Abstract
Background: Cardioskeletal myopathy is thought to contribute to exercise intolerance, and reduced quality of life (QOL) in Barth syndrome (BTHS). The objectives of this study were to examine: (1) skeletal muscle strength/performance in adolescents and young adults with BTHS and (2) the safety, feasibility, and initial efficacy of 12 weeks of progressive resistance exercise training (RET) on muscle strength, mass, and performance, bone mineral density, exercise tolerance, cardiac function, and QOL in individuals with BTHS.
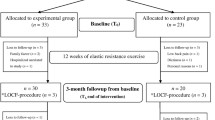
Methods: Individuals with BTHS (n = 9, 23 ± 6 years), and age-, sex-, and activity level-matched unaffected Controls (n = 7, 26 ± 5 years) underwent baseline testing to assess muscle performance, exercise capacity, cardiac structure and function, body composition, and health-related QOL. Subsequently, n = 3 participants with BTHS performed 12 weeks of supervised RET (60 min per session, 3 sessions/week). All testing was repeated post-RET.
Results: BTHS had lower strength and lean muscle mass compared to Controls (all p < 0.05). BTHS also had diminished lower extremity, upper extremity, thoracic spine, lumbar spine, and pelvic bone mineral density (all p < 0.05) and reduced exercise capacity (p < 0.001) compared to Controls. RET was well-tolerated and attended, was not associated with any adverse events, and significantly increased muscle strength (p < 0.05).
Conclusions: Individuals with BTHS demonstrate reduced muscle strength and mass, bone mineral density, and exercise capacity. RET appears safe and well-tolerated in BTHS and promotes increased muscle strength. Larger studies are needed to confirm these improvements and to fully determine the effects of RET in individuals with BTHS.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Barth syndrome (BTHS) is a rare, X-linked disorder caused by recessive mutations in the gene encoding for tafazzin (TAZ) that leads to pathological remodeling of mitochondrial phospholipid cardiolipin (Schlame et al. 2003). As cardiolipin functions to maintain mitochondrial structure and stabilize respiratory chain supercomplexes to facilitate mitochondrial energy production, BTHS-related deficits in cardiolipin result in cardioskeletal myopathy, exercise intolerance, and reduced cardioskeletal oxidative function (McKenzie et al. 2006; Clarke et al. 2013; Wang et al. 2014). BTHS is also characterized by left ventricular non-compaction, endocardial fibroelastosis, prominent left ventricular trabeculations, and hypertrophic cardiomyopathy (rarely) (Clarke et al. 2013).
Previously, our lab identified that both cardiac and skeletal muscle impairments contributed to exercise intolerance in BTHS (Spencer et al. 2011), and that reduced skeletal muscle (soleus) ATP production through oxidative metabolism was directly related to exercise intolerance (VO2peak) (Bashir et al. 2017). Given the metabolic and functional sequela of BTHS, endurance exercise training may be an important non-pharmacologic intervention capable of enhancing cardioskeletal muscle health.
Preliminary evidence from our group has demonstrated that a 12-week, progressive, supervised endurance (i.e., aerobic) exercise training program only modestly (~5%) increased exercise tolerance (VO2peak) in participants with BTHS (Cade et al. 2017), whereas other non-BTHS cardiomyopathies typically fare better with endurance exercise training (~15–25%) (De Maeyer et al. 2013). Endurance exercise training typically results in increased mitochondrial density and enzyme function (primarily in type I oxidative > glycolytic muscle fibers) in non-BTHS populations; however, in BTHS, due to X-linked inherited mitochondrial dysfunction, endurance exercise training might only result in the generation of more impaired mitochondria, thus limiting any beneficial effect on exercise tolerance. Therefore, it might be more beneficial to target type II muscle fibers (glycolytic > oxidative capacity) with exercise training in BTHS.
