Abstract
Type III secretion systems are used by some Gram-negative bacteria to inject effector proteins into targeted eukaryotic cells for the benefit of the bacterium. The type III secretion injectisome is a complex nanomachine comprised of four main substructures including a cytoplasmic sorting platform, an envelope-spanning basal body, an extracellular needle and an exposed needle tip complex. Upon contact with a host cell, secretion is induced, resulting in the formation of a translocon pore in the host membrane. Translocon formation completes the conduit needed for effector secretion into the host cell. Control of type III secretion occurs in response to environmental signals, with the final signal being host cell contact. Secretion control occurs primarily at two sites—the cytoplasmic sorting platform, which determines secretion hierarchy, and the needle tip complex, which is critical for sensing and responding to environmental signals. The best-characterized injectisomes are those from Yersinia, Shigella and Salmonella species where there is a wealth of information on the tip complex and the two translocator proteins. Of these systems, the best characterized from a secretion regulation standpoint is Shigella. In the Shigella system, the tip complex and the first secreted translocon both contribute to secretion control and, thus, both are considered components of the tip complex. In this review, all three of these type III secretion systems are described with discussion focused on the structure and formation of the injectisome tip complex and what is known of the transition from nascent tip complex to assembled translocon pore.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The importance of type III secretion systems (T3SS) for the pathogenesis of Gram-negative human pathogens was acknowledged before they were recognized as actual secretion systems and long before visualization of the type III secretion nanomachine (the injectisome). The effects of calcium as it is related to the V antigen in Yersinia (Pasteurella) pestis were described more than a half-century ago (Bacon and Burrows 1956; Lawton et al. 1963) with similar observations in Yersinia pseudotuberculosis (Burrows and Bacon 1960). The observed calcium effects came to be known as the low calcium response (LCR) and it was found to have profound effects on the expression of the Yersinia V antigen. For the LCR, it was determined that in the absence of calcium Y. pestis growth was arrested at 37 °C, with no such growth arrest at 26 °C (Kupferberg and Higuchi 1958). This effect was reversed by including calcium at millimolar concentrations in the growth medium, restoring production of the Yersinia W and V antigens (Brubaker and Surgalla 1964; Lawton et al. 1963). Excitingly, the V antigen had been demonstrated to provide protection against bacterial challenge (Lawton et al. 1963), however, it was not immediately understood why or how this secreted antigen was protective. The V antigen later came to be known as LcrV and it was found to be an essential virulence factor for Y. pestis as well as other Yersinia species known to infect humans (i.e., Y. enterocolitica and Y. pseudotuberculosis) (Bhaduri et al. 1990; Goguen et al. 1986; Sample et al. 1987; Skrzypek and Straley 1995). Later studies described bacteria that had lost a large plasmid and become avirulent and no longer responded to the LCR (Ferber and Brubaker 1981). This long line of research would eventually lead investigators to identify the role of the plasmid-encoded Yersinia T3SS in evasion of host innate immunity (Michiels et al. 1990; Rosqvist et al. 1991) and the eventual study of the injectisome from both a functional and structural perspective. Injectisome needles were observed about a decade later (Hoiczyk and Blobel 2001) with higher resolution models of the Yersinia injectisome described shortly thereafter (Cornelis 2002). Yersinia continues to be a prime model system for studying type III secretion and it was in this system that identification of the first injectisome needle tip complex was made (described in more detail below).
Like Yersinia, Shigella species possess a plasmid that is essential for virulence (Sansonetti et al. 1981) and their ability to invade host cells within the gastrointestinal tract (Sansonetti et al. 1982). As with Yersinia, key Shigella antigens were found to be secreted into culture supernatants under conditions in which the virulence plasmid was active and the organism fully invasive (e.g., growth at 37 °C). In this case, the identified secreted proteins were called the invasion plasmid antigen (Ipa) proteins and they were considered to be potential protective antigens based on anecdotal information provided at that time (Buysse et al. 1987; Oaks et al. 1986). The first reports regarding the potentially complex structures of the Shigella injectisome structure were in 1999 (Blocker et al. 1999) with much more detailed structural information soon following (Blocker et al. 2001). Along with the Yersinia T3SS, this secretion system in Shigella has become a second model for improving our understanding of the structural and functional features of this important virulence determinant. Identification of the Shigella injectisome needle tip complex was reported shortly after identification of the analogous structure in Yersinia (Espina et al. 2006a). This is also discussed in further detail below.
A third pathogen that has become a paradigm for the study of type III secretion and injectisome structure–function relationships is Salmonella enterica. Unlike Yersinia and Shigella, Salmonella encodes many of its virulence determinants on pathogenicity islands (SPI) that are located chromosomally rather than on a large virulence plasmid. Two of these, SPI-1 and SPI-2, were found to encode T3SS that contribute to this pathogen’s complex lifestyle as an extracellular and intracellular pathogen (Mills et al. 1995; Ochman et al. 1996; Shea et al. 1996). Kaniga and colleagues identified homologs of the secreted Ipa proteins from Shigella that were expressed from SPI-1, which were important for Salmonella entry into host cells (Kaniga et al. 1995a, b). This observation was followed up by the Galan and Miller groups who reported the first high-quality images of the Salmonella injectisome (Kimbrough and Miller 2000; Kubori et al. 1998). Taken together, the Shigella, Yersinia and Salmonella T3SS arguably represent the best-characterized T3SS with regard to our understanding of architecture, substructure and function. From these organisms, there is an evolving understanding of the assembly and dynamics of the individual components, including the most exposed portions of the apparatus, the needle and its associated tip complex (TC). The TC is the major focus of this review.
2 The Type III Secretion Apparatus or Injectisome (The Injectisome)
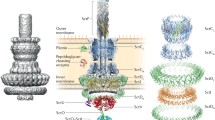
The injectisome is a complex nanomachine that functions through the combined actions of distinct substructures to promote the secretion of proteins (termed effectors) into target eukaryotic cells for the benefit of the infecting pathogen. These systems appear to be used exclusively by bacteria to communicate with eukaryotic cells for the benefit of the bacteria and have been identified in many plant- and animal-associated Gram-negative bacteria (Bergman et al. 1994; Galan et al. 1992; Gough et al. 1993; Wei and Beer 1993). It was initially unclear what types of structures were responsible for type III secretion, however, filamentous surface appendages (diameter of 6–8 nm) associated with pathogenesis were observed in the plant pathogen Pseudomonas syringae and called Hrp pili because of their roles in the plant hypersensitivity response and pathogenicity (Roine et al. 1997). Likewise, extracellular appendages that might be associated with type III secretion were identified in the human pathogen Salmonella following bacterial contact with host cells (Ginocchio et al. 1994). From various types of transmission electron micrograph analyses and high-resolution structure studies, we now have a reasonable understanding of the overall architecture of the injectisome (see Fig. 1) (Hu et al. 2015; Deng et al. 2017). While T3SS fall into broad groups that include multiple pathogen types (e.g., the Mxi/Spa/Ipa family includes Salmonella and Shigella), there are some general features common among all the known injectisomes. Each system contains a fixed basal body spanning the entire Gram-negative cell envelope (IM-cell wall-OM) (Marlovits et al. 2004). The basal body is sandwiched between an extracellular needle composed of a polymer of a small, helix-turn-helix needle protein (Cordes et al. 2005; Deane et al. 2006a; Demers et al. 2014; Fujii et al. 2012; Zhang et al. 2006; Wang et al. 2008) and a cytoplasmic sorting platform. The sorting platform is comprised of the export gate, an energy source (ATPase) and an associated hexameric unit that is the structural equivalent of the flagellar C ring (Hu et al. 2017; Lara-Tejero et al. 2011; Hu et al. 2015). Recognition of secretion substrates and determination of secretion hierarchy most likely occurs within this cytoplasmic portion of the injectisome. Induction of secretion occurs as a result of external signals such as changes in the bacterium’s environment (e.g., host cell membrane contact, change in pH or changes extracellular calcium levels) which potentially implicates the needle and/or the complex of proteins located at the exposed end of the injectisome needle, the tip complex (TC), in sensing such signals. Once the environmental signals are encountered, however, the switch to effector secretion involves complexes that contain gatekeeper proteins such as SsaL for the SPI-2 injectisome of Salmonella (Yu et al. 2010, 2018), SepL in enteropathogenic Escherichia coli (Shaulov et al. 2017) or YopN in Yersinia (Bamyaci et al. 2018). The regulators of needle length have also been implicated in these switches to effector protein secretion. The focus here will be a description of the TC and a summary of how it may be involved in secretion control and sensing the environment.

Panels A and B are adapted from (Hu et al. 2015). Copyright National Academy of Sciences. Panel C is PDB ID: 2J0O, (Johnson et al. 2007)
The proposed architecture of the Shigella injectisome. In panel A, a side view of the injectisome is shown with the major basal body components indicated (in blue hues). Beneath the bacterial inner membrane is the cytoplasmic sorting platform with the main components indicated. At the top is the extracellular needle comprised of a polymer of MxiH. Panel B shows a bottom view of the sorting platform depicting the sixfold symmetry of this complex. The tip complex is not indicated here, but panel C shows the crystal structure of IpaD, which is the main component of the tip complex. IpaD resides as the most distal structure from the surface of the bacterium—presumably as a pentamer in the nascent injectisome.
