Abstract
Metabolism is a widely recognized facet of all host–pathogen interactions. Knowledge of its roles in pathogenesis, however, remains comparatively incomplete. Existing studies have emphasized metabolism as a cell autonomous property of pathogens used to fuel replication in a quantitative, rather than qualitatively specific, manner. For Mycobacterium tuberculosis, however, matters could not be more different. M. tuberculosis is a chronic facultative intracellular pathogen that resides in humans as its only known host. Within humans, M. tuberculosis resides chiefly within the macrophage phagosome, the cell type, and compartment most committed to its eradication. M. tuberculosis has thus evolved its metabolic network to both maintain and propagate its survival as a species within a single host. The specific ways in which its metabolic network serves these distinct, through interdependent, functions, however, remain incompletely defined. Here, we review existing knowledge of the M. tuberculosis–host interaction, highlighting the distinct phases of its natural life cycle and the diverse microenvironments encountered therein.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Most bacterial pathogens reside in polymicrobial niches and seek to replicate as quickly as possible to overtake their host niche. However, humans are the only known host and reservoir of M. tuberculosis. M. tuberculosis has thus evolved within a niche, in which either unrestrained replication or perfect symbiosis threaten its very existence as a species. Instead, the M. tuberculosis lifecycle consists in a prolonged period of replicative quiescence tolerant to host immunity and conventional chemotherapy, and while sometimes lasting decades, if not the lifetime of the host, is followed by an obligatory episode of replication, required for transmission to a new host. M. tuberculosis thus both replicates and persists within the same host species.
Interest in metabolic aspects of the M. tuberculosis–host interaction dates to Koch’s discovery of M. tuberculosis and its distinct chemical staining properties among the “heaping nuclei and detritus” of tuberculous lesions in animals that had died from tuberculosis several weeks earlier, suggesting the existence of specific metabolic differences (Sakula 1983). This discovery prompted several decades of human autopsy, experimental animal, bacteriologic, and biochemical studies that focused chiefly on histopathologic descriptions of the host niches occupied by M. tuberculosis, its crude biochemical composition, and cell autonomous growth capabilities (Rich 1946; Youmans 1979). The advent of protein biochemistry later shifted attention to descriptions of its enzymatic activities as reported by whole cell lysates and indirect chemical reporters (Murthy et al. 1962; Wheeler and Blanchard 2005). Based on such studies, Segal and Bloch adduced seminal evidence that M. tuberculosis grown in vitro and recovered from the lungs of infected animals existed in distinct metabolic states, in the latter of which it was found to preferentially respire on fatty acids rather than on carbohydrates more typically used to cultivate M. tuberculosis in vitro (Bloch and Segal 1956). Following publication of the M. tuberculosis genome sequence in 1998, studies of the M. tuberculosis–host interaction turned heavily, if not exclusively, to the use of comparative genomics, transcriptional profiling, homology-based bioinformatic modeling, and gene knockout approaches (Cole et al. 1998; Sassetti et al. 2001; Rhee et al. 2011; Griffin et al. 2011). These studies afforded unprecedented genome-scale insight into the biology of M. tuberculosis with single nucleotide resolution.
Notwithstanding, knowledge of the M. tuberculosis–host interaction remains incomplete. Unlike the case for other cellular processes, metabolism consists of reactions whose substrates and products are atomic, rather than molecular, in diversity and often cannot be inherently deduced from genetic information. As a result, homology-based comparison have failed to suggest a function for nearly 40 % of M. tuberculosis annotated genes, while up to 30 % of detected enzymatic activities have not been ascribed to a known gene (Cole et al. 1998; Chen 2007). Their levels, connectivities, and directionalities remain similarly undefined due to regulation by multiple pathways and regulatory circuits, many of which are nongenetic in nature. In this regard, efforts to infer the in vivo metabolic state of M. tuberculosis based on its transcriptional profile or survival of gene knockouts in animal models of tuberculosis have been hindered by fundamental biochemical ambiguities. While emergent metabolomics technologies have begun to resolve such ambiguities, these same technologies have simultaneously revealed unique properties of its metabolic network that have prompted a re-evaluation current experimental knowledge.
All tools and approaches have inherent strengths and limitations. Classical bacteriology and biochemistry have provided insight into the cell autonomous traits and capabilities of M. tuberculosis and its proteins but also been limited by the artificial culture conditions used to study them. Genetic approaches, by contrast, have afforded a singular specificity but relied almost completely on the accuracy and completeness of their accompanying bioinformatic gene annotations. Metabolomics, defined as the simultaneous measurement of all metabolites in a biological system under a given set of conditions, is the youngest of systems level disciplines and begun to deliver unique insights into the intracellular biochemistry of M. tuberculosis and its response to perturbation. However, while metabolites represent the integrated product of a cell’s genome, proteome and environment, and most direct reporters of a cell’s metabolic state, technical limitations have thus far precluded the ability to profile M. tuberculosis recovered from host cells or tissues (Rhee et al. 2011).
Technical limitations aside, knowledge of the M. tuberculosis–host interaction has been limited by biological dissociations between M. tuberculosis life cycle in vitro and in vivo. As a result, experimental studies of M. tuberculosis pathogenesis have primarily focused on its ability to persist, while its physiology has been most extensively studied when replicating at maximal growth rates.
Here, we review and assimilate existing knowledge of M. tuberculosis physiologic and pathophysiologic traits within the context of its natural life cycle and primary experimental evidence.
2 The Mycobacterium tuberculosis Host Niche: Down the Rabbit Hole
Following aerosol transmission to a new host, M. tuberculosis first encounters the terminal airspace, or alveolus, of the lung where it is phagocytosed by tissue resident alveolar macrophages and dendritic cells. M. tuberculosis is then believed to undergo a period of unrestricted replication, migrate to the local draining lymph node, and spread throughout the bloodstream, infecting additional macrophages and reseeding additional regions of the lung to establish a systemic infection until contained by the onset of cellular immunity. Activation of the adaptive immune response results in the production of interferon-γ (IFN-γ), which enables macrophages to kill or restrain replication of M. tuberculosis and marks the onset of a period in which M. tuberculosis persists in a non- or slowly-replicating state that often lasts decades, if not the lifetime of the host (Russell 2001; Barry et al. 2009; Philips and Ernst 2012).
Mycobacterium tuberculosis has been reported to infect and/or be found in both phagocytic and nonphagocytic cell types, including myeloid dendritic cells, neutrophils, adipocytes, and epithelial cells (Neyrolles et al. 2006; Wolf et al. 2007; Nathan 2009). However, macrophages constitute the quantitatively largest and the most significant cellular reservoir of M. tuberculosis during the establishment and maintenance of a chronic infection. Moreover, not all macrophages, nor their phagosomes, are the same. Though microscopically well defined, phagosomes are both diverse and dynamic. Following internalization of a particle or pathogen, phagosomes undergo a series of rapid and extensive changes in their biochemical composition and function that can be further modulated by immune activation. Nascent phagosomes, for example, while hypoxic and nutrient poor, are generally not competent to kill intracellular bacteria (James et al. 1995; Vieira et al. 2002). However, subsequent fusion with endomembrane compartments, such as lysosomes, leads to a progressive acidification, alteration in ionic composition, and accumulation of lytic enzymes, oxygenated lipids, and reactive oxygen species that can be further intensified with immune activation by cytokines. Cytokines such as IFN-γ can further modify this chemistry with the coordinated production and trafficking of reactive oxygen and nitrogen intermediates, antimicrobial peptides and mediators of autophagy, such as inducible nitric oxide synthase (iNOS), the immunity-related GTPase LRG-47, and guanylate-binding proteins (GBPs) (MacMicking et al. 1997; MacMicking 2003; Gutierrez et al. 2004; Alonso et al. 2007; Purdy and Russell 2007; Kim et al. 2011; Shenoy et al. 2012).
Mycobacterium tuberculosis-containing phagosomes have long been noted to exhibit an exceptional degree of heterogeneity (Russell 2001; Rohde et al. 2007). Unlike the case for pyogenic bacteria, some M. tuberculosis-containing phagosomes are remarkably long lived, lasting days, rather than hours, in vitro, and years, rather than days, in vivo, while others succumb to their replication, and yet others undergo apoptosis (Fratazzi et al. 1999). In addition, considerable biochemical and cell biologic evidence has established that M. tuberculosis can actively regulate the phagosome, by preventing its fusion with the lysosome and antagonizing its acidification (Sturgill-Koszycki et al. 1994; Schaible et al. 1998). More recent studies have suggested that M. tuberculosis may even escape the phagosome to reside in the cytosol (van der Wel et al. 2007; Houben et al. 2012).