In contrast to endurance exercise, resistance exercise (a form of exercise characterized by the contraction of muscle against an external force aimed to increase strength) relies heavily on non-oxidative, glycolytic metabolism (Egan and Zierath 2013). Resistance exercise training (RET) has also been associated with fiber type switching to type II, which may reduce the reliance on dysfunctional oxidative (type 1) fibers while improving strength in BTHS (Wilson et al. 2012). RET also increases bone mineral density (Menkes et al. 1993); a feature shown to be decreased in BTHS. In non-BTHS heart failure populations, RET reverses deconditioning, improves muscle strength and physical function, and increases exercise tolerance and stamina, health status, and quality of life (QOL) (De Maeyer et al. 2013). RET may, therefore, avoid exacerbation of myopathic symptoms while facilitating cardiovascular adaptations that occur independent of mitochondrial changes (e.g., improved endothelial function, reduced sympathetic tone, increased nitric oxide production, improved vital capacity and tidal volume, and improved skeletal muscle calcium handling) (Gielen et al. 2010). However, to-date no studies have been performed to examine the effects of RET in BTHS.
Therefore, the objectives of this pilot study were: (1) to compare skeletal muscle strength/performance and bone mineral density in adolescents and young adults with BTHS (n = 9) and healthy, unaffected controls (n = 7) and (2) to examine the safety, feasibility, and initial efficacy of 12 weeks of progressive RET on muscle strength, mass, and performance, bone mineral density, exercise tolerance, heart function, and QOL in one adolescent and two young adults with BTHS (n = 3).
Methods
Participants
Sixteen (n = 16) participants were recruited for this study: n = 9 individuals with BTHS, and n = 7 healthy non-affected controls matched for age, height, weight, BMI, and activity level. All participants were considered sedentary defined as participation in routine exercise ≤2×/week. Medications used by participants with BTHS are provided in Supplemental Table 1. Participants with BTHS were recruited from the Barth Syndrome Foundation Registry located at the University of Florida. Control participants were recruited from the Volunteers for Health at Washington University School of Medicine, and the surrounding St. Louis community. All participants performed a baseline visit at the Washington University Institute for Clinical and Translational Sciences (ICTS) Clinical Research Unit to obtain a medical history and to perform body composition, fasting blood chemistries, exercise and physical activity testing, and echocardiography. All participants enrolled were cardiac stable at the time of baseline testing, and throughout the training and post-testing period. Following baseline testing, n = 3 individuals with BTHS performed 12 weeks of supervised RET, and then completed post-testing (identical to pre-testing). All measures and training parameters are outlined below. Studies were approved by the Human Studies Committee at Washington University in St. Louis and all participants and parents (i.e., adolescents) provided written informed consent.
Body Composition
Each participant underwent whole-body dual energy X-ray absorptiometry (DXA) scans (Hologic Discovery GDR 1000/W, software version 12.6.2 OD; Waltham, Massachusetts) to assess regional and composite lean and fat mass (kg). Scans were also analyzed for bone mineral density (g/cm2) of the spine, upper and lower extremities, and pelvis. Image analysis and subregion (thigh, leg, trunk, and upper extremities) composition quantification was completed using Hologic GDR software version 12.6.2.
Skeletal Muscle Index
The skeletal muscle index was used as a measure of sarcopenia and was calculated as shown below (Merriwether et al. 2012). Appendicular lean mass (ALM) was calculated as the sum of upper and lower extremity lean mass (kg). Values denoting the classification for sarcopenia were implemented as outlined by Janssen et al., where scores ≤37% for men indicate classification as sarcopenic (Janssen et al. 2004).
Plasma Hormone and Metabolite Analyses
Fasting blood for plasma hormone and metabolite analysis was collected from an antecubital vein prior to exercise testing. Samples were immediately chilled on ice, centrifuged at 2,000xg for 10 min, and the supernatant collected and frozen at −80°C until analysis. Plasma glucose was analyzed using an automated glucose analyzer (Yellow Springs Instruments Co, Yellow Springs, OH, USA). A complete blood count (CBC), basic metabolic panel (BMP), pro-brain natriuretic peptide, creatine kinase, and lipid panel were performed on isolated plasma in the Washington University ICTS Core Lab for Clinical Studies.
Echocardiography
All participants underwent conventional two-dimensional (2D), M-mode, pulsed-wave Doppler, tissue Doppler echocardiography, and 2D speckle-tracking global longitudinal strain (GLS) (GE Healthcare Vivid E9; Waukesha, WI, USA) as previously described (Bashir et al. 2017). Ejection fraction, fractional shortening, and global strain measures were made on the left ventricle. The E/A ratio was measured across the mitral valve.