3 The Injectisome Needle Tip Complex
3.1 Discovery of the Nascent Tip Complex Protein and Overall Structure
It was noted long ago that the Yersinia V antigen (LcrV) had the potential for inducing protective immunity in animals (Lawton et al. 1963). Eventually, recombinant LcrV was specifically studied for its strong potential as a subunit vaccine component (Leary et al. 1995; Motin et al. 1994) and it continues to be a component of some of the lead candidate vaccines being explored for protection against plague (Verma and Tuteja 2016). The Cornelis group reported in 1998 that LcrV was required for proper secretion of what later were identified as the Yersinia T3SS translocator proteins (the Yersinia Outer Proteins or Yops) YopB and YopD (Sarker et al. 1998). Once the structural architecture for the injectisome began to be revealed, it was found that antibodies against LcrV could disrupt proper translocon assembly in targeted cells (in this case erythrocytes). Strikingly, the same was true for antibodies against the LcrV-homolog PcrV from the closely related Pseudomonas aeruginosa T3SS (Goure et al. 2005). The mechanistic basis for these phenomena did not become entirely clear until the V antigen was definitively localized to the tip of the injectisome needle in three related pathogens (Y. pestis, P. aeruginosa and Aeromonas salmonicida) (Mueller et al. 2005). It was at this time that LcrV was designated as the Yersinia injectisome needle tip complex protein. Shortly afterward, IpaD was identified as being the needle tip protein for the Shigella injectisome (Espina et al. 2006b). The crystal structure of LcrV had previously been reported to 2.2 Å resolution in 2004 (Derewenda et al. 2004) and this structure was used to model the LcrV tip protein as a pentameric needle “tip complex” (TC) atop the Yersinia YscF needle filament (Broz et al. 2007).
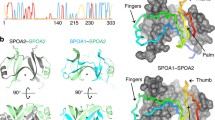
While advances were being made regarding LcrV as the Yersinia needle TC, parallel achievements were occurring for a needle TC from a distinct injectisome family. IpaD was identified as the Shigella needle tip protein in 2006 (Espina et al. 2006b) and its crystal structure was determined shortly thereafter (see Figs. 1 and 2), along with a TC homolog (BipD) from the same injectisome family and produced by Burkholderia pseudomallei (Erskine et al. 2006; Johnson et al. 2007). In addition to LcrV and IpaD, a number of homologous TC proteins have now been described and compared using biophysical analyses to identify their shared structural features (Deng et al. 2017; Espina et al. 2007; Sato and Frank 2011). One major feature of all the TC proteins characterized to date is the presence of an extended coiled-coil (green region within Fig. 2) that provides the scaffold upon which the rest of the protein is built. While the coiled-coil domain appears important for protein–protein interactions across all T3SS-possessing pathogens, the remainder of the TC protein structure appears to vary somewhat and may be adapted to more pathogen-specific functions.
Crystal structure of IpaD and the camelid single chain (VHH) antibody designated 20ipaD. The crystal structure of IpaD (colored based on individual domains: N-domain (blue), central coiled-coil (green), distal domain (red) (Johnson et al. 2007) is depicted in cartoon ribbon format in complex with the neutralizing VHH 20ipaD (colored gray). Complex [PDB ID: 5VXM, (Barta et al. 2017b)]. Inset, IpaD residues within hydrogen bonding distance (2.5–3.5 Å) are depicted as balls-and-sticks (magenta)
The structural basis for the interaction between cognate needle tip protein and the actual needle proteins has been described for some of these systems, most notably for the IpaD-MxiH combination, respectively, from Shigella and the homologous combination (SipD-PrgI) from Salmonella (Rathinavelan et al. 2011, 2014; Zhang et al. 2007). It is likely that the interactions between the TC and needle itself play an instrumental role in regulation of type III secretion, which is clearly the case for Shigella (Deane et al. 2006b; Picking et al. 2005; Kenjale et al. 2005). It has been shown that mutations within the MxiH needle protein of the Shigella T3SS can have profound effects on secretion kinetics and, in some cases, secretion substrate hierarchy (Deane et al. 2006b; Kenjale et al. 2005). Additional studies have shown that the C-terminus of IpaD is required for anchoring it to the injectisome needle tip (Picking et al. 2005) with deletion of only a few C-terminal amino acids resulting in secretion of uncontrolled amounts of IpaD into the culture supernatant. The stabilizing coiled-coil of IpaD has been implicated in generating secretion signals (Barta et al. 2012b; Roehrich et al. 2013; Stensrud et al. 2008) along with anchoring it to the tip of the Shigella injectisome needle (the IpaD C-terminus is part of the coiled-coil). If the IpaD coiled-coil does indeed interact with the needle protein, then it is not surprising that mutations within this region that influence contact (and thus communication) between these structures are able to influence secretion control. Additional structure/function features of IpaD are presented in Sect. 4.
Elucidation of the atomic-level structure of IpaD enabled modeling of IpaD as a pentameric TC at the distal end of the Shigella MxiH needle where it was later directly observed by transmission electron microscopy (Sani et al. 2007). Since this work, multiple models of the Shigella TC have been proposed with an additional hypothesis that the TC is a heteropentamer consisting of four copies of IpaD and one of the first secreted translocator protein IpaB (Veenendaal et al. 2007). In the initial description of the IpaD tip complex, it was noted that while IpaB was not detected on the surface of the nascent injectisome, it could be detected by immunoblot analysis of artificially long needles (generated by overexpressing MxiH). This suggested that IpaB could be associated with needles, but probably was not physically located at the nascent injectisome needle tip at this stage (Espina et al. 2006b). It is clear that IpaB is not required for IpaD to reside at the tip of the nascent injectisome needle (Olive et al. 2007) and in ipaB null strains it exists as a homopentamer (Epler et al. 2012; Espina et al. 2006b; Cheung et al. 2015). Conversely, IpaB has never been found to associate with the injectisome needle tip in the absence of IpaD, even though it is secreted at elevated levels, thus confirming that IpaD is the anchor for IpaB binding (Dickenson et al. 2013a; Espina et al. 2007; Olive et al. 2007). Furthermore, even for wild-type Shigella, the majority of the TC appear to exist as a pentamer of IpaD (Cheung et al. 2015), perhaps indicating that detection of IpaB within some TC may be due to an intermediate stage of the secretion process.
3.2 Maturation of the Tip Complex in Shigella
As described above, the IpaD TC protein and IpaB, a Shigella T3SS translocator protein, have long been known to control Shigella secretion. This role is seen as substantially elevated levels of Ipa and effector proteins in culture supernatants for null mutants of either (Menard et al. 1994). It is worth noting, however, that Shigella does not control its T3SS as stringently as many other systems do in the absence of extracellular signals for secretion induction. Such signals for Shigella could include incubation with the dye Congo red or contact with a host cell. This low level of background or steady-state secretion has not been specifically described for other T3SS, which implies that there is phylogenetic variation in the control mechanisms used by these pathogens. Consistent with system-to-system variation, similar roles in secretion control have been described for IpaD and its homolog (SipD) from Salmonella (Kaniga et al. 1995b), however, no such regulatory role has been reported for SipB, the IpaB-homolog in Salmonella (Kaniga et al. 1995b). This suggests that differences in the way that type III secretion is controlled can be seen even for closely related pathogens within the same T3SS family (e.g., Shigella and Salmonella SPI-1). As more distantly related injectisomes are considered, even greater differences in the mechanisms by which secretion is induced are observed. For example, Yersinia expresses their T3SS at 37 °C but only secretes when depletion of calcium is detected and this process may be under the control of multiple genes including the injectisome needle protein YscF (Torruellas et al. 2005). This is distinct from Shigella where cation concentrations in the media have not been reported to influence type III secretion, while the addition of small amphipathic dyes such as Congo red or Evans blue elicits a strong secretion phenotype (Bahrani et al. 1997).
Thus far, the discrete steps of secretion induction have only been described for the T3SS of Shigella species. For the Shigella injectisome, the nascent apparatus is primarily comprised of a pentameric IpaD TC population (Cheung et al. 2015; Espina et al. 2006b). As mentioned above, there may also be a minor TC population that is composed of only four copies of IpaD with the fifth position being filled with IpaB (Cheung et al. 2015; Veenendaal et al. 2007). Neither complex composition can be ruled out as being physiologically relevant, but within the context of the low background secretion phenotype of Shigella alluded to above, it is possible that this heteropentameric TC represents an intermediate state where IpaB is being recruited into the complex. Active recruitment of IpaB into the TC appears to require additional external signals, and these will be described in more detail below.
Enteric bacteria have evolved numerous mechanisms to allow survival and colonization of the human gastrointestinal tract (Merritt and Donaldson 2009). Among the many chemical defenses in the GI tract are bile salts that act to reduce the bacterial burden in the small intestine in addition to their contributions to nutrient uptake and metabolism (Schubert et al. 2017). It has been shown that bile salts influence a variety of Shigella behaviors, including increased: adherence to mammalian cells, invasiveness and protein secretion during laboratory propagation (Olive et al. 2007; Pope et al. 1995; Faherty et al. 2012). We were able to demonstrate that after a short (≤30 min) incubation in the presence of the bile salt deoxycholate (DOC), Shigella invasiveness increased significantly (Olive et al. 2007). Unexpectedly, this DOC-mediated virulence increase did not correlate with upregulated protein effector secretion, but in fact, resulted in the recruitment of IpaB to the tip of the Shigella injectisome needle (Olive et al. 2007) where it was stably maintained. IpaB was unable to localize to the needle tip for an ipaD null mutant, strongly suggesting an interaction within the TC for these two proteins. Meanwhile, in an ipaB null mutant, IpaD could still associate with the needle tip, however, incubation of these bacteria with DOC resulted in decreased levels of IpaD at this site (Olive et al. 2007). Considered in toto, these observations suggested that DOC acts directly on IpaD. Furthermore, the fact that IpaD and IpaB were both genetically identified as major players controlling the Shigella T3SS suggests they are able to physically communicate with each other and this correlates with identification of a stable interaction at the exposed injectisome tip.
