Abstract
We describe two half-siblings with monocarboxylate transporter 1 (MCT1, SLC16A1) deficiency, a defect on ketone body utilization, that has only recently been identified (van Hasselt et al., N Engl J Med, 371:1900–1907, 2014) as a cause for recurrent ketoacidoses. Our index patient is a boy with non-consanguineous parents who had presented acutely with impaired consciousness and severe metabolic ketoacidosis following a 3-day history of gastroenteritis at age 5 years. A 12.5-year-old half-brother who shared the proband’s mother also had a previous history of recurrent ketoacidoses. Results of mutation and enzyme activity analyses in proband samples advocated against methylacetoacetyl-coenzyme A thiolase (“beta-ketothiolase”) and succinyl-coenzyme A: 3-oxoacyl coenzyme A transferase (SCOT) deficiencies. A single heterozygous c.982C>T transition in the SLC16A1 gene resulting in a stop mutation (p.Arg328Ter) was detected in both boys. It was shared by their healthy mother and by the proband’s half-sister, but was absent in the proband’s father. MCT1 deficiency may be more prevalent than is apparent, as clinical manifestations can occur both in individuals with bi- and monoallelic mutations. It may be an important differential diagnosis in recurrent ketoacidosis with or without hypoglycemia, particularly in the absence of any specific metabolic profiles in blood and urine. Early diagnosis may enable improved disease management. Careful identification of potential triggers of metabolic decompensations in individuals even with single heterozygous mutations in the SLC16A1 gene is indicated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The ketone bodies acetoacetate and d-3-hydroxy-n-butyric acid are derived from fatty acids and ketogenic amino acids such as leucine. They are important vectors of energy transport from the liver to extrahepatic tissues during prolonged fasting or in cases of enhanced energy requirements (Sass 2012; Fukao et al. 2014). Ketone bodies play a key role in glucose-sparing energy supply, particularly in the brain, which is unable to utilize fatty acids directly (Mitchell et al. 1995). The interconversion of acetoacetate with d-3-hydroxy-n-butyric acid is catalyzed by d-3-hydroxy-n-butyrate dehydrogenase. It reflects the oxidation status of the mitochondrial matrix.
Ketolysis (ketone body utilization) occurs in extrahepatic tissues. Its first and rate-limiting step requires the enzyme succinyl-coenzyme A: 3-oxoacyl coenzyme A transferase (SCOT; EC 2.8.3.5) which activates acetoacetate to acetoacetyl-coenzyme A. A mitochondrial acetoacetyl-coenzyme A thiolase (“beta-ketothiolase”; EC 2.3.1.9) then catalyzes the formation of two acetyl-coenzyme A (acetyl-CoA) molecules per molecule acetoacetyl-CoA. Since this enzyme has also a role in isoleucine catabolism, where it catalyzes the thiolytic cleavage of methylacetoacetyl-coenzyme A, it is more unambiguously named methylacetoacetyl-CoA thiolase (MAT; EC 2.3.1.9) (Sass 2012).
Defects in the genes encoding for SCOT (OXCT1) and MAT (ACAT1) are the cause of the established inborn errors of ketolysis (OMIM 245050 and OMIM 203750) (Mitchell et al. 1995; Sass 2012; Fukao et al. 2014). Patients usually present with ketoacidotic episodes, which may be life-threatening. Due to accumulating isoleucine metabolites, the laboratory diagnosis of MAT deficiency can be rather straightforward by analyses of urinary organic acids and blood acylcarnitines, as long as a defect of the preceding step in the isoleucine pathway is considered in the differential diagnosis (Sass 2012). In contrast there is no specific metabolite marker for SCOT deficiency. This ketolysis defect is suspected in cases of unexplained pronounced or frequent ketoacidotic episodes and in some cases is associated with persistent ketonuria (Fukao et al. 2004). Enzyme activity testing in blood cells or cultivated fibroblasts may clarify whether SCOT deficiency is present or not. Sequence analysis of the OXCT1 gene is another option.