Muscle Strength and Function
Maximal isometric torque as well as isokinetic torque, power, and work of the knee extensors and flexors was measured at 180°/sec using a Biodex System 3 Isokinetic Dynamometer (Shirley, NY, USA). Each isokinetic movement was repeated three times, with the average of the three trials used in the final analysis. Additionally, one repetition maximum (1RM) assessments were performed on a Hoist single pod machine (San Diego, CA, USA) according to guidelines established by the American College of Sports Medicine (2000). The maximum weight lifted through the full range of motion with proper form was recorded for the leg press, bench press, biceps curl, seated row, knee extension, and shoulder press.
Quality of Life
Participants with BTHS were asked to complete the Minnesota Living with Heart Failure Questionnaire (MLWHFQ) – a 21-item survey that asks individuals with heart failure to describe the effects of symptoms, functional limitations, and psychological distress on their QOL (Rector and Cohn 1992). Responses are graded on a 6-point Likert scale from 0 (having no effect on QOL) to 5 (very much affecting QOL).
Exercise Testing
Graded exercise testing was performed using a ramped protocol on a recumbent cycle ergometer (Lode, The Netherlands). Work rate on the ergometer was increased by 10 W/min (BTHS) or 20 W/min (CON) cycling at 60 rpm (RPM) until volitional exhaustion. 12-lead ECG, blood pressure, ratings of perceived exertion, oxygen consumption (VO2), carbon dioxide production (VCO2), ventilation (VE), and respiratory exchange ratio (RER) (ParvoMedics, Sandy, UT, USA) were continuously measured during testing. Exercise testing was considered peak with attainment of ≥85% predicted peak heart rate (220 − age) and/or RER ≥ 1.10 according to the American College of Sports Medicine (2000).
Resistance Exercise Training
Three participants (ages 24, 23, and 19) with BTHS participated in a 12-week supervised, progressive RET regimen performed at a local physical therapy or cardiac rehabilitation clinic near the participant’s home. Participants trained 3×/week for 60 min at 60% 1RM for the first 18 sessions, with the intensity increased to 70% 1RM for the last 18 sessions as tolerated. All participants were instructed to use a 3-s concentric, and 3-s eccentric lifting cadence, and performed three sets of six to ten repetitions with 2 min of rest between sets for eight lifts: knee extension, knee flexion, leg press, ankle plantar flexion, chest press, seated row, biceps curl, and overhead press. The participant’s 1RM was retested every ten sessions, with the weight on each lift increased to maintain the prescribed intensity. All sessions were supervised by a licensed physical therapist or exercise physiologist, who monitored participants’ heart rate, blood pressure, and levels of perceived exertion throughout training.
Post-testing
Post-testing consisted of the same items as outlined above. Post-testing was completed at Washington University within 48–72 h of the final exercise session.
Statistical Analysis
Baseline differences between BTHS and CON for outcomes were determined using independent t-tests. Differences between pre-testing and post-testing outcomes for body composition, muscle function, blood metabolites, QOL, exercise tolerance, and heart function were determined using paired t-tests. Significance was determined at p ≤ 0.05.
Results
Baseline Barth Syndrome vs. Controls Comparison
Participants were appropriately matched for age, sex, height, weight, and BMI (Table 1). Compared to CON, participants with BTHS had lower 1RM strength of the upper and lower extremities as well as reduced lower extremity muscle torque, work, and power (Fig. 1a, b). Participants with BTHS had greater regional and whole-body fat, and lower regional and whole-body lean tissue compared to Controls (Table 1). Moreover, all participants with BTHS were classified as sarcopenic according to the SMI. Individuals with BTHS also had significantly lower leg, arm, thoracic spine, lumbar spine, and pelvic bone mineral density as well as reduced VO2peak and exercise capacity than Controls (Table 1). Mean bone mineral density was <1 standard deviation below age-matched normative values as reported by the 1999–2006 National Health and Nutrition Examination Survey (NHANES), classifying them as osteopenic (Batsis et al. 2015). In addition to muscle function and body composition, individuals with BTHS had significantly lower serum creatinine, total cholesterol (p = 0.01), high-density lipoprotein (HDL, p = 0.01), and low-density lipoprotein (LDL, p = 0.02) (Table 1).
