Abstract
Cryptococcus neoformans is a human pathogenic yeast that causes hundreds of thousands of deaths worldwide among susceptible individuals, in particular, HIV+ patients. This yeast has developed several adaptation mechanisms that allow replication within the host. During decades, this yeast has been well known for a very peculiar and unique structure that contributes to virulence, a complex polysaccharide capsule that surrounds the cell wall. In contrast to other fungal pathogens, such as Candida albicans or Aspergillus fumigatus, the role of morphological transitions has not been studied in the virulence of Cryptococcus neoformans since this yeast does not form hyphae during infection. However, in the last years, different groups have described the ability of this fungus to change its size during infection. In particular, Cryptococcus can form “titan cells,” which are blastoconidia of an abnormal large size. Since their discovery, there is increasing evidence that these cells contribute, not only to long-term persistence in the host, but they can also actively participate in the development of the disease. Recently, several groups have simultaneously described different media that induce the appearance of titan cells in laboratory conditions. Using these conditions, new inducing factors and signaling pathways involved in this transition have been described. In this article, we will review the main phenotypic features of these cells, factors, and transduction pathways that induce cell growth, and how titan cells contribute to the disease caused by this pathogen.
Rocío García-Rodas and Haroldo Cesar de Oliveira, both authors have equally contributed to this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Microscopic fungi are distributed worldwide and can be isolated from most of the environmental niches. They play important roles in multiple processes, since they are commensals of multiple organisms and participate in bioremediation and ecological balance. Furthermore, they are a frequent cause of disease in plants and animals.
In the case of humans, fungi are commensals that can be isolated from the skin, mucosa, and gastrointestinal tract. Commonly, fungi can cause superficial infections. However, in immunosuppressed patients they can cause severe and disseminated diseases (Lamps et al. 2014; McNeil et al. 2001; Singh 2001). The increasing incidence of invasive fungal infections has several important problems associated, such as the high mortality, the economical costs of prolonged hospital stays and the frequent neurological sequelae (Brown et al. 2012; Erjavec et al. 2009; Rueping et al. 2009).
The management of these diseases is complicated for many reasons. Despite the development of new diagnostic tools, such as MALDI-TOF, still the lack of availability of early detection tests, the increasing selection of resistant strains due to the massive use of antifungals and the poor knowledge on the virulence mechanisms constitute a problem (Ruhnke and Schwartz 2016). In this context, the main factor that determines the outcome of fungal infections is the immune system of the host (Casadevall and Pirofski 2003, 2009; Pirofski and Casadevall 2008, 2017). However microbial elements have also a key role in the development of the disease. In particular, the ability to survive in the host is most probably the main factor that determines the ability of a fungus to behave as commensal or symbiotic, or even as a pathogen in immunosuppressed patients.
There are many mechanisms that allow the proliferation of pathogenic fungi within the human, such as the adaptation to the nutritional, osmotic and oxidative environment, growth at corporal temperature and the capacity to evade killing by macrophages (Alvarez et al. 2009; Fu et al. 2018; Johnston and May 2013; Robert and Casadevall 2009; Zaragoza et al. 2008). In addition, most fungi can also induce morphological transitions that contribute to the evasion of the immune response, dissemination through the organism and tissue invasion (May et al. 2016; Mayer et al. 2013). Most of these morphological transitions involve a change in the shape of the cells, and in particular the formation of hyphae and pseudohyphae. The role of these morphotypes during infection has been extensively described and reviewed in the literature [reviewed in (Huang 2012; Kadosh 2013; Mayer et al. 2013; Mitchell 1998; San-Blas et al. 2000; Sudbery 2011; Trevijano-Contador et al. 2016)].
However, in some fungi, morphogenesis does not involve a change in the shape of the cell but in the size. This is the case of the pathogenic yeast Cryptococcus neoformans, which is widely distributed in the environment. This fungus has a high prevalence among HIV infected patients, and it is the cause of hundreds of thousands of deaths per year (Park et al. 2009; Rajasingham et al. 2017). Cryptococcus neoformans is an excellent model to investigate fungal pathogenesis, since it has developed some characteristics that have a determinant role during infection (Alspaugh 2015; Coelho et al. 2014; Esher et al. 2018). For example, this fungus has a polysaccharide capsule around the cell that protects it against multiple stress factors, but that also has deleterious effects in the host [reviewed in (Agustinho et al. 2018; Ding et al. 2016; Doering 2009; O’Meara and Alspaugh 2012; Vecchiarelli et al. 2013; Zaragoza et al. 2009)].
Regarding morphological changes, C. neoformans also possesses a complex morphogenetic program that results in the appearance of a heterogeneous population in the lung (Feldmesser et al. 2001). Although C. neoformans can form pseudohyphae during infection, this is a rare phenomenon, and most of the cells present a spherical shape. However, the size of the cells is very heterogeneous, in particular, in the lung (Zaragoza 2011). The regular size of cryptococcal size is around 5–8 μ. However, in vivo, the diameter of the cells can vary from 1 to 100 μ (Fig. 1). This review will focus on the transition that results in the significant cellular enlargement that leads to the formation of the so called “titan cells”.
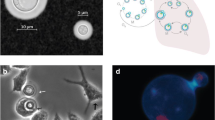
Cryptococcal titan cells. Cells from C. neoformans obtained in Sabouraud-rich medium in vitro (a, c and e) and titan cells isolated from infected mice (b, d, and f) were suspended in India Ink and pictures were taken with different objectives: 20x (a and b), 40x (c and d), and 63x (e and f). In consequence, a and b, c and d, and e and f have the same magnification. Scale bars denote 25 μ in a, 40 μ in b and 10 μ in c, d, e, and f
2 Cryptococcal Titan Cells. A Dramatic Morphological Change
The most typical morphological transition in C. neoformans consists of a significant enlargement of both the capsule and cell body size (Okagaki et al. 2010; Zaragoza et al. 2010). The magnitude of the cell body increase can reach over ten-fold the regular cell size, but there are different definitions of titan cells. As recently reviewed by Zhou and Ballou, titan cells can be defined in different ways (Zhou and Ballou 2018). The initial criteria defined titan cells as those with a cell body diameter over 15 μ or total size (including the capsule) over 30 μ (Zaragoza and Nielsen 2013). However, some other authors suggest that a cell body diameter above 10 μ is enough to consider those cells as titan. Other definitions of titan cells are based on ploidy or phenotypic features, such as large vacuole or thickened cell wall. In fact, most definitions have arguments against, because there could be situations in which titan cells are found without all the classical phenotypic characteristics. Furthermore, there are some cases in which huge cells are formed due to a massive growth of the capsule, and not so much of the cell body, and these cells could also be considered as titan cells because they are also cells with a significant large size difficult to remove by the immune system.