Recently, van Hasselt et al. (2014) have revealed homozygous and heterozygous mutations in the SLC16A1 gene, which encodes the monocarboxylate transporter 1 (MCT1), in ketoacidotic patients with a suspected defect in ketolysis, but normal enzyme activities of SCOT and MAT. Such a finding may have major impact on the diagnostics of ketoacidosis, but so far awaits confirmation in other patients. Here we report a family with two symptomatic boys and a pedigree which supports the opinion that even a single heterozygous mutation can result in clinically relevant symptoms and that biallelic mutations in the SLC16A1 gene are not always required for clinical symptoms.
Case Reports
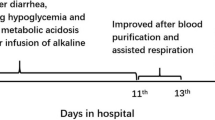
A 5-year-old boy born to non-consanguineous British parents presented acutely with impaired consciousness following a 3-day history of gastroenteritis while holidaying in Croatia. He was initially managed with oral rehydration solutions for the first 24 h; however, due to unrelenting vomiting, he presented to an emergency clinic. His capillary blood glucose was normal; however, no other blood or urine tests were done at that stage. After initiation of intravenous normal saline maintenance infusion, with sips of sweet drinks, his vomiting reduced. By the third day, he became extremely lethargic and was referred to a regional hospital. Shortly upon presentation to the hospital, he deteriorated rapidly and became encephalopathic. He was tachypnoeic and mildly dehydrated but afebrile with no localizing neurological signs. His arterial blood pH was 7.13, bicarbonate 7.3 mmol/L, BE −19.2 mmol/L, and anion gap 22.7 mmol/L. His plasma 3-hydroxy-n-butyrate was increased at 5.2 mmol/L, and significant urinary excretion of ketones was demonstrated. His plasma lactate, ammonia, glucose, and liver function remained normal. He was treated with a normal saline bolus, followed by continuous infusion of dextrose at 9 mg/kg/min with sodium and potassium supplements. His condition improved over the next few hours with fluid and caloric replacement, and he was discharged the next day. His urine metabolic screen showed massive excretions of 3-hydroxy-n-butyrate and acetoacetate and detectable amounts of 2-methyl-3-hydroxybutyrate (2M3HB), 2-methylacetoacetate (2MeAA), and tiglylglycine (TG), leading to an initial suspicion of mitochondrial MAT deficiency. A Guthrie card collected during the crisis was not analyzed then, but provided to the parents and studied later (see below). Back home in Perth, Western Australia, the proband was referred to the metabolic service. Upon further recollection, his parents reported a possible previous history of recurrent ketotic episodes associated with fruity odor on his breath and fast breathing during intercurrent infections or unwell periods. Clinical examination was unremarkable. He was a well-thrived child with age-appropriate developmental milestones. Interestingly, a similar history of recurrent ketoacidosis was elicited in an elder 11.5-year-old half-brother who shared the proband’s mother. He was previously managed at a local hospital in the United Kingdom, having first presented at 18 months of age, following a day of profuse vomiting and diarrhea. He had significant metabolic acidosis with a pH of 7.24, bicarbonate of 5 mmol/L, and BE of −13 mmol/L. He was discharged home after a short stay, but readmitted 10 days later with gastroenteritis. His blood pH was 7.33, bicarbonate 15 mmol/L, and BE −7 mmol/L. His blood glucose, plasma ammonia, and urine organic acids were reported to be normal. He was reported to be ketotic; however, a documented level was not provided. At 2 years of age, while holidaying in Tenerife, he had another vomiting illness and became apathetic, but responded to antiemetics. At 3.5 years of age, an occipital skull fracture sustained from a fall off play equipment resulted in recurrent vomiting necessitating hospital admission. His blood pH was 7.20, bicarbonate 9.5 mmol/L, and BE −15.5 mmol/L. He responded well after the commencement of an intravenous glucose infusion. Neuroimaging did not reveal any intracranial hemorrhages or cerebral concussions. He was discharged soon thereafter.
