Abstract
Mucopolysaccharidosis IIIA (MPS IIIA) is a neurodegenerative lysosomal storage disorder characterised by progressive loss of learned skills, sleep disturbance and behavioural problems. Reduced activity of lysosomal sulfamidase results in accumulation of heparan sulfate and secondary storage of glycolipids in the brain. Intra-cisternal sulfamidase infusions reduce disease-related neuropathology; however, repeated injections may subject patients to the risk of infection and tissue damage so alternative approaches are required. We undertook a proof-of-principle study comparing the ability of slow/continual or repeat/bolus infusion to ameliorate neuropathology in MPS IIIA mouse brain. Six-week-old MPS IIIA mice were implanted with subcutaneously located mini-osmotic pumps filled with recombinant human sulfamidase (rhSGSH) or vehicle, connected to lateral ventricle-directed cannulae. Pumps were replaced at 8 weeks of age. Additional MPS IIIA mice received intra-cisternal bolus infusions of the same amount of rhSGSH (or vehicle), at 6 and 8 weeks of age. Unaffected mice received vehicle via each strategy. All mice were euthanised at 10 weeks of age and the brain was harvested to assess the effect of treatment on neuropathology. Mice receiving pump-delivered rhSGSH exhibited highly significant reductions in lysosomal storage markers (lysosomal integral membrane protein-2, GM3 ganglioside and filipin-positive lipids) and neuroinflammation (isolectin B4-positive microglia, glial fibrillary acidic protein-positive astroglia). MPS IIIA mice receiving rhSGSH via bolus infusion displayed reductions in these markers, but the effectiveness of the strategy was inferior to that seen with slow/pump-based delivery. Continual low-dose infusion may therefore be a more effective strategy for enzyme delivery in MPS IIIA.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Mucopolysaccharidosis type IIIA (MPS IIIA) is an inherited lysosomal storage disorder that results from the absence or defective function of lysosomal sulfamidase, which is involved in the stepwise degradation of heparan sulfate, resulting in the accumulation of heparan sulfate in lysosomes and subsequent clinical disease. The main feature of this disorder is central nervous system (CNS) pathology, with progressive neurodegeneration and subsequent mental decline resulting in a greatly shortened lifespan, often <20 years (Neufeld and Muenzer 2001).
We have identified a naturally occurring mouse model of MPS IIIA (Crawley et al. 2006), which exhibits similar neuropathological features to the human condition, and have used it to investigate therapy options for this condition. Whilst intravenous enzyme replacement therapy is useful for reducing lysosomal storage in non-CNS tissues, the blood–brain barrier prevents access of conventional doses of sulfamidase delivered intravenously (Gliddon and Hopwood 2004). Enzyme uptake into the brain parenchyma and subsequent reductions in lysosomal storage and related neurodegenerative changes, together with improvements in clinical function have, however, been observed in MPS IIIA mice receiving repeated injection of sulfamidase into the cisternal cerebrospinal fluid (CSF) (e.g. Hemsley et al. 2007, 2008, 2009). This treatment is also efficacious at ameliorating neuropathology in the larger brain of the MPS IIIA Huntaway dog (Crawley et al. 2011).
Similar observations have been made in MPS I dogs (Kakkis et al. 2004), Krabbe mice (Lee et al. 2007), late-infantile neuronal ceroid lipofuscinosis mice (LINCL; Chang et al. 2008), Niemann–Pick A mice (Dodge et al. 2009), Sandhoff disease mice (Tsuji et al. 2011) and fucosidosis dogs (Kondagari et al. 2011). Therefore, this approach appears to be a disease-spanning therapeutic strategy, and the movement towards application in MPS IIIA patients (www.clinicaltrials.gov #NCT01299727, #02060526) appears both rational and justified.