Given this extensive heterogeneity, it is likely, if not certain, that M. tuberculosis encounters phagosomal environments that vary according to the specific cell type, location, and time of infection. Indeed, bone marrow-derived macrophages, alveolar macrophages, and dendritic cells have all been shown to be capable of producing similar levels of nitric oxide (NO) (the major determinant of immune control of M. tuberculosis in mice), upon immune stimulation with IFN-γ (MacMicking et al. 1997; Bodnar et al. 2001; Choi et al. 2002; Roy et al. 2004). Yet, infection of each is associated with a distinct physiologic outcome. For example, alveolar macrophages support sufficient replication to enable M. tuberculosis to establish infection, while dendritic cells achieve sufficient control to enable maturation of a T cell response and bone marrow-derived macrophages, which are believed to predominate during the chronic or persistent phase of infection, and characterized by their unique ability to restrict, if not kill, M. tuberculosis over prolonged periods of time. Such differences thus highlight key, yet unresolved, differences in the cellular microenvironments associated with the M. tuberculosis lifecycle.
From an experimental perspective, it is important to note that biochemical studies of the host milieu of M. tuberculosis have relied heavily, if not exclusively, on the in vitro use of murine bone marrow-derived macrophages or immortalized cell lines (Vogt and Nathan 2011) that, when used, have been challenged by methodologic shortfalls associated with identifying their constituents and preserving their concentrations during subcellular fractionation.
Limitations aside, experimental studies of M. tuberculosis-infected macrophages of immunocompetent mice or humans have provided direct biochemical (rather than inferred genetic) evidence for: a transient burst of ROI mediated by assembly of the phagocyte oxidase complex accompanying formation of the phagocytic cup; decreased oxygen; a pH of about 6.3 in resting macrophages, due to exclusion of the vacuolar proton-exchanging ATPase, that can be overcome with IFN-γ-stimulation to achieve a pH of approximately 4.5; and NO generated upon IFN-γ-induced production and vesicular localization of iNOS as well as from chemical dismutation of its auto-oxidation product, nitrite (Nathan and Shiloh 2000; MacMicking 2003; Vandal et al. 2008). The relevance of NO-derived species in human TB has been specifically affirmed by the demonstration of co-localizing immunoreactivity of iNOS and nitrotyrosine, a biochemical footprint of NO reactivity (Choi et al. 2002). Transcriptional profiling studies of M. tuberculosis recovered from macrophages in vitro and from the lungs of infected mice have been used to infer the availability of fatty acids, and lack carbohydrates, lysine, leucine, and iron in resting and immune-activated phagosomes (Sambandamurthy et al. 2002; Schnappinger et al. 2003; Timm et al. 2003; Voskuil et al. 2003). However, direct biochemical support for such inferences remains lacking. Interestingly, studies of M. tuberculosis’ bioactive cell surface lipids and secreted proteins have documented trafficking into host cytosol and extracellular vesicles, while electron microscopy has demonstrated co-localization of M. tuberculosis-containing phagosomes with host lipid bodies, suggesting a potential biochemical connectivity (Russell 2001; Russell et al. 2009).
Looking more macroscopically, both human patient and animal studies have extended this heterogeneity to include broader changes in the tissue surrounding M. tuberculosis-infected macrophages. For example, while the initial infection of alveolar macrophages triggers a localized inflammatory response consisting in tumor necrosis factor (TNF)-α and IFN-γ, the concurrent release of inflammatory chemokines initiates the formation of a granuloma, the pathologic hallmark of tuberculosis (Russell 2006). Initially the granuloma, is an amorphous mass of macrophages, monocytes, and neutrophils, but over time undergoes differentiation giving rise to specialized cell types, including multinucleated giant cells, foamy macrophages and epithelioid macrophages, that organize into a multicellular structure consisting in a macrophage-rich center surrounded by a mantle of lymphocytes and fibrous sheath. This microanatomic heterogeneity is accompanied by further variations in vascular supply and oxygen tension that can range from neovascularized to hypoxic necrotic granulomas within the same host (Via et al. 2008; Russell et al. 2010). Moreover, studies of rabbit and human tuberculous granulomas have shown M. tuberculosis to exist in anatomically distinct transcriptional states within a single lesion (Kaplan et al. 2003).
Albeit brief, M. tuberculosis also traverses key extracellular environments that are critical to completion of its life cycle. As granulomas progress, their fibrous sheath becomes more marked, while their vasculature wanes and the number of foamy macrophages increases. These changes are accompanied by further increases in hypoxia, as reported by in situ staining with pimonidazole, and presumptive gradients of other blood borne nutrients, that culminate in necrosis, a central caseation that is sometimes surrounded by calcification, and accumulation of extracellular bacilli (Boshoff and Barry 2005; Russell 2006; Via et al. 2008). Biochemical analysis of the major lipid species within this caseum has revealed an abundance of cholesterol ester, cholesterol, and triacylglycerol, and also a high level of lactosylceramide (Kim et al. 2010). Ultimately, such granulomas rupture into the airways, perhaps via an increase in levels of matrix metalloproteinase 9 (MMP9), and spill thousands of viable bacilli, poised to re-enter cell cycle (Russell 2006; Elkington and D’Armiento 2011). Once released into the airways, M. tuberculosis completes its lifecycle via infectious sputum aerosol and transmission to a new host. Though generally considered aerated mucus polysaccharides and epithelial cell debris of the lower respiratory tract, cytologic studies of M. tuberculosis-containing sputum have identified a significant number of expelled bacteria within neutrophils, while bacteriologic and transcriptional profiling studies of M. tuberculosis have revealed distinct subpopulations of nonreplicating as well as viable but not culturable subpopulations suggestive of a more complex environment (Garton et al. 2008; Eum et al. 2010; Mukamolova et al. 2010). Sputum aerosol studies have further established a distinct array of physical characteristics associated with droplets of different size that may further contribute to the heterogeneity of this critical niche (Rieder 1999).
3 The Mycobacterium tuberculosis Looking Glass: Current Gaps in Knowledge
3.1 Eating and Sleeping on an Atkins Diet?
Looking from the pathogen seminal studies from Segal and Bloch taught that M. tuberculosis isolated from the lungs of infected mice preferentially metabolized fatty acids over carbohydrates implicating fatty acids as the predominant carbon source encountered by M. tuberculosis in vivo (Bloch and Segal 1956). This view was later enforced with the completion of the M. tuberculosis genome and discovery of its extensive duplications of genes encoding enzymes associated with beta-oxidation (Cole et al. 1998). Genome wide transcriptome analyses of M. tuberculosis recovered from primary mouse macrophages, mouse lungs and TB patients similarly revealed that genes encoding enzymes required for lipid and fatty acid metabolism were induced in the host niche (Schnappinger et al. 2003; Timm et al. 2003).
The specific lipid and fatty acid substrates accessible to M. tuberculosis remain incompletely defined. However, M. tuberculosis expresses a number of lipases and phospholipases capable of catalyzing the release of fatty acids from host lipids (Côtes et al. 2008; Singh et al. 2010; Dedieu et al. 2012). Accordingly, an M. tuberculosis strain lacking three phospholipase C enzymes failed to persist normally during mouse infection indicating a potential demand for lipid degradation in the later phase of infection (Raynaud et al. 2002). In addition, a large fraction of bacilli isolated from tuberculosis patient sputum samples have been shown to contain lipid bodies similar to those observed in hypoxic, nonreplicating M. tuberculosis (Garton et al. 2002, 2008; Daniel et al. 2004; Deb et al. 2006). The dominant component of these lipid bodies consists of triacylglycerol (TAG), which intracellular bacilli seem to synthesize directly from fatty acids released from host TAG (Daniel et al. 2011). Mycobacterium tuberculosis-infected macrophages have similarly been found to undergo foamy maturation followed by migration of M. tuberculosis-containing phagosomes toward newly formed intracellular lipid bodies, which ultimately encapsulate the pathogen (Peyron et al. 2008). Such encapsulation provides M. tuberculosis with potential access to nutrients in the form of fatty acids from host TAG that can be stored in its own lipid bodies as potential source of energy, even when not replicating. LipY a TAG hydrolase (Deb et al. 2006; Mishra et al. 2007) has been detected in the M. tuberculosis cytosol and on the bacterial surface suggesting that it is involved in hydrolysis of intrabacterial, stored TAGs as well as TAGs from host lipid bodies (Dedieu et al. 2012).
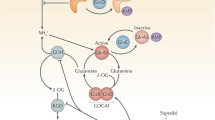
Studies of genetically engineered M. tuberculosis mutant strains have provided similar evidence of fatty acids in the host niche. Bacteria grown on fatty acid-derived acetyl-CoA rely on gluconeogenesis and the anaplerotic glyoxylate shunt (Fig. 1). Accordingly, M. tuberculosis mutants lacking the glyoxylate shunt enzyme isocitrate lyase (ICL) or phosphoenolpyruvate carboxykinase (PEPCK), catalyzing the first committed of gluconeogenesis, were shown to be incapable of growing on fatty acids in vitro and found to be profoundly attenuated during the acute and chronic phases of mouse infections (McKinney et al. 2000; Muñoz-Elías and McKinney 2005; Marrero et al. 2010; Blumenthal et al. 2010). Together, these mutants implicated fatty acid metabolism as essential for growth and survival of M. tuberculosis in vivo.
