Abstract
Clinical and pre-clinical studies have demonstrated an important role of neuroinflammation in the etiology of schizophrenia. While the underlying mechanisms remain poorly understood, there are some studies demonstrating an association between maternal immune activation and behavioral changes in adult offspring and identifying early life infection as a trigger for schizophrenia; in addition, inflammatory markers were found to be increased in the schizophrenic post-mortem brain. During maternal immune activation, pro-inflammatory mediators such as cytokines, chemokines, antibodies, and acute-phase proteins are released in the maternal bloodstream, thus increasing the permeability of the placental barrier and the fetal blood-brain barrier, allowing the inflammatory mediators to enter the fetal brain. In the central nervous system (CNS), these pro-inflammatory mediators are able to activate microglial cells that can release pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6. As a consequence, circulating immune cells may infiltrate the brain, increasing cytokine levels and releasing antibodies that aggravate the neuroinflammation. Neuroinflammation may affect processes that are pivotal for normal brain maturation such as myelination, synaptic pruning, and neuronal remodeling. Microglial cell activation and pro-inflammatory mediators have been extensively studied in schizophrenic post-mortem brain samples. Some results of these investigations demonstrated an increase in microglial activation markers, cytokines, and chemokines in post-mortem brain samples from individuals with schizophrenia. In contrast, there are studies that have demonstrated low levels of microglial activation makers in the schizophrenic post-mortem brain. Thus, based on the important role of neuroinflammation as a trigger in the development of schizophrenia, this chapter aims (1) to enumerate evidence of neuroinflammation and microglial activation from pre-clinical schizophrenia models, (2) to show links between schizophrenia and neuroinflammation in clinical studies, and (3) to identify mechanisms by which microglial activation may influence in the development of schizophrenia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Clinical and pre-clinical studies have demonstrated an important role of neuroinflammation in the etiology of schizophrenia. While the underlying mechanisms remain poorly understood, there are some studies showing evidence of microglial activation and increased levels of cytokines and chemokines in post-mortem schizophrenic brain samples, as well as in fetal and adult brains of offspring subjected to maternal immune activation during fetal life. In 1999, the first evidence of microglial and macrophage activation in the brains of patients with psychiatric disorders was reported. In the study, 3 of the 14 samples of post-mortem brains from patients with schizophrenia presented immunoreactivity to human leukocyte antigen-antigen D related (HLA-DR) protein in the frontal cortex and the hippocampus (Bayer et al. 1999). After that, a number of studies showed an increase in microglial markers in post-mortem schizophrenic brains, whereas few studies found no effect or a decrease in microglial markers. The studies that followed demonstrated an important role of maternal immune activation in releasing cytokines, chemokines, antibodies, and C-reactive protein (CRP) as an inductor of schizophrenia rather than the pathogen involved in maternal infection (Feigenson et al. 2014; Khandaker et al. 2014a, b). Pre-clinical studies have shown that during maternal immune activation, cytokines, chemokines, antibodies, and acute-phase proteins are released into the maternal bloodstream, thus increasing the permeability of the placental barrier and the fetal blood-brain barrier and allowing the inflammatory mediators to reach the fetal brain. In the central nervous system (CNS), these pro-inflammatory mediators are able to activate microglia that can release pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6. In addition, circulating immune cells may infiltrate the brain, increasing the cytokine levels and releasing antibodies that aggravate the neuroinflammation (Garay et al. 2013; Feigenson et al. 2014; van den Eynde et al. 2014; Reus et al. 2017). Thus, based on the important role of neuroinflammation as a trigger for the development of schizophrenia, this chapter aims (1) to enumerate evidence of neuroinflammation and microglial activation in pre-clinical schizophrenia models, (2) to highlight links between schizophrenia and neuroinflammation in clinical studies, and (3) to identify mechanisms by which microglial activation may influence the development of schizophrenia.
2 Evidence of Neuroinflammation and Microglial Activation from Pre-clinical and Clinical Schizophrenia Studies
2.1 Microglia Overview
Microglia comprise approximately 10–15% of all glial cells and are tissue-resident macrophages that present important functions in the CNS, including in supporting newborn neurons, cell death and clearance, homeostasis, and regulation of neuronal and synaptic plasticity (Salter and Stevens 2017). Microglia are derived from primitive myeloid progenitors emanating from the embryonic yolk sac during development and then populate the CNS (embryonic day 8.5 in mice) prior to its blood vessel formation (Ginhoux et al. 2010). Resting microglia have a small cell body and possess long branching; after being activated, the cells replace their ramified branches with highly amoeboid, motile protrusions (Stence et al. 2001). A modern transcriptome profiling of microglia in mice showed that the response phenotypes fail to conform to M1 or M2 patterns, though the functional significance and ontogeny of microglia had not yet been characterized (Ransohoff 2016; Salter and Stevens 2017). Microglia present class I (HLA-A, HLA-B, HLA-C) and class II (HLA-DR, HLA-DP, HLA-DQ) major histocompatibility complex (MHC) molecules. The MHC class II is found only on antigen-presenting cells, such as microglia, dendritic cells, mononuclear phagocytes, and B cells, because these cells are essential in initiating an immune response. Microglia are an important component of the innate immune system, and during their resting states, they are active with extremely motile processes and protrusions; thus, this cell type is referred to as a “housekeeper” in the adult brain (Nimmerjahn et al. 2005) (see Fig. 1).
