Abstract
Programmed cell death-1 (PD-1) is a member of the CD28 superfamily that delivers negative signals upon interaction with its two ligands, PD-L1 or PD-L2. PD-1 and its ligands are broadly expressed and exert a wider range of immunoregulatory roles in T cells activation and tolerance compared with other CD28 family members. Subsequent studies show that PD-1–PD-L interaction regulates the induction and maintenance of peripheral tolerance and protect tissues from autoimmune attack. PD-1 and its ligands are also involved in attenuating infectious immunity and tumor immunity, and facilitating chronic infection and tumor progression. The biological significance of PD-1 and its ligand suggests the therapeutic potential of manipulation of PD-1 pathway against various human diseases. In this review, we summarize our current understanding of PD-1 and its ligands ranging from discovery to clinical significance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Experimental Autoimmune Encephalomyelitis
- Simian Immunodeficiency Virus
- Myelin Oligodendrocyte Glycoprotein
- Chronic Viral Infection
- Anergy Induction
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Identification of PD-1 and Its Ligands
In 1992, programmed cell death-1 (PD-1) was identified as a molecule whose expression was strongly induced upon apoptotic stimuli (Ishida et al. 1992). Based on the observation that LyD9 (hematopoietic progenitor cell line) and 2B4.11 cell lines (T-cell hybridoma) died of apoptosis upon interleukin-3 deprivation and phorbol 12-myristate 13-acetate (PMA) and ionomycin treatment, respectively, and that apoptosis of both cell lines required de novo RNA and protein synthesis, Honjo and colleagues performed subtractive-hybridization to identify the gene(s) that plays critical roles in apoptosis. A complementary DNA (cDNA) library of dying LyD9 cells subtracted with messenger RNA (mRNA) of resting LyD9 cells was screened by a labeled cDNA library of dying 2B4.11 cells subtracted with mRNA of resting LyD9 cells. Four clones were isolated and all of them encoded PD-1 cDNA. Deduced amino acid sequence predicted that PD-1 is a type I transmembrane protein with a single IgV domain in the extracellular region. However, overexpression of PD-1 cDNA in these cell lines failed to induce apoptosis (Agata et al. 1996) and the function of PD-1 was intangible for many years. In 1999, Honjo and colleagues found that PD-1 negatively regulates immune responses based on the observation that PD-1-deficient mice spontaneously developed lupus-like arthritis and glomerulonephritis (Nishimura et al. 1999).
The identification of PD-1 ligands rests on several chance events. Honjo and colleagues collaborated with Genetic Institute to identify the ligand of PD-1 using the Biacore system. At that time, Freeman in Harvard University identified a B7-like molecule (clone 129) by database search and collaborated with Genetic Institute independently. The group in Genetic Institute accidentally found that these molecules interacted with each other. T cells from PD-1 sufficient but not PD-1-deficient mice showed lower proliferative response against anti-CD3 antibody stimulation in the presence of 129-Ig chimeric protein. Based on these physical and functional experiments, clone 129 was confirmed to be the ligand of PD-1 and was named PD-L1 (Pdcd1lg1) for programmed cell death 1 ligand 1 (Freeman et al. 2000). Later, PD-L1 was endowed CD number 274. The collaboration further identified another ligand, PD-L2 (Pdcd1lg2, CD273) (Latchman et al. 2001). The identification of PD-L1 added PD-1 to the list of CD28 family (Fig.1). At the same time, the other groups reported B7-H1 and B7-DC, which were identical to PD-L1 and PD-L2, respectively, costimulated T cells, the mechanisms of which still remain unknown (Dong et al. 1999; Tseng et al. 2001).
2 Structure of PD-1 and Its Ligands
PD-1 is a 50–55kDa type I transmembrane glycoprotein composed of an IgV domain sharing 21–33% sequence identity with CTLA-4, CD28, and inducible costimulatory molecule. Unlike CTLA-4 that forms homodimer, PD-1 lacks the membrane proximal cysteine residue required for homodimerization of other members of the CD28 family and is supposed to exist as monomer on the cell surface (Zhang et al. 2004). The PD-1 cytoplasmic region has two tyrosine residues, the membrane–proximal one constitutes an immunoreceptor tyrosine-based inhibitory motif (ITIM) and the other an immunoreceptor tyrosine-based switch motif (ITSM), which is essential for the inhibitory function of PD-1 (Long 1999; Sidorenko and Clark 2003). The ligands of PD-1 (PD-L1 and PD-L2, PD-Ls) are type I transmembrane glycoproteins composed of IgC and IgV domains. The amino acid identity between PD-L1 and PD-L2 is about 40%, while the amino acid identity between PD-Ls and B7s is about 20%. The amino acid identities between human and murine orthologues of PD-Ls are about 70%. B7-1 is also reported to associate with PD-L1 and transduce inhibitory signal (Butte et al. 2007). However, the precise mechanism how PD-L1 transduces inhibitory signals is currently unknown.
Recently, the crystal structures of PD-1 and PD-Ls were clarified (Fig.2) (Zhang et al. 2004; Lazar-Molnar et al. 2008; Lin et al. 2008). In contrast to CTLA-4 that uses a hydrophobic sequence, MYPPY, in the loop connecting F and G β-strands (FG loop, corresponding to CDR3 region of antigen receptors) of its IgV domain to bind B7-1 and B7-2, PD-1 uses its front β-face (AGFCC′ β-strands) to bind to the β-face of PD-L1 (AGFCC′) or PD-L2 (AGFC). This face-to-face interaction of PD-1 and PD-Ls makes the contact area (1,870Å2) larger than that of CTLA-4 and B7s (~1,200Å2) and makes the PD-1/PD-Ls complex more compact (76Å and 100Å for PD-1/PD-Ls and B7-1/CTLA-4, respectively). The total length of receptors and ligands should be compatible with the dimension of pMHC/TCR complex (~140Å) in the immunological synapse. The longer linker segment connecting PD-1 IgV domain and transmembrane region (20 a.a. and 6 a.a. for PD-1 and CTLA-4, respectively) may help adjust the total size of the PD-1/PD-Ls complex allowing its appropriate co-localization with pMHC/TCR complex. The structure of PD-1 and PD-Ls showed high similarity to those of T-cell receptor (TCR), antibody, and CD8 dimer. Especially, CDR-like loops (BC, C′C″, and FG loops for CDR1, 2, and 3, respectively) of PD-1/PD-Ls are in positions similar to those of the antigen-binding loops of TCR and antibody. Because TCRs and antibodies bind antigens and CD8 dimers bind major histocompatibility complex (MHC) class I using these loops, it is possible that these loops of PD-1/PD-Ls complex may bind another molecule.