Baseline differences in muscle function between individuals with Barth syndrome (BTHS) and Controls. (a) Lower extremity knee extension and knee flexion isometric and isokinetic peak torque, work, and power measured at 180°/s. (b) One repetition maximum (1RM) strength for six exercises. Iso isometric, Isokin isokinetic. *Denotes significant difference BTHS vs. CON (p < 0.05)
Safety of Resistance Exercise Training
RET was well-tolerated with participants completing all 36 exercise visits. Participants experienced no adverse events or complications during testing or training. Mean plasma creatine kinase or brain natriuretic peptide did not increase with RET (Table 2). Mean absolute neutrophil count did not decrease but trended upward following RET (Table 2).
Muscle Strength and Function with Resistance Exercise Training
RET significantly increased muscle strength for the chest press, biceps curl, and shoulder press on 1RM testing from pre-test levels (Fig. 2a–d). While not significant, training also promoted consistent increases across almost all isometric and isokinetic tests of knee extension and flexion torque, work, and power, with the largest changes observed in knee extension and flexion power (Fig. 2e–h).
Changes in pre–post 1RM strength and muscle performance in individuals with BTHS (n = 3) following 12 weeks of resistance exercise training (RET). (a) Changes in knee extension 1RM. (b) Changes in shoulder press 1RM. (c) Changes in bench press 1RM. (d) Changes in biceps curl 1RM. (e) Changes in knee extension isokinetic torque measured at 180°/s. (f) Changes in knee extension isometric torque. (g) Changes in knee extension power measured at 180°/s. (h) Changes in knee flexion isokinetic torque measured at 180°/s. *Denotes mean significant difference from pre-training to post-training (p < 0.05). Iso isometric, Isokin isokinetic, KE knee extension, KF knee flexion
Body Composition and Bone Mineral Density with Resistance Exercise Training
RET tended to increase whole-body and tended to improve arm, leg, lumbar spine, and pelvis bone mineral density (Table 2) in individuals with BTHS.
Cardiac Function, Exercise Tolerance, and Quality of Life with Resistance Exercise Training
Individuals with BTHS had significantly higher heart rates and reduced systolic and diastolic blood pressure, though none of these measures were abnormal (Table 1). There was also a trend toward reduced fractional shortening among participants with BTHS (Table 1). Cardiovascular parameters including blood pressure and echocardiographic measures of systolic (ejection fraction, GSL) and diastolic function (early to late diastolic filling ratio (E/A ratio)) did not change following RET (Table 2). RET did not improve mean VO2peak, but tended to increase exercise test time (~25%). The mean total score on the MLWHFQ increased by an average of eight points following RET (Table 2) but this change was not significant.
Discussion
The primary finding of this pilot study was that adolescents and young adults with BTHS had reduced muscle strength and function that could be safely improved by 12 weeks of RET. This is the first study to demonstrate the potential efficacy of RET in improving skeletal muscle strength and function in individuals with BTHS. Despite the small sample size (n = 3), 12 weeks of RET significantly improved 1RM strength in both upper and lower extremity exercise and tended to improve muscle isometric and isokinetic torque, work, and power. The effects of RET on exercise tolerance (e.g., VO2peak), cardiac function (e.g., ejection fraction and fractional shortening), and QOL are less clear as no significant changes in these outcomes occurred with training. Larger studies with more sustained interventions are needed to fully detail the effects of RET on these variables.
