Abstract
The goals of animal research in post-traumatic stress disorder (PTSD) include better understanding the neurophysiological etiology of PTSD, identifying potential targets for novel pharmacotherapies, and screening drugs for their potential use as PTSD treatment in humans. Diagnosis of PTSD relies on a patient interview and, as evidenced by changes to the diagnostic criteria in the DSM-5, an adequate description of this disorder in humans is a moving target. Therefore, it may seem insurmountable to model the construct of PTSD in animals such as rodents. Fortunately, the neural circuitry involved in fear and anxiety, thought to be essential to the etiology of PTSD in humans, is highly conserved throughout evolution. Furthermore, many symptoms can be modeled using behavioral tests that have face, construct, and predictive validity. Because PTSD is precipitated by a definite traumatic experience, animal models can simulate the induction of PTSD, and test causal factors with longitudinal designs. Accordingly, several animal models of physical and psychological trauma have been established. This review discusses the widely used animal models of PTSD in rodents, and overviews their strengths and weaknesses in terms of face, construct, and predictive validity.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Animal Models of Traumatic Stress
Diagnostic criteria for PTSD according to DSM-5 include expression – for over 1 month – of debilitating symptoms from four clusters following exposure to traumatic events such as threat of death, or actual or threatened serious injury or violence:
-
Cluster B: Intrusion (e.g., nightmares, flashbacks, intrusive thoughts, and physiological reactions to trauma reminders).
-
Cluster C: Avoidance (intentionally avoiding trauma-related people, places, or activities).
-
Cluster D: Negative alterations in cognitions and mood (e.g., dissociative amnesia, negative perception of self and world, anhedonia, social withdrawal).
-
Cluster E: Alterations in arousal and reactivity (e.g., irritability, aggression, problems concentrating, sleep disturbances, and hypervigilance).
The goal of this chapter is to critically review current animal models of trauma exposure and how the behavioral and physiological outcomes of these models translate to these symptom clusters.
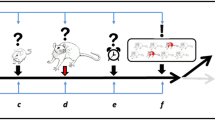
For models of PTSD to reach high translational value, essential requirements have been defined (Siegmund and Wotjak 2006): (1) the trauma must be severe, (2) a relatively short duration should be sufficient to provoke PTSD-like symptoms, (3) the intensity of the trauma should predict the severity of outcome, and (4) the stressor should induce persistent or progressive PTSD-like alterations with (5) significant interindividual variability in outcomes. Existing models apply stressors of a physical, psychological, social, or combined nature.
1.1 Physical Stressors
Restraint stress, underwater holding, and electric shock are all stressors of a physical nature. However, it is generally accepted that these lead to a combined physical and psychological stress from the rodents’ perspective. Application of these stressors varies greatly between laboratories in terms of duration and severity. Although most of these can also be applied as chronic stressors, this discussion focuses on acute stressors in keeping with the above second criteria for an animal model of PTSD.
1.1.1 Restraint/Immobilization Stress
Both restraint and immobilization stress involve placing rodents in an enclosed chamber allowing for minimal or no movement. Total immobilization can be considered the most severe of the restraint methods. Two hours of complete immobilization has been shown to increase anxiety-like behavior in the elevated plus maze and open-field tests (Andero et al. 2013; Mitra et al. 2005), increase compulsive-like behavior in the marble-burying test (Kedia and Chattarji 2014), reduce declarative memory performance in the water maze task, increase fear learning (Andero et al. 2013), and increase REM sleep (Meerlo et al. 2001). Importantly, avoidance and morphological changes mostly manifested after a significant delay (i.e., 10 days) (Andero et al. 2013; Mitra et al. 2005; Kedia and Chattarji 2014). In line with endocrine data from PTSD patients (Yehuda et al. 2006), when immobilization stress was followed 1, 7, or 13 days later by a 20-min reminder restraint, significant hypoactivity of the hypothalamic pituitary adrenal (HPA) axis was induced, as measured by decreased concentrations of ACTH and corticosterone (Harvey et al. 2006).
1.1.2 Underwater Holding
In rats, underwater trauma typically involves 40 s of forced swimming followed by a 20-s forced submersion (Richter-Levin 1998). Rats exposed to this stress exhibit increased startle reactivity and anxiety-like behavior in the elevated plus maze both immediately, 7, and 30 days later with reduced corticosterone levels 7 days after stress (Richter-Levin 1998; Cohen et al. 2004; Moore et al. 2012). Additionally, contextual fear with altered limbic activity persists for over a month in this model (Ritov et al. 2016).
1.1.3 Single Prolonged Stress
Single prolonged stress (SPS) paradigms typically involve three stressors: 2-h restraint followed by forced swim, followed by ether anesthesia. These stressors induce psychological, physiological, and endocrine stress, respectively. Rats exposed to this SPS procedure exhibit increased anxiety-like behavior in the elevated plus maze, enhanced fear acquisition, and reduced fear extinction learning (Imanaka et al. 2006; Knox et al. 2012; Takahashi et al. 2006; Wang et al. 2008; Yamamoto et al. 2009). Halothane-combined SPS exposure also increased anxiety-like behavior; corticosterone concentration was elevated 1 day following SPS but normalized by day 7 (Harvey et al. 2006). Interestingly, when rats were exposed to a reminder restraint, increased anxiety-like behavior was associated with a decreased corticosterone response, suggesting that the initial trauma is insufficient to produce PTSD-like symptoms in the absence of a reminder (Harvey et al. 2006). Increased anxiety-like behavior in the open field and increased immobility in the forced swim test 1 and 7 (but not 4) days were also apparent following SPS (Wu et al. 2016). The authors suggest that relevant compensatory changes occur in the first week following SPS exposure explain the timing of these effects (Wu et al. 2016).
Similar effects have been observed in mice: restraint, group swim, rat bedding (as a predator exposure) followed by ether exposure until unconsciousness led to enhanced cue-induced freezing, reduced extinction, and increased HPA axis negative feedback in the dexamethasone-suppression test, presumably due to elevated hippocampal expression of the glucocorticoid receptor (Perrine et al. 2016).
PTSD-like disruptions in sleep have also been observed in rats exposed to SPS. EEG recordings revealed that on the day of SPS exposure, rats exhibited increased REM sleep and decreased wakefulness (Vanderheyden et al. 2015). While these changes were not sustained over time, a decrease in non-REM sleep was observed during the active phase up to 7 days following trauma (Vanderheyden et al. 2015).
Morphological changes were also observed in SPS models including apoptotic volume loss in the hippocampus and the dorsal raphe nucleus (Han et al. 2013; Liu et al. 2012a) that may correspond with human findings reporting volumetric decreases in these brain regions in PTSD (Carrion et al. 2009; Gilbertson et al. 2002).