Crystal structure of PD-1/PD-L1 and PD-1/PD-L2 complexes. (a) The structure of PD-1/PD-L1 complex; the loop at the ends of the PD-1 domain and the first domain of PD-L1 are located on the same side of the complex. The strands of the two β-sheets of PD-1 are labeled ABED and A′GFCC′C″. The strands of the two β-sheets of PD-L1 V domain are labeled AGFCC′C″ and BED. (b) The structure of PD-1/PD-L2 complex; the face-to-face interaction of PD-1 and PD-L2 makes the large contact area and the PD-1/PD-L2 complex more compact. The strands of PD-1 and PD-L2 are labeled in red and blue, respectively. Figures are taken from the original paper (Lazar-Molnar et al. 2008; Lin et al. 2008) with permission
3 Regulation of PD-1 Expression
PD-1 can be expressed on CD4 and CD8 T cells, B cells, monocytes, natural killer (NK) cells, and dendritic cells (DCs) (Agata et al. 1996; Yamazaki et al. 2002; Keir et al. 2008). PD-1 is not expressed on resting T cells but is inducibly expressed within 24h after stimulation (Chemnitz et al. 2004). PD-1 expression can also be induced on antigen-presenting cells (APCs) on myeloid CD11c+ DCs and monocytes (Petrovas et al. 2006). Recently, it was found that PD-1 expression was elevated on NKT cells in response to CD1d-restricted lipid antigen (Moll et al. 2009). The two known ligands for PD-1, PD-L1 (B7-H1; CD274), and PD-L2 (B7-DC; CD273) have differential expression. PD-L1 is constitutively expressed on T and B cells, DCs, macrophages, mesenchymal stem cells, and bone marrow-derived mast cells (Yamazaki et al. 2002). In addition, PD-L1 is expressed on a wide variety of nonhematopoietic cells including lung, vascular endothelium, liver nonparenchymal cells, mesenchymal stem cells, pancreatic islets, and keratinocytes (Keir et al. 2008). In contrast, the expression of PD-L2 is restricted to activated DCs, macrophages, bone marrow-derived mast cells, and more than 50% of peritoneal B1 cells (Zhong et al. 2007). The comparative studies with PD-L1- or PD-L2-deficient mice and with blocking antibody against PD-L1 and PD-L2 demonstrate overlapping inhibitory functions on APCs and different features on tissues for these two ligands (Kanai et al. 2003; Matsumoto et al. 2004; Keir et al. 2006; Habicht et al. 2007).
The broader expressions of PD-1 and its ligands suggest that PD-1 regulates a wider spectrum of immune response compared with other CD28 family members. The spontaneous development of autoimmune diseases by PD-1-deficient mice implies that PD-1 is involved in the establishment and maintenance of immunological tolerance (Nishimura et al. 1999, 2001). In the thymus, PD-L1 is expressed broadly in the cortex, whereas PD-L2 expression is restricted to medullary stromal cells (Brown et al. 2003; Liang et al. 2003). PD-1 is expressed on CD4−CD8− double-negative thymocytes and is required for the normal selection of thymocytes (Nishimura et al. 2000). PD-1 expression is upregulated after TCR ligation on CD4+CD8+ double-positive thymocytes, and PD-1 can participate in selection of the αβ TCR repertoire by controlling TCR signaling thresholds. PD-1–PD-L1 interactions modulate positive selection (Keir et al. 2005), and PD-1 also regulates negative selection (Blank et al. 2003). Collectively, these findings implicate that PD-1 and its ligand play a crucial role in central as well as peripheral tolerance. PD-L1 and PD-L2 is also expressed on placental syncytiotrophoblasts and vascular endothelial cells, respectively (Guleria et al. 2005). PD-L1 is differentially expressed across gestation and functions in the placenta to induce fetal–maternal tolerance (Guleria et al. 2005; Holets et al. 2006). PD-L1 is expressed constitutively in the cornea, and PD-1–PD-L1 interaction protects the eye from activated T cells (Hori et al. 2006; Meng et al. 2006; Watson et al. 2006; Sugita et al. 2009). So, PD-1-PD-L pathway may protect immune-privileged sites, such as the placenta and the eye, from immune responses. Given that PD-L is found on various tumor cells and PD-1 expression is upregulated and sustained on virus-specific T cells during chronic viral infection, PD-1-PD-L pathway may play important roles in tumor immunity and infectious immunity, which are addressed in depth in the following section of this review.
