiddie et al. 1997). The activation of postsynaptic metabotropic receptors by GABA and ATP/adenosine has not been investigated so far in the context of cotransmission. However, we have recently shown that corelease of ATP and GABA might finely regulate their own release by acting synergistically at presynaptic GABAB and A1 adenosine receptors (Hugel and Schlichter 2003) (see below).
11.2.4.1 Presynaptic P2X Receptors
P2X receptors are present on the presynaptic terminals of GABAergic neurones but not of glutamatergic neurones in cultures of neonatal spinal cord dorsal horn neurones (Fig. 11.1a) (Hugel and Schlichter 2000). These receptors might be the target of synaptically released ATP but this issue is difficult to address due to the absence of selective antagonists of the P2X receptors expressed in the central nervous system. Nevertheless, it appears that presynaptic P2X receptors are preferentially expressed on GABAergic neurones coreleasing GABA and ATP (Hugel and Schlichter 2000).
11.2.4.2 Presynaptic GABAB and A1 Autoreceptors
In cultured neurones from the dorsal horn of the spinal cord, the synaptic corelease of ATP and GABA is controlled by both released transmitters and by ATP hyrdrolysis products such as adenosine (Jo and Schlichter 1999; Hugel and Schlichter 2000).
In control conditions, presynaptic GABAB and A1 receptors are tonically inhibiting both the ATPergic and the GABAergic component of the cotransmission (Hugel and Schlichter 2003). During repetitive stimulation of the presynaptic neurone coreleasing GABA and ATP, GABAB and A1 autoreceptors are recruited to inhibit evoked transmitter release. This phasic control exerted by the released GABA and adenosine (via ATP hydrolysis) is not observed in the case of neurones releasing only GABA (Hugel and Schlichter 2003) (Fig. 11.5 ). This suggests that GABAB and A1 receptors are acting in synergy to control presynaptically the ATP/GABA cotransmission, possibly by converging on the same transduction pathway. In addition to presynaptic A1 receptors, we have shown that postsynaptic A1 receptors are inhibiting selectively GABAA receptors mediated currents in dorsal horn neurones (Jo and Schlichter 1999).
Mixed ATPergic/GABAergic spontaneous miniature PSCs recorded in cultured chick lateral hypothalamus neurones. (a) averaged traces of bicuculline-sensitive and bicuculline-resistant mPSCs. (b) The decay time of the mixed events was best-fitted by a biexponential function. Flunitrazepam (Flu, 100 nM), a positive allosteric modulator of GABAA receptors had no effect on the time constant of the fast component (τfast) whereas it increases the time constant of the slow exponential function (τslow), confirming the GABAergic nature of the slow component. Modified from Jo and Role, 2002b. The Journal of Neuroscience, Copyright 2002 by the Society for Neuroscience
Presynaptic adenosine, P2X and GABAB receptors seem therefore able to sense and to regulate the corelease of ATP and GABA (Hugel and Schlichter 2000, 2003). In the hippocampus, synaptically released GABA is also detected by perisynaptic astrocytes which express GABAB receptors and respond to the synaptic release of GABA by an elevation in the intracellular calcium concentration (Kang et al. 1998). ATP has recently been established as an important gliotransmitter, and astrocytes express functional P2X and P2Y receptors (Haydon 2001; Nedergaard et al. 2003; Haydon and Carmignoto 2006). These receptors participate in the propagation of calcium waves in the astrocytic network (Haydon 2001; Nedergaard et al. 2003; Haydon and Carmignoto 2006). However, it is not known whether ATP released from neurones can activate P2X or P2Y receptors expressed by perisynaptic astrocytes.
11.2.4.3 Conclusion on Corelease Costorage and Codetection
As discussed above, synaptic corelease of ATP and GABA from the same presynaptic neurone has been clearly established in cultures of rat dorsal horn spinal cord neurones and lateral hypothalamic neurones of chick and mouse. However, the issue of ATP/GABA corelease from the same vesicles is difficult to assess in the absence of recordable miniature P2X-receptor-mediated PSCs in most of preparations. It is hoped that conditions revealing such miniature currents can be found in order to fully answer this question which is of fundamental importance for understanding the role of cotransmissions involving ATP, as well as the general organization of pre- and postsynaptic elements in this context.