Microglial activation. Resting microglial cell with ramified shape is activated by DAMPs, PAMPs, or pro-inflammatory mediators. After microglial activation, these cells present with highly amoeboid motile protrusions and release cytokines and chemokines. API-1 apoptosis inhibitor gene-1; ASC caspase-recruitment domain; DAMPS damage-associated molecular patterns; IFN-α, IFN-β interferon-α, interferon-β; IKKs IκB kinase complex; IL-1β, IL-6, IL-8, IL-18 interleukin-1β, interleukin-6, interleukin-8, and interleukin-18; IRF-3, IRF-7 interferon regulatory factor-3, interferon regulatory factor-7; MAPKs mitogen-activated protein kinases; MDA-5 melanoma differentiation-associated gene 5; MyD88 myeloid differentiation factor 88; NF-κB nuclear factor kappa B; NLRP-3 NLR family pyrin domain containing-3; PAMPs pathogen-associated molecular patterns; RIG-1 retinoic acid-inducible gene-1; TIRAP domain-containing adaptor protein; TLR Toll-like receptor; TNF-α tumor necrosis factor alpha; TRAF-3, TRAF-6 TNF receptor-associated factor-3, TNF receptor-associated factor-6; TRIF Toll/IL-1 receptor domain-containing adaptor-inducing interferon-β
2.2 Evidence of Neuroinflammation and Microglial Activation in Pre-clinical Schizophrenia Models
Several pre-clinical studies have demonstrated and supported evidence for the role of neuroinflammation in the development of schizophrenia. Among the different pre-clinical models that aim at recapitulating the development of schizophrenia, a subset of these is based on gestational exposure to maternal immune activation, a clinically relevant risk factor for schizophrenia. The experimental maternal immune activation induced by polyinosinic-polycytidylic acid (Poly I:C) mimics a viral infection because this chemical compound is a synthetic analogue of double-stranded ribonucleic acid (ssRNA). A multitude of studies that implement this model have observed long-lasting alterations of microglial markers, suggesting persistent microglial activation in adult animals exposed to gestational Poly I:C. For example, a study evaluated ionized calcium-binding adapter molecule-1 (Iba1), a microglia- and macrophage-specific calcium-binding protein that has actin-bundling activity and participates in membrane ruffling and phagocytosis in activated microglia. On gestation day 15, pregnant dams were given a single i.v. injection to the tail vein of Poly I:C or saline. The number of Iba1-positive cells was increased in the Poly I:C offspring’s hippocampus and nucleus accumbens but was unchanged in the prefrontal cortex. In addition, MHC class II expression in microglia increased in the Poly I:C prefrontal cortex, but not in the hippocampus of adult male offspring at 18 weeks of life (Hadar et al. 2017). Similarly, Mattei et al. observed an increase in Iba1 immunoreactivity in the proximity of the hippocampal dentate gyrus of adult mice on PND 60 that were subjected to maternal immune activation by Poly I:C at embryonic day 15 compared with control offspring in adult life (Mattei et al. 2017). Further, using the same microglial marker, Iba1, the offspring of mice exposed to Poly I:C at embryonic day 9 were shown to have an elevated number of activated microglial cells in the hippocampus and striatum, but not in the frontal cortex, on PND 30 (Juckel et al. 2011). In another study, OX-42, an antibody designed to detect CD11b, was used as a marker of microglia in the brain. OX-42 immunoreactivity was detected on postnatal day (PND) 180 in adult Poly I:C offspring, showing an increase in the concentration of OX-42-positive staining and microglial density, and reduced microglia ramifications, indicating that the microglia were in the activated state in all brain regions. Additionally, there was a difference in the form of an overall significant increase in microglia score in the corpus callosum, hippocampus, and thalamus; however, this difference was not found in the pons, cortex, or striatum obtained from adult offspring of dams treated with Poly I:C on embryonic day 15 (Van den Eynde, Missault et al. 2014).
Activated microglial cells can increase the production and expression of pro-inflammatory cytokines, such as TNF-α and IL-1β, and neurotoxic substances, resulting in neuroinflammatory and neurodegenerative processes. Adult mice subjected to maternal immune activation by Poly I:C during the fetal stage presented high expression of proteins involved in the Toll-like receptor (TLR)-3 signaling pathway, such as signal transducer and activator of transcription-1 (STAT-1), Toll/IL-1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and phosphorylated-IRF3 (pIRF3), in the frontal cortex. Increased oxidative and nitrosative stress, as evidenced by increased malondialdehyde (MDA) and inducible nitric oxide synthase (iNOS), and increased levels of TNF-α, interferon (IFN)-α, and IFN-β in the frontal cortex were also observed (MacDowell et al. 2017a). In another study, mice were subjected to maternal immune activation on embryonic day 12.5. On PNDs 0, 7, 14, 30, and 60, the offspring brains were removed, and the frontal cortex, cingulate cortex, and hippocampus were used to evaluate the presence of cytokines and chemokines. On PND 0, the frontal cortex showed increased levels of IL-1β, IL-10, IL-12, and granulocyte/monocyte colony-stimulating factor (GM-CSF). On PND 7, the levels of granulocyte colony-stimulating factor (G-CSF) were increased, and on PND 60, the levels of IL-1α, IL-6, IL-9, and IL-10 were also increased. On PND 0, the cingulate cortex showed increased levels of IFN-γ, IL-12, and monocyte chemoattractant protein-1 (MCP-1). On PND 7, the levels of IL-17 increased, and on PND 60, the levels of IL-10 and IFN-γ increased. On PND 0, the hippocampus showed an increase in the level of IL-6. On PND 7, increased levels of IL-9, keratinocyte chemoattractant (KC), and macrophage inflammatory protein-1 alpha (MIP-1α) were observed, and on PND 14, increased levels of IL-1α and IL-6 were found (Garay et al. 2013).
Pratt et al. injected pregnant mice on embryonic day 12.5 with Poly I:C, and fetal brains were collected at embryonic day 16.5 to evaluate the inflammatory profile of microglial cells, which included cytokine and chemokine expression. Fetal microglia expressed high levels of cytokines and chemokines such as IL-1α, IL-4, IL-6, IL-9, GM-CSF, and M-CSF, which were regulated upon activation by normal T-cell expressed and secreted (RANTES), lipopolysaccharide-induced CXC chemokine (LIX), exotoxin, and MIP-1β (Pratt et al. 2013). Using another approach, Arad et al. injected dams with Poly I:C on day 4 after birth, and the offspring were breastfed. Two hours after Poly I:C injection, the milk of the dams presented elevated levels of IL-1β, IL-6, and corticosterone. At 6 and 24 h after the dams received the Poly I:C injection, the male offspring presented high levels of IL-6 and IFN-γ in the hippocampus. Twenty-four hours after the dams received the Poly I:C injection, both male and female offspring presented high levels of TNF-α in the hippocampus. In addition, lactational Poly I:C exposure triggered behavioral abnormalities in the adult offspring (PND 90 to 120), with male, but not female, offspring exhibiting attentional and executive function abnormalities (manifested in persistent latent inhibition and slow reversal) and female, but not male, offspring exhibiting despair and anhedonia (Arad et al. 2017).
A subset of studies aimed at characterizing the role of single cytokines. For example, Smith et al. demonstrated the important role of IL-6 in schizophrenia-like behavior. Specifically, an intraperitoneal injection of IL-6 on embryonic day 12.5 in pregnant mice triggered prepulse inhibition and latent inhibition deficits in the adult offspring, but IFN-γ maternal injection did not affect the schizophrenia-like behavior of adult offspring (Smith et al. 2007). The section above highlights several studies demonstrating that TLR-3 activation and pro-inflammatory cytokines could influence the development of schizophrenia-like behavior in adult offspring. In contrast to other studies, on PND 90 to 104, adult offspring did not present any significant difference in the level of microglial activation compared to the control adult offspring (Missault et al. 2014). In this study, despite the confirmation of systemic inflammation in the pregnant mice, there was no difference in fetal microglial cell density or in the activation level on embryonic days 11.5–17.5 between the control and Poly I:C group (Smolders et al. 2015); see Table 1.