Curiously, these cells have been almost neglected for the scientific community for decades. Although some articles reported cryptococcal cells of abnormal size in clinical specimen (Cruickshank et al. 1973; Love et al. 1985), titan cells were not described in detail until 2010. Since then, this morphotype has been the focus of multiple studies. Besides, they have been found in further studies in clinical samples from patients with cryptococcal pneumonia (Wang et al. 2014). The main purpose of this review is to illustrate the main aspects of this type of cryptococcal cells, how they are formed, and how they contribute to adaptation to the host and to the development of disease.
2.1 Phenotypical Features of Cryptococcal Titan Cells
The first phenotypical characteristic of titan cells is their huge size compared to the cells obtained in rich media in laboratory conditions and/or isolated from the environment. The increase in diameter goes from 6 μ in vitro to around 40–70 μ in vivo (Fig. 1). Cells with even a diameter of 100 μ have been described in the lungs of infected mice (Okagaki et al. 2010; Zaragoza et al. 2010). This is a dramatic change, in particular, if the increase in volume is calculated, since it can be 1000 times larger in titan cells compared to cells of regular size. The increase in size is due to growth of both the capsule and the cell body. In addition to their large size, titan cells also present other phenotypic differences. The capsule consists of much denser polysaccharide fibbers compared to that of cells obtained in vitro, and it has reduced permeability (Zaragoza et al. 2010). Furthermore, the cell wall is also enlarged. If cells in vitro have a cell wall thickness of around 200 nm, in titan cells it can reach up to 3 μ. Titan cells cell wall contains increased amounts of chitin, significantly higher glucosamine and lower glucose than cell walls from in vitro regular size cells, which has been associated with a detrimental Th2 response in mice (Mukaremera et al. 2018; Wiesner et al. 2015).
Intracellularly, titan cells have also a large vacuole, and it has been hypothesized that this could represent a mechanism by which these cells do not need to augment their cytoplasmic content (such as mitochondria, DNA, proteins) in direct proportion to their size (Zaragoza et al. 2010). However, these cells are still polyploid (Okagaki et al. 2010; Zaragoza et al. 2010) and it has been argued that they are formed by endoreduplication. In this way, there would be several rounds of S phase without mitosis, so the cells would go several times through G1 and G2 phases, which are the phases in which there is a significant increase of the cell size, without separating the genetic content into a daughter cell. Titan cells have not lost their capacity to replicate, and in fact, when isolated from the lungs and placed in laboratory conditions, these cells can originate a large number of daughter cells (Gerstein et al. 2015; Zaragoza et al. 2010).
2.2 Which Factors Trigger Titan Cell Formation? In Vivo Studies
One of the main aspects that have been the focus in the research of titan cells is to elucidate the factors and signaling pathways that trigger their induction. Initial studies were based on in vivo experiments, in which mice were infected with different mutants and the proportion of titan cells was investigated in the lungs. Using this approach, it was first discovered that mutants lacking adenylate cyclase (Cac1) and that cannot synthesize cAMP cannot form titan cells, indicating that this pathway is required for this process (Zaragoza et al. 2010). Furthermore, the proportion of titan cells in vivo increased when mice were coinfected with strains of different mating type, and mutants defective in the pheromone receptor Ste3 were defective in this transition (Okagaki et al. 2010, 2011). This receptor is coupled to the G-protein Gpa1, which activates the activity of the adenylate cyclase (Alspaugh et al. 2002). However, mutants that do not produce cAMP are avirulent, so in vivo studies might be influenced by the decreased fitness of the yeasts. In one of these early studies, it was found that C. neoformans can produce a small proportion of titan cells in vitro after prolonged incubation of the yeasts in minimal media, and using this approach, it was confirmed that cAMP mutants are defective in titan cell production (Zaragoza et al. 2010). This result also suggested that titan cells are formed in response to nutritional stress. In agreement, overexpression of PKA1 which encodes protein kinase A produces increase in cell size and ploidy of cryptococcal cells (Choi et al. 2012).
Elegant studies by Okagaki et al. demonstrated that other elements of the cAMP pathway regulate cell growth in C. neoformans. Among them, they identified a G-coupled receptor Gpr5 as a positive regulator of titan cell formation. Interestingly, this receptor mediates signaling through Gpa1 and Cac1. These authors also found that transcription factor Rim101 is required for titan cell development. This transcription factor is activated by cAMP and plays pleiotropic effects in the virulence of C. neoformans. One of the proteins that were also found to be required for titan cell formation was the cyclin Pcl1, which confirmed that this transition is regulated by cell cycle elements (Okagaki et al. 2011).
The host environment also plays an important role in the cryptococcal morphogenesis in vivo. Despite it is not fully clear which specific environmental elements trigger titan cell formation, this transition does not happen in the same way in different mouse strains that elicit different immune responses. For example, in mice that induce Th2-type immunity, the proportion of titan cells is higher than in mice that induce a Th1 response (García-Barbazán et al. 2016). This result suggests that non-protective immune responses against C. neoformans result in a less aggressive environment that favors cellular growth of the yeasts.
3 Titan Cells Obtained In Vitro: New Factors and Signaling Pathways Elucidated
The studies that aimed a better molecular characterization of titan cells faced a great challenge since researchers did not have an effective way to obtain them in vitro. Initially, investigation of titan cells required infecting mice and then isolating the fungal cells from the lungs of the animals. This approach has been very effective, but it presents two important limitations: (1) the low amount of titan cells obtained and (2) the ethical issues and economic costs associated to the use of animals for research.