PD-1 expression is induced by TCR- or B-cell receptor (BCR)-mediated signaling and is augmented by stimulation with tumor necrosis factor (TNF) (Nakae et al. 2006). Particularly, the expression of PD-1 on virus-specific CD8 T cells is dependent upon continued epitope recognition during chronic infections (Blattman et al. 2009). However, molecular mechanism for the regulation of PD-1 expression relatively remains unclear. Recent study demonstrates that NFATc1 is a critical transcription factor in promoting the induction of PD-1 expression following T cells activation (Oestreich et al. 2008), and interferon (IFN)-sensitive responsive element and STAT1/2 are primarily responsible for the regulation of constitutive and IFN-α-mediated PD-1 expression on macrophages (Cho et al. 2008). PD-L1 is upregulated by IFN-α, IFN-β, and IFN-γ (Eppihimer et al. 2002; Schreiner et al. 2004). IL-4 and granulocyte macrophage colony-stimulating factor stimulate the expression of PD-L2 on DCs, and IL-10 can induce the expression of PD-L1 on monocytes (Selenko-Gebauer et al. 2003). Analyses of PD-L1 promoter show that PD-L1 expression is dependent on two IFN regulatory factor-1 binding sites (Lee et al. 2006b). In addition, MyD88, TRAF6, MEK, and JAK2 have been implicated in signaling pathway for PD-L1 expression (Lee et al. 2006b; Liu et al. 2007; Parsa et al. 2007).
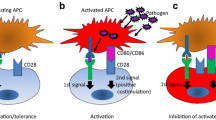
4 PD-1 Signaling: Molecular Mechanisms of Inhibition
ITSM (TxYxxL) is essential for the inhibitory function of PD-1. The inhibitory mechanism of PD-1 was initially analyzed in B cell line, IIA1.6 by using a chimeric molecule of PD-1 cytoplasmic region and FcγRIIB extracellular region (FcPD) (Okazaki et al. 2001). In IIA1.6 cells, crosslinking of BCRs induced strong Ca2+ mobilization, which was almost completely suppressed by co-crosslinking of FcPD and BCR. Tyrosine residues in both ITIM and ITSM of PD-1 were phosphorylated by Lyn upon BCR crosslinking and the phosphorylated tyrosine residue in ITSM but not ITIM recruited SHP-2 (SH2-domain containing tyrosine phosphatase 2) through its SH2 domain (Fig.3a). Then SHP-2 dephosphorylated BCR-proximal signaling molecules including Igα/β and Syk, which attenuated the activation of downstream molecules including PLCγ2, PI3K, vav, and ERK1/2. Later, two groups reported that PD-1 inhibited TCR signaling by similar mechanisms (Fig.3b) (Chemnitz et al. 2004; Sheppard et al. 2004; Parry et al. 2005). Interestingly, one of them reported that phosphorylated ITSM recruited SHP-1 in addition to SHP-2 in T cells, while the other group reported that SHP-1 associated with phosphorylated ITSM only in the artificial system using synthetic peptide. SHP-1 may also bind to phosphorylated ITSM of PD-1 but its contribution to the inhibitory function of PD-1 can be much less compared to that of SHP-2. ITSM has been first defined in CD150 (SLAM) for its dual function (Sidorenko and Clark 2003). CD150 preferentially recruits either SHIP or SHP-2 in the presence or absence of SAP (SH2D1A), a small SH2-domain-containing adaptor protein, respectively. Because ITSM of PD-1 has been reported to be unable to bind SAP, it is currently unknown whether ITSM of PD-1 can really “switch” to transduce positive signal or not.
Signaling of programmed cell death-1 (PD-1) pathway. (a) Upon B-cell receptor (BCR) crosslinking, the phosphorylated tyrosine residue in immunoreceptor tyrosine-based switch motif (ITSM) recruits SHP-2. And then, SHP-2 dephosphorylates BCR-proximal signaling molecules including Syk, which attenuates the activation of downstream molecules, such as PLCγ2, PI3K, and ERK. (b) PD-1 engagement on T cell surface also leads to phosphorylation of PD-1 cytoplasmic tyrosines and increases SHP-2 association with the ITSM of PD-1. Recruitment of SHPP-2 dephosphorylates signaling through the PI3K or Zap70 pathways
PD-1 signal has been shown to play critical roles in the induction of anergy and the development of induced regulatory T cells (iTregs). Recently, Francisco et al. reported that engagement of PD-1 preferentially induced the development of iTregs (Francisco et al. 2009). In addition, PD-1 signal maintained FoxP3 expression and enhanced the efficiency of their suppressive function. During the induction of iTregs by PD-1, they found the downregulation of phopho-Akt, mTOR, S6, and ERK2 and concomitant upregulation of PTEN, suggesting that PD-1 signal promoted the development of iTregs by antagonizing the Akt-mTOR signaling pathway. The mechanism of anergy induction is also becoming clearer. Chikuma et al. reported that PD-1-sufficient but not PD-1-deficient 2C CD8 T cells could be anergyzed by single injection of an antigenic peptide in vivo (Chikuma et al. 2009). Interestingly, PD-1-deficient 2C T cells preferentially produced IL-2 upon anergy induction. The blockade of IL-2 during the anergy induction phase prevented the anergy resistance of PD-1-deficient 2C T cells and the IL-2 complementation resulted in anergy resistance in PD-1-sufficient 2C T cells. Therefore, PD-1 may induce anergy by negatively regulating autonomous production of IL-2 by CD8 T cells. Bishop et al. also found that PD-1 induced anergy on CD4 T cells by regulating IL-2 production using an in vitro model of A.E7 T cells (Bishop et al. 2009). Further studies are required to understand the precise molecular mechanisms how PD-1 regulate the production of IL-2.
5 Biological Significance of PD-1
As mentioned above, PD-1 and its ligands are broadly expressed and exert a vital and diverse range of immunoregulatory roles in T cells activation and tolerance. In this section, to address the biological significance of PD-1 pathway, we summarize our current understanding of the roles of PD-1 and its ligands in disease model including autoimmunity, chronic viral infection, and tumor.