The Gunn rat is another animal model of schizophrenia (Gunn 1944). Gunn rats present behavioral abnormalities, deficits in prepulse inhibition, and neuropathological changes that are similar to the characteristics of schizophrenia-like behavior (Liaury et al. 2012). CD11b immunoreactivity is increased in microglial cells of the hippocampal dentate gyrus of Gunn rats (Liaury et al. 2012, 2014). Gunn rats showed a prepulse inhibition deficit compared to Wistar rats. The amount of CD11b microglial cell marker increased in the hippocampus of Gunn rats compared to the same brain structure of the Wistar rats (Limoa et al. 2016).
3 Evidence of Neuroinflammation from Schizophrenic Patients
3.1 Microglia Evaluation in Post-mortem Schizophrenic Brain
Brain samples from 3 of 14 patients with schizophrenia exhibited HLA-DR-positive tests in the frontal cortex and hippocampus (Bayer et al. 1999). HLA-DR is an MHC class II cell surface receptor that interacts with antigen-presenting cells such as microglia, mononuclear phagocytes, dendritic cells, and B cells. The microglial marker HLA-DR was increased in paranoid schizophrenic hippocampal samples compared with residual schizophrenic and matched control samples. In the same study, higher expression levels of CD3+ and CD20+ lymphocytes were found in the hippocampus of residual schizophrenics compared with paranoid schizophrenics and matched controls (Busse et al. 2012). The density of HLA-DR cells that were morphologically similar to microglia was increased in the dorsolateral prefrontal cortex of individuals with schizophrenia (Fillman et al. 2013). In a previous study, the frontal and temporal lobes of chronic schizophrenics presented greater microglial cell activation compared with control brains. However, the first layer of the cerebral cortex presented the same amounts of well-developed ramifications (RM), degenerative traits, and damaged processes (DM), and the number of DM cells in the remaining regions was higher than that of the RM cells (Wierzba-Bobrowicz et al. 2005). Another study evaluated 12 brains of female chronic schizophrenics. The schizophrenic frontal and temporal lobe samples presented ramified microglial cells with expression of MHC class II. Most cells presented with cytoplasm shrinkage, thinning, shortening and fragmentation of their processes, and apoptotic changes. Several microglial cells presented phagosomes and/or degenerated mitochondria (Wierzba-Bobrowicz et al. 2004). The dorsolateral prefrontal cortex, anterior cingulate cortex, hippocampus, and mediodorsal thalamus were evaluated in 16 schizophrenic brain samples. HLA-DR-positive cell expression was not different between the schizophrenia and control groups. The post-mortem interval correlated with the ramified cell numbers in the anterior cingulate cortex and the dorsolateral prefrontal cortex and with the amoeboid cell density in the hippocampus. Two schizophrenic patients who had committed suicide during acute psychosis presented highly elevated microglial cell numbers in the anterior cingulate cortex and the mediodorsal thalamus (Steiner et al. 2006). In another study from the same research group, microglial HLA-DR expression was evaluated in the dorsolateral prefrontal cortex, anterior cingulate cortex, mediodorsal thalamus, and hippocampus of 16 schizophrenic patients. Microglial HLA-DR expression did not presently affect the diagnosis of microglial density in the dorsolateral prefrontal cortex, anterior cingulate cortex, mediodorsal thalamus, and hippocampus. However, the study found microgliosis in the dorsolateral prefrontal cortex, cingulate cortex, and mediodorsal thalamus of the schizophrenic suicide patients (Steiner et al. 2008). In a study by Sinkus et al., the mRNA levels for the MHC class I antigen HLA-B was increased in schizophrenic nonsmokers, while the levels for smokers were indistinguishable from those of controls. HLA-A was expressed in a pattern where inflammatory illness was associated with increased expression in controls but not in subjects with schizophrenia (Sinkus et al. 2013). Radewicz et al. found an increase of HLA-DR expression in the dorsolateral prefrontal cortex in eight schizophrenics compared with ten controls. Regarding the superior temporal gyrus, there was an increase in microglia in seven schizophrenics compared with ten controls. In the anterior cingulate gyrus, the results did not reach significance (Radewicz et al. 2000). Calprotectin is a calcium-binding protein of the S100 family and is a nonspecific inflammatory marker. Samples of post-mortem brain tissue from Brodmann area 9 were obtained from the prefrontal cortices of subjects with schizophrenia and of controls. Calprotectin presented higher levels in the schizophrenic brains (Brodmann area 9 from prefrontal cortex) compared to the controls, and this protein was found to localize in microglial cells (Foster et al. 2006).
Through investigation of the microglial activation using Iba1 antibody marker, which is expressed in macrophages and microglia and is upregulated during the activation of these cells, the brain samples presented unaltered immunoreactivity in the cingulate white matter (Connor et al. 2009) and the dorsolateral prefrontal cortex in the post-mortem schizophrenic brain (Hercher et al. 2014). Moreover, in another study, the regional differences in the ependymal and subventricular zone cytoarchitecture were unchanged in schizophrenic brain samples (Comte et al. 2012). CD68 was evaluated for resting and active microglia in the caudate nucleus and the mediodorsal nucleus of the thalamus in a post-mortem study of 11 elderly people with schizophrenia. No differences were found between the schizophrenic and control subjects (Falke et al. 2000). HLA-DRA did not present any differences in the dorsolateral prefrontal cortex or the parietal cortex samples between the schizophrenic and control groups (Nakatani et al. 2006). Messenger RNA expression of HLA-A did not present any differences in the frontal cortex of schizophrenic subjects compared to control subjects (Saetre et al. 2007). The temporal cortex of the schizophrenic brain samples did not present differences in HLA-DRB3 and HLA-DPA1 expression compared with control brain samples (Schmitt et al. 2011). Another study evaluated the MHC class I and complement protein C3 expression in two frontal cortical regions of post-mortem brains of schizophrenic patients. MHC class I protein expression was decreased in the dorsolateral prefrontal cortex, but the protein expression did not present any change in the orbitofrontal cortex of nonsmoking schizophrenic patients, and this study did not find any association between schizophrenia and changes in C3 mRNA expression (Kano et al. 2011). A subsequent study presented a reduction in microglial immunoreactivity for the endogenous NMDA receptor agonist, quinolinic acid, in the hippocampus of schizophrenic patients and presented no difference in HLA-DR expression between schizophrenic and the control group brain samples (Gos et al. 2014). The MHC class II receptors HLA-DR and HLA-DRBA were downregulated in the temporal lobe of schizophrenic post-mortem brain samples (Durrenberger et al. 2015). No differences were found in the CD40 and HLA-DP/DQ/DR markers in four brain samples of schizophrenic patients (Togo et al. 2000). CD68 for resting and active microglia was evaluated in the entorhinal cortex, the subiculum and CA1 of the hippocampus, midfrontal cortex, orbitofrontal cortex, and calcarine cortex in schizophrenic brain samples. There were no differences between the schizophrenic and the control brain samples in the densities of any of the markers (Arnold et al. 1998). Kurumaji et al. evaluated [3H] PK 11195 as a ligand for the translocator protein (TSPO) receptor in the cerebral cortex, thalamus, and extrapyramidal system of the post-mortem brains of 13 chronic schizophrenics and 10 control subjects. The [3H] PK 11195-specific binding was decreased in the superior parietal cortex, primary visual area, and putamen of schizophrenics, although there were no changes in this binding in the other brain areas (Kurumaji et al. 1997); see Table 2.