In the last years, several articles described that titan cells can occasionally appear in some media. In this way, and as stated above, Zaragoza et al. described that prolonged incubation of cryptococcal cells in minimal media resulted in the appearance of titan cells in vitro (Zaragoza et al. 2010). Then, it was found that other conditions, such as overexpression of PKA1 or exposure to ameba or macrophage extracts also result in the appearance of titan cells in vitro (Choi et al. 2012; Chrisman et al. 2011). However, it has not been until recently that three different groups have described in vitro conditions that consistently result in the appearance of C. neoformans titan cells that resemble the ones found in vivo, with body size diameters close to 15 µm, enlarged capsule, increased nuclear DNA content, thicker cell wall, and capacity to generate a normal size progeny (Dambuza et al. 2018; Hommel et al. 2018; Trevijano-Contador et al. 2018). Percentage of titan cells obtained in vitro is variable depending on the protocol followed and the strains tested ranging from 14 to 70%. In two of these articles, the main induction factor was addition of serum to limited nutrient media in the presence of CO2 (Dambuza et al. 2018; Trevijano-Contador et al. 2018). The other protocol involves prolonged incubation (3–5 days) in hypoxia in minimal media (Hommel et al. 2018), which resembles in part the conditions initially described by (Zaragoza et al. 2010). Figure 2 shows a scheme of the different methods described that induce titan cells in vitro. The possibility to obtain titan cells in vitro has opened new perspectives in this field and has also elucidated new signaling factors and signaling pathways involved in this morphological transition.
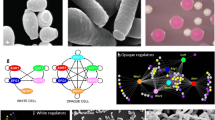
Scheme of the methods to obtain titan cells in vitro. The figure shows a scheme of three methods published in 2018 by Trevijano-Contador et al. (2018) (yellow), Dambuza et al. (2018) (gray), and Hommel et al. (2018) (blue) to obtain titan cells in vitro. The methods are based on two steps: pre-growth at 30 °C with agitation (150 rpm) on Sabouraud overnight (yellow), YNB overnight (gray), or YPD for 22 h (blue), followed by an incubation on the different inducing medium, in different conditions
3.1 Regulation by Serum, CO2, and Nutritional Limitation
The in vitro conditions involved nutrient limitation by transferring yeasts from a rich to a poor medium (Dambuza et al. 2018; Hommel et al. 2018; Trevijano-Contador et al. 2018). Furthermore, the addition of several inducing factors contributes to titan cell formation.
Mammalian serum, as described by Trevijano-Contador et al. and Dambuza et al., is an essential inducing factor to obtain titan cells in vitro. The addition of 5 and 10% of bovine fetal serum led to a significant increase in cell size (Dambuza et al. 2018; Trevijano-Contador et al. 2018). The same did not occur when the serum was added to rich medium, showing the importance of the nutrient limitation in this process (Trevijano-Contador et al. 2018). These two groups also found that serum components are capable to induce titan cell formation in vitro. Polar lipids isolated from the serum were able to induce the formation of the titan cells. They also could evaluate that phosphatidylcholine (PC), induced a partial increase in cell size in vitro, but which was not of the same magnitude as of whole serum, indicating that other components are also important in this process. This is in agreement with previous reports in the literature, where is it was found that PC promotes cell growth in vitro (Chrisman et al. 2011), although these author found the effect at higher PC concentrations. However, phospholipase B seems to play a paradoxical role in titan cell formation, since mutants lacking this enzyme induce titan cell formation inside phagocytic cells (Evans et al. 2015). Pharmacological inhibition of PKC abolished the formation of titan cells (Trevijano-Contador et al. 2018). In C. neoformans, PKC is activated by diacylglycerol (DAG), which is produced by degradation of phospholipids by phospholipases. For this reason, these authors hypothesized that serum effect was in part due to the activation of PKC signaling in the cell (Trevijano-Contador et al. 2018).
Dambuza et al. found that two serum fractions composed of at least nine different compounds with sugar and amino acids structures, induced titan cell formation in vitro (Dambuza et al. 2018). However, Hommel et al. showed that addition of FBS to minimal medium decreased the cell size and the same was observed with the addition of phosphatidylcholine showing that titan cells can be obtained in vitro through parallel or independent pathways (Hommel et al. 2018). It is noteworthy that the time to produce in vitro titan cells following the above-mentioned protocol is very different, and this could clearly determine the different response to the same stimuli. Besides, since quorum sensing plays a role in titan cell formation, different initial cell densities could also promote different response to the same stimuli.
Oxygen limitation also led to an increase of C. neoformans cell size in vitro. Addition of sodium azide, which inhibits complex IV of the respiratory chain, and statistic incubation of the cells leads to a higher proportion of cells with enlarged sizes (Trevijano-Contador et al. 2018). This result suggests that mitochondrial damage can induce a stress signal that results in cell cycle arrest and cell growth. Similarly, Hommel et al. found that induced hypoxia, either physical (lack of shaking) or chemical by adding 1 nM COCl2 favors the formation of titan cells (Hommel et al. 2018; Trevijano-Contador et al. 2018). Oxygen limitation may reduce the respiration rate of the cell, which can trigger a stress signal that influences cell cycle. Furthermore, inhibition of respiration induces activation of cAMP pathway in different fungi (Fuller and Rhodes 2012), which may contribute to titan cell formation.
CO2, which induces capsule growth in C. neoformans (Granger et al. 1985), also induced cell body enlargement in vitro (Dambuza et al. 2018; Trevijano-Contador et al. 2018). In C. neoformans, CO2 is converted into \( {\text{HCO}}_{ 3}^{\text{ - }} \) by the action of two carbonic anhydrases, Can1 and Can2, being Can2 the most expressed. Bicarbonate can activate adenylate cyclase (Klengel et al. 2005). In consequence, it was argued that CO2 induces titan cell production through the activation of the cAMP pathway.
3.2 Regulation by Microbiome
Titan cells are mainly found in the lungs, and Dambuza et al. found that bronchial alveolar lavage (BAL) can also induce titan cell formation (Dambuza et al. 2018). BAL extracts contained lung-resident bacteria, so they also tested the impact of the host microbiome in the formation of the titan cells. Live or heat-killed Escherichia coli and live Streptomyces pneumonia, when co-incubated with C. neoformans in YNB, induced the formation of titan cells. The importance of the host microbiome was also evaluated in vivo. Infection of mice with C. neoformans previously treated with penicillin and streptomycin resulted in a reduction of titan cells in the lungs compared with non-treated animals, suggesting that the interaction between cryptococcal cells and bacteria induces cell growth of this fungal pathogen (Dambuza et al. 2018).