Despite being matched for age, height, and weight, individuals with BTHS presented with abnormal body composition including lower lean mass, lower bone mineral density, and elevated fat mass. Elevations in fat mass and reductions in lean mass have been previously documented in individuals with BTHS (Cade et al. 2013; Bashir et al. 2017) and are also common in other metabolic diseases, including obesity and type 2 diabetes (Heshka et al. 2008; Batsis et al. 2015). Our group previously reported that adipose tissue lipolytic rate, when normalized to fat free mass, is reduced in BTHS, which may contribute to the accumulation of adipose tissue as lipids are spared in favor of amino acid (from muscle catabolism, potentially for gluconeogenesis) or glucose utilization (Cade et al. 2013). In this same study, we reported elevated leucine rate of appearance (a measure of protein catabolism) in BTHS, which may contribute to muscle wasting and cardioskeletal myopathy (Cade et al. 2013). Indeed, participants with BTHS in the current study were sarcopenic; a condition defined by a loss of muscle mass that is commonly associated with functional declines and difficulties with basic activities of daily living (Merriwether et al. 2012). RET tended to increase lean muscle mass and reduce fat mass in the arms and legs but did not rectify the notable reduction in lean mass. However, the ability to increase lean mass following resistance training may not be diminished in BTHS. Previous studies in healthy adults have reported 0.3–1 kg increases in lean mass after training periods ranging from 24 weeks to 8 months (Lo et al. 2011; Willis et al. 2012). Individuals with BTHS in this study, on average, gained 0.4 kg of whole-body lean mass after only 12 weeks of training, which suggests that their ability to increase lean mass may not be impaired.
Following RET, participants with BTHS also demonstrated significant increases in upper and lower extremity muscle strength during 1RM testing, with the gains in chest press, biceps curl, and shoulder press 1RM reaching significance in this small sample. RET also produced trends toward improvement in lower extremity muscle toque, work, and power. Previous studies in other populations have demonstrated analogous results – identifying adaptations in 1RM strength without significant improvements in isokinetic performance. In 2012, Toth et al. reported significant increases in 1RM strength, with minimal improvements in knee extensor torque production at 180°/s in a group of individuals with chronic heart failure (Toth et al. 2012). The improvement in muscle strength measured by 1RM (ranging from 7 to 65% per lift) is consistent with previous training studies in healthy adults, further confirming that despite lower absolute strength, individuals with BTHS retain their capacity to adapt to strength training (Aarskog et al. 2012).
The findings from this study have not only confirmed the previous finding of lower extremity muscle weakness in BTHS (Thompson et al. 2016) but have also expanded these data by demonstrating upper extremity weakness and deficits in lower extremity torque, work, and power production. Normative data by Brinks et al. (1995) found that among 20 males knee extension and knee flexion isokinetic torque measured at 180°/s was 140.3 Nm and 79.7 Nm, respectively. These results are: (1) similar to the performance of age-matched unaffected controls and (2) substantially higher than the knee extension and knee flexion torque produced by participants with BTHS in our study. Individuals with BTHS have been described as “clumsy,” which may be related to declines in muscle power output identified in this study (Barth Syndrome Foundation 2006). Importantly, 12 weeks of RET tended to improve isokinetic and isometric muscle performance, with the greatest improvements occurring in knee extension isometric and isokinetic torque, and knee extension power. However, even after training, individuals with BTHS maintained notably diminished lower extremity muscle torque, work, and power relative to age-matched, untrained controls.
Muscle mass is one of the most important determinants of muscle force production and thus declines in muscle torque are not unexpected in BTHS, and might account for declines in exercise tolerance and stamina. Likewise, reductions in lower extremity muscle work may be due to a loss of ability to maintain maximal or near-maximal force output throughout the tested range of motion, which may contribute to difficulties with activities of daily living or higher-level physical activities (e.g., sports). Harrington et al. found that in young men with non-BTHS chronic heart failure, quadriceps cross sectional area was an independent and significant predictor of exercise tolerance, concluding that muscle atrophy negatively affects exercise capacity (Harrington et al. 1997). These results suggest that reductions in leg, arm, and whole-body lean mass may exacerbate exercise intolerance by increasing the demand of even low-level activities.
In addition to deficits in muscle performance, we also identified lower lumbar spine, leg, and arm bone mineral density in individuals with BTHS. Indeed, bone mineral density in these participants was <1 standard deviation below normative values for their age, classifying them as osteopenic (Batsis et al. 2015). RET in the current study tended to improve bone mineral density in all regions assessed in the three participants. RET is known to increase bone mineral density in other populations secondary to increased strain from muscle contraction and bone/joint loading during exercise (Braith et al. 1996; Martyn-St James and Carroll 2010).
As noted above, many of the adaptations following RET identified in this study were small, suggesting that further intervention may be needed to augment RET-induced adaptation, including nutritional supplementation. For individuals with BTHS, a disease characterized by cardioskeletal myopathy, loss of lean muscle mass, reduced exercise tolerance, and abnormal amino acid metabolism, protein supplementation might be a beneficial adjunct to exercise training regimens.