Central metabolic pathways of M. tuberculosis. Enzymes and their encoding genes are color coded to reflect their dedicated pathways: glycolysis (light blue), gluconeogenesis (red), glycolysis and gluconeogenesis (purple), TCA cycle (green), glyoxylate shunt (orange), and methylcitrate cycle (dark blue). Reactions that convert α-ketoglutarate into succinate are detailed in Fig. 2
More detailed interpretations of these phenotypes, however have proven challenging. For example, recent work showed that M. tuberculosis ICL functions as a bifunctional isocitrate and methylisocitrate lyase (Muñoz Elías et al. 2006), thus making it unclear which of its bifunctional metabolic roles in the glyoxylate shunt and methylcitrate cycles explains the profound attenuation of ICL-deficient M. tuberculosis in vivo. Loss of methylisocitrate lyase activity is predicted to result in the accumulation of toxic propionyl-CoA metabolites (Upton and McKinney 2007), while loss of isocitrate lyase activity is expected to impair carbon assimilation through inactivation of the glyoxylate shunt. While disruption of the methylcitrate cycle via deletion of the downstream enzymes, prpDC encoding methylcitrate synthase and methylcitrate dehydratase (Fig. 1), attenuated M. tuberculosis growth on propionate and in isolated macrophages, replication and persistence in mice was unimpaired, suggesting the primacy of the glyoxylate shunt (Muñoz Elías et al. 2006). However, absence of methylcitrate cycle activity can also be compensated for by the vitamin B12-dependent methylmalonyl pathway (Savvi et al. 2008; Griffin et al. 2012), while the ability of M. tuberculosis to scavenge vitamin B12 from the host or synthesize it during infection remains to be investigated.
The failure of M. tuberculosis lacking PEPCK to replicate in resting macrophages or during acute mouse infections further supports the view that M. tuberculosis relies on gluconeogenesis for biomass during growth within a host. However, the profound death following PEPCK deletion or silencing in vivo at early and at late stages of the infection remains unexplained. In contrast to the ICL deficient mutant, the growth defect of M. tuberculosis lacking PEPCK in media containing fatty acids could be rescued by supplementation with glucose or glycerol (Marrero et al. 2010). Lack of PEPCK may, however, cause metabolite perturbations that might sensitize M. tuberculosis to stresses encountered within the host and lead to its death. Together, these examples illustrate that, while powerful, the use of genetically engineered M. tuberculosis strains as a bioprobes of the host–pathogen interaction can be complicated by their limited biochemical resolution, as exemplified by their inability to determine if the attenuation of a given enzymatic mutant is due to depletion of its product or an intoxication arising from the accumulation of an upstream or distantly related metabolite. Nonetheless, that fatty acids and lipids are relevant carbon substrates during M. tuberculosis growth and persistence in macrophages and during infections remain the prevailing paradigm.
3.2 Good Cholesterol?
Cholesterol is an essential constituent of mammalian cell membranes where it plays important structural and regulatory roles (Munro 2003; Brown and Goldstein 2008; Yuan et al. 2012). It is also a component of lipid droplets, which accumulate in alveolar macrophages of tuberculosis patients and in foamy macrophages of mouse granulomas (Russell et al. 2009). In fact, M. tuberculosis can induce the formation of foamy, lipid-loaded macrophages, and electron microscopy showed that the bacilli containing phagosomes are found in close proximity to intracellular nonmembrane bound lipid bodies (Peyron et al. 2008; Melo and Dvorak 2012). These foamy macrophages appear to provide a hospitable niche for M. tuberculosis as they protect the bacilli from direct contact with lymphocytes, appear to have lost bactericidal activity and might facilitate the acquisition of lipid and fatty acid nutrients. That M. tuberculosis utilizes cholesterol in vivo is supported by a wealth of data. It encodes a dedicated cholesterol uptake system and the complete pathway for its degradation (van der Geize et al. 2007), can catabolize cholesterol in vitro and use it as sole carbon source for replication (Pandey and Sassetti 2008; Griffin et al. 2012). Catabolism of cholesterol is predicted to yield propionyl-CoA, acetyl-CoA, and pyruvate (van der Geize et al. 2007) and cholesterol contributes to M. tuberculosis propionyl-CoA pool in vitro and when it resides in macrophages (Yang et al. 2009; Griffin et al. 2012).
Mycobacterium tuberculosis mutants whose ability to degrade cholesterol is impaired via deletion of the mce4-encoded cholesterol transporter, or abolished due to lack of enzymes required for cholesterol catabolism, fail to persist normally in the chronic phase of mouse and guinea pig infections (Pandey and Sassetti 2008; Nesbitt et al. 2009; Yam et al. 2009; Hu et al. 2010). This suggested that cholesterol is an important nutrient in vivo, but also indicates access to alternative carbon sources prior to the onset of adaptive immunity. A mutant lacking the “intracellular growth (igr)” locus, that encodes enzymes important for early steps in cholesterol degradation was similarly attenuated during early mouse infections (Chang et al. 2009). This phenotype has been attributed to a presumptive intoxication from cholesterol metabolites as disruption of the Mce4 cholesterol transporter suppressed the in vivo growth defect of the irg mutant. In contrast, M. tuberculosis lacking 3β-hyroxysteroid dehydrogenase (HSD), the enzyme catalyzing the first step in cholesterol catabolism, grew normally in guinea pig lungs and persisted normally for up to 7 weeks suggesting that cholesterol is not an essential nutrient source for M. tuberculosis during infection of guinea pigs (Yang et al. 2011). The dispensability of HSD could be explained if M. tuberculosis has access not only to intact cholesterol, but also the product of HSD, cholest-4-en-3-one; or it expresses a second, regulated HSD that is only induced during infection. However, while these data further highlight the complexity of interpreting the phenotypes of gene deletion mutants, they clearly support a role for cholesterol as a “good” carbon source for M. tuberculosis during chronic infection.
3.3 Carb Counting and the Role of Sugar Transporters
While the weight of prevailing evidence has clearly implicated fatty acids as an essential carbon source for M. tuberculosis in vivo, roles for other carbon sources remain conspicuously unresolved. Recent work showed that, unlike most bacterial pathogens, M. tuberculosis is capable of utilizing multiple carbon substrates simultaneously (de Carvalho et al. 2010a; Rhee et al. 2011). In addition, genetic studies have clearly demonstrated that the LpqY-SugA-SugB-SugC carbohydrate transporter is required for normal growth of M. tuberculosis in mouse lungs and spleens (Kalscheuer et al. 2010). This transporter was shown to be highly specific for uptake of the disaccharide trehalose, which is not present in mammals, but can be released by M. tuberculosis from trehalose-containing cell wall glycolipids (Kalscheuer et al. 2010). The intracellular fate of recycled trehalose remains to be identified; it might serve as a precursor for α-glucans, for trehalose-containing glycolipids, which are very prevalent in the mycolic acid cell wall, and as a reservoir of glucose. Trehalose biosynthesis is mediated by three biosynthetic pathways (De Smet et al. 2000) and is required for growth of M. smegmatis, likely because it serves as an essential precursor for cell wall biosynthesis. In vitro, M. tuberculosis can grow with trehalose as sole carbon source (Kalscheuer et al. 2010) presumably due to conversion of trehalose to glucose by trehalase (Carroll et al. 2007). Trehalose hydrolysis by trehalase yields two molecules of glucose, while trehalose phosphorylase catalyzes the phosphorolytic cleavage of trehalose into glucose 1-phosphate and glucose (Argüelles 2000). A phosphate dependent trehalase yet, has been purified from M. smegmatis and M. tuberculosis contains a homolog. Yet, despite the requirement for phosphate, there was no evidence of phosphorolytic cleavage, suggesting that the enzyme produces two molecules of glucose. No matter how it is metabolized, it is intriguing to speculate that trehalose serves as carbon store in M. tuberculosis in form of cell wall glycolipids such as trehalose dimycolate. Within the host, M. tuberculosis may also have a direct access to glucose and glucose-phosphate. Salmonella typhimurium uses primarily glucose when replicating in the phagosome (Bowden et al. 2009), while glucose-6-phosphate serves as a carbon source for intracellular Listeria monocytogenes (Chico-Calero et al. 2001). Uptake of glucose-6-phosphate is mediated by a specific hexose-phosphate transporter, which is present in several intracellular pathogens such as L. monocytogenes, Shigella flexneri, Salmonella enterica, and Chlamydia pneumonia (Schwoppe et al. 2002; Fuchs et al. 2011), but has not been identified in M. tuberculosis. Glucose-6-phosphate is an important precursor for anabolic processes, especially cell wall and nucleotide biosynthesis and serves as a source of reducing power in mycobacteria (Hasan et al. 2010). Whether glucose metabolism is important for the generation of biomass during in vivo growth remains to be shown. M. tuberculosis expresses two hexokinases (Cole et al. 1998). Transposon mutant analysis suggested that one of them, encoded by ppgK, is important for replication in mouse spleens (Sassetti and Rubin 2003), but this awaits confirmation with gene deletion mutants. Thus, while it is clear that M. tuberculosis relies on trehalose recycling during mouse infections, the intracellular fate of trehalose remains to be determined and the importance of host-derived glucose or glucose-6-phosphate at any stage of the infection unresolved.
