Abstract
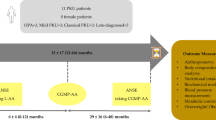
Background: As part of the German Collaborative Study on Phenylketonuria (PKU)/Hyperphenylalaninaemia (HPA) Study Protocol, a Blaskovics protein loading test (180 mg phenylalanine (phe) protein equivalent per kg body weight and day for 72 h) had been applied to 145 children at the age of 6 months. For investigating possible age-related changes of metabolic phenotype, 51 of them received a 2nd loading test at 5 years of age.
Methods: Besides the analysis of blood phe levels, acidic phe transamination metabolites were quantified in urine.
Results: Compared to the 6-month data, the mean blood phe level 72 h after start of loading (Phe72) was found to be decreased by 7% (P = 0.06), whereas the mean urinary excretion (per 1.73 m2 body surface and day) of 2-hydroxyphenylacetic acid was increased 1.9-fold (P < 0.01). Corresponding with these analytical data, the kinetic model constant k out of metabolic plus renal phe disposal was found increased 1.3-fold in mean (P < 0.01).
In 3 of the 51 patients, Phe72 was very high at 6 months while in the medium range at 5 years, suggesting that catabolic states may mimic a more severe metabolic defect.
The blood phe level response of mild PKU (type II) was assigned identically at both ages in 7/9 patients. Diverging results were (i) response type III (mild hyperphenylalaninaemia) at 6 months and type II at 5 years and (ii) type II at 6 months and type III at age 5.
Conclusion: Renal elimination of OHPAA and phe tolerance increase significantly between the age of 6 months and 5 years, suggesting that, at least in childhood, formation and/or renal disposal of phe transamination metabolites may be major distal determinants of phe tolerance.
Competing interests: None declared
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Phenylketonuria (PKU) due to deficient activity of phenylalanine hydroxylase (PAH) is characterized by a very high degree of molecular heterogeneity, with 872 variants of PAH presently known (Blau et al. 2015). Among the first hints at genetic heterogeneity of this disease was the observation that children with measurable PAH activity in their liver had a higher phe tolerance than children with undetectable PAH activity (Bartholomé et al. 1975). These results of microenzyme assay on liver needle biopsy specimens are at the root of the German Collaborative Study on Phenylketonuria (PKU)/Hyperphenylalaninaemia (HPA) (Collaborative study of children treated for phenylketonuria (PKU) in the Federal Republic of Germany 1990) which was designed in 1976. On the whole, it consisted of studies in three fields: (i) differential diagnosis of subtypes of HPA by analysis of the clinical, metabolic and molecular phenotype as an aid to rational treatment; (ii) therapeutic outcome in terms of intellectual, behavioural and neurological development; and (iii) decisions on suspending or continuing dietary treatment at given ages (Lutz et al. 1990).
For defining their metabolic phenotype, at the age of 6 months of life (6ML), patients with PKU/HPA were loaded with natural protein, corresponding to 180 mg phe/kg bw/day over a period of three consecutive days (72 h). The analysis of acidic transamination metabolites in urine was made part of this test because possible benefits of such data for investigating heterogeneity and pathogenesis of PKU were still contemplated at that time (Chalmers and Watts 1974). Building on a preceding study (Lutz et al. 1982), the blood phe level 72 h after start of loading (Phe72) was identified in these studies as a reliable surrogate parameter of residual PAH enzyme activity as estimated by in vitro analysis of PKU cell lines of defined genotype. This finding strongly supported the hypothesis of a molecular basis of phenotypic heterogeneity of PKU/HPA and opened the way for predicting metabolic and clinical phenotypes from molecular data (Okano et al. 1991).
To investigate the stability of metabolic phenotype beyond 6 months of age, e.g. excluding possible cases of delayed PAH maturation, the same type of loading was repeated at the age of 5 years of life (5YL) in a subset of the 6ML patients. After description of the 6ML data (Lutz et al. 1990; Mönch et al. 1990; Langenbeck et al. 2009) and of preliminary results of 5YL (Schmidt et al. 1989), we here present for all 5YL study patients the analytical results of phe disposal in blood and of urinary excretion of acidic phe transamination metabolites. Synopsis of the 5YL blood and urine data suggests maturation of phe transamination and/or renal metabolite transport between 6 months and 5 years as the reason for both the predictability of phe tolerance from 2 years on (van Spronsen et al. 2009) and reliable classification of PKU/HPA through age 5 phe tolerance data (Güttler and Guldberg 1996).