Selective serotonin reuptake inhibitors (SSRIs), a common treatment in PTSD patients (Steckler and Risbrough 2012), have been shown to reduce or prevent PTSD-like behavioral and endocrine symptoms in some SPS paradigms (Wang et al. 2008; Perrine et al. 2016) but not others (Takahashi et al. 2006). This difference could be due to laboratory differences in SPS design, SSRI administration, or behavioral testing. Of note, SSRIs are also ineffective in a portion of human PTSD patients (Stein et al. 2002); therefore successful symptom alleviation with SSRIs may not represent a meaningful concern from a validity perspective. Another possibility is that while SSRIs successfully treat comorbid anxiety and depression in PTSD patients, they may not truly address the core symptoms. As evidence for this hypothesis, a recent study demonstrated that the SSRI escitalopram reduced depressive-like and avoidance symptoms, but not fear extinction deficits in mice exposed to SPS (Lin et al. 2016).
1.1.4 Electric Shock
Uncontrollable and unpredictable foot shock is the most common method of stress exposure and even a single exposure can lead to long-lasting behavioral changes (reviewed in Bali and Jaggi 2015). Significant advantages of electric shock are (1) reliable delivery and precise control of the number and amperage of the shocks, length of the session, and inter-shock intervals; (2) well-defined and reproducible context and environmental cues by using standard boxes with lights and speakers; and (3) adjustable changes in contextual modalities, cues, and reminders. Another potential advantage of using electric shock is that, compared to restraint stress, there is less habituation (Siegmund and Wotjak 2007a). A potential limitation of electric shock as a model of PTSD-like trauma is that compared to human subjects and other animal trauma models, there is relatively little individual variability in terms of sensitivity and resiliency; the vast majority of subjects exposed to electric shock will go on to display behavioral consequences to stress (reviewed in Bali and Jaggi 2015).
The underlying vulnerability, progressive development, and persistence of PTSD symptoms can only be modeled using long-term testing covering multiple symptom domains. Consistent with clinical criteria, several rodent studies reveal progressive development and persistence (3–5 weeks following single or multiple shocks, with or without subsequent reminders) of PTSD-like symptoms, including social withdrawal or avoidance, defensive behavior, hypervigilance, sleep disturbances, and generalization of fear (Siegmund and Wotjak 2007a; Cullen et al. 2015; Louvart et al. 2005; Mikics et al. 2008a, b; Philbert et al. 2011; Pynoos et al. 1996). Importantly, these studies applied somewhat higher amperage (0.8–3.0 mA) compared to most conditioned fear paradigms focusing on fear learning characteristics on a short term (~0.5–1.5 mA). Increased fear response over time in vulnerable individuals has been conceptualized as “incubation of fear” (Siegmund and Wotjak 2007a; Elharrar et al. 2013; Eysenck 1968; Milad et al. 2009) that refers to recurrent recalls leading to sensitization of fear and stress responses, and reconsolidation of traumatic memories, in contrast with the progressive habituation and extinction of traumatic memories in well-adjusting (aka resistant) individuals. Because exposure therapies in clinical settings correspond well with reexposures of animals to shock context or cues, animal models will likely provide important mechanistic information about how cue-based, out-of-context (i.e., reminders in therapy office), and “in-the-context” (virtual reality) exposure therapies are mediated and may be enhanced effectively.
It is important to note some confusion between paradigms that use electric shock as a single excessive traumatic stressor to induce lasting symptoms of PTSD and those that induce conditioned fear to understand the elements of fear learning. Electric shock as a trauma exposure, as described above, focuses on long-term outcomes on multiple PTSD domains (e.g., freezing, startle, avoidance, endocrine, and neuromorphological outcomes), and as such, it is more specific to PTSD. In contrast, fear conditioning, described below, investigates neurobiological mechanisms underlying learning processes relevant to fear and anxiety disorders, uses short-term protocols, and measures conditioned fear response (i.e., freezing) or enhanced unconditioned fear response (i.e., fear-potentiated startle).
1.1.4.1 Shock-Induced Conditioned Fear
Conditioned fear models are based on the classical findings of Pavlov, who originally described that an association is formed between a neutral “conditioned” stimulus and an unconditioned stimulus following repeated co-presentations. Fear conditioning investigates elements of fear learning: fear acquisition, recall, extinction, and reinstatement and renewal of fear memories (Quirk et al. 2010; VanElzakker et al. 2014).
Most conditioned fear studies deliver scrambled electric shocks via metal grid floor in acoustically isolated plastic boxes with no escape. Major parameters (e.g., conditioned stimulus intensity, number of acquisition trials, intensity of shock) can be modified to optimize phenotypic outcome to avoid ceiling/floor effects. These modifications are essential given the significant differences across species (mice vs. rats) and strains in fear learning, presumably due to differences in nociception, exploratory activity, or other background characteristics (Bolivar et al. 2001; Keeley et al. 2015; Keum et al. 2016; Schaap et al. 2013; Stiedl et al. 1999). Shock exposure typically ranges from one to ten 0.3–3 mA shocks lasting between 1 and 2 s each, administered during a 5- to 30-min session (Mikics et al. 2008b; Aliczki and Haller 2015; Davis and Gould 2007; Lehner et al. 2010; Roozendaal et al. 2006; Sanford et al. 2010; Shoji et al. 2014; Siegmund and Wotjak 2007b). For cued associations, shocks are commonly co-presented with novel and salient cues such as sound, light, or odor. These specific cues can be used to dissect context- and cue-dependent fear recall, which are mediated in part by unique neural circuits (Marschner et al. 2008; Phillips and LeDoux 1992). Importantly, these neural circuits are highly conserved across species such that morphological and functional changes in animal models are analogous to those in PTSD patients (Acheson et al. 2012). An overview of conditioned fear literature is beyond the limits of this review; for more detailed reviews see Herry and Johansen (2014); Milad and Quirk (2012); and VanElzakker et al. (2014).
There are two major arguments in support of simple conditioned fear models in PTSD research. First, its face validity is high given that a single shock session as traumatic experience induces fear response with strong associative memory. Second, several reports show alterations of fear conditioning characteristics in PTSD including enhanced fear acquisition, blunted extinction, and relapse (Acheson et al. 2015; Jovanovic et al. 2012; Norrholm et al. 2011). Indeed, conditioned fear paradigms are translatable between animal and human laboratories by using similar procedural protocols (Parsons and Ressler 2013; Risbrough et al. 2016; Singewald et al. 2015). Accordingly, fruitful interactions between animal and human conditioning models have been reported, particularly in endeavors to develop effective pharmacological enhancers of extinction learning (Bowers and Ressler 2015; Johnson et al. 2012). Animal studies also advanced our understanding of how associative components of fear memories are acquired, consolidated, and extinguished spontaneously or following repeated exposures to trauma-related stimuli. There exists a growing body of evidence mapping the specific prefrontal cortex, hippocampal, and amygdala neurocircuits that modulate fear learning and inhibition via distinct neurotransmitter systems (Haubensak et al. 2010; Liu et al. 2012b; Rozeske et al. 2015). In support of translational efforts, these studies suggest that fear circuitry is comparable between rodents and humans (Mahan and Ressler 2012).