Limitations
All participants with BTHS were taking beta blockers during this study, which may have affected maximal heart rate and therefore peak oxygen consumption. However, we previously investigated if graded exercise testing differed between those on or off beta-blocker therapy (Spencer et al. 2011). We found that there were no differences in contractility or heart rate between participants with BTHS on and off the medications. Based on these findings, we believe that these medications did not significantly influence the outcomes in this study. Additionally, although healthy controls reported a sedentary lifestyle, it is possible that participants with BTHS were more sedentary, which could have affected the results. However, given the very large group differences in muscle strength, muscle performance, and VO2, this was not likely to be solely due to differences in physical activity level.
Conclusion
Individuals with BTHS demonstrate lower lean mass muscle and increased fat mass, diminished upper and lower body muscle strength and function, bone mineral density, exercise tolerance, and oxygen utilization relative to healthy, age- and activity level-matched unaffected controls. Twelve weeks of individualized, progressive RET was safe and feasible, and individuals with BTHS were able to significantly improve upper and lower extremity muscle strength; larger studies are needed to determine the effects of RET on exercise tolerance, bone mineral density, and body composition.
Change history
03 August 2019
The name of the author, W. Todd Cade, was incorrectly captured as:
References
Aarskog R, Wisnes A, Wilhelmsen K, Skogen A, Bjordal JM (2012) Comparison of two resistance training protocols, 6RM versus 12RM, to increase the 1RM in healthy young adults. A single-blind, randomized controlled trial. Physiother Res Int 17:179–186
American College of Sports Medicine (2000) ACSM’s guidelines for exercise testing and prescription. Lippincott, Williams & Wilkins, Baltimore
Bashir A, Bohnert KL, Reeds DN et al (2017) Impaired cardiac and skeletal muscle bioenergetics in children, adolescents, and young adults with Barth syndrome. Physiol Rep 5:e13130
Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ (2015) Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999-2004. Nutr Res 35:1031–1039
Barth Syndrome Foundation (2006) Neurological manifestation of Barth syndrome. https://www.barthsyndrome.org/file_download/dd9faf11-7782-49cb-9163-264747605f2c
Braith RW, Mills RM, Welsch MA, Keller JW, Pollock ML (1996) Resistance exercise training restores bone mineral density in heart transplant recipients. J Am Coll Cardiol 28:1471–1477
Brinks K, DeLong R, Stout T (1995) The relationship among isokinetic knee parameters and three functional tests. Masters Theses 237. http://scholarworks.gvsu.edu/theses/237
Cade WT, Spencer CT, Reeds DN et al (2013) Substrate metabolism during basal and hyperinsulinemic conditions in adolescents and young-adults with Barth syndrome. J Inherit Metab Dis 36:91–101
Cade WT, Reeds DN, Peterson LR et al (2017) Endurance exercise training in young adults with Barth syndrome: a pilot study. JIMD Rep 32:15–24
Clarke SL, Bowron A, Gonzalez IL et al (2013) Barth syndrome. Orphanet J Rare Dis 8:23
De Maeyer C, Beckers P, Vrints CJ, Conraads VM (2013) Exercise training in chronic heart failure. Ther Adv Chronic Dis 4:105–117
Egan B, Zierath JR (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17:162–184
Gielen S, Schuler G, Adams V (2010) Cardiovascular effects of exercise training: molecular mechanisms. Circulation 122:1221–1238
Harrington D, Anker SD, Chua TP et al (1997) Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol 30(7):1758–1764
Heshka S, Ruggiero A, Bray GA et al (2008) Altered body composition in type 2 diabetes mellitus. Int J Obes 32:780–787
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R (2004) Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 159:413–421
Lo MS, Lin LL, Yao WJ, Ma MC (2011) Training and detraining effects of the resistance vs. endurance program on body composition, body size, and physical performance in young men. J Strength Cond Res 25:2246–2254
Martyn-St James M, Carroll S (2010) Effects of different impact exercise modalities on bone mineral density in premenopausal women: a meta-analysis. J Bone Miner Metab 28:251–267