11.3 Physiological Role of ATP/GABA Cotransmission: From Facts to Speculations
An important question concerning synaptic ATP and GABA corelease is related to its role under physiological and/or pathological situations. In particular, it is important to know whether this phenomenon is only transiently observed during development in immature systems or if it is still present in adult organisms.
11.3.1 ATP/GABA Cotransmission During Development and in the Adult
Purines act as signalling molecules in the CNS (Fields and Stevens 2000; Fields and Burnstock 2006; Haydon and Carmignoto 2006) but they play also an important role as trophic factors for neurones and glial cells (Rathbone et al. 1999). Therefore it is legitimate to ask whether synthesis and release of purines is prominent only in developing organisms. So far, ATP and GABA corelease has been described in cultures or slices from immature animals. However, in the dorsal spinal cord or the lateral hypothalamus of adult rodents P2X receptors are still expressed (Collo et al. 1996) along with GABAA receptors, suggesting that release and codetection of ATP and GABA release might still be possible in these regions in adults. Moreover, expression of functional P2X receptors increases during postnatal development in lamina V of the spinal cord (Shiokawa et al. 2006). We have preliminary evidence that a functional synaptic transmission involving ATP and postsynaptic P2X receptors still occurs in 3 to 4 weeks old rats (unpublished observations), but the issue of corelease of ATP with GABA could not be addressed for the moment. Indeed, identification of ATP/GABA cotransmission in the absence of detectable miniature P2X receptor-mediated PSCs requires to identify and to selectively stimulate electrically a single presynaptic neurone. This is difficult in slices because: (1) even minimal local extracellular stimulation will activate several presynaptic fibres due to the dense fibre network in the superficial layers of the spinal cord and (2) unitary connections within lamina II of the spinal cord are difficult to identify rendering paired recordings problematic (Lu and Perl 2003).
11.3.2 Role in Synaptogenesis and in the Modulation of GABAA Receptor Function
An important process taking place during development is the formation of functional synaptic contacts. This phenomenon seems to depend on local increases in intracellular calcium at postsynaptic sites where synapses are to be established. During this phase of synaptogenesis, GABAA receptor activation triggers a membrane depolarization which facilitates the activation of voltage dependent Ca2+ channels (Fitzgerald 2005). It is only later in development that GABAA receptor-mediated responses become hyperpolarising, a phenomenon due to the progressive expression of the K+/Cl– cotransporter KCC2 (Owens et al. 1996; Rivera et al. 1999; Ben-Ari 2002; Baccei and Fitzgerald 2004). Therefore, at early stages at which the GABAA responses are still depolarising, activation of P2X receptors by ATP coreleased with GABA at future synaptic sites could amplify the rise in intracellular calcium concentration at the post- synaptic membrane, thereby facilitating the establishment and the stabilization of synaptic contacts. Once GABAA responses are hyperpolarising, activation of P2X receptors might finely tune the activity of GABAA receptors via calcium-dependent mechanisms. Indeed, GABAA receptors are directly modulated by increases in intracellular free calcium concentration and the sign of the modulation depends on the amplitude of the calcium signal (Inoue et al. 1986; Llano et al. 1991; Mouginot et al. 1991). In addition, intracellular calcium can activate calcium-dependent protein kinases and phosphatases which are known to modulate GABAA receptors (Moss and Smart 1996, 2001). Therefore, P2X receptors might provide a means for activity- and calcium-dependent modulation of synaptic GABAA receptors. In a slightly different context, ATP might also act in concert with GABA in facilitating the integration and differentiation of newborn neurones (Owens and Kriegstein 2002).