3.3 The Translocon
The ultimate goal of the T3SS is the formation of a pore (the translocon) in the target cell membrane, thereby allowing the subsequent passage of effector proteins into the cell where they commandeer normal cellular functions for the benefit of the pathogen (Pizarro-Cerda et al. 2016). In generating the translocon, a difficulty that must be overcome by the pathogen is maintaining proteins having a significant hydrophobic character in the cytoplasm until they can be recognized for secretion, at which point they become imbedded within the targeted cell’s membrane. Preventing interactions with the bacterial cytoplasmic membrane is critical and this is ensured by maintaining stability and solubility as a complex with a chaperone. For example, in Shigella the chaperone for the IpaB and IpaC is IpgC (Birket et al. 2007) and in Salmonella the chaperone for SipB and is SicA (Tucker and Galan 2000). In addition to this feature, it has been shown that the proteins secreted by the T3SS that are destined to become transmembrane proteins in the host cell possess an inherent balance of their transmembrane segments that prevent them from targeting to the bacterial cytoplasmic membrane (Krampen et al. 2018).
In Shigella, IpaB is the first of the two translocators to be secreted. As such it seems to have two roles—as a TC protein following exposure to bile salts and as a component of the translocon pore following host cell contact. A hallmark of all T3SS is the presence of a TC protein and two translocator proteins that function together to form the translocon pore (Deng et al. 2017). In the Shigella system, the translocon is formed by IpaB and IpaC (Blocker et al. 1999; Terry et al. 2008) and one assay for monitoring the formation of the complete translocon is measuring contact-mediated hemolysis (Picking et al. 2005). Based on its hydrophobicity, a potential role of IpaB in translocon formation from its position at the injectisome needle tip is sensing contact with the host cell membrane. Indeed, IpaB has been shown to interact with lipid membranes in vitro (De Geyter et al. 2000), as has the second hydrophobic translocator IpaC (Kueltzo et al. 2003). Furthermore, contact with host cells was found to trigger the release of Ipa translocators as a prelude to invasion (Watarai et al. 1995) and lipid-based signaling has been shown to trigger the secretion of effector proteins. These host-pathogen interactions were proposed to occur at cholesterol-rich cellular lipid rafts (van der Goot et al. 2004) and are consistent with the final step of TC maturation/activation being triggered by host cell lipids.
Red blood cell ghosts and liposomes having a defined composition were used to demonstrate that the final step of T3SS TC maturation is triggered by tip-localized IpaB sensing contact with host cell membranes (Epler et al. 2009). Treating Shigella with DOC followed by liposomes resulted in the recruitment of IpaC to the bacterial surface. Not surprisingly, recruitment of IpaC to the surface coincided with full induction of type III secretion. Unlike IpaB, which appears largely to be limited to the TC once it is recruited to the Shigella surface, the secreted IpaC was found to stick to many surfaces once it was in the extracellular milieu. IpaC was found as part of the needle TC, however, it was also found in complexes not associated with the bacteria, on the bacterial membrane surface and bound to almost anything with which it could interact. In these studies, it was demonstrated that maximal liposome-induced secretion occurred when cholesterol and sphingomyelin were present, which is in agreement with previous observations that IpaB is a cholesterol-binding protein (Hume et al. 2003) and Shigella invasion most likely occurs at lipid rafts (van der Goot et al. 2004). Similar to IpaB, purified IpaC is able to insert spontaneously into phospholipid membranes (Kueltzo et al. 2003; De Geyter et al. 1997) and has been shown to possess important effector functions that contribute to host cell entry (Jaumouille et al. 2008; Marquart et al. 1996; Terry et al. 2008; Tran Van Nhieu et al. 1999). IpaC’s effector function is essential for Shigella entry into host cells (Terry et al. 2008), while its ability to insert into and disrupt membranes may also be responsible for Shigella escape into the cytoplasmic niche in which it replicates (Osiecki et al. 2001; Du et al. 2016). Unfortunately, most of the information obtained on the translocon is from indirect biochemical analyses of purified or partially purified proteins because visualization of the translocon has been difficult. This may be changing since Park and colleagues have now used cryo-electron tomography of mini-cells interacting with host cells to generate images of the in situ Salmonella translocon at the pathogen-host interface (Park et al. 2018).
Little information has been obtained on the step-wise TC maturation or secretion induction for other T3SS, however, interaction between the Yersinia TC protein LcrV and one of the Yersinia translocon proteins (YopD) has been reported as being important for type III secretion by this pathogen (Costa et al. 2010). Additionally, LcrV has been implicated in control of type III secretion, but this control has not been described as occurring from the injectisome needle tip (Hamad and Nilles 2007; Matson and Nilles 2001), but possibly via interactions with LcrG in the bacterial cytoplasm which serve to influence the mobilization of the YopB translocator (Nilles et al. 1998). Most importantly, LcrV is implicated in directing the formation of translocon pores in Yersinia (Mota 2006), suggesting that intermediates between LcrV TC formation and translocon assembly most likely do exist. It should be noted that alternative pathways for effector entry into target cells by Y. pseudotuberculosis have been proposed that might not necessarily even require TC contact with the traditional model of a translocon (Akopyan et al. 2011). However, this review will be limited to events expected to occur only at the injectisome needle tip and that are involved in the regulation of secretion induction. Because of this and the fact that the best-understood sequence of events related to T3SS induction are those that have been observed in the Shigella system, the remainder of this review will mostly focus on the Shigella TC.
3.4 Where Does the Tip Complex End and the Translocon Begin
In many cases, the proteins that make up the needle TC and the hydrophobic proteins that give rise to the translocon pore are collectively referred to as the “translocator proteins” (Deng et al. 2017). This is understandable since all three proteins are needed for proper translocation of effector proteins into target cells. In the same respect, the conduit that assembles to become the injectisome needle is also needed for proper translocation. Thus, for the purpose of this review, a distinction is made between the needle, the TC and the translocon pore. IpaD clearly falls into the category of TC protein for the Shigella injectisome since it is the protein initially placed at the tip of the MxiH needle once it is formed and, perhaps more importantly, because it is essential for controlling or limiting secretion from this position. In this respect, IpaB in the Shigella system could also be considered a needle TC protein because it is also essential for controlling secretion. Moreover, IpaB can also be found stably associated with the injectisome needle tip prior to secretion induction. It is in this role that IpaB most likely triggers type III secretion induction by sensing contact with the host cell membrane. The presence of translocator proteins as part of the TC has only been described in detail for the Shigella system (Cheung et al. 2015; Murillo et al. 2016; Olive et al. 2007). Simultaneous with or in transition from its function as a regulatory TC component, IpaB becomes essential for the formation of a fully functional translocon in conjunction with IpaC, which has no role in the control of type III secretion in Shigella. In fact, once IpaC secretion is triggered, the full cascade of secretion induction is completed. For these reasons, IpaD and IpaB are grouped here as the two components of the Shigella regulatory TC and IpaB and IpaC are separately grouped as the two translocator proteins in this organism. These are not arbitrary groupings from the Shigella perspective, but they may be difficult to apply precisely to other T3SS. While IpaD homologs from other systems have been implicated in controlling type III secretion and are found at the needle tip, this cannot be said of IpaB homologs. Nevertheless, the sections that follow will focus on IpaD as the initial needle TC protein and IpaB, which joins IpaD once the TC is primed to sense contact with host cells.
4 Sensing the Signals Responsible for Type III Secretion Induction in Shigella
4.1 Invasion Plasmid Antigen D
By now it is clear that IpaD is an essential virulence determinant for Shigella with a role in controlling type III secretion (Menard et al. 1993). Once nonpolar knockouts of the Ipa proteins became available it was learned that IpaB, along with IpaD, was responsible for controlling type III secretion in this pathogen (Menard et al. 1994). It was not until more than 10 years later, however, that IpaD was recognized as being a controlling unit that resides atop the Shigella injectisome needle (Espina et al. 2006b; Sani et al. 2007). Structurally, IpaD was initially proposed to form a homopentamer at the needle tip much like LcrV is proposed to do at the tip of the Yersinia injectisome needle (Deane et al. 2006b; Epler et al. 2012), however, alternative TC compositions have been suggested that include four copies of IpaD and one copy of IpaB (Blocker et al. 2008). While most of the TC found on the Shigella surface are clearly composed of five copies of IpaD (Cheung et al. 2015), it cannot be ruled out that alternative states exist that represent intermediates related to TC maturation and the onset of secretion induction. Unlike the T3SS described for many other bacteria, Shigella displays a low level of background secretion that is readily measured by monitoring Ipa protein secretion in overnight cultures. Under such conditions, a small percentage of injectisomes could be in a state that lies between quiescence (having five copies of IpaD making up the TC) and primed (having a TC complex that contains a reduced number of IpaD moieties in combination with one or more copies of IpaB). The physiological importance for such intermediates cannot be ruled out because of the Shigella background secretion phenotype, however, it does appear that the first static state or checkpoint in the assembly or maturation of the newly made Shigella injectisome TC gives rise to a TC comprised of a pentamer of IpaD.