McKenzie M, Lazarou M, Thorburn DR, Ryan MT (2006) Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. J Mol Biol 361:462–469
Menkes A, Mazel S, Redmond RA et al (1993) Strength training increases regional bone mineral density and bone remodeling in middle-aged and older men. J Appl Physiol (1985) 74:2478–2484
Merriwether EN, Host HH, Sinacore DR (2012) Sarcopenic indices in community-dwelling older adults. J Geriatr Phys Ther 35:118–125
Rector TS, Cohn JN (1992) Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J 124:1017–1025
Schlame M, Kelley RI, Feigenbaum A et al (2003) Phospholipid abnormalities in children with Barth syndrome. J Am Coll Cardiol 42:1994–1999
Spencer CT, Byrne BJ, Bryant RM et al (2011) Impaired cardiac reserve and severely diminished skeletal muscle O(2) utilization mediate exercise intolerance in Barth syndrome. Am J Physiol Heart Circ Physiol 301:H2122–H2129
Thompson WR, DeCroes B, McClellan R et al (2016) New targets for monitoring and therapy in Barth syndrome. Genet Med 18:1001–1010
Toth MJ, Miller MS, VanBuren P et al (2012) Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol 590:1243–1259
Wang G, McCain ML, Yang L et al (2014) Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 20:616–623
Willis LH, Slentz CA, Bateman LA et al (2012) Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol (1985) 113:1831–1837
Wilson JM, Loenneke JP, Jo E, Wilson GJ, Zourdos MC, Kim JS (2012) The effects of endurance, strength, and power training on muscle fiber type shifting. J Strength Cond Res 26:1724–1729
Acknowledgements
This work was supported by the Barth Syndrome Foundation, Foundation for Physical Therapy and National Institutes of Health R01HL107406-01, P30DK056341, P30DK020579, and UL1TR000448 from the National Center for Research Resources and NIH Roadmap for Medical Research. Echocardiographic imaging was supported by NIH grant S10RR024532 and a grant from the Barnes-Jewish Hospital Foundation to the Cardiovascular Imaging and Clinical Research Core Laboratory.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Verena Peters
Electronic Supplementary Material
Supplemental Table 1
Medications used and ICD status for participants with Barth Syndrome (DOCX 103 kb)
Appendices
Synopsis
Progressive resistance exercise training (RET) is safe and feasible for individuals with BTHS, and results in significant improvements in upper and lower extremity muscle strength.
Author Contributions
WTC, LRP, DNR, and BJB were responsible for the concept and design of the study. AJB, KLB, LRP, LD, MC, and WTC were responsible for data collection. AJB, KLB, LD, CLT, and WTC performed data analysis and interpretation. AJB and WTC drafted the manuscript. KLB, DNR, LRP, LD, MC, CLT, and BJB contributed to editing the manuscript.
Guarantor
W. Todd Cade, PT, PhD.
Competing Interests
None of the authors have conflicts of interest to declare and have not received significant financial support for this work that could have influenced its outcome.
Funding
This work was supported by the Barth Syndrome Foundation, Foundation for Physical Therapy and National Institutes of Health R01HL107406-01, P30DK056341, P30DK020579, and UL1TR000448 from the National Center for Research Resources and NIH Roadmap for Medical Research. Echocardiographic imaging was supported by NIH grant S10RR024532 and a grant from the Barnes-Jewish Hospital Foundation to the Cardiovascular Imaging and Clinical Research Core Laboratory. The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Ethics Approval
Studies were approved by the Human Studies Committee at Washington University in St. Louis on 6/7/12.
Consent Statement
All participants and parents (i.e. adolescents) provided written informed consent prior to all study procedures.
Rights and permissions
Copyright information
© 2018 Society for the Study of Inborn Errors of Metabolism (SSIEM)
About this chapter
Cite this chapter
Bittel, A.J. et al. (2018). Reduced Muscle Strength in Barth Syndrome May Be Improved by Resistance Exercise Training: A Pilot Study. In: Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V. (eds) JIMD Reports, Volume 41. JIMD Reports, vol 41. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2018_102
Download citation
DOI: https://doi.org/10.1007/8904_2018_102
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-58080-6
Online ISBN: 978-3-662-58081-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)