Despite the advantages listed above, there exist major concerns about short-term conditioned fear paradigms as models of PTSD. Namely, most models ignore the temporal and differential diagnostic characteristics of PTSD. According to the 5th edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association 2013), acute symptoms must be separated from long-term, chronic symptomatology. The former is defined as “acute stress disorder” and may be an adaptive stress-coping response in well-adjusting individuals, whereas in vulnerable individuals, PTSD symptoms are progressive and persistent and develop after a delay (DSM-5). Moreover, PTSD is no longer categorized as an “anxiety disorder” in DSM-5; rather, it is defined as “trauma- and stressor-related disorder.” Accordingly, differential diagnostic criteria of PTSD require multiple-domain testing, especially given the heterogeneity of PTSD.
Fear-based reexperiencing (or intrusive memories) is a core symptom and is thought to be best modeled by fear conditioning in animals and humans. Acute and chronic phases of fear learning may have differential mechanisms and potential treatment targets; there is differential neuronal activation in the prefrontal cortex, amygdala, hippocampus, and monoaminergic systems during acute (24 h post-trauma) vs. remote (3–5 weeks) cued fear recall (Cullen et al. 2015; Mikics et al. 2008b; Frankland et al. 2004; Goshen et al. 2011; Tulogdi et al. 2012). Models focusing exclusively on acute phenotypic changes, short-term fear learning characteristics, and associative memory components of PTSD may have questionable construct validity and specificity. Considering the long-lasting behavioral consequences following a single shock session in several studies (Siegmund and Wotjak 2007a), conditioned fear models could potentially detect long-term PTSD-like outcomes if testing were extended.
1.2 Social and Psychological Stressors
1.2.1 Social Defeat Stress (SDS)
Social defeat stress (SDS) has been used as a robust stressor in both rat (Koolhaas et al. 2013) and mouse models (Golden et al. 2011) to induce depression (reviewed in Krishnan and Nestler 2011) and PTSD-like symptoms (reviewed in Daskalakis and Yehuda 2014). Evidence for SDS as a valid model for PTSD includes the following: a single defeat can lead to anxiety-like behavior, and behavioral abnormalities continue long after initial defeat (Pulliam et al. 2010).
As with other stress models, the intensity and duration of SDS can be modified. Most mouse SDS models follow a protocol in which experimental subjects as “intruders” are exposed to aggressive resident mice for 5–10 min, followed by 24 h of sensory (but not physical) exposure (Golden et al. 2011). This procedure is repeated for 5–10 days during which the intruder is exposed to different aggressor (Golden et al. 2011). PTSD-like symptoms following this social defeat exposure include decreased social exploration, anhedonia, and increased anxiety-like behavior, which can be reduced with chronic but not acute treatment with antidepressant drugs (Berton et al. 2006; Der-Avakian et al. 2014). Importantly, the PTSD-like symptoms are expressed by only 60–70% of exposed subjects, providing an inherent model of sensitivity and resiliency (Golden et al. 2011). Similar rates of sensitivity and resiliency have been achieved by 10 days of “witnessed social defeat stress.” In this modification, the test mouse witnesses a peer exposed to an aggressive resident and then spends 24 h in sensory contact with that aggressor (Warren et al. 2013).
Another modified SDS model employs 6 h of indirect exposure of a partially restrained intruder mouse to an aggressive resident; within those 6 h, researchers randomly allow 1–3 direct exposures of 1 min each (Hammamieh et al. 2012). The authors argue that this model increases the unpredictability and uncontrollability of the stress exposure; this hypothesis is supported by data showing increased freezing behavior 28 days following a 10-day exposure, and increased grooming behavior 42 days following a 10-day exposure (Hammamieh et al. 2012; Muhie et al. 2015).
Although most SDS models violate the criteria of a single traumatic exposure (Yehuda and Antelman 1993), SDS may be relevant for socially induced or combat-related PTSD, which typically do involve multiple exposures. SDS may also be particularly relevant for comorbidity with depression. An important limitation of the SDS model is the lack of protocol for social stress in females. It has been suggested that ovariectomized female mice from an aggressive strain could be primed with male steroids and used as an appropriate resident for an SDS model in females (Daskalakis and Yehuda 2014), indicating that future studies are needed to establish models in females.
1.2.2 Predator Stress
In predator stress models, rodents are exposed to their natural predators or to their odor. In live predator exposure, the predator is well fed and habituated to rodents; therefore no physical injuries occur. In contrast to the physical stressors, which apply well-defined and precise traumatic experience, predator stress models focus on ecological validity to increase translatability.
Predator exposure immediately elicits unconditioned fear and marked stress responses in rodents (Adamec et al. 2006; Adamec and Shallow 1993; Blanchard and Blanchard 1989; Blanchard et al. 1998; Cohen et al. 2003; Dielenberg and McGregor 2001; Zoladz et al. 2008). A single predator exposure (1) increases avoidance behavior of both novel and predator-related stimuli (cluster C in DSM-5; Cohen et al. 2004; Toth et al. 2016), (2) induces cognitive impairments and negative mood-like states as measured by spatial learning in the Morris water maze (cluster D; Diamond et al. 2006), and (3) increases hyperarousal and hypervigilance as measured by startle reactivity (cluster E; Adamec et al. 2010). Depending on the specific predator stress model employed, enduring behavioral changes can last up to a month or more after exposure, replicating the chronic nature of PTSD (Adamec and Shallow 1993; Zoladz et al. 2012). These behavioral changes coincide with long-term structural changes of the hippocampus and the amygdala that are in line with circuit changes observed in PTSD patients (Diamond et al. 2006; Adamec et al. 2012; Mitra et al. 2009).
In a modified predator exposure, rodents are in a porous container to allow sensory but not physical exposure to live cats (Diamond et al. 1999; Mesches et al. 1999). The authors argue that besides offering physical protection, the container can enhance fear by increasing uncontrollability and helplessness. The exposure in this model is 30–75 min long. To maximize the predator interest across the session, food is placed on top of the container in which the rodent is held. This single exposure can induce long-term memory and cognitive deficits in rats (Diamond et al. 1999; Mesches et al. 1999; Woodson et al. 2003). In a version of this model, rodents are also reexposed to the same procedure 10 days later. Because the reexposure is identical to the traumatic event itself, it is not clear if this modification simulates “flashback-like memory retrieval” as the authors indicate or simply increases the “dose” of trauma. According to a recent study, a single reexposure instigated PTSD-like symptoms while a second reexposure was not able to further increase predator-induced effects and actually caused a slight decrease in PTSD-like symptoms (Zoladz et al. 2015), potentially due to habituation.