Maturation or priming of the Shigella needle TC, as defined by the recruitment of IpaB to become a major component of the TC, can be elicited by exposure to bile salts such as DOC (Olive et al. 2007). A mechanistic equivalent step for Salmonella has not been described, mostly because bile salts appear to actually inhibit SPI-1 expression (Eade et al. 2016). Nevertheless, bile salts have been shown to bind to the Salmonella TC protein SipD (Chatterjee et al. 2011). In Shigella, the mechanistic basis for bile salt-induced recruitment of IpaB into the TC appears to be a direct interaction between DOC and IpaD (Stensrud et al. 2008) with this binding causing a change in the structural features of the central coiled-coil of IpaD (Barta et al. 2012b). DOC binding occurs at the hydrophobic interface between helix 3 and helix 7 (the stabilizing coiled-coil that has also been implicated in anchoring IpaD at the needle tip) involving residues L134, K137, I138 and L315. Furthermore, mutation of some of these residues (L134 and L315) was shown to eliminate the organism’s enhanced invasiveness that was seen following incubation with DOC (Barta et al. 2012b). Interestingly, the binding of DOC by IpaD was found to occur concomitant with an exacerbation of a kink found in helix 3, which results in a movement of ~10 Å for the end of helix 7 near the C-terminus of the protein. Such a change in the conformational dynamics at this region is expected to significantly affect the interaction between IpaD and the underlying needle assembly, as well as between the IpaD subunits within the TC. Mutagenesis studies have also implicated the C-terminal helix (helix 7) of IpaD in its ability to control type III secretion (Roehrich et al. 2013), however, the residues implicated here were further away from IpaD’s proposed C-terminal anchor than were the residues involved in DOC binding. In this case, the mutations were selected for their ability to resist rapid induced secretion caused by incubation with Congo red.
As repeatedly mentioned in the above sections, IpaD has been proposed to exist as a pentamer as part of the Shigella needle TC. This is based on the structures seen for Yersinia, P. aeruginosa and A. salmonicida (Mueller et al. 2005) and what was initially proposed for Shigella (Deane et al. 2006b; Sani et al. 2007). Variations on the initially proposed IpaD pentamer include a four (IpaD) plus one (IpaB) model, along with a model in which an IpaD pentamer is located at the needle tip with the so-called distal domain (red domain in Fig. 2) extended outward (Epler et al. 2012). An interesting finding related to this last model is that when cleavage sites for tobacco etch virus (TEV) protease were placed on both sides of the distal domain and the distal domain was removed by site-specific cleavage, a loss of secretion control was observed, thus indicating that the distal domain has some role in controlling secretion. While alone this result is not confirmatory with regard to the position of the distal domain within the TC, it does provide something to consider when considered alongside two other findings. First, IpaD was shown to be able to associate with a stable coiled-coil derived from the N-terminal part of IpaB whose crystal structure has been solved (Barta et al. 2012a) (see Fig. 3), but only if bile salts are present (Dickenson et al. 2013a). More importantly, association of IpaD with the IpaB fragment resulted in movement of a fluorescent probe located within the distal domain away from a second probe located at IpaD position 322, near the region that anchors IpaD to the tip of the needle (Dickenson et al. 2013a). These findings not only implicate the distal domain in interactions with IpaB, but also suggest that IpaD has a dynamic structure that can accommodate multiple conformations, including one in which the distal domain moves relative to the rest of the protein. IpaD had already been shown to consist of multiple folding units (Espina et al. 2006a), but this was the first biochemical evidence that there is flexibility in the distal domain. A second piece of evidence for the IpaD distal domain being important for type III signaling processes was derived from its interaction with specific camelid single-domain (VHH) antibodies generated by vaccination against recombinant IpaD, which behaves as a highly soluble monomer in solution. From a panel of IpaD-specific VHH antibodies, two populations were identified—one population exhibited significant neutralizing activity with regard to cellular invasion and contact-hemolysis while the other population was non-neutralizing. The former were found to uniformly recognize the IpaD distal domain (helix 4) as shown for one of the VHH (called 20ipaD) in Fig. 2 (Barta et al. 2017b). Recently, NMR studies have further indicated that interactions occur between the distal domain of IpaD and the purified N-terminal coiled-coil of IpaB (McShan et al. 2016). This study also found that the same phenomenon occurred for the Ipa homologs from Salmonella, SipD and SipB, and that mutations within the SipD distal domain equivalent (e.g., helix 4 in IpaD) led to a reduced ability to invade cultured Henle 407 cells (McShan et al. 2016).
Crystal Structure of T3SS Translocator Coiled-coil Fragments. Left, Cartoon ribbon diagram of the IpaB74−224 crystal structure [PDB ID: 3U0C, (Barta et al. 2012a)], colored blue (N-terminus) to red (C-terminus). Middle, Structural alignment of the IpaB (residues 120-224) and SipB (residues 126-226) coiled-coil motifs align with an RMSD of 1.42 Å over 93/94 Cα atoms. Right, Cartoon ribbon diagram of the SipB82−226 crystal structure [PDB ID: 3TUL, (Barta et al. 2012a)], colored gray
4.2 Invasion Plasmid Antigen B
Because of its hydrophobic nature, IpaB has been more difficult to work with as a recombinant protein than IpaD (Barta et al. 2017a) and this has slowed efforts to fully appreciate its biochemistry and prevented determination of its atomic-level structure. It has been possible, however, to purify N-terminal IpaB fragments that have contributed substantially to our current level of understanding of IpaB. Crystal structures of stable (and soluble) N-terminal domains of both IpaB (residues 74-224) and SipB (residues 82-226) were determined and are shown in Fig. 3 (Barta et al. 2012a). Both fragments have been shown to be capable of interacting with their cognate needle tip protein as mentioned above (McShan et al. 2016), although no crystal structure is available to describe this interaction. A slightly longer IpaB fragment (residues 28-226) has been shown to strongly associate with its chaperone IpgC (Adam et al. 2012). It does appear that chaperone binding alters the IpaB fragment’s structure, however, the precise influence of chaperone binding on the structure of the IpaB coiled-coil is not known. On the other hand, a co-crystal structure has been solved for an N-terminal fragment of the IpaB/SipB homolog AopB from Aeromonas in complex with its chaperone AcrH (Nguyen et al. 2015) and this structure indicates that the coiled-coil is specifically bent by association with the N-terminal groove of AcrH (colored purple in Fig. 4). This suggests that chaperone binding not only involves the immediate N-terminus of IpaB and its homologs, but it may also specifically perturb the stable coiled-coil structure found near the N-terminus. No structural information is yet available on the hydrophobic portions of this translocator protein family and it is this portion that is expected to be involved in membrane recognition and penetration. Intriguingly, current data support a model in which IpaB association with its chaperone in the bacterial cytoplasm occurs through its N-terminal region, however, this would be expected to leave the hydrophobic portion of the protein exposed. Despite this, the IpaB-IpgC complex is unable to associate, even peripherally, with phospholipid membranes (Dickenson et al. 2013b). Thus, it is clear that there is still much to learn about IpaB structure and function, even before it leaves the Shigella cytoplasm.
Crystal structure of AcrH/AopB40−264. Cartoon ribbon diagram of the AcrH/AopB40−264 crystal structure [PDB ID: 3WXX, (Nguyen et al. 2015)], with AcrH colored blue (N-terminus) to red (C-terminus) and AopB colored gray. Structure rotated 90° about the horizontal axis on the right. The AopB coiled-coil motif (residues 123-157; colored purple) is bent and rests in a groove created by the AcrH N-terminus
Based on what is known about the stable IpaB N-terminal domain, it has been hypothesized that the coiled-coil allows IpaB (or SipB) interaction with IpaD (or SipD) via the distal domain of the later (Dickenson et al. 2013a; McShan et al. 2016). This would position the hydrophobic portion of IpaB (SipB) so that it is available for recognition of and interaction with the host cell membrane (Dickenson et al. 2013b). The previously described neutralizing anti-IpaD VHHs support this model because their interaction with the IpaD distal domain appears to be interfering with steps involved in TC function such as IpaB recruitment or binding to the TC (Barta et al. 2017b). Other groups suggest a similar orientation for the IpaB hydrophobic domain, but favor a model where it is anchored to IpaD via its C-terminus based on decreased detection of IpaB within the TC as residues are removed from the end of the protein (Shen et al. 2010). To be clear, IpaB’s initial interaction with the host cell membrane is a pivotal event in triggering the final step of type III secretion induction and for the recruitment of IpaC, which is needed for formation of the translocon pore. There will be additional discussion of IpaB’s interaction with membranes below.
Based on homology with pore-forming toxins, it was proposed that the IpaB coiled-coil was involved in anchoring this protein to the needle TC following its recruitment to the bacterial surface. Such a scenario might equate to the role of these coiled-coil structures in a family of bactericidal toxins produced by some bacteria to gain an advantage against competing bacteria (Jakes 2012). For example, colicin E3 and colicin Ia share a similar coiled-coil structure with IpaB and SipB and these extended structures are used by the colicins to span the periplasm to bridge the domain needed for binding to an outer membrane receptor with the domain that interacts with the target cell cytoplasmic membrane following an outer membrane translocation step (Wiener et al. 1997). Because of the parallel need for presentation of a membrane active moiety at a distance, it would seem logical that the translocator coiled-coil could be used in a similar manner. Intriguingly, this same coiled-coil structure has been heavily implicated in the control of Shigella type III secretion (Murillo et al. 2016). In an extensive mutagenesis study, seven mutations were identified within the IpaB N-terminal portion that affected Shigella’s ability to respond to Congo red induction of secretion and all but one of these resided within the coiled-coil (Murillo et al. 2016). These secretion phenotypes fell into two groups, those having mild and those having strong defects in sensing Congo red. As with other aspects of IpaB function, however, there is still much to learn about these phenotypes since defects in sensing Congo red did not strictly correlate with defects in contact-mediated hemolysis or invasion of HeLa cells (Murillo et al. 2016).
