Abstract
We report the third case of Glycogen Storage Disease type 1b (GSD 1b) with Giant Cell Tumour (GCT) of the mandible, associated with Granulocyte Colony Stimulating Factor (G-CSF) use. G-CSF in GSD 1b is indicated for persistent neutropaenia, sepsis, inflammatory bowel disease and severe diarrhoea. Our patient was 12 years old at GCT diagnosis and had been treated with G-CSF from 5 years of age. He underwent therapy with interferon followed by local resection which was successful in initial control of the disease. Histology demonstrated spindle shaped stromal cells together with numerous interspersed multinuclear osteoclastic giant cells. G-CSF has been hypothesized to induce osteoclastic differentiation and thus may be involved in the pathogenesis of GCT formation. At age 19 years he required a repeat operation for local recurrence. He currently continues on G-CSF and was commenced on denosumab for control of the GCT with no recurrence to date. A cause and effect relationship between G-CSF therapy and the development of GCT in GSD type 1b remains to be established.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Glycogen storage disorder 1b (GSD 1b) is due to recessive mutations in the SLC37A4 gene expressing glucose-6-phosphate translocase. In addition to hypoglycaemia, GSD 1b is complicated by hyperlipidaemia, hyperuricaemia, growth retardation, hepatosplenomegaly, renal dysfunction and neutropaenia with other hematologic abnormalities (Burda and Hochuli 2015; Chou et al. 2015). Here we report the third case associating GSD 1b and Giant Cell Tumour (GCT) of the mandible while the patient was on Granulocyte Colony Stimulating Factor (G-CSF).
Case Report
Our patient was born following a normal pregnancy at term weighing 3.3 kg. He had transient hypoglycaemia in the newborn period and was discharged home on day 14 of life. He represented at age 5 months with a diarrhoeal illness and hypoglycaemia. Hepatomegaly was noted at that time. Following a number of further admissions with hypoglycaemia during intercurrent illness, a liver biopsy was performed which reportedly demonstrated glycogen storage disease (although the details of this result are not available to us). Persistent neutropenia was apparent in the first year of life. Genetic sequencing demonstrated an apparently homozygous variant reported as pathogenic in the SLC37A4 gene (c.1179G>A; p.Trp393*). Metabolic control was not optimal during the first years of life, with multiple hospital admissions and documented hypoglycaemia. Disease complications have included episodes of hypoglycaemia leading to intellectual disability, multiple episodes of sepsis, neutropenia, haemolytic anaemia, splenectomy and multiple hepatic adenomas. G-CSF was commenced at 5 years of age. His current dose of G-CSF is 300 mcg (5 mcg/kg) four times weekly, with Australian guidelines (Australian Medicines Handbook) suggesting 5 mcg/kg daily in cases of idiopathic or cyclic neutropenia.
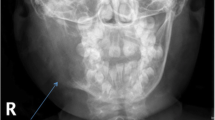
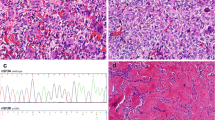
He was diagnosed with GCT of the mandible at age 12 years. Interferon therapy for post excision management of his tumour was continued for 3 years post resection. He re-presented with increased gum bleeding 7 years later. A CT and biopsy of the site confirmed recurrence and tumour excision surgery was performed. Histology of the lesion revealed large giant cells consistent with giant cell tumour (Figs. 1 and 2). He has been placed on denosumab therapy for postoperative control of the giant cell tumour.
Discussion
GSD 1b and GCT of the mandible have previously been described in two other patients (Mortellaro et al. 2005; Amaral et al. 2009). Both were receiving G-CSF at the time of tumour occurrence. To our knowledge, this is the third case of a patient with GSD 1b and a giant cell tumour on G-CSF, suggesting a causative association.
Giant cell granulomas of the jaw are benign, locally aggressive tumours of the jaw of unknown aetiology but their occurrence has been observed in Neurofibromatosis type 1, Noonan syndrome and cherubism (O’Connell et al. 2015). These lesions arise from the supporting connective tissue of the marrow and are characterised histologically by ovoid stromal or spindle shaped cells interspersed with multinuclear cells and tend to recur locally despite surgery (Amanatullah et al. 2014; O’Connell et al. 2015).
Although the origins of giant cell tumours are unclear, it has been suggested that they may be derived from osteoclast progenitors (Liu et al. 2003). It has been hypothesized that long term use of G-CSF promotes osteoclastogenesis resulting in giant cell tumours (Amaral et al. 2009). G-CSF activates extracellular membrane receptors such as c-fms. These receptors then activate a signalling pathway that differentiates progenitor myeloid cells into osteoclasts (Kitaura et al. 2005).
It is interesting to note that the other two cases of GSD 1b who developed giant cell tumours were also on G-CSF (Mortellaro et al. 2005; Amaral et al. 2009). While generally accepted to be safe, a potential for carcinogenicity due to the presence of G-CSF receptors in tumour cell lines exists (Schipperus et al. 1990). There have been reports of three patients with GSD 1b developing acute myelogenous leukaemia, two on G-CSF and one not on G-CSF (Simmons et al. 1984; Pinsk et al. 2002; Schroeder et al. 2008). In fact, previous consensus guidelines for GSD 1 management included annual bone marrow examination with cytologic studies in patients on G-CSF, although this is no longer included in the current consensus guidelines (Visser et al. 2002; Kishnani et al. 2014). The role of GSD1b and resultant aberrant glycogen in GCT tumourigenesis remains to be defined.