3.2 Cytokine and Chemokine Evaluation in Post-mortem Schizophrenic Brain Samples
In a clinical study, the mRNA expression of IL-6, IL-8, and SERPINA-3 presented higher levels in the dorsolateral prefrontal cortex of individuals with schizophrenia compared with their controls (Fillman et al. 2013). IL-6, IL-1β, IL-8, and SERPINA-3 mRNA levels were quantified in the contralateral fresh frozen orbitofrontal cortex. The volumes of the cortical gray matter and the superior frontal gyrus had a significant negative correlation with IL-1β, IL-6, and SERPINA-3 mRNA levels in the schizophrenic group. Thus, cortical gray matter volume reduction in schizophrenic patients was associated with neuroinflammation, and the researchers also found that the expression of inflammatory mRNA in the orbitofrontal cortex was correlated with those found by Fillman et al. (2013), in the dorsolateral prefrontal cortex, except for IL-8 (Zhang et al. 2016). SERPINA-3 mRNA was also present at high levels in the dorsolateral prefrontal cortex of individuals with schizophrenia (Fillman et al. 2014). IFN-γ, as evaluated by the ELISA technique, was elevated in the BA10 brain region of schizophrenic patients (Harris et al. 2012). Schizophrenic subjects presented markedly higher mRNA levels of IL-6, IFN-β, and NF-κB transcription factor in the prefrontal cortex compared with the control group (Volk et al. 2015). TNF receptor 1 (TNFR1) mRNA was significantly increased in both Brodmann areas 24 (BA24) and 46 (BA46) in patients with schizophrenia (Dean et al. 2013). In contrast, IL-1β mRNA levels were not changed in post-mortem brain tissues of the prefrontal or parietal cortices, putamen, or the hypothalamus. In addition, endogenous IL-1 receptor antagonist (IL-1RA) decreased in the prefrontal cortex of schizophrenic patients (Toyooka et al. 2003). IL-13 receptor alpha-1 (IL-13RA1) was downregulated in the temporal lobe of schizophrenic patients (Durrenberger et al. 2015). Chemokine (C-C motif) ligand 3 (CCL3) gene expression was also downregulated in the dorsolateral prefrontal cortex and parietal cortex (Nakatani et al. 2006), and in the temporal cortex, the expression of IL-1α and IL-8 was downregulated in schizophrenic brain samples (Schmitt et al. 2011); see Table 2.
4 Mechanisms by Which Neuroinflammation Could Influence the Development of Schizophrenia
During pregnancy or early life infection, replication of microorganisms and the release of their immunogenic compounds can occur. These immunogenic compounds derived from microorganisms are denominated as pathogen-associated molecular patterns (PAMPs), and they are recognized by the immune system through equipped receptors denominated as pattern-recognition receptors (PRRs) (Barichello et al. 2015; Morris et al. 2018). These receptors, such as Toll-like receptors (TLR), nucleotide-binding oligomerization domain (NOD)-like receptors (NLR), C-type lectin receptors (CLR), retinoic acid-inducible gene-1 (RIG-1)-like receptors (RLR), receptors for advanced glycation end products (RAGE), and intra-cytosolic deoxyribonucleic acid (DNA) sensors, are crucial components in the activation of the innate immune system (Keestra-Gounder and Tsolis 2017; Zhou et al. 2017). The PRRs can also recognize a broader array of endogenous danger signals such as adenosine 5-triphosphate (ATP), heat shock proteins (HSPs), and high mobility group box-1 proteins (HMGB-1) that are denominated as damage-associated molecular patterns (DAMPs) (Nakahira et al. 2015; Wilkins et al. 2017); see Figs. 1 and 2.
Maternal immune activation and a possible mechanism in which neuroinflammation could influence the development of schizophrenia. TLRs, MDA-5, and RIG-1 are innate immune sensors involved in the detection of microorganisms. TLR-3, RIG-1, and MDA-5 promote the expression of type I and type III IFNs and the NF-kappa B-dependent expression of pro-inflammatory cytokines. Maternal immune activation increases the levels of cytokines such as IL-6, TNF-α, and IL-1β in the serum, as well as in the amniotic fluid, placenta, and fetal brain. ASC caspase-recruitment domain; IFITM-1, IFITM-2 interferon-induced transmembrane protein-1, interferon-induced transmembrane protein-2; IFN-α, IFN-β interferon-α, interferon-β; IκB inhibitors of NF-κB; IL-1β, IL-6, IL-18 interleukin-1β, interleukin-6, interleukin-18; IRAK-4 interleukin receptor-associated kinase-4; IRF-1, IRF-3, IRF-7 interferon regulatory factor-1, interferon regulatory factor-3, interferon regulatory factor-7; MDA-5 melanoma differentiation-associated gene 5; MyD88 myeloid differentiation factor 88; NF-κB nuclear factor kappa B; NLRP-3 NLR family pyrin domain containing-3; RIG-1 retinoic acid-inducible gene-1; SERPINA-3 serpin family A member-3; TIRAP domain-containing adaptor protein; TLR Toll-like receptor; TNF-α tumor necrosis factor alpha; TRAF-3, TRAF-6 TNF receptor-associated factor-3, TNF receptor-associated factor-6; TRIF Toll/IL-1 receptor domain-containing adaptor-inducing interferon-β
TLR receptors are divided into two groups, of which one is expressed on the cell membrane for ligand recognition (TLR-1, TLR-2, TLR-4, TLR-5, TLR-6, and TLR-10) and the other is localized in the intracellular endosomal space for the recognition of pathogen nucleic acids: TLR-3, TLR-7, TLR-8, and TLR-9 (Kigerl et al. 2014). TLR-3 can signal through a TRIF-dependent pathway that recruits the TNF receptor-associated factor-3 (TRAF-3), thus resulting in the activation of interferon regulatory factor-3 (IRF-3) and IRF-7. This pathway triggers the production of type I interferons, such as IFN-α or IFN-β. In another pathway, TLR-3 activates TRIF, AP1, and NF-κB, inducing the expression of pro-inflammatory cytokine genes. TLR-3 serves as a sensor of dsRNA produced during the replication of single-stranded RNA (ssRNA) and is also activated by a synthetic chemical compound analogue of dsRNA, Poly I:C (Verma and Bharti 2017). TLR-3 is an essential sensor of the host’s immune responses to protect it against viral infections. A pre-clinical model of schizophrenia demonstrated high expression of TLR-3 signaling, IFN-α, and IFN-β in the frontal cortex of adult offspring subjected to maternal immune activation by Poly I:C during fetal life (MacDowell et al. 2017a). In addition, TLR-3 activation inhibited embryonic neuronal stem cell replication and population of the superficial layers of the neocortex by neurons (de Miranda et al. 2010).