Social instability after the initial trauma has been used as a chronic stressor to exacerbate PTSD-like behaviors (Zoladz et al. 2008). Three weeks after this procedure, increased anxiety-like behavior, startle hyperreactivity, and spatial memory deficits are observed (Zoladz et al. 2008, 2012). This combined paradigm also results in reduced thymus and adrenal weights, reduced basal glucocorticoid levels, and increased dexamethasone suppression of the HPA axis (Zoladz et al. 2008, 2012), physiological and endocrine changes corresponding with HPA abnormalities in PTSD (Yehuda et al. 1993). This paradigm was also shown to impair spatial memory, presumably secondary to structural and functional plasticity changes in the hippocampus (Diamond et al. 2006; Mesches et al. 1999; Sandi et al. 2005). These symptoms and structural changes are similar to observations in PTSD patients (O'Doherty et al. 2015).
Exposure only to olfactory cues from a predator can also induce behavioral signs of fear in rats and mice, and activate brain regions mediating defensive, fear, and anxiety-like responses, such as the lateral septum, extended amygdala, paraventricular nucleus of the hypothalamus, hypothalamic circuits mediating defensive reaction, and periaqueductal gray (Dielenberg and McGregor 2001; Canteras et al. 2015; Takahashi 2014). Predator odor methods use litter or bedding from a predator, fur and/or urine, a cat-worn collar, or trimethylthiazoline, a synthetic component of fox feces. Based on data from field and laboratory studies, the fear-eliciting efficacy of predator odor exposure is highly variable; fur and used litter appear to be the most effective odor stimuli (Takahashi 2014; Apfelbach et al. 2005).
In support of the predictive validity of predator stress paradigms, benzodiazepines, SSRIs, and the tricyclic antidepressant tianeptine have all been shown to efficiently reduce some PTSD-like symptoms of predator exposure (Dielenberg and McGregor 2001; Vouimba et al. 2006; Zoladz et al. 2013).
2 Operationalizing PTSD-Like Symptoms in Animal Models
The DSM-5 divides PTSD symptoms into four major symptom clusters: intrusion, avoidance, negative alterations in cognitions and mood, and alterations in arousal and reactivity. There exist numerous validated behavioral tests in rodents designed to operationalize these constructs. These behavioral tests can be used to assess sensitivity or resiliency to trauma exposure and to screen novel pharmaceutical treatments.
Assessing intrusive (i.e., reexperiencing) symptoms (Cluster B) is not possible in animal models; however measuring physiological reactions to trauma-related cues can operationalize fear memory processes thought to underlie reexperiencing symptoms. Approach-avoidance conflict tests such as the elevated plus maze, elevated zero maze, open-field arena, and light-dark box are designed to measure avoidance behavior (Cluster C) and generalized-like anxiety in different contexts, and they are widely used in preclinical research with reasonable predictive validity, although there are limitations in their use (Adamec 1997; Bailey and Crawley 2009; Belzung and Griebel 2001; File and Seth 2003; McCormick and Green 2013; Olson et al. 2011). Trauma-specific avoidance can also be measured via introduction of trauma-related cues, e.g., predator scent in the case of predator stress, novel conspecific in the case of social defeat stress, and training context in the case of active and passive avoidance.
Negative alterations in mood (depression-like symptoms; Cluster D) are commonly measured by tests of “behavioral despair,” i.e., reduced activity in forced swim and tail suspension tests or decreased preference of sucrose as a sign of anhedonia, or reduced intracranial self-stimulation. Predominantly, these tests are based on their predictive validity for antidepressant medications which are also somewhat effective in PTSD (Cryan et al. 2005). Developing additional measures of negative mood in rodents should be an active area of continued research to expand reliability and validity and minimize locomotion and memory confounds.
Modeling negative alterations in cognition (Cluster D) is highly limited in animals as these symptoms are manifested in distorted, negatively tuned self- and environment interpretations. However, more general cognitive deficits are commonly measured, i.e., attention, spatial learning, and social recognition. For spatial learning and memory, Morris water maze became a golden standard test (Vorhees and Williams 2006), although less stressful versions, i.e., Barnes maze, T-maze, or radial maze (Sharma et al. 2010), are also widely used and may be advantageous in certain paradigms where testing with minimal stress is crucial. Attention and more complex learning characteristics can be reliably assessed in rodents using the five-choice paradigm (Chudasama and Robbins 2004); there is no data available on this specific symptom domain in the most common models of PTSD (see Table 1). As specific neurocircuits are involved in these tasks with documented changes in PTSD (Gilbertson et al. 2002; Moser and Moser 1998), identification of neurobiological changes including structural and functional alterations of the prefrontal and hippocampal networks can increase the construct validity of these models.
Trauma-induced hyperarousal and hyperreactivity can be assessed by startle amplitude, its habituation, and prepulse inhibition (Adamec et al. 2010). Hypervigilance can be measured using object-burying paradigms or locomotor changes (Mikics et al. 2008a; Philbert et al. 2011). Irritability-impulsivity can be approximated using the resident-intruder test, and delayed discounting paradigm (Fodor et al. 2014; Mar and Robbins 2007); however, none of the described models have been shown to result in such externalizing-like symptoms. Sleep disturbances (altered structure or quantitative changes in stages) can be measured using EEG signals (Pawlyk et al. 2008). For instance, sleep disturbances in trauma-exposed rats correlated with other behavioral symptoms; rats with the greatest increase in REM sleep following SPS exposure also had the largest percent of freezing behavior in a fear conditioning paradigm (Vanderheyden et al. 2015).
To outline a current status of preclinical models of PTSD, Table 1 summarizes which clusters and symptoms have been induced in particular models, also indicating symptoms, which need to be addressed as data are not available. Noteworthy, we did not include models and symptoms, where repeated stress exposure is required to induce phenotypic changes, e.g., anhedonia or exaggerated startle following repeated exposures, to be more specific to PTSD, and eliminate mixed (e.g., depression-like) models.
3 The “Cutoff Behavioral Criteria” Model of PTSD: Focus on Vulnerability and Resiliency Factors
Large epidemiological studies estimated the incidence of PTSD following traumatic events around 10–20% with significant sex differences (i.e., 8–13% for men and 20–30% for women), although these rates are highly dependent on the type of the trauma (Breslau et al. 1991, 1997; Kessler et al. 1995). Accordingly, animal models have sought to use individual variability as a means to identify the neurobiological risk factors and underpinnings of PTSD.
The “cutoff behavioral criteria” established by Cohen and colleagues (2006a, 2012) used predator stress as the trauma; according to predefined behavioral “cutoff” criteria, traumatized animals are assigned to “extreme behavioral response” (EBR; aka vulnerable) and “minimal behavioral response” (MBR; aka resilient) groups based on their post-stress startle reactivity and performance on the elevated plus maze. Although potentially other behavioral, endocrine, or physiological measurements could be used as cutoff criteria, startle reactivity and avoidance on the elevated plus maze detect changes in measures of hyper-alertness and overall behavioral avoidance/anxiety, modeling two main symptom clusters of PTSD. To maximize effect size and interpretability of the data, the group of rodents between EBR and MBR is considered to have a “partial behavioral response,” and not investigated (Cohen et al. 2006a).