Patients and Methods
Selection of Patients
For the 6ML study, 165 patients with PKU/HPA had been enrolled, and 155 received a protein loading test in the years 1978–1984. Because of incomplete sampling, only 145 loads were analysed, with 14 classified as response type II (Lutz et al. 1990). The detailed analysis of complete test samples included 134 study cases, and 40 more patients were tested during routine diagnostic workup in the years 1996–1998 and 2007 (Langenbeck et al. 2009). The protein loading of the study patients was repeated between 1983 and 1989 at age 5 years in 51 of the original patients and is described in the present communication.
Analytical Methods
Blood phe levels were determined by an amino acid analyser (Lutz et al. 1982). Urinary organic acids were quantified as trimethylsilyl derivatives with gas chromatography in a sample of the 24 h urine collection of day 3 (Mönch et al. 1990). The PAH gene mutations were determined as previously described (Zschocke and Hoffmann 1999).
Classification and Kinetic Analysis of Phenylalanine Blood Level Response
The protein loading test elicits three main types of phe blood level response (O’Flynn et al. 1980; Lutz et al. 1990) which in typical cases can be recognized from the data pattern over the 72 h observation period: response type I (classical PKU) shows a continuous increase beyond 1,200 μmol/L, and type II (mild PKU) starts with an increase of blood phe concentration up to around 1,200 μmol/L followed by a spontaneous decrease well below 1,200 μmol/L starting at 36–48 h of the loading, i.e. when intake of high doses of phe is still continued. Type III (mild hyperphenylalaninaemia) shows a data pattern fluctuating around 600 μmol/L. Therefrom, classification of HPA is done on the basis of the Phe72 values as >1,200 μmol/L (type I), 600–1,200 μmol/L (type II) and <600 μmol/L (type III), respectively.
For kinetic analysis of blood phe level response during dietary treatment and protein loading tests, a model was built with the Windows™-based simulation and model analysis package ModelMaker 4 of Cherwell Scientific Ltd. (2000, now available at modelkinetix.com and exetersoftware.com). It comprises a single compartment with a single input, i.e. alimentary phe on 3 meals per day, and dual output, i.e. zero-order net protein synthesis and first-order, time-dependent metabolic plus renal phe disposal (dphe/dt = phe × k out). In the following, this original model (Langenbeck et al. 2001) without activation is called kinetic model type 1. It fits reaction types I and III. Kinetic model type 2 with time-dependent, exponentially saturated activation of phe disposal (dphe/dt = phe × A[1 − e B × t]) (see Keen and Spain 1992) and maximal k out = A × [1 − e B × 3] 72 h after start of loading fits reaction type II (Langenbeck et al. 2009).
For the present analysis, model parameters were set as follows: (i) mean body weight of girls and boys combined (bw), 7.63 and 18.94 kg at 6 months and 5 years, respectively (Neuhauser et al. 2013); (ii) net protein synthesis, 35 and 21 mg phe/kg bw/day at 6 months and 5 years, respectively (van Spronsen et al. (2009); (iii) predicted total body water, i.e. volume of distribution of phe, 0.588 and 0.602 L/kg bw at 6 months and 5 years, respectively (Wells et al. 2005); and (IV) rate of intestinal phe uptake k in (d−1): 2.1. At this latter value, the model fit for the mean time courses of types I, II and III was found closest, with the metabolic plus renal rate reaching a plateau.
To statistically distinguish kinetic types 1 and 2, the variable parameters of both models were estimated in parallel with the loading data by initial Simplex and final Marquardt optimization. For model 1, the initial phe blood level (mg/dL) and rate of metabolic plus renal disposal/loss k out (d−1) were optimized together. For model 2, A was empirically set to 5, and B was optimized together with initial phe blood level.
Urinary Excretion of Acidic Phenylalanine Metabolites
Spontaneous morning urine was collected on days 1, 2 and 5. A 24 h urine was collected from morning of day 3 till morning of day 4. Data of the latter sample are presented here.