Bacterial components can also induce titan cell formation. Muramyl tetrapeptides (MTP) are peptidoglycan subunits that are found in the cell wall of Gram-positive, Gram-negative, and mycobacteria and that induce the yeast-to-hypha transition in C. albicans (Xu et al. 2008). Dambuza et al. found that FBS contained muramyl dipeptide (MDP) and N-Acetylmuramyl-L-alanyl-D-isoglutamine (NMAiGn), which are similar to MTP, induce titan cell formation (Dambuza et al. 2018). The effect of these peptidoglycans was absent in a mutant defective in cAMP signaling, suggesting that these compounds induce titan cell formation through activation of the cAMP pathway (Dambuza et al. 2018). All together, these data highlight that titan cells in C. neoformans could be formed in response to the interaction with bacteria. In addition, these findings also indicate that the interaction with the microorganisms in the host can be a determinant factor influencing microbial virulence.
3.3 Regulation by Quorum Sensing
Another factor found to be important in titan cell formation in vitro is the cell density of the cultures. When cultures are inoculated at low cellular densities, the proportion of titan cells in the cultures was much higher compared to cultures inoculated with higher cryptococcal cell concentrations (Trevijano-Contador et al. 2018). Similarly, Dambuza et al. also found that larger cells are found at higher proportion when cultures were inoculated at lower optical densities (Dambuza et al. 2018).
These findings suggest that this process is regulated by quorum sensing (QS), which is a mechanism of cell-to-cell communication regulated by molecules that are released to the medium. Trevijano-Contador et al. found that cell-free medium from C. neoformans cultures inhibits the formation of titan cells in the presence of other inducing factors, such as CO2 and serum. These data suggest that molecules released by the cells of regular size negatively regulate the process of cellular enlargement.
Some molecules where previously described as QS molecules in C. neoformans. The peptide Qsp1 was described as an important QS molecule required for virulence (Homer et al. 2016; Lee et al. 2007), and more recently, as an important regulator for sexual reproduction of C. neoformans (Tian et al. 2018). Trevijano-Contador et al. and Hommel et al. found that addition of Qsp1 to the titan cell inducing media inhibits cell enlargement in a dose-dependent manner. However, a qsp1 mutant strain formed titan cells in vitro (Dambuza et al. 2018; Trevijano-Contador et al. 2018). This result suggests that although Qsp1 negatively regulates titan cell formation, other QS molecules could be involved. Another molecule involved in the QS phenomenon described in C. neoformans is pantothenic acid (PA) (Albuquerque et al. 2013), and Hommel et al. also found that PA increased the proportion of titan cells in vitro (Hommel et al. 2018). Together, these data suggest that cell-to-cell communication is an important factor that regulates titan cells formation.
3.4 New Factors Discovered Through Genomic Analysis and Gene Expression Experiments
Recently, genomic approaches have been also carried out to find genes and pathways involved in titan cell formation. Trevijano-Contador et al. compared the gene expression of regular and titan cells in vitro after 7 and 18 h of incubation in titan cell inducing conditions and found that during the development of titan cells, there is a repression of genes that encode proteins involved in cell cycle regulation (DNA repair and chromosomal condensation) which is in agreement with the idea that titan cell induction is the result of cell cycle alterations. In this analysis, they also found that in titan cells, there is an increase in the expression of genes that encode proteins from the tricarboxylic cycle, glycolysis and involved in stress response, suggesting that there is a metabolic (Trevijano-Contador et al. 2018). Interestingly, there was also an increase in genes involved in protein trafficking (vesicle secretion, COPI vesicle coating and proteins from Golgi apparatus and Golgi membrane).
One of the genes that had increased expression at both times was the one encoding calnexin (Cne1), which is a chaperone from the ER required for proper folding of glycoproteins (Ellgaard and Helenius 2003). Paradoxically, calnexin mutants had an abnormal large size even in rich medium. This result revealed an unexpected role for regulatory elements of intracellular trafficking pathways in cell size. In agreement, atg7 mutants that are defective in recycling proteins at the proteasome have also increased cell size in vitro (Oliveira et al. 2016).
Another gene that was found upregulated in titan cells was CIG1, which encodes a glycoprotein involved in heme and iron uptake. In C. neoformans, iron limitation induces capsule growth (Lian et al. 2005). Depletion of iron from the media induces an increase in titan cell formation (Trevijano-Contador et al. 2018). Iron limitation is sensed by the cell through the G-protein Gpa1, which also activates cAMP production (Alspaugh et al. 1997). These data confirm that iron limitation is another factor that triggers this morphological transition in C. neoformans. Iron is required for many processes in yeasts and since its concentration is low in the host environment, microbes and the host have to compete to uptake this element.
In parallel, genome sequencing of strains with different capacity to form titan cells has been carried out. Using this approach, it was found that PKR1 gene, a cAMP-dependent protein kinase regulatory subunit and whose disruption results in constitutive activation of this pathway, was mutated in an isolate able to overproduce titan cells (Hommel et al. 2018). This result confirms that constitutive activation of the cAMP pathways and PKA induce titan cell development.
Hommel et al. also found that a clinical isolate that had a truncation of USV101 produced more titan cells than the wild-type strain (Dambuza et al. 2018; Hommel et al. 2018). In agreement, an usv101Δ mutant strain had a significantly increase in the cell size in vitro. Usv101 is a C2H2 transcription factor required for virulence that regulates melanin production and capsule formation (Gish et al. 2016). These findings revealed a new role for Usv101 as a negative regulator of titan cells formation. Tetraspanin 2 (TSP2) plays an important role in the integrity of plasma membrane (Li et al. 2012) and appears to negatively regulate titan cells development in C. neoformans. Clinical isolates with a truncated TSP2 gene have cells with larger cell size, and a similar phenotype is found in tsp2 mutants generated in the laboratory (Hommel et al. 2018). Tsp2 is a plasma membrane that in C. neoformans is involved in the repression of laccase, and this phenotype is reverted by the addition of cAMP (Li et al. 2012). For this reason, Hommel et al. argued that the inhibitory effect of Tsp2 on titan cell formation is mediated through inhibition of the cAMP signaling pathway (Hommel et al. 2018).
The genome sequence of different isolates of the wild-type H99 strain that has undergone microevolution in different laboratories (Janbon et al. 2014) with different abilities to form titan cells has been also analyzed (Hommel et al. 2018). In this way, the authors associated SNPs/indels with two genes, SGF29 (which encodes a transcription factor (Bian et al. 2011) and LMP1 (which is required for cryptococcal virulence) with cellular enlargement (Hommel et al. 2018).