A conclusive diagnosis for the recurrent ketoacidosis had not been reached. It was suggested that he may outgrow the ketotic episodes and was advised to have sweetened drinks when unwell. He was subsequently discharged from the pediatric clinic, and the family migrated to Australia shortly thereafter.
Investigations performed on both well boys aged 5.5 and 12 years included plasma glucose, amino acids, free carnitine, acylcarnitines, 3-hydroxy-n-butyrate, free fatty acids, urate, blood gas, and liver function tests which were all normal. The crisis bloodspot collected from the index patient in Croatia was analyzed, but did not show any abnormalities; of specific relevance, the C5:1 was 0.02 μmol/L (normal range <0.04) and C5OH was 0.30 μmol/L (normal range <0.44). The levels of 2M3HB, 2MeAA, and TG were all undetectable in noncrisis urine samples from both boys.
The index patient has subsequently had a few mild episodes of ketonuria when unwell or if he has exerted himself during strenuous activities. He has, however, responded well with early initiation of increased caloric intake using glucose polymers, mild protein restriction during unwell periods, and early use of antiemetics when nonobstructive vomiting is a predominant feature.
Interestingly, the initially suspected resolution of ketotic episodes that the parents had reported in the elder half-brother was proven otherwise when suggested home monitoring displayed intermittent mild ketonuria when unwell. His mother had previously monitored his urine ketones when he was younger, but ceased over the last couple of years.
The other family members including the boys’ biological fathers and their mother were all reported to be healthy. The proband’s almost 15-year-old half-sister had poor weight gain in early childhood but caught up later and was otherwise well. The absence of relevant symptomology in the mother and half-sister was particularly interesting as they too carried the same mutation.
Material and Methods
Enzyme Activity Assays
Assays for acetoacetyl-CoA thiolases (with MAT being the only such enzyme activated by potassium ions) and for SCOT were performed as described, based on spectrophotometric monitoring of conversion of the substrate acetoacetyl-CoA spectrophotometrically at 303 nm (Fukao et al. 1996; Sakazaki et al. 1995).
Molecular Testing
Molecular genetic investigations were performed by PCR and sequence analysis of genomic DNA. We tested all exons of the ACAT1 gene of the index patient plus flanking intronic regions. Reference sequence for the ACAT1 gene was ENSG00000075239. Reference sequence for the ACAT1 cDNA was NM_000019.3. We tested all four coding exons of the SLC16A1 gene plus flanking noncoding sequences in the parents and children indicated in the pedigree (Fig. 1). Reference sequence for the SLC16A1 gene was ENSG00000155380; reference sequence for the SLC16A1 cDNA was NM_003051.3.
Pedigree. The index patient is indicated with an arrow. Black and hatched black-and-white fills indicate a monoallelic c.982C>T (p.Arg328Ter) mutation in the SLC16A1 gene of symptomatic (full black) and asymptomatic (hatched) family members, respectively (Grey fills indicate the investigated individuals)
Immunoblot Analysis
In Western blot analysis, three fibroblast samples of the index patient (different passages) were analyzed on one or two gels each, and the mean value ± standard deviation of intensity ratios was calculated from the (mean) value obtained for each of the three samples. In parallel to the patient samples, 18 control samples were investigated. They comprised cells of individuals with various confirmed metabolic disorders. Samples of patients with a suspected defect in ketone body utilization were only included as controls if such a defect had been confirmed (n = 1 SCOT deficiency; n = 2 MAT deficiency), but not if any suspicion for an undiagnosed defect on ketone body utilization was still pending. One of the 18 controls was analyzed on all three gels; in that case, the mean values were used for the calculation.