Urinary excretion data are normalized to 1.73 m2 body surface (bs) because this format has been shown in the Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study to be an age-independent parameter of renal net organic acid excretion in healthy subjects (Manz and Wentz 2000; Berkemeyer and Remer 2006), thus allowing identification of disease-related changes depending on age. Mean bs at 6 months and 5 years is taken as 0.3904 and 0.7809 m2, respectively, by applying the height and body weight data of the German Health Survey for Children and Adolescents (KiGGS) Study (Neuhauser et al. 2013) and the mean from five different equations for estimating bs (www.halls.md/body-surface-area/refs.htm).
Data Analysis
After parallel optimization of the type 1 and 2 kinetic models of phe disposal, the kinetic type was assigned by comparing with Z statistics the R 2 values of model fit (Langenbeck et al. 2009). Data with statistically assigned kinetic type 2 are classified as response type II, else as response type I or III, respectively. With response type III data, because of their flat profile over time, it is not possible, as a rule, to detect statistically possible cases of activation.
The 6ML and 5YL analytical and model data were tested for normality by the non-parametric one-sample Kolmogorov-Smirnov test. If normality is not excluded, the data are compared by linear least squares regression with and without intercept (Phe 72, urinary OHPAA). The significance of observed quantitative changes is computed with the non-parametric Wilcoxon matched-pairs signed-rank test (Forthofer et al. 2007).
The SYSTAT 11 program package (2004) of Systat Software GmbH, D-40699 Erkrath, Germany, was used for statistical analysis and graphics.
Results
The study of the 51 5YL patients yielded 48 Phe72, 30 urinary OHPAA and 51 model constant k out values. From these same patients are available at 6ML 48 Phe72, 44 urinary OHPAA and 49 model constant k out data. Due to missing values in either series and exclusion of three 6ML patients because of their excessive Phe72 levels (see below), 43 Phe72, 24 urinary OHPAA and 46 k out values remained for the final 6ML versus 5YL comparison.
Analysis of Phenylalanine Disposal
Two parameters characterize the time course of blood phe during protein loading tests, the observed Phe72 (Lutz et al. 1982; Okano et al. 1991) and the estimated first-order rate constant k out of metabolic plus renal phe disposal. Both are closely correlated, with lower values of Phe72 corresponding to higher ones of k out (Langenbeck et al. 2009).
The 43 6ML and 5YL Phe72 values are found in the same range. Linear least square regression (Fig. 1) indicates a 7% decrease of Phe72 at 5YL (Wilcoxon Z = 1.89, P 2-tail = 0.06). In contrast, the Phe0 values (μmol/L; mean ± s.d.) at the beginning of the test are almost identical in 6ML and 5YL: 448.4 ± 276.6 vs. 550.3 ± 245.2, respectively.
Consistent with the lower Phe72 values at 5 years of age, there is a 1.30-fold increase of k out at this age (mean ± s.d.): 0.8946 ± 0.6111 vs. 1.1609 ± 0.7872, N = 46. In 38 of the 46 cases, k out was found higher in 5YL, a highly significant difference (Wilcoxon Z = 4.09, P 2-tailed < 0.01).
Not included in this statistical analysis are three patients with Phe72 >1.200 μmol/L higher at 6ML than at 5YL. For one of them (Fig. 2), the genotype p.R261Q/c.1066-11G>A, with an AV of 3 (Guldberg et al. 1998), and the Phe72 at age 5 indicate a moderate to mild PKU. The genotypes of the two other patients are not known. Catabolic states at the time of the first test (instead of delayed PAH maturation) most probably explain these observations.
Urinary Phenylalanine Metabolites
The urinary levels of the phe transamination metabolites phenylpyruvic acid (PPA), phenyllactic acid (PLA) and 2-hydoxyphenylacetic acid (OHPAA) are linearly related in older children to the logarithm of their corresponding plasma levels (Langenbeck et al. 1992). Analytically valid quantitative urine data of these metabolites may therefore be taken as proxy of the endogenous phe transamination capacity.
Besides PLA, which attains urinary concentrations in the range of PPA only after prolonged phe loads (Langenbeck et al. 1992), OHPAA is established as the analytically most stable phe transamination metabolite in urine (Dhondt et al. 1974) when gas chromatography with traditional derivatives is applied (Langenbeck et al. 1980). Therefore, urinary OHPAA is taken in the present communication as an indicator of phe transamination during the protein loading tests. Complete 6ML findings were reported by Mönch et al. (1990).