While a combination of Congo red phenotypes found for IpaD and IpaB mutants can be used to develop a model for secretion control in Shigella, it should be kept in mind that the way Congo red works is still not entirely clear. Congo red has been known to induce protein unfolding in some cases, possibly after penetrating regions possessing a somewhat intrinsically unfolded state (Zhang et al. 2009; Kim et al. 2003). It is this propensity for binding to unfolded regions of proteins which has made it useful in staining of amyloid fibrils for diagnostic purposes (Serpell et al. 1997). Furthermore, in preferentially associating with partially unfolded regions of proteins, Congo red can shift a protein’s folding toward an unfolded state. If this is occurring within the Shigella injectisome needle TC, then it is difficult to confidently assign a physiological role to Congo red-induced secretion. Any disruptions within the TC, especially where there are interfacial interactions, such as between IpaD and IpaB might well be expected to give rise to changes in secretion status. This is only said as a cautionary statement in considering TC functions using artificial inducers and to point out that there continues to be much to learn about the dynamics of IpaD and IpaB within the TC. A similar statement might be said with regard to studying translocon pore formation using contact-mediated hemolysis, however, such artificial systems have thus far been invaluable in reaching our current level of understanding of type III secretion in Shigella.
In addition to being a TC component, IpaB is also a translocator protein. While this review does not consider the translocon pore as a part of the injectisome needle TC, it probably is important to consider the potential state of IpaB as it encounters the host cell membrane. Shigella mutants harboring an ipaC null mutation do not suffer defects in type III secretion control, however, they are completely noninvasive. The inability to invade cells is due to the inability to form a functional translocon for effector delivery and to the absence of IpaC’s early effector functions (Terry et al. 2008). Nevertheless, ipaC null mutants are still able to induce a low level of contact-mediated hemolysis (~10% relative to wild-type), indicating that something has been inserted into the target cell membrane that is able to compromise membrane integrity (Blocker et al. 1999). Because a functional translocon does not form for these mutants, IpaB can be considered to continue to be a part of the needle TC following host cell contact, but it should be noted that it is still potentially membrane active. It may be here that the ability to work with purified IpaB has proven most useful. IpaB was first purified efficiently as a complex with its chaperone IpgC (Birket et al. 2007), but it is readily separated from its chaperone using mild detergents (Barta et al. 2017a). It is important, however, that these detergents be continually present in the preparation to maintain IpaB solubility. While this has made detailed structural analysis of IpaB difficult, it was recently found that the detergent used to prepare IpaB can have a profound effect on its biochemical properties.
IpaB clearly has an intrinsic ability to interact with membranes and this property is shared with its homolog from Salmonella (De Geyter et al. 2000; Hume et al. 2003). When prepared in the detergent lauryl-dimethylamine-N-oxide (LDAO), IpaB exists as a monomer in solution, however, when prepared in the alternative detergent N-octyl-poly-oxyethylene (OPOE) it forms a tetramer (Dickenson et al. 2013b). Both of these detergents are so mild that they do not disrupt phospholipid vesicles at or slightly above their critical micelle concentrations, which allows them to be present when looking at IpaB-membrane interactions. In either detergent, IpaB can associate with phospholipid vesicles, however, only the oligomeric form of IpaB is able to cause the release of small molecules from these liposomes and this release shows the hallmarks of being the result of pore formation (Adam et al. 2014; Dickenson et al. 2013b). Thus, even before orchestrating the formation of an active translocon pore through interactions with IpaC, IpaB itself is proposed to insert into target cell membranes. This provides the trigger for IpaC recruitment, translocon formation and secretion induction (Epler et al. 2009; van der Goot et al. 2004). However, based on background hemolysis levels and biochemical analysis of the IpaB-membrane interaction, it is possible that this insertion event results in the formation of what might be termed a pre-translocon pore composed of an oligomeric complex of IpaB (Dickenson et al. 2013b). While such a pore would not be a fully functional translocon, it could provide a platform into which IpaC can be incorporated, leading to formation of the active translocon pore. It is at this point in wild-type Shigella that IpaB becomes an integral part of the translocon while maintaining a bridge to IpaD at the tip of the injectisome needle.
5 Concluding Remarks
Much of what is known about type III secretion control from the TC has been determined based upon the tractability of the Shigella system. It is clear that, despite a high degree of conservation within the injectisomes from a wide array of pathogens, there are still differences in the cargo they inject into host cells, subtle variations in their assembly and function of their component pieces, and in how they respond to external stimuli. Perhaps their greatest unifying feature is the formation of a translocon pore upon host cell contact, enabling translocation of host altering effector proteins that are maintained within the bacterial cytoplasm in association with cognate chaperones. The chaperone then is instrumental in targeting the effectors to the sorting platform for ultimate delivery through the translocon and into the cytoplasm of the host cell. The resulting host-pathogen intercommunication can lead to a variety of outcomes: colonization of a host cell surface through effacing lesions; invasion of macrophages, epithelial cells or lymphocytes; or killing of macrophages. Yet, despite their many differences, it makes sense that there are significant mechanistic similarities that are guided by the general architecture of these amazing nanomachines. The four major macromolecular assemblies within the injectisome (sorting platform with ATPase, envelope-spanning basal body, extracellular needle and needle TC) may display subtle differences but they largely appear to be consistent from one system to the next.
It is generally accepted that all injectisome needle TC are essential for creating a continuous conduit from the bacterium through the host cell membrane, suggesting they share important mechanistic characteristics. This would seem to be borne out of the conservation of key structural features for all the extracellular portions of the injectisome that are involved in controlling secretion (Deng et al. 2017). The structure of the needle protein monomer has consistently been shown to be a relatively small helix-turn-helix protein (Deane et al. 2006b; Zhang et al. 2007; Wang et al. 2007). The initial needle TC protein can vary in overall structure, but is consistently built upon a stable anti-parallel helical coiled-coil scaffold (Derewenda et al. 2004; Espina et al. 2007; Johnson et al. 2007). And finally, it is the first hydrophobic translocator protein that, at least for Shigella and Salmonella, possesses an elongated helical coiled-coil that may provide a contact interface with the nascent TC protein (Barta et al. 2012a). Altogether, it appears that these protein–protein interfaces, likely involving extensive contributions from coiled-coil motifs, are critical to the assembly, communication and overall function of the injectisome.
Thus, while a significant portion of this review has focused on the Shigella injectisome needle tip complex and its role in regulating type III secretion, it is likely that there are lessons to be learned here that will hold true in many, or perhaps most, other systems. Nevertheless, it is certainly a mistake to view the injectisome needle TC as a static object that progresses from one rigid form to the next. For Shigella, the TC is a fluid structure that has yielded transitions that can be teased apart to reveal what can be described as discrete steps in type III secretion induction. Part of what has made this so, may be the one confounding feature of the Shigella T3SS—the fact that it is in a low, but continual, steady-state secretion state. The same progression of steps may very well occur for other T3SS, but due to stringent control prior to host cell contact, these steps may be difficult, if not impossible, to dissect. Over time, other systems will be discovered or methods devised to determine how universal the process of step-wise type III secretion induction actually is.