3.4 The Glycerol Paradox
Early studies showed that M. tuberculosis growth was most strongly enhanced with the inclusion of glycerol in the culture medium. Glycerol-fed M. tuberculosis grows faster and the bacilli reach a higher density than when metabolizing other carbon sources such as glucose or fatty acids, which has thus led to the use of glycerol in virtually all standard mycobacterial growth media (Dubos 1947; Edson 1951). Recent work, however, demonstrated that M. tuberculosis lacking glycerol kinase GlpK, which is essential for the first step in glycerol catabolism (Fig. 1), was unable to utilize glycerol as sole carbon source in vitro, yet replicated and persisted like wild type M. tuberculosis in mouse lungs suggesting that glycerol is not a critical carbon source for M. tuberculosis, at least in the mouse model (Pethe et al. 2010). After uptake, glycerol is phosphorylated by glycerol kinase and glycerol-3-phosphate dehydrogenase converts the resulting glycerol-3-phosphate (glycerol-3-P) to dihydroxyacetone phosphate, which enters glycolysis and gluconeogenesis (Fig. 1). The possibility of a redundant glycerol kinase induced in the intracellular environment during infection thus remains to be evaluated. An alternative model consistent with the foregoing observations is that M. tuberculosis utilizes glycerol-3-P liberated from phospholipids and metabolizes it via glycerol-3-P dehydrogenase into dihydroxyacetone phosphate, at the same time perhaps metabolizing the released free fatty acid chains. E. coli expresses glycerophosphodiester phosphodiesterase, which can convert phospholipid derived glycerophosphodiesters into glycerol-3-P (Larson et al. 1983), however this enzyme has not been identified in M. tuberculosis. The pathogen might instead utilize host-derived glycerol-phosphate consistent with the observation that intracellular M. tuberculosis induced expression of the ugp operon encoding a putative ABC transporter for glycerol-3-P (Schnappinger et al. 2003). M. tuberculosis contains two genes encoding probable glycerol-3-phosphate dehydrogenases, glpD1 and glpD2 (Fig. 1) and the latter is required for normal growth on solid media containing glycerol and glucose (Griffin et al. 2011). Their importance during host infection has not yet been investigated. The phagosome might not provide easy access to glycerol-3-P; however, M. tuberculosis that escaped to the cytosol (van der Wel et al. 2007; Houben et al. 2012) or to the extracellular space might face a less restricted nutritional environment. Of note, there is ample evidence that glycerol and glycerol-3-P serve as major carbon sources for several cytosolic pathogens including Shigella flexneri and cytosolic Listeria monocytogenes (Eisenreich et al. 2010). In summary, while M. tuberculosis does not appear to utilize glycerol to replicate or persist during mouse infections, glycerol-3-P potentially derived from host phospholipids might serve as alternative carbon source in vivo.
3.5 Eating and Breathing at the Same Time: Effects of Oxygen and Carbon Dioxide Tensions on Mycobacterium tuberculosis’s Central Carbon Metabolism?
Putting aside the specific nutrients consumed in the host, M. tuberculosis resides within granulomas, which are frequently hypoxic (Via et al. 2008) and situated in the CO2-rich environment of the lung. Not surprisingly, M. tuberculosis’s metabolic activity has been found strongly influenced by oxygen tension and CO2 availability. That M. tuberculosis can utilize CO2 as source of carbon has been known for many years (Nishihara 1954); however, the functional relevance of this was unknown until recently. 13C metabolic flux analysis revealed that in a carbon-limited chemostat M. tuberculosis dissimilated pyruvate via the glyoxylate shunt and incorporated CO2 into central carbon metabolism as shown by CO2-derived 13C incorporation into several amino acids and produced succinyl-CoA (Beste et al. 2011). Importantly, in conditions of low oxygen tension, at levels that mimic those identified in lung granulomas, M. tuberculosis generated fumarate though the reductive TCA cycle, assimilated CO2, and used fumarate as an electron sink (Watanabe et al. 2011). Reduction of fumarate generates succinate, which M. tuberculosis actively secretes into the extracellular environment thereby maintaining an energized membrane (Watanabe et al. 2011). This led to the hypothesis that, in hypoxic granulomas, M. tuberculosis might reverse its TCA cycle, incorporate CO2 and accumulate and secrete succinate to maintain a state of persistence, if provided a glycolytic carbon source. While PEPCK was found to catalyze the conversion of oxaloacetate to PEP in vitro in oxygenated conditions, it is possible that it may also function in the reverse direction, especially during nonreplicative persistence, contributing to CO2 utilization and energy generation instead of anabolism and biomass production (Zhang et al. 2010; Watanabe et al. 2011).
These studies separately emphasize the inherent dependence of all genetic studies of M. tuberculosis metabolism on the limitations of the specific host model used. The mouse strains most commonly used for such studies do not form necrotic and caseating granulomas in response to M. tuberculosis infection (McMurray et al. 1996; Rhoades et al. 1997; Flynn 2006). Accordingly granulomas from C57BL/6 mice were not severely hypoxic in contrast to those in guinea pigs, rabbits, and nonhuman primates (Aly et al. 2006; Tsai et al. 2006; Via et al. 2008). This difference in pathology and oxygen tension might be associated with an altered nutritional environment and different metabolic adaptations of the bacilli within the lung of mice compared to humans, nonhuman primates, rabbits, and guinea pigs. Notwithstanding, hypoxia is likely not the only cue triggering CO2 utilization as this is also associated with slow growth (Beste et al. 2011); even in mouse lesions the oxygen concentration may be reduced and growth of M. tuberculosis is significantly slowed with the onset of adaptive immunity so that metabolic adaptations become essential that enable the pathogen to maintain its energy metabolism during phases of persistence.
3.6 Adapting to Nutritionally Diverse Environments
As described above, M. tuberculosis encounters a diverse and dynamic array of microenvironments during infection that require the pathogen to adapt its carbon and energy metabolism accordingly. While the intracellular phagosomal environment is clearly one of the chief niches M. tuberculosis resides in, the bacilli can actively escape into the cytosol (van der Wel et al. 2007; Houben et al. 2012), are found in the acellular center of caseating granulomas (Kaplan et al. 2003), and must survive at least for some time in air during transmission. In addition, when the host responds to infection, the intracellular niches change. Several examples indicate that M. tuberculosis is metabolically flexible and well capable of utilizing and realigning different metabolic pathways to optimally exploit available nutrients or adapt to nutrient deficiency. Many bacteria use available carbons substrates selectively: when presented with a mix of carbon substrates, they first catabolize their preferred carbon source, which in model organisms such as E. coli and Bacillus subtilis is glucose and only when this preferred substrate is exhausted will they catabolize secondary substrates (Görke and Stülke 2008). This carbon catabolite repression is mediated by different sophisticated regulatory mechanisms and represents one of the most studied global control systems in bacteria. Similar to some highly host-adapted pathogens such as Chlamydia trachomatis, M. tuberculosis lacks classical carbon catabolite repression and instead regulates its growth through simultaneous co-catabolism of multiple carbon sources (Nicholson and Chiu 2004; de Carvalho et al. 2010a). When fed with 13C-labled glucose and acetate, M. tuberculosis metabolized glucose preferentially through glycolysis and the pentose phosphate pathway and converted acetate mostly into intermediates of the TCA cycle. Yet, at the same time, it incorporated low amounts of acetate-derived carbon into glycolytic/gluconeogenic and pentose phosphate intermediates and dextrose-derived carbon into intermediates of the TCA cycle. M. tuberculosis thus metabolizes different carbon substrates simultaneously to feed different pathways and is capable to segregate carbon flow through the same metabolic pathway but in opposite directions. This unusual metabolic network topology likely benefits the capacity of M. tuberculosis to adapt to different host niches.
Other examples of metabolic plasticity come from M. tuberculosis’s TCA cycle enzymes. An M. tuberculosis mutant, whose pyruvate dehydrogenase (PDH) complex was inactivated due to the deletion of dlaT, coding for dihydrolipoamide dehydrogenase, the E2 of PDH, upregulated a branched-chain keto acid dehydrogenase (BCKADH) complex (Venugopal et al. 2011). BCKADH activity prevented accumulation of pyruvate, branched-chain amino acids, and branched-chain keto acids in DlaT deficient M. tuberculosis. The genes encoding BCKADH were also upregulated in response to nutrient starvation (Betts et al. 2002) suggesting that catabolism of branched-chain keto and/or amino acids in nutrient restricted environments might fuel M. tuberculosis’s energy metabolism.