Fibroblasts were homogenized in RIPA buffer (25 mM Tris–HCl (pH 7.6), 150 mM NaCl, 1% (v/v) NP-40, 1% (w/v) sodium deoxycholate, 0.1% (w/v) SDS) containing protease inhibitors (Protease Inhibitor Cocktail, Sigma). Aliquots of 30 μg of total protein were separated on 4–20% gradient Mini Protean TGX Gels (Biorad) and transferred onto PVDF membranes by semidry blotting using the Trans Blot Turbo system (Biorad). Membranes were blocked with 4% nonfat dry milk in TBS/0.05% Tween 20 (TTBS) for 1 h at room temperature and then incubated overnight at 4°C with affinity purified rabbit polyclonal antibody against MCT1 (Millipore, AB3538P) diluted 1:1,000 in 5% nonfat dry milk/TTBS. As a loading control, the membranes were stripped and reprobed with a polyclonal antibody against GAPDH (GeneTex, #GTX100118) diluted 1:10,000 in 5% nonfat dry milk/TTBS. After incubation with a horseradish-peroxidase-conjugated secondary antibody (GE Healthcare, NA934V) diluted 1:10,000 in 5% nonfat dry milk/TTBS for 1 h at room temperature, the antigen-antibody complexes were visualized by use of Super Signal West Femto Maximum Sensitivity Substrate (Pierce, #34094) for MCT1 detection and ECL (GE Healthcare, RPN2106) for GAPDH detection in an imaging apparatus (My ECL Imager, Fisher Scientific). Ratios of signal intensities of the MCT1 and GAPDH bands were determined with the program Image J (http://rsbweb.nih.goc/ij/download.html).
Results
MAT and SCOT enzyme activities were assessed in fibroblast homogenates (Table 1). MAT activity was considered normal, since thiolase activity was clearly increased if potassium ions were added, thus reflecting the results in negative control samples, while the positive control for MAT deficiency showed no such increase. SCOT activity was also normal. Since small signals of isoleucine metabolites had been noted in the urinary organic acids analyzed during that metabolic decompensation which prompted the metabolic work-up, a special effort was made to exclude MAT deficiency. Although MAT deficiency would be expected to be identified by fibroblast analysis, the focus on potential MAT deficiency was due to the fact that metabolite abnormalities may be subtle (Fukao et al. 2011). Therefore, mutation analysis was added by Sanger sequencing of the ACAT1 gene of the patient, but also revealed no abnormality. Thus, it was concluded that neither MAT nor SCOT deficiency was the cause for the metabolic decompensations in the index patient. The small signals of urinary isoleucine metabolites which were not identified in any other urine samples of the patient and his brother and were not reflected by abnormalities in the acylcarnitine pattern during the crisis very likely represent nonspecific changes during a heavy metabolic crisis, as it is especially known for 2M3HB.
Sanger sequencing of the SLC16A1 gene was performed in the index patient and his parents and half-siblings. The mother and all children were found to be heterozygous for the c.982C>T mutation which is predicted to result in a premature stop of protein synthesis (p.Arg328Ter) (Fig. 1, Table 1). This was not identified in the DNA of the father of the index case. In homozygous form, this mutation has already been reported by van Hasselt et al. (2014) in the ketoacidotic son of consanguineous Turkish parents. Since c.982C>T was identified both in symptomatic and asymptomatic individuals, we also studied the distribution of single nucleotide polymorphisms (SNPs) of the SLC16A1 gene among the five individuals to reveal a possible contribution of one of these SNPs to disease manifestation (Table 2).
The immunoblot analysis of the three fibroblast homogenate samples of the index patient did not yield a relative decrease of the MCT1/GAPDH signal ratio compared with control fibroblasts (n = 18); mean values ± standard deviations were 0.70 ± 0.15 for the patient cells and 0.49 ± 0.19 for the controls.