References
Adam PR, Dickenson NE, Greenwood JC 2nd, Picking WL, Picking WD (2014) Influence of oligomerization state on the structural properties of invasion plasmid antigen B from Shigella flexneri in the presence and absence of phospholipid membranes. Proteins 82(11):3013–3022. https://doi.org/10.1002/prot.24662
Adam PR, Patil MK, Dickenson NE, Choudhari S, Barta M, Geisbrecht BV, Picking WL, Picking WD (2012) Binding affects the tertiary and quaternary structures of the Shigella translocator protein IpaB and its chaperone IpgC. Biochemistry 51(19):4062–4071. https://doi.org/10.1021/bi300243z
Akopyan K, Edgren T, Wang-Edgren H, Rosqvist R, Fahlgren A, Wolf-Watz H, Fallman M (2011) Translocation of surface-localized effectors in type III secretion. Proc Natl Acad Sci USA 108(4):1639–1644. https://doi.org/10.1073/pnas.1013888108
Bacon GA, Burrows TW (1956) The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol 37(5):481–493
Bahrani FK, Sansonetti PJ, Parsot C (1997) Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect Immun 65(10):4005–4010
Bamyaci S, Ekestubbe S, Nordfelth R, Erttmann SF, Edgren T, Forsberg A (2018) YopN is required for efficient effector translocation and virulence in Yersinia pseudotuberculosis. Infect Immun 86(8). https://doi.org/10.1128/iai.00957-17
Barta ML, Adam PR, Dickenson NE (2017a) Recombinant expression and purification of the Shigella Translocator IpaB. Methods Mol Biol 1531:173–181. https://doi.org/10.1007/978-1-4939-6649-3_15
Barta ML, Dickenson NE, Patil M, Keightley A, Wyckoff GJ, Picking WD, Picking WL, Geisbrecht BV (2012a) The structures of coiled-coil domains from type III secretion system translocators reveal homology to pore-forming toxins. J Mol Biol 417(5):395–405. https://doi.org/10.1016/j.jmb.2012.01.026
Barta ML, Guragain M, Adam P, Dickenson NE, Patil M, Geisbrecht BV, Picking WL, Picking WD (2012b) Identification of the bile salt binding site on IpaD from Shigella flexneri and the influence of ligand binding on IpaD structure. Proteins 80(3):935–945
Barta ML, Shearer JP, Arizmendi O, Tremblay JM, Mehzabeen N, Zheng Q, Battaile KP, Lovell S, Tzipori S, Picking WD, Shoemaker CB, Picking WL (2017b) Single-domain antibodies pinpoint potential targets within Shigella invasion plasmid antigen D of the needle tip complex for inhibition of type III secretion. J Biol Chem 292(40):16677–16687. https://doi.org/10.1074/jbc.M117.802231
Bergman T, Erickson K, Galyov E, Persson C, Wolf-Watz H (1994) The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J Bacteriol 176(9):2619–2626
Bhaduri S, Turner-Jones C, Taylor MM, Lachica RV (1990) Simple assay of calcium dependency for virulent plasmid-bearing clones of Yersinia enterocolitica. J Clin Microbiol 28(4):798–800
Birket SE, Harrington AT, Espina M, Smith ND, Terry CM, Darboe N, Markham AP, Middaugh CR, Picking WL, Picking WD (2007) Preparation and characterization of translocator/chaperone complexes and their component proteins from Shigella flexneri. Biochemistry 46(27):8128–8137. https://doi.org/10.1021/bi700099c
Blocker A, Gounon P, Larquet E, Niebuhr K, Cabiaux V, Parsot C, Sansonetti P (1999) The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J Cell Biol 147(3):683–693
Blocker A, Jouihri N, Larquet E, Gounon P, Ebel F, Parsot C, Sansonetti P, Allaoui A (2001) Structure and composition of the Shigella flexneri “needle complex”, a part of its type III secreton. Mol Microbiol 39(3):652–663
Blocker AJ, Deane JE, Veenendaal AK, Roversi P, Hodgkinson JL, Johnson S, Lea SM (2008) What’s the point of the type III secretion system needle? Proc Natl Acad Sci USA 105(18):6507–6513. https://doi.org/10.1073/pnas.0708344105
Broz P, Mueller CA, Muller SA, Philippsen A, Sorg I, Engel A, Cornelis GR (2007) Function and molecular architecture of the Yersinia injectisome tip complex. Mol Microbiol 65(5):1311–1320. https://doi.org/10.1111/j.1365-2958.2007.05871.x
Brubaker RR, Surgalla MJ (1964) The effect of Ca++ and Mg++ on lysis, growth, and production of virulence antigens by Pasteurella pestis. J Infect Dis 114:13–25
Burrows TW, Bacon GA (1960) V and W antigens in strains of Pasteurella pseudotuberculosis. Br J Exp Pathol 41:38–44
Buysse JM, Stover CK, Oaks EV, Venkatesan M, Kopecko DJ (1987) Molecular cloning of invasion plasmid antigen (ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J Bacteriol 169(6):2561–2569
Chatterjee S, Zhong D, Nordhues BA, Battaile KP, Lovell S, De Guzman RN (2011) The crystal structures of the Salmonella type III secretion system tip protein SipD in complex with deoxycholate and chenodeoxycholate. Protein Sci 20(1):75–86. https://doi.org/10.1002/pro.537
Cheung M, Shen DK, Makino F, Kato T, Roehrich AD, Martinez-Argudo I, Walker ML, Murillo I, Liu X, Pain M, Brown J, Frazer G, Mantell J, Mina P, Todd T, Sessions RB, Namba K, Blocker AJ (2015) Three-dimensional electron microscopy reconstruction and cysteine-mediated crosslinking provide a model of the type III secretion system needle tip complex. Mol Microbiol 95(1):31–50. https://doi.org/10.1111/mmi.12843
Cordes FS, Daniell S, Kenjale R, Saurya S, Picking WL, Picking WD, Booy F, Lea SM, Blocker A (2005) Helical packing of needles from functionally altered Shigella type III secretion systems. J Mol Biol 354(2):206–211. https://doi.org/10.1016/j.jmb.2005.09.062
Cornelis GR (2002) The Yersinia Ysc-Yop ‘type III’ weaponry. Nat Rev Mol Cell Biol 3(10):742–752. https://doi.org/10.1038/nrm932
Costa TR, Edqvist PJ, Broms JE, Ahlund MK, Forsberg A, Francis MS (2010) YopD self-assembly and binding to LcrV facilitate type III secretion activity by Yersinia pseudotuberculosis. J Biol Chem 285(33):25269–25284. https://doi.org/10.1074/jbc.M110.144311
De Geyter C, Vogt B, Benjelloun-Touimi Z, Sansonetti PJ, Ruysschaert JM, Parsot C, Cabiaux V (1997) Purification of IpaC, a protein involved in entry of Shigella flexneri into epithelial cells and characterization of its interaction with lipid membranes. FEBS Lett 400(2):149–154
De Geyter C, Wattiez R, Sansonetti P, Falmagne P, Ruysschaert JM, Parsot C, Cabiaux V (2000) Characterization of the interaction of IpaB and IpaD, proteins required for entry of Shigella flexneri into epithelial cells, with a lipid membrane. Eur J Biochem 267(18):5769–5776
Deane JE, Cordes FS, Roversi P, Johnson S, Kenjale R, Picking WD, Picking WL, Lea SM, Blocker A (2006a) Expression, purification, crystallization and preliminary crystallographic analysis of MxiH, a subunit of the Shigella flexneri type III secretion system needle. Acta Crystallogr Sect F Struct Biol Cryst Commun 62(Pt 3):302–305. https://doi.org/10.1107/S1744309106006555
Deane JE, Roversi P, Cordes FS, Johnson S, Kenjale R, Daniell S, Booy F, Picking WD, Picking WL, Blocker AJ, Lea SM (2006b) Molecular model of a type III secretion system needle: implications for host-cell sensing. Proc Natl Acad Sci USA 103(33):12529–12533. https://doi.org/10.1073/pnas.0602689103
Demers JP, Habenstein B, Loquet A, Kumar Vasa S, Giller K, Becker S, Baker D, Lange A, Sgourakis NG (2014) High-resolution structure of the Shigella type-III secretion needle by solid-state NMR and cryo-electron microscopy. Nat Commun 5:4976. https://doi.org/10.1038/ncomms5976
Deng W, Marshall NC, Rowland JL, McCoy JM, Worrall LJ, Santos AS, Strynadka NCJ, Finlay BB (2017) Assembly, structure, function and regulation of type III secretion systems. Nat Rev Microbiol 15(6):323–337. https://doi.org/10.1038/nrmicro.2017.20
Derewenda U, Mateja A, Devedjiev Y, Routzahn KM, Evdokimov AG, Derewenda ZS, Waugh DS (2004) The structure of Yersinia pestis V-antigen, an essential virulence factor and mediator of immunity against plague. Structure 12(2):301–306. https://doi.org/10.1016/j.str.2004.01.010
Dickenson NE, Arizmendi O, Patil MK, Toth RTt, Middaugh CR, Picking WD, Picking WL (2013a) N-terminus of IpaB provides a potential anchor to the Shigella type III secretion system tip complex protein IpaD. Biochemistry 52(49):8790–8799. https://doi.org/10.1021/bi400755f
Dickenson NE, Choudhari SP, Adam PR, Kramer RM, Joshi SB, Middaugh CR, Picking WL, Picking WD (2013b) Oligomeric states of the Shigella translocator protein IpaB provide structural insights into formation of the type III secretion translocon. Protein Sci 22(5):614–627. https://doi.org/10.1002/pro.2245
Du J, Reeves AZ, Klein JA, Twedt DJ, Knodler LA, Lesser CF (2016) The type III secretion system apparatus determines the intracellular niche of bacterial pathogens. Proc Natl Acad Sci USA 113(17):4794–4799. https://doi.org/10.1073/pnas.1520699113
Eade CR, GungCC, Bullard B, Gonzalez-Escobedo G, Gunn JS, Altier C (2016) Bile acids function synergistically to repress invasion gene expression in Salmonella by destabilizing the invasion regulator HilD. Infect Immun 84(8):2198–2208. https://doi.org/10.1128/IAI.00177-16
Epler CR, Dickenson NE, Bullitt E, Picking WL (2012) Ultrastructural analysis of IpaD at the tip of the nascent MxiH type III secretion apparatus of Shigella flexneri. J Mol Biol 420(1–2):29–39. https://doi.org/10.1016/j.jmb.2012.03.025
Epler CR, Dickenson NE, Olive AJ, Picking WL, Picking WD (2009) Liposomes recruit IpaC to the Shigella flexneri type III secretion apparatus needle as a final step in secretion induction. Infect Immun 77(7):2754–2761. https://doi.org/10.1128/IAI.00190-09