Metabolic capabilities aside, the nature of M. tuberculosis’s TCA cycle has proven surprisingly difficult to dissect. It was thought to lack a functional α-ketoglutarate dehydrogenase (KDH) complex (Tian et al. 2005b) and the predicted E1 enzyme was found to function as a thiamine diphosphate-dependent α-ketoglutarate decarboxylase (KGD) (Tian et al. 2005a). This enzyme also has carboligase activity and produces 2- hydroxy-3-oxoadipate (HOA) from α-ketoglutarate and glyoxylate (de Carvalho et al. 2010b), thereby perhaps detoxifying the cell from high glyoxylate concentrations or producing biosynthetic precursors. The discovery of an aero-tolerant anaerobic-type α-KG ferredoxin oxidoreductase (KOR) that could link the oxidative and reductive branch of the TCA cycle and produce succinyl-CoA from α-KG and CoA-SH further supported the model that M. tuberculosis lacks KDH (Baughn et al. 2009). Recent work, however, revealed that M. tuberculosis’s KGD is in fact multifunctional and in vitro capable of catalyzing KG dehydrogenase, KG decarboxylase, and HOA synthase activities (Wagner et al. 2011) (Fig. 2). KGD and KOR together could enable M. tuberculosis to convert α-ketoglutarate into succinate via three parallel pathways linking the oxidative and reductive branch of the TCA cycle. M. tuberculosis lacking KOR grew like wt when provided with either heightened CO2 concentration, in the absence of fatty acids, or when the glyoxylate cycle was inhibited (Baughn et al. 2009). KOR thus appears to be necessary to provide succinyl-CoA, CO2 and reducing equivalents during growth on fatty acids, thereby fueling gluconeogenesis. A different not mutually exclusive interpretation of the data is that KOR is important when the reverse TCA cycle, which requires CO2, is active. Operation of the reverse TCA cycle has only been demonstrated with glycolytic carbon sources and in a hypoxic environment (Watanabe et al. 2011), however it is plausible that additional metabolic cues and environmental conditions trigger reversal of the cycle. Notwithstanding, these alternative functional pathways of the TCA cycle might provide metabolic flexibility required for growth and survival in nutritionally diverse environments and allow the pathogen to consume various carbon sources.
M. tuberculosis’s TCA cycle reactions. α-Ketoglutarate (α-KG) can be converted to succinate via (1) α-KG ferredoxin oxidoreductase (KOR, Rv2254c, Rv2455c), (2) α-KG decarboxylase (KGD, Rv1248c) and (3) α-KG dehydrogenase complex (KDH) consisting of KGD, dihydrolipoamide dehydrogenase (DlaT, Rv2215) and lipoamide dehydrogenase (LpdC, Rv0462). KGD also functions as 2-hydroxy-3-oxoadipate synthase (HOAS). GabD, succinic semialdehyde dehydrogenase (GabD1, Rv0234c; GabD2, Rv1731); SucC/D, succinyl- CoA synthetase (SucC, Rv0951; SucD, Rv0952)
3.7 How Do Others Do It?
Elegant work has investigated the intracellular lifestyle and metabolic requirements of S. typhimurium (reviewed in Eisenreich et al. 2010; Dandekar 2012), a pathogen that remains within a vacuole throughout its life inside the host. This revealed that metabolism of carbohydrates including glucose via glycolysis is essential for intracellular growth of Salmonella in macrophages and during mouse infections (Bowden et al. 2009; Paterson et al. 2009; Götz and Goebel 2010). Fatty acid catabolism and gluconeogenesis, by contrast, were dispensable for virulence of S. typhimurium in mice (Tchawa Yimga et al. 2006). This differs significantly from the metabolic pathways required by M. tuberculosis and suggests that intracellular pathogens, despite sharing the intraphagosomal habitat, have evolved distinctive carbon acquisition strategies. Host-derived amino acids are essential for the intracellular growth of Legionella pneumophila, which parasitizes various protozoan species, but can also be transmitted to humans where it infects and replicates in alveolar macrophages causing Legionnaires’ disease (Sauer et al. 2005; Wieland et al. 2005; Newton et al. 2010). L. pneumophila replicates within a specialized endosome-derived vacuole that maintains a neutral pH and acquires characteristics of the endoplasmic reticulum. While it seems to depend on metabolism of amino acids (George et al. 1980), it can also utilize glucose via the Entner-Doudoroff pathway (Eylert et al. 2010). Recent work revealed that Legionella ‘hijacks’ the host proteasome to generate amino acids from polyubiquitinated proteins (Price et al. 2011). Whether this strategy is exploited by other intracellular pathogens remains to be seen; M. tuberculosis might rely on its own proteasome for the generation of amino acids as carbon substrates during nutrient starvation, as a proteasome mutant was unable to survive in stationary phase, during chronic mouse infections and in a model of strict carbon starvation (Gandotra et al. 2010).
It is possible that some of the differences in nutrient dependence between intracellular M. tuberculosis and Salmonella or Legionella may be explained by the ability of M. tuberculosis to gain access to the cytosol. L. monocytogenes, which replicates in the cytosol, relies predominantly on glycerol for intracellular growth and also utilizes sugar phosphates (Eisenreich et al. 2010; Fuchs et al. 2012). In contrast, glucose seems not to play a role (Stoll and Goebel 2010) and there is no evidence that L. monocytogenes accesses lipids or catabolizes fatty acids inside a host. In fact, L. monocytogenes lacks the genes for β–oxidation and the glyoxylate shunt (Eisenreich et al. 2010); its TCA cycle is interrupted (Eisenreich et al. 2006) and it requires pyruvate carboxylase (PYC) for the synthesis of oxaloacetate from pyruvate bypassing TCA cycle activity (Schar et al. 2010). This is essential for in vivo growth as a mutant lacking PYC was strongly attenuated in a mouse sepsis model (Schar et al. 2010). Major differences thus exist with respect to both the carbon sources that different pathogens scavenge from their host cells and the metabolic pathways they employ for biomass and energy generation.
It is similarly possible that pathogens may manipulate host metabolism to directly or indirectly modifying the metabolic environment they are confronted with. For example, macrophage activation by IFN-γ and proinflammatory cytokines induces NADPH oxidase and iNOS. NADPH oxidase consumes ATP, whose production requires flux through glycolysis; generation of NO requires arginine, is accompanied by recycling of citruline and inhibition of ornithine and polyamine biosynthesis (Gordon 2003; Mori and Gotoh 2004; Naderer and McConville 2007). In contrast, macrophages activated by Th2-type cytokines IL-4 or IL-13 are characterized by increased arginase activity and production of polyamine biosynthesis precursors (Gordon 2003). Leishmania parasites induce a Th2 response in susceptible mouse strains (Sacks and Anderson 2004) and benefit from the increased availability of essential amino acids and polyamines supporting their intracellular replication (Naderer and McConville 2007).
Finally, hormone-, growth factor-, and nutrient-regulated signaling pathways control the metabolism of mammalian host cells (Levine and Puzio-Kuter 2010). Pathogens might directly interfere or modulate such signaling pathways in pathogen-specific ways thereby altering host cell metabolism and controlling nutrient accessibility.
4 Concluding Remarks
Multiple lines of evidence have implicated M. tuberculosis’ metabolic network as a central mediator of its pathogenicity. However, knowledge of how it achieves this remains incomplete. Most textbooks depict metabolism as a housekeeping activity of all cells relegated to bulk restocking of biosynthetic precursors and energy. We believe that such views are both outdated and incomplete. They misequate evolutionary conservation with biological law, neglect the selective pressure of an organism’s ecologic and nutritional niche on its physiology, and dissociate metabolism from cell physiology. Moreover, it remains a fact that cells have evolved an extensive array of mechanisms to regulate the activity of individual pathways and that metabolism serves as the chemical arbiter of all cellular reactions.
Existing studies have focused on compiling a molecular inventory and homology-based reconstruction of M. tuberculosis’ metabolic network. However, facts do not equate into knowledge nor knowledge into understanding. Key challenges thus await. Chief among them is a more detailed understanding of the specific biochemical needs served by M. tuberculosis’ metabolic network during each phase of its nutrient-limited life cycle. Studies of the M. tuberculosis cell surface components have revealed their distinct host regulatory activities, ranging from phagocytosis by alveolar macrophages to phagolysosomal maturation of bone marrow-derived macrophages to secretion of pro-inflammatory cytokines. These are associated with specific cell wall lipids and carbohydrates, and presumably derive from distinct metabolic pathways that await elucidation (Torrelles and Schlesinger 2010; Mishra et al. 2011; Neyrolles and Guilhot 2011). Knowledge of such links, however, is likely to require more integrated views of metabolism that include balanced homeostasis of essential noncarbon metabolites, such as sulfur, nitrogen, and phosphorous, as well as metals including but not limited to Fe, Cu, Zn, Mg, Mn, and Mo.
An equally important area of M. tuberculosis metabolism in need of further study is its nutrient uptake and transport mechanisms (Niederweis 2008). Considerable knowledge about lipid transport across the cytoplasmic membrane has been gained (Jackson and Stadthagen 2007), and transporters of nitrogen, phosphate, sulfates, and several metal ions have been identified using bio-informatic methods (Niederweis 2008). Such analyses, for example, helped predict five carbohydrate transporters in M. tuberculosis, whereas M. smegmatis was predicted to encode 28 such transporters, underscoring important differences in their physiology (Titgemeyer et al. 2007). Direct biochemical and structural understanding of these and most other nutrient transport processes however remains scarce, while transport across the outer membrane of M. tuberculosis is even less well understood (Niederweis 2008; Niederweis et al. 2010).