5.1 PD-1 in Autoimmunity
Involvement of PD-1 in autoimmunity was first demonstrated by the autoimmune phenotype of PD-1-deficient mice (Nishimura et al. 1999, 2001; Okazaki et al. 2003). PD-1 deficiency results in the development of a spontaneous, late-onset lupus-like disease with deposition of IgG3 in the glomeruli and a dilated cardiomyopathy owing to the production of an autoantibody against cardiac troponin-I. Interestingly, different target organs were affected by the autoimmune response when the genetic background of the PD-1-deficient mice was replaced by backcrossing. For example, PD-1-deficient mice developed lupus-like glomerulonephritis and arthritis, dilated cardiomyopathy and gastritis, subacute type I diabetes, and lethal myocarditis on the C57BL/6, BALB/c, nonobese diabetic (NOD), and MRL backgrounds, respectively (Nishimura et al. 1996, 2001; Wang et al. 2005, 2010). Studies of mouse models of autoimmunity further emphasize important immunoregulatory functions for PD-1 and its ligands. In the NOD mouse model of autoimmune diabetes, PD-L1 is upregulated in the pancreas on islet cells (Liang et al. 2003). Administration of anti-PD-1 or anti-PD-L1 to prediabetic NOD mice leads to rapid and exacerbated diabetes, which is associated with accelerated insulitis and proinflammatory cytokine production by T cells (Ansari et al. 2003). Compared to the blockade of CTLA-4, which exaggerates diabetes only in the neonates, PD-1/PD-L1 blockade exaggerates diabetes both in neonates and in older mice, indicating that PD-1–PD-L1 interactions regulate both the initiation and the progression of autoimmune diabetes in NOD mice. In the experimental autoimmune encephalomyelitis (EAE) model, PD-1 and its ligand also suppress EAE. The administration of anti-PD-1 during the induction of EAE accelerates the onset and increases the severity of EAE with increased frequency of IFN-γ-producing myelin oligodendrocyte glycoprotein (MOG)-reactive T cells and more MOG-specific antibodies in serum (Salama et al. 2003). Interestingly, the blockade of PD-L1 but not PD-L2 exacerbates EAE in BALB/c and SJL/J mice, whereas only PD-L2 blockade markedly worsen EAE in C57BL/6 mice (Salama et al. 2003; Zhu et al. 2006). This strain-specific effect of antibody-mediated blockade of PD-1 ligand cannot be explained by expression of PD-L1 or PD-L2 because their expressions vary little in lymphoid APCs and spinal cord tissues among different strains (Zhu et al. 2006). However, adoptive transfer studies demonstrate the critical role of PD-L1 on T cells and host tissues in restraining myelin-reactive pathogenic effector T cells in EAE (Latchman et al. 2004).
The involvement of PD-1 in human autoimmune diseases has also become evident. Prokunina et al. reported that the allele A of a single nucleotide polymorphisms (SNPs) named PD1.3 (PD1.3A) in intron 4 of PD-1 gene was associated with the development of systemic lupus erythematosus (SLE) in Europeans (relative risk = 2.6) and Mexicans (relative risk = 3.5) but not African Americans (Prokunina et al. 2002). To date, the PD1.3 and several other SNPs on PD-1 gene have been reported to link with the development of various autoimmune diseases including type I diabetes, progressive multiple sclerosis (MS), rheumatoid arthritis, Graves disease, and ankylosing spondylitis (Nielsen et al. 2003; Kroner et al. 2005; Lee et al. 2006a; Okazaki and Honjo 2007). The PD1.3 locates on the binding site for the runt-related transcription factor 1 (RUNX1) and PD1.3A interferes the binding of RUNX1 resulting in the impaired induction of PD-1 (Bertsias et al. 2009). This polymorphism may alter PD-1 mRNA stability or expression level and is associated with reduced PD-1-mediated inhibition of IFN-γ production in MS patients (Kroner et al. 2005). Studies of human with autoimmune diseases also suggest important regulatory function of PD-1 and its ligands. Patients with MS treated with IFN-β, the principle immune-modulatory agent for the treatment of MS, in vivo for 6months have eightfold more PD-L1 mRNA transcript than before treatment, suggesting that part of the anti-inflammatory effect of IFN-β treatment is due to PD-L1 expression (Schreiner et al. 2004). Autoantibodies to PD-L1 have been found in patients with rheumatoid arthritis and correlate with the progression of disease, indicating that autoantibodies can block the inhibitory function of the PD-1-PD-L pathway and thus contribute to the development of autoimmune disease (Dong et al. 2003).
Based on the important role of PD-1-PD-L pathway in autoimmunity, this pathway has become a new therapeutic target for ameliorating autoimmune disease by increasing the expression of PD-L or triggering PD-1. Even though these approaches are just beginning in animal models, the results appear promising. Hirata et al. genetically modified mouse embryonic stem (ES) cells to express surface PD-L1 and MOG in MHC class II (Hirata et al. 2005). They next differentiated the cells to DCs and transferred the cells into mice with EAE. These genetically modified DCs overexpressing PD-L1 and MOG dramatically ameliorated clinical EAE and reduced severity of central nervous system inflammation. Furthermore, Ding et al. tried to suppress lupus-like syndrome in BXSB mice by delivering recombinant adenovirus expressing full-length PD-L1 gene (rAd.PD-L1) (Ding et al. 2006). Intravenous injection of rAd.PD-L1 partially prevented the development of nephritis as evidenced by the lower frequency of proteinuria, reduced amount of serum anti-dsDNA IgG, and better renal pathology. Further analyses may enable us to establish new therapeutic strategies for autoimmune disease by manipulating the PD-1-PD-L pathway.