In summary, the development of new tools and media that allow the production of titan cells in vitro has allowed the identification of new genes and signaling pathways required for this morphological transition. Figure 3 represents a suggestive scheme on how all these signals and pathways could be integrated at the moment. However, we acknowledge that future work is required to fully elucidate the mechanisms of titan cell formation. For this reason, the availability of different methods that reproduce the phenomenon in vitro will open new research lines that will contribute to characterize this process.
Summary of different signals and pathways involved in titan cell formation. In red, we denote negative regulators of titan cell formation. See text for all the details. BAL, bronchoalveolar lavage. Cac1, adenylate cyclase. Pkai, Protein kinase A inactive bound to the regulatory subunit Pkr. After binding of cAMP (small black circles) to Pkr, Pka becomes free and active (Pkaa). Can2, carbonic anhydrase 2. Tsp2, tetraspanin 2. Qsp1, quorum sensing peptide. Ste3a, G-coupled pheromone receptor. Gpr5, G-coupled receptor 5. Gpa1, G-protein. Cne1, calnexin
4 Importance for Cryptococcal Virulence
One of the main questions that have been the focus of different investigations is how titan cells contribute to the virulence of C. neoformans. One of the main processes that result in the cryptococcal disease is the dissemination from the lung to the brain. Titan cells are too big, and it is believed that they cannot cross-biological barriers nor disseminate. However, different evidences indicate that these cells are important, not only for the survival of the fungus in the host, but also because they actively participate in different pathogenic processes.
4.1 Resistance to Stress Factors
Titan cells are thought to be produced by endoreduplication, and therefore, they are polyploid while their progenies have shown different aneuploidies (Gerstein et al. 2015; Okagaki et al. 2010; Zaragoza et al. 2010). Analysis of titan cells showed that they are more resistant to stress factors and antifungals, such as H2O2, nitrosative oxidants, and fluconazole (Gerstein et al. 2015; Zaragoza et al. 2010). Interestingly, the progeny of titan cells is also more resistance to those stresses (Gerstein et al. 2015), which suggests that titan cells can actively participate during the pathogenesis of the disease, by producing a population of daughter cells more adapted to the host environment and to antifungal treatments.
4.2 Titan Cells Are Not Phagocytosed
Macrophages are the first line of attack of the immune system once microorganisms reach the alveoli. Cryptococcus neoformans has developed several mechanisms that avoid killing by phagocytic cells. This fungus is a facultative intracellular pathogen, and therefore, it is able to survive and replicate inside phagocytic cells [reviewed in (DeLeon-Rodriguez and Casadevall 2016; Feldmesser et al. 2000; Garcia-Rodas and Zaragoza 2012)]. In addition, it has also acquired some traits that difficult the internalization by macrophages. One of them is the presence of the capsule, which “hides” the main cell wall epitopes that are recognized by phagocytic cells (Kozel and Gotschlich 1982). And of course, another mechanism that inhibits phagocytosis is the production of titan cells, because they cannot be phagocytosed due to their large size. In consequence, it is reasonable to suggest that production of titan cells could be important for cryptococcal virulence.
Interaction of titan cells and macrophages has been studied by microscopy time lapse in vitro (Zaragoza et al. 2010). In this sense, different studies have shown that titan cell production results in overall lowered phagocytosis. Okagaki and Nielsen proved that there was a nonlinear relationship between titan cell formation and phagocytosis using several mutants with different capacities of producing titan cells during murine infections. Furthermore, titan cells also protect cells of regular size from phagocytosis (Okagaki and Nielsen 2012). Overall, these data suggest that production of titan cells confers cross-protection to regular size cryptococcal cells.
In agreement to this, in vitro titan-like cells are not phagocytosed (Zaragoza et al. 2010). However, titan-like cells did not impair nor modulate phagocytosis of regular size cryptococcal cells, which means that phagocytosis and activation of macrophages in the lungs may be regulated by different factors not present in vitro (Trevijano-Contador et al. 2018).
4.3 Proliferation and Dissemination Mediated by Titan Cells
Titan cells constitute an active source of infection since they are able to produce a progeny of regular size cells (Gerstein et al. 2015; Zaragoza et al. 2010), and therefore, it may provide an advantage during establishment of infection.
Titan cells isolated from lungs of infected mice can kill the invertebrate Galleria mellonella similarly to cryptococcal cells of regular size (Garcia-Rodas et al. 2011), and in this model, it was described the appearance of a progeny of cells of normal size in the larvae. In mice, however, the gpr4∆gpr5∆ strain, with limited titan cell production, showed attenuated virulence when compared to infection produced by the wild type strain resulting in lower fungal burdens (Crabtree et al. 2012). This observation suggests that production of titan cells promotes survival and proliferation of cryptococcal cells in the lungs. In agreement to this, the otc1∆ strain, which overproduces titan cells, is able to persist in the lungs of mice for more than two months despite that this strain has impaired in vivo replication. Therefore, titan cell production promotes cryptococcal cell survival in the host (Crabtree et al. 2012).
Furthermore, mice infected with a gpr4∆gpr5∆ mutant showed lower fungal burden in the brain compared to those infected with the wt strain. However, no differences in fungal burden between the strains were observed during intracerebral infections. These results indicate that limited titan cell production correlates with reduced dissemination (Crabtree et al. 2012).
4.4 Polarization Toward a Th2 Response
Titan cell production increases the number of eosinophils in the lungs (García-Barbazán et al. 2016). Increased recruitment of eosinophils is characteristic of a Th2 response, typically observed during extracellular pathogens infection. However, in the case of fungal pathogens, Th2 immunity is associated with non-effective responses and lack of protection. In the particular case of C. neoformans, as infection progresses, induction of Th2 immune response may result in a more permissive environment for the yeast that favors fungal survival and replication. Furthermore, the immune polarization would also facilitate the survival of the yeasts inside phagocytic cells and their replication.