At present, application of enzyme to MPS IIIA patients occurs via bolus injection using an indwelling intrathecal drug-delivery cannula, with enzyme administered over a short period. Indeed, the majority of the preclinical studies described above have administered the respective enzyme over similar time frames (i.e. minutes). To explore the impact of varying the enzyme supply rate on the amelioration of neuropathology in the MPS IIIA mouse brain, we have compared the effectiveness of continually supplying low-concentration recombinant human sulfamidase (rhSGSH) via a subcutaneous mini-osmotic pump device connected to a cannula directed at the right lateral ventricle, with repeated high-concentration bolus delivery to the cisternal CSF. The total quantity of enzyme supplied to both groups was the same (200 μg over 1 month).
Materials and Methods
Enzyme
RhSGSH was provided by Shire at 25 μg/μL and stored at −70°C until used. The enzyme was diluted to 1.2 μg/μL in 10 mM sodium phosphate and 138 mM sodium chloride (pH 7) and injected into the pump (Alzet; pump rate 0.25 μL/h; Jomar Bioscience, Australia) >48 h prior to surgery. During this time, the pumps were stored at 37°C under sterile conditions. Vehicle was infused in the same manner. Enzyme activity was determined using a natural tritiated tetrasaccharide substrate (Hopwood and Elliott 1982).
Mice
Congenic C57BL/6 MPS IIIA mice (Crawley et al. 2006), or unaffected −/+, +/+ littermates (hereafter referred to as “Normal” mice), were bred, housed and maintained in the institutional Animal House, with all breeding and experimental procedures undertaken with the approval of the Women’s and Children’s Health Network Animal Ethics Committee, with regard to the guidelines of the National Health and Medical Research Council of Australia on the Use and Care of Experimental Animals. The mice were genotyped using previously established methods (Gliddon and Hopwood 2004).
Intra-cisternal CSF Injections
All methods have been previously described in full (Hemsley et al. 2009). Briefly, 6-week-old mice were anesthetised with ketamine (87 mg/kg; Parnell Laboratories, Australia)/xylazine (13 mg/kg; Troy Laboratories, Australia) (i.p.). Four microlitres of rhSGSH (25 μg/μL) or vehicle was injected into the cerebellomedullary cistern using a 27G dental needle attached to an infusion pump via plastic tubing. MPS IIIA mice received rhSGSH or vehicle injections according to the schedule shown in Fig. 1a (n = 3/group); unaffected mice received vehicle only (n = 3). All mice received butorphanol tartrate (2 mg/kg; Fort Dodge, NSW Australia) following surgery. Figure 1c shows the position of the injection site.
Experimental plan. Mice received either (a) bolus injection of 100 μg recombinant human sulfamidase (rhSGSH) via the cisterna magna (CM) at each of 6 and 8 weeks of age, with euthanasia at 10 weeks of age, or (b) implantation of a mini-osmotic pump and cannula, directed at the right lateral ventricle (LV) at 6 weeks of age. The pump was removed and replaced at 8 weeks of age and mice were euthanised at 10 weeks of age. During the experimental period, the pump delivered rhSGSH (1.2 μg/μL) at 0.25 μL/h, delivering 100 μg over each 2-week period. (c) Illustrates the injection region in each treatment strategy and shows a photo of a pump-/cannula-implanted mouse
Cannula/Pump Implantation and Pump Replacement
A further nine mice were anaesthetised as above, secured in a stereotaxic frame (David Kopf Instruments, California, USA), and an incision was made in the scalp. A small hole was made above the right lateral ventricle with a 0.5 mm hand drill bit (Flintware, Adelaide, Australia). The coordinates in reference to bregma and the midline were 0.5 mm posterior and 1.0 mm lateral (Paxinos and Franklin 2001). The cannula (Alzet Brain Infusion Kit 3 fitted with two 0.5 mm spacers; Jomar Bioscience, Australia) was inserted into the brain and secured to the skull with glue. The enzyme/vehicle-filled pump was inserted through the scalp incision and positioned subcutaneously on the flank. The skin wound was sutured and all mice received butorphanol tartrate for analgesia (2 mg/kg; Fort Dodge, Baulkham Hills, NSW, Australia), following surgery. Pump replacement was performed at 8 weeks of age (see experimental plan, Fig. 1b) and required a skin incision above the pump site (under anaesthesia as above), to remove the pump and replace it with a new one. The wound was sutured and analgesia administered as above. Figure 1c shows a photo of a mouse fitted with a pump/cannula and the location of the cannula in the right hemisphere of the brain.