TLR-4, CD14, and myeloid differentiation protein-2 (MD-2) form a complex heteromer that, after activation, recruits the MyD88 adapter-like (Mal) and the TIR domain-containing adaptor protein (TIRAP). Mal/TIRAP recruits myeloid differentiation primary response gene 88 (MYD88) adaptor. The MyD88 adaptor molecule connects with the serine/threonine kinase IL-1 receptor-associated protein leading to phosphorylation of IRAK-1 and IRAK-2 and the recruitment of TNF receptor-associated factor-6 (TRAF-6) adaptor. TRAF-6 activates inhibitory IκB kinases (IkBα and IkBβ) and mitogen-activated protein kinases (MAPKs), resulting in NF-κB and activator protein-1 (AP-1) transcription factor activation and production of cytokines. In parallel, the TLR4 complex also recruits TRIF-related adaptor molecules that interact with TRIF adaptor and activate the interferon regulatory factor-3 (IRF-3) transcription factor. The post-mortem cerebellum of human schizophrenic subjects presented an increase in protein expression of TLR-4, MyD88, and IκBα. In contrast, NF-κB activity was reduced, iNOS expression was not changed, while cyclooxygenase-2 (COX-2) protein levels were increased and there were no changes in lipid peroxidation (MDA). In the post-mortem schizophrenic prefrontal cortex, TLR-4, MyD88, and IκBα protein levels were lower in schizophrenic patients, while nuclear transcription NF-κB activity, COX-2 expression, and malondialdehyde (MDA) were increased (MacDowell et al. 2017b). Another study found evidence of alterations in the expression of the TLR-4 signaling and MyD88 and NF-κB in the prefrontal cortex of patients with schizophrenia. However, there were no changes in the IκBα protein levels, IL-1β, and IL-6 mRNA levels in the prefrontal cortex. An additional study evaluated the effect of antipsychotic treatment on schizophrenic post-mortem brain samples. The antipsychotic treatment schizophrenic group presented higher levels of TLR-4, MyD88 protein, and MyD88 mRNA compared to control samples. An MDA decrease was observed in the antipsychotic-free group compared to the control and antipsychotic treatment groups, but the antipsychotic-free group presented high levels of NF-κB protein compared with controls. This study demonstrated that it is necessary to pay special attention to the potentially confounding factor of antipsychotic treatment, because these alterations seem to depend on the presence or absence of antipsychotic treatment at death (Garcia-Bueno et al. 2016).
A number of studies have shown an increase in expression of SERPINA-3 and its gene in schizophrenic brain samples (Arion et al. 2007; Fillman et al. 2013, 2014; Zhang et al. 2016). The transcriptome signature of altered genes related to immune function may be a consequence of high levels of pro-inflammatory cytokines such as IL-6, TNF-α, IL-1β, or IFNs, during the stages of prenatal development or during early life. These pro-inflammatory mediators could not only alter brain development but also be responsible for these immune-/inflammation-related genes such as SERPINA-3, interferon-induced transmembrane protein (IFITM)-1, IFITM-2, and IFITM-3 that are found in the schizophrenic adult brain (Arion et al. 2007; Saetre et al. 2007; Hwang et al. 2013; Volk et al. 2015); see Tables 2 and 3.
5 Conclusion
There are a significant number of studies showing an increase in microglial markers and pro-inflammatory gene expression in the post-mortem brains of schizophrenic patients compared with controls. The transcriptome signature of altered genes related to immune function may be a consequence of high levels of pro-inflammatory cytokines during the stages of prenatal development or during early life. These pro-inflammatory mediators could not only alter brain development but also be responsible for these immune/inflammation-related genes found in the schizophrenic adult brain.