Erskine PT, Knight MJ, Ruaux A, Mikolajek H, Wong Fat Sang N, Withers J, Gill R, Wood SP, Wood M, Fox GC, Cooper JB (2006) High resolution structure of BipD: an invasion protein associated with the type III secretion system of Burkholderia pseudomallei. J Mol Biol 363(1):125–136. https://doi.org/10.1016/j.jmb.2006.07.069
Espina M, Ausar SF, Middaugh CR, Baxter MA, Picking WD, Picking WL (2007) Conformational stability and differential structural analysis of LcrV, PcrV, BipD, and SipD from type III secretion systems. Protein Sci 16(4):704–714. https://doi.org/10.1110/ps.062645007
Espina M, Ausar SF, Middaugh CR, Picking WD, Picking WL (2006a) Spectroscopic and calorimetric analyses of invasion plasmid antigen D (IpaD) from Shigella flexneri reveal the presence of two structural domains. Biochemistry 45(30):9219–9227. https://doi.org/10.1021/bi060625v
Espina M, Olive AJ, Kenjale R, Moore DS, Ausar SF, Kaminski RW, Oaks EV, Middaugh CR, Picking WD, Picking WL (2006b) IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect Immun 74(8):4391–4400. https://doi.org/10.1128/IAI.00440-06
Faherty CS, Redman JC, Rasko DA, Barry EM, Nataro JP (2012) Shigella flexneri effectors OspE1 and OspE2 mediate induced adherence to the colonic epithelium following bile salts exposure. Mol Microbiol 85(1):107–121. https://doi.org/10.1111/j.1365-2958.2012.08092.x
Ferber DM, Brubaker RR (1981) Plasmids in Yersinia pestis. Infect Immun 31(2):839–841
Fujii T, Cheung M, Blanco A, Kato T, Blocker AJ, Namba K (2012) Structure of a type III secretion needle at 7-A resolution provides insights into its assembly and signaling mechanisms. Proc Natl Acad Sci USA 109(12):4461–4466. https://doi.org/10.1073/pnas.1116126109
Galan JE, Ginocchio C, Costeas P (1992) Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol 174(13):4338–4349
Ginocchio CC, Olmsted SB, Wells CL, Galan JE (1994) Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell 76(4):717–724
Goguen JD, Walker WS, Hatch TP, Yother J (1986) Plasmid-determined cytotoxicity in Yersinia pestis and Yersinia pseudotuberculosis. Infect Immun 51(3):788–794
Gough CL, Genin S, Lopes V, Boucher CA (1993) Homology between the HrpO protein of Pseudomonas solanacearum and bacterial proteins implicated in a signal peptide-independent secretion mechanism. Mol Gen Genet 239(3):378–392
Goure J, Broz P, Attree O, Cornelis GR, Attree I (2005) Protective anti-V antibodies inhibit Pseudomonas and Yersinia translocon assembly within host membranes. J Infect Dis 192(2):218–225. https://doi.org/10.1086/430932
Hamad MA, Nilles ML (2007) Roles of YopN, LcrG and LcrV in controlling Yops secretion by Yersinia pestis. Adv Exp Med Biol 603:225–234. https://doi.org/10.1007/978-0-387-72124-8_20
Hoiczyk E, Blobel G (2001) Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc Natl Acad Sci USA 98(8):4669–4674. https://doi.org/10.1073/pnas.071065798
Hu B, Lara-Tejero M, Kong Q, Galan JE, Liu J (2017) In situ molecular architecture of the Salmonella type III secretion machine. Cell 168(6):1065–1074, e1010. https://doi.org/10.1016/j.cell.2017.02.022
Hu B, Morado DR, Margolin W, Rohde JR, Arizmendi O, Picking WL, Picking WD, Liu J (2015) Visualization of the type III secretion sorting platform of Shigella flexneri. Proc Natl Acad Sci USA 112(4):1047–1052. https://doi.org/10.1073/pnas.1411610112
Hume PJ, McGhie EJ, Hayward RD, Koronakis V (2003) The purified Shigella IpaB and Salmonella SipB translocators share biochemical properties and membrane topology. Mol Microbiol 49(2):425–439
Jakes KS (2012) Translocation trumps receptor binding in colicin entry into Escherichia coli. Biochem Soc Trans 40(6):1443–1448. https://doi.org/10.1042/BST20120207
Jaumouille V, Francetic O, Sansonetti PJ, Tran Van Nhieu G (2008) Cytoplasmic targeting of IpaC to the bacterial pole directs polar type III secretion in Shigella. EMBO J 27(2):447–457. https://doi.org/10.1038/sj.emboj.7601976
Johnson S, Roversi P, Espina M, Olive A, Deane JE, Birket S, Field T, Picking WD, Blocker AJ, Galyov EE, Picking WL, Lea SM (2007) Self-chaperoning of the type III secretion system needle tip proteins IpaD and BipD. J Biol Chem 282(6):4035–4044. https://doi.org/10.1074/jbc.M607945200
Kaniga K, Trollinger D, Galan JE (1995a) Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol 177(24):7078–7085
Kaniga K, Tucker S, Trollinger D, Galan JE (1995b) Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol 177(14):3965–3971
Kenjale R, Wilson J, Zenk SF, Saurya S, Picking WL, Picking WD, Blocker A (2005) The needle component of the type III secreton of Shigella regulates the activity of the secretion apparatus. J Biol Chem 280(52):42929–42937. https://doi.org/10.1074/jbc.M508377200
Kim YS, Randolph TW, Manning MC, Stevens FJ, Carpenter JF (2003) Congo red populates partially unfolded states of an amyloidogenic protein to enhance aggregation and amyloid fibril formation. J Biol Chem 278(12):10842–10850. https://doi.org/10.1074/jbc.M212540200
Kimbrough TG, Miller SI (2000) Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc Natl Acad Sci USA 97(20):11008–11013. https://doi.org/10.1073/pnas.200209497
Krampen L, Malmsheimer S, Grin I, Trunk T, Luhrmann A, de Gier JW, Wagner S (2018) Revealing the mechanisms of membrane protein export by virulence-associated bacterial secretion systems. Nat Commun 9(1):3467. https://doi.org/10.1038/s41467-018-05969-w
Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galan JE, Aizawa SI (1998) Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280(5363):602–605
Kueltzo LA, Osiecki J, Barker J, Picking WL, Ersoy B, Picking WD, Middaugh CR (2003) Structure-function analysis of invasion plasmid antigen C (IpaC) from Shigella flexneri. J Biol Chem 278(5):2792–2798. https://doi.org/10.1074/jbc.M208383200
Kupferberg LL, Higuchi K (1958) Role of calcium ions in the stimulation of growth of virulent strains of Pasteurella pestis. J Bacteriol 76(1):120–121
Lara-Tejero M, Kato J, Wagner S, Liu X, Galan JE (2011) A sorting platform determines the order of protein secretion in bacterial type III systems. Science 331(6021):1188–1191. https://doi.org/10.1126/science.1201476
Lawton WD, Erdman RL, Surgalla MJ (1963) Biosynthesis and purification of V and W antigen in Pasteurella pestis. J Immunol 91:179–184
Leary SE, Williamson ED, Griffin KF, Russell P, Eley SM, Titball RW (1995) Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect Immun 63(8):2854–2858
Marlovits TC, Kubori T, Sukhan A, Thomas DR, Galan JE, Unger VM (2004) Structural insights into the assembly of the type III secretion needle complex. Science 306(5698):1040–1042. https://doi.org/10.1126/science.1102610
Marquart ME, Picking WL, Picking WD (1996) Soluble invasion plasmid antigen C (IpaC) from Shigella flexneri elicits epithelial cell responses related to pathogen invasion. Infect Immun 64(10):4182–4187
Matson JS, Nilles ML (2001) LcrG-LcrV interaction is required for control of Yops secretion in Yersinia pestis. J Bacteriol 183(17):5082–5091
McShan AC, Kaur K, Chatterjee S, Knight KM, De Guzman RN (2016) NMR identification of the binding surfaces involved in the Salmonella and Shigella Type III secretion tip-translocon protein-protein interactions. Proteins 84(8):1097–1107. https://doi.org/10.1002/prot.25055
Menard R, Sansonetti P, Parsot C, Vasselon T (1994) Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 79(3):515–525
Menard R, Sansonetti PJ, Parsot C (1993) Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol 175(18):5899–5906
Merritt ME, Donaldson JR (2009) Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J Med Microbiol 58(Pt 12):1533–1541. https://doi.org/10.1099/jmm.0.014092-0
Michiels T, Wattiau P, Brasseur R, Ruysschaert JM, Cornelis G (1990) Secretion of Yop proteins by Yersiniae. Infect Immun 58(9):2840–2849
Mills DM, Bajaj V, Lee CA (1995) A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol Microbiol 15(4):749–759
Mota LJ (2006) Type III secretion gets an LcrV tip. Trends Microbiol 14(5):197–200. https://doi.org/10.1016/j.tim.2006.02.010
Motin VL, Nakajima R, Smirnov GB, Brubaker RR (1994) Passive immunity to Yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect Immun 62(10):4192–4201
Mueller CA, Broz P, Muller SA, Ringler P, Erne-Brand F, Sorg I, Kuhn M, Engel A, Cornelis GR (2005) The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310(5748):674–676. https://doi.org/10.1126/science.1118476
Murillo I, Martinez-Argudo I, Blocker AJ (2016) Genetic dissection of the signaling cascade that controls activation of the Shigella Type III secretion system from the needle tip. Sci Rep 6:27649. https://doi.org/10.1038/srep27649
Nguyen VS, Jobichen C, Tan KW, Tan YW, Chan SL, Ramesh K, Yuan Y, Hong Y, Seetharaman J, Leung KY, Sivaraman J, Mok YK (2015) Structure of AcrH-AopB chaperone-translocator complex reveals a role for membrane hairpins in type III secretion system translocon assembly. Structure 23(11):2022–2031. https://doi.org/10.1016/j.str.2015.08.014
Nilles ML, Fields KA, Straley SC (1998) The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J Bacteriol 180(13):3410–3420