Necropsy and Sample Collection
Mice were euthanised via CO2 asphyxiation at 10 weeks of age, blood was sampled, and mice were fixation-perfused for light microscopy examination (4% paraformaldehyde in PBS, pH 7.4). The brain was removed and each hemisphere was sectioned in a sagittal plane at 1 mm from the midline (i.e. at the lateral coordinate of the cannula), producing a medial and lateral portion of each hemisphere. Medial portions of the left and right hemisphere were embedded in paraffin; lateral portions of the left and right hemispheres were infiltrated with 30% sucrose and snap-frozen in OCT.
Measurement of Anti-rhSGSH Antibodies in Sera
Previously published methods were used to determine the presence of anti-rhSGSH antibody titres in mouse sera (Hemsley et al. 2009).
Histochemistry, Immunohistochemistry and Quantification of Neuropathology Using Light Microscopy
Reagents for Staining
Rabbit polyclonal antibodies against glial fibrillary acidic protein (GFAP, #Z334) were purchased from Dako (Glostrup, Denmark). A monoclonal anti-lysosomal integral membrane protein (LIMP-2) antibody (Hemsley et al. 2008), a mouse monoclonal antibody to rhSGSH (Shire) and a mouse monoclonal antibody against GM3 (Seikagaku, Japan) were used. Peroxidase-conjugated lectin from Bandeiraea (Griffonia) simplicifolia (isolectin B4, BSI-B4, #L5391; Sigma, Missouri, USA), which recognises α-galactosyl groups, was used as a marker for activated microglia. Filipin complex from Streptomyces filipinensis (Sigma #F9765) was resuspended in N,N-dimethylformamide. Biotinylated secondary antibodies, donkey anti-rabbit IgG and donkey anti-mouse IgG were purchased from Jackson ImmunoResearch (West Grove, PA, USA), and goat anti-mouse IgM was obtained from Vector Laboratories (Burlingame, CA USA).
Immunohistochemistry
All procedures and post-staining image analyses were undertaken by an experimenter blinded to genotype/treatment status, and all methods have been previously described (Hemsley et al. 2009; Lau et al. 2010). Briefly, 6 micron thick sagittal sections of fixed paraffin-embedded brain tissue were deparaffinised and exposed to heat-induced epitope retrieval. Tissues were blocked and incubated with primary antibodies (anti-GFAP 1:12,000; anti-LIMP-2 1:800; anti-rhSGSH 1:50). Endogenous peroxidases were quenched prior to addition of a species-specific biotinylated secondary antibody (1:2,000). Sections were visualised using a Vectastain ABC Kit (PK-6100; Vector Laboratories, California, USA) and diaminobenzidine (DAB; #K3468; Dako). BSI-B4 (1 mg/mL) was diluted 1:80 in TRIS buffer and reactions were visualised with DAB. Sections were batched for each stain. Histochemical detection of filipin- or GM3-positive inclusions was carried out on frozen sections (6 μm), with anti-GM3 antibodies applied overnight (1:750) at 4°C. Visualisation was as for the paraffin sections. Filipin staining was carried out using previously published methods (Lau et al. 2010).
Sections were viewed on either an Olympus BX41 or BX61 microscope; digital images were collected using either an Olympus UC50 or Colorview III camera. Several brain regions were imaged and analysed, with all regions defined using a mouse brain atlas (Paxinos and Franklin 2001). All parameters of imaging and calibration remained constant for each comparative region and stain. Images were analysed using AnalySIS LifeScience software (version 2.8, Build 1235). Thresholding, based on the optical density of positive immunostaining for LIMP-2, GFAP and GM3, was applied to the images in a consistent manner. Data are reported as percentage immunoreactivity. The number of BSI-B4-stained activated microglia (per mm2) was determined; rhSGSH immunostaining was qualitatively assessed, and the accumulation of filipin-positive inclusions was examined semi-quantitatively (i.e. number of cells with positively stained inclusions), using a +/++/+++ scale.