Abbreviations
- ATP:
-
Adenosine 5-triphosphate
- CCL3:
-
Chemokine (C-C motif) ligand 3
- CLR:
-
C-type lectin receptors
- CNS:
-
Central nervous system
- COX-2:
-
Cyclooxygenase-2
- CRP:
-
C-reactive protein
- DAMPS:
-
Damage-associated molecular patterns
- DM:
-
Damaged processes
- DNA:
-
Deoxyribonucleic acid
- G-CSF:
-
Granulocyte colony-stimulating factor
- GM-CSF:
-
Granulocyte/monocyte colony-stimulating factor
- HLA-DR:
-
Human leukocyte antigen-antigen D related
- HMGB-1:
-
High mobility group box-1 protein
- HSPs:
-
Heat shock proteins
- Iba1:
-
Ionized calcium-binding adaptor molecule
- IFITM:
-
Interferon-induced transmembrane protein
- IFN:
-
Interferon
- IkB:
-
Inhibitors of NF-B
- IL:
-
Interleukin
- IL-13RA1:
-
IL-13 receptor alpha-1
- IL-1RA:
-
IL-1 receptor antagonist
- iNOS:
-
Inducible nitric oxide synthase
- IRF:
-
Interferon regulatory factor
- KC:
-
Keratinocyte chemoattractant
- LIX:
-
Lipopolysaccharide-induced CXC chemokine
- Mal:
-
MyD88 adapter-like
- MAPKs:
-
Mitogen-activate protein kinases
- MCP-1 :
-
Monocyte chemoattractant protein-1
- MD-2:
-
Myeloid differentiation protein-2
- MDA:
-
Malondialdehyde
- MHC:
-
Major histocompatibility complex
- MIP-1:
-
Macrophage inflammatory protein-1
- mRNA :
-
Messenger ribonucleic acid
- MYD88:
-
Myeloid differentiation factor 88
- NF-κB:
-
Nuclear factor kappa B
- NLR:
-
NOD-like receptors
- PAMPs:
-
Pathogen-associated molecular patterns
- pIRF3:
-
Phosphorylated-IRF3
- PK 11195:
-
1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinoline carboxamide
- PND:
-
Postnatal day
- Poly I:C:
-
Polyinosinic-polycytidylic acid
- PRRs:
-
Pattern-recognition receptors
- RAGE:
-
Receptors for advanced glycation end products
- RANTES:
-
Regulated upon activation normal T-cell expressed and secreted
- RIG-1:
-
Retinoic acid-inducible gene-1
- RLR:
-
RIG-1-like receptors
- RM:
-
Ramification
- RNA:
-
Ribonucleic acid
- SERPINA-3:
-
Serpin family A member-3
- ssRNA:
-
Double-stranded ribonucleic acid
- STAT-1:
-
Signal transducer and activator of transcription-1
- TIR:
-
Toll/IL-1 receptor
- TIRAP:
-
Domain-containing adaptor protein
- TLR:
-
Toll-like receptor
- TNFR1:
-
TNF receptor 1
- TNF-α:
-
Tumor necrosis factor alpha
- TRAF:
-
Tumor necrosis factor receptor-associated factor
- TRIF:
-
Toll/IL-1 receptor domain-containing adaptor-inducing interferon-β
- TSPO:
-
Translocator protein
References
Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, Pogorelov V, Ladenheim B, Yang C, Krasnova IN, Cadet JL, Pardo C, Mori S, Kamiya A, Vogel MW, Sawa A, Ross CA, Pletnikov MV (2010) Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry 68(12):1172–1181
Arad M, Piontkewitz Y, Albelda N, Shaashua L, Weiner I (2017) Immune activation in lactating dams alters sucklings’ brain cytokines and produces non-overlapping behavioral deficits in adult female and male offspring: a novel neurodevelopmental model of sex-specific psychopathology. Brain Behav Immun 63:35–49
Arion D, Unger T, Lewis DA, Levitt P, Mirnics K (2007) Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry 62(7):711–721
Arnold SE, Trojanowski JQ, Gur RE, Blackwell P, Han LY, Choi C (1998) Absence of neurodegeneration and neural injury in the cerebral cortex in a sample of elderly patients with schizophrenia. Arch Gen Psychiatry 55(3):225–232
Barak V, Barak Y, Levine J, Nisman B, Roisman I (1995) Changes in interleukin-1 beta and soluble interleukin-2 receptor levels in CSF and serum of schizophrenic patients. J Basic Clin Physiol Pharmacol 6(1):61–69
Barichello T, Generoso JS, Goularte JA, Collodel A, Pitcher MR, Simoes LR, Quevedo J, Dal-Pizzol F (2015) Does infection-induced immune activation contribute to dementia? Aging Dis 6(5):342–348
Bayer TA, Buslei R, Havas L, Falkai P (1999) Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett 271(2):126–128
Brisch R, Steiner J, Mawrin C, Krzyzanowska M, Jankowski Z, Gos T (2017) Microglia in the dorsal raphe nucleus plays a potential role in both suicide facilitation and prevention in affective disorders. Eur Arch Psychiatry Clin Neurosci 267(5):403–415
Busse S, Busse M, Schiltz K, Bielau H, Gos T, Brisch R, Mawrin C, Schmitt A, Jordan W, Muller UJ, Bernstein HG, Bogerts B, Steiner J (2012) Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun 26(8):1273–1279
Comte I, Kotagiri P, Szele FG (2012) Regional differences in human ependymal and subventricular zone cytoarchitecture are unchanged in neuropsychiatric disease. Dev Neurosci 34(4):299–309
Connor CM, Guo Y, Akbarian S (2009) Cingulate white matter neurons in schizophrenia and bipolar disorder. Biol Psychiatry 66(5):486–493
Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C, Hayes LN, Schretlen DJ, Dannals RF, Kassiou M, Sawa A, Pomper MG (2016) In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 12(6):40
de Miranda J, Yaddanapudi K, Hornig M, Villar G, Serge R, Lipkin WI (2010) Induction of Toll-like receptor 3-mediated immunity during gestation inhibits cortical neurogenesis and causes behavioral disturbances. MBio 1(4):e00176–e00110
Dean B, Gibbons AS, Tawadros N, Brooks L, Everall IP, Scarr E (2013) Different changes in cortical tumor necrosis factor-alpha-related pathways in schizophrenia and mood disorders. Mol Psychiatry 18(7):767–773
Durrenberger PF, Fernando FS, Kashefi SN, Bonnert TP, Seilhean D, Nait-Oumesmar B, Schmitt A, Gebicke-Haerter PJ, Falkai P, Grunblatt E, Palkovits M, Arzberger T, Kretzschmar H, Dexter DT, Reynolds R (2015) Common mechanisms in neurodegeneration and neuroinflammation: a BrainNet Europe gene expression microarray study. J Neural Transm (Vienna) 122(7):1055–1068
el-Mallakh RS, Suddath RL, Wyatt RJ (1993) Interleukin-1 alpha and interleukin-2 in cerebrospinal fluid of schizophrenic subjects. Prog Neuro-Psychopharmacol Biol Psychiatry 17(3):383–391
Falke E, Han LY, Arnold SE (2000) Absence of neurodegeneration in the thalamus and caudate of elderly patients with schizophrenia. Psychiatry Res 93(2):103–110
Feigenson KA, Kusnecov AW, Silverstein SM (2014) Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev 38:72–93
Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS (2013) Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 18(2):206–214
Fillman SG, Sinclair D, Fung SJ, Webster MJ, Shannon Weickert C (2014) Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl Psychiatry 4:e365