In vivo studies have shown that titan cell formation depends on the dose of infection and the cryptococcal strain (Okagaki et al. 2010, 2011; Zaragoza et al. 2010). Furthermore, it is known that the percentage of cryptococcal titan cells in the population of mouse infected lungs varies during the course of infection and that the proportion of titan cells is higher when the mice are infected with low doses (Zaragoza et al. 2010). Cryptococcal cell growth also depends on host factors. It has been shown that the size of C. neoformans is larger in the lungs of mice deficient of B cells (Szymczak et al. 2013). In agreement, mice that develop Th2-type responses are more susceptible to the infection and present higher percentages of titan cells in the lungs (García-Barbazán et al. 2016). This Th2-polarized response consists of low levels of IFN-γ and TNF-α and a poor recruitment of T and B cells in the lungs (García-Barbazán et al. 2016). Besides, consistent with previous data, these mice showed more infiltrates of eosinophils instead of neutrophils. These data indicate that titan cells might induce a polarization to non-protective responses, which highlight another mechanism by which these cells contribute to the disease.
5 Future Perspectives
Cryptococcus neoformans is one of the most interesting models to investigate fungal virulence due to its ability to adapt to the lung environment. Multiple factors contribute to this adaptation, such as the polysaccharide capsule, production of melanin and growth at 37 °C. The formation of titan cells is a dramatic morphological change that can also facilitate the survival of the yeasts in the hosts. At the moment, this transition has been mainly described in the lungs (Okagaki et al. 2010; Wang et al. 2014; Zaragoza et al. 2010), but it is not known whether these cells also appear in other target organs, such as the brain or spleen, which are usually inflamed during the course of infection. Due to their significant capacity to evade the immune response, future studies are required to investigate whether titan cells also contribute to fungal colonization of different organs and niches in the human.
The recent description of conditions that promote titan cell formation in vitro can become a key contribution to better understand the biology of these cells. So far, three different groups have described conditions to induce this process, and interestingly, some of the inducing factors (serum, cell density effect, and quorum sensing) have been described simultaneously in these laboratories. One of the main conclusions of these works is that cellular enlargement is a response to multiple signals, so it is still possible that new factors will be found in the future. These approaches are limited by the fact that titan cell formation is highly dependent on the genetic background of the strain and microevolution in different laboratories. However, we anticipate that the involvement of multiple research groups in this topic and standardization of the different protocols will allow the full identification of the pathways involved in titan cell development.
Finally, the role of the immune response on cryptococcal morphology is another factor of great importance on this field. Apart from some in vivo factors, such as serum or bacterial components, there is increasing evidence that different polarization of the immune response has a determinant influence on titan cell development. This is an important aspect when extrapolated to human patients, because different persons might have different predisposition to develop titan cells. The identification of the factors of the immune response that influence cryptococcal morphology might in consequence help to predict the susceptibility of different individuals to this infection. Furthermore, future research on this topic will also contribute to anticipate the efficacy of different antifungal treatments on different patients.
References
Agustinho DP, Miller LC, Li LX, Doering TL (2018) Peeling the onion: the outer layers of Cryptococcus neoformans. Mem Inst Oswaldo Cruz 113:e180040. https://doi.org/10.1590/0074-02760180040
Albuquerque P, Nicola AM, Nieves E, Paes HC, Williamson PR, Silva-Pereira I, Casadevall A (2013) Quorum sensing-mediated, cell density-dependent regulation of growth and virulence in Cryptococcus neoformans. MBio 5:e00986–e00913. https://doi.org/10.1128/mbio.00986-13
Alspaugh JA (2015) Virulence mechanisms and Cryptococcus neoformans pathogenesis. Fungal Genet Biol 78:55–58. https://doi.org/10.1016/j.fgb.2014.09.004
Alspaugh JA, Perfect JR, Heitman J (1997) Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev 11:3206–3217
Alspaugh JA et al (2002) Adenylyl cyclase functions downstream of the Galpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot Cell 1:75–84. https://doi.org/10.1128/EC.1.1.75-84.2002
Alvarez M, Burn T, Luo Y, Pirofski LA, Casadevall A (2009) The outcome of Cryptococcus neoformans intracellular pathogenesis in human monocytes. BMC Microbiol 9:51. https://doi.org/10.1186/1471-2180-9-51
Bian C et al (2011) Sgf29 binds histone H3K4me2/3 and is required for SAGA complex recruitment and histone H3 acetylation. EMBO J 30:2829–2842. https://doi.org/10.1038/emboj.2011.193
Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC (2012) Hidden killers: human fungal infections. Sci Transl Med 4:165rv113. https://doi.org/10.1126/scitranslmed.3004404
Casadevall A, Pirofski LA (2003) The damage-response framework of microbial pathogenesis. Nat Rev Microbiol 1:17–24. https://doi.org/10.1038/nrmicro732
Casadevall A, Pirofski LA (2009) Virulence factors and their mechanisms of action: the view from a damage-response framework. J Water Health 7(Suppl 1):S2–S18. https://doi.org/10.2166/wh.2009.036
Choi J, Vogl AW, Kronstad JW (2012) Regulated expression of cyclic AMP-dependent protein kinase A reveals an influence on cell size and the secretion of virulence factors in Cryptococcus neoformans. Mol Microbiol 85:700–715. https://doi.org/10.1111/j.1365-2958.2012.08134.x
Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A (2011) Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog 7:e1002047. https://doi.org/10.1371/journal.ppat.1002047
Coelho C, Bocca AL, Casadevall A (2014) The tools for virulence of Cryptococcus neoformans. Adv Appl Microbiol 87:1–41. https://doi.org/10.1016/B978-0-12-800261-2.00001-3