A larger mystery awaiting investigation is how M. tuberculosis senses various nutrients and different forms of nutrient limitations. Some bacteria can regulate their cell size in response to nutrient availability (Schaechter et al. 1958; Chien et al. 2012). In B. subtilis, the glucosyl transferase UgtP serves as UDP-glucose dependent metabolic sensor of nutrient availability to coordinate cell size with growth rate thus linking nutritional information from the environment with cell division (Weart et al. 2007). Whether M. tuberculosis employs similar nutritional regulatory pathways remains to be investigated. Addressing these questions may thus help further elucidate the intracellular lifestyle of M. tuberculosis and its interaction with the host.
References
Alonso S, Pethe K, Russell DG, Purdy GE (2007) Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci USA 104:6031–6036. doi:10.1073/pnas.0700036104
Aly S, Wagner K, Keller C et al (2006) Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J Pathol 210:298–305. doi:10.1002/path.2055
Argüelles JC (2000) Physiological roles of trehalose in bacteria and yeasts: a comparative analysis. Arch Microbiol 174:217–224. doi:10.1007/s002030000192
Barry CE, Boshoff HI, Dartois V et al (2009) The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Micro 7:845–855. doi:10.1038/nrmicro2236
Baughn AD, Garforth SJ, Vilchèze C, Jacobs WR (2009) An anaerobic-type alpha-ketoglutarate ferredoxin oxidoreductase completes the oxidative tricarboxylic acid cycle of Mycobacterium tuberculosis. PLoS Pathog 5:e1000662. doi:10.1371/journal.ppat.1000662
Beste DJV, Bonde B, Hawkins N et al (2011) C metabolic flux analysis identifies an unusual route for pyruvate dissimilation in Mycobacteria which requires isocitrate lyase and carbon dioxide fixation. PLoS Pathog 7:e1002091. doi:10.1371/journal.ppat.1002091
Betts JC, Lukey PT, Robb LC et al (2002) Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43:717–731
Bloch H, Segal W (1956) Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J Bacteriol 72:132–141
Blumenthal A, Trujillo C, Ehrt S, Schnappinger D (2010) Simultaneous analysis of multiple Mycobacterium tuberculosis knockdown mutants in vitro and in vivo. PLoS One 5:e15667. doi:10.1371/journal.pone.0015667
Bodnar KA, Serbina NV, Flynn JL (2001) Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect Immun 69:800–809. doi:10.1128/IAI.69.2.800-809.2001
Boshoff H, Barry C (2005) Tuberculosis—metabolism and respiration in the absence of growth. Nat Rev Micro 3:70–80
Bowden S, Rowley G, Hinton J, Thompson A (2009) Glucose and glycolysis are required for the successful infection of macrophages and mice by Salmonella enterica serovar Typhimurium. Inf Immun 77:3117
Brown MS, Goldstein JL (2008) Cholesterol feedback: from Schoenheimer’s bottle to Scap’s MELADL. J Lipid Res 50:S15–S27. doi:10.1194/jlr.R800054-JLR200
Carroll JD, Pastuszak I, Edavana VK et al (2007) A novel trehalase from Mycobacterium smegmatis—purification, properties, requirements. FEBS J 274:1701–1714. doi:10.1111/j.1742-4658.2007.05715.x
Chang JC, Miner MD, Pandey AK et al (2009) Igr genes and Mycobacterium tuberculosis cholesterol metabolism. J Bacteriol 191:5232–5239. doi:10.1128/JB.00452-09
Chen L, Vitkup D (2007) Distribution of orphan metabolic activities. Trends Biotechnol 25:343–348. doi:10.1016/j.tibtech.2007.06.001
Chico-Calero I, Suárez M, González-Zorn B et al (2001) Hpt, a bacterial homolog of the microsomal glucose- 6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc Natl Acad Sci USA 99:431–436. doi:10.1073/pnas.012363899
Chien A-C, Hill NS, Levin PA (2012) Cell size control in bacteria. Curr Biol 22:R340–R349. doi:10.1016/j.cub.2012.02.032
Choi H-S, Rai PR, Chu HW et al (2002) Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am J Respir Crit Care Med 166:178–186
Cole ST, Brosch R, Parkhill J et al (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544
Côtes K, Bakala N’Goma JC, Dhouib R et al (2008) Lipolytic enzymes in Mycobacterium tuberculosis. Appl Microbiol Biotechnol 78:741–749. doi:10.1007/s00253-008-1397-2
Dandekar T, Astrid F, Jasmin P, Hensel M (2012) Salmonella enterica: a surprisingly well-adapted intracellular lifestyle. Front Microbiol 3:164. doi:10.3389/fmicb.2012.00164
Daniel J, Deb C, Dubey VS et al (2004) Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol 186:5017–5030. doi:10.1128/JB.186.15.5017-5030.2004
Daniel J, Maamar H, Deb C et al (2011) Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog 7:e1002093. doi:10.1371/journal.ppat.1002093.t003
de Carvalho LPS, Fischer SM, Marrero J et al (2010a) Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem Biol 17:1122–1131. doi:10.1016/j.chembiol.2010.08.009
de Carvalho LPS, Zhao H, Dickinson CE et al (2010b) Activity-based metabolomic profiling of enzymatic function: identification of Rv1248c as a mycobacterial 2-Hydroxy-3-oxoadipate synthase. Chem Biol 17:323–332. doi:10.1016/j.chembiol.2010.03.009
De Smet KA, Weston A, Brown IN et al (2000) Three pathways for trehalose biosynthesis in mycobacteria. Microbiology 146(Pt 1):199–208
Deb C, Daniel J, Sirakova TD et al (2006) A novel lipase belonging to the hormone-sensitive lipase family induced under starvation to utilize stored triacylglycerol in Mycobacterium tuberculosis. J Biol Chem 281:3866–3875. doi:10.1074/jbc.M505556200
Dedieu L, Serveau-Avesque C, Kremer L, Canaan S (2012) Mycobacterial lipolytic enzymes: a gold mine for tuberculosis research. Biochimie 95(1):66–73. doi:10.1016/j.biochi.2012.07.008 (in press)
Dubos R (1947) Media for tubercle bacilli. Am Rev Tuberc 56:334–345
Edson NL (1951) The intermediary metabolism of the mycobacteria. Bacteriol Rev 15:147–182
Eisenreich W, Dandekar T, Heesemann J, Goebel W (2010) Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat Rev Micro 8:401–412
Eisenreich W, Slaghuis J, Laupitz R et al (2006) 13C isotopologue perturbation studies of Listeria monocytogenes carbon metabolism and its modulation by the virulence regulator PrfA. Proc Natl Acad Sci USA 103:2040–2045. doi:10.1073/pnas.0507580103
Elkington PT, D’Armiento JM, Friedland JS (2011) Tuberculosis immunopathology: the neglected role of extracellular matrix destruction. Sci Transl Med 3:71ps6. doi:10.1126/scitranslmed.3001847
Eum S-Y, Kong J-H, Hong M-S et al (2010) Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137:122–128. doi:10.1378/chest.09-0903
Eylert E, Herrmann V, Jules M et al (2010) Isotopologue Profiling of Legionella pneumophila: role of serine and glucose as carbon substrates. J Biol Chem 285:22232–22243. doi:10.1074/jbc.M110.128678
Flynn JL (2006) Lessons from experimental Mycobacterium tuberculosis infections. Microbes Inf 8:1179–1188. doi:10.1016/j.micinf.2005.10.033
Fratazzi C, Arbeit RD, Carini C et al (1999) Macrophage apoptosis in mycobacterial infections. J Leukoc Biol 66:763–764
Fuchs T, Eisenreich W, Heesemann J, Goebel W (2011) Metabolic adaptation of human pathogenic and related nonpathogenic bacteria to extra-and intracellular habitats. FEMS Microbiol Rev 36:435–462. doi:10.1111/j.1574-6976.2011.00301.x
Fuchs TM, Eisenreich W, Kern T, Dandekar T (2012) Toward a systemic understanding of Listeria monocytogenes metabolism during infection. Front Microbiol. doi:10.1186/1471-2105-11-77
Gandotra S, Lebron MB, Ehrt S (2010) The Mycobacterium tuberculosis proteasome active site threonine is essential for persistence yet dispensable for replication and resistance to nitric oxide. PLoS Pathog 6:e1001040. doi:10.1371/journal.ppat.1001040
Garton NJ, Christensen H, Minnikin DE et al (2002) Intracellular lipophilic inclusions of mycobacteria in vitro and in sputum. Microbiology 148:2951–2958
Garton NJ, Waddell SJ, Sherratt AL et al (2008) Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med 5:e75. doi:10.1371/journal.pmed.0050075
George J, Pine L, Reeves M, Harrell WK (1980) Amino acid requirements of Legionella pneumophila. J Clin Microbiol 11:286–291
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35. doi:10.1038/nri978
Görke B, Stülke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Micro 6:613–624. doi:10.1038/nrmicro1932
Götz A, Goebel W (2010) Glucose and glucose 6-phosphate as carbon sources in extra-and intracellular growth of enteroinvasive Escherichia coli and Salmonella enterica. Microbiology 156:1176
Griffin JE, Gawronski JD, Dejesus MA et al (2011) High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7:e1002251. doi:10.1371/journal.ppat.1002251