11.3.3 Cross-inhibition of P2X and GABAA Receptors
It has been shown recently that P2X and GABAA receptors display a reciprocal functional inhibition due to direct protein-protein interactions between the two receptors (Sokolova et al. 2001; Boue-Grabot et al. 2004; Karanjia et al. 2006). Interestingly, the presence of a γ subunit, which confers sensitivity to benzodiazepines, in the composition of the GABAA receptor allows inhibition of P2X receptors by GABAA receptors but precludes inhibition of GABAA receptors by P2X receptor activation. In the dorsal horn of the spinal cord and in the lateral hypothalamus, synaptic GABAA receptors are positively modulated by benzodiazepines indicating that they include a γ subunit (Chery and de Koninck 1999; Jo and Role 2002b; Keller et al. 2004). This situation will certainly limit the action of P2X receptors on synaptic GABAA receptors which are in close association with them. However, ultrastructural studies have shown that P2X receptors have rather a perisynaptic localization (Rubio and Soto 2001) and it is not known whether these perisynaptic P2X receptors are in close association with γ subunit-containing GABAA receptors. By contrast, extrasynaptic GABAA receptors do generally not include γ subunits (Mohler et al. 2004) rendering a reciprocal inhibition between extrasynaptic GABAA and P2X receptors possible. Such an interaction could occur during spillover of GABA and ATP and might finely regulate, either positively or negatively, the excitability of neurones in which such an interaction occurs (Semyanov et al. 2004). Under these circumstances, the sign of the modulatory effect on excitability will essentially depend on the balance between the two transmitters, i.e. the relative activation of both types of receptors.
11.3.4 ATP/GABA Cotransmission and Pathological Situations
One could speculate that ATP and GABA corelease is prominent during early development, that this corelease might “disappear” at later stages, and could subsequently “reappear” under particular physiological and/or pathological conditions. There are examples for such a situation in the case of other synaptic coreleases. For instance, synaptic corelease of GABA and glutamate is observed at synapses between mossy fibre and CA3 pyramidal neurones in the developing hippocampus (Gutierrez et al. 2003; Gutierrez 2005). These synapses subsequently become purely glutamatergic, but following epileptic seizures the mixed phenotype reappears due to the reexpression of glutamic acid decarboylase, the biosynthetic enzyme for GABA, in the mossy fibre terminals (Gutierrez 2000, 2002, 2005). In this case, the cotransmission plasticity is of presynaptic origin. In the same line, GABA and glycine corelease is present at a subset of inhibitory synapses in laminae I and II of the dorsal horn of the spinal cord during the early postnatal period (Chery and de Koninck 1999; Keller et al. 2004). Subsequently, these synapses become exclusively glycinergic in lamina I and GABAergic and glycinergic in lamina II, i.e. in lamina II one detects only “pure” GABAergic or “pure” glycinergic events in the same neurone but never mixed GABA/glycine synaptic currents (Chery and de Koninck 1999; Keller et al. 2004). The “disappearance” of mixed GABA/glycine mIPSCs is not due to a “disappearance” of corelease but most probably to the redistribution of synaptic GABAA receptors which become extrasynaptic at the synapses that corelease GABA and glycine. Indeed, application of positive allosteric modulators of GABAA receptors such as benzodiazepines or 3α5α-reduced neurosteroids leads to the reappearance of mixed GABA/glycine events (Chery and de Koninck 1999; Keller et al. 2004). In this case, the apparent downregulation of cotransmission involves postsynaptic modifications. A similar “reappearance” of mixed GABA/glycine events is observed in lamina II following the induction of an inflammatory pain and this phenomenon is related to the stimulation the local production of 3α5α-reduced neurosteroids (Poisbeau et al. 2005). One could therefore speculate that ATP/GABA cotransmission might be subjected to similar regulations.