Discussion
Our investigations on a family with affected half-siblings further support that even a single heterozygous mutation in the SLC16A1 gene can lead to clinical symptoms of MCT1 deficiency (OMIM 616095). In their study, van Hasselt et al. (2014) had confirmed heterozygosity of mutations by ruling out exon-sized deletions and confirmed biallelic expression by cDNA sequencing. In our family, the identification of only the maternal mutation in both symptomatic boys with different fathers further supports that a monoallelic mutation in the SLC16A1 gene can be the only relevant change in this gene. The presence of the mutation in the asymptomatic mother and the patients’ (half) sister underlines that additional triggers are needed for the development of ketoacidotic episodes. The study of SNPs in the SLC16A1 gene of the five individuals provided no strong evidence for an association of any of the analyzed SNPs with the clinical phenotype, although one SNP (c.362-14_362-11delTATT; rs149491709) is present in heterozygous form in the affected half-brothers, but in homozygous form in the mother and her daughter. However, this is not only a frequent SNP (MAF = 0.123) but also localized in an intron and unlikely to result in functional interference with the truncating mutation c.982C>T (p.Arg328Ter).
The relevance of polymorphisms in the SLC16A1 gene has recently been highlighted by Fei et al. (2015) who have shown that in genomic DNA obtained from blood, the SNP rs1049434 was significantly associated with the death risk of colorectal cancer patients. However, in our family, there was no consistent difference regarding this SNP if the symptomatic boys were compared with their nonsymptomatic relatives.
It is noteworthy that our immunoblot analyses, for which we used the same polyclonal antibody against MCT1 protein as van Hasselt et al. (2014), did not yield a decreased amount of MCT1/GAPDH ratio in the index patient, who carries p.Arg328Ter mutation. Van Hasselt et al. (2014) reported a significantly reduced relative level of MCT1 protein in fibroblasts from patients with other heterozygous truncating mutations. However, they have studied fibroblasts with the p.Arg328Ter mutation in homozygous form only and showed a decreased ratio between MCT1 and their reference protein tubulin. The discrepancy is surprising, so far it is not clear whether it reflects a functional difference between heterozygosity for the p.Arg328Ter mutation and that for other truncating mutations in the SLC16A1 gene, differences in the sensitivity of the immunoblots performed in different laboratories or has a yet undefined cause.
A variety of causes of ketoacidoses are known (for review, see Sass 2012). Thus far, however ketoacidosis in subjects without associated hypoglycemia, with normal blood lactate, and in the absence of any specific metabolic markers in blood or urine analysis has usually prompted diagnostic work-up for SCOT deficiency. MCT1 deficiency should be considered in the differential diagnosis. Awareness of those diseases is crucial for taking early preventive measures and thus minimizing the damaging effects of ketoacidotic episodes. In case of MCT1 deficient patients with some residual activity, as expected in heterozygotes, one may speculate, whether treatment with methionine precursor DL-2-hydroxy-(4-methylthio)butanoic acid (HMTBA) might have a supporting effect. Further studies should be carried out to investigate whether HMTBA, which is widely used as a supplemental methionine source in animals, will enhance MCT1 expression in vivo, after this has successfully been demonstrated in vitro with immortalized human epithelial colorectal adenocarcinoma cells (Caco-2) (Martín-Venegas et al. 2012).