Foster R, Kandanearatchi A, Beasley C, Williams B, Khan N, Fagerhol MK, Everall IP (2006) Calprotectin in microglia from frontal cortex is up-regulated in schizophrenia: evidence for an inflammatory process? Eur J Neurosci 24(12):3561–3566
Garay PA, Hsiao EY, Patterson PH, McAllister AK (2013) Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 31:54–68
Garcia-Bueno B, Gasso P, MacDowell KS, Callado LF, Mas S, Bernardo M, Lafuente A, Meana JJ, Leza JC (2016) Evidence of activation of the Toll-like receptor-4 proinflammatory pathway in patients with schizophrenia. J Psychiatry Neurosci 41(3):E46–E55
Garver DL, Tamas RL, Holcomb JA (2003) Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology 28(8):1515–1520
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330(6005):841–845
Gos T, Myint AM, Schiltz K, Meyer-Lotz G, Dobrowolny H, Busse S, Muller UJ, Mawrin C, Bernstein HG, Bogerts B, Steiner J (2014) Reduced microglial immunoreactivity for endogenous NMDA receptor agonist quinolinic acid in the hippocampus of schizophrenia patients. Brain Behav Immun 41:59–64
Gunn CK (1944) Hereditary acholuric jaundice in the rat. Can Med Assoc J 50(3):230–237
Hadar R, Dong L, Del-Valle-Anton L, Guneykaya D, Voget M, Edemann-Callesen H, Schweibold R, Djodari-Irani A, Goetz T, Ewing S, Kettenmann H, Wolf SA, Winter C (2017) Deep brain stimulation during early adolescence prevents microglial alterations in a model of maternal immune activation. Brain Behav Immun 63:71–80
Harris LW, Pietsch S, Cheng TM, Schwarz E, Guest PC, Bahn S (2012) Comparison of peripheral and central schizophrenia biomarker profiles. PLoS One 7(10):30
Hercher C, Chopra V, Beasley CL (2014) Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci 39(6):376–385
Hwang Y, Kim J, Shin JY, Kim JI, Seo JS, Webster MJ, Lee D, Kim S (2013) Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl Psychiatry 3:e321
Juckel G, Manitz MP, Brune M, Friebe A, Heneka MT, Wolf RJ (2011) Microglial activation in a neuroinflammational animal model of schizophrenia – a pilot study. Schizophr Res 131(1–3):96–100
Kano S, Nwulia E, Niwa M, Chen Y, Sawa A, Cascella N (2011) Altered MHC class I expression in dorsolateral prefrontal cortex of nonsmoker patients with schizophrenia. Neurosci Res 71(3):289–293
Katila H, Hurme M, Wahlbeck K, Appelberg B, Rimon R (1994) Plasma and cerebrospinal fluid interleukin-1 beta and interleukin-6 in hospitalized schizophrenic patients. Neuropsychobiology 30(1):20–23
Keestra-Gounder AM, Tsolis RM (2017) NOD1 and NOD2: beyond peptidoglycan sensing. Trends Immunol 38(10):758–767
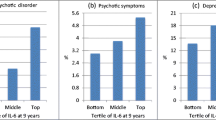
Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB (2014a) Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiat 71(10):1121–1128
Khandaker GM, Zammit S, Lewis G, Jones PB (2014b) A population-based study of atopic disorders and inflammatory markers in childhood before psychotic experiences in adolescence. Schizophr Res 152(1):139–145
Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW (2014) Pattern recognition receptors and central nervous system repair. Exp Neurol 258:5–16
Kurumaji A, Wakai T, Toru M (1997) Decreases in peripheral-type benzodiazepine receptors in postmortem brains of chronic schizophrenics. J Neural Transm 104(11–12):1361–1370
Liaury K, Miyaoka T, Tsumori T, Furuya M, Wake R, Ieda M, Tsuchie K, Taki M, Ishihara K, Tanra AJ, Horiguchi J (2012) Morphological features of microglial cells in the hippocampal dentate gyrus of Gunn rat: a possible schizophrenia animal model. J Neuroinflammation 9:56
Liaury K, Miyaoka T, Tsumori T, Furuya M, Hashioka S, Wake R, Tsuchie K, Fukushima M, Limoa E, Tanra AJ, Horiguchi J (2014) Minocycline improves recognition memory and attenuates microglial activation in Gunn rat: a possible hyperbilirubinemia-induced animal model of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 50:184–190
Licinio J, Seibyl JP, Altemus M, Charney DS, Krystal JH (1993) Elevated CSF levels of interleukin-2 in neuroleptic-free schizophrenic patients. Am J Psychiatry 150(9):1408–1410
Limoa E, Hashioka S, Miyaoka T, Tsuchie K, Arauchi R, Azis IA, Wake R, Hayashida M, Araki T, Furuya M, Liaury K, Tanra AJ, Horiguchi J (2016) Electroconvulsive shock attenuated microgliosis and astrogliosis in the hippocampus and ameliorated schizophrenia-like behavior of Gunn rat. J Neuroinflammation 13(1):230
MacDowell KS, Munarriz-Cuezva E, Caso JR, Madrigal JL, Zabala A, Meana JJ, Garcia-Bueno B, Leza JC (2017a) Paliperidone reverts Toll-like receptor 3 signaling pathway activation and cognitive deficits in a maternal immune activation mouse model of schizophrenia. Neuropharmacology 116:196–207
MacDowell KS, Pinacho R, Leza JC, Costa J, Ramos B, Garcia-Bueno B (2017b) Differential regulation of the TLR4 signalling pathway in post-mortem prefrontal cortex and cerebellum in chronic schizophrenia: relationship with SP transcription factors. Prog Neuropsychopharmacol Biol Psychiatry 79(Pt B):481–492
Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossio LF, Goetz T, Matyash M, Kettenmann H, Winter C, Wolf SA (2014) Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun 38:175–184
Mattei D, Ivanov A, Ferrai C, Jordan P, Guneykaya D, Buonfiglioli A, Schaafsma W, Przanowski P, Deuther-Conrad W, Brust P, Hesse S, Patt M, Sabri O, Ross TL, Eggen BJL, Boddeke E, Kaminska B, Beule D, Pombo A, Kettenmann H, Wolf SA (2017) Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl Psychiatry 7(5):e1120
Missault S, van den Eynde K, van den Berghe W, Fransen E, Weeren A, Timmermans JP, Kumar-Singh S, Dedeurwaerdere S (2014) The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav Immun 42:138–146
Morris G, Barichello T, Stubbs B, Kohler CA, Carvalho AF, Maes M (2018) Zika virus as an emerging neuropathogen: mechanisms of neurovirulence and neuro-immune interactions. Mol Neurobiol 55:4160–4184
Nakahira K, Hisata S, Choi AM (2015) The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal 23(17):1329–1350
Nakatani N, Hattori E, Ohnishi T, Dean B, Iwayama Y, Matsumoto I, Kato T, Osumi N, Higuchi T, Niwa S, Yoshikawa T (2006) Genome-wide expression analysis detects eight genes with robust alterations specific to bipolar I disorder: relevance to neuronal network perturbation. Hum Mol Genet 15(12):1949–1962
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318
Ning H, Wang H, Zhao L, Zhang C, Li XY, Chen YH, Xu DX (2008) Maternally-administered lipopolysaccharide (LPS) increases tumor necrosis factor alpha in fetal liver and fetal brain: its suppression by low-dose LPS pretreatment. Toxicol Lett 176(1):13–19