Statistics
Data were assessed using GraphPad Prism v6.05 software. Data were log transformed Y = log (Y + 1) and examined using the analysis of variance and post-hoc testing with Bonferroni correction. Mice were compared with their respective control groups (i.e. Normal v MPS cisternal vehicle v MPS cisternal enzyme and Normal v MPS pump vehicle v MPS pump enzyme). P < 0.05 was considered to be statistically significant. Data are shown as mean ± SEM.
Results
Anti-rhSGSH Antibodies in Sera
All MPS IIIA mice receiving pump-delivered rhSGSH developed serum anti-rhSGSH antibodies (titres of 1/3,200 to 1/51,200 in enzyme-treated mice cf. titres of ≤1/100 in vehicle-treated mice). The sera from the cisternal high-dose bolus group were unfortunately unable to be analysed; however, we have consistently observed anti-rhSGSH antibodies in the sera from mice treated in this manner (Hemsley et al. 2007, 2008, 2009). There was no apparent clinical effect of the humoral immune response on the mice.
Enzyme Distribution in the Brain Following Cisternal Bolus or Pump Infusion
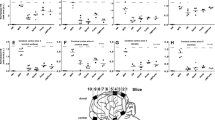
Immunohistochemical detection of rhSGSH in 10-week-old pump-infused MPS IIIA mouse brain revealed very intense immunostaining in many regions of the brain (Fig. 2b–g). Immunopositive puncta were observed in the cerebral cortex overlying the lateral ventricle and the adjacent hippocampus and in the olfactory bulb. Diffusion from the ventricle was evident, with cells in the thalamus, retrosplenial cerebral cortex and cerebellum displaying rhSGSH-positive puncta. Morphologically, cells containing positive immunoreactivity appeared to include neurons, glia, endothelial cells and macrophages. In contrast, cisternal bolus enzyme-treated MPS IIIA mouse brain revealed low numbers of immunopositive cells in the outer layers of the posterior lobes of the cerebellum (Fig. 2h) and pontine nucleus. Some positive staining was also seen in the ventral meninges. All other areas of the brain, including the cerebral cortex, hippocampus, thalamus, and the colliculi, were negative.
Immunohistochemical localisation of rhSGSH in regions shown in (a) within the pump-/cannula-implanted (b–g) and the cisternally injected (h) MPS IIIA mouse brain at 10 weeks of age. Staining in both left (L) and right (R) hemispheres of brain is shown for the cannulae-implanted mice (c–g). The photo in (b) shows a high power view of the immunopositive puncta seen in the cerebral cortex of a pump-/cannula-implanted MPS IIIA mouse. The staining is presumptively in endo-/lysosomes
Impact of rhSGSH Treatment on Storage-Related Neuropathology
Three brain regions were examined to determine the effect of rhSGSH delivery on MPS IIIA-related neuropathology. On the basis of previously described disease-related changes and proximity to each of the injection sites, we selected the rostral aspect of the cerebral cortex, thalamus or subiculum of the hippocampus (stain dependent) and the inferior colliculus. The regions and their relationship to the injection locations are shown in Fig. 3.
Quantification of immunohistochemical staining of lysosomal integral membrane protein-2 (LIMP-2; a–c) and GM3 (d–f) and semi-quantification of filipin-stained inclusions (g–i) in 10-week-old MPS IIIA and unaffected mouse brain following cisternal bolus (CM) or pump-delivered enzyme treatment. The location of the brain regions analysed with respect to the injection sites is shown in the sagittal brain section (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 cf. Normal; #p < 0.05, ##p < 0.01, ###p < 0.001, ###p < 0.0001 cf. MPS vehicle)
Sustained delivery of rhSGSH to the MPS IIIA mouse brain resulted in complete normalisation of two of the three disease markers (LIMP-2 and GM3) in the rostral cerebral cortex (right hemisphere; Fig. 3a, d) after 4 weeks of treatment. Near-normalisation of filipin staining was also observed in the deeper pyramidal cell layers III/IV in this brain region at this time (Fig. 3g). The ventricular infusion site is proximal to the right rostral cortex; however, the impact of treatment on LIMP-2 and GM3 staining was bilateral, with both cortices exhibiting similar reductions in inclusion number at 10 weeks of age. Further, even in brain areas distant from the ventricle (e.g. inferior colliculus), pump-delivered rhSGSH mediated a significant and bilateral reduction in (LIMP-2) or near-complete amelioration of GM3 and filipin-stained inclusions (Fig. 3c, f, i). Reductions in these secondarily stored substrates were also observed in the subiculum of the hippocampus (bilaterally). This structure is located caudal but adjacent to the injection region.