The phenotype of a mixed ATP/GABA synapse will depend on: (1) the relative amount of GABA and ATP released, (2) the density and the properties of the postsynaptic receptors activated and (3) the functional interactions (cross-inhibition) between postsynaptic P2X and GABAA receptors. Our observations suggest that under resting conditions, the GABAA component is always dominant indicating that the phenotype of these synapses is inhibitory. However, under conditions of differential release of the two transmitters or of differential regulation of postsynaptic P2X and GABAA receptors, the inhibitory weight of the synapses is likely to change. In an extreme situation, one could even envisage that the synapse becomes excitatory when the P2X component becomes dominant with respect to the GABAA component. This could occur during plastic states associated with inflammatory or neuropathic pain (see below) and could in part explain the changes that take place in the processing of nociceptive and non-nociceptive messages in the dorsal horn of the spinal cord in the absence of major changes in anatomical connections. It is important to underline that changes in the processing of somatosensory information by the dorsal horn neuronal networks do no necessarily imply that the sign of the mixed ATP/GABA synapse has to switch to an excitatory phenotype. Indeed, the postsynaptic neurone receiving these mixed synapses also receives excitatory and inhibitory synaptic inputs mediated by glutamate and GABA or glycine (Fig. 11.1c). The postsynaptic neurone integrates these inputs and the mixed (ATP/GABA) synapses contribute to the balance between excitation and inhibition. In the case of a reduction in the inhibitory power of the mixed synapse (relative increase of the P2X component), this would result in a net excitation of the postsynaptic neurone due to a deficit in inhibition. In cultures of neurones from the lateral hypothalamus, Jo and Role have described a differential modulation of the GABAergic and the purinergic components of the ATP/GABA cotransmission by stimulation of nicotinic and muscarinic ACh receptors, respectively (Fig. 11.3) (Jo and Role 2002a). This modulation takes place at the presynaptic level. A differential control of this cotransmission would also occur in the case of a differential modulation of postsynaptic P2X and GABAA receptors. We have recently shown that a peripheral inflammation stimulates the production of 3α5α-reduced neurosteroids in lamina II of the dorsal horn of the spinal cord (Poisbeau et al. 2005). These 3α5α-reduced neurosteroids are selective positive allosteric modulators of GABAA receptors (Belelli and Lambert 2005; Schlichter et al. 2006) that do not affect the function P2X receptors (unpublished observation). Therefore, the production of 3α5α-reduced neurosteroids would selectively potentiate the GABAergic component of the ATP/GABA cotransmission, leaving the purinergic component unaffected. However, one cannot exclude the production of other neurosteroids such as dehydroepiandrosterone (DHEA) and its derivatives in lamina II (Kibaly et al. 2005). Interestingly DHEA potentiates the activity of P2X receptors including the P2X2 subunit and has either no effect on or inhibits GABAA receptor-mediated currents (De Roo et al. 2003). The P2X2 subunit is expressed in lamina II of the dorsal horn of the spinal cord and the lateral hypothalamus (Collo et al. 1996) and we have described the presence of post- and presynaptic P2X receptors having the properties of P2X2-containing receptors in cultures of superficial dorsal horn neurones (Hugel and Schlichter 2000). Therefore, in case of a local production of DHEA, a selective potentiation of the P2X receptor-mediated component can be expected whereas the GABAergic component will be unaffected or inhibited. These considerations suggest that a differential modulation of the GABAergic and the purinergic components of the ATP/GABA cotransmission is theoretically possible. An important issue to follow is to know under which physiological or pathological situations such differential modulations are likely to occur. As discussed above, changes in the local production of neurosteroids might achieve such conditions. GABAA receptors play an important role in the processing of pain messages in the dorsal horn of the spinal cord because intrathecal administration of the GABAA receptor antagonist bicuculline induces states of hyperalgesia and allodynia (Millan 1999; Yaksh 1999). Similarly, P2X receptors are implicated in the detection and transmission of peripheral pain messages. However, up to now, studies on the role of P2X receptors in nociception have essentially concentrated on receptors expressed by primary afferent neurones and little is known about the plasticity in the expression and the function of P2X receptors in the dorsal horn of the spinal cord. There are indications that these receptors might be implicated in inflammatory pain processing in the dorsal horn (Stanfa et al. 2000). In the case of neuropathic pain, neuronal P2X receptors seem to be less involved (Stanfa et al. 2000) but P2X4 receptors expressed by microglial cells play an important role in the development of the pathological situation (Tsuda et al. 2003).
Interestingly, P2X and GABAA receptors are also colocalized on the central terminals of primary nociceptors within the spinal cord (Labrakakis et al. 2003). Therefore, corelease of ATP and GABA might contribute to the fine tuning of synaptic glutamate release at the first relay of nociceptive messages in the dorsal horn of the spinal cord.