Compared to the 6ML findings, the 24 h urinary excretion of OHPAA on day 3 is increased 1.9-fold at 5YL, comprising 427 (18–1,409; 42) and 806 (51–1,761; 27) μmol per 1.73 m2 bs area and day (mean; range; N), respectively; see Fig. 3. This positive difference is highly significant (Wilcoxon Z = 3.11, N = 24, P 2-tailed < 0.01) and consistent with the apparent age-dependent rise of phe disposal, as indicated by the changes of Phe72 and k out shown above.
Relation between urinary excretions of 2-hydroxyphenylacetic acid (μmol per 1.73 m2 body surface area) on day 3 at 6 months and 5 years. Linear least squares regression with intercept, the 3 Phe72 outliers ignored: (OHPAA at 5 years) = (450.0 ± 119.8 s.e.) + (0.7025 ± 0.1927 s.e.) × [OHPAA at 6 months]; N = 24, mult. R = 0.61, P < 0.01
Recognition of the Response Type II
The blood phe level response type II was assigned statistically to 6 and visually to 2 of the 51 5YL data sets. One patient with response type II at 5YL was type III at 6ML, and one case with response type II at 6ML was response type III at 5YL. Two more patients were assigned response type III (mild hyperphenylalaninaemia) at both 6ML and 5YL. In summary, the blood phe level response type II was assigned identically to both ages in 7/9 patients, and no case of delayed PAH maturation was detected.
As shown in Table 1, the response type II patients carry genotypes characteristic of mild PKU, i.e. with AVs of 5–7 (Guldberg et al. 1998) and residual PAH activities around 60% (Blau et al. 2015). Of the 51 5YL patients, 9 (17.6%) manifested response type II at one or both ages which is close to the 6ML study data (19/125 = 15.2%), implying absence of a significant sampling bias in recruitment of the 5YL patients.
Discussion
As documented above, renal elimination of OHPAA and phe tolerance increase significantly between the age of 6 months and 5 years. Mechanistically, this could be explained with maturation of either phe transamination or renal organic acid transport. Also concomitant evolution of both systems may take place.
Phenylpyruvate is cleared in the kidney through proximal tubular secretion, mediated by the para-aminohippuric acid (pAH) transporter (Vink and Kroes 1961). The activity for its pAH substrate is low in newborns and reaches the normal values of older children only at the end of the first year (Bertram et al. 1970). In wild-type mice, the pAH clearance increases till 10 weeks of age. In adult mice, OAT1 is the principal pAH transporter (Sweeney et al. 2011).
If all phe transamination metabolites shared this transport system, their excretion in early life is expected to be low. Rey et al. (1974) first described the influence of age on excretion of OHPAA in children with PKU and found adult threshold values only after 2 years. Accordingly, metabolic data of PKU children older than 2 years conform with respective findings in juvenile patients (Langenbeck et al. 1980, 1992), and phe tolerance at 10 years of age can be predicted reliably from the respective data at 2 years (van Spronsen et al. 2009).
Whether delayed maturation of phe transaminase, as suggested by Rey et al. (1974), and/or delayed maturation of the pAH transporter explains the data could be decided by knowledge of phe metabolite blood levels. However, we only know of three such observations: Partington and Vickery (1974), using the enol-borate method for quantifying PPA, found in a 3-week-old infant plasma PPA levels in the range seen in older children. The urinary PPA levels, however, were very low. In contrast, using the trimethylsilyl-quinoxalinol method, Bebehani and Langenbeck (1980, unpublished) found in two 11-day-old infants very low plasma PPA levels in relation to plasma phe (1,501 vs. 11 and 2,700 vs. 50 μmol/L) but more ‘normal’ PPA excretion in relation to plasma PPA (39 and 291 μmol/mmol creatinine, respectively). Only detailed clearance studies in PKU infants and young children could resolve the joint effects of these mechanisms.
The intricate interplay of factors determining phe tolerance is documented in a study by Treacy et al. (1996) of two siblings with mild PKU (p.R408W/p.I65T). The child with the higher phe tolerance at 42 months (600 vs. 350 mg phe/day) excreted higher amounts of phe transamination metabolites into urine (10.9 vs. 4.9 μmol/kg × h), whereas the sibling with much lower excretion had plasma metabolite levels in the toxic range (220 vs. 134 μmol/L; cf. Langenbeck et al. 1992).