In contrast, whilst reductions in LIMP-2 immunoreactivity and filipin-stained inclusion levels were noted in the inferior colliculus of bolus-injected mice at 10 weeks of age (i.e. 2 weeks after the last injection), LIMP-2 and GM3 staining in the thalamus and hippocampus (respectively) was not significantly different to that seen in bolus vehicle-infused MPS IIIA mice (Fig. 3b, e). The impact of bolus cisternal enzyme delivery on neuropathology in the rostral cerebral cortex was statistically negligible, although two of the three cisternally injected MPS IIIA mice exhibited reductions in GM3 and filipin-positive lipid accumulation in this region.
Impact of rhSGSH Treatment on Micro- and Astrogliosis
Microgliosis was completely ameliorated in the rostral cortex in both hemispheres (Fig. 4a) after 4 weeks of sustained delivery of low-dose rhSGSH. A small, non-statistically significant reduction in microgliosis was observed in this region after bolus delivery. In the thalamus, microglia appeared to be completely deactivated on the injected side (right hemisphere; Fig. 4b), and a small (non-statistically significant) reduction in the number of activated microglia was observed in the non-injected (left) hemisphere of the brain in pump-treated MPS IIIA mice. The reason for the disparity between left and right hemispheres is unknown.
Quantification of immunohistochemical staining of isolectin B4 (activated microglia; a–c) and glial fibrillary acidic protein (GFAP; activated astrocytes; d–f) in 10-week-old MPS IIIA and unaffected mouse brain. The location of the brain regions analysed, with respect to the injection sites, is shown in the sagittal section of brain (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 cf. Normal; #p < 0.05, ##p < 0.01, ###p < 0.001, ###p < 0.0001 cf. MPS vehicle)
In contrast, there was no impact on microgliosis in this region when mice receiving bolus delivery of rhSGSH were examined at 10 weeks of age (2 weeks after the last injection). Neither treatment strategy initiated large reductions in microgliosis in the inferior colliculus (Fig. 4c), and astrogliosis remained at vehicle-treated MPS IIIA mouse levels in all regions of the brain examined following both high-dose bolus and low-dose continual enzyme delivery (Fig. 4d–f).
Stability of rhSGSH
At the end of each 2-week period, the remaining enzyme was removed from the pump and its activity was determined against a natural substrate. The rhSGSH removed from pumps explanted at 8 weeks of age exhibited 95.0 ± 5.0% of its original, undiluted activity, and enzyme removed from pumps taken at the end of the experiment retained 78.7 ± 2.0% of its original activity.
Discussion
In this proof-of-principle study, we evaluated the efficacy of continuous low-dose rhSGSH delivery to the ventricular CSF of MPS IIIA mice. Outcomes have been compared with those from MPS IIIA mice receiving equivalent amounts of rhSGSH (200 μg) over the same time frame (1 month) via intra-cisternal bolus infusions. Until now, all injections into MPS IIIA animals have been performed using bolus injection over the course of several minutes, and this is the methodology presently used in MPS IIIA patients enrolled in a clinical trial of this therapy (www.clinicaltrials.gov #NCT 01155778 and 01299727).
Data presented here suggest the superiority of low-dose continual enzyme supply over high-dose bolus injection, in both achieving and maintaining a low pathology burden in the MPS IIIA mouse brain. We hypothesise that continual delivery enables the maintenance of brain SGSH concentration at or above the effective concentration required for substrate catabolism. In contrast, bolus delivery may result in an initially high SGSH concentration, but this could be followed by a period of time during which the amount of SGSH falls below the effective concentration. As this is a proof-of principle study, this hypothesis is yet to be proven.