5.2 PD-1 in Chronic Viral Infection
During chronic viral infection, virus-specific CD8 T cells become unresponsive to viral antigens and persist in a nonfunctional exhausted state (Wherry and Ahmed 2004). These exhausted CD8 T cells are characterized by a hierarchical and progressive loss of function with cytotoxicity and IL-2 production lost first, followed by TNF-α and IFN-γ cytokine production (Wherry and Ahmed 2004). Since CD8 T-cell exhaustion was characterized in the murine lymphocyte choriomeningitis virus (LCMV), such a functional impairment has been a common feature in human chronic viral infections such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) (Wherry et al. 2003; Klenerman and Hill 2005; Shin and Wherry 2007). These functional defects in responding T cells are probably a primary reason for failure of immunological control of these persisting pathogens. We initially have shown that PD-1 is highly expressed on exhausted CD8 T cells in LCMV system, and that PD-1-PD-L pathway plays a major role in regulating T cells exhaustion during this infection (Barber et al. 2006). PD-1 is transiently expressed by virus-specific CD8 T cells after acute LCMV infection and rapidly downregulated, whereas CD8 T cells retain high PD-1 expression in lymphoid and nonlymphoid tissues throughout chronic LCMV infection. Subsequently, several groups have shown that PD-1 is highly expressed on simian immunodeficiency virus (SIV)-specific (Velu et al. 2007), HIV-specific (Day et al. 2006; Petrovas et al. 2006; Trautmann et al. 2006), HBV-specific (Boettler et al. 2006; Boni et al. 2007), and HCV-specific (Urbani et al. 2006; Kasprowicz et al. 2008) T cells. The level of PD-1 expression per cell is important in regulating T-cell exhaustion during chronic viral infections (Freeman et al. 2006). The percentage of HIV-specific CD8 T cells expressing PD-1 correlates with viral load, declining CD4 counts, and decreased capacity of CD8 T cell to proliferate (Day et al. 2006; D’Souza et al. 2007). HCV-specific CD8 T cells during persistent infection also display impaired ability of T cells to proliferate and produce cytokines, which correlate with PD-1 expression level (Golden-Mason et al. 2007; Nakamoto et al. 2008). So, PD-1 may serve as a useful marker on virus-specific CD8 T cells to indicate the degree of T-cell exhaustion and disease severity.
The mechanisms of PD-1 regulation in exhausted T cells are still poorly defined. In a longitudinal study of HIV-infected subjects, PD-1 expression declined on T cells specific for epitopes that had undergone mutational escape, whereas PD-1 expression was highly increased over time on those specific for conserved epitope (Streeck et al. 2008). These data indicate that continued antigen-specific TCR stimulation plays an important role in modulating PD-1 expression in HIV infection. In addition, viral protein is known to contribute to TCR-independent upregulation of PD-1. The accessory Nef protein of HIV was shown to upregulate PD-1 through a p38 mitogen-activated protein kinase-dependent mechanism during infection (Muthumani et al. 2008). Furthermore, HCV-core protein binding to the complement receptor gC1q is responsible for inducing the expression of PD-1 on T cells (Yao et al. 2007).
The key role of PD-1-PD-L pathway in CD8 T-cell exhaustion during chronic viral infections drive development of strategies to manipulate the interaction of PD-1 and its ligands for the reversal of exhausted CD8 T cells and viral control. In mice, blocking PD-1 pathway with anti-PD-L1 antibody restored cytokine production, augmented the generation of LCMV-specific T cells and, most importantly, led to a dramatic reduction in viral load (Barber et al. 2006). During SIV infection in nonhuman primate, PD-1 blockade using anti-PD-1 antibody resulted in rapid expansion of virus-specific CD8 T cell with improved functionality, which was associated with significant reduction in plasma viral load (Velu et al. 2009). Furthermore, blocking PD-L1 with a monoclonal antibody led to increased HIV-specific T-cell proliferation, and production of TNF-α, IFN-γ, and granzyme B (Day et al. 2006; Petrovas et al. 2006; Trautmann et al. 2006). However, given that blocking or eliminating PD-1 or its ligands can accelerate autoimmune disease, we must better understand the immunoregulatory roles of PD-1-PD-L pathway to determine how to modulate this pathway to effectively activate virus-specific T cells while minimizing the risk of immunopathology.
5.3 PD-1 in Antitumor Immunity
There are accumulating evidences that tumors exploit PD-1-dependent immune suppression for immune evasion. The expression of PD-L1 and PD-L2 has been found on a wide variety of solid tumors and hematologic malignancies. In addition, PD-1 expression on tumor infiltrating lymphocytes has been reported, suggesting that these T cells are functionally exhausted. Strikingly, a strong correlation between PD-Ls expression on tumor cells and unfavorable prognosis has been demonstrated for various cancers including kidney, ovarian, esophageal, bladder, gastric, and pancreatic cancers and melanoma (Thompson et al. 2004; Ohigashi et al. 2005; Wu et al. 2006; Hamanishi et al. 2007; Nakanishi et al. 2007; Nomi et al. 2007; Hino et al. 2010). Thompson et al. have analyzed the expression of PD-L1 on renal cell carcinoma and found that patients with high tumor and/or lymphocyte PD-L1 levels were 4.5 times more likely to die of their cancer than patients exhibiting low levels of PD-L1 expression (Thompson et al. 2004). Hamanishi et al. reported that patients with tumors positive for both PD-L1 and PD-L2 showed dramatically lower survival rate than patients with tumors negative for both of these ligands (46% vs. 83% for 5-year survival) (Hamanishi et al. 2007).
To date, many groups reported that PD-Ls on tumor cells suppressed antitumor immunity by inhibiting T-cell activation and lysis of tumor cells, or inducing apoptosis of tumor-specific T cells (Iwai et al. 2002; Curiel et al. 2003; Hirano et al. 2005). PD-Ls on tumor-associated DCs also suppressed the activation of antitumor T cells. Accordingly, the efficacy of PD-1 blockade in tumor eradication has been demonstrated in various experimental systems. The blocking strategies used include blocking antibodies against PD-1 and PD-L1, DNA vaccination of the extracellular region of PD-1, genetic ablation of PD-1 gene, RNA interference, and recombinant protein of the extracellular region of PD-1 and PD-L1 (Iwai et al. 2002, 2005; Curiel et al. 2003; Blank et al. 2004; He et al. 2004; Hirano et al. 2005; Terawaki et al. 2007; Borkner et al. 2010).