Oaks EV, Hale TL, Formal SB (1986) Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun 53(1):57–63
Ochman H, Soncini FC, Solomon F, Groisman EA (1996) Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA 93(15):7800–7804
Olive AJ, Kenjale R, Espina M, Moore DS, Picking WL, Picking WD (2007) Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect Immun 75(5):2626–2629. https://doi.org/10.1128/IAI.01599-06
Osiecki JC, Barker J, Picking WL, Serfis AB, Berring E, Shah S, Harrington A, Picking WD (2001) IpaC from Shigella and SipC from Salmonella possess similar biochemical properties but are functionally distinct. Mol Microbiol 42(2):469–481
Park D, Lara-Tejero M, Waxham MN, Li W, Hu B, Galan JE, Liu J (2018) Visualization of the type III secretion mediated Salmonella-host cell interface using cryo-electron tomography. Elife 7. https://doi.org/10.7554/elife.39514
Picking WL, Nishioka H, Hearn PD, Baxter MA, Harrington AT, Blocker A, Picking WD (2005) IpaD of Shigella flexneri is independently required for regulation of Ipa protein secretion and efficient insertion of IpaB and IpaC into host membranes. Infect Immun 73(3):1432–1440. https://doi.org/10.1128/IAI.73.3.1432-1440.2005
Pizarro-Cerda J, Charbit A, Enninga J, Lafont F, Cossart P (2016) Manipulation of host membranes by the bacterial pathogens Listeria, Francisella, Shigella and Yersinia. Semin Cell Dev Biol 60:155–167. https://doi.org/10.1016/j.semcdb.2016.07.019
Pope LM, Reed KE, Payne SM (1995) Increased protein secretion and adherence to HeLa cells by Shigella spp. following growth in the presence of bile salts. Infect Immun 63(9):3642–3648
Rathinavelan T, Lara-Tejero M, Lefebre M, Chatterjee S, McShan AC, Guo DC, Tang C, Galan JE, De Guzman RN (2014) NMR model of PrgI-SipD interaction and its implications in the needle-tip assembly of the Salmonella type III secretion system. J Mol Biol 426(16):2958–2969. https://doi.org/10.1016/j.jmb.2014.06.009
Rathinavelan T, Tang C, De Guzman RN (2011) Characterization of the interaction between the Salmonella type III secretion system tip protein SipD and the needle protein PrgI by paramagnetic relaxation enhancement. J Biol Chem 286(6):4922–4930. https://doi.org/10.1074/jbc.M110.159434
Roehrich AD, Guillossou E, Blocker AJ, Martinez-Argudo I (2013) Shigella IpaD has a dual role: signal transduction from the type III secretion system needle tip and intracellular secretion regulation. Mol Microbiol 87(3):690–706. https://doi.org/10.1111/mmi.12124
Roine E, Wei W, Yuan J, Nurmiaho-Lassila EL, Kalkkinen N, Romantschuk M, He SY (1997) Hrp pilus: an hrp-dependent bacterial surface appendage produced by Pseudomonas syringae pv. tomato DC3000. Proc Natl Acad Sci USA 94(7):3459–3464
Rosqvist R, Forsberg A, Wolf-Watz H (1991) Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun 59(12):4562–4569
Sample AK, Fowler JM, Brubaker RR (1987) Modulation of the low-calcium response in Yersinia pestis via plasmid-plasmid interaction. Microb Pathog 2(6):443–453
Sani M, Botteaux A, Parsot C, Sansonetti P, Boekema EJ, Allaoui A (2007) IpaD is localized at the tip of the Shigella flexneri type III secretion apparatus. Biochim Biophys Acta 1770(2):307–311. https://doi.org/10.1016/j.bbagen.2006.10.007
Sansonetti PJ, Kopecko DJ, Formal SB (1981) Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun 34(1):75–83
Sansonetti PJ, Kopecko DJ, Formal SB (1982) Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun 35(3):852–860
Sarker MR, Neyt C, Stainier I, Cornelis GR (1998) The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol 180(5):1207–1214
Sato H, Frank DW (2011) Multi-functional characteristics of the Pseudomonas aeruginosa type III needle-tip protein, PcrV; comparison to orthologs in other gram-negative bacteria. Front Microbiol 2:142. https://doi.org/10.3389/fmicb.2011.00142
Schubert K, Olde Damink SWM, von Bergen M, Schaap FG (2017) Interactions between bile salts, gut microbiota, and hepatic innate immunity. Immunol Rev 279(1):23–35. https://doi.org/10.1111/imr.12579
Serpell LC, Sunde M, Blake CC (1997) The molecular basis of amyloidosis. Cell Mol Life Sci 53(11–12):871–887
Shaulov L, Gershberg J, Deng W, Finlay BB, Sal-Man N (2017) The ruler protein EscP of the enteropathogenic Escherichia coli type III secretion system is involved in calcium sensing and secretion hierarchy regulation by interacting with the gatekeeper protein SepL. MBio 8(1). https://doi.org/10.1128/mbio.01733-16
Shea JE, Hensel M, Gleeson C, Holden DW (1996) Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA 93(6):2593–2597
Shen DK, Saurya S, Wagner C, Nishioka H, Blocker AJ (2010) Domains of the Shigella flexneri type III secretion system IpaB protein involved in secretion regulation. Infect Immun 78(12):4999–5010. https://doi.org/10.1128/IAI.00470-10
Skrzypek E, Straley SC (1995) Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol 177(9):2530–2542
Stensrud KF, Adam PR, La Mar CD, Olive AJ, Lushington GH, Sudharsan R, Shelton NL, Givens RS, Picking WL, Picking WD (2008) Deoxycholate interacts with IpaD of Shigella flexneri in inducing the recruitment of IpaB to the type III secretion apparatus needle tip. J Biol Chem 283(27):18646–18654. https://doi.org/10.1074/jbc.M802799200
Terry CM, Picking WL, Birket SE, Flentie K, Hoffman BM, Barker JR, Picking WD (2008) The C-terminus of IpaC is required for effector activities related to Shigella invasion of host cells. Microb Pathog 45(4):282–289. https://doi.org/10.1016/j.micpath.2008.06.003
Torruellas J, Jackson MW, Pennock JW, Plano GV (2005) The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol Microbiol 57(6):1719–1733. https://doi.org/10.1111/j.1365-2958.2005.04790.x
Tran Van Nhieu G, Caron E, Hall A, Sansonetti PJ (1999) IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J 18(12):3249–3262. https://doi.org/10.1093/emboj/18.12.3249
Tucker SC, Galan JE (2000) Complex function for SicA, a Salmonella enterica serovar typhimurium type III secretion-associated chaperone. J Bacteriol 182(8):2262–2268
van der Goot FG, Tran van Nhieu G, Allaoui A, Sansonetti P, Lafont F (2004) Rafts can trigger contact-mediated secretion of bacterial effectors via a lipid-based mechanism. J Biol Chem 279(46):47792–47798. https://doi.org/10.1074/jbc.M406824200
Veenendaal AK, Hodgkinson JL, Schwarzer L, Stabat D, Zenk SF, Blocker AJ (2007) The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol Microbiol 63(6):1719–1730. https://doi.org/10.1111/j.1365-2958.2007.05620.x
Verma SK, Tuteja U (2016) Plague vaccine development: current research and future trends. Front Immunol 7:602. https://doi.org/10.3389/fimmu.2016.00602
Wang Y, Ouellette AN, Egan CW, Rathinavelan T, Im W, De Guzman RN (2007) Differences in the electrostatic surfaces of the type III secretion needle proteins PrgI, BsaL, and MxiH. J Mol Biol 371(5):1304–1314. https://doi.org/10.1016/j.jmb.2007.06.034
Wang Y, Zhang L, Picking WL, Picking WD, De Guzman RN (2008) Structural dissection of the extracellular moieties of the type III secretion apparatus. Mol BioSyst 4(12):1176–1180. https://doi.org/10.1039/b808271p
Watarai M, Tobe T, Yoshikawa M, Sasakawa C (1995) Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J 14(11):2461–2470
Wei ZM, Beer SV (1993) HrpI of Erwinia amylovora functions in secretion of harpin and is a member of a new protein family. J Bacteriol 175(24):7958–7967
Wiener M, Freymann D, Ghosh P, Stroud RM (1997) Crystal structure of colicin Ia. Nature 385(6615):461–464. https://doi.org/10.1038/385461a0
Yu XJ, Grabe GJ, Liu M, Mota LJ, Holden DW (2018) SsaV interacts with SsaL to control the translocon-to-effector switch in the Salmonella SPI-2 type three secretion system. MBio 9(5). https://doi.org/10.1128/mbio.01149-18
Yu XJ, McGourty K, Liu M, Unsworth KE, Holden DW (2010) pH sensing by intracellular Salmonella induces effector translocation. Science 328(5981):1040–1043. https://doi.org/10.1126/science.1189000
Zhang L, Wang Y, Olive AJ, Smith ND, Picking WD, De Guzman RN, Picking WL (2007) Identification of the MxiH needle protein residues responsible for anchoring invasion plasmid antigen D to the type III secretion needle tip. J Biol Chem 282(44):32144–32151. https://doi.org/10.1074/jbc.M703403200
Zhang L, Wang Y, Picking WL, Picking WD, De Guzman RN (2006) Solution structure of monomeric BsaL, the type III secretion needle protein of Burkholderia pseudomallei. J Mol Biol 359(2):322–330. https://doi.org/10.1016/j.jmb.2006.03.028
Zhang YZ, Xiang X, Mei P, Dai J, Zhang LL, Liu Y (2009) Spectroscopic studies on the interaction of Congo Red with bovine serum albumin. Spectrochim Acta A Mol Biomol Spectrosc 72(4):907–914. https://doi.org/10.1016/j.saa.2008.12.007
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Picking, W.D., Barta, M.L. (2019). The Tip Complex: From Host Cell Sensing to Translocon Formation. In: Wagner, S., Galan, J. (eds) Bacterial Type III Protein Secretion Systems. Current Topics in Microbiology and Immunology, vol 427. Springer, Cham. https://doi.org/10.1007/82_2019_171
Download citation
DOI: https://doi.org/10.1007/82_2019_171
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52122-6
Online ISBN: 978-3-030-52123-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)