There are few reports only on the further development of juvenile, transitional and adult patients. Further studies would therefore be welcomed on therapeutically relevant aspects like the possible influence of obesity on phe tolerance (MacLeod et al. 2009), the degree of oxidative stress at elevated blood phe levels (Okano and Nagasaka 2013), the value of self-management in improving the patients’ individual responsibility and adherence (ten Hoedt et al. 2011), the development and production of more palatable and satiating low-phe food products (van Calcar and Ney 2012) and the practicality of home monitoring (Wendel and Langenbeck 1996). Progress in these fields would contribute to converting a cumbersome disease into an aspect of self-determined life, like diabetes mellitus.
Abbreviations
- AV:
-
Assigned value of PKU phenotype (Guldberg et al. 1998)
- bs:
-
Body surface
- HPA:
-
Hyperphenylalaninaemia
- k out :
-
First-order kinetic constant of metabolic plus renal loss/disposal
- OHPAA:
-
2-Hydroxyphenylacetic acid
- phe:
-
Phenylalanine
- PAH:
-
Phenylalanine hydroxylase (E.C. 1.14.16.1)
- pAH:
-
Para-aminohippuric acid
- Phe72:
-
Blood phenylalanine 72 h after start of the loading test (morning of day 4)
- PKU:
-
Phenylketonuria (OMIM +261600)
- PLA:
-
Phenyllactic acid
- PPA:
-
Phenylpyruvic acid
- t 1/2 :
-
Phenylalanine half-life (50% elimination time)
- 6ML:
-
Loading test at age of 6 months
- 5YL:
-
Loading test at age of 5 years
References
Bartholomé K, Lutz P, Bickel H (1975) Determination of phenylalanine hydroxylase activity in patients with phenylketonuria and hyperphenylalaninemia. Pediatr Res 9:899–903. doi:10.1203/00006450-197512000-00006
Berkemeyer S, Remer T (2006) Anthropometrics provide a better estimate of urinary organic acid anion excretion than a dietary mineral intake-based estimate in children, adolescents and adults. J Nutr 136:1203–1208
Bertram D, Rind H, Gladtke E (1970) Die Elimination von para-Aminohippursäure beim Kind. Z Kinderheilk 108:208–220. doi:10.1007/BF00447033
Blau N, Yue W, Perez B (2015) PAHvdb. http://www.biopku.org/pah/. Assessed 30 Mar 2015
Chalmers RA, Watts RWE (1974) Quantitative studies on the urinary excretion of unconjugated aromatic acids in phenylketonuria. Clin Chim Acta 55:281–294. doi:10.1016/0009-8981(74)90002-3
Collaborative study of children treated for phenylketonuria (PKU) in the Federal Republic of Germany (1990) Eur J Pediatr 149(Suppl 1):S3–S51
Dhondt JL, Cartigny B, Farriaux JP (1974) Intérêts du dosage de l’acide ortho-hydroxyphénylacétique urinaire dans les hyperphénylalaninémies. Ann Biol Clin 32:499–506
Forthofer RN, Lee ES, Hernandez M (2007) Biostatistics. A guide to design, analysis, and discovery, 2nd edn. Elsevier Academic, Amsterdam
Guldberg P, Rey F, Zschocke J et al (1998) A European multicenter study for phenylalanine hydroxylase deficiency: classification of 105 mutations and a general system for genotype-based prediction of metabolic phenotype. Am J Hum Genet 63:71–79. doi:10.1086/301920
Güttler F, Guldberg P (1996) The influence of mutations on enzyme activity and phenylalanine tolerance in phenylalanine hydroxylase deficiency. Eur J Pediatr 155(Suppl 1):S6–S10. doi:10.1007/pl00014253
Keen RE, Spain JD (1992) Computer simulation in biology. A BASIC introduction. Wiley-Liss, New York
Langenbeck U, Behbehani A, Mench-Hoinowski A, Petersen M (1980) Absence of a significant renal threshold for two aromatic acids in phenylketonuric children over two years of age. Eur J Pediatr 134:115–118; corr. 135:118
Langenbeck U, Behbehani A, Mench-Hoinowski A (1992) A synopsis of the unconjugated acidic transamination metabolites of phenylalanine in phenylketonuria. J Inherit Metab Dis 15:136–144. doi:10.1007/BF01800355
Langenbeck U, Zschocke J, Wendel U, Hönig V (2001) Modelling the phenylalanine blood level response during treatment of phenylketonuria. J Inherit Metab Dis 24:805–814. doi:10.1023/A:1013946006155