Once stability of the enzyme within the pump can be assured, increased duration experiments would need to be carried out to determine the long-term ability of this infusion method to both achieve and maintain low storage pathology burdens and to elucidate the impact of continuous delivery of rhSGSH on neurological function in MPS IIIA.
Two clinically feasible strategies accessing different CSF injection points were utilised in this study. To have compared ventricularly infused pump-delivered rhSGSH to a ventricular bolus strategy, we would have needed to have undertaken repeated intracerebral (lateral ventricle) injections. This is an invasive method and would have caused considerable damage to the overlying brain tissue. Similarly, we were unable to direct infusion cannulae (connected to the pumps) towards the cisterna magna, to enable comparison with cisternally infused bolus rhSGSH. The difference in CSF infusion points may/may not have biased the outcomes towards one infusion strategy or the other. Further investigation of the effectiveness of bolus versus slow delivery of rhSGSH via a single infusion site is now warranted, and these studies would be feasible in a larger animal model.
Pump-infused mice exhibited anti-rhSGSH antibodies in sera at post-mortem; however, we were unable to study the humoral response in the present cohort of cisternal bolus-infused mice. We have previously shown that repeat bolus rhSGSH-treated animals exhibit anti-rhSGSH antibodies in sera (Hemsley et al. 2007, 2008, 2009). In all instances (i.e. in both previously bolus-treated and the current pump-infused mice), there is no apparent clinical change in the mice. Thus, as anti-rhSGSH antibodies are found in the sera of rhSGSH-treated mice regardless of treatment method, we suggest that this is not a factor contributing to the superiority of one method of enzyme delivery over another.
To our knowledge, this is the first study to directly compare bolus and sustained-release enzyme delivery to the brain in a lysosomal storage disorder animal model. Other neurodegenerative lysosomal storage disorders that have been treated with slow, continuous infusion of enzyme into the CSF are late-infantile neuronal ceroid lipofuscinosis mice (Chang et al. 2008), metachromatic leukodystrophy mice (Stroobants et al. 2011) and MPS II mice (Sohn et al. 2013); Niemann–Pick A mice have received repeated slow (but not continuous) infusions of enzyme (Dodge et al. 2009). In the Chang et al. study (2008), 100 μg of recombinant human tripeptidyl peptidase (TPP1) was delivered over 14 days with no subsequent pump replacement. Euthanasia was carried out 45 days after treatment initiation, and post-mortem analyses revealed bilateral enzyme distribution, with 2- to 18-fold heterozygote levels of TPP1 detected in all ten 1 mm coronal slices of mouse brain at euthanasia, reduced cortical gliosis and autofluorescence, which was able to initiate an improved tremor phenotype. Whilst anti-TPP1 antibodies were recorded in treated mice, as also observed here, mouse health was not adversely affected. The influence of the antibodies on enzyme uptake and internalisation was not explored.
Mice with metachromatic leukodystrophy received recombinant human arylsulfatase A into the lateral ventricle over 4 weeks (Stroobants et al. 2011) using the same methodology employed here. The authors observed significant reductions in sulfatide storage and improved gait parameters. It was reported that various brain cell types responded differently to enzyme infusion, with macrophages most readily treated, as judged by catabolism of stored sulfatide. Intra-CSF enzyme-infused mice did not develop anti-arylsulfatase antibodies, in contrast to those animals enrolled in previous intravenous enzyme delivery studies (Matzner et al. 2005, 2008).