Griffin JE, Pandey AK, Gilmore SA et al (2012) Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem Biol 19:218–227. doi:10.1016/j.chembiol.2011.12.016
Gutierrez M, Master S, Singh S et al (2004) Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119:753–766
Hasan MR, Rahman M, Jaques S et al (2010) Glucose 6-phosphate accumulation in mycobacteria: implications for a novel F420-dependent anti-oxidant defense system. J Biol Chem 285:19135–19144. doi:10.1074/jbc.M109.074310
Houben D, Demangel C, van Ingen J et al (2012) ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol 14:1287–1298. doi:10.1111/j.1462-5822.2012.01799.x
Hu Y, van der Geize R, Besra GS et al (2010) 3-Ketosteroid 9Î ± -hydroxylase is an essential factor in the pathogenesis of Mycobacterium tuberculosis. Mol Microbiol 75:107–121. doi:10.1111/j.1365-2958.2009.06957.x
Jackson M, Stadthagen G, Gicquel B (2007) Long-chain multiple methyl-branched fatty acid-containing lipids of Mycobacterium tuberculosis: biosynthesis, transport, regulation and biological activities. Tuberculosis 87:78–86. doi:10.1016/j.tube.2006.05.003
James PE, Grinberg OY, Michaels G, Swartz HM (1995) Intraphagosomal oxygen in stimulated macrophages. J Cell Physiol 163:241–247. doi:10.1002/jcp.1041630204
Kalscheuer R, Weinrick B, Veeraraghavan U et al (2010) Trehalose-recycling ABC transporter LpqY-SugA-SugB-SugC is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci 107:21761–21766. doi:10.1073/pnas.1014642108
Kaplan G, Post FA, Moreira AL et al (2003) Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Inf Immun 71:7099–7108. doi:10.1128/IAI.71.12.7099-7108.2003
Kim BH, Shenoy AR, Kumar P et al (2011) A family of IFN- -Inducible 65-kD GTPases protects against bacterial infection. Science 332:717–721. doi:10.1126/science.1201711
Kim M-J, Wainwright HC, Locketz M et al (2010) Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med 2:258–274. doi:10.1002/emmm.201000079
Larson TJ, Ehrmann M, Boos W (1983) Periplasmic glycerophosphodiester phosphodiesterase of Escherichia coli, a new enzyme of the glp regulon. J Biol Chem 258:5428–5432
Levine AJ, Puzio-Kuter AM (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330:1340–1344. doi:10.1126/science.1193494
MacMicking JD (2003) Immune control of tuberculosis by IFN-γ-Inducible LRG-47. Science 302:654–659. doi:10.1126/science.1088063
MacMicking JD, North RJ, LaCourse R et al (1997) Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA 94:5243–5248
Marrero J, Rhee KY, Schnappinger D et al (2010) Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc Natl Acad Sci 107:9819–9824. doi:10.1073/pnas.1000715107
McKinney J, zu Bentrup K, Muñoz-Elías E et al (2000) Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735–738
Melo RCN, Dvorak AM (2012) Lipid body–phagosome interaction in macrophages during infectious diseases: host defense or pathogen survival strategy? PLoS Pathog 8:e1002729. doi:10.1371/journal.ppat.1002729.t001
McMurray DN, Collins FM, Dannenberg AMJ, Smith DW (1996) Pathogenesis of experimental tuberculosis in animal models. Curr Top Microbiol Immunol 215:157–179
Mishra AK, Driessen NN, Appelmelk BJ, Besra GS (2011) Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol Rev 35:1126–1157. doi:10.1111/j.1574-6976.2011.00276.x
Mishra KC, De Chastellier C, Narayana Y et al (2007) Functional role of the PE domain and immunogenicity of the Mycobacterium tuberculosis triacylglycerol hydrolase LipY. Inf Immun 76:127–140. doi:10.1128/IAI.00410-07
Mori M, Gotoh T (2004) Arginine metabolic enzymes, nitric oxide and infection. J Nutr 134:2820S–2825S
Mukamolova GV, Turapov O, Malkin J et al (2010) Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med 181:174–180. doi:10.1164/rccm.200905-0661OC
Munro S (2003) Lipid rafts: elusive or illusive? Cell 115:377–388
Muñoz Elías E, Upton A, Cherian J, McKinney J (2006) Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol 60:1109–1122
Muñoz-Elías E, McKinney J (2005) Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med 11:638–644
Murthy PS, Sirsi M, Ramakrishnan T (1962) Tricarboxylic acid cycle and related enzymes in cell-free extracts of Mycobacterium tuberculosis H37Rv. Biochem J 84:263
Naderer T, McConville MJ (2007) The Leishmania-macrophage interaction: a metabolic perspective. Cell Microbiol 10:301–308. doi:10.1111/j.1462-5822.2007.01096.x
Nathan C (2009) Taming tuberculosis: a challenge for science and society. Cell Host Microbe 5:220–224. doi:10.1016/j.chom.2009.02.004
Nathan C, Shiloh MU (2000) Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA 97:8841–8848
Nesbitt NM, Yang X, Fontan P et al (2009) A thiolase of Mycobacterium tuberculosis is required for virulence and production of androstenedione and androstadienedione from cholesterol. Inf Immun 78:275–282. doi:10.1128/IAI.00893-09
Newton HJ, Ang DKY, van Driel IR, Hartland EL (2010) Molecular pathogenesis of infections caused by legionella pneumophila. Clin Microbiol Rev 23:274–298. doi:10.1128/CMR.00052-09
Neyrolles O, Guilhot C (2011) Recent advances in deciphering the contribution of Mycobacterium tuberculosis lipids to pathogenesis. Tuberculosis 91:187–195. doi:10.1016/j.tube.2011.01.002
Neyrolles O, Hernández-Pando R, Pietri-Rouxel F et al (2006) Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS One 1:e43. doi:10.1371/journal.pone.0000043.t002
Nicholson TL, Chiu K, Stephens RS (2004) Chlamydia trachomatis lacks an adaptive response to changes in carbon source availability. Inf Immun 72:4286–4289. doi:10.1128/IAI.72.7.4286-4289.2004
Niederweis M (2008) Nutrient acquisition by mycobacteria. Microbiology 154:679–692. doi:10.1099/mic.0.2007/012872-0
Niederweis M, Danilchanka O, Huff J et al (2010) Mycobacterial outer membranes: in search of proteins. Trends Microbiol 18:109–116. doi:10.1016/j.tim.2009.12.005
Nishihara H (1954) Studies on the metabolism of the tubercle bacillus with the use of radioactive substrates in the presence and absence of streptomycin. J Biochem 41:167–181
Pandey A, Sassetti C (2008) Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci 105:4376
Paterson GK, Cone DB, Northen H et al (2009) Deletion of the gene encoding the glycolytic enzyme triosephosphate isomerase (tpi) alters morphology of Salmonella entericaserovar Typhimurium and decreases fitness in mice. FEMS Microbiol Lett 294:45–51. doi:10.1111/j.1574-6968.2009.01553.x
Pethe K, Sequeira PC, Agarwalla S et al (2010) A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nature Commun 1:1–8. doi:10.1038/ncomms1060
Peyron P, Vaubourgeix J, Poquet Y et al (2008) Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog 4:e1000204. doi:10.1371/journal.ppat.1000204.t003
Philips JA, Ernst JD (2012) Tuberculosis pathogenesis and immunity. Annu Rev Pathol Mech Dis 7:353–384. doi:10.1146/annurev-pathol-011811-132458
Price CTD, Al-Quadan T, Santic M et al (2011) Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 334:1553–1557. doi:10.1126/science.1212868
Purdy GE, Russell DG (2007) Lysosomal ubiquitin and the demise of Mycobacterium tuberculosis. Cell Microbiol 9:2768–2774. doi:10.1111/j.1462-5822.2007.01039.x
Raynaud C, Guilhot C, Rauzier J et al (2002) Phospholipases C are involved in the virulence of Mycobacterium tuberculosis. Mol Microbiol 45:203–217
Rhee KY, de Carvalho LPS, Bryk R et al (2011) Central carbon metabolism in Mycobacterium tuberculosis: an unexpected frontier. Trends Microbiol 19:307–314. doi:10.1016/j.tim.2011.03.008
Rhoades ER, Frank AA, Orme IM (1997) Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis 78:57–66
Rich AR (1946) The pathogenesis of tuberculosis, 2nd ed. Charles C Thomas, Publisher, Springfied, III, Illinois
Rieder HL (1999) Epidemiologic basis of tuberculosis control 1–162 In: international union against tuberculosis and lung disease (IUATLD)
Rohde K, Yates RM, Purdy GE, Russell DG (2007) Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev 219:37–54. doi:10.1111/j.1600-065X.2007.00547.x
Roy S, Sharma S, Sharma M et al (2004) Induction of nitric oxide release from the human alveolar epithelial cell line A549: an in vitro correlate of innate immune response to Mycobacterium tuberculosis. Immunology 112:471–480. doi:10.1046/j.1365-2567.2004.01905.x
Russell D, Barry C, Flynn J (2010) Tuberculosis: what we don’t know can, and does, hurt us. Science 328:852
Russell DG (2001) Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol 2:569–577. doi:10.1038/35085034