Currently, two monoclonal antibodies against PD-1 are in clinical trials for cancer and hepatitis C virus infection. ONO-4538/MDX-1106 is a fully human IgG4 monoclonal antibody against human PD-1. Phase I clinical trial of ONO-4538/MDX-1106 has been performed on 39 patients with nonsmall-cell lung cancer, renal cell carcinoma, colorectal cancer, melanoma, and prostate cancer (Brahmer et al. 2010). ONO-4538/MDX-1106 was well tolerated although one serious adverse event, inflammatory colitis was observed. One patient with colorectal cancer had a complete response and two patients with renal cell carcinoma and melanoma had partial responses (>30% regression) (Fig.4). In addition, significant lesional tumor regressions not meeting PR criteria were observed in two patients with melanoma and nonsmall-cell lung cancer. CT-011, which was originally generated as a monoclonal antibody against B lymphoblastoid cells and developed as a drug for cancer for its lymphocyte-activating and tumor-regressing activities, turned out to recognize PD-1 (Berger et al. 2008). Phase I clinical trial of CT-011 has been performed on six patients (follicular B-cell lymphoma, chronic lymphocytic leukemia, Hodgkin’s lymphoma, multiple myeloma, and acute myeloid leukemia) and no severe adverse events were observed. Clinical benefit was observed in 33% of the patients with one patient with follicular B-cell lymphoma showed complete remission. One minimal response was observed in an acute myeloid leukemia patient. The other four patients have shown stable disease for >35weeks.
Tumor regression in patient with metastatic renal cell carcinoma (RCC) and melanoma after repeated dosing with anti-PD-1 monoclonal antibody (MDX-1106). (a) Patient with RCC with regression of metastases in mediastinal lymph nodes after receiving three doses of MDX-1106. (b) Patient with melanoma experienced a partial response after receiving 11 doses of MDX-1106. Biopsies of a regressing axillary lymph node metastasis infiltrated with CD8 T cells. Figures are taken from the original paper (Brahmer et al. 2010) with permission
Further clinical studies are expected to reveal the efficacy of PD-1 blocking antibodies for the treatment of cancer. A low-molecular compound that can block PD-1 signal more efficiently may also appear based on the crystal structure of PD-1/PD-Ls. In addition, combinatorial treatments of PD-1 blockade and other immunotherapies including cytokine therapies, vaccination with tumor-associated antigens, blockade of other immunoregulatory molecules, infusion of activated DCs, and depletion of Tregs may help further improve therapeutic potential against tumors.
6 Conclusions
Since PD-1 was initially identified in 1992, accumulating evidences suggest that PD-1 and its ligands are key regulators in T cells activation and tolerance followed by induction and maintenance of peripheral tolerance. Subsequently, it became clear that PD-1 pathway plays crucial roles in the regulation of autoimmunity, transplantation immunity, infectious immunity, and tumor immunity. This biological significance of PD-1 and its ligands sheds light on the development of therapeutic strategies against clinical incurable diseases by manipulating the PD-1 pathway. Indeed, many groups are trying to develop not only PD-1 antagonists for the treatment of cancer and infectious disease but also PD-1 agonist for the treatment of autoimmune disease and transplantation rejection (Fig.5). However, it still remains unclear how the expressions of PD-1 and its ligands are spatially and temporally regulated and what are the molecular mechanisms of signaling through PD-1 and its ligands. It is important to understand how PD-1 pathway mediates its inhibitory pathways. Recent observations have revealed that other inhibitory receptors, such as 2B4, LAG-3, CTLA-4, PirB, GP49, and CD160, were co-expressed on exhausted CD8 T cells during chronic infections (Blackburn et al. 2009). There is considerable diversity in the number and type of inhibitory receptors that can be expressed by T cells during virus infection, and these diverse inhibitory pathways appear to cooperate with PD-1 pathway in regulating T-cell function. Thus, further studies are required to address whether these diverse inhibitory receptors as well as PD-1 are also involved in the coregulation of autoimmunity and tumor immunity. Such studies will not only provide a better mechanistic understanding of the PD-1 pathway in regulating T cell responses but will also facilitate precise manipulation of this pathway therapeutically.