11.4 Conclusion
The simultaneous corelease and postsynaptic codetection of ATP with GABA has been demonstrated in cultures from two different structures of the central nervous system: the dorsal horn of the spinal cord and the lateral hypothalamus. The lack of miniature ATPergic PSC together with the difficulty to identify pairs of synaptically connected neurones has, for the moment, precluded the identification of corelease in slice preparations from spinal cord and hypothalamus. It is however probable that this corelease persists in the adult dorsal horn of the spinal cord where it might participate in the modulation of neuronal excitability observed in inflammatory pain situations. In such a context, the modulation of ATP/GABA cotransmission might contribute to the modulation or alteration of the processing of nervous messages without reorganization of the anatomical connections. In particular, it will be interesting to determine whether extracellular mediators (e.g. inflammatory mediators) released in these structures under specific physiological or pathological situations will reveal, potentiate or differentially modulate this intriguing mixed excitatory/inhibitory cotransmission.
Abbreviations
- ACh:
-
Acetylcholine
- ADP:
-
adenosine 5’-diphosphate
- AMP:
-
adenosine 5’-monophosphate
- ATP:
-
adenosine 5’-triphosphate
- CNQX:
-
6-cyano-7-nitroquinoxaline-2,3-dione
- D-APV:
-
D-amino-phosphonovaleric acid
- ECl :
-
equilibrium potential for Cl- ions
- Ecations :
-
equilibrium potential for cations
- ePSC:
-
electrically-evoked postsynaptic current
- eEPSC:
-
electrically-evoked excitatory postsynaptic current
- eIPSC:
-
electrically-evoked inhibitory postsynaptic current
- DHEA:
-
dehydroepiandrosterone
- DPCPX:
-
8-Cyclopentyl-1,3-dipropylxanthine
- GABA:
-
γ-aminobutyric acid
- HP:
-
holding potential
- mPSC:
-
miniature postsynaptic current
- mEPSC:
-
miniature excitatory postsynaptic current
- mIPSC:
-
miniature inhibitory postsynaptic current
- NA:
-
noradrenaline
- NMDA:
-
N-methyl-D-Aspartic acid
References
Baccei ML, Fitzgerald M (2004) Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci 24:4749–4757
Belelli D, Lambert JJ (2005) Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 6:565–575
Ben-Ari Y (2002) Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3:728–739
Boue-Grabot E, Toulme E, Emerit MB et al. (2004) Subunit-specific coupling between gamma-aminobutyric acid type A and P2X2 receptor channels. J Biol Chem 279:52517–52525
Braun M, Wendt A, Karanauskaite J et al. (2007) Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic beta cells. J Gen Physiol 129:221–231
Burnstock G (1976) Do some nerve cells release more than one transmitter? Neuroscience 1:239–248
Burnstock G (2004) Cotransmission. Curr Opin Pharmacol 4:47–52
Burnstock G (2006) Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci 27:166–176
Chery N, de Koninck Y (1999) Junctional versus extrajunctional glycine and GABA(A) receptor-mediated IPSCs in identified lamina I neurons of the adult rat spinal cord. J Neurosci 19:7342–7355
Collo G, North RA, Kawashima E et al. (1996) Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 16:2495–2507
Dale HH (1935) Pharmacology and nerve endings. Proc Roy Soc Med 18:319–322
De Roo M, Rodeau JL, Schlichter R (2003) Dehydroepiandrosterone potentiates native ionotropic ATP receptors containing the P2X2 subunit in rat sensory neurones. J Physiol 552:59–71
Duarte CB, Santos PF, Carvalho AP (1999) Corelease of two functionally opposite neurotransmitters by retinal amacrine cells: experimental evidence and functional significance. J Neurosci Res 58:475–479
Dunwiddie TV, Diao L, Proctor WR (1997) Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci 17:7673–7682
Edwards FA, Gibb AJ, Colquhoun D (1992) ATP receptor-mediated synaptic currents in the central nervous system. Nature 359:144–147
Fields RD, Stevens B (2000) ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci 23:625–633
Fields RD, Burnstock G (2006) Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci 7:423–436