Finally, in the Dodge et al. study (2009), freely moving Niemann–Pick A mice were treated with recombinant human acid sphingomyelinase (rhASM) either once with one of three doses (7.5, 25, 250 μg) at 14 weeks of age or every 2 weeks from 6 to 30 weeks of age. Slow and repeated enzyme administration (25 μg) occurred over the course of 6 h. Treated mice exhibited intracellular (presumptively lysosomal) uptake of rhASM throughout the rostro-caudal axis, and dose-dependent reductions in sphingomyelin in the brain, liver and lung were noted. Cholesterol accumulation began to be ameliorated within 24 h post-infusion of rhASM, with levels subsequently re-accumulating to reach those of untreated affected mice by 3 weeks post-infusion. This is similar to the time course for pathology amelioration/re-accumulation in the MPS IIIA mouse (Hassiotis et al. 2014). Repeated treatment of Niemann–Pick A mice normalised forelimb and near-normalised hind-limb gait and improved performance in a foot-fault assay, but did not improve grip strength.
The results of the present study provide proof-of-principle support for continued evaluation of sustained delivery of lysosomal enzyme as a treatment for MPS IIIA. This strategy may significantly reduce the invasiveness of lifelong enzyme treatment and improve patient and family quality of life by reducing hospital/clinic visits.
Abbreviations
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- DAB:
-
Diaminobenzidine
- GFAP:
-
Glial fibrillary acidic protein
- LIMP-2:
-
Lysosomal integral membrane protein
- MPS:
-
Mucopolysaccharidosis
- OCT:
-
Optimal cutting temperature compound
- rhSGSH:
-
Recombinant human sulfamidase
References
Chang M, Cooper JD, Sleat DE et al (2008) Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol Ther 16:649–656
Crawley AC, Gliddon BL, Auclair D et al (2006) Characterization of a C57BL/6 congenic mouse strain of mucopolysaccharidosis type IIIA. Brain Res 1104:1–17
Crawley AC, Marshall N, Beard H et al (2011) Enzyme replacement reduces neuropathology in MPS IIIA dogs. Neurobiol Dis 43:422–434
Dodge JC, Clarke J, Treleaven CM et al (2009) Intracerebroventricular infusion of acid sphingomyelinase corrects CNS manifestations in a mouse model of Niemann–Pick A disease. Exp Neurol 215:349–357
Gliddon BL, Hopwood JJ (2004) Enzyme-replacement therapy from birth delays the development of behavior and learning problems in mucopolysaccharidosis type IIIA mice. Pediatr Res 56:65–72
Hassiotis S, Beard H, Luck A et al (2014) Disease stage determines the efficacy of treatment of a paediatric neurodegenerative disease. Eur J Neurosci 39:2139–2150
Hemsley KM, King B, Hopwood JJ (2007) Injection of recombinant human sulfamidase into the CSF via the cerebellomedullary cistern in MPS IIIA mice. Mol Genet Metab 90:313–328
Hemsley KM, Beard H, King BM et al (2008) Effect of high dose, repeated intra-CSF injection of sulfamidase on neuropathology in MPS IIIA mice. Genes Brain Behav 7:740–753
Hemsley KM, Luck AJ, Crawley AC et al (2009) Examination of intravenous and intra-CSF protein delivery for treatment of neurological disease. Eur J Neurosci 29:1197–1214
Hopwood JJ, Elliott H (1982) Diagnosis of Sanfilippo type A syndrome by estimation of sulfamidase activity using a radiolabelled tetrasaccharide substrate. Clin Chim Acta 123:241–250
Kakkis E, McEntee M, Vogler C et al (2004) Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol Genet Metab 83:163–174
Kondagari GS, King BM, Thomson PC et al (2011) Treatment of canine fucosidosis by intracisternal enzyme infusion. Exp Neurol 230:218–226
Lau AA, Hannouche H, Rozaklis T et al (2010) Allogeneic stem cell transplantation does not improve neurological deficits in mucopolysaccharidosis type IIIA mice. Exp Neurol 225:445–454
Lee WC, Tsoi YK, Troendle FJ et al (2007) Single-dose intracerebroventricular administration of galactocerebrosidase improves survival in a mouse model of globoid cell leukodystrophy. FASEB J 21:2520–2527
Matzner U, Herbst E, Hedayati KK et al (2005) Enzyme replacement improves nervous system pathology and function in a mouse model for metachromatic leukodystrophy. Hum Mol Genet 14:1139–1152
Matzner U, Matthes F, Weigelt C et al (2008) Non-inhibitory antibodies impede lysosomal storage reduction during enzyme replacement therapy of a lysosomal storage disease. J Mol Med (Berl) 86:433–442