The cutoff criteria described by Cohen and colleagues are fairly conservative, yet the rate of EBR varies substantially between strains. For rodents to meet EBR criteria they must spend the entire 5-min elevated plus session in the closed arms, and exhibit >800 units startle amplitude without habituation. MBR criteria required rodents to spend less than 1 of 5 min in closed arms of the elevated plus, and to exhibit <700 units startle amplitude with significant habituation over time (Matar et al. 2013). Using these criteria with Sprague-Dawley rats, EBR rates 24 h following predator stress may be as high as 90%. However, similar to humans, acute-anxiety symptoms fade in well-adapted animals; the EBR rate drops sharply in the days immediately following predator stress (Cohen et al. 2004). For Sprague-Dawley rats, EBR incidence settles at around 25% (Cohen et al. 2003), which is comparable with human epidemiological data (Breslau et al. 1991; Kessler et al. 1995). The EBR phenotype can persist for more than 3 weeks in the 25% of experimental subjects classified as EBR (Cohen et al. 2004), which is again compatible with the temporal characteristic of human PTSD (Cohen et al. 2004). However, EBR rates vary significantly between strains in rats (10% and 50% in Lewis and in Fisher rats, respectively) (Cohen et al. 2006a, b), and mice (from 6% to 55% in the most widely used strains, DBA/2J and C57Bl/6J, as two extremes of the spectrum, respectively) (Cohen et al. 2008). Importantly, extreme behavioral response at baseline (without stress exposure) is hardly detectable in experimental populations, i.e., 1.3% (Cohen et al. 2012), confirming that EBR is clearly induced by trauma exposure, and lasts for weeks only in a vulnerable subpopulation (i.e., 25%).
Studies using these behavioral cutoff criteria reported enhanced stress reactivity in EBR subjects as measured by increased heart rate and sympathetic activity, and higher plasma corticosterone and adrenocorticotropic hormone concentrations (Cohen et al. 2003, 2005). EBR subjects also exhibited additional plasticity-related changes in the hippocampus, such as reduced expression of BDNF, synaptophysin, and ERK1/2 pathway elements, and elevated expression of glucocorticoid receptor and postsynaptic density protein-95 mRNA (Matar et al. 2013; Cohen et al. 2014; Kozlovsky et al. 2007). Importantly, acute and 7-day-long treatment with the SSRI sertraline was able to decrease anxiety, reduce startle reactivity, and significantly decrease the number of rodents meeting EBR criteria (Matar et al. 2006).
Importantly, this cutoff criteria approach can be applied to other PTSD models to compare vulnerable and resilient subjects. An underwater trauma model found that EBR rats exhibited lasting anxiety and hyperarousal (Cohen et al. 2004). In another study, mice were exposed to electric shock (14× 1-s-long shocks of 1 mA with variable intervals over 85-min period) followed 24 h later by a shock “trigger” (5× 1-s-long shocks of 0.7 mA with fixed intervals over 5 min) and tested behavioral and endocrine outcomes 7–16 days later. Based on a cumulative 5-test criteria system (upper quintiles in the marble-burying test, acoustic startle, pre-pulse inhibition, light-dark box, and home cage locomotor activity), researchers categorized subjects as “resilient” or “PTSD-like” (20–20% of the population). Compared to resilient subjects, mice with PTSD-like phenotype exhibited blunted corticosterone response to acute restraint stress with significant upregulation of hippocampal glucocorticoid receptors (Lebow et al. 2012). Moreover, PTSD-like phenotype was associated with altered expression of a number of “PTSD candidate” genes (i.e., previously reported as risk polymorphisms in human studies) in the bed nucleus of stria terminalis (Lebow et al. 2012).
In summary, despite low genetic variability, animal models of PTSD using inbred strains provide substantial individual variability for application of cutoff criteria. Models that can reliably and robustly identify resistant and vulnerable populations may be highly valuable to test the causal contribution of developmental risk factors and candidate mechanisms identified by human studies (e.g., single polymorphisms).
4 Special Challenges
While the animal models described above have strong face and predictive validity, reaching full diagnosis (based on human DSM-5 criteria) in a single animal model may not be feasible. Most of the symptoms operationalized in rodent PTSD models such as associative fear, avoidance, anhedonia, or hypervigilance are also described in other anxiety or mood disorders; thus specificity of a given model for PTSD is a challenge. Additionally, some symptoms are difficult (if not impossible) to model in animals, e.g., intrusive memories and flashbacks, feeling emotional numb, or detachment. These challenges might mean that we cannot create a rat or mouse model that is comprehensive and specific to PTSD. However, it is relevant to note that the symptoms of PTSD have significant overlap with mood and anxiety disorders and, furthermore, PTSD is frequently found comorbid with mood, anxiety, and substance-use disorders (Sareen 2014). PTSD patients also show extensive heterogeneity in symptom presentation, with over 20 possible symptoms described in DSM-5. To account for this heterogeneity, preclinical and clinical characterizations have shifted focus from diagnosis to domain-based conception of disorders based on biological mechanisms and their domain-like organization (i.e., Research Domain Criteria-RDoC proposed by NIMH), e.g., distinct and definite mechanisms underlying reexperiencing and hyperarousal in PTSD (Cuthbert 2014; Cuthbert and Insel 2013). This approach may increase translatability of animal findings in the clinics in the future, and benefit for our understanding of the pathogenesis of PTSD.
Although the above-described models have been found to successfully model some aspects of PTSD, lack of data on particular symptoms in particular models (e.g., externalizing or attention symptoms), negative findings (likely significant amount unpublished), and inconsistencies between laboratories still exist, which is difficult to interpret as experimental procedures show high variability between laboratories. Each laboratory has a unique method of trauma exposure and optimized behavioral test battery and specific optimized protocols to assess symptoms. For example “incubation time” periods between trauma and testing (~1–5 weeks), testing time (light or dark cycle), or the order of tests in a battery, and the specific tests included in a battery, all vary between laboratories.
5 Conclusions and Future Perspectives
Development of valid animal models with high translational values is a constant challenge for preclinical research and needs recurrent updates to maximize efficacy. On the one hand, diagnostic criteria are constantly shifting targets as clinical and epidemiological data are accumulating. Additionally, identification and interpretation of measured behaviors in animal models is a complex task as variables or behavioral symptoms must be ecologically valid in the behavioral repertoire of the experimental species and still translatable to humans.
To better understand the complex etiology and mechanisms of PTSD and specific contributions of different symptom domains will require wider screening of phenotypic changes in different models. From a translational standpoint, it would be particularly important to identify pathways linked to vulnerability and resilience against traumatic stress either causally or as robust and measurable predictive markers (e.g., blood-based markers). In this respect, the “cutoff behavioral criteria” strategy and comparative studies contrasting biological characteristics of resilient and vulnerable populations provide an essential approach either to clarify the causal role of candidate mechanisms identified by humans studies (e.g., polymorphisms) or to implicate new targets to test them in human studies.