Fitzgerald M (2005) The development of nociceptive circuits. Nat Rev Neurosci 6:507–520
Gandhi SP, Stevens CF (2003) Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature 423:607–613
Ghildyal P, Palani D, Manchanda R (2006) Post- and prejunctional consequences of ecto-ATPase inhibition: electrical and contractile studies in guinea-pig vas deferens. J Physiol 575:469–480
Gualix J, Pintor J, Miras-Portugal MT (1999) Characterization of nucleotide transport into rat brain synaptic vesicles. J Neurochem 73:1098–1104
Gutierrez R (2000) Seizures induce simultaneous GABAergic and glutamatergic transmission in the dentate gyrus-CA3 system. J Neurophysiol 84:3088–3090
Gutierrez R (2002) Activity-dependent expression of simultaneous glutamatergic and GABAergic neurotransmission from the mossy fibers in vitro. J Neurophysiol 87:2562–2570
Gutierrez R (2005) The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci 28:297–303
Gutierrez R, Romo-Parra H, Maqueda J et al. (2003) Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J Neurosci 23:5594–5598
Haydon PG (2001) GLIA: listening and talking to the synapse. Nat Rev Neurosci 2:185–193
Haydon PG, Carmignoto G (2006) Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86:1009–1031
Hugel S, Schlichter R (2000) Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci 20:2121–2130
Hugel S, Schlichter R (2003) Convergent control of synaptic GABA release from rat dorsal horn neurones by adenosine and GABA autoreceptors. J Physiol 551:479–489
Inoue M, Oomura Y, Yakushiji T et al. (1986) Intracellular calcium ions decrease the affinity of the GABA receptor. Nature 324:156–158
Jo YH, Schlichter R (1999) Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci 2:241–245
Jo YH, Role LW (2002a) Cholinergic modulation of purinergic and GABAergic cotransmission at in vitro hypothalamic synapses. J Neurophysiol 88:2501–2508
Jo YH, Role LW (2002b) Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J Neurosci 22:4794–4804
Jo YH, Stoeckel ME, Schlichter R (1998a) Electrophysiological properties of cultured neonatal rat dorsal horn neurons containing GABA and met-enkephalin-like immunoreactivity. J Neurophysiol 79:1583–1586
Jo YH, Stoeckel ME, Freund-Mercier MJ et al. (1998b) Oxytocin modulates glutamatergic synaptic transmission between cultured neonatal spinal cord dorsal horn neurons. J Neurosci 18:2377–2386
Johnson MD (1994) Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron 12:433–442
Kang J, Jiang L, Goldman SA et al. (1998) Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci 1:683–692
Karanjia R, Garcia-Hernandez LM, Miranda-Morales M et al. (2006) Cross-inhibitory interactions between GABAA and P2X channels in myenteric neurones. Eur J Neurosci 23:3259–3268
Keller AF, Breton JD, Schlichter R et al. (2004) Production of 5alpha-reduced neurosteroids is developmentally regulated and shapes GABA(A) miniature IPSCs in lamina II of the spinal cord. J Neurosci 24:907–915
Keller AF, Coull JA, Chery N et al. (2001) Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. J Neurosci 21:7871–7880
Khakh BS, North RA (2006) P2X receptors as cell-surface ATP sensors in health and disease. Nature 442:527–532
Kibaly C, Patte-Mensah C, Mensah-Nyagan AG (2005) Molecular and neurochemical evidence for the biosynthesis of dehydroepiandrosterone in the adult rat spinal cord. J Neurochem 93:1220–1230
Labrakakis C, Tong CK, Weissman T et al. (2003) Localization and function of ATP and GABAA receptors expressed by nociceptors and other postnatal sensory neurons in rat. J Physiol 549:131–142
Llano I, Leresche N, Marty A (1991) Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron 6:565–574
Lu Y, Perl ER (2003) A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci 23:8752–8758
Millan MJ (1999) The induction of pain: an integrative review. Prog Neurobiol 57:1–164
Mohler H, Fritschy JM, Crestani F et al. (2004) Specific GABA(A) circuits in brain development and therapy. Biochem Pharmacol 68:1685–1690
Mori M, Heuss C, Gahwiler BH et al. (2001) Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J Physiol 535:115–123