We also consider the possibility that the periodontitis and resulting chronic inflammation, as well as the aberrant glycogen seen in GSD1b may represent an additive effect together with G-CSF that results in abnormal cellular differentiation and growth. Oral manifestations of GSD 1b (periodontitis, recurrent oral ulceration, bleeding diatheses and dental caries) are thought to be related to neutropaenia and/or neutrophil dysfunction; similar oral manifestations are seen in other neutropaenic states (Barrett et al. 1990). This case of GCT in a patient with GSD1b on G-CSF highlights a possible association, and indicates that vigilance for this disease complication is warranted.
Conclusion
We describe the third reported case of GCT of the mandible recurring in a patient with GSD1b whilst the patient was on G-CSF therapy. Histology and imaging supported the diagnosis of GCT of the mandible. It remains to be established whether there is a cause and effect relationship between G-CSF therapy and the development of GCT in GSD type 1b.
References
Amanatullah DF, Clark TR, Lopez MJ, Borys D, Tamurian RM (2014) Giant cell tumor of bone. Orthopedics 37:112–120
Amaral FR, Carvalho VM, Fraga MG, Amaral TM, Gomes CC, Gomez RS (2009) Oral giant cell granuloma in a patient with glycogen storage disease. Open Dent J 3:144–146
Barrett AP, Buckley DJ, Katelaris CH (1990) Oral complications in type 1B glycogen storage disease. Oral Surg Oral Med Oral Pathol 69:174–176
Burda P, Hochuli M (2015) Hepatic glycogen storage disorders: what have we learned in recent years? Curr Opin Clin Nutr Metab Care 18:415–421
Chou JY, Jun HS, Mansfield BC (2015) Type I glycogen storage diseases: disorders of the glucose-6-phosphatase/glucose-6-phosphate transporter complexes. J Inherit Metab Dis 38:511–519
Kishnani PS, Austin SL, Abdenur JE et al (2014) Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med 16:e1
Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL (2005) M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest 115:3418–3427
Liu B, Yu SF, Li TJ (2003) Multinucleated giant cells in various forms of giant cell containing lesions of the jaws express features of osteoclasts. J Oral Pathol Med 32:367–375
Mortellaro C, Garagiola U, Carbone V, Cerutti F, Marci V, Bonda PL (2005) Unusual oral manifestations and evolution in glycogen storage disease type Ib. J Craniofac Surg 16:45–52
O’Connell JE, Bowe C, Murphy C, Toner M, Kearns GJ (2015) Aggressive giant cell lesion of the jaws: a review of management options and report of a mandibular lesion treated with denosumab. Oral Surg Oral Med Oral Pathol Oral Radiol 120:e191–e198
Pinsk M, Burzynski J, Yhap M, Fraser RB, Cummings B, Ste-Marie M (2002) Acute myelogenous leukemia and glycogen storage disease 1b. J Pediatr Hematol Oncol 24:756–758
Schipperus MR, Sonneveld P, Lindemans J et al (1990) The combined effects of Il-3, GM-CSF and G-CSF on the in vitro growth of myelodysplastic myeloid progenitor cells. Leuk Res 14:1019–1025
Schroeder T, Hildebrandt B, Mayatepek E, Germing U, Haas R (2008) A patient with glycogen storage disease type Ib presenting with acute myeloid leukemia (AML) bearing monosomy 7 and translocation t(3;8)(q26;q24) after 14 years of treatment with granulocyte colony-stimulating factor (G-CSF): a case report. J Med Case Rep 2:319
Simmons PS, Smithson WA, Gronert GA, Haymond MW (1984) Acute myelogenous leukemia and malignant hyperthermia in a patient with type 1b glycogen storage disease. J Pediatr 105:428–431
Visser G, Rake JP, Labrune P et al (2002) Consensus guidelines for management of glycogen storage disease type 1b – European Study on Glycogen Storage Disease Type 1. Eur J Pediatr 161(Suppl 1):S120–S123
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Avihu Boneh, MD, PhD, FRACP
Appendices
Conflict of Interest
Raajiv Prasad, Jane Estrella, John Christodoulou, Geoffrey McKellar and Michel C. Tchan have no disclosures related to this manuscript.
Informed Consent
Informed consent was obtained from the patient and his parents for being included in this study.
Contributions of the Individual Authors
Raajiv Prasad and Jane Estrella have contributed equally to the writing of this manuscript. All authors have contributed to the planning, conduct and reporting of the work described in this chapter and all authors have approved the manuscript.
Rights and permissions
Copyright information
© 2017 Society for the Study of Inborn Errors of Metabolism (SSIEM)
About this chapter
Cite this chapter
Prasad, R., Estrella, J., Christodoulou, J., McKellar, G., Tchan, M.C. (2017). A Third Case of Glycogen Storage Disease IB and Giant Cell Tumour of the Mandible: A Disease Association or Iatrogenic Complication of Therapy. In: Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V. (eds) JIMD Reports, Volume 42. JIMD Reports, vol 42. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2017_67
Download citation
DOI: https://doi.org/10.1007/8904_2017_67
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-58364-7
Online ISBN: 978-3-662-58365-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)