Langenbeck U, Burgard P, Wendel U, Lindner M, Zschocke J (2009) Metabolic phenotypes of phenylketonuria. Kinetic and molecular evaluation of the Blaskovics protein loading test. J Inherit Metab Dis 32:506–513. doi:10.1007/s10545-009-1152-6
Lutz P, Schmidt H, Frey G, Bickel H (1982) Standardized loading test with protein for the differentiation of phenylketonuria from hyperphenylalaninaemia. J Inherit Metab Dis 5:29–35. doi:10.1007/BF01799751
Lutz P, Schmidt H, Batzler U (1990) Study design and description of patients. Eur J Pediatr 149(Suppl 1):S5–S12
MacLeod EL, Gleason ST, van Calcar SC, Ney DM (2009) Reassesment of phenylalanine tolerance in adults with phenylketonuria is needed as body mass changes. Mol Genet Metab 98:331–337. doi:10.1016/j.ymgme.2009.07.016
Manz F, Wentz A (2000) Renal net acid excretion related to body surface area in children and adolescents. Pediatr Nephrol 15:101–104. doi:10.1007/s004670000424
Mönch E, Kneer J, Jakobs J et al (1990) Examination of urine metabolites in the newborn period and during protein loading tests at 6 months of age. Eur J Pediatr 149(Suppl 1):17–24. doi:10.1007/BF02126294
Neuhauser H, Schienkiewitz A, Schaffrath Rosario A, Dortschy R et al (2013) Referenzperzentile für anthropometrische Maßzahlen aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KIGGS). 2. erw. Aufl., Robert-Koch-Institut, Berlin
O’Flynn ME, Holtzman NA, Blaskovics M et al (1980) The diagnosis of phenylketonuria. A report from the collaborative study of children treated for phenylketonuria. Am J Dis Child 134:769–774. doi:10.1001/archpedi.1980.02130200039013
Okano Y, Nagasaka H (2013) Optimal serum phenylalanine for adult patients with phenylketonuria. Mol Genet Metab 110:424–430. doi:10.1016/j.ymgme.2013.09.007
Okano Y, Eisensmith RC, Güttler F et al (1991) Molecular basis of phenotypic heterogeneity in phenylketonuria. N Engl J Med 324:1232–1238. doi:10.1056/NEJM199105023241802
Partington MW, Vickery SK (1974) Phenylketonemia in phenylketonuria. Neuropädiatrie 5:125–137. doi:10.1055/s-0028-1091695
Rey F, Pellié C, Sivy M et al (1974) Influence of age on ortho-hydroxyphenylacetic acid excretion in phenylketonuria and its genetic variants. Pediat Res 8:540–545. doi:10.1203/00006450-197405000-00002
Schmidt H, Lutz P, Batzler U (1989) Differentialdiagnose des erhöhten Phenylalanin-Blutspiegels im Säuglingsalter. Ergebnisse der deutschen Verbundstudie über Phenylketonurie (PKU)/Hyperphenylalaninämie (HPA). Mschr Kinderheilk 137:86–92
Sweeney DE, Vallon V, Rieg T et al (2011) Functional maturation of drug transporters in the developing, neonatal and postnatal kidney. Mol Pharmacol 80:147–154. doi:10.1124/mol.110.070680
ten Hoedt AE, Hollak CEM, Boelen CCA et al (2011) “My PKU”: increasing self-management in patients with phenylketonuria. A randomized controlled trial. Orphanet J Rare Dis 6:48. doi:10.1186/1750-1172-6-48
Treacy E, Pitt JJ, Seller K et al (1996) In vivo disposal of phenylalanine in phenylketonuria: a study of two siblings. J Inher Metab Dis 19:595–602. doi:10.1007/BF01799832
van Calcar SC, Ney DM (2012) Food products made with glycomacropeptide, a low-phenylalanine whey protein, provide a new alternative to amino acid-based medical foods for nutrition management of phenylketonuria. J Acad Nutr Diet 112:1201–1210. doi:10.1016/j.jand.2012.05.004
van Spronsen FJ, van Rijn M, Dorgelo B, Hoeksma M et al (2009) Phenylalanine tolerance can already reliably be assessed at the age of 2 years in patients with PKU. J Inherit Metab Dis 32:27–31. doi:10.1007/s10545-008-0937-3
Vink CLJ, Kroes AA (1961) The renal clearance of phenylpyuvate. Clin Chim Acta 6:813–818
Wells JCK, Fewtrell MS, Davies PSW, Williams JE et al (2005) Prediction of total body water in infants and children. Arch Dis Child 90:965–971. doi:10.1136/adc.2005.067538