Crabtree JN, Okagaki LH, Wiesner DL, Strain AK, Nielsen JN, Nielsen K (2012) Titan cell production enhances the virulence of Cryptococcus neoformans. Infect Immun 80:3776–3785. https://doi.org/10.1128/IAI.00507-12
Cruickshank JG, Cavill R, Jelbert M (1973) Cryptococcus neoformans of unusual morphology. Appl Microbiol 25:309–312
Dambuza IM et al (2018) The Cryptococcus neoformans Titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog 14:e1006978. https://doi.org/10.1371/journal.ppat.1006978
DeLeon-Rodriguez CM, Casadevall A (2016) Cryptococcus neoformans: tripping on acid in the phagolysosome. Front Microbiol 7:164. https://doi.org/10.3389/fmicb.2016.00164
Ding H, Mayer FL, Sanchez-Leon E, de SAGR, Frases S, Kronstad JW (2016) Networks of fibers and factors: regulation of capsule formation in Cryptococcus neoformans. F1000Res 5. https://doi.org/10.12688/f1000research.8854.1
Doering TL (2009) How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu Rev Microbiol 63:223–247. https://doi.org/10.1146/annurev.micro.62.081307.162753
Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4:181–191. https://doi.org/10.1038/nrm1052
Erjavec Z, Kluin-Nelemans H, Verweij PE (2009) Trends in invasive fungal infections, with emphasis on invasive aspergillosis. Clin Microbiol Infect 15:625–633. https://doi.org/10.1111/j.1469-0691.2009.02929.x
Esher SK, Zaragoza O, Alspaugh JA (2018) Cryptococcal pathogenic mechanisms: a dangerous trip from the environment to the brain. Mem Inst Oswaldo Cruz 113:e180057. https://doi.org/10.1590/0074-02760180057
Evans RJ, Li Z, Hughes WS, Djordjevic JT, Nielsen K, May RC (2015) Cryptococcal phospholipase B1 is required for intracellular proliferation and control of titan cell morphology during macrophage infection. Infect Immun 83:1296–1304. https://doi.org/10.1128/IAI.03104-14
Feldmesser M, Kress Y, Novikoff P, Casadevall A (2000) Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun 68:4225–4237. https://doi.org/10.1128/IAI.68.7.4225-4237.2000
Feldmesser M, Kress Y, Casadevall A (2001) Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 147:2355–2365. https://doi.org/10.1099/00221287-147-8-2355
Fu MS et al (2018) Cryptococcus neoformans urease affects the outcome of intracellular pathogenesis by modulating phagolysosomal pH. PLoS Pathog 14:e1007144. https://doi.org/10.1371/journal.ppat.1007144
Fuller KK, Rhodes JC (2012) Protein kinase A and fungal virulence: a sinister side to a conserved nutrient sensing pathway. Virulence 3:109–121. https://doi.org/10.4161/viru.19396
García-Barbazán I et al (2016) The formation of titan cells in Cryptococcus neoformans depends on the mouse strain and correlates with induction of Th2-type responses. Cell Microbiol 18:111–124. https://doi.org/10.1111/cmi.12488
Garcia-Rodas R, Zaragoza O (2012) Catch me if you can: phagocytosis and killing avoidance by Cryptococcus neoformans. FEMS Immunol Med Microbiol 64:147–161. https://doi.org/10.1111/j.1574-695X.2011.00871.x
Garcia-Rodas R, Casadevall A, Rodriguez-Tudela JL, Cuenca-Estrella M, Zaragoza O (2011) Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS ONE 6:e24485. https://doi.org/10.1371/journal.pone.0024485
Gerstein AC et al (2015) Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. MBio 6:e01340-01315. https://doi.org/10.1128/mBio.01340-15
Gish SR et al (2016) Computational analysis reveals a key regulator of cryptococcal virulence and determinant of host response. MBio 7:e00313–e00316. https://doi.org/10.1128/mBio.00313-16
Granger DL, Perfect JR, Durack DT (1985) Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest 76:508–516. https://doi.org/10.1172/JCI112000
Homer CM et al (2016) Intracellular action of a secreted peptide required for fungal virulence. Cell Host Microbe 19:849–864. https://doi.org/10.1016/j.chom.2016.05.001
Hommel B et al (2018) Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog 14:e1006982. https://doi.org/10.1371/journal.ppat.1006982
Huang G (2012) Regulation of phenotypic transitions in the fungal pathogen Candida albicans. Virulence 3:251–261. https://doi.org/10.4161/viru.20010
Janbon G et al (2014) Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS Genet 10:e1004261. https://doi.org/10.1371/journal.pgen.1004261
Johnston SA, May RC (2013) Cryptococcus interactions with macrophages: evasion and manipulation of the phagosome by a fungal pathogen. Cell Microbiol 15:403–411. https://doi.org/10.1111/cmi.12067
Kadosh D (2013) Shaping up for battle: morphological control mechanisms in human fungal pathogens. PLoS Pathog 9:e1003795. https://doi.org/10.1371/journal.ppat.1003795
Klengel T et al (2005) Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol 15:2021–2026. https://doi.org/10.1016/j.cub.2005.10.040
Kozel TR, Gotschlich EC (1982) The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol 129:1675–1680
Lamps LW, Lai KK, Milner DA Jr (2014) Fungal infections of the gastrointestinal tract in the immunocompromised host: an update. Adv Anat Pathol 21:217–227. https://doi.org/10.1097/PAP.0000000000000016
Lee H, Chang YC, Nardone G, Kwon-Chung KJ (2007) TUP1 disruption in Cryptococcus neoformans uncovers a peptide-mediated density-dependent growth phenomenon that mimics quorum sensing. Mol Microbiol 64:591–601. https://doi.org/10.1111/j.1365-2958.2007.05666.x
Li Z, Bi J, Yang J, Pan J, Sun Z, Zhu X (2012) Requirement of a Tsp2-type tetraspanin for laccase repression and stress resistance in the basidiomycete Cryptococcus neoformans. Appl Environ Microbiol 78:21–27. https://doi.org/10.1128/AEM.06072-11
Lian T et al (2005) Iron-regulated transcription and capsule formation in the fungal pathogen Cryptococcus neoformans. Mol Microbiol 55:1452–1472. https://doi.org/10.1111/j.1365-2958.2004.04474.x
Love GL, Boyd GD, Greer DL (1985) Large Cryptococcus neoformans isolated from brain abscess. J Clin Microbiol 22:1068–1070
May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K (2016) Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol 14:106–117. https://doi.org/10.1038/nrmicro.2015.6
Mayer FL, Wilson D, Hube B (2013) Candida albicans pathogenicity mechanisms. Virulence 4:119–128. https://doi.org/10.4161/viru.22913
McNeil MM, Nash SL, Hajjeh RA, Phelan MA, Conn LA, Plikaytis BD, Warnock DW (2001) Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin Infect Dis 33:641–647. https://doi.org/10.1086/322606
Mitchell AP (1998) Dimorphism and virulence in Candida albicans. Curr Opin Microbiol 1:687–692. https://doi.org/10.1016/S1369-5274(98)80116-1