Pratt L, Ni L, Ponzio NM, Jonakait GM (2013) Maternal inflammation promotes fetal microglial activation and increased cholinergic expression in the fetal basal forebrain: role of interleukin-6. Pediatr Res 74(4):393–401
Radewicz K, Garey LJ, Gentleman SM, Reynolds R (2000) Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol 59(2):137–150
Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19(8):987–991
Rapaport MH, McAllister CG, Pickar D, Tamarkin L, Kirch DG, Paul SM (1997) CSF IL-1 and IL-2 in medicated schizophrenic patients and normal volunteers. Schizophr Res 25(2):123–129
Reus GZ, Simoes LR, Colpo GD, Scaini G, Oses JP, Generoso JS, Prossin AR, Kaddurah-Daouk R, Quevedo J, Barichello T (2017) Ketamine potentiates oxidative stress and influences behavior and inflammation in response to lipolysaccharide (LPS) exposure in early life. Neuroscience 353:17–25
Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E (2007) Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry 7:46
Salter MW, Stevens B (2017) Microglia emerge as central players in brain disease. Nat Med 23(9):1018–1027
Sasayama D, Hattori K, Wakabayashi C, Teraishi T, Hori H, Ota M, Yoshida S, Arima K, Higuchi T, Amano N, Kunugi H (2013) Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res 47(3):401–406
Schmitt A, Leonardi-Essmann F, Durrenberger PF, Parlapani E, Schneider-Axmann T, Spanagel R, Arzberger T, Kretzschmar H, Herrera-Marschitz M, Gruber O, Reynolds R, Falkai P, Gebicke-Haerter PJ (2011) Regulation of immune-modulatory genes in left superior temporal cortex of schizophrenia patients: a genome-wide microarray study. World J Biol Psychiatry 12(3):201–215
Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, Finn A, Bhat M, Samuelsson M, Lundberg K, Dahl ML, Sellgren C, Schuppe-Koistinen I, Svensson C, Erhardt S, Engberg G (2015) Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia – significance for activation of the kynurenine pathway. J Psychiatry Neurosci 40(2):126–133
Sinkus ML, Adams CE, Logel J, Freedman R, Leonard S (2013) Expression of immune genes on chromosome 6p21.3-22.1 in schizophrenia. Brain Behav Immun 32:51–62
Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007) Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27(40):10695–10702
Smolders S, Smolders SM, Swinnen N, Gartner A, Rigo JM, Legendre P, Brone B (2015) Maternal immune activation evoked by polyinosinic:polycytidylic acid does not evoke microglial cell activation in the embryo. Front Cell Neurosci 9:301
Soderlund J, Schroder J, Nordin C, Samuelsson M, Walther-Jallow L, Karlsson H, Erhardt S, Engberg G (2009) Activation of brain interleukin-1beta in schizophrenia. Mol Psychiatry 14(12):1069–1071. https://doi.org/10.1038/mp.2009.52
Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein HG, Bogerts B (2006) Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol 112(3):305–316
Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B (2008) Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 42(2):151–157
Stence N, Waite M, Dailey ME (2001) Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33(3):256–266
Togo T, Akiyama H, Kondo H, Ikeda K, Kato M, Iseki E, Kosaka K (2000) Expression of CD40 in the brain of Alzheimer’s disease and other neurological diseases. Brain Res 885(1):117–121
Toyooka K, Watanabe Y, Iritani S, Shimizu E, Iyo M, Nakamura R, Asama K, Makifuchi T, Kakita A, Takahashi H, Someya T, Nawa H (2003) A decrease in interleukin-1 receptor antagonist expression in the prefrontal cortex of schizophrenic patients. Neurosci Res 46(3):299–307
Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH (2001) Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res 47(1):27–36
van den Eynde K, Missault S, Fransen E, Raeymaekers L, Willems R, Drinkenburg W, Timmermans JP, Kumar-Singh S, Dedeurwaerdere S (2014) Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav Brain Res 258:179–186
Vawter MP, Dillon-Carter O, Issa F, Wyatt RJ, Freed WJ (1997) Transforming growth factors beta 1 and beta 2 in the cerebrospinal fluid of chronic schizophrenic patients. Neuropsychopharmacology 16(1):83–87
Verma R, Bharti K (2017) Toll like receptor 3 and viral infections of nervous system. J Neurol Sci 372:40–48
Volk DW, Chitrapu A, Edelson JR, Roman KM, Moroco AE, Lewis DA (2015) Molecular mechanisms and timing of cortical immune activation in schizophrenia. Am J Psychiatry 172(11):1112–1121
Wierzba-Bobrowicz T, Lewandowska E, Kosno-Kruszewska E, Lechowicz W, Pasennik E, Schmidt-Sidor B (2004) Degeneration of microglial cells in frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol 42(3):157–165
Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepien T, Pasennik E (2005) Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol 43(2):81–89
Wilkins HM, Weidling IW, Ji Y, Swerdlow RH (2017) Mitochondria-derived damage-associated molecular patterns in neurodegeneration. Front Immunol 8:508
Zhang Y, Catts VS, Sheedy D, McCrossin T, Kril JJ, Shannon Weickert C (2016) Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry 6(12):e982
Zhou Y, He C, Wang L, Ge B (2017) Post-translational regulation of antiviral innate signaling. Eur J Immunol 47(9):1414–1426
Acknowledgments
The Translational Psychiatry Program (USA) is funded by the Department of Psychiatry and Behavioral Sciences, McGovern Medical School, The University of Texas Health Science Center at Houston (UTHealth). The Laboratory of Neurosciences (Brazil) is one of the centers of the National Institute for Molecular Medicine (INCT-MM) and one of the members of the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC). This research is supported by grants from CNPq (TB, JQ), FAPESC (TB, JQ), Instituto Cérebro e Mente (JQ), and UNESC (TB and JQ).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Barichello, T., Simoes, L.R., Quevedo, J., Zhang, X.Y. (2019). Microglial Activation and Psychotic Disorders: Evidence from Pre-clinical and Clinical Studies. In: Khandaker, G., Meyer, U., Jones, P. (eds) Neuroinflammation and Schizophrenia. Current Topics in Behavioral Neurosciences, vol 44. Springer, Cham. https://doi.org/10.1007/7854_2018_81
Download citation
DOI: https://doi.org/10.1007/7854_2018_81
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39140-9
Online ISBN: 978-3-030-39141-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)