Wendel U, Langenbeck U (1996) Towards self-monitoring and self-treatment in phenylketonuria – a way to better diet compliance. Eur J Pediatr 155(Suppl 1):S105–S107. doi:10.1007/pl00014224
Zschocke J, Hoffmann GF (1999) Phenylketonuria mutations in Germany. Hum Genet 104:390–398. doi:10.1007/s004390050973
Acknowledgement
The collaborative study of children treated for phenylketonuria (PKU) in the Federal Republic of Germany (1978 to 1996) was headed until 1989 by the late Professor Horst Bickel, thereafter by Professor Hans Joachim Bremer. It received financial support from Stiftung Volkswagenwerk and Bundesministerium für Forschung und Technologie (BMBF). The following paediatric centres participated in the study: Berlin (E. Mönch), Düsseldorf (Hildegard Przyrembel, U.Wendel), Göttingen (A.W. Behbehani, W. Voss), Hamburg (P. Koepp, P. Clemens), Heidelberg (Hildgund Schmidt, P. Lutz, K. Bartholomé, F.K. Trefz), München (J. Schaub, W. Endres), Münster (H. Gröbe, K. Ullrich) and Ulm (Dorothea Leupold). Thanks are due to Sylvia Körner for her excellent administrative work and data handling. Additional acknowledgements appeared in 1990 in Eur J Pediatr 149 (Suppl 1): S3–S4. The helpful comments of the referees are gratefully acknowledged.
Author information
Authors and Affiliations
Consortia
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Nenad Blau, PhD
Appendices
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (at the time of data collection) as revised in 2000 (at the time of data analysis). Prior to study enrolment, all families were informed in written and oral form that the clinical-therapeutic study was in technical and formal accordance with legal data protection regulations, and informed proxy consent for participation was obtained from all of them. All data obtained were stored in pseudonomized form in a central database.
Conflict of Interest
Peter Burgard has received speaker honoraria from Vitaflo Ltd., Merck Serono GmbH and Swedish Orphan Biovitrum GmbH. Eberhard Mönch has received speaker honoraria from Vitaflo Pharma GmbH., metaX Institut für Diätetik GmbH, Cytonet GmbH & Co. KG and Swedish Orphan Biovitrum GmbH. Johannes Zschocke has received financial support for educational, research and diagnostic activities from Nutricia (Milupa) and Merck Serono. Udo Wendel and Ulrich Langenbeck declare that they have no conflict of interest.
Contributions of Individual Authors
P.B. curates the collaborative study’s database and designed and edited the present communication, E.M. supervised the collection and analysis of urine samples, J.Z. analysed and verified the DNA data and U.W. helped interpret the clinical data and test results. U.L. developed and performed the model calculations. U.L. and P.B. drafted the report, and all other authors critically reviewed the report. All authors saw and approved the final submitted version. As the corresponding author, U.L. confirms that he had full access to the data and had final responsibility for the decision to submit for publication.
Animal Rights
This article does not contain any studies with animal subjects performed by any of the authors.
Rights and permissions
Copyright information
© 2015 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Burgard, P., Mönch, E., Zschocke, J., Wendel, U., Langenbeck, U., On behalf of the German Collaborative Study of Phenylketonuria (PKU)/Hyperphenylalaninaemia (HPA). (2015). Development of Metabolic Phenotype in Phenylketonuria: Evaluation of the Blaskovics Protein Loading Test at 5 Years of Age. In: Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V. (eds) JIMD Reports, Volume 29. JIMD Reports, vol 29. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2015_508
Download citation
DOI: https://doi.org/10.1007/8904_2015_508
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53277-5
Online ISBN: 978-3-662-53278-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)