Neufeld EF, Muenzer J (2001) The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Valle D, Sly WS (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 3421–3452
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic co-ordinates. Academic, USA
Sohn YB, Lee J, Cho SY et al (2013) Improvement of CNS defects via continuous intrathecal enzyme replacement by osmotic pump in mucopolysaccharidosis type II mice. Am J Med Genet A 161A:1036–1043
Stroobants S, Gerlach D, Matthes F et al (2011) Intracerebroventricular enzyme infusion corrects central nervous system pathology and dysfunction in a mouse model of metachromatic leukodystrophy. Hum Mol Genet 20:2760–2769
Tsuji D, Akeboshi H, Matsuoka K et al (2011) Highly phosphomannosylated enzyme replacement therapy for GM2 gangliosidosis. Ann Neurol 69:691–701
Acknowledgements
We acknowledge Hanan Hannouche for genotyping the mice, the WCHN Animal Care Facility staff for caring for our mice and Shire for the rhSGSH and anti-rhSGSH antibody used in the study. The Australian National Health and Medical Research Council (Grant #565074 to JJH and KMH) and Shire are acknowledged for provision of funds to support the study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Maurizio Scarpa, M.D, Ph.D
Appendices
Conflict of Interest Statement
Role of Commercial Funding Source
Shire provided the rhSGSH and the anti-rhSGSH antibody. Shire did not have any role in study design, data collection, data analysis, interpretation of data, writing of the report or in the decision to submit the paper for publication. Shire was given the opportunity to review the manuscript for scientific accuracy and legal/IP compliance, but the views expressed remain those of the authors.
Disclosure Statement
An international patent is held by JJH and others for mammalian sulfamidase and genetic sequences encoding it, for use in the investigation, diagnosis and treatment of subjects suspected of suffering from sulfamidase deficiency (US Patent # 5,863,782).
Synopsis
Continual rhSGSH infusion is superior to repeat bolus CSF injection.
Compliance with Ethics Guidelines
Conflict of Interest
Helen Beard, Sofia Hassiotis, Amanda J. Luck and Tina Rozaklis declare that they have no conflict of interest.
John J. Hopwood has received research grant funding from Shire and the Australian National Health and Medical Research Council for this and other studies. He and others hold an international patent for mammalian sulfamidase and genetic sequences encoding it, for use in the investigation, diagnosis and treatment of subjects suspected of suffering from sulfamidase deficiency (US Patent # 5,863,782). Kim M. Hemsley has received research grant funding from Shire and the Australian National Health and Medical Research Council for this and other studies.
Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Details of the Contributions of Individual Authors
Helen Beard and Sofia Hassiotis carried out tissue processing, undertook the immunohistochemical and histochemical studies outlined in the manuscript, analysed and interpreted data and edited the manuscript for accuracy. Amanda J. Luck carried out the mouse husbandry, assisted with the surgical procedures, monitored the mice post-surgery, carried out post-mortems/tissue collection, interpreted the data and edited the manuscript for accuracy. Tina Rozaklis carried out the enzyme activity and antibody titre assays analysed and interpreted the data and edited the manuscript for accuracy. John J. Hopwood conceived the study, obtained funding and edited the manuscript for accuracy. Kim M. Hemsley conceived and designed the study, obtained required consents, performed the surgery, analysed and interpreted data and wrote the manuscript.
Rights and permissions
Copyright information
© 2015 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Beard, H., Hassiotis, S., Luck, A.J., Rozaklis, T., Hopwood, J.J., Hemsley, K.M. (2015). Continual Low-Dose Infusion of Sulfamidase Is Superior to Intermittent High-Dose Delivery in Ameliorating Neuropathology in the MPS IIIA Mouse Brain. In: Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V. (eds) JIMD Reports, Volume 29. JIMD Reports, vol 29. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2015_495
Download citation
DOI: https://doi.org/10.1007/8904_2015_495
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53277-5
Online ISBN: 978-3-662-53278-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)