Biological significance of PD-1-PD-L pathway and its clinical application. Blockade of PD-1 pathway with antagonist antibody, such as anti-PD-1 or anti-PD-L, augment immune responses and may be useful in the treatment of cancer and infectious disease. Triggering PD-1 pathway with agonist antibody or by delivery of PD-L gene/protein suppress adverse immune responses and can be used for the treatment of autoimmune disease, allergy, and transplant rejection
References
Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T (1996) Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 8:765–772
Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H Jr, Sayegh MH (2003) The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 198:63–69
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687
Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A (2008) Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14:3044–3051
Bertsias GK, Nakou M, Choulaki C, Raptopoulou A, Papadimitraki E, Goulielmos G, Kritikos H, Sidiropoulos P, Tzardi M, Kardassis D, Mamalaki C, Boumpas DT (2009) Genetic, immunologic, and immunohistochemical analysis of the programmed death 1/programmed death ligand 1 pathway in human systemic lupus erythematosus. Arthritis Rheum 60:207–218
Bishop KD, Harris JE, Mordes JP, Greiner DL, Rossini AA, Czech MP, Phillips NE (2009) Depletion of the programmed death-1 receptor completely reverses established clonal anergy in CD4(+) T lymphocytes via an interleukin-2-dependent mechanism. Cell Immunol 256:86–91
Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ (2009) Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29–37
Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF (2003) Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J Immunol 171:4574–4581
Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF (2004) PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res 64:1140–1145
Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R (2009) Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol 83:4386–4394
Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE, Thimme R (2006) Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol 80:3532–3540
Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C (2007) Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 81:4215–4225
Borkner L, Kaiser A, van de Kasteele W, Andreesen R, Mackensen A, Haanen JB, Schumacher TN, Blank C (2010) RNA interference targeting programmed death receptor-1 improves immune functions of tumor-specific T cells. Cancer Immunol Immunother 59(8):1173–1183
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28:3167–3175
Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ (2003) Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 170:1257–1266
Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ (2007) Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27:111–122
Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL (2004) SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 173:945–954
Chikuma S, Terawaki S, Hayashi T, Nabeshima R, Yoshida T, Shibayama S, Okazaki T, Honjo T (2009) PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol 182:6682–6689
Cho HY, Lee SW, Seo SK, Choi IW, Choi I (2008) Interferon-sensitive response element (ISRE) is mainly responsible for IFN-alpha-induced upregulation of programmed death-1 (PD-1) in macrophages. Biochim Biophys Acta 1779:811–819
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W (2003) Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9:562–567
Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354
Ding H, Wu X, Wu J, Yagita H, He Y, Zhang J, Ren J, Gao W (2006) Delivering PD-1 inhibitory signal concomitant with blocking ICOS co-stimulation suppresses lupus-like syndrome in autoimmune BXSB mice. Clin Immunol 118:258–267
Dong H, Zhu G, Tamada K, Chen L (1999) B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5:1365–1369
Dong H, Strome SE, Matteson EL, Moder KG, Flies DB, Zhu G, Tamura H, Driscoll CL, Chen L (2003) Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest 111:363–370
D'Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, Wilson CC, Connick E, Palmer BE (2007) Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol 179:1979–1987
Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, Leonard JP (2002) Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 9:133–145
Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 206:3015–3029
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034
Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH (2006) Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med 203:2223–2227
Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR (2007) Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol 81:9249–9258
Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH (2005) A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med 202:231–237
Habicht A, Kewalaramani R, Vu MD, Demirci G, Blazar BR, Sayegh MH, Li XC (2007) Striking dichotomy of PD-L1 and PD-L2 pathways in regulating alloreactive CD4(+) and CD8(+) T cells in vivo. Am J Transplant 7:2683–2692
Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S (2007) Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 104:3360–3365
He YF, Zhang GM, Wang XH, Zhang H, Yuan Y, Li D, Feng ZH (2004) Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J Immunol 173:4919–4928
Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y (2010) Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 116:1757–1766
Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L (2005) Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65:1089–1096
Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y (2005) Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J Immunol 174:1888–1897
Holets LM, Hunt JS, Petroff MG (2006) Trophoblast CD274 (B7-H1) is differentially expressed across gestation: influence of oxygen concentration. Biol Reprod 74:352–358
Hori J, Wang M, Miyashita M, Tanemoto K, Takahashi H, Takemori T, Okumura K, Yagita H, Azuma M (2006) B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol 177:5928–5935
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11:3887–3895
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99:12293–12297
Iwai Y, Terawaki S, Honjo T (2005) PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol 17:133–144
Kanai T, Totsuka T, Uraushihara K, Makita S, Nakamura T, Koganei K, Fukushima T, Akiba H, Yagita H, Okumura K, Machida U, Iwai H, Azuma M, Chen L, Watanabe M (2003) Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. J Immunol 171:4156–4163
Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, McMahon C, Reyor LL, Elias N, Kwok WW, McGovern BG, Freeman G, Chung RT, Klenerman P, Lewis-Ximenez L, Walker BD, Allen TM, Kim AY, Lauer GM (2008) High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol 82:3154–3160
Keir ME, Latchman YE, Freeman GJ, Sharpe AH (2005) Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J Immunol 175:7372–7379
Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH (2006) Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 203:883–895
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Klenerman P, Hill A (2005) T cells and viral persistence: lessons from diverse infections. Nat Immunol 6:873–879
Kroner A, Mehling M, Hemmer B, Rieckmann P, Toyka KV, Maurer M, Wiendl H (2005) A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol 58:50–57
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2:261–268
Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH (2004) PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA 101:10691–10696
Lazar-Molnar E, Yan Q, Cao E, Ramagopal U, Nathenson SG, Almo SC (2008) Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci USA 105:10483–10488
Lee SH, Lee YA, Woo DH, Song R, Park EK, Ryu MH, Kim YH, Kim KS, Hong SJ, Yoo MC, Yang HI (2006a) Association of the programmed cell death 1 (PDCD1) gene polymorphism with ankylosing spondylitis in the Korean population. Arthritis Res Ther 8:R163
Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, Oh S, Shin JG, Yao S, Chen L, Choi IH (2006b) Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett 580:755–762
Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH (2003) Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol 33:2706–2716
Lin DY, Tanaka Y, Iwasaki M, Gittis AG, Su HP, Mikami B, Okazaki T, Honjo T, Minato N, Garboczi DN (2008) The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci USA 105:3011–3016
Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B (2007) Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood 110:296–304
Long EO (1999) Regulation of immune responses through inhibitory receptors. Annu Rev Immunol 17:875–904
Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, Tsushima F, Hoshino T, Aizawa H, Akiba H, Pardoll D, Hara N, Yagita H, Azuma M, Nakanishi Y (2004) B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J Immunol 172:2530–2541
Meng Q, Yang P, Li B, Zhou H, Huang X, Zhu L, Ren Y, Kijlstra A (2006) CD4+PD-1+ T cells acting as regulatory cells during the induction of anterior chamber-associated immune deviation. Invest Ophthalmol Vis Sci 47:4444–4452
Moll M, Kuylenstierna C, Gonzalez VD, Andersson SK, Bosnjak L, Sonnerborg A, Quigley MF, Sandberg JK (2009) Severe functional impairment and elevated PD-1 expression in CD1d-restricted NKT cells retained during chronic HIV-1 infection. Eur J Immunol 39:902–911
Muthumani K, Choo AY, Shedlock DJ, Laddy DJ, Sundaram SG, Hirao L, Wu L, Thieu KP, Chung CW, Lankaraman KM, Tebas P, Silvestri G, Weiner DB (2008) Human immunodeficiency virus type 1 Nef induces programmed death 1 expression through a p38 mitogen-activated protein kinase-dependent mechanism. J Virol 82:11536–11544
Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ (2006) Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol 176:2238–2248
Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM (2008) Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology 134:1927–1937
Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S (2007) Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother 56:1173–1182
Nielsen C, Hansen D, Husby S, Jacobsen BB, Lillevang ST (2003) Association of a putative regulatory polymorphism in the PD-1 gene with susceptibility to type 1 diabetes. Tissue Antigens 62:492–497
Nishimura H, Agata Y, Kawasaki A, Sato M, Imamura S, Minato N, Yagita H, Nakano T, Honjo T (1996) Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int Immunol 8:773–780
Nishimura H, Nose M, Hiai H, Minato N, Honjo T (1999) Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11:141–151
Nishimura H, Honjo T, Minato N (2000) Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J Exp Med 191:891–898
Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T (2001) Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291:319–322
Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y (2007) Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 13:2151–2157
Oestreich KJ, Yoon H, Ahmed R, Boss JM (2008) NFATc1 regulates PD-1 expression upon T cell activation. J Immunol 181:4832–4839
Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 11:2947–2953
Okazaki T, Honjo T (2007) PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 19:813–824
Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T (2001) PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA 98:13866–13871
Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T (2003) Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 9:1477–1483
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL (2005) CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25:9543–9553
Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO (2007) Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 13:84–88
Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA (2006) PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 203:2281–2292
Prokunina L, Castillejo-Lopez C, Oberg F, Gunnarsson I, Berg L, Magnusson V, Brookes AJ, Tentler D, Kristjansdottir H, Grondal G, Bolstad AI, Svenungsson E, Lundberg I, Sturfelt G, Jonssen A, Truedsson L, Lima G, Alcocer-Varela J, Jonsson R, Gyllensten UB, Harley JB, Alarcon-Segovia D, Steinsson K, Alarcon-Riquelme ME (2002) A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet 32:666–669
Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, Azuma M, Yagita H, Sayegh MH, Khoury SJ (2003) Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med 198:71–78
Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, Wiendl H (2004) Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol 155:172–182
Selenko-Gebauer N, Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, Knapp W, Stockl J (2003) B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol 170:3637–3644
Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, Chaudhary D (2004) PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett 574:37–41
Shin H, Wherry EJ (2007) CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol 19:408–415
Sidorenko SP, Clark EA (2003) The dual-function CD150 receptor subfamily: the viral attraction. Nat Immunol 4:19–24
Streeck H, Brumme ZL, Anastario M, Cohen KW, Jolin JS, Meier A, Brumme CJ, Rosenberg ES, Alter G, Allen TM, Walker BD, Altfeld M (2008) Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med 5:e100
Sugita S, Usui Y, Horie S, Futagami Y, Yamada Y, Ma J, Kezuka T, Hamada H, Usui T, Mochizuki M, Yamagami S (2009) Human corneal endothelial cells expressing programmed death-ligand 1 (PD-L1) suppress PD-1+ T helper 1 cells by a contact-dependent mechanism. Invest Ophthalmol Vis Sci 50:263–272
Terawaki S, Tanaka Y, Nagakura T, Hayashi T, Shibayama S, Muroi K, Okazaki T, Mikami B, Garboczi DN, Honjo T, Minato N (2007) Specific and high-affinity binding of tetramerized PD-L1 extracellular domain to PD-1-expressing cells: possible application to enhance T cell function. Int Immunol 19:881–890
Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED (2004) Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA 101:17174–17179
Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP (2006) Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 12:1198–1202
Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H (2001) B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 193:839–846
Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C (2006) PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol 80:11398–11403
Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, Ahmed R, Amara RR (2007) Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol 81:5819–5828
Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR (2009) Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458:206–210
Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T (2005) Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA 102:11823–11828
Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, Hiai H, Honjo T, Okazaki T (2010) PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol 22:443–452
Watson MP, George AJ, Larkin DF (2006) Differential effects of costimulatory pathway modulation on corneal allograft survival. Invest Ophthalmol Vis Sci 47:3417–3422
Wherry EJ, Ahmed R (2004) Memory CD8 T-cell differentiation during viral infection. J Virol 78:5535–5545
Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R (2003) Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 77:4911–4927
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N (2006) Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 108:19–24
Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H (2002) Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 169:5538–5545
Yao ZQ, King E, Prayther D, Yin D, Moorman J (2007) T cell dysfunction by hepatitis C virus core protein involves PD-1/PDL-1 signaling. Viral Immunol 20:276–287
Zhang X, Schwartz JC, Guo X, Bhatia S, Cao E, Lorenz M, Cammer M, Chen L, Zhang ZY, Edidin MA, Nathenson SG, Almo SC (2004) Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 20:337–347
Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL (2007) PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol 37:2405–2410
Zhu B, Guleria I, Khosroshahi A, Chitnis T, Imitola J, Azuma M, Yagita H, Sayegh MH, Khoury SJ (2006) Differential role of programmed death-ligand 1 [corrected] and programmed death-ligand 2 [corrected] in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J Immunol 176:3480–3489
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Jin, HT., Ahmed, R., Okazaki, T. (2010). Role of PD-1 in Regulating T-Cell Immunity. In: Ahmed, R., Honjo, T. (eds) Negative Co-Receptors and Ligands. Current Topics in Microbiology and Immunology, vol 350. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2010_116
Download citation
DOI: https://doi.org/10.1007/82_2010_116
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-19544-0
Online ISBN: 978-3-642-19545-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)