Although large animal numbers and extensive screening are necessary to produce behavioral extremes with the necessary statistical power for reliable and reproducible results, the highly variable outcomes observed after trauma exposure in humans clearly warrant the application of such responder/nonresponder classification approaches based on the fact that only a portion of trauma-exposed individuals develop PTSD symptoms.
As a final comment, the past decade has seen a substantial increase in PTSD research in both humans and animal models. Building upon this literature is essential to developing tools that can actually improve the human experience. Although sex differences were beyond the scope of this review, to effectively study this topic will require expanding the subject pool more consistently to include females. The NIMH mandate to include female cells, tissue, and subjects in preclinical research will certainly push the field towards addressing this gap.
References
Acheson DT, Gresack JE, Risbrough VB (2012) Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology 62:674–685
Acheson DT, Geyer MA, Baker DG, Nievergelt CM, Yurgil K, Risbrough VB, Team M-I (2015) Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty Marines. Psychoneuroendocrinology 51:495–505
Adamec R (1997) Transmitter systems involved in neural plasticity underlying increased anxiety and defense—implications for understanding anxiety following traumatic stress. Neurosci Biobehav Rev 21:755–765
Adamec RE, Shallow T (1993) Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav 54:101–109
Adamec R, Head D, Blundell J, Burton P, Berton O (2006) Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol Behav 88:12–29
Adamec R, Fougere D, Risbrough V (2010) CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. Int J Neuropsychopharmacol 13:747–757
Adamec R, Hebert M, Blundell J, Mervis RF (2012) Dendritic morphology of amygdala and hippocampal neurons in more and less predator stress responsive rats and more and less spontaneously anxious handled controls. Behav Brain Res 226:133–146
Aliczki M, Haller J (2015) Electric shock as model of post-traumatic stress disorder in rodents. In: Martin CR, Preedy VR, Patel VB (eds) Comprehensive guide to post-traumatic stress disorder. Springer International Publishing, Cham
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Washington, DC
Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, Bannister TD, Almli L, Stevens JS, Bradley B et al (2013) Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci Transl Med 5:188ra173
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29:1123–1144
Bailey KR, Crawley JN (2009) Anxiety-related behaviors in mice. In: Buccafusco JJ (ed) Methods of behavior analysis in neuroscience. CRC Press, Boca Raton, FL
Bali A, Jaggi AS (2015) Electric foot shock stress adaptation: does it exist or not? Life Sci 130:97–102
Belzung C, Griebel G (2001) Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res 125:141–149
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M et al (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868
Blanchard RJ, Blanchard DC (1989) Antipredator defensive behaviors in a visible burrow system. J Comp Psychol 103:70–82
Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC (1998) Behavioral and endocrine change following chronic predatory stress. Physiol Behav 63:561–569
Bolivar VJ, Pooler O, Flaherty L (2001) Inbred strain variation in contextual and cued fear conditioning behavior. Mamm Genome 12:651–656
Bowers ME, Ressler KJ (2015) An overview of translationally informed treatments for posttraumatic stress disorder: animal models of pavlovian fear conditioning to human clinical trials. Biol Psychiatry 78:E15–E27
Breslau N, Davis GC, Andreski P, Peterson E (1991) Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry 48:216–222
Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR (1997) Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry 54:1044–1048
Canteras NS, Pavesi E, Carobrez AP (2015) Olfactory instruction for fear: neural system analysis. Front Neurosci 9:276
Carrion VG, Weems CF, Watson C, Eliez S, Menon V, Reiss AL (2009) Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res 172:226–234
Cassella JV, Davis M (1985) Fear-enhanced acoustic startle is not attenuated by acute or chronic imipramine treatment in rats. Psychopharmacology (Berl) 87:278–282
Chudasama Y, Robbins TW (2004) Psychopharmacological approaches to modulating attention in the five-choice serial reaction time task: implications for schizophrenia. Psychopharmacology (Berl) 174:86–98
Cohen H, Zohar J, Matar M (2003) The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol Psychiatry 53:463–473
Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G (2004) Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology 29:1962–1970
Cohen H, Zohar J, Matar MA, Kaplan Z, Geva AB (2005) Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biol Psychiatry 58:640–650
Cohen H, Matar MA, Richter-Levin G, Zohar J (2006a) The contribution of an animal model toward uncovering biological risk factors for PTSD. Ann N Y Acad Sci 1071:335–350
Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z (2006b) Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry 59:1208–1218
Cohen H, Geva AB, Matar MA, Zohar J, Kaplan Z (2008) Post-traumatic stress behavioural responses in inbred mouse strains: can genetic predisposition explain phenotypic vulnerability? Int J Neuropsychopharmacol 11:331–349
Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z (2012) Animal model for PTSD: from clinical concept to translational research. Neuropharmacology 62:715–724
Cohen H, Kozlovsky N, Matar MA, Zohar J, Kaplan Z (2014) Distinctive hippocampal and amygdalar cytoarchitectural changes underlie specific patterns of behavioral disruption following stress exposure in an animal model of PTSD. Eur Neuropsychopharmacol 24:1925–1944
Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625
Cullen PK, Gilman TL, Winiecki P, Riccio DC, Jasnow AM (2015) Activity of the anterior cingulate cortex and ventral hippocampus underlie increases in contextual fear generalization. Neurobiol Learn Mem 124:19–27
Cuthbert BN (2014) The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatryn 13:28–35
Cuthbert BN, Insel TR (2013) Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 11:126
Daskalakis NP, Yehuda R (2014) Principles for developing animal models of military PTSD. Eur J Psychotraumatol 5
Davis M (1989) Sensitization of the acoustic startle reflex by footshock. Behav Neurosci 103:495–503
Davis JA, Gould TJ (2007) beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 190:343–352
Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A (2014) Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry 76:542–549
Diamond DM, Park CR, Heman KL, Rose GM (1999) Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 9:542–552
Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF (2006) Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus 16:571–576
Dielenberg RA, McGregor IS (2001) Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev 25:597–609
Elharrar E, Warhaftig G, Issler O, Sztainberg Y, Dikshtein Y, Zahut R, Redlus L, Chen A, Yadid G (2013) Overexpression of corticotropin-releasing factor receptor type 2 in the bed nucleus of stria terminalis improves posttraumatic stress disorder-like symptoms in a model of incubation of fear. Biol Psychiatry 74:827–836
Eysenck HJ (1968) A theory of the incubation of anxiety-fear responses. Behav Res Ther 6:309–321
File SE, Seth P (2003) A review of 25 years of the social interaction test. Eur J Pharmacol 463:35–53