Mukaremera L, Lee KK, Wagener J, Wiesner DL, Gow NAR, Nielsen K (2018) Titan cell production in Cryptococcus neoformans reshapes the cell wall and capsule composition during infection. Cell Surf 1:15–24. https://doi.org/10.1016/j.tcsw.2017.12.001
O’Meara TR, Alspaugh JA (2012) The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev 25:387–408. https://doi.org/10.1128/CMR.00001-12
Okagaki LH, Nielsen K (2012) Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot Cell 11:820–826. https://doi.org/10.1128/EC.00121-12
Okagaki LH et al (2010) Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog 6:e1000953. https://doi.org/10.1371/annotation/1b59fd9e-9ac9-4ea8-a083-14c413c80b03
Okagaki LH et al (2011) Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli. Eukaryot Cell 10:1306–1316. https://doi.org/10.1128/EC.05179-11
Oliveira DL et al (2016) The putative autophagy regulator Atg7 affects the physiology and pathogenic mechanisms of Cryptococcus neoformans. Future Microbiol 11:1405–1419. https://doi.org/10.2217/fmb-2016-0090
Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 23:525–530. https://doi.org/10.1097/QAD.0b013e328322ffac
Pirofski LA, Casadevall A (2008) The damage-response framework of microbial pathogenesis and infectious diseases. Adv Exp Med Biol 635:135–146. https://doi.org/10.1007/978-0-387-09550-9_11
Pirofski LA, Casadevall A (2017) Immune-mediated damage completes the parabola: Cryptococcus neoformans pathogenesis can reflect the outcome of a weak or strong immune response. MBio 8 https://doi.org/10.1128/mbio.02063-17
Rajasingham R et al (2017) Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. https://doi.org/10.1016/S1473-3099(17)30243-8
Robert VA, Casadevall A (2009) Vertebrate endothermy restricts most fungi as potential pathogens. J Infect Dis 200:1623–1626. https://doi.org/10.1086/644642
Rueping MJ, Vehreschild JJ, Cornely OA (2009) Invasive candidiasis and candidemia: from current opinions to future perspectives. Expert Opin Investig Drugs 18:735–748. https://doi.org/10.1517/13543780902911440
Ruhnke M, Schwartz S (2016) Recent developments in the management of invasive fungal infections in patients with oncohematological diseases. Ther Adv Hematol 7:345–359. https://doi.org/10.1177/2040620716656381
San-Blas G et al (2000) Fungal morphogenesis and virulence. Med Mycol 38(Suppl 1):79–86. https://doi.org/10.1080/mmy.38.s1.79.86
Singh N (2001) Trends in the epidemiology of opportunistic fungal infections: predisposing factors and the impact of antimicrobial use practices. Clin Infect Dis 33:1692–1696. https://doi.org/10.1086/323895
Sudbery PE (2011) Growth of Candida albicans hyphae. Nat Rev Microbiol 9:737–748. https://doi.org/10.1038/nrmicro2636
Szymczak WA, Davis MJ, Lundy SK, Dufaud C, Olszewski M, Pirofski LA (2013) X-linked immunodeficient mice exhibit enhanced susceptibility to Cryptococcus neoformans infection. MBio 4 https://doi.org/10.1128/mbio.00265-13
Tian X et al (2018) Cryptococcus neoformans sexual reproduction is controlled by a quorum sensing peptide. Nat Microbiol 3:698–707. https://doi.org/10.1038/s41564-018-0160-4
Trevijano-Contador N, Rueda C, Zaragoza O (2016) Fungal morphogenetic changes inside the mammalian host. Semin Cell Dev Biol 57:100–109. https://doi.org/10.1016/j.semcdb.2016.04.008
Trevijano-Contador N et al (2018) Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog 14:e1007007. https://doi.org/10.1371/journal.ppat.1007007
Vecchiarelli A, Pericolini E, Gabrielli E, Kenno S, Perito S, Cenci E, Monari C (2013) Elucidating the immunological function of the Cryptococcus neoformans capsule. Future Microbiol 8:1107–1116. https://doi.org/10.2217/fmb.13.84
Wang JM et al (2014) Clinicopathological features of pulmonary cryptococcosis with cryptococcal titan cells: a comparative analysis of 27 cases. Int J Clin Exp Pathol 7:4837–4846
Wiesner DL et al (2015) Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog 11:e1004701. https://doi.org/10.1371/journal.ppat.1004701
Xu XL et al (2008) Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe 4:28–39. https://doi.org/10.1016/j.chom.2008.05.014
Zaragoza O (2011) Multiple disguises for the same party: the concepts of morphogenesis and phenotypic variations in Cryptococcus neoformans. Front Microbiol 2:181. https://doi.org/10.3389/fmicb.2011
Zaragoza O, Nielsen K (2013) Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr Opin Microbiol 16:409–413. https://doi.org/10.1016/j.mib.2013.03.006
Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A (2008) Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol 10:2043–2057. https://doi.org/10.1111/j.1462-5822.2008.01186.x
Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A (2009) The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol 68:133–216. https://doi.org/10.1016/S0065-2164(09)01204-0
Zaragoza O, Garcia-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A (2010) Fungal cell gigantism during mammalian infection. PLoS Pathog 6:e1000945. https://doi.org/10.1371/annotation/0675044c-d80f-456f-bb63-4f85fb1d0c33
Zhou X, Ballou ER (2018) The Cryptococcus neoformans titan cell: from in vivo phenomenon to in vitro model. Curr Clin Microbiol Reports. https://doi.org/10.1007/s40588-018-0107-9 (in press)
Acknowledgements
OZ is funded by grant SAF2014-54336-R and SAF2017-86192-R1 from the Spanish Ministry for Economics, Industry, and Competitivity. HCdO is funded by postdoctoral fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-BEPE 2016/20631-3). RG-R is funded by a “Juan de la Cierva-Incorporación” Contract from the Spanish Ministry for Economics, Industry, and Competitivity (reference: IJCI-2015-25683).
Dedicatory. HCdO wishes to dedicate this article to the memory of his beloved mother Irene Aparecida Pinto de Oliveira, who passed away during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
García-Rodas, R., de Oliveira, H., Trevijano-Contador, N., Zaragoza, O. (2018). Cryptococcal Titan Cells: When Yeast Cells Are All Grown up. In: Rodrigues, M. (eds) Fungal Physiology and Immunopathogenesis . Current Topics in Microbiology and Immunology, vol 422. Springer, Cham. https://doi.org/10.1007/82_2018_145
Download citation
DOI: https://doi.org/10.1007/82_2018_145
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30236-8
Online ISBN: 978-3-030-30237-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)