References
Fei F, Guo X, Chen Y, Liu X, Tu J, Xing J, Chen Z, Ji J, He X (2015) Polymorphisms of monocarboxylate transporter genes are associated with clinical outcomes in patients with colorectal cancer. J Cancer Res Clin Oncol 141:1095–1102
Fukao T, Song XQ, Yamaguchi S, Hashimoto T, Orii T, Kondo N (1996) Immunotitration analysis of cytosolic acetoacetyl-coenzyme A thiolase activity in human fibroblasts. Pediatr Res 39:1055–1058
Fukao T, Shintaku H, Kusubae R, Zhang X-Q, Nakamura K, Kondo M, Kondo N (2004) Patients homozygous for the T435N mutation of succinyl-CoA:3-ketoacid CoA transferase (SCOT) do not show permanent ketosis. Pediatr Res 56:858–863
Fukao T, Maruyama S, Ohura T, Hasegawa Y, Toyoshima M, Haapalainen AM, Kuwada N, Imamuram I, Yuasa I, Wierenga RK, Yamaguchi S, Kondo N (2011) Three Japanese patients with beta-ketothiolase deficiency who share a mutation, c.431A>C (H144P) in ACAT1. Subtle abnormality in urinary organic acid analysis and blood acylcarnitine analysis using tandem mass spectrometry. JIMD Rep 3:107–115
Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y (2014) Ketone body metabolism and its defects. J Inherit Metab Dis 37:541–551
Martín-Venegas R, Brufau MT, Mañas-Cano O, Mercier Y, Nonis MK, Ferrer R (2012) Monocarboxylate transporter 1 is up-regulated in Caco-2 cells by the methionine precursor DL-2-hydroxy-(4-methylthio)butanoic acid. Vet J 202:555–560
Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, Robert MF, Wang SP, Ashmarina L, Lambert M, Lapierre P, Potier E (1995) Medical aspects of ketone body metabolism. Clin Invest Med 18:193–216
Sakazaki H, Hirayama K, Murakami S, Yonezawa S, Shintaku H, Sawada Y, Fukao T, Watanabe H, Orii T, Isshiki G (1995) A new Japanese case of succinyl-CoA: 3-ketoacid CoA-transferase deficiency. J Inherit Metab Dis 18:323–325
Sass JO (2012) Inborn errors of ketogenesis and ketone body utilization. J Inherit Metab Dis 35:23–28
van Hasselt PM, Ferdinandusse S, Monroe GR, Ruiter JP, Turkenburg M, Geerlings MJ, Duran K, Harakalova M, van der Zwaag B, Monavari AA, Okur I, Sharrard MJ, Cleary M, O’Connell N, Walker V, Rubio-Gozalbo ME, de Vries MC, Visser G, Houwen RH, van der Smagt JJ, Verhoeven-Duif NM, Wanders RJ, van Haaften G (2014) Monocarboxylate transporter 1 deficiency and ketone utilization. N Engl J Med 371:1900–1907
Acknowledgments
J.O. Sass is grateful for the financial support by the Hans Hermann Voss-Stiftung (Wipperfürth, Germany) and by the Fondation Claude et Giuliana (Vaduz, Liechtenstein) and thanks Ms. Corinne Gemperle-Britschgi and Ms. Lisa Stübbe for the analytical support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Robert Steiner
Appendices
One-Sentence Take-Home Message
MCT1 deficiency is an only recently described cause of recurrent ketoacidosis with clinical manifestation observed both in individuals with bi- or monoallelic mutations.
Compliance with Ethics Guidelines
Conflict of Interest
Shanti Balasubramaniam, Barry Lewis, Lawrence Greed, David Meili, Annegret Flier, Raina Yamamoto, Karmen Bilić, Claudia Till, and Jörn Oliver Sass declare have no conflict of interest.
Informed Consent and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
The investigated family has agreed to the publication and provided informed consent. This article does not contain any studies with animal subjects performed by the any of the authors.
Details of the Contributions of Individual Authors
SB was the physician in charge of the family. BL, LG, DM, AF, RY, KB, CT, and JOS performed/supervised/interpreted laboratory investigations. SB and JOS have drafted the manuscript. All authors have read/critically revised the manuscript.
Rights and permissions
Copyright information
© 2015 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Balasubramaniam, S. et al. (2015). Heterozygous Monocarboxylate Transporter 1 (MCT1, SLC16A1) Deficiency as a Cause of Recurrent Ketoacidosis. In: Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V. (eds) JIMD Reports, Volume 29. JIMD Reports, vol 29. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2015_519
Download citation
DOI: https://doi.org/10.1007/8904_2015_519
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53277-5
Online ISBN: 978-3-662-53278-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)