Moss SJ, Smart TG (1996) Modulation of amino acid-gated ion channels by protein phosphorylation. Int Rev Neurobiol 39:1–52
Moss SJ, Smart TG (2001) Constructing inhibitory synapses. Nat Rev Neurosci 2:240–250
Mouginot D, Feltz P, Schlichter R (1991) Modulation of GABA-gated chloride currents by intracellular Ca2+ in cultured porcine melanotrophs. J Physiol 437:109–132
Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26:523–530
Owens DF, Kriegstein AR (2002) Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 3:715–727
Owens DF, Boyce LH, Davis MB et al. (1996) Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci 16:6414–6423
Pankratov Y, Lalo U, Verkhratsky A et al. (2007) Quantal release of ATP in mouse cortex. J Gen Physiol 129:257–265
Poisbeau P, Patte-Mensah C, Keller AF et al. (2005) Inflammatory pain upregulates spinal inhibition via endogenous neurosteroid production. J Neurosci 25:11768–11776
Rathbone MP, Middlemiss PJ, Gysbers JW et al. (1999) Trophic effects of purines in neurons and glial cells. Prog Neurobiol 59:663–690
Rivera C, Voipio J, Payne JA et al. (1999) The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–255
Robertson SJ, Edwards FA (1998) ATP and glutamate are released from separate neurones in the rat medial habenula nucleus: frequency dependence and adenosine-mediated inhibition of release. J Physiol 508 (Pt 3):691–701
Rubio ME, Soto F (2001) Distinct Localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci 21:641–653
Schlichter R, Keller AF, De Roo M et al. (2006) Fast nongenomic effects of steroids on synaptic transmission and role of endogenous neurosteroids in spinal pain pathways. J Mol Neurosci 28:33–51
Semyanov A, Walker MC, Kullmann DM et al. (2004) Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci 27:262–269
Shiokawa H, Nakatsuka T, Furue H et al. (2006) Direct excitation of deep dorsal horn neurones in the rat spinal cord by the activation of postsynaptic P2X receptors. J Physiol 573:753–763
Sokolova E, Nistri A, Giniatullin R (2001) Negative cross talk between anionic GABAA and cationic P2X ionotropic receptors of rat dorsal root ganglion neurons. J Neurosci 21:4958–4968
Sperlagh B, Vizi ES (1996) Neuronal synthesis, storage and release of ATP. Sem Neurosci 8:175–186
Stanfa LC, Kontinen VK, Dickenson AH (2000) Effects of spinally administered P2X receptor agonists and antagonists on the responses of dorsal horn neurones recorded in normal, carrageenan-inflamed and neuropathic rats. Br J Pharmacol 129:351–359
Todorov LD, Mihaylova-Todorova S, Westfall TD et al. (1997) Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature 387:76–79
Tsuda M, Shigemoto-Mogami Y, Koizumi S et al. (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424:778–783
Westfall DP, Todorov LD, Mihaylova-Todorova ST (2002) ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J Pharmacol Exp Ther 303:439–444
Wong AY, Burnstock G, Gibb AJ (2000) Single channel properties of P2X ATP receptors in outside-out patches from rat hippocampal granule cells. J Physiol 527 Pt 3:529–547
Yaksh TL (1999) Spinal systems and pain processing: development of novel analgesic drugs with mechanistically defined models. Trends Pharmacol Sci 20:329–337
Zimmermann H (1996) Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol 49:589–618
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362:299–309
Acknowledgments
The authors would like to acknowledge financial support from Centre National de la Recherche Scientifique (CNRS), French Ministry of Education and Research, University Louis Pasteur, Institut UPSA de la Douleur, DRTC and American Diabetes Association.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Hugel, S., Jo, Y., Schlichter, R. (2009). Synaptic Co-Release of ATP and GABA. In: Gutierrez, R. (eds) Co-Existence and Co-Release of Classical Neurotransmitters. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-09622-3_11
Download citation
DOI: https://doi.org/10.1007/978-0-387-09622-3_11
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-0-387-09621-6
Online ISBN: 978-0-387-09622-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)