Russell DG (2006) Who puts the tubercle in tuberculosis? Nat Rev Micro 5:39–47. doi:10.1038/nrmicro1538
Russell DG, Cardona P-J, Kim M-J et al (2009) Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol 10:943–948. doi:10.1038/ni.1781
Sacks D, Anderson C (2004) Re-examination of the immunosuppressive mechanisms mediating non-cure of Leishmania infection in mice. Immunol Rev 201:225–238. doi:10.1111/j.0105-2896.2004.00185.x
Sakula A (1983) Robert Koch: Centenary of the discovery of the tubercle bacillus, 1882. Can Vet J 24:127–131
Sambandamurthy VK, Wang X, Chen B et al (2002) A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat Med 8:1171–1174. doi:10.1038/nm765
Sassetti CM, Boyd DH, Rubin EJ (2001) Comprehensive identification of conditionally essential genes in mycobacteria. Proc Natl Acad Sci USA 98:12712–12717. doi:10.1073/pnas.231275498
Sassetti CM, Rubin EJ (2003) Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci USA 100:12989–12994. doi:10.1073/pnas.2134250100
Sauer J-D, Bachman MA, Swanson MS (2005) The phagosomal transporter A couples threonine acquisition to differentiation and replication of Legionella pneumophila in macrophages. Proc Natl Acad Sci USA 102:9924–9929. doi:10.1073/pnas.0502767102
Savvi S, Warner DF, Kana BD et al (2008) Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids. J Bacteriol 190:3886–3895. doi:10.1128/JB.01767-07
Schaechter M, Maaløe O, Kjeldgaard NO (1958) Dependency on medium and temperature of cell size and chemical composition during balanced growth of Salmonella typhimurium. J Gen Microbiol 19:592–606. doi:10.1099/00221287-19-3-592
Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG (1998) Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol 160:1290–1296
Schar J, Stoll R, Schauer K et al (2010) Pyruvate carboxylase plays a crucial role in carbon metabolism of extra- and intracellularly replicating Listeria monocytogenes. J Bacteriol 192:1774–1784. doi:10.1128/JB.01132-09
Schnappinger D, Ehrt S, Voskuil MI et al (2003) Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J Exp Med 198:693–704. doi:10.1084/jem.20030846
Schwoppe C, Winkler HH, Neuhaus HE (2002) Properties of the Glucose-6-Phosphate transporter from Chlamydia pneumoniae (HPTcp) and the glucose-6-phosphate sensor from Escherichia coli (UhpC). J Bacteriol 184:2108–2115. doi:10.1128/JB.184.8.2108-2115.2002
Shenoy AR, Wellington DA, Kumar P et al (2012) GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336:481–485. doi:10.1126/science.1217141
Singh G, Singh G, Jadeja D, Kaur J (2010) Lipid hydrolizing enzymes in virulence: Mycobacterium tuberculosisas a model system. Crit Rev Microbiol 36:259–269. doi:10.3109/1040841X.2010.482923
Stoll R, Goebel W (2010) The major PEP-phosphotransferase systems (PTSs) for glucose, mannose and cellobiose of Listeria monocytogenes, and their significance for extra- and intracellular growth. Microbiology 156:1069–1083. doi:10.1099/mic.0.034934-0
Sturgill-Koszycki S, Schlesinger PH, Chakraborty P et al (1994) Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678–681
Tchawa Yimga M, Leatham MP, Allen JH et al (2006) Role of Gluconeogenesis and the Tricarboxylic Acid Cycle in the Virulence of Salmonella enterica Serovar Typhimurium in BALB/c Mice. Inf Immun 74:1130–1140. doi:10.1128/IAI.74.2.1130-1140.2006
Tian J, Bryk R, Itoh M et al (2005a) Variant tricarboxylic acid cycle in Mycobacterium tuberculosis: Identification of α-ketoglutarate decarboxylase. Proc Natl Acad Sci USA 102:10670
Tian J, Bryk R, Shi S et al (2005b) Mycobacterium tuberculosis appears to lack α-ketoglutarate dehydrogenase and encodes pyruvate dehydrogenase in widely separated genes. Mol Microbiol 57:859–868. doi:10.1111/j.1365-2958.2005.04741.x
Timm J, Post FA, Bekker L-G et al (2003) Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc Natl Acad Sci USA 100:14321–14326. doi:10.1073/pnas.2436197100
Titgemeyer F, Amon J, Parche S et al (2007) A genomic view of sugar transport in Mycobacterium smegmatis and Mycobacterium tuberculosis. J Bacteriol 189:5903
Torrelles JB, Schlesinger LS (2010) Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis 90:84–93. doi:10.1016/j.tube.2010.02.003
Tsai MC, Chakravarty S, Zhu G et al (2006) Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol 8:218–232. doi:10.1111/j.1462-5822.2005.00612.x
Upton A, McKinney J (2007) Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology 153:3973
van der Geize R, Yam K, Heuser T et al (2007) A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci USA 104:1947–1952. doi:10.1073/pnas.0605728104
van der Wel N, Hava D, Houben D et al (2007) M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129:1287–1298. doi:10.1016/j.cell.2007.05.059
Vandal OH, Pierini LM, Schnappinger D et al (2008) A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med 14:849–854. doi:10.1038/nm.1795
Venugopal A, Bryk R, Shi S et al (2011) Virulence of Mycobacterium tuberculosis depends on lipoamide dehydrogenase, a member of three multienzyme complexes. Cell Host Microbe 9:21–31. doi:10.1016/j.chom.2010.12.004
Via LE, Lin PL, Ray SM et al (2008) Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Inf Immun 76:2333–2340. doi:10.1128/IAI.01515-07
Vieira OV, Botelho RJ, Grinstein S (2002) Phagosome maturation: aging gracefully. Biochem J 366:689–704. doi:10.1042/BJ20020691
Vogt G, Nathan C (2011) In vitro differentiation of human macrophages with enhanced antimycobacterial activity. J Clin Investig 121:3889–3901. doi:10.1172/JCI57235
Voskuil MI, Schnappinger D, Visconti KC et al (2003) Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198:705–713. doi:10.1084/jem.20030205
Wagner T, Bellinzoni M, Wehenkel A et al (2011) Functional plasticity and allosteric regulation of α-ketoglutarate decarboxylase in central mycobacterial metabolism. Chem Biol 18:1011–1020. doi:10.1016/j.chembiol.2011.06.004
Watanabe S, Zimmermann M, Goodwin MB et al (2011) Fumarate reductase activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis. PLoS Pathog 7:e1002287. doi:10.1371/journal.ppat.1002287
Weart RB, Lee AH, Chien A-C et al (2007) A metabolic sensor governing cell size in bacteria. Cell 130:335–347. doi:10.1016/j.cell.2007.05.043
Wheeler P, Blanchard J (2005) General Metabolism and biochemical pathways of tubercle bacilli. In: Cole ST, Eisenach KD, McMurray DN, Jacobs WR Jr (ed) Tuberculosis and the tubercle bacillus. ASM Press, Washington DC
Wieland H, Ullrich S, Lang F, Neumeister B (2005) Intracellular multiplication of Legionella pneumophila depends on host cell amino acid transporter SLC1A5. Mol Microbiol 55:1528–1537. doi:10.1111/j.1365-2958.2005.04490.x
Wolf AJ, Linas B, Trevejo-Nuñez GJ et al (2007) Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol 179:2509–2519
Yam KC, D’angelo I, Kalscheuer R et al (2009) Studies of a ring-cleaving dioxygenase illuminate the role of cholesterol metabolism in the pathogenesis of Mycobacterium tuberculosis. PLoS Pathog 5:e1000344. doi:10.1371/journal.ppat.1000344.t003
Yang X, Gao J, Smith I et al (2011) Cholesterol is not an essential source of nutrition for Mycobacterium tuberculosis during infection. J Bacteriol 193:1473–1476. doi:10.1128/JB.01210-10
Yang X, Nesbitt NM, Dubnau E et al (2009) Cholesterol metabolism increases the metabolic pool of propionate in Mycobacterium tuberculosis. Biochemistry 48:3819–3821. doi:10.1021/bi9005418
Youmans GP (1979) Tuberculosis. W.B. Saunders Company, Philadelphia
Yuan Y, Li P, Ye J (2012) Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein Cell 3:173–181. doi:10.1007/s13238-012-2025-6
Zhang X, Shanmugam KT, Ingram LO (2010) Fermentation of glycerol to succinate by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 76:2397–2401. doi:10.1128/AEM.02902-09
Acknowledgments
We thank Werner Goebel for critical reading of the manuscript, insightful comments, and valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Ehrt, S., Rhee, K. (2012). Mycobacterium Tuberculosis Metabolism and Host Interaction: Mysteries and Paradoxes. In: Pieters, J., McKinney, J. (eds) Pathogenesis of Mycobacterium tuberculosis and its Interaction with the Host Organism. Current Topics in Microbiology and Immunology, vol 374. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2012_299
Download citation
DOI: https://doi.org/10.1007/82_2012_299
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40231-9
Online ISBN: 978-3-642-40232-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)