Fodor A, Barsvari B, Aliczki M, Balogh Z, Zelena D, Goldberg SR, Haller J (2014) The effects of vasopressin deficiency on aggression and impulsiveness in male and female rats. Psychoneuroendocrinology 47:141–150
Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ (2004) The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304:881–883
Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK (2002) Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 5:1242–1247
Golden SA, Covington HE 3rd, Berton O, Russo SJ (2011) A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6:1183–1191
Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K (2011) Dynamics of retrieval strategies for remote memories. Cell 147:678–689
Hammamieh R, Chakraborty N, De Lima TC, Meyerhoff J, Gautam A, Muhie S, D’Arpa P, Lumley L, Carroll E, Jett M (2012) Murine model of repeated exposures to conspecific trained aggressors simulates features of post-traumatic stress disorder. Behav Brain Res 235:55–66
Han F, Yan S, Shi Y (2013) Single-prolonged stress induces endoplasmic reticulum-dependent apoptosis in the hippocampus in a rat model of post-traumatic stress disorder. PLoS One 8:e69340
Harvey BH, Brand L, Jeeva Z, Stein DJ (2006) Cortical/hippocampal monoamines, HPA-axis changes and aversive behavior following stress and restress in an animal model of post-traumatic stress disorder. Physiol Behav 87:881–890
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM et al (2010) Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468:270–276
Herry C, Johansen JP (2014) Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci 17:1644–1654
Imanaka A, Morinobu S, Toki S, Yamawaki S (2006) Importance of early environment in the development of post-traumatic stress disorder-like behaviors. Behav Brain Res 173:129–137
Johnson LR, McGuire J, Lazarus R, Palmer AA (2012) Pavlovian fear memory circuits and phenotype models of PTSD. Neuropharmacology 62:638–646
Jovanovic T, Kazama A, Bachevalier J, Davis M (2012) Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62:695–704
Kedia S, Chattarji S (2014) Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J Neurosci Methods 233:150–154
Keeley RJ, Bye C, Trow J, McDonald RJ (2015) Strain and sex differences in brain and behaviour of adult rats: learning and memory, anxiety and volumetric estimates. Behav Brain Res 288:118–131
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995) Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52:1048–1060
Keum S, Park J, Kim A, Park J, Kim KK, Jeong J, Shin HS (2016) Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav 15:231–242
Khan S, Liberzon I (2004) Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl) 172:225–229
Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I (2012) Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem 19:43–49
Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ (2013) The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp e4367
Kozlovsky N, Matar MA, Kaplan Z, Kotler M, Zohar J, Cohen H (2007) Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. Int J Neuropsychopharmacol 10:741–758
Krishnan V, Nestler EJ (2011) Animal models of depression: molecular perspectives. Curr Top Behav Neurosci 7:121–147
Lebow M, Neufeld-Cohen A, Kuperman Y, Tsoory M, Gil S, Chen A (2012) Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. J Neurosci 32:6906–6916
Lehner M, Wislowska-Stanek A, Maciejak P, Szyndler J, Sobolewska A, Krzascik P, Plaznik A (2010) The relationship between pain sensitivity and conditioned fear response in rats. Acta Neurobiol Exp (Wars) 70:56–66
Lin CC, Tung CS, Liu YP (2016) Escitalopram reversed the traumatic stress-induced depressed and anxiety-like symptoms but not the deficits of fear memory. Psychopharmacology (Berl) 233(7):1135–1146
Liu D, Xiao B, Han F, Wang E, Shi Y (2012a) Single-prolonged stress induces apoptosis in dorsal raphe nucleus in the rat model of posttraumatic stress disorder. BMC Psychiatry 12:211
Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S (2012b) Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484:381–385
Louvart H, Maccari S, Ducrocq F, Thomas P, Darnaudery M (2005) Long-term behavioural alterations in female rats after a single intense footshock followed by situational reminders. Psychoneuroendocrinology 30:316–324
Mahan AL, Ressler KJ (2012) Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 35:24–35
Mar AC, Robbins TW (2007) Delay discounting and impulsive choice in the rat. Curr Protoc Neurosci Chapter 8, Unit 8 22
Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C (2008) Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci 28:9030–9036
Matar MA, Cohen H, Kaplan Z, Zohar J (2006) The effect of early poststressor intervention with sertraline on behavioral responses in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 31:2610–2618
Matar MA, Zohar J, Cohen H (2013) Translationally relevant modeling of PTSD in rodents. Cell Tissue Res 354:127–139
McCormick CM, Green MR (2013) From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience 249:242–257
Meerlo P, Easton A, Bergmann BM, Turek FW (2001) Restraint increases prolactin and REM sleep in C57BL/6 J mice but not in BALB/cJ mice. Am J Physiol Regul Integr Comp Physiol 281:R846–R854
Mesches MH, Fleshner M, Heman KL, Rose GM, Diamond DM (1999) Exposing rats to a predator blocks primed burst potentiation in the hippocampus in vitro. J Neurosci 19:RC18
Mikics E, Baranyi J, Haller J (2008a) Rats exposed to traumatic stress bury unfamiliar objects—a novel measure of hyper-vigilance in PTSD models? Physiol Behav 94:341–348
Mikics E, Toth M, Varju P, Gereben B, Liposits Z, Ashaber M, Halasz J, Barna I, Farkas I, Haller J (2008b) Lasting changes in social behavior and amygdala function following traumatic experience induced by a single series of foot-shocks. Psychoneuroendocrinology 33:1198–1210
Milad MR, Quirk GJ (2012) Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 63:129–151
Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL (2009) Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66:1075–1082
Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S (2005) Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A 102:9371–9376
Mitra R, Adamec R, Sapolsky R (2009) Resilience against predator stress and dendritic morphology of amygdala neurons. Behav Brain Res 205:535–543
Moore NL, Gauchan S, Genovese RF (2012) Differential severity of anxiogenic effects resulting from a brief swim or underwater trauma in adolescent male rats. Pharmacol Biochem Behav 102:264–268
Moser MB, Moser EI (1998) Distributed encoding and retrieval of spatial memory in the hippocampus. J Neurosci 18:7535–7542
Muhie S, Gautam A, Meyerhoff J, Chakraborty N, Hammamieh R, Jett M (2015) Brain transcriptome profiles in mouse model simulating features of post-traumatic stress disorder. Mol Brain 8:14
Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ (2011) Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry 69:556–563
O'Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J (2015) A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res 232:1–33
Olson VG, Rockett HR, Reh RK, Redila VA, Tran PM, Venkov HA, Defino MC, Hague C, Peskind ER, Szot P, Raskind MA (2011) The role of norepinephrine in differential response to stress in an animal model of posttraumatic stress disorder. Biol Psychiatry 70:441–448
Parsons RG, Ressler KJ (2013) Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci 16:146–153
Pawlyk AC, Morrison AR, Ross RJ, Brennan FX (2008) Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev 32:99–117
Perrine SA, Eagle AL, George SA, Mulo K, Kohler RJ, Gerard J, Harutyunyan A, Hool SM, Susick LL, Schneider BL et al (2016) Severe, multimodal stress exposure induces PTSD-like characteristics in a mouse model of single prolonged stress. Behav Brain Res 303:228–237
Philbert J, Pichat P, Beeske S, Decobert M, Belzung C, Griebel G (2011) Acute inescapable stress exposure induces long-term sleep disturbances and avoidance behavior: a mouse model of post-traumatic stress disorder (PTSD). Behav Brain Res 221:149–154
Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106:274–285
Pulliam JV, Dawaghreh AM, Alema-Mensah E, Plotsky PM (2010) Social defeat stress produces prolonged alterations in acoustic startle and body weight gain in male Long Evans rats. J Psychiatr Res 44:106–111
Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I (1996) A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry 39:129–134
Quirk GJ, Pare D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A (2010) Erasing fear memories with extinction training. J Neurosci 30:14993–14997
Richter-Levin G (1998) Acute and long-term behavioral correlates of underwater trauma—potential relevance to stress and post-stress syndromes. Psychiatry Res 79:73–83
Risbrough VB, Glenn DE, Baker DG (2016) On the road to translation for PTSD treatment: theoretical and practical considerations of the use of human models of conditioned fear for drug development. Curr Top Behav Neurosci 28:173–196
Ritov G, Boltyansky B, Richter-Levin G (2016) A novel approach to PTSD modeling in rats reveals alternating patterns of limbic activity in different types of stress reaction. Mol Psychiatry 21(5):630–641. doi:10.1038/mp.2015.169
Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM (2006) Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol Learn Mem 86:249–255
Rozeske RR, Valerio S, Chaudun F, Herry C (2015) Prefrontal neuronal circuits of contextual fear conditioning. Genes Brain Behav 14:22–36
Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E (2006) Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res 174:188–192
Sandi C, Woodson JC, Haynes VF, Park CR, Touyarot K, Lopez-Fernandez MA, Venero C, Diamond DM (2005) Acute stress-induced impairment of spatial memory is associated with decreased expression of neural cell adhesion molecule in the hippocampus and prefrontal cortex. Biol Psychiatry 57:856–864
Sanford LD, Yang L, Wellman LL, Liu X, Tang X (2010) Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep 33:621–630
Sareen J (2014) Posttraumatic stress disorder in adults: impact, comorbidity, risk factors, and treatment. Can J Psychiatry 59:460–467
Schaap MW, van Oostrom H, Doornenbal A, van’t Klooster J, Baars AM, Arndt SS, Hellebrekers LJ (2013) Nociception and conditioned fear in rats: strains matter. PLoS One 8:e83339
Sharma S, Rakoczy S, Brown-Borg H (2010) Assessment of spatial memory in mice. Life Sci 87:521–536
Shoji H, Takao K, Hattori S, Miyakawa T (2014) Contextual and cued fear conditioning test using a video analyzing system in mice. J Vis Exp (85). doi:10.3791/50871
Siegmund A, Wotjak CT (2006) Toward an animal model of posttraumatic stress disorder. Ann N Y Acad Sci 1071:324–334
Siegmund A, Wotjak CT (2007a) A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res 41:848–860
Siegmund A, Wotjak CT (2007b) Hyperarousal does not depend on trauma-related contextual memory in an animal model of posttraumatic stress disorder. Physiol Behav 90:103–107
Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ (2015) Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther 149:150–190
Steckler T, Risbrough V (2012) Pharmacological treatment of PTSD—established and new approaches. Neuropharmacology 62:617–627
Stein MB, Kline NA, Matloff JL (2002) Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 159:1777–1779
Stiedl O, Radulovic J, Lohmann R, Birkenfeld K, Palve M, Kammermeier J, Sananbenesi F, Spiess J (1999) Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav Brain Res 104:1–12
Takahashi LK (2014) Olfactory systems and neural circuits that modulate predator odor fear. Front Behav Neurosci 8:72
Takahashi T, Morinobu S, Iwamoto Y, Yamawaki S (2006) Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology (Berl) 189:165–173
Toth M, Flandreau EI, Deslauriers J, Geyer MA, Mansuy IM, Merlo Pich E, Risbrough VB (2016) Overexpression of forebrain CRH during early life increases trauma susceptibility in adulthood. Neuropsychopharmacology 41:1681–1690
Tulogdi A, Soros P, Toth M, Nagy R, Biro L, Aliczki M, Klausz B, Mikics E, Haller J (2012) Temporal changes in c-Fos activation patterns induced by conditioned fear. Brain Res Bull 88:359–370
Vanderheyden WM, George SA, Urpa L, Kehoe M, Liberzon I, Poe GR (2015) Sleep alterations following exposure to stress predict fear-associated memory impairments in a rodent model of PTSD. Exp Brain Res 233:2335–2346
VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM (2014) From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem 113:3–18
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Vouimba RM, Munoz C, Diamond DM (2006) Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress 9:29–40
Wang W, Liu Y, Zheng H, Wang HN, Jin X, Chen YC, Zheng LN, Luo XX, Tan QR (2008) A modified single-prolonged stress model for post-traumatic stress disorder. Neurosci Lett 441:237–241
Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolanos-Guzman CA (2013) Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry 73:7–14
Woodson JC, Macintosh D, Fleshner M, Diamond DM (2003) Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn Mem 10:326–336
Wu Z, Tian Q, Li F, Gao J, Liu Y, Mao M, Liu J, Wang S, Li G, Ge D et al (2016) Behavioral changes over time in post-traumatic stress disorder: insights from a rat model of single prolonged stress. Behav Processes 124:123–129
Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I (2009) Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety 26:1110–1117
Yehuda R, Antelman SM (1993) Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry 33:479–486
Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW (1993) Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry 150:83–86
Yehuda R, Yang RK, Buchsbaum MS, Golier JA (2006) Alterations in cortisol negative feedback inhibition as examined using the ACTH response to cortisol administration in PTSD. Psychoneuroendocrinology 31:447–451
Zoladz PR, Conrad CD, Fleshner M, Diamond DM (2008) Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress 11:259–281
Zoladz PR, Fleshner M, Diamond DM (2012) Psychosocial animal model of PTSD produces a long-lasting traumatic memory, an increase in general anxiety and PTSD-like glucocorticoid abnormalities. Psychoneuroendocrinology 37:1531–1545
Zoladz PR, Fleshner M, Diamond DM (2013) Differential effectiveness of tianeptine, clonidine and amitriptyline in blocking traumatic memory expression, anxiety and hypertension in an animal model of PTSD. Prog Neuropsychopharmacol Biol Psychiatry 44:1–16
Zoladz PR, Park CR, Fleshner M, Diamond DM (2015) Psychosocial predator-based animal model of PTSD produces physiological and behavioral sequelae and a traumatic memory four months following stress onset. Physiol Behav 147:183–192
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Flandreau, E.I., Toth, M. (2017). Animal Models of PTSD: A Critical Review. In: Vermetten, E., Baker, D.G., Risbrough, V.B. (eds) Behavioral Neurobiology of PTSD. Current Topics in Behavioral Neurosciences, vol 38. Springer, Cham. https://doi.org/10.1007/7854_2016_65
Download citation
DOI: https://doi.org/10.1007/7854_2016_65
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94823-2
Online ISBN: 